Dietary Acid Load and Potassium Intake Associate with Blood Pressure and Hypertension Prevalence in a Representative Sample of the German Adult Population
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Dietary Intake
- PRAL (mEq/day) = 0.49 × protein (g/day)
- +0.037 × phosphorus (mg/day)
- −0.021 × potassium (mg/day)
- −0.026 × magnesium (mg/day)
- −0.013 × calcium (mg/day).
2.3. Measurements and Laboratory Analyses
2.4. Other Variables
2.5. Statistical Analyses
3. Results
3.1. Descriptive Data
3.2. Linear Regression
3.3. Logistic Regression
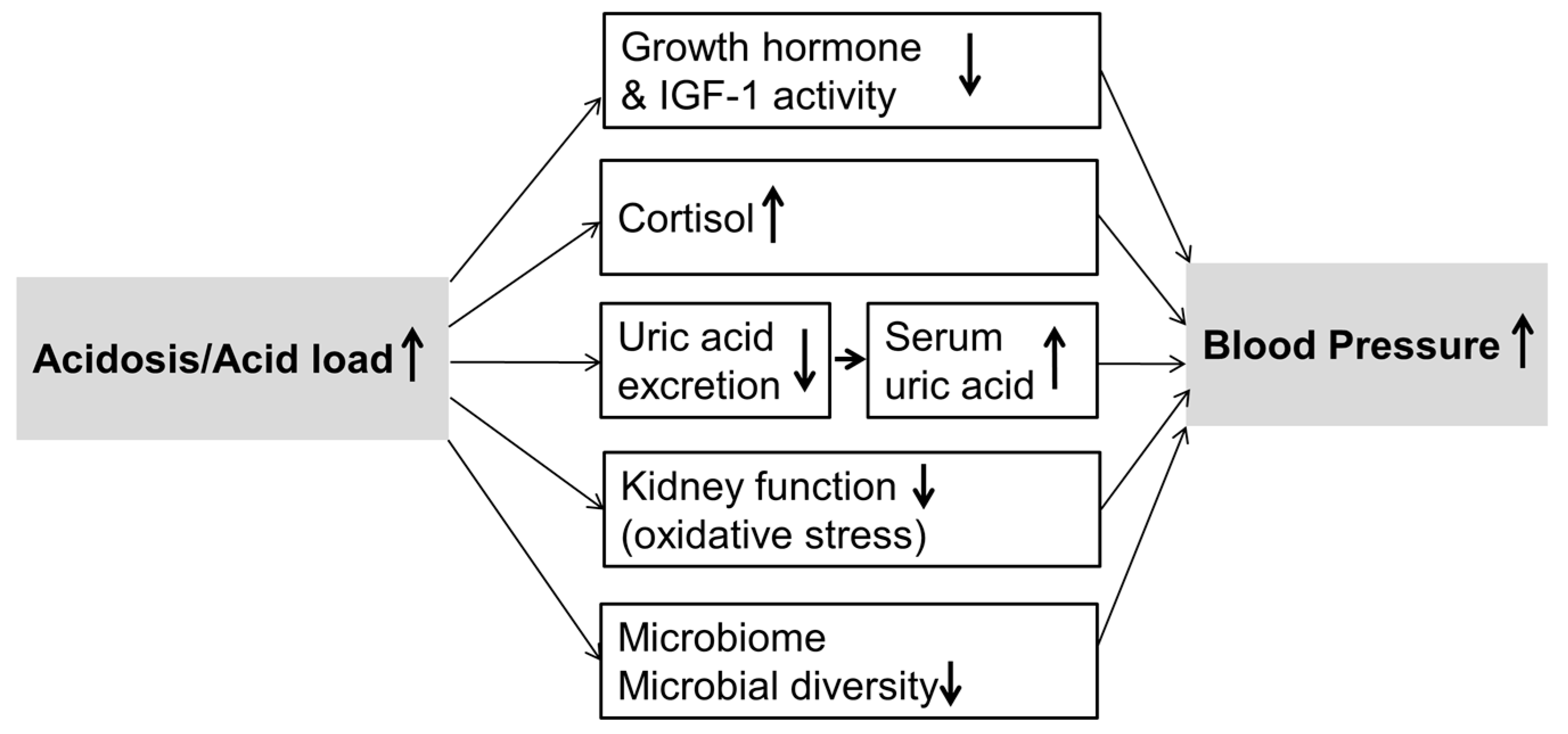
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Mancia, G.; Fagard, R.; Narkiewicz, K.; Redon, J.; Zanchetti, A.; Bohm, M.; Christiaens, T.; Cifkova, R.; de Backer, G.; Dominiczak, A.; et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: The task force for the management of arterial hypertension of the European Society Of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur. Heart J. 2013, 34, 2159–2219. [Google Scholar] [PubMed]
- Boeing, H.; Bechthold, A.; Bub, A.; Ellinger, S.; Haller, D.; Kroke, A.; Leschik-Bonnet, E.; Muller, M.J.; Oberritter, H.; Schulze, M.; et al. Critical review: Vegetables and fruit in the prevention of chronic diseases. Eur. J. Nutr. 2012, 51, 637–663. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, Y.; Nishimura, K.; Barnard, N.D.; Takegami, M.; Watanabe, M.; Sekikawa, A.; Okamura, T.; Miyamoto, Y. Vegetarian diets and blood pressure: A meta-analysis. JAMA Intern. Med. 2014, 174, 577–587. [Google Scholar] [CrossRef] [PubMed]
- Gay, H.C.; Rao, S.G.; Vaccarino, V.; Ali, M.K. Effects of different dietary interventions on blood pressure: Systematic review and meta-analysis of randomized controlled trials. Hypertension 2016, 67, 733–739. [Google Scholar] [CrossRef] [PubMed]
- Haddy, F.J.; Vanhoutte, P.M.; Feletou, M. Role of potassium in regulating blood flow and blood pressure. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006, 290, R546–R552. [Google Scholar] [CrossRef] [PubMed]
- Penton, D.; Czogalla, J.; Loffing, J. Dietary potassium and the renal control of salt balance and blood pressure. Pflugers Arch. Eur. J. Physiol. 2015, 467, 513–530. [Google Scholar] [CrossRef] [PubMed]
- Alexy, U.; Remer, T.; Manz, F.; Neu, C.M.; Schoenau, E. Long-term protein intake and dietary potential renal acid load are associated with bone modeling and remodeling at the proximal radius in healthy children. Am. J. Clin. Nutr. 2005, 82, 1107–1114. [Google Scholar] [PubMed]
- Banerjee, T.; Crews, D.C.; Wesson, D.E.; Tilea, A.; Saran, R.; Rios Burrows, N.; Williams, D.E.; Powe, N.R. Dietary acid load and chronic kidney disease among adults in the United States. BMC Nephrol. 2014, 15, 137. [Google Scholar] [CrossRef] [PubMed]
- Kiefte-de Jong, J.C.; Li, Y.; Chen, M.; Curhan, G.C.; Mattei, J.; Malik, V.S.; Forman, J.P.; Franco, O.H.; Hu, F.B. Diet-dependent acid load and type 2 diabetes: Pooled results from three prospective cohort studies. Diabetologia 2017, 60, 270–279. [Google Scholar] [CrossRef] [PubMed]
- Murakami, K.; Sasaki, S.; Takahashi, Y.; Uenishi, K. Association between dietary acid-base load and cardiometabolic risk factors in young Japanese women. Br. J. Nutr. 2008, 100, 642–651. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Curhan, G.C.; Forman, J.P. Diet-dependent net acid load and risk of incident hypertension in United States women. Hypertension 2009, 54, 751–755. [Google Scholar] [CrossRef] [PubMed]
- Krupp, D.; Shi, L.; Remer, T. Longitudinal relationships between diet-dependent renal acid load and blood pressure development in healthy children. Kidney Int. 2014, 85, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Akter, S.; Eguchi, M.; Kurotani, K.; Kochi, T.; Pham, N.M.; Ito, R.; Kuwahara, K.; Tsuruoka, H.; Mizoue, T.; Kabe, I.; et al. High dietary acid load is associated with increased prevalence of hypertension: The Furukawa Nutrition and Health Study. Nutrition 2015, 31, 298–303. [Google Scholar] [CrossRef] [PubMed]
- Engberink, M.F.; Bakker, S.J.L.; Brink, E.J.; van Baak, M.A.; van Rooij, F.J.; Hofman, A.; Witteman, J.C.M.; Geleijnse, J.M. Dietary acid load and risk of hypertension: The Rotterdam Study. Am. J. Clin. Nutr. 2012, 95, 1438–1444. [Google Scholar] [CrossRef] [PubMed]
- Luis, D.; Huang, X.; Riserus, U.; Sjogren, P.; Lindholm, B.; Arnlov, J.; Cederholm, T.; Carrero, J.J. Estimated dietary acid load is not associated with blood pressure or hypertension incidence in men who are approximately 70 years old. J. Nutr. 2015, 145, 315–321. [Google Scholar] [CrossRef] [PubMed]
- Pizzorno, J.; Frassetto, L.A.; Katzinger, J. Diet-induced acidosis: Is it real and clinically relevant? Br. J. Nutr. 2010, 103, 1185–1194. [Google Scholar] [CrossRef] [PubMed]
- Hietavala, E.-M.; Stout, J.R.; Hulmi, J.J.; Suominen, H.; Pitkanen, H.; Puurtinen, R.; Selanne, H.; Kainulainen, H.; Mero, A.A. Effect of diet composition on acid-base balance in adolescents, young adults and elderly at rest and during exercise. Eur. J. Clin. Nutr. 2015, 69, 399–404. [Google Scholar] [CrossRef] [PubMed]
- Taylor, E.N.; Mount, D.B.; Forman, J.P.; Curhan, G.C. Association of prevalent hypertension with 24-h urinary excretion of calcium, citrate, and other factors. Am. J. Kidney Dis. Off. J. Natl. Kidney Found. 2006, 47, 780–789. [Google Scholar] [CrossRef] [PubMed]
- Taylor, E.N.; Forman, J.P.; Farwell, W.R. Serum anion gap and blood pressure in the national health and nutrition examination survey. Hypertension 2007, 50, 320–324. [Google Scholar] [CrossRef] [PubMed]
- Mandel, E.I.; Forman, J.P.; Curhan, G.C.; Taylor, E.N. Plasma bicarbonate and odds of incident hypertension. Am. J. Hypertens. 2013, 26, 1405–1412. [Google Scholar] [CrossRef] [PubMed]
- Batlle, D.; Redon, J.; Gutterman, C.; LaPointe, M.; Saleh, A.; Sharma, A.; Rombola, G.; Ye, M.; Alsheikha, W.; Gomez, L. Acid-base status and intracellular pH regulation in lymphocytes from rats with genetic hypertension. J. Am. Soc. Nephrol. 1994, 5, S12–S22. [Google Scholar] [PubMed]
- Kim, S.; Yang, J.Y.; Jung, E.S.; Lee, J.; Heo, N.J.; Lee, J.W.; Na, K.Y.; Han, J.S. Effects of sodium citrate on salt sensitivity and kidney injury in chronic renal failure. J. Korean Med. Sci. 2014, 29, 1658–1664. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.M.; Kribben, A.; Schattenfroh, S.; Cetto, C.; Distler, A. Salt sensitivity in humans is associated with abnormal acid-base regulation. Hypertension 1990, 16, 407–413. [Google Scholar] [CrossRef] [PubMed]
- Scheidt-Nave, C.; Kamtsiuris, P.; Gosswald, A.; Holling, H.; Lange, M.; Busch, M.A.; Dahm, S.; Dolle, R.; Ellert, U.; Fuchs, J.; et al. German health interview and examination survey for adults (DEGS)—Design, objectives and implementation of the first data collection wave. BMC Public Health 2012, 12, 730. [Google Scholar] [CrossRef] [PubMed]
- Haftenberger, M.; Heuer, T.; Heidemann, C.; Kube, F.; Krems, C.; Mensink, G.B.M. Relative validation of a food frequency questionnaire for national health and nutrition monitoring. Nutr. J. 2010, 9, 36. [Google Scholar] [CrossRef] [PubMed]
- Remer, T.; Manz, F. Estimation of the renal net acid excretion by adults consuming diets containing variable amounts of protein. Am. J. Clin. Nutr. 1994, 59, 1356–1361. [Google Scholar] [CrossRef] [PubMed]
- Deutsches Ernährungsberatungs- & Informationsnetz (DEBInet). Available online: http://ernaehrung.de/lebensmittel/ (accessed on 12 August 2016).
- European Food Safety Authority (EFSA). The EFSA Comprehensive European Food Consumption Database. Available online: https://www.efsa.europa.eu/de/food-consumption/comprehensive-database (accessed on 12 August 2016).
- Burger, M.; Mensink, G.B.M. High alcohol consumption in Germany: Results of the German National Health Interview and Examination Survey 1998. Public Health Nutr. 2004, 7, 879–884. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mensink, G.B.M.; Beitz, R. Food and nutrient intake in East and West Germany, 8 years after the reunification—The German Nutrition Survey 1998. Eur. J. Clin. Nutr. 2004, 58, 1000–1010. [Google Scholar] [CrossRef] [PubMed]
- Levey, A.S.; Coresh, J.; Greene, T.; Marsh, J.; Stevens, L.A.; Kusek, J.W.; van Lente, F. Expressing the modification of diet in renal disease study equation for estimating glomerular filtration rate with standardized serum creatinine values. Clin. Chem. 2007, 53, 766–772. [Google Scholar] [CrossRef] [PubMed]
- Johner, S.A.; Boeing, H.; Thamm, M.; Remer, T. Urinary 24-h creatinine excretion in adults and its use as a simple tool for the estimation of daily urinary analyte excretion from analyte/creatinine ratios in populations. Eur. J. Clin. Nutr. 2015, 69, 1336–1343. [Google Scholar] [CrossRef] [PubMed]
- Lampert, T.; Kroll, L.; Muters, S.; Stolzenberg, H. Messung des soziookonomischen Status in der Studie zur Gesundheit Erwachsener in Deutschland (DEGS1). Bundesgesundheitsblatt Gesundh. Gesundh. 2013, 56, 631–636. [Google Scholar] [CrossRef] [PubMed]
- Wills, A.K.; Lawlor, D.A.; Matthews, F.E.; Sayer, A.A.; Bakra, E.; Ben-Shlomo, Y.; Benzeval, M.; Brunner, E.; Cooper, R.; Kivimaki, M.; et al. Life course trajectories of systolic blood pressure using longitudinal data from eight UK cohorts. PLoS Med. 2011, 8, e1000440. [Google Scholar] [CrossRef] [PubMed]
- Lucas, P.A.; Lacour, B.; McCarron, D.A.; Drueke, T. Disturbance of acid-base balance in the young spontaneously hypertensive rat. Clin. Sci. 1987, 73, 211–215. [Google Scholar] [CrossRef] [PubMed]
- Conen, K.; Scanni, R.; Gombert, M.-T.; Hulter, H.N.; Krapf, R. Effects of potassium citrate or potassium chloride in patients with combined glucose intolerance: A placebo-controlled pilot study. J. Diabetes Complicat. 2016, 30, 1158–1161. [Google Scholar] [CrossRef] [PubMed]
- Vongpatanasin, W.; Peri-Okonny, P.; Velasco, A.; Arbique, D.; Wang, Z.; Ravikumar, P.; Adams-Huet, B.; Moe, O.W.; Pak, C.Y.C. Effects of Potassium magnesium citrate supplementation on 24-h ambulatory blood pressure and oxidative stress marker in prehypertensive and hypertensive subjects. Am. J. Cardiol. 2016, 118, 849–853. [Google Scholar] [CrossRef] [PubMed]
- Mente, A.; O’Donnell, M.J.; Rangarajan, S.; McQueen, M.J.; Poirier, P.; Wielgosz, A.; Morrison, H.; Li, W.; Wang, X.; Di, C.; et al. Association of urinary sodium and potassium excretion with blood pressure. N. Engl. J. Med. 2014, 371, 601–611. [Google Scholar] [CrossRef] [PubMed]
- Augusto, J.-F.; Teboul, J.-L.; Radermacher, P.; Asfar, P. Interpretation of blood pressure signal: Physiological bases, clinical relevance, and objectives during shock states. Intensiv. Care Med. 2011, 37, 411–419. [Google Scholar] [CrossRef] [PubMed]
- Weiner, I.D. Untangling the complex relationship between dietary acid load and glucocorticoid metabolism. Kidney Int. 2016, 90, 247–249. [Google Scholar] [CrossRef] [PubMed]
- Esche, J.; Shi, L.; Sánchez-Guijo, A.; Hartmann, M.F.; Wudy, S.A.; Remer, T. Higher diet-dependent renal acid load associates with higher glucocorticoid secretion and potentially bioactive free glucocorticoids in healthy children. Kidney Int. 2016, 90, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Maurer, M.; Riesen, W.; Muser, J.; Hulter, H.N.; Krapf, R. Neutralization of Western diet inhibits bone resorption independently of K intake and reduces cortisol secretion in humans. Am. J. Physiol. Renal Physiol. 2003, 284, F32–F40. [Google Scholar] [CrossRef] [PubMed]
- Buehlmeier, J.; Remer, T.; Frings-Meuthen, P.; Maser-Gluth, C.; Heer, M. Glucocorticoid activity and metabolism with NaCl-induced low-grade metabolic acidosis and oral alkalization: Results of two randomized controlled trials. Endocrine 2016, 52, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Di Dalmazi, G.; Pasquali, R.; Beuschlein, F.; Reincke, M. Subclinical hypercortisolism: A state, a syndrome, or a disease? Eur. J. Endocrinol. 2015, 173, M61–M71. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Qin, T.; Chen, J.; Li, Y.; Wang, L.; Huang, H.; Li, J. Hyperuricemia and risk of incident hypertension: A systematic review and meta-analysis of observational studies. PLoS ONE 2014, 9, e114259. [Google Scholar] [CrossRef] [PubMed]
- Kanbara, A.; Miura, Y.; Hyogo, H.; Chayama, K.; Seyama, I. Effect of urine pH changed by dietary intervention on uric acid clearance mechanism of pH-dependent excretion of urinary uric acid. Nutr. J. 2012, 11, 39. [Google Scholar] [CrossRef] [PubMed]
- David, L.A.; Maurice, C.F.; Carmody, R.N.; Gootenberg, D.B.; Button, J.E.; Wolfe, B.E.; Ling, A.V.; Devlin, A.S.; Varma, Y.; Fischbach, M.A.; et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2014, 505, 559–563. [Google Scholar] [CrossRef] [PubMed]
- Jose, P.A.; Raj, D. Gut microbiota in hypertension. Curr. Opin. Nephrol. Hypertens. 2015, 24, 403–409. [Google Scholar] [CrossRef] [PubMed]
- Rebholz, C.M.; Coresh, J.; Grams, M.E.; Steffen, L.M.; Anderson, C.A.M.; Appel, L.J.; Crews, D.C. Dietary acid load and incident chronic Kidney Disease: Results from the ARIC study. Am. J. Nephrol. 2015, 42, 427–435. [Google Scholar] [CrossRef] [PubMed]
- Goraya, N.; Simoni, J.; Jo, C.-H.; Wesson, D.E. Treatment of metabolic acidosis in patients with stage 3 chronic kidney disease with fruits and vegetables or oral bicarbonate reduces urine angiotensinogen and preserves glomerular filtration rate. Kidney Int. 2014, 86, 1031–1038. [Google Scholar] [CrossRef] [PubMed]
- van den Berg, E.; Hospers, F.A.P.; Navis, G.; Engberink, M.F.; Brink, E.J.; Geleijnse, J.M.; van Baak, M.A.; Gans, R.O.B.; Bakker, S.J.L. Dietary acid load and rapid progression to end-stage renal disease of diabetic nephropathy in Westernized South Asian people. J. Nephrol. 2011, 24, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Mirmiran, P.; Yuzbashian, E.; Bahadoran, Z.; Asghari, G.; Azizi, F. Dietary acid-base load and risk of chronic Kidney Disease in adults: Tehran lipid and glucose study. Iran. J. Kidney Dis. 2016, 10, 119–125. [Google Scholar] [PubMed]
- Akter, S.; Eguchi, M.; Kuwahara, K.; Kochi, T.; Ito, R.; Kurotani, K.; Tsuruoka, H.; Nanri, A.; Kabe, I.; Mizoue, T. High dietary acid load is associated with insulin resistance: The Furukawa Nutrition and health study. Clin. Nutr. 2016, 35, 453–459. [Google Scholar] [CrossRef] [PubMed]
- Johner, S.A.; Thamm, M.; Schmitz, R.; Remer, T. Current daily salt intake in Germany: Biomarker-based analysis of the representative DEGS study. Eur. J. Nutr. 2015, 54, 1109–1115. [Google Scholar] [CrossRef] [PubMed]
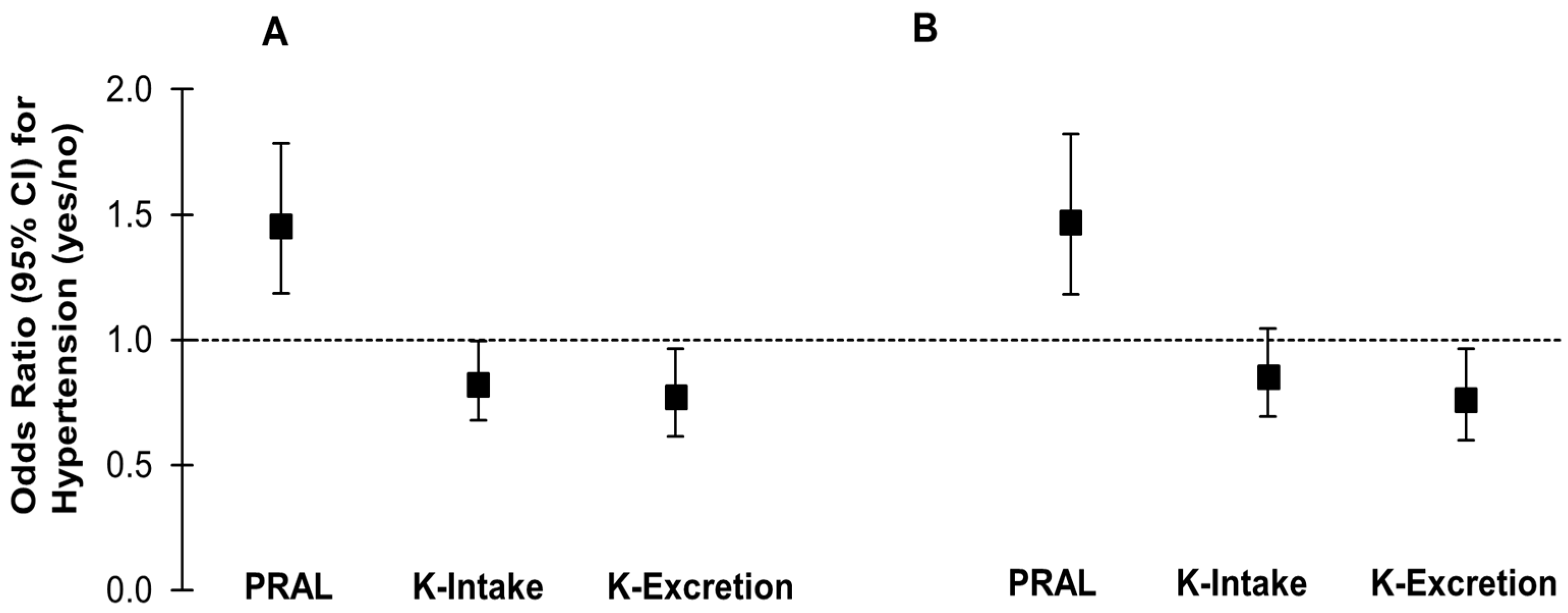
| Median PRAL, mEq/Day | −30.8 (−44.3, −23.7) | −12.7 (−16.9, −7.9) | −4.5 (−7.7, 0.4) | 3.9 (−0.6, 7.8) | 15.5 (9.3, 23.2) | p |
|---|---|---|---|---|---|---|
| n | 1356 | 1358 | 1358 | 1358 | 1358 | |
| Women, % | 49.4 (46.2, 52.7) | 51.2 (47.9, 54.5) | 52.4 (48.9, 55.9) | 49.7 (46.1, 53.4) | 49.4 (46.1, 52.7) | 0.7 |
| Age, years a | 49.9 (48.9, 50.9) | 52.5 (51.3, 53.7) | 50.1 (49.0, 51.3) | 45.7 (44.6, 46.8) | 40.4 (39.4, 41.3) | <0.0001 |
| BMI, kg/m2 a | 27.0 (26.6, 27.3) | 26.9 (26.6, 27.3) | 27.0 (26.6, 27.3) | 26.9 (26.6, 27.3) | 26.4 (26.0, 26.8) | 0.1 |
| Systolic BP, mmHg a | 123.8 (122.8, 124.7) | 124.7 (123.5, 125.9) | 124.6 (123.5, 125.6) | 123.9 (122.9, 124.9) | 123.7 (122.7, 124.7) | 0.5 |
| Diastolic BP, mmHg a | 73.4 (72.7, 74.0) | 73.5 (72.8, 74.2) | 73.5 (72.9, 74.1) | 73.1 (72.5, 73.7) | 72.9 (72.2, 73.6) | 0.7 |
| Hypertension prevalence b, % | 31.8 (28.8, 34.8) | 36.3 (32.7, 40.0) | 34.9 (31.7, 38.1) | 32.2 (28.7, 35.7) | 24.6 (22.0, 27.3) | <0.0001 |
| Diuretic use, % | 4.4 (3.3, 5.5) | 5.6 (4.1, 7.1) | 5.4 (3.8, 7.0) | 5.4 (3.8, 6.9) | 2.4 (1.6, 3.1) | 0.001 |
| Beta blocker use, % | 15.1 (12.9, 17.3) | 17.8 (15.2, 20.4) | 15.3 (12.8, 17.8) | 14.5 (11.9, 17.0) | 9.4 (7.8, 11.0) | <0.0001 |
| Total cholesterol, mg/dL a | 203.6 (200.6, 206.6) | 207.7 (204.4, 210.9) | 205.1 (201.3, 209.0) | 201.6 (198.5, 204.7) | 196.2 (193.2, 199.2) | <0.0001 |
| Estimated GFR c, mL/min/1.73 m2 a | 93.3 (91.5, 95.1) | 89.6 (87.8, 91.5) | 92.5 (90.5, 94.4) | 93.6 (91.8, 95.4) | 99.0 (96.9, 101.1) | <0.0001 |
| Smoking | ||||||
| Daily or occasionally, % | 32.6 (29.2, 36.0) | 22.8 (19.9, 25.8) | 25.9 (23.0, 28.9) | 30.7 (27.3, 34.0) | 34.0 (30.6, 37.4) | <0.0001 |
| Former smoker, % | 27.3 (24.4, 30.2) | 32.0 (28.9, 35.0) | 32.1 (29.3, 35.0) | 27.8 (24.7, 31.0) | 23.2 (20.4, 26.1) | |
| Never smoker, % | 40.1 (36.6, 43.6) | 45.2 (42.0, 48.4) | 41.9 (38.6, 45.2) | 41.5 (38.0, 45.0) | 42.7 (39.4, 46.1) | |
| Sports activity | ||||||
| No sports activity, % | 32.3 (28.8, 35.8) | 31.8 (28.2, 35.3) | 31.3 (27.8, 34.8) | 32.4 (28.9, 36.0) | 34.8 (31.7, 38.0) | 0.2 |
| <2 h per week, % | 38.8 (35.3, 42.4) | 43.5 (40.1, 46.9) | 42.6 (39.2, 46.0) | 44.2 (40.8, 47.6) | 40.4 (37.3, 43.5) | |
| >2 h per week, % | 28.9 (25.3, 32.4) | 24.8 (21.9, 27.7) | 26.1 (22.8, 29.4) | 23.4 (20.3, 26.6) | 24.8 (21.9, 27.7) | |
| Socioeconomic Status (SES) | ||||||
| Low | 18.4 (15.7, 21.0) | 17.1 (14.1, 20.1) | 18.9 (15.9, 22.0) | 16.3 (13.5, 19.1) | 22.8 (20.0, 25.7) | <0.0001 |
| Medium | 62.0 (58.5, 65.5) | 58.4 (54.7, 62.0) | 58.9 (55.5, 62.4) | 62.6 (59.3, 66.0) | 61.9 (59.1, 64.6) | |
| High | 19.6 (16.8, 22.4) | 24.5 (21.3, 27.7) | 22.2 (19.0, 25.3) | 21.1 (18.3, 23.9) | 15.3 (13.0, 17.6) | |
| Alcohol | ||||||
| 0 g/day, % | 15.7 (13.2, 18.2) | 11.3 (8.7, 13.9) | 16.4 (13.7, 19.1) | 13.2 (11.0, 15.5) | 16.3 (13.5, 19.0) | 0.04 |
| <10/20 g/day, % | 67.9 (64.8, 71.1) | 73.8 (70.8, 76.9) | 67.0 (63.7, 70.3) | 71.6 (68.5, 74.6) | 67.6 (64.6, 70.5) | |
| >10/20 g/day, % | 16.4 (13.6, 19.1) | 14.9 (12.5, 17.2) | 16.6 (14.1, 19.0) | 15.2 (13.0, 17.5) | 16.2 (14.1, 18.3) | |
| Estimated urinary Na-Excretion, mmol/day d | 161.1 (103.2, 242.5) | 156.0 (97.1, 232.4) | 157.1 (102.5, 227.2) | 160.7 (98.9, 232.9) | 165.1 (103.9, 236.2) | 0.2 |
| Estimated salt intake, g/day d | 9.4 (6.0, 14.2) | 9.1 (5.7, 13.6) | 9.2 (6.0, 13.3) | 9.4 (5.8, 13.6) | 9.6 (6.1, 13.8) | 0.2 |
| Estimated K-Excretion, mmol/day d | 93.1 (67.9, 121.9) | 84.9 (64.5, 112.2) | 85.2 (59.9, 113.8) | 78.9 (60.4, 106.0) | 73.9 (54.2, 102.1) | <0.0001 |
| Estimated K-Intake, mg/day d | 4403 (3540, 5664) | 3120 (2595, 3785) | 2700 (2185, 3407) | 2619 (1975, 3207) | 2793 (2196, 3606) | <0.0001 |
| Meat consumption e, g/day d | 66.4 (37.9, 103.2) | 66.2 (40.0, 97.8) | 67.6 (42.6, 97.8) | 81.3 (49.2, 117.9) | 118.0 (78.2, 183.3) | <0.0001 |
| Milk product consumption f, g/day d | 314.6 (142.9, 616.3) | 262.1 (130.8, 457.6) | 242.2 (127.1, 428.9) | 242.6 (128.5, 425.6) | 245.6 (127.9, 457.3) | <0.0001 |
| Fruit and vegetable consumption, g/day d | 461.1 (232.4, 827.9) | 357.7 (213.5, 530.7) | 269.8 (166.1, 420.5) | 211.6 (128.1, 330.3) | 179.3 (97.2, 303.8) | <0.0001 |
| Total Sample (n = 6788) | ||||
|---|---|---|---|---|
| Predictor | Outcome | β (95% CI) | Ptrend | R2 |
| Systolic blood pressure | ||||
| PRAL (FFQ), mEq/day | Basic model a | 0.0486 (0.0216, 0.0756) | 0.0005 c | 0.1570 |
| Adjusted model b | 0.0521 (0.0250, 0.0792) | 0.0002 c | 0.1927 | |
| K-Intake (FFQ), g/day | Basic model a | −0.3327 (−0.7114, 0.0461) | 0.08 | 0.1551 |
| Adjusted model b | −0.3969 (−0.7734, −0.0204) | 0.04 c | 0.1906 | |
| K-Excretion, mmol/day | Basic model a | −0.0119 (−0.0235, −0.0003) | 0.04 c | 0.1551 |
| Adjusted model b | −0.0330 (−0.0455, −0.0205) | <0.0001 c | 0.1944 | |
| Diastolic blood pressure | ||||
| PRAL (FFQ), mEq/day | Basic model a | 0.0119 (−0.0070, 0.0308) | 0.2 | 0.1032 |
| Adjusted model b | 0.0148 (−0.0038, 0.0334) | 0.1 | 0.1481 | |
| K-Intake (FFQ), g/day | Basic model a | −0.1727 (−0.4088, 0.0634) | 0.2 | 0.1033 |
| Adjusted model b | −0.2546 (−0.4891, −0.0202) | 0.03 c | 0.1484 | |
| K-Excretion, mmol/day | Basic model a | −0.0069 (−0.0141, 0.0004) | 0.06 | 0.1034 |
| Adjusted model b | −0.0154 (−0.0236, −0.0071) | 0.0003 c | 0.1500 | |
| Sample without Antihypertensive Medication (n = 4677) | ||||
|---|---|---|---|---|
| Predictor | Outcome | β (95% CI) | Ptrend | R2 |
| Systolic blood pressure | ||||
| PRAL (FFQ), mEq/day | Basic model a | 0.0375 (0.0094, 0.0657) | 0.009 c | 0.2059 |
| Adjusted model b | 0.0375 (0.0086, 0.0664) | 0.01 c | 0.2385 | |
| K-Intake (FFQ), g/day | Basic model a | −0.2648 (−0.685, 0.1553) | 0.2 | 0.2045 |
| Adjusted model b | −0.2210 (−0.6379, 0.1960) | 0.3 | 0.2370 | |
| K-Excretion, mmol/day | Basic model a | −0.0128 (−0.0257, 0.0002) | 0.05 | 0.2050 |
| Adjusted model b | −0.0280 (−0.0420, −0.0140) | 0.0001 c | 0.2406 | |
| Diastolic blood pressure | ||||
| PRAL (FFQ), mEq/day | Basic model a | 0.0068 (−0.0119, 0.0256) | 0.5 | 0.1878 |
| Adjusted model b | 0.0064 (−0.0129, 0.0258) | 0.5 | 0.2132 | |
| K-Intake (FFQ), g/day | Basic model a | −0.1954 (−0.4718, 0.0810) | 0.2 | 0.1882 |
| Adjusted model b | −0.1880 (−0.4597, 0.0836) | 0.2 | 0.2136 | |
| K-Excretion, mmol/day | Basic model a | −0.0085 (−0.0167, −0.0002) | 0.04 c | 0.1886 |
| Adjusted model b | −0.0144 (−0.0238, −0.0049) | 0.003 c | 0.2154 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krupp, D.; Esche, J.; Mensink, G.B.M.; Klenow, S.; Thamm, M.; Remer, T. Dietary Acid Load and Potassium Intake Associate with Blood Pressure and Hypertension Prevalence in a Representative Sample of the German Adult Population. Nutrients 2018, 10, 103. https://doi.org/10.3390/nu10010103
Krupp D, Esche J, Mensink GBM, Klenow S, Thamm M, Remer T. Dietary Acid Load and Potassium Intake Associate with Blood Pressure and Hypertension Prevalence in a Representative Sample of the German Adult Population. Nutrients. 2018; 10(1):103. https://doi.org/10.3390/nu10010103
Chicago/Turabian StyleKrupp, Danika, Jonas Esche, Gert Bernardus Maria Mensink, Stefanie Klenow, Michael Thamm, and Thomas Remer. 2018. "Dietary Acid Load and Potassium Intake Associate with Blood Pressure and Hypertension Prevalence in a Representative Sample of the German Adult Population" Nutrients 10, no. 1: 103. https://doi.org/10.3390/nu10010103
APA StyleKrupp, D., Esche, J., Mensink, G. B. M., Klenow, S., Thamm, M., & Remer, T. (2018). Dietary Acid Load and Potassium Intake Associate with Blood Pressure and Hypertension Prevalence in a Representative Sample of the German Adult Population. Nutrients, 10(1), 103. https://doi.org/10.3390/nu10010103





