Effect of Branched-Chain Amino Acid Supplementation on Recovery Following Acute Eccentric Exercise
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
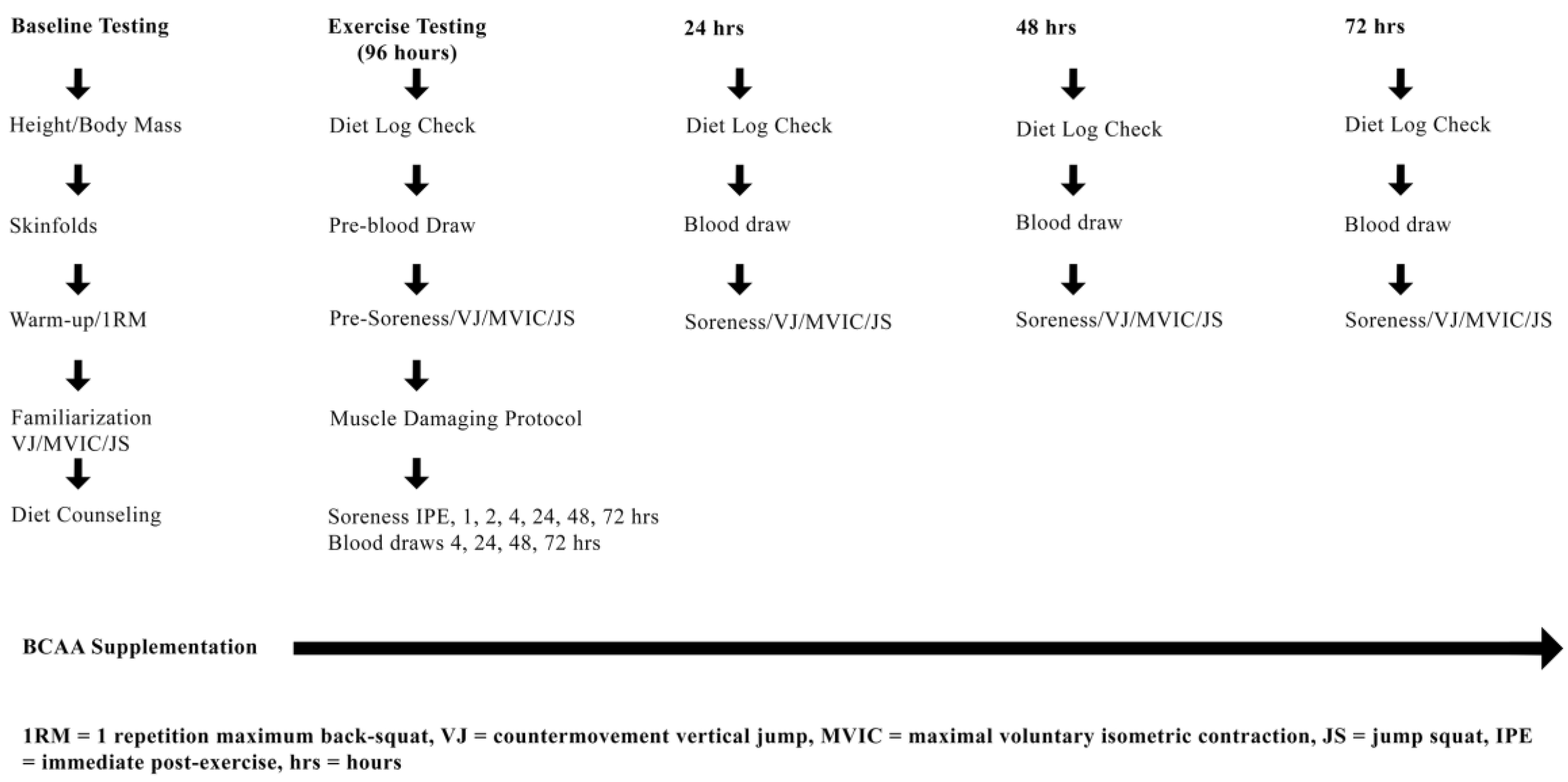
2.2. Experimental Design
2.3. Baseline Testing: Enrollment, 1RM, Familiarization and Dietary Counseling
2.4. Anthropometric Measurements
2.5. One-Repetition Maximum (1RM)
2.6. Supplementation, Diet and Diet Tracking
2.7. Experimental Protocol (Muscle Damaging Exercise Visit)
2.8. Muscle Damaging Protocol
2.9. Markers of Muscle Performance and Soreness
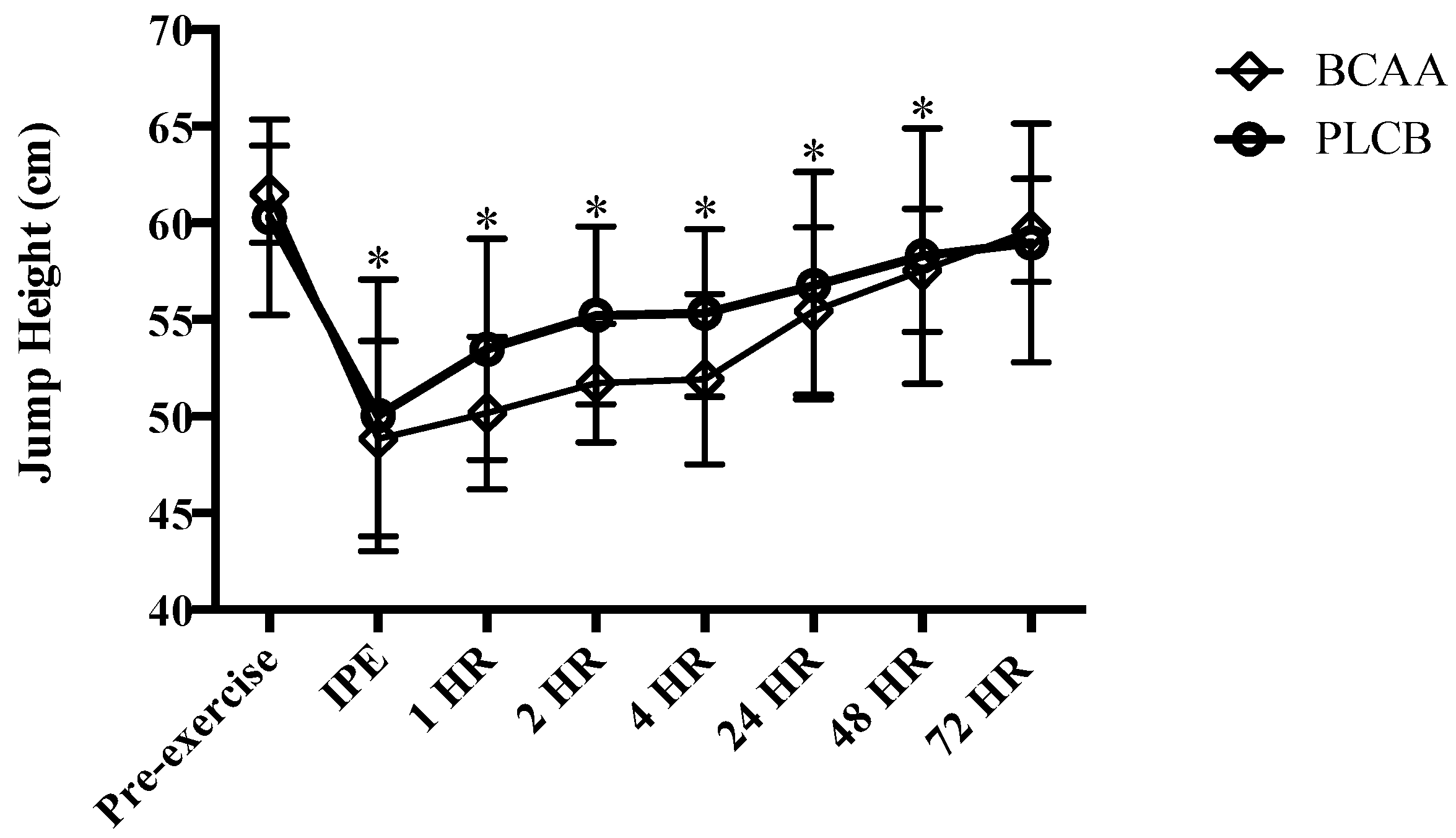
2.9.1. Vertical Jump
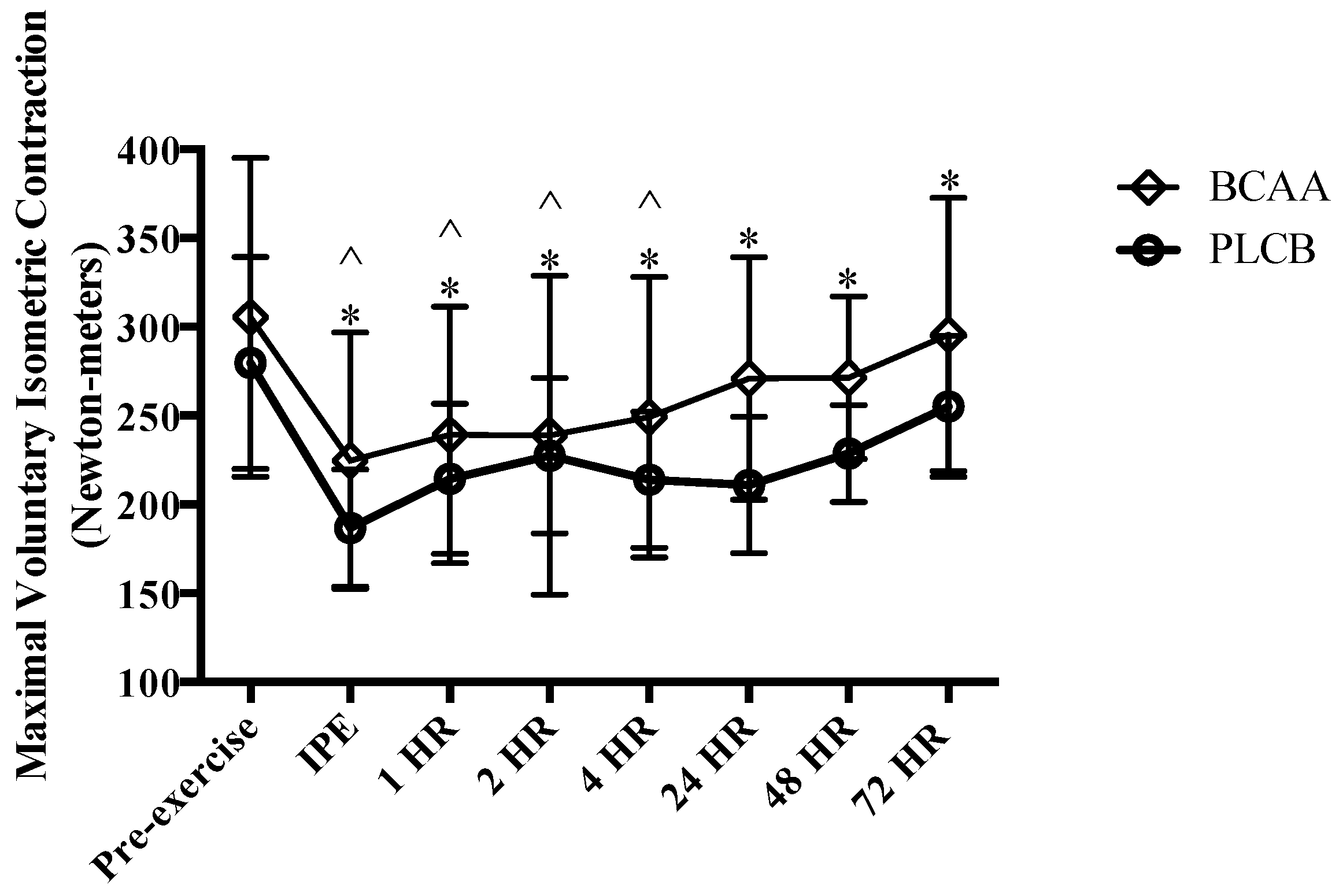
2.9.2. Maximal Voluntary Isometric Contraction
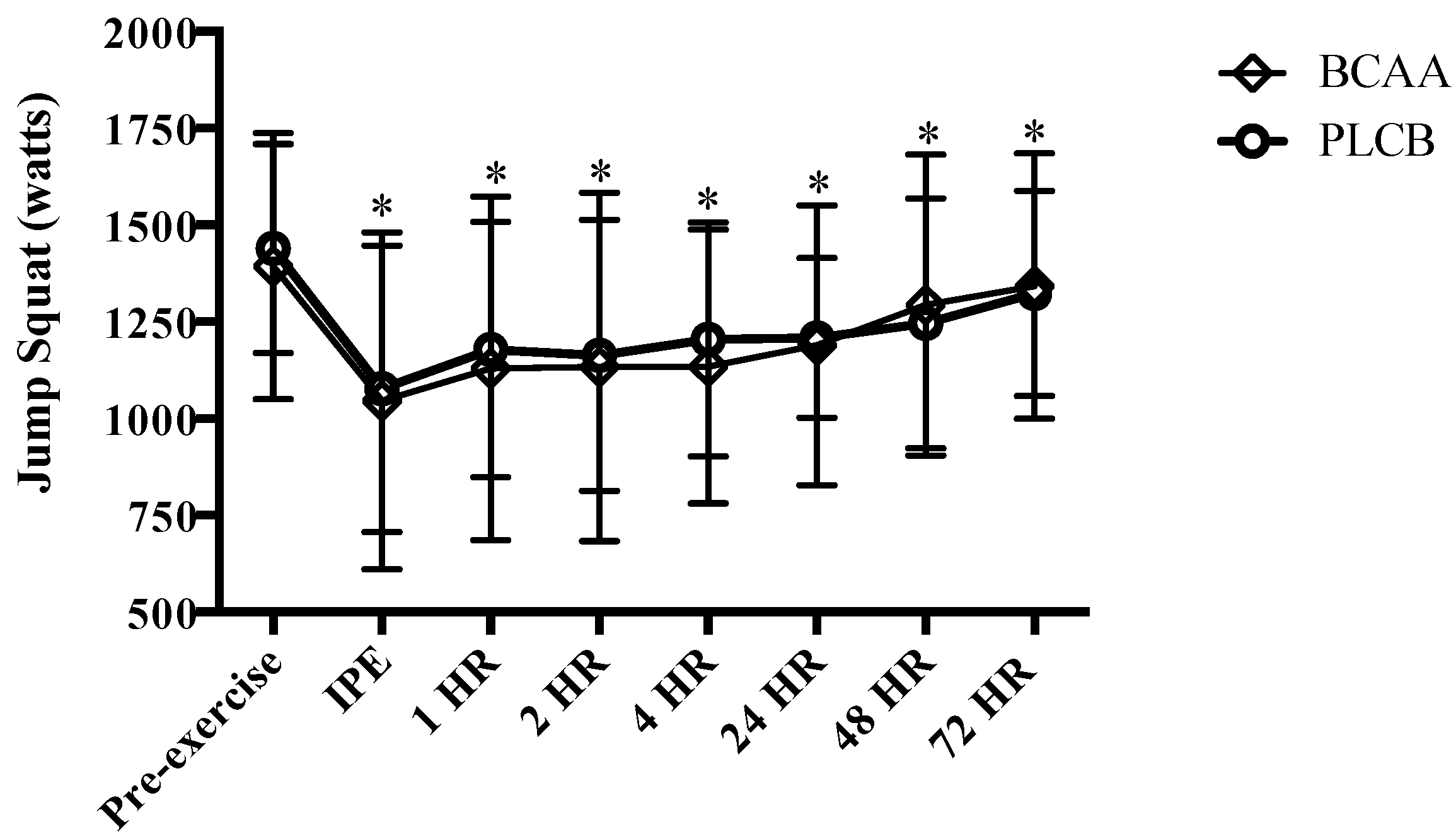
2.9.3. Jump Squat
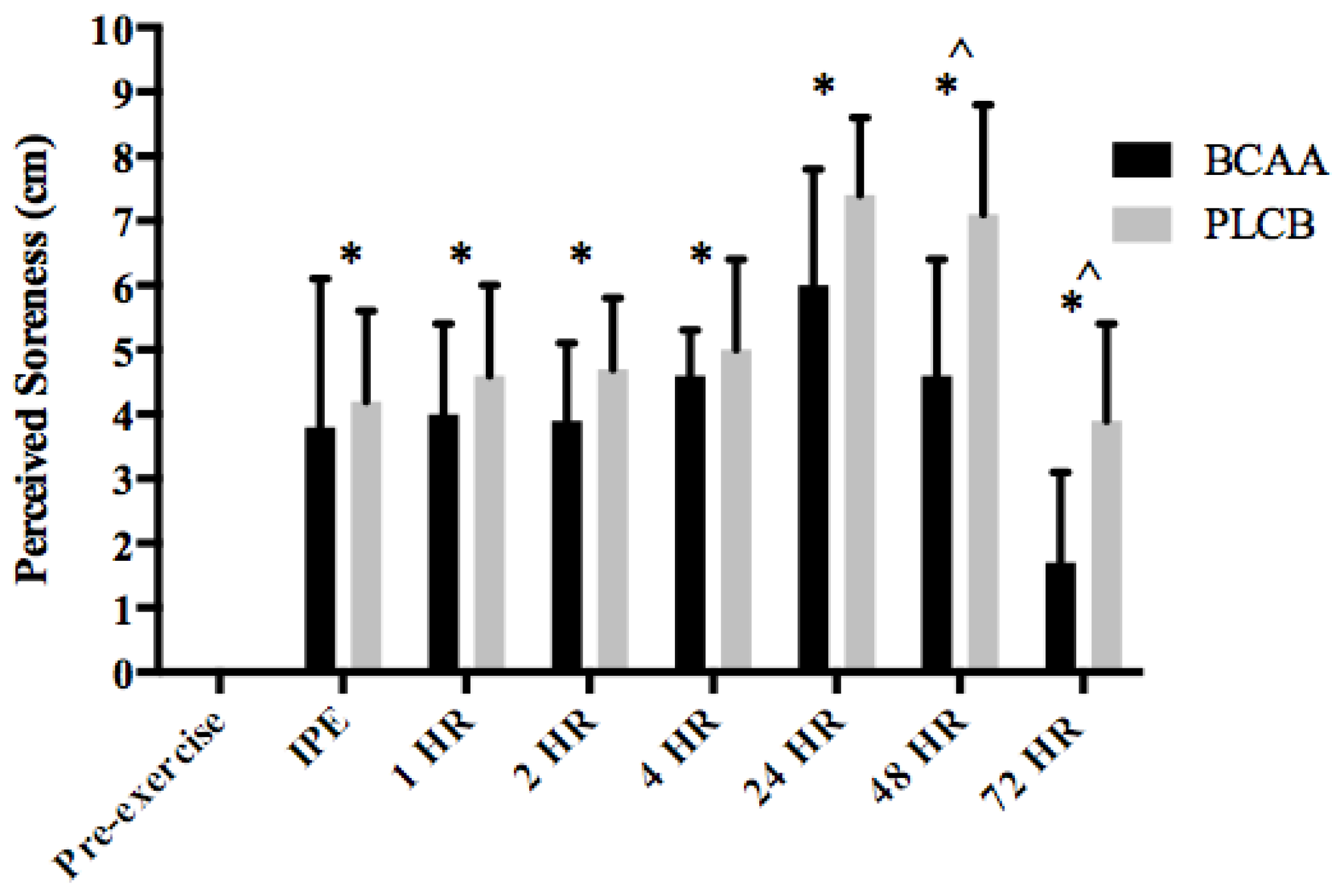
2.9.4. Perceived Soreness
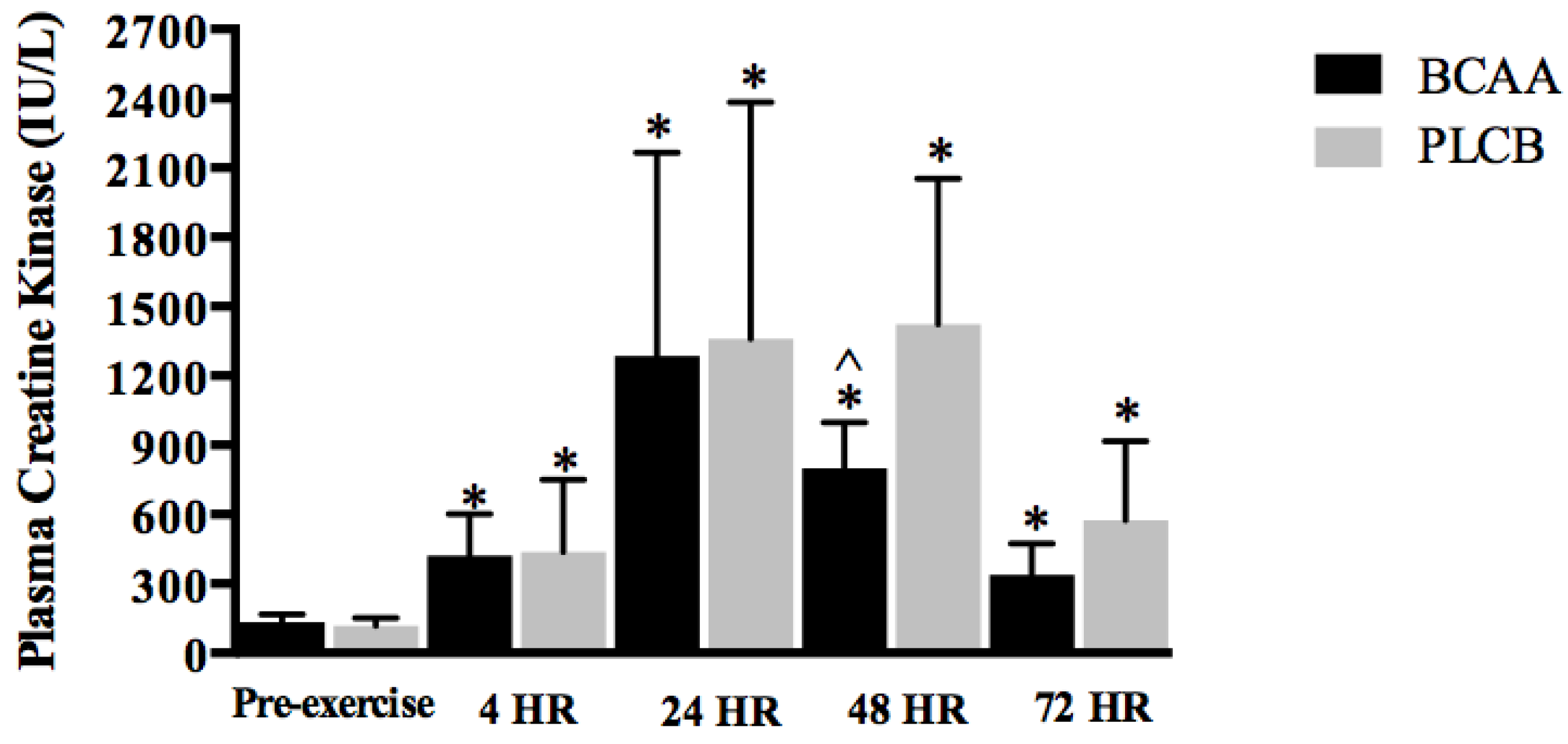
2.10. Blood Sampling and Analysis
2.11. Statistical Analysis
3. Results
3.1. Muscular Performance: Vertical Jump
3.2. Muscular Performance: Maximal Voluntary Isometric Contraction
3.3. Muscular Performance: Jump Squat
3.4. Muscle Soreness
3.5. Blood Parameter: Creatine Kinase
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Howatson, G.; van Someren, K.A. The prevention and treatment of exercise-induced muscle damage. Sports Med. 2008, 38, 483–503. [Google Scholar] [CrossRef] [PubMed]
- Da Luz, C.R.; Nicastro, H.; Zanchi, N.E.; Chaves, D.F.; Lancha, A.H. Potential therapeutic effects of branched-chain amino acids supplementation on resistance exercise-based muscle damage in humans. J. Int. Soc. Sports Nutr. 2011, 8, 23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kreider, R.B.; Wilborn, C.D.; Taylor, L.; Campbell, B.; Almada, A.L.; Collins, R.; Cooke, M.; Earnest, C.P.; Greenwood, M.; Kalman, D.S.; et al. ISSN exercise & sport nutrition review: Research & recommendations. J. Int. Soc. Sports Nutr. 2010, 7. [Google Scholar] [CrossRef]
- Pasiakos, S.M.; Lieberman, H.R.; McLellan, T.M. Effects of protein supplements on muscle damage, soreness and recovery of muscle function and physical performance: A systematic review. Sports Med. 2014, 44, 655–670. [Google Scholar] [CrossRef] [PubMed]
- Nosaka, K.; Sacco, P.; Mawatari, K. Effects of Amino Acid Supplementation on Muscle Soreness and Damage. Int. J. Sport Nutr. Exerc. Metab. 2006, 16, 620–635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harris, R.A.; Joshi, M.; Jeoung, N.H. Mechanisms responsible for regulation of branched-chain amino acid catabolism. Biochem. Biophys. Res. Commun. 2004, 313, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Shimomura, Y.; Murakami, T.; Nakai, N.; Nagasaki, M.; Harris, R.A. Exercise promotes BCAA catabolism: Effects of BCAA supplementation on skeletal muscle during exercise. J. Nutr. 2004, 134, 1583S–1587S. [Google Scholar] [CrossRef] [PubMed]
- Coombes, J.S.; McNaughton, L.R. Effects of branched-chain amino acid supplementation on serum creatine kinase and lactate dehydrogenase after prolonged exercise. J. Sports Med. Phys. Fitness 2000, 40, 240–246. [Google Scholar] [PubMed]
- Ra, S.-G.; Miyazaki, T.; Ishikura, K.; Nagayama, H.; Komine, S.; Nakata, Y.; Maeda, S.; Matsuzaki, Y.; Ohmori, H. Combined effect of branched-chain amino acids and taurine supplementation on delayed onset muscle soreness and muscle damage in high-intensity eccentric exercise. J. Int. Soc. Sports Nutr. 2013, 10, 51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Howatson, G.; Hoad, M.; Goodall, S.; Tallent, J.; Bell, P.G.; French, D.N. Exercise-induced muscle damage is reduced in resistance-trained males by branched chain amino acids: A randomized, double-blind, placebo controlled study. J. Int. Soc. Sports Nutr. 2012, 9, 20. [Google Scholar] [CrossRef] [PubMed]
- Sharp, C.P.; Pearson, D.R. Amino acid supplements and recovery from high-intensity resistance training. J. Strength Cond. Res. 2010, 24, 1125–1130. [Google Scholar] [CrossRef] [PubMed]
- Greer, B.K.; Woodard, J.L.; White, J.P.; Arguello, E.M.; Haymes, E.M. Branched-chain amino acid supplementation and indicators of muscle damage after endurance exercise. Int. J. Sport Nutr. Exerc. Metab. 2007, 17, 595–607. [Google Scholar] [CrossRef] [PubMed]
- Blomstrand, E. A role for branched-chain amino acids in reducing central fatigue. J. Nutr. 2006, 136, 544S–547S. [Google Scholar] [CrossRef] [PubMed]
- Newsholme, E.A.; Blomstrand, E. Branched-chain amino acids and central fatigue. J. Nutr. 2006, 136, 274S–276S. [Google Scholar] [CrossRef] [PubMed]
- Jackman, S.R.; Witard, O.C.; Jeukendrup, A.E.; Tipton, K.D. Branched-chain amino acid ingestion can ameliorate soreness from eccentric exercise. Med. Sci. Sports Exerc. 2010, 42, 962–970. [Google Scholar] [CrossRef] [PubMed]
- Fouré, A.; Nosaka, K.; Gastaldi, M.; Mattei, J.-P.; Boudinet, H.; Guye, M.; Vilmen, C.; Le Fur, Y.; Bendahan, D.; Gondin, J. Effects of branched-chain amino acids supplementation on both plasma amino acids concentration and muscle energetics changes resulting from muscle damage: A randomized placebo controlled trial. Clin. Nutr. 2016, 35, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Areces, F.; Salinero, J.J.; Abian-Vicen, J.; González-Millán, C.; Gallo-Salazar, C.; Ruiz-Vicente, D.; Lara, B.; Del Coso, J. A 7-day oral supplementation with branched-chain amino acids was ineffective to prevent muscle damage during a marathon. Amino Acids 2014, 46, 1169–1176. [Google Scholar] [CrossRef] [PubMed]
- Shimomura, Y.; Murakami, T.; Nagasaki, M.; Honda, T.; Goto, H.; Kotake, K.; Kurokawa, T.; Nonami, T. Regulation of branched-chain amino acid metabolism and pharmacological effects of branched-chain amino acids. Hepatol. Res. 2004, 30, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Kreider, R.B.; Miriel, V.; Bertun, E. Amino acid supplementation and exercise performance. Analysis of the proposed ergogenic value. Sports Med. 1993, 16, 190–209. [Google Scholar] [CrossRef] [PubMed]
- Williams, M.H. Facts and fallacies of purported ergogenic amino acid supplements. Clics Sports Medice 1999, 18, 633–649. [Google Scholar] [CrossRef]
- Jager, R.; Kerksick, C.M.; Campbell, B.I.; Cribb, P.J.; Wells, S.D.; Skwiat, T.M.; Purpura, M.; Ziegenfuss, T.N.; Ferrando, A.A.; Arent, S.M. International society of sports nutrition position stand: Protein and exercise. J. Int. Soc. Sports Nutr. 2017, 14. [Google Scholar] [CrossRef] [PubMed]
- Morton, R.W.; Murphy, K.T.; McKellar, S.R.; Schoenfeld, B.J.; Henselmans, M.; Helms, E.; Aragon, A.A.; Devries, M.C.; Banfield, L.; Krieger, J.W.; et al. A systematic review, meta-analysis and meta-regression of the effect of protein supplementation on resistance training-induced gains in muscle mass and strength in healthy adults. Br. J. Sports Med. 2018, 52, 376–384. [Google Scholar] [CrossRef] [PubMed]
- Shimomura, Y.; Harris, R.A. Metabolism and physiological function of branched-chain amino acids: Discussion of session 1. J. Nutr. 2006, 136, 232S–233S. [Google Scholar] [CrossRef] [PubMed]
- Shimomura, Y.; Yamamoto, Y.; Bajotto, G.; Sato, J.; Murakami, T.; Shimomura, N.; Kobayashi, H.; Mawatari, K. Nutraceutical effects of branched-chain amino acids on skeletal muscle. J. Nutr. 2006, 136, 529S–532S. [Google Scholar] [CrossRef] [PubMed]
- Greenhaff, P.L.; Casey, A.; Short, A.H.; Harris, R.; Soderlund, K.; Hultman, E. Influence of oral creatine supplementation of muscle torque during repeated bouts of maximal voluntary exercise in man. Clin. Sci. 1993, 84, 565–571. [Google Scholar] [CrossRef] [PubMed]
- Jackson, A.S.; Pollock, M.L. Generalized equations for predicting body density of men. Br. J. Nutr. 1978, 40, 497–504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siri, W.E. Body composition from fluid spaces and density: Analysis of methods. 1961. Nutrition 1993, 9, 480–491. [Google Scholar] [PubMed]
- Kraemer, W.J.; Fry, A.C. ACSM’s Guidelines for Exercise Testing and Prescription, 6th ed.; Human Kinetics: Champaign, IL, USA, 1995. [Google Scholar]
- Thompson, B.J.; Ryan, E.D.; Sobolewski, E.J.; Conchola, E.C.; Cramer, J.T. Age related differences in maximal and rapid torque characteristics of the leg extensors and flexors in young, middle-aged and old men. Exp. Gerontol. 2013, 48, 277–282. [Google Scholar] [CrossRef] [PubMed]
- Hasson, C.J.; Dugan, E.L.; Doyle, T.L.A.; Humphries, B.; Newton, R.U. Neuromechanical strategies employed to increase jump height during the initiation of the squat jump. J. Electromyogr. Kinesiol. 2004, 14, 515–521. [Google Scholar] [CrossRef] [PubMed]
- Dugan, E.L.; Doyle, T.L.; Humphries, B.; Hasson, C.J.; Newton, R.U. Determining the optimal load for jump squats: A review of methods and calculations. J. Strength Cond. Res. 2004, 18, 668–674. [Google Scholar] [PubMed]
- Baird, M.F.; Graham, S.M.; Baker, J.S.; Bickerstaff, G.F. Creatine-kinase- and exercise-related muscle damage implications for muscle performance and recovery. J. Nutr. Metab. 2012, 2012. [Google Scholar] [CrossRef] [PubMed]
- Nikolaidis, M.G.; Jamurtas, A.Z.; Paschalis, V.; Fatouros, I.G.; Koutedakis, Y.; Kouretas, D. The effect of muscle-damaging exercise on blood and skeletal muscle oxidative stress: Magnitude and time-course considerations. Sports Med. 2008, 38, 579–606. [Google Scholar] [CrossRef] [PubMed]
- Hyldahl, R.D.; Hubal, M.J. Lengthening our perspective: Morphological, cellular and molecular responses to eccentric exercise. Muscle Nerve 2013, 49, 155–170. [Google Scholar] [CrossRef] [PubMed]
- Atherton, P.J.; Kumar, V.; Selby, A.L.; Rankin, D.; Hildebrandt, W.; Phillips, B.E.; Williams, J.P.; Hiscock, N.; Smith, K. Enriching a protein drink with leucine augments muscle protein synthesis after resistance exercise in young and older men. Clin. Nutr. 2016, 36, 888–895. [Google Scholar] [CrossRef] [PubMed]
- Reidy, P.T.; Rasmussen, B.B. Role of ingested amino acids and protein in the promotion of resistance exercise-induced muscle protein anabolism. J. Nutr. 2016, 146, 155–183. [Google Scholar] [CrossRef] [PubMed]
- Morton, R.W.; McGlory, C.; Phillips, S.M. Nutritional interventions to augment resistance training-induced skeletal muscle hypertrophy. Front Physiol. 2015, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kirby, T.J.; Triplett, N.T.; Haines, T.L.; Skinner, J.W.; Fairbrother, K.R.; McBride, J.M. Effect of leucine supplementation on indices of muscle damage following drop jumps and resistance exercise. Amino Acids 2012, 42, 1987–1996. [Google Scholar] [CrossRef] [PubMed]
- Shimomura, Y.; Inaguma, A.; Watanabe, S.; Yamamoto, Y.; Muramatsu, Y.; Bajotto, G.; Sato, J.; Shimomura, N.; Kobayashi, H.; Mawatari, K. Branched-chain amino acid supplementation before squat exercise and delayed-onset muscle soreness. Int. J. Sport Nutr. Exerc. Metab. 2010, 20, 236–244. [Google Scholar] [CrossRef] [PubMed]
- Ohtani, M.; Sugita, M.; Maruyama, K. Amino acid mixture improves training efficiency in athletes. J. Nutr. 2006, 136, 538S–543S. [Google Scholar] [CrossRef] [PubMed]
- Margaritelis, N.V.; Theodorou, A.A.; Baltzopoulos, V.; Maganaris, C.N.; Paschalis, V.; Kyparos, A.; Nikolaidis, M.G. Muscle damage and inflammation after eccentric exercise: Can the repeated bout effect be removed? Physiol. Rep. 2015, 3, e12648. [Google Scholar] [CrossRef] [PubMed]
- Kamandulis, S.; Skurvydas, A.; Brazaitis, M.; Škikas, L.; Duchateau, J. The repeated bout effect of eccentric exercise is not associated with changes in voluntary activation. Eur. J. Appl. Physiol. 2009, 108, 1065–1074. [Google Scholar] [CrossRef] [PubMed]
- Meldrum, D.; Cahalane, E.; Keogan, F.; Hardiman, O. Maximum voluntary isometric contraction: Investigation of reliability and learning effect. Amyotroph. Lateral. Scler. Other Motor Neuron Disord. 2003, 4, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Warren, G.L.; Lowe, D.A.; Armstrong, R.B. Measurement tools used in the study of eccentric contraction-induced injury. Sports Med. 1999, 27, 43–59. [Google Scholar] [CrossRef] [PubMed]
- Cheung, K.; Hume, P.; Maxwell, L. Delayed onset muscle soreness: Treatment strategies and performance factors. Sports Med. 2003, 33, 145–164. [Google Scholar] [CrossRef] [PubMed]
- Lewis, P.B.; Ruby, D.; Bush-Joseph, C.A. Muscle soreness and delayed-onset muscle soreness. Clin. Sports Med. 2012, 31, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Deyhle, M.R.; Gier, A.M.; Evans, K.C.; Eggett, D.L.; Nelson, W.B.; Parcell, A.C.; Hyldahl, R.D. Skeletal muscle inflammation following repeated bouts of lengthening contractions in humans. Front. Physiol. 2016, 6. [Google Scholar] [CrossRef] [PubMed]
- Nicastro, H.; da Luz, C.R.; Chaves, D.F.S.; Bechara, L.R.G.; Voltarelli, V.A.; Rogero, M.M.; Lancha, A.H. Does branched-chain amino acids supplementation modulate skeletal muscle remodeling through inflammation modulation? Possible mechanisms of action. J. Nutr. Metab. 2012, 2012. [Google Scholar] [CrossRef] [PubMed]
- Greer, B.K.; White, J.P.; Arguello, E.M.; Haymes, E.M. Branched-chain amino acid supplementation lowers perceived exertion but does not affect performance in untrained males. J. Strength Cond. Res. 2011, 25, 539–544. [Google Scholar] [CrossRef] [PubMed]
- Howatson, G.; van Someren, K.A. Evidence of a contralateral repeated bout effect after maximal eccentric contractions. Eur. J. Appl. Physiol. 2007, 101, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Ratamess, N.A.; Kraemer, W.J.; Volek, J.S.; Rubin, M.R.; Gómez, A.L.; French, D.N.; Sharman, M.J.; McGuigan, M.M.; Scheett, T.; Häkkinen, K. The effects of amino acid supplementation on muscular performance during resistance training overreaching. J. Strength Cond. Res. 2003, 17, 250–258. [Google Scholar] [PubMed]
- Ohtani, M.; Kawada, S.; Seki, T.; Okamoto, Y. Amino acid and vitamin supplementation improved health conditions in elderly participants. J. Clin. Biochem. Nutr. 2012, 50, 162–168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marg, A.; Schoewel, V.; Timmel, T.; Schulze, A.; Shah, C.; Daumke, O.; Spuler, S. Sarcolemmal repair is a slow process and includes ehd2. Traffic 2012, 13, 1286–1294. [Google Scholar] [CrossRef] [PubMed]
- Hyldahl, R.D.; Chen, T.C.; Nosaka, K. Mechanisms and Mediators of the Skeletal Muscle Repeated Bout Effect. Exerc. Sport Sci. Rev. 2017, 45, 24–33. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | BCAA | PLCB |
|---|---|---|
| Participant # | 10 | 10 |
| Age (yr) | 23.0 ± 1.2 | 21.5 ± 1.5 |
| Height (cm) | 177.6 ± 7.1 | 173.2 ± 6.2 |
| Body Mass (kg) | 86.6 ± 15.2 | 86.2 ± 16.8 |
| Body Fat% | 12.3 ± 3.8 | 11.7 ± 4.3 |
| 1RM Squat (kg) | 154.8 ± 31.7 | 155.0 ± 32.0 |
| RT Experience (yr) | 5.6 ± 2.3 | 5.0 ± 1.9 |
| RT Experience (hrs/week) | 7.00 ± 2.30 | 7.65 ± 2.05 |
| Protein Intake (g/kg/d) | 1.29 ± 0.12 | 1.25 ± 0.09 |
| Average Calorie Intake (kcal) | 2555 ± 324 | 2638 ± 309 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
VanDusseldorp, T.A.; Escobar, K.A.; Johnson, K.E.; Stratton, M.T.; Moriarty, T.; Cole, N.; McCormick, J.J.; Kerksick, C.M.; Vaughan, R.A.; Dokladny, K.; et al. Effect of Branched-Chain Amino Acid Supplementation on Recovery Following Acute Eccentric Exercise. Nutrients 2018, 10, 1389. https://doi.org/10.3390/nu10101389
VanDusseldorp TA, Escobar KA, Johnson KE, Stratton MT, Moriarty T, Cole N, McCormick JJ, Kerksick CM, Vaughan RA, Dokladny K, et al. Effect of Branched-Chain Amino Acid Supplementation on Recovery Following Acute Eccentric Exercise. Nutrients. 2018; 10(10):1389. https://doi.org/10.3390/nu10101389
Chicago/Turabian StyleVanDusseldorp, Trisha A., Kurt A. Escobar, Kelly E. Johnson, Matthew T. Stratton, Terence Moriarty, Nathan Cole, James J. McCormick, Chad M. Kerksick, Roger A. Vaughan, Karol Dokladny, and et al. 2018. "Effect of Branched-Chain Amino Acid Supplementation on Recovery Following Acute Eccentric Exercise" Nutrients 10, no. 10: 1389. https://doi.org/10.3390/nu10101389
APA StyleVanDusseldorp, T. A., Escobar, K. A., Johnson, K. E., Stratton, M. T., Moriarty, T., Cole, N., McCormick, J. J., Kerksick, C. M., Vaughan, R. A., Dokladny, K., Kravitz, L., & Mermier, C. M. (2018). Effect of Branched-Chain Amino Acid Supplementation on Recovery Following Acute Eccentric Exercise. Nutrients, 10(10), 1389. https://doi.org/10.3390/nu10101389







