Effect of Chlorogenic Acids on Cognitive Function: A Randomized, Double-Blind, Placebo-Controlled Trial
Abstract
:1. Introduction
2. Materials and Methods
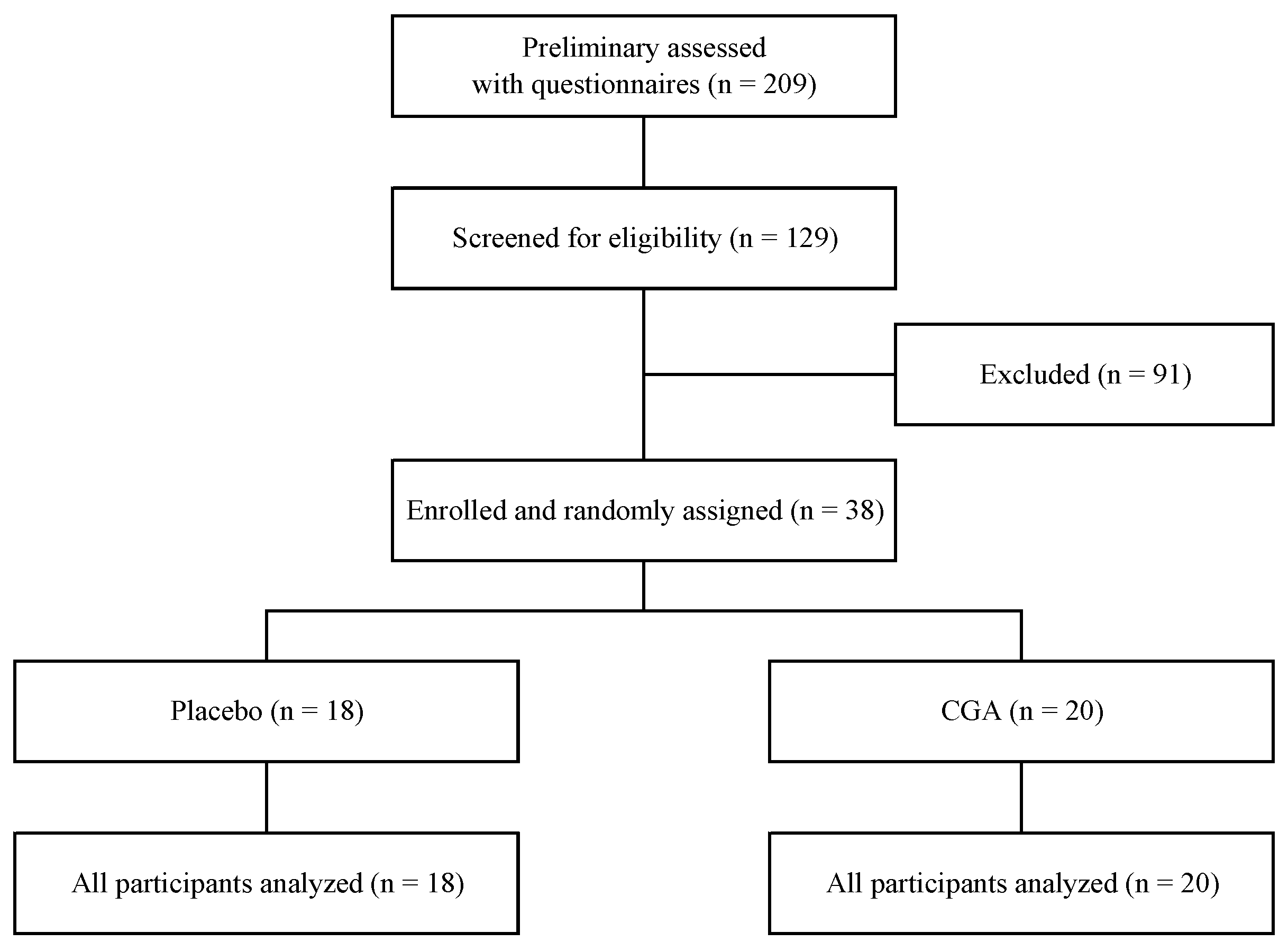
2.1. Participants
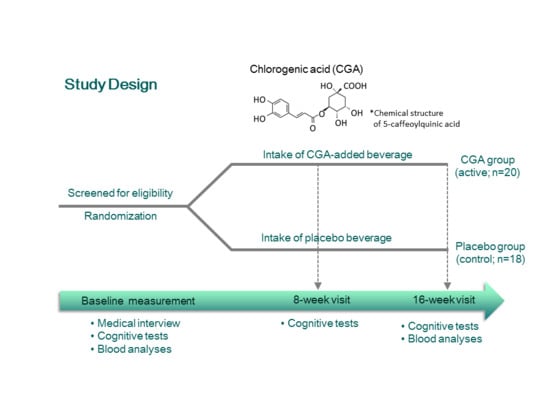
2.2. Experimental Design
2.3. Materials
2.4. Cognitive Function Assessment
2.5. Blood Analysis
2.6. Statistical Analysis
3. Results
3.1. Participants and Baseline Characteristics
3.2. Cognitive Functions
3.3. Blood Analysis
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Meguro, K. Clinical features of mild cognitive impairment and dementia in a community: An update of the Osaki-Tajiri Project. Tohoku J. Exp. Med. 2008, 215, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Saraçlı, Ö.; Akca, A.S.; Atasoy, N.; Önder, Ö.; Şenormancı, Ö.; Kaygisız, İ.; Atik, L. The Relationship between Quality of Life and Cognitive Functions, Anxiety and Depression among Hospitalized Elderly Patients. Clin. Psychopharmacol. Neurosci. 2015, 13, 194–200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wesnes, K.A.; Edgar, C.J. The role of human cognitive neuroscience in drug discovery for the dementias. Curr. Opin. Pharmacol. 2014, 14, 62–73. [Google Scholar] [CrossRef] [PubMed]
- Depp, C.A.; Harmell, A.; Vahia, I.V. Successful cognitive aging. Curr. Top. Behav. Neurosci. 2012, 10, 35–50. [Google Scholar] [PubMed]
- Rovio, S.; Kåreholt, I.; Helkala, E.L.; Viitanen, M.; Winblad, B.; Tuomilehto, J.; Soininen, H.; Nissinen, A.; Kivipelto, M. Leisure-time physical activity at midlife and the risk of dementia and Alzheimer’s disease. Lancet Neurol. 2005, 4, 705–711. [Google Scholar] [CrossRef]
- Kramer, A.F.; Erickson, K.I.; Colcombe, S.J. Exercise, cognition, and the aging brain. J. Appl. Physiol. 2006, 101, 1237–1242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anstey, K.J.; von Sanden, C.; Salim, A.; O’Kearney, R. Smoking as a risk factor for dementia and cognitive decline: A meta-analysis of prospective studies. Am. J. Epidemiol. 2007, 166, 367–378. [Google Scholar] [CrossRef] [PubMed]
- Vauzour, D.; Camprubi-Robles, M.; Miquel-Kergoat, S.; Andres-Lacueva, C.; Bánáti, D.; Barberger-Gateau, P.; Bowman, G.L.; Caberlotto, L.; Clarke, R.; Hogervorst, E.; et al. Nutrition for the ageing brain: Towards evidence for an optimal diet. Ageing Res. Rev. 2017, 35, 222–240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lourida, I.; Soni, M.; Thompson-Coon, J.; Purandare, N.; Lang, I.A.; Ukoumunne, O.C.; Llewellyn, D.J. Mediterranean diet, cognitive function, and dementia: A systematic review. Epidemiology 2013, 24, 479–489. [Google Scholar] [CrossRef] [PubMed]
- Rabassa, M.; Cherubini, A.; Zamora-Ros, R.; Urpi-Sarda, M.; Bandinelli, S.; Ferrucci, L.; Andres-Lacueva, C.J. Low levels of a urinary biomarker of dietary polyphenol are associated with substantial cognitive decline over a 3-year period in older adults: The invecchiare in Chianti study. J. Am. Geriatr. Soc. 2015, 63, 938–946. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nurk, E.; Refsum, H.; Drevon, C.A.; Tell, G.S.; Nygaard, H.A.; Engedal, K.; Smith, A.D. Intake of flavonoid-rich wine, tea, and chocolate by elderly men and women is associated with better cognitive test performance. J. Nutr. 2009, 139, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Commenges, D.; Scotet, V.; Renaud, S.; Jacqmin-Gadda, H.; Barberger-Gateau, P.; Dartigues, J.F. Intake of flavonoids and risk of dementia. Eur. J. Epidemiol. 2000, 16, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Spagnuolo, C.; Napolitano, M.; Tedesco, I.; Moccia, S.; Milito, A.; Russo, G.L. Neuroprotective role of natural polyphenols. Curr. Top. Med. Chem. 2016, 16, 1943–1950. [Google Scholar] [CrossRef] [PubMed]
- Heitman, E.; Ingram, D.K. Cognitive and neuroprotective effects of chlorogenic acid. Nutr. Neurosci. 2017, 20, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Van Gelder, B.M.; Buijsse, B.; Tijhuis, M.; Kalmijn, S.; Giampaoli, S.; Nissinen, A.; Kromhout, D. Coffee consumption is inversely associated with cognitive decline in elderly European men: The FINE Study. Eur. J. Clin. Nutr. 2007, 61, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Eskelinen, M.H.; Ngandu, T.; Tuomilehto, J.; Soininen, H.; Kivipelto, M. Midlife coffee and tea drinking and the risk of late-life dementia: A population-based CAIDE study. J. Alzheimer’s Dis. 2009, 16, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Tajik, N.; Tajik, M.; Mack, I.; Enck, P. The potential effects of chlorogenic acid, the main phenolic components in coffee, on health: A comprehensive review of the literature. Eur. J. Nutr. 2017, 56, 2215–2244. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Lee, S.; Shim, J.; Kim, H.W.; Kim, J.; Jang, Y.J.; Yang, H.; Park, J.; Choi, S.H.; Yoon, J.H.; et al. Caffeinated coffee, decaffeinated coffee, and the phenolic phytochemical chlorogenic acid up-regulate NQO1 expression and prevent H2O2-induced apoptosis in primary cortical neurons. Neurochem. Int. 2012, 60, 466–474. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, Y.; Shimazawa, M.; Mishima, S.; Hara, H. Water extract of propolis and its main constituents, caffeoylquinic acid derivatives, exert neuroprotective effects via antioxidant actions. Life Sci. 2007, 80, 370–377. [Google Scholar] [CrossRef] [PubMed]
- Ito, H.; Sun, X.L.; Watanabe, M.; Okamoto, M.; Hatano, T. Chlorogenic acid and its metabolite m-coumaric acid evoke neurite outgrowth in hippocampal neuronal cells. Biosci. Biotechnol. Biochem. 2008, 72, 885–888. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.H.; Lee, H.K.; Kim, J.A.; Hong, S.I.; Kim, H.C.; Jo, T.H.; Park, Y.I.; Lee, C.K.; Kim, Y.B.; Lee, S.Y.; et al. Neuroprotective effects of chlorogenic acid on scopolamine-induced amnesia via anti-acetylcholinesterase and anti-oxidative activities in mice. Eur. J. Pharmacol. 2010, 649, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Miyamae, Y.; Shigemori, H.; Isoda, H. Neuroprotective effect of 3,5-di-O-caffeoylquinic acid on SH-SY5Y cells and senescence-accelerated-prone mice 8 through the up-regulation of phosphoglycerate kinase-1. Neuroscience 2010, 169, 1039–1045. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cropley, V.; Croft, R.; Silber, B.; Neale, C.; Scholey, A.; Stough, C.; Schmitt, J. Does coffee enriched with chlorogenic acids improve mood and cognition after acute administration in healthy elderly? A pilot study. Psychopharmacology 2012, 219, 737–749. [Google Scholar] [CrossRef] [PubMed]
- Camfield, D.A.; Silber, B.Y.; Scholey, A.B.; Nolidin, K.; Goh, A.; Stough, C.A. Randomised placebo-controlled trial to differentiate the acute cognitive and mood effects of chlorogenic acid from decaffeinated coffee. PLoS ONE 2013, 8, e82897. [Google Scholar] [CrossRef] [PubMed]
- Kato, M.; Ochiai, R.; Kozuma, K.; Sato, H.; Katsuragi, Y. Effect of chlorogenic acid intake on cognitive function in the elderly: A pilot study. Evid. Based Complement. Altern. Med. 2018, 2018, 8608497. [Google Scholar] [CrossRef] [PubMed]
- Tsoi, K.K.; Chan, J.Y.; Hirai, H.W.; Wong, S.Y.; Kwok, T.C. Cognitive tests to detect dementia: A systematic review and meta-analysis. JAMA. Intern. Med. 2015, 175, 1450–1458. [Google Scholar] [CrossRef] [PubMed]
- Randolph, C.; Tierney, M.C.; Mohr, E.; Chase, T.N. The Repeatable Battery for the Assessment of Neuropsychological Status (RBANS): Preliminary clinical validity. J. Clin. Exp. Neuropsychol. 1998, 20, 310–319. [Google Scholar] [CrossRef] [PubMed]
- Park, I.; Ochiai, R.; Ogata, H.; Kayaba, M.; Hari, S.; Hibi, M.; Katsuragi, Y.; Satoh, M.; Tokuyama, K. Effects of subacute ingestion of chlorogenic acids on sleep architecture and energy metabolism through activity of the autonomic nervous system: A randomised, placebo-controlled, double-blinded cross-over trial. Br. J. Nutr. 2017, 117, 979–984. [Google Scholar] [CrossRef] [PubMed]
- Ochiai, R.; Sugiura, Y.; Shioya, Y.; Otsuka, K.; Katsuragi, Y.; Hashiguchi, T. Coffee polyphenols improve peripheral endothelial function after glucose loading in healthy male adults. Nutr. Res. 2014, 34, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Martín, E.D.; Buño, W. Caffeine-mediated presynaptic long-term potentiation in hippocampal CA1 pyramidal neurons. J. Neurophysiol. 2003, 89, 3029–3038. [Google Scholar] [CrossRef] [PubMed]
- Roehrs, T.; Roth, T. Caffeine: Sleep and daytime sleepiness. Sleep. Med. Rev. 2008, 12, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Gualtieri, C.T.; Johnson, L.G. Reliability and validity of a computerized neurocognitive test battery, CNS Vital Signs. Arch. Clin. Neuropsychol. 2006, 21, 623–643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- CNS Vital Signs. Brief Interpretation Guide. Available online: http://www.cnsvs.com/WhitePapers/CNSVS-BriefInterpretationGuide.pdf (accessed on 30 July 2018).
- Crowgey, T.; Peters, K.B.; Hornsby, W.E.; Lane, A.; McSherry, F.; Herndon, J.E.; West, M.J.; Williams, C.L.; Jones, L.W. Relationship between exercise behavior, cardiorespiratory fitness, and cognitive function in early breast cancer patients treated with doxorubicin-containing chemotherapy: A pilot study. Appl. Physiol. Nutr. Metab. 2014, 39, 724–729. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, T.E.; Harvey, P.D.; Wesnes, K.A.; Snyder, P.J.; Schneider, L.S. Practice effects due to serial cognitive assessment: Implications for preclinical Alzheimer’s disease randomized controlled trials. Alzheimer’s Dement. 2015, 1, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Littleton, A.C.; Register-Mihalik, J.K.; Guskiewicz, K.M. Test-retest reliability of a computerized concussion test: CNS Vital Signs. Sports Health 2015, 7, 443–447. [Google Scholar] [CrossRef] [PubMed]
- Rabinowitz, I.; Lavner, Y. Association between finger tapping, attention, memory, and cognitive diagnosis in elderly patients. Percept. Mot. Skills 2014, 119, 259–278. [Google Scholar] [CrossRef] [PubMed]
- Sheridan, L.K.; Fitzgerald, H.E.; Adams, K.M.; Nigg, J.T.; Martel, M.M.; Puttler, L.I.; Wong, M.M.; Zucker, R.A. Normative Symbol Digit Modalities Test performance in a community-based sample. Arch. Clin. Neuropsychol. 2006, 21, 23–28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hindmarch, I. Psychomotor function and psychoactive drugs. Br. J. Clin. Pharm. 1980, 10, 189–209. [Google Scholar] [CrossRef]
- Era, P.; Sainio, P.; Koskinen, S.; Ohlgren, J.; Härkänen, T.; Aromaa, A. Psychomotor speed in a random sample of 7979 subjects aged 30 years and over. Aging Clin. Exp. Res. 2011, 23, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Houx, P.J.; Jolles, J. Age-related decline of psychomotor speed: Effects of age, brain health, sex, and education. Percept. Mot. Skills 1993, 76, 195–211. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Aranda, C.; Waterloo, K.; Sparr, S.; Sundet, K.J. Age-related psychomotor slowing as an important component of verbal fluency: Evidence from healthy individuals and Alzheimer’s patients. J. Neurol. 2006, 253, 1414–1427. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.Y.; Peronto, C.L.; Edwards, J.D. Cognitive function as a prospective predictor of falls. J. Gerontol. B Psychol. Sci. Soc. Sci. 2012, 67, 720–728. [Google Scholar] [CrossRef] [PubMed]
- Gloeckner, S.F.; Meyne, F.; Wagner, F.; Heinemann, U.; Krasnianski, A.; Meissner, B.; Zerr, I. Quantitative analysis of transthyretin, tau and amyloid-beta in patients with dementia. J. Alzheimer’s Dis. 2008, 14, 17–25. [Google Scholar] [CrossRef]
- Castaño, E.M.; Roher, A.E.; Esh, C.L.; Kokjohn, T.A.; Beach, T. Comparative proteomics of cerebrospinal fluid in neuropathologically-confirmed Alzheimer’s disease and non-demented elderly subjects. Neurol. Res. 2006, 28, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Uchida, K.; Shan, L.; Suzuki, H.; Tabuse, Y.; Nishimura, Y.; Hirokawa, Y.; Mizukami, K.; Akatsu, H.; Meno, K.; Asada, T. Amyloid-β sequester proteins as blood-based biomarkers of cognitive decline. Alzheimer’s Dement. 2015, 1, 270–280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watanabe, T.; Arai, Y.; Mitsui, Y.; Kusaura, T.; Okawa, W.; Kajihara, Y.; Saito, I. The blood pressure-lowering effect and safety of chlorogenic acid from green coffee bean extract in essential hypertension. Clin. Exp. Hypertens. 2006, 28, 439–449. [Google Scholar] [CrossRef] [PubMed]
- Soga, S.; Ota, N.; Shimotoyodome, A. Stimulation of postprandial fat utilization in healthy humans by daily consumption of chlorogenic acids. Biosci. Biotechnol. Biochem. 2013, 77, 1633–1636. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Kang, H.; Xu, Q.; Chen, M.J.; Liao, Y.; Thiyagarajan, M.; O’Donnell, J.; Christensen, D.J.; Nicholson, C.; Iliff, J.J.; et al. Sleep drives metabolite clearance from the adult brain. Science 2013, 342, 373–377. [Google Scholar] [CrossRef] [PubMed]
| Cognitive Domains | Score Calculations |
|---|---|
| Neurocognitive Index (NCI) | Average of Five Domain Scores: Composite Memory, Psychomotor Speed, Reaction Time, Complex Attention, Cognitive Flexibility |
| Composite Memory | VBM (Correct Hits + Correct Passes) + VIM (Correct Hits + Correct Passes) |
| Verbal Memory | VBM (Correct Hits + Correct Passes) |
| Visual Memory | VIM (Correct Hits + Correct Passes) |
| Psychomotor Speed | SDC (Correct Responses) + FTT (Right Tap Average + Left Tap Average) |
| Reaction Time | ST (Complex Reaction Time + Stroop Reaction Time) |
| Complex Attention | ST (Stroop Commission Errors) + SAT (Errors) + CPT (Commission Errors) |
| Cognitive Flexibility | SAT (Correct Responses − Errors) − ST (Stroop Commission Errors) |
| Processing Speed | SDC (Correct Responses − Errors) |
| Executive Function | SAT (Correct Responses − Errors) |
| Simple Attention | CPT (Correct Responses − Commission Errors) |
| Motor Speed | FTT (Right Taps Average + Left Taps Average) |
| Group | Placebo | CGA |
|---|---|---|
| Number of participants (male) | 18 (9) | 20 (12) |
| Age, y | 58.5 ± 6.3 | 59.3 ± 5.0 |
| Body weight, kg | 63.1 ± 11.4 | 60.6 ± 11.5 |
| MMSE score | 26.6 ± 1.5 | 26.7 ± 1.4 |
| RBANS total score | 49.0 ± 10.7 | 50.0 ± 16.2 |
| Standardized Scores | ANCOVA (p-Values) | LMM (p-Values) | ||||||
|---|---|---|---|---|---|---|---|---|
| Baseline (0 W) | 8 W | 16 W | T (8 W–16 W) | G (8 W–16 W) | T × G (8 W–16 W) | T × G (0 W–16 W) | ||
| Neurocognitive Index (NCI) | Placebo | 82.7 ± 20.5 | 92.2 ± 23.2 | 95.7± 8.8 | <0.05 | 0.251 | 0.539 | 0.970 |
| CGA | 87.5 ± 23.0 | 97.6 ± 15.9 | 102.0 ± 9.0 | |||||
| Composite Memory | Placebo | 86.3 ± 17.2 | 104.7 ± 13.2 | 97.6 ± 11.0 | 0.190 | 0.327 | 0.107 | 0.269 |
| CGA | 84.3 ± 25.4 | 94.8 ± 24.6 | 99.5 ± 12.9 | |||||
| Verbal Memory | Placebo | 84.3 ± 13.9 | 105.2 ± 13.0 | 98.4 ± 16.4 | <0.05 | 0.356 | 0.212 | 0.594 |
| CGA | 81.3 ± 25.1 | 96.8 ± 24.0 | 98.1 ± 19.1 | |||||
| Visual Memory | Placebo | 94.2 ± 19.5 | 102.4 ± 13.3 | 98.3 ± 13.0 | 0.770 | 0.629 | 0.109 | 0.254 |
| CGA | 92.8 ± 19.3 | 95.8 ± 17.8 | 101.3 ± 8.8 | |||||
| Psychomotor Speed | Placebo | 94.9 ± 21.2 | 99.3 ± 20.6 | 95.5 ± 11.9 | 0.141 | 0.684 | <0.05 | <0.05 |
| CGA | 91.9 ± 23.9 | 92.3 ± 28.7 | 103.4 ± 13.8 # | |||||
| Reaction Time | Placebo | 82.8 ± 23.4 | 90.4 ± 13.5 | 90.7 ± 10.6 | 0.708 | 0.305 | 0.461 | 0.273 |
| CGA | 101.3 ± 36.5 | 99.6 ± 27.7 | 94.4 ± 11.8 | |||||
| Processing Speed | Placebo | 102.3 ± 14.4 | 106.2 ± 13.6 | 110.1 ± 11.4 | 0.946 | 0.158 | 0.938 | 0.937 |
| CGA | 107.7 ± 13.9 | 112.6 ± 9.3 | 116.4 ± 13.1 | |||||
| Motor Speed | Placebo | 91.5 ± 25.7 | 94.8 ± 21.1 | 86.8 ± 14.3 | 0.290 | 0.838 | <0.05 | <0.05 |
| CGA | 85.5 ± 25.2 | 84.6 ± 28.2 | 94.4 ± 14.8 | |||||
| Complex Attention | Placebo | 81.9 ± 29.4 | 91.7 ± 29.5 | 98.1 ± 20.7 | <0.05 | 0.132 | 0.832 | 0.914 |
| CGA | 88.3 ± 330 | 101.6 ± 22.3 | 108.1 ± 12.6 | |||||
| Simple Attention | Placebo | 91.6 ± 27.8 | 85.8 ± 31.9 | 90.9 ± 29.6 | 0.284 | 0.242 | 0.567 | 0.316 |
| CGA | 84.8 ± 35.4 | 89.3 ± 30.3 | 100.7 ± 14.1 | |||||
| Cognitive Flexibility | Placebo | 80.8 ± 23.4 | 95.9 ± 16.4 | 96.1 ± 14.4 | <0.05 | 0.641 | 0.087 | 0.279 |
| CGA | 91.9 ± 18.4 | 101.0 ± 16.7 | 104.9 ± 11.9 | |||||
| Executive Function | Placebo | 81.2 ± 23.1 | 97.0 ± 16.2 | 96.6 ± 14.6 | <0.05 | 0.794 | <0.05 | 0.145 |
| CGA | 91.8 ± 18.1 | 100.6 ± 16.8 | 104.9 ± 11.1 # | |||||
| Standardized Scores | ANCOVA (p-Values) | LMM (p-Values) | ||||||
|---|---|---|---|---|---|---|---|---|
| Baseline (0 W) | 8 W | 16 W | T (8 W–16 W) | G (8 W–16 W) | T × G (8 W–16 W) | T × G (0 W–16 W) | ||
| Verbal Memory Test (VBM)—Immediate | ||||||||
| Correct Hits | Placebo | 89.6 ± 27.4 | 100.6 ± 23.6 | 100.2 ± 16.7 | 0.181 | 0.685 | 0.375 | 0.545 |
| CGA | 82.6 ± 33.6 | 94.8 ± 34.2 | 102.0 ± 15.8 | |||||
| Correct Passes | Placebo | 103.7 ± 8.0 | 105.3 ± 10.9 | 104.3 ± 7.0 | 0.203 | 0.093 | 0.639 | 0.739 |
| CGA | 99.8 ± 16.5 | 101.5 ± 10.0 | 97.5 ± 14.3 | |||||
| Verbal Memory Test (VBM)—Delay | ||||||||
| Correct Hits | Placebo | 78.4 ± 20.9 | 104.6 ± 12.2 | 95.1 ± 15.7 | 0.154 | 0.643 | 0.590 | 0.669 |
| CGA | 82.2 ± 24.8 | 99.9 ± 21.7 | 96.0 ± 25.8 | |||||
| Correct Passes | Placebo | 102.2 ± 11.1 | 104.7 ± 7.7 | 100.7 ± 10.9 | 0.665 | 0.244 | 0.244 | 0.418 |
| CGA | 99.3 ± 15.6 | 97.0 ± 16.0 | 100.4 ± 14.6 | |||||
| Visual Memory Test (VIM)—Immediate | ||||||||
| Correct Hits | Placebo | 83.5 ± 27.0 | 97.4 ± 18.3 | 90.2 ± 17.7 | 0.289 | 0.591 | 0.081 | 0.146 |
| CGA | 77.1 ± 32.1 | 86.3 ± 32.6 | 94.8 ± 13.1 | |||||
| Correct Passes | Placebo | 106.9 ± 19.6 | 106.1 ± 16.5 | 110.2 ± 10.0 | 0.636 | 0.629 | 0.189 | 0.374 |
| CGA | 107.0 ± 16.8 | 108.0 ± 12.8 | 105.3 ± 11.6 | |||||
| Visual Memory Test (VIM)—Delay | ||||||||
| Correct Hits | Placebo | 85.3 ± 21.9 | 98.7 ± 19.4 | 93.3 ± 12.4 | 0.315 | 0.976 | 0.121 | 0.303 |
| CGA | 85.8 ± 31.5 | 93.8 ± 21.9 | 98.1 ± 15.7 | |||||
| Correct Passes | Placebo | 109.0 ± 19.4 | 102.4 ± 17.2 | 100.8 ± 17.7 | 0.919 | 0.879 | 0.542 | 0.825 |
| CGA | 110.4 ± 15.9 | 102.3 ± 14.8 | 103.4 ± 14.4 | |||||
| Finger Tapping Test (FTT) | ||||||||
| Right Taps Average | Placebo | 92.3 ± 28.0 | 97.2 ± 17.8 | 87.4 ± 12.4 | 0.100 | 0.922 | <0.05 | <0.05 |
| CGA | 87.0 ± 25.7 | 84.8 ± 31.8 | 96.0 ± 16.0 # | |||||
| Left Taps Average | Placebo | 93.8 ± 17.2 | 92.9 ± 23.2 | 87.9 ± 18.5 | 0.403 | 0.535 | 0.126 | 0.054 |
| CGA | 85.0 ± 25.4 | 86.0 ± 25.2 | 93.5 ± 13.4 | |||||
| Symbol Digit Coding (SDC) | ||||||||
| Correct Responses | Placebo | 102.8 ± 13.0 | 106.9 ± 11.4 | 110.6 ± 9.6 | 0.655 | 0.261 | 0.618 | 0.922 |
| CGA | 107.7 ± 12.6 | 112.6 ± 9.2 | 115.3 ± 12.9 | |||||
| Errors | Placebo | 94.8 ± 23.6 | 95.3 ± 24.0 | 93.1 ± 18.0 | <0.05 | 0.080 | 0.066 | 0.390 |
| CGA | 98.8 ± 20.4 | 99.1 ± 17.0 | 106.5 ± 9.1 | |||||
| Stroop Test (ST) | ||||||||
| Simple Reaction Time | Placebo | 76.8 ± 25.4 | 88.6 ± 14.7 | 86.6 ± 19.6 | 0.667 | 0.310 | 0.337 | 0.642 |
| CGA | 64.9 ± 32.5 | 76.1 ± 26.9 | 80.6 ± 25.3 | |||||
| Complex Reaction Time | Placebo | 82.3 ± 21.5 | 87.1 ± 14.5 | 89.2 ± 9.4 | 0.080 | 0.225 | 0.860 | 0.367 |
| CGA | 74.9 ± 26.9 | 89.6 ± 18.6 | 92.0 ± 11.2 | |||||
| Stroop Reaction Time | Placebo | 85.8 ± 23.3 | 94.9 ± 12.0 | 93.9 ± 11.2 | 0.245 | 0.721 | 0.169 | 0.251 |
| CGA | 81.4 ± 29.7 | 91.9 ± 21.4 | 97.3 ± 11.3 | |||||
| Stroop Commission Errors | Placebo | 94.8 ± 17.5 | 90.7 ± 19.3 | 96.2 ± 15.8 | 0.920 | 0.105 | 0.075 | 0.133 |
| CGA | 99.2 ± 13.9 | 102.9 ± 8.1 | 99.3 ± 14.8 | |||||
| Shifting Attention Test (SAT) | ||||||||
| Correct Responses | Placebo | 79.3 ± 20.7 | 94.6 ± 14.9 | 94.0 ± 13.9 | <0.05 | 0.779 | <0.05 | 0.165 |
| CGA | 88.3 ± 17.7 | 97.9 ± 17.2 | 102.2 ± 12.3 # | |||||
| Errors | Placebo | 88.8 ± 22.5 | 101.8 ± 14.9 | 102.1 ± 14.0 | <0.05 | 0.733 | 0.074 | 0.168 |
| CGA | 99.8 ± 16.5 | 105.2 ± 13.2 | 108.7 ± 7.9 | |||||
| Correct Reaction Time | Placebo | 91.3 ± 14.4 | 101.6 ± 13.9 | 101.9 ± 12.8 | 0.189 | 0.530 | 0.214 | 0.518 |
| CGA | 94.9 ± 15.2 | 104.5 ± 16.6 | 107.8 ± 14.7 | |||||
| Continuous Performance Test (CPT) | ||||||||
| Correct Responses | Placebo | 90.0 ± 27.8 | 78.8 ± 34.5 | 87.0 ± 32.2 | 0.561 | 0.348 | 0.960 | 0.434 |
| CGA | 81.0 ± 36.4 | 83.7 ± 33.4 | 92.8 ± 20.8 | |||||
| Commission Errors | Placebo | 91.6 ± 28.9 | 92.6 ± 28.5 | 96.3 ± 22.2 | 0.561 | 0.348 | 0.960 | 0.867 |
| CGA | 94.8 ± 27.4 | 97.2 ± 23.0 | 103.5 ± 9.4 | |||||
| Reaction Time | Placebo | 78.8 ± 17.4 | 84.8 ± 19.0 | 84.8 ± 8.4 | <0.05 | 0.401 | 0.607 | 0.965 |
| CGA | 78.4 ± 18.4 | 86.3 ± 11.7 | 85.9 ± 9.1 | |||||
| Baseline (0 W) | 16 W | ∆ Values | ||
|---|---|---|---|---|
| Apo A1, mg/dL | Placebo | 161.1 ± 29.0 | 155.2 ± 29.6 | −5.9 ± 15.1 |
| CGA | 161.9 ± 27.6 | 165.9 ± 31.7 | 4.0 ± 17.4 # | |
| TTR, mg/dL | Placebo | 25.9 ± 4.5 | 24.7 ± 3.8 | −1.2 ± 2.5 |
| CGA | 25.7 ± 4.7 | 26.5 ± 5.1 | 0.7 ± 2.5 * |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saitou, K.; Ochiai, R.; Kozuma, K.; Sato, H.; Koikeda, T.; Osaki, N.; Katsuragi, Y. Effect of Chlorogenic Acids on Cognitive Function: A Randomized, Double-Blind, Placebo-Controlled Trial. Nutrients 2018, 10, 1337. https://doi.org/10.3390/nu10101337
Saitou K, Ochiai R, Kozuma K, Sato H, Koikeda T, Osaki N, Katsuragi Y. Effect of Chlorogenic Acids on Cognitive Function: A Randomized, Double-Blind, Placebo-Controlled Trial. Nutrients. 2018; 10(10):1337. https://doi.org/10.3390/nu10101337
Chicago/Turabian StyleSaitou, Katsuyoshi, Ryuji Ochiai, Kazuya Kozuma, Hirotaka Sato, Takashi Koikeda, Noriko Osaki, and Yoshihisa Katsuragi. 2018. "Effect of Chlorogenic Acids on Cognitive Function: A Randomized, Double-Blind, Placebo-Controlled Trial" Nutrients 10, no. 10: 1337. https://doi.org/10.3390/nu10101337
APA StyleSaitou, K., Ochiai, R., Kozuma, K., Sato, H., Koikeda, T., Osaki, N., & Katsuragi, Y. (2018). Effect of Chlorogenic Acids on Cognitive Function: A Randomized, Double-Blind, Placebo-Controlled Trial. Nutrients, 10(10), 1337. https://doi.org/10.3390/nu10101337