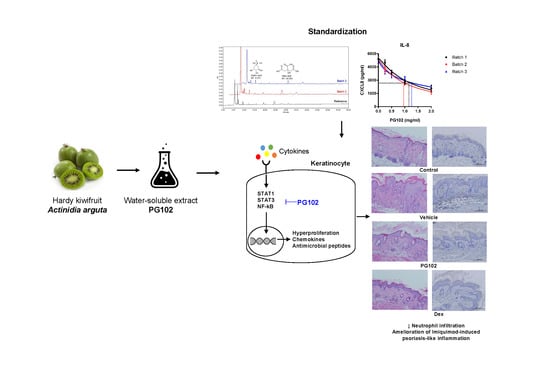
A Water-Soluble Extract from Actinidia arguta Ameliorates Psoriasis-Like Skin Inflammation in Mice by Inhibition of Neutrophil Infiltration
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of PG102
2.2. Cell Culture and Reagents
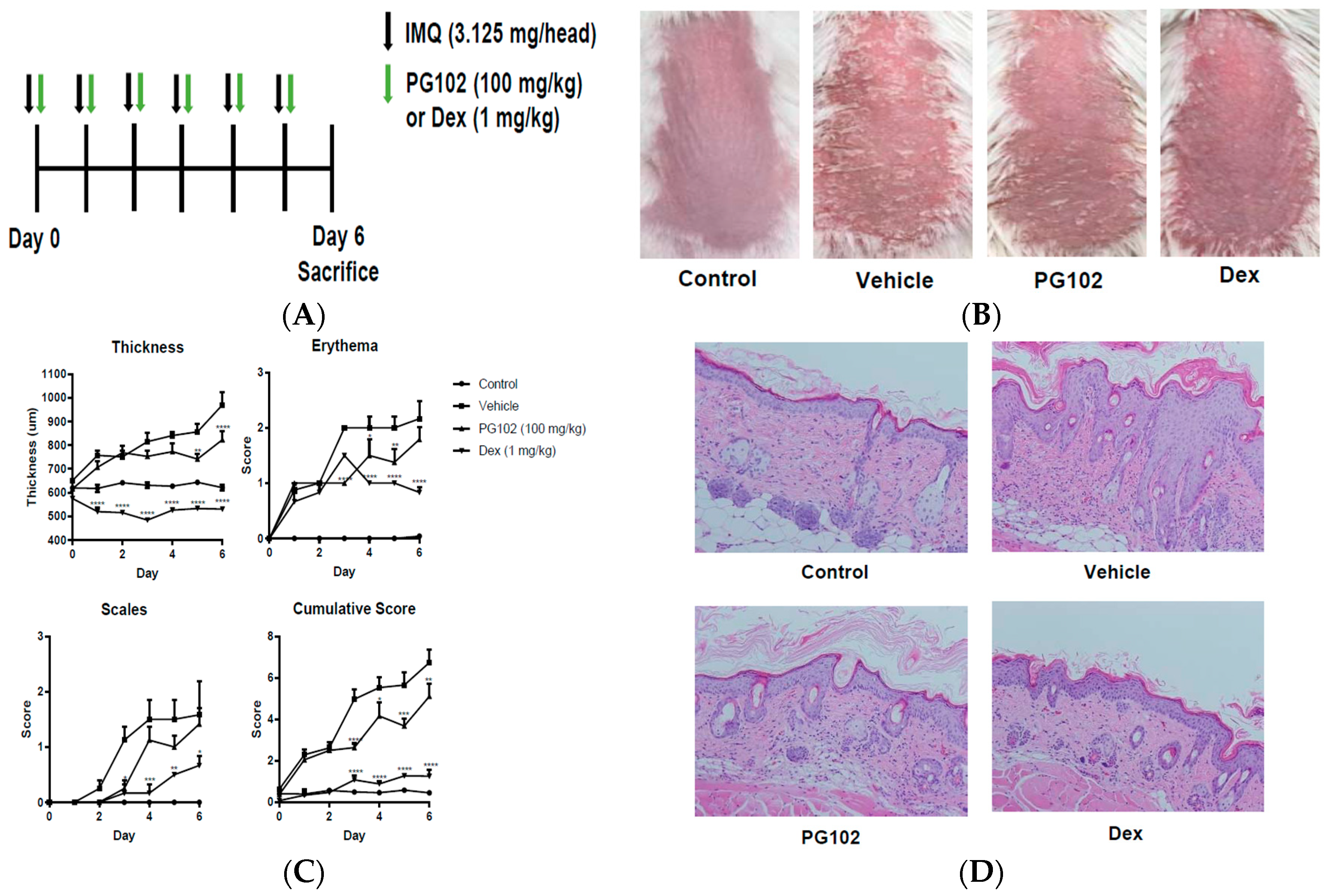
2.3. Mice and Treatment
2.4. Scoring Severity of Skin Inflammation
2.5. Cell Isolation and Stimulation
2.6. Tissue Preparation and Immunohistochemistry (IHC)
2.7. Measurement of Cell Proliferation
2.8. RNA Extraction and Quantitative Real-Time Polymerase Chain Reaction (RT-PCR)
2.9. Enzyme-Linked Immunosorbent Assay (ELISA)
2.10. Western Blot Analysis
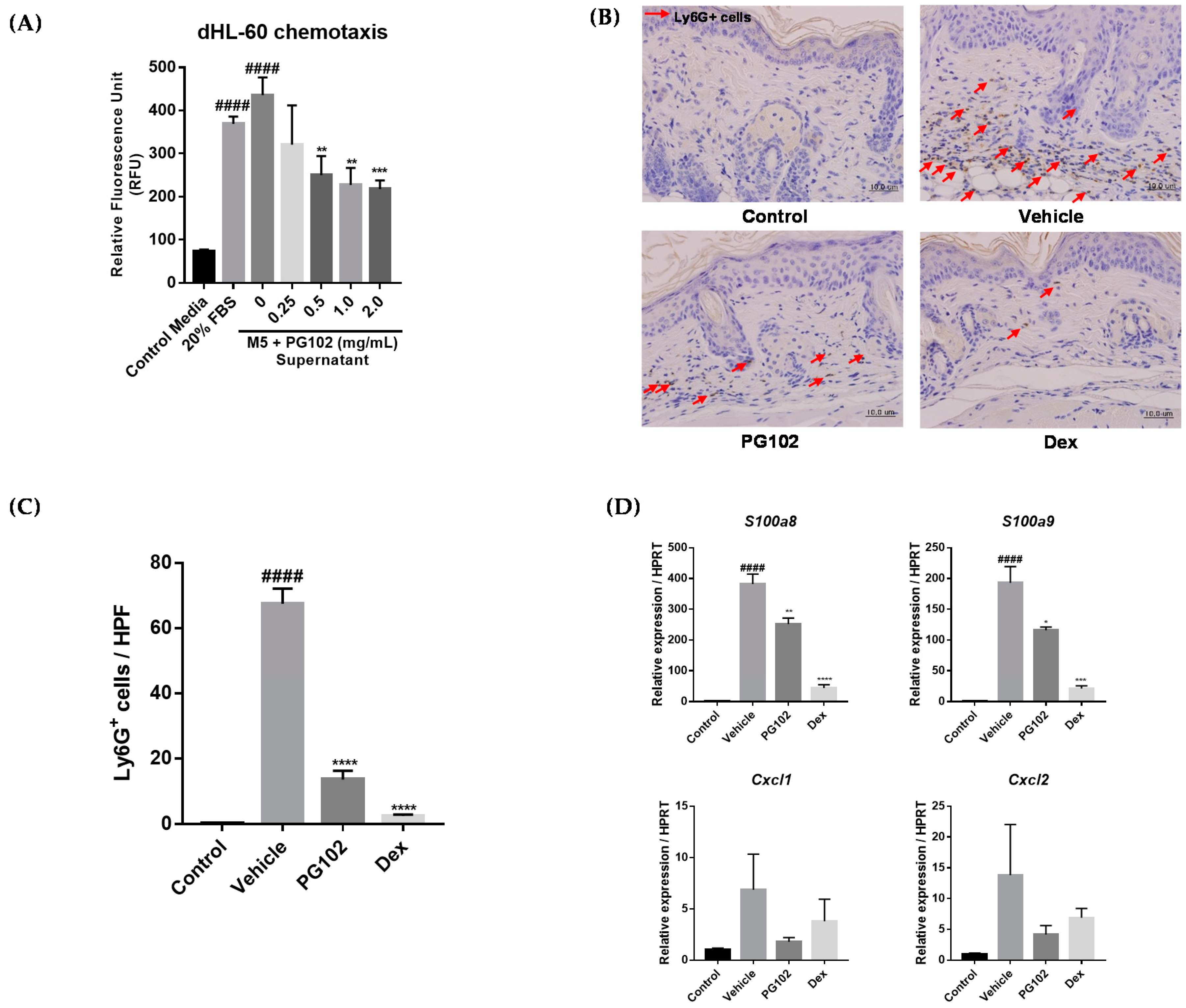
2.11. Neutrophil Chemotaxis Assay
2.12. Statistical Analysis
3. Results
3.1. Quality of PG102 is Standardized by Quantification of Marker Compounds and Cell-Based IL-8 Bioassay
3.2. Topical Application of PG102 Ameliorates Clinical Symptoms of IMQ-Induced Psoriasis-Like Skin Inflammation
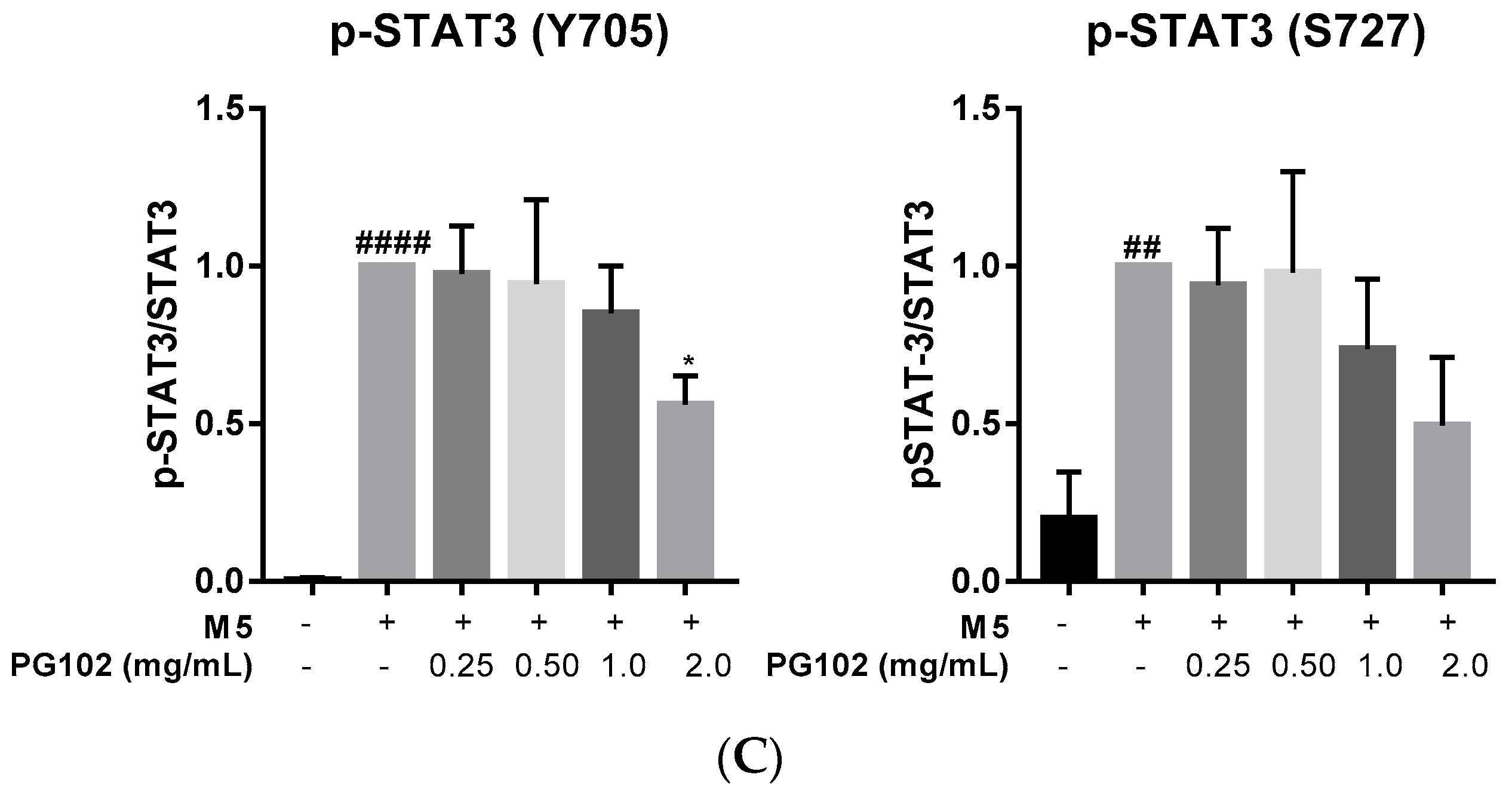
3.3. PG102 Inhibits Hyperproliferation of Hacat Cells and Phosphorylation of STAT3
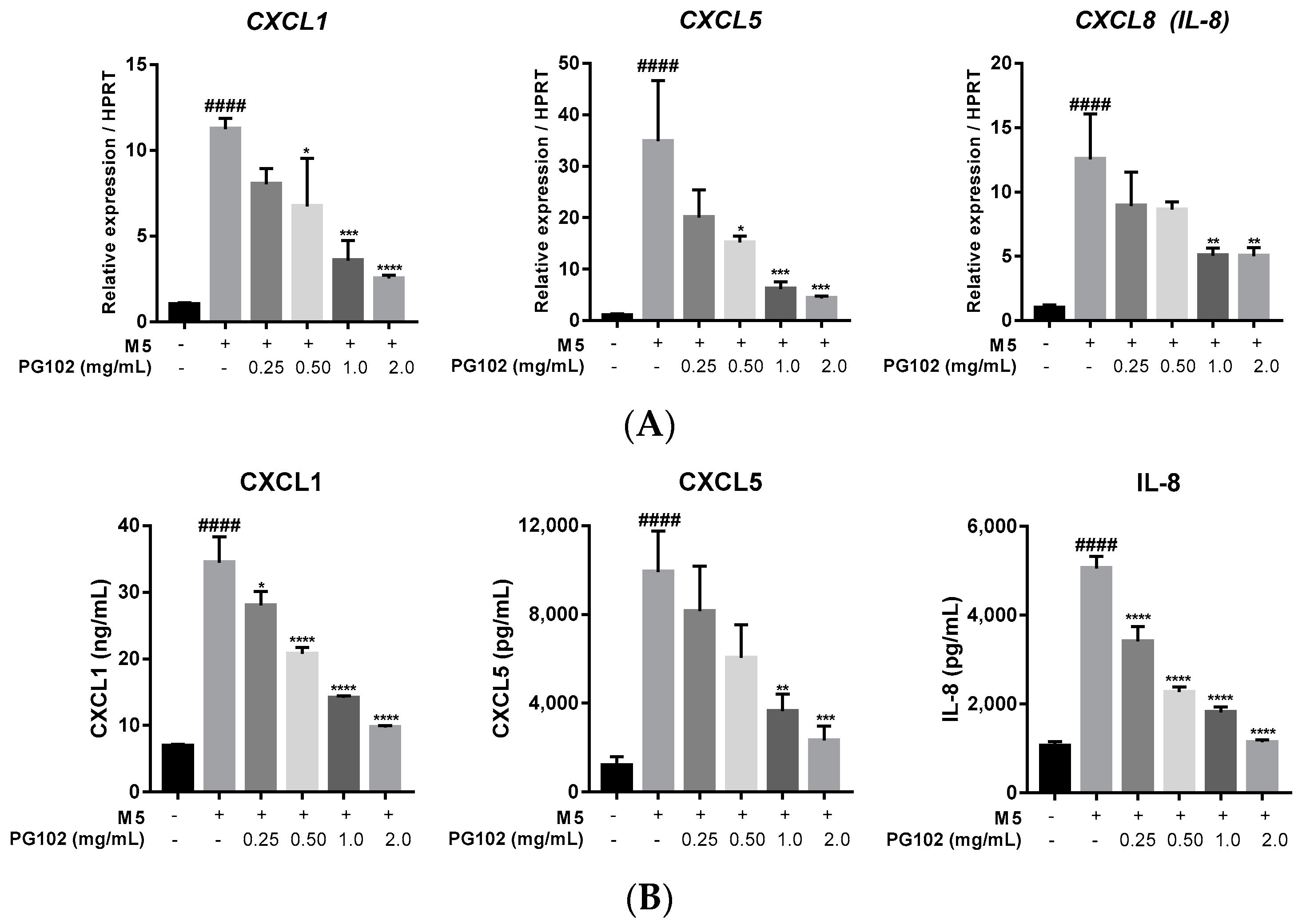
3.4. PG102 Downregulates Expression of Chemokines in HaCaT Cells
3.5. PG102 Significantly Suppresses Expression of Antimicrobial Peptides in HaCaT Cells
3.6. PG102 Exerts Anti-Inflammatory Effects through Inhibition of STAT and NF-κB Signaling
3.7. PG102 Suppresses Neutrophil Chemotaxis in vitro and Neutrophil Infiltration to Skin in IMQ-Treated Mice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Greb, J.E.; Goldminz, A.M.; Elder, J.T.; Lebwohl, M.G.; Gladman, D.D.; Wu, J.J.; Mehta, N.N.; Finlay, A.Y.; Gottlieb, A.B. Psoriasis. Nat. Rev. Dis. Primers 2016, 2, 16082. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boehncke, W.H.; Schon, M.P. Psoriasis. Lancet 2015, 386, 983–994. [Google Scholar] [CrossRef]
- Gottlieb, A.B.; Chao, C.; Dann, F. Psoriasis comorbidities. J. Dermatol. Treat. 2008, 19, 5–21. [Google Scholar] [CrossRef] [PubMed]
- De Korte, J.; Sprangers, M.A.; Mombers, F.M.; Bos, J.D. Quality of life in patients with psoriasis: A systematic literature review. J. Investig. Dermatol. Symp. Proc. 2004, 9, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, C.E.M.; van der Walt, J.M.; Ashcroft, D.M.; Flohr, C.; Naldi, L.; Nijsten, T.; Augustin, M. The global state of psoriasis disease epidemiology: A workshop report. Br. J. Dermatol. 2017, 177, e4–e7. [Google Scholar] [CrossRef] [PubMed]
- Lande, R.; Gregorio, J.; Facchinetti, V.; Chatterjee, B.; Wang, Y.H.; Homey, B.; Cao, W.; Wang, Y.H.; Su, B.; Nestle, F.O.; et al. Plasmacytoid dendritic cells sense self-DNA coupled with antimicrobial peptide. Nature 2007, 449, 564–569. [Google Scholar] [CrossRef] [PubMed]
- Fitch, E.; Harper, E.; Skorcheva, I.; Kurtz, S.E.; Blauvelt, A. Pathophysiology of psoriasis: Recent advances on IL-23 and Th17 cytokines. Curr. Rheumatol. Rep. 2007, 9, 461–467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rohrl, J.; Yang, D.; Oppenheim, J.J.; Hehlgans, T. Human beta-defensin 2 and 3 and their mouse orthologs induce chemotaxis through interaction with CCR2. J. Immunol. 2010, 184, 6688–6694. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Jang, S.; Min, J.K.; Lee, K.; Sohn, K.C.; Lim, J.S.; Im, M.; Lee, H.E.; Seo, Y.J.; Kim, C.D.; et al. S100a8 and s100a9 are messengers in the crosstalk between epidermis and dermis modulating a psoriatic milieu in human skin. Biochem. Biophys. Res. Commun. 2012, 423, 647–653. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.C.-S.; Yu, H.S.; Yen, F.L.; Lin, C.L.; Chen, G.-S.; Lan, C.-C.E. Neutrophil extracellular trap formation is increased in psoriasis and induces human beta-defensin-2 production in epidermal keratinocytes. Sci. Rep. 2016, 6, 31119. [Google Scholar] [CrossRef] [PubMed]
- Uva, L.; Miguel, D.; Pinheiro, C.; Antunes, J.; Cruz, D.; Ferreira, J.; Filipe, P. Mechanisms of action of topical corticosteroids in psoriasis. Int. J. Endocrinol. 2012, 2012, 561018. [Google Scholar] [CrossRef] [PubMed]
- Carrascosa, J.M.; van Doorn, M.B.; Lahfa, M.; Nestle, F.O.; Jullien, D.; Prinz, J.C. Clinical relevance of immunogenicity of biologics in psoriasis: Implications for treatment strategies. J. Eur. Acad. Dermatol. Venereol. 2014, 28, 1424–1430. [Google Scholar] [CrossRef] [PubMed]
- Matich, A.J.; Young, H.; Allen, J.M.; Wang, M.Y.; Fielder, S.; McNeilage, M.A.; MacRae, E.A. Actinidia arguta: Volatile compounds in fruit and flowers. Phytochemistry 2003, 63, 285–301. [Google Scholar] [CrossRef]
- Leontowicz, H.; Leontowicz, M.; Latocha, P.; Jesion, I.; Park, Y.S.; Katrich, E.; Barasch, D.; Nemirovski, A.; Gorinstein, S. Bioactivity and nutritional properties of hardy kiwi fruit actinidia arguta in comparison with actinidia deliciosa ‘hayward’ and actinidia eriantha ‘bidan’. Food Chem. 2016, 196, 281–291. [Google Scholar] [CrossRef] [PubMed]
- Park, E.J.; Kim, B.; Eo, H.; Park, K.; Kim, Y.; Lee, H.J.; Son, M.; Chang, Y.S.; Cho, S.H.; Kim, S.; et al. Control of igE and selective T(H)1 and T(H)2 cytokines by PG102 isolated from actinidia arguta. J. Allergy Clin. Immunol. 2005, 116, 1151–1157. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Kim, S.H.; Park, E.J.; Kang, C.Y.; Cho, S.H.; Kim, S. Anti-allergic effects of PG102, a water-soluble extract prepared from actinidia arguta, in a murine ovalbumin-induced asthma model. Clin. Exp. Allergy 2009, 39, 280–289. [Google Scholar] [CrossRef] [PubMed]
- Bae, M.J.; Lim, S.; Lee, D.S.; Ko, K.R.; Lee, W.; Kim, S. Water soluble extracts from actinidia arguta, PG102, attenuates house dust mite-induced murine atopic dermatitis by inhibiting the mTOR pathway with Treg generation. J. Ethnopharmacol. 2016, 193, 96–106. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Kim, S.; Lee, S.H.; Park, H.W.; Chang, Y.S.; Min, K.U.; Cho, S.H. The effects of PG102, a water-soluble extract from actinidia arguta, on serum total ige levels: A double-blind, randomized, placebo-controlled exploratory clinical study. Eur. J. Nutr. 2011, 50, 523–529. [Google Scholar] [CrossRef] [PubMed]
- Link, A.; Vogt, T.K.; Favre, S.; Britschgi, M.R.; Acha-Orbea, H.; Hinz, B.; Cyster, J.G.; Luther, S.A. Fibroblastic reticular cells in lymph nodes regulate the homeostasis of naive t cells. Nat. Immunol. 2007, 8, 1255–1265. [Google Scholar] [CrossRef] [PubMed]
- Millius, A.; Weiner, O.D. Chemotaxis in neutrophil-like hl-60 cells. Methods Mol. Biol. 2009, 571, 167–177. [Google Scholar] [PubMed]
- Gillitzer, R.; Berger, R.; Mielke, V.; Muller, C.; Wolff, K.; Stingl, G. Upper keratinocytes of psoriatic skin lesions express high levels of NAP-1/IL-8 mrna in situ. J. Investig. Dermatol. 1991, 97, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Guilloteau, K.; Paris, I.; Pedretti, N.; Boniface, K.; Juchaux, F.; Huguier, V.; Guillet, G.; Bernard, F.X.; Lecron, J.C.; Morel, F. Skin inflammation induced by the synergistic action of IL-17a, IL-22, oncostatin M, IL-1{alpha}, and TNF-{alpha} recapitulates some features of psoriasis. J. Immunol. 2010, 184, 5263–5270. [Google Scholar] [CrossRef] [PubMed]
- Van der Fits, L.; Mourits, S.; Voerman, J.S.; Kant, M.; Boon, L.; Laman, J.D.; Cornelissen, F.; Mus, A.M.; Florencia, E.; Prens, E.P.; et al. Imiquimod-induced psoriasis-like skin inflammation in mice is mediated via the IL-23/IL-17 Axis. J. Immunol. 2009, 182, 5836–5845. [Google Scholar] [CrossRef] [PubMed]
- Orecchia, V.; Regis, G.; Tassone, B.; Valenti, C.; Avalle, L.; Saoncella, S.; Calautti, E.; Poli, V. Constitutive stat3 activation in epidermal keratinocytes enhances cell clonogenicity and favours spontaneous immortalization by opposing differentiation and senescence checkpoints. Exp. Dermatol. 2015, 24, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Ha, H.; Debnath, B.; Neamati, N. Role of the CXCL8-CXCR1/2 axis in cancer and inflammatory diseases. Theranostics 2017, 7, 1543–1588. [Google Scholar] [CrossRef] [PubMed]
- Schonthaler, H.B.; Guinea-Viniegra, J.; Wculek, S.K.; Ruppen, I.; Ximenez-Embun, P.; Guio-Carrion, A.; Navarro, R.; Hogg, N.; Ashman, K.; Wagner, E.F. S100a8–S100a9 protein complex mediates psoriasis by regulating the expression of complement factor C3. Immunity 2013, 39, 1171–1181. [Google Scholar] [CrossRef] [PubMed]
- Wehkamp, J.; Harder, J.; Wehkamp, K.; Wehkamp-von Meissner, B.; Schlee, M.; Enders, C.; Sonnenborn, U.; Nuding, S.; Bengmark, S.; Fellermann, K.; et al. Nf-kappab- and AP-1-mediated induction of human beta defensin-2 in intestinal epithelial cells by escherichia coli nissle 1917: A novel effect of a probiotic bacterium. Infect. Immun. 2004, 72, 5750–5758. [Google Scholar] [CrossRef] [PubMed]
- Hsu, K.; Chung, Y.M.; Endoh, Y.; Geczy, C.L. Tlr9 ligands induce S100a8 in macrophages via a stat3-dependent pathway which requires IL-10 and PGE2. PLoS ONE 2014, 9, e103629. [Google Scholar] [CrossRef] [PubMed]
- Park, E.J.; Park, K.C.; Eo, H.; Seo, J.; Son, M.; Kim, K.H.; Chang, Y.S.; Cho, S.H.; Min, K.U.; Jin, M.; et al. Suppression of spontaneous dermatitis in NC/Nga murine model by PG102 isolated from actinidia arguta. J. Investig. Dermatol. 2007, 127, 1154–1160. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Choi, J.; Kim, M.J.; Kim, S.H.; Cho, S.H.; Kim, S. Reconstitution of anti-allergic activities of PG102 derived from actinidia arguta by combining synthetic chemical compounds. Exp. Biol. Med. 2013, 238, 631–640. [Google Scholar] [CrossRef] [PubMed]
- Wolf, R.; Orion, E.; Ruocco, E.; Ruocco, V. Abnormal epidermal barrier in the pathogenesis of psoriasis. Clin. Dermatol. 2012, 30, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.; Lin, Z.; Zhang, L.; Qin, X.; Zhang, Y.; Sun, L. Calcipotriol inhibits proliferation of human keratinocytes by downregulating stat1 and stat3 signaling. J. Investig. Med. 2017, 65, 376–381. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Rizo, V.; Martinez-Guzman, M.A.; Iniguez-Gutierrez, L.; Garcia-Orozco, A.; Alvarado-Navarro, A.; Fafutis-Morris, M. Neutrophil extracellular traps and its implications in inflammation: An overview. Front. Immunol. 2017, 8, 81. [Google Scholar] [CrossRef] [PubMed]
- Reich, K.; Papp, K.A.; Matheson, R.T.; Tu, J.H.; Bissonnette, R.; Bourcier, M.; Gratton, D.; Kunynetz, R.A.; Poulin, Y.; Rosoph, L.A.; et al. Evidence that a neutrophil-keratinocyte crosstalk is an early target of IL-17a inhibition in psoriasis. Exp. Dermatol. 2015, 24, 529–535. [Google Scholar] [CrossRef] [PubMed]
- Toichi, E.; Tachibana, T.; Furukawa, F. Rapid improvement of psoriasis vulgaris during drug-induced agranulocytosis. J. Am. Acad. Dermatol. 2000, 43, 391–395. [Google Scholar] [CrossRef] [PubMed]
- Keijsers, R.R.M.C.; Hendriks, A.G.M.; van Erp, P.E.J.; van Cranenbroek, B.; van de Kerkhof, P.C.M.; Koenen, H.J.P.M.; Joosten, I. In vivo induction of cutaneous inflammation results in the accumulation of extracellular trap-forming neutrophils expressing rorgammat and IL-17. J. Investig. Dermatol. 2014, 134, 1276–1284. [Google Scholar] [CrossRef] [PubMed]
- Richmond, A. Nf-kappa B, chemokine gene transcription and tumour growth. Nat. Rev. Immunol. 2002, 2, 664–674. [Google Scholar] [CrossRef] [PubMed]
- Albanesi, C.; Fairchild, H.R.; Madonna, S.; Scarponi, C.; De Pita, O.; Leung, D.Y.; Howell, M.D. IL-4 and IL-13 negatively regulate TNF-alpha- and IFN-gamma-induced beta-defensin expression through stat-6, suppressor of cytokine signaling (socs)-1, and socs-3. J. Immunol. 2007, 179, 984–992. [Google Scholar] [CrossRef] [PubMed]





| Target Gene | Primer Sequence (5′-3′) | |
|---|---|---|
| Forward | Reverse | |
| hBD-2 | 5′-GGTGTTTTTGGTGGTATAGGC-3′ | 5′-AGGGCAAAAGACTGGATGACA-3′ |
| hCXCL1 | 5′-AATCCTGCATCCCCCATA-3′ | 5′-TGTCTCTCTTTCCTCTTCTGTTCCT-3′ |
| hCXCL5 | 5′-TGCGTTGCGTTTGTTTACAG-3′ | 5′-GAAAAGGGGCTTCTGGATCA-3′ |
| hCXCL8 (IL-8) | 5′-ATGACTTCCAAGCTGGCCGTG-3′ | 5′-TTATGAATTCTCAGCCCTCTTCAAAAACTTCTC-3′ |
| hHPRT | 5′-TATGGCGACCCGCAGCCCT-3′ | 5′-CATCTCGAGCAAGACGTTCAG-3′ |
| hS100A8 | 5′-GGGAATTTCCATGCCGTCT-3′ | 5′-CCTTTTTCCTGATATACTGAGGAC-3′ |
| hS100A9 | 5′-CAGCTGGAACGCAACATAGA-3′ | 5′-TCAGCTGCTTGTCTGCATTT-3′ |
| mCXCL1 | 5′-CACCTCAAGAACATCCAGAGCT-3′ | 5′-CAAGCAGAACTGAACTACCATCG-3′ |
| mDEFB4 | 5′-GCTTCAGTCATGAGGATCCAT-3′ | 5′-CTTGCTGGTTCTTCGTCTTTT-3′ |
| mHPRT | 5′-CACAGGACTAGAACACCTGC-3′ | 5′-GCTGGTGAAAAGGACCTCT-3′ |
| mCXCL2 | 5′-TTGCCTTGACCCTGAAGCCCCC-3′ | 5′-GGCACATCAGGTACGATCCAGGC-3′ |
| mS100A8 | 5′-AAATCACCATGCCCTCTACAAG-3′ | 5′-CCCACTTTTATCACCATCGCAA-3′ |
| mS100A9 | 5′-ATACTCTAGGAAGGAAGGACACC-3′ | 5′-TCCATGATGTCATTTATGAGGGC-3′ |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.-k.; Bae, M.J.; Lim, S.; Lee, W.; Kim, S. A Water-Soluble Extract from Actinidia arguta Ameliorates Psoriasis-Like Skin Inflammation in Mice by Inhibition of Neutrophil Infiltration. Nutrients 2018, 10, 1399. https://doi.org/10.3390/nu10101399
Kim H-k, Bae MJ, Lim S, Lee W, Kim S. A Water-Soluble Extract from Actinidia arguta Ameliorates Psoriasis-Like Skin Inflammation in Mice by Inhibition of Neutrophil Infiltration. Nutrients. 2018; 10(10):1399. https://doi.org/10.3390/nu10101399
Chicago/Turabian StyleKim, Hyun-keun, Min Jung Bae, Seonung Lim, Wonwoo Lee, and Sunyoung Kim. 2018. "A Water-Soluble Extract from Actinidia arguta Ameliorates Psoriasis-Like Skin Inflammation in Mice by Inhibition of Neutrophil Infiltration" Nutrients 10, no. 10: 1399. https://doi.org/10.3390/nu10101399
APA StyleKim, H.-k., Bae, M. J., Lim, S., Lee, W., & Kim, S. (2018). A Water-Soluble Extract from Actinidia arguta Ameliorates Psoriasis-Like Skin Inflammation in Mice by Inhibition of Neutrophil Infiltration. Nutrients, 10(10), 1399. https://doi.org/10.3390/nu10101399