Land Cover, Land Use, and Climate Change Impacts on Endemic Cichlid Habitats in Northern Tanzania
Abstract
:1. Introduction
2. Materials and Methods
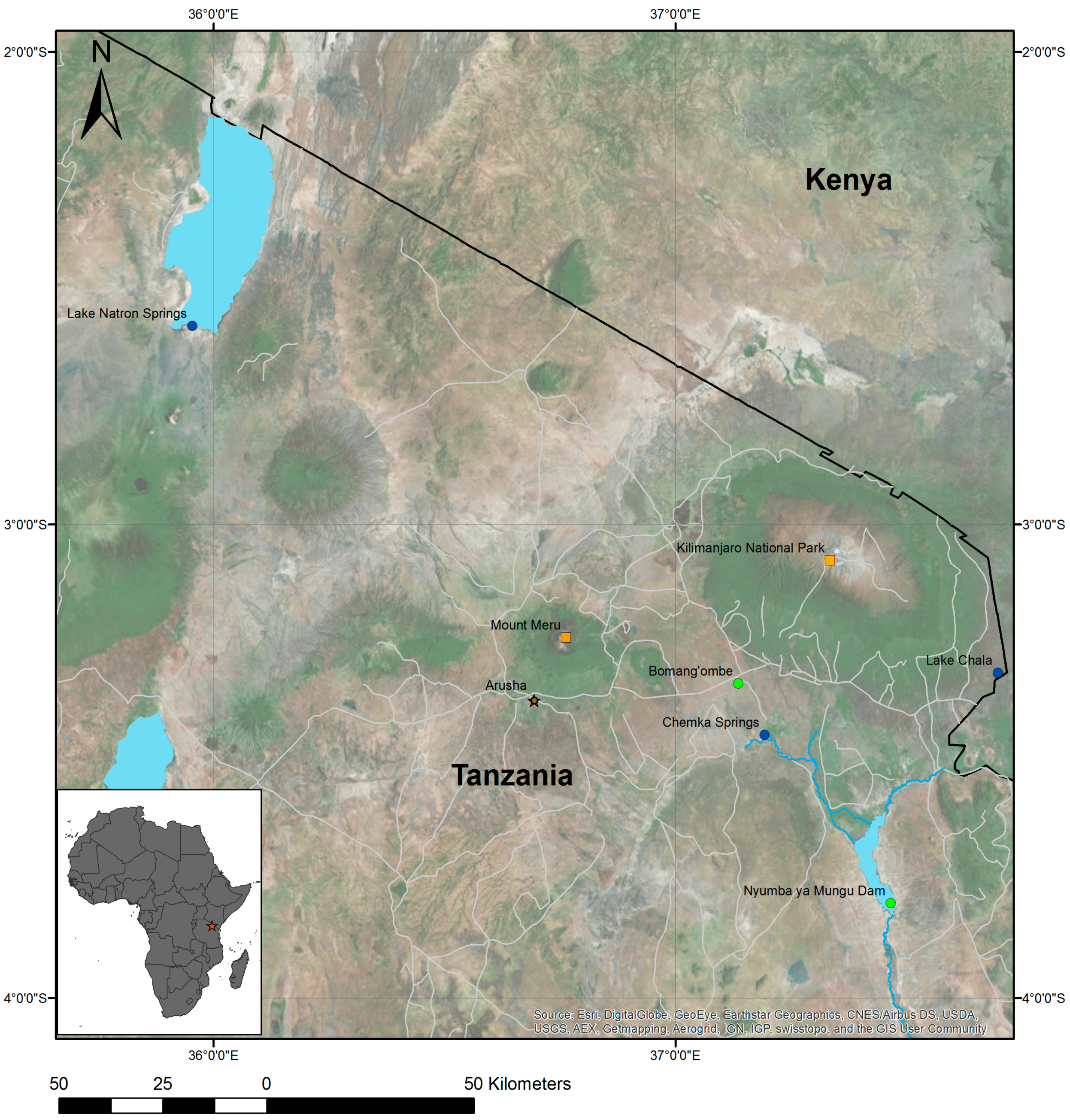
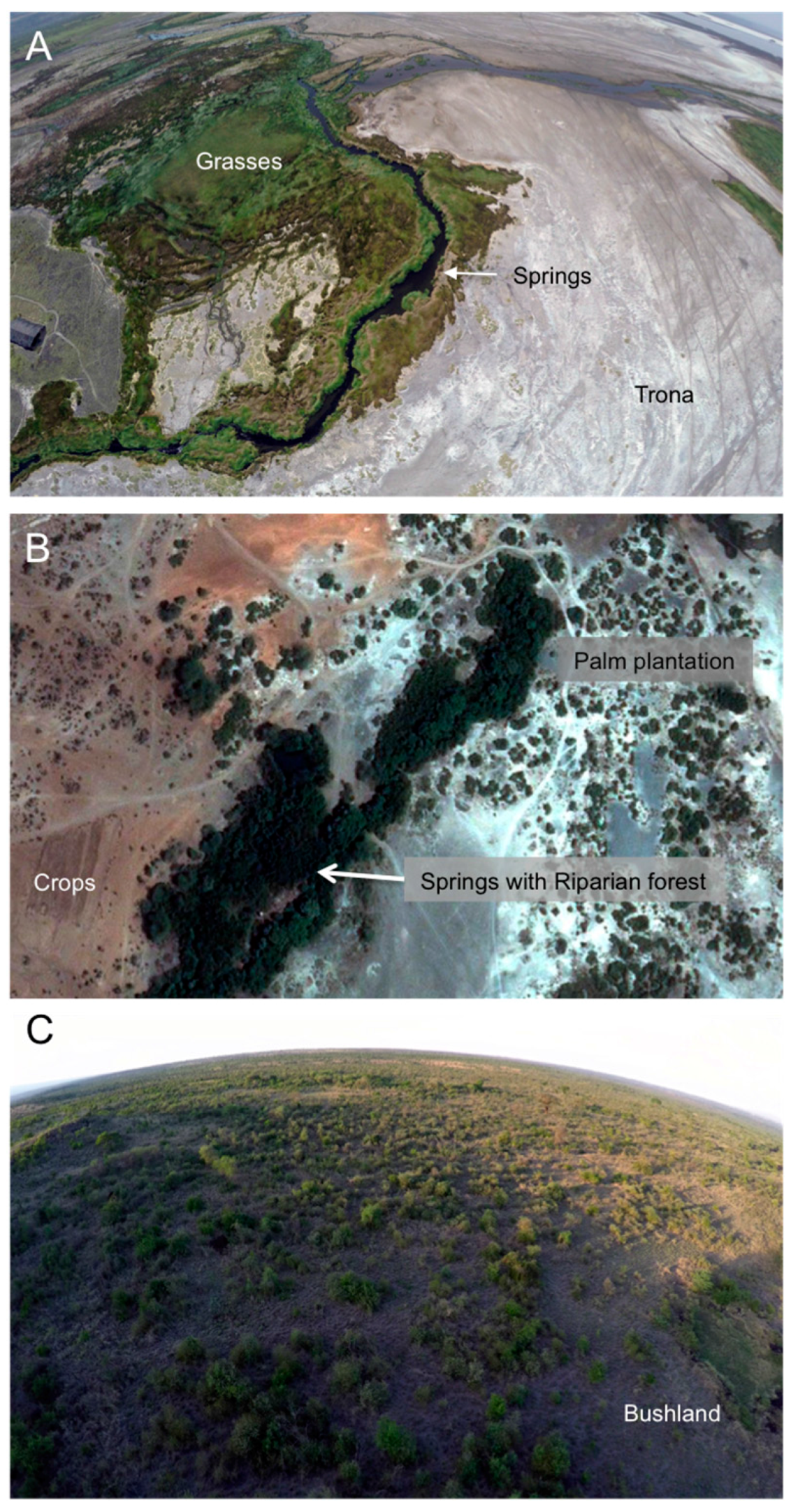
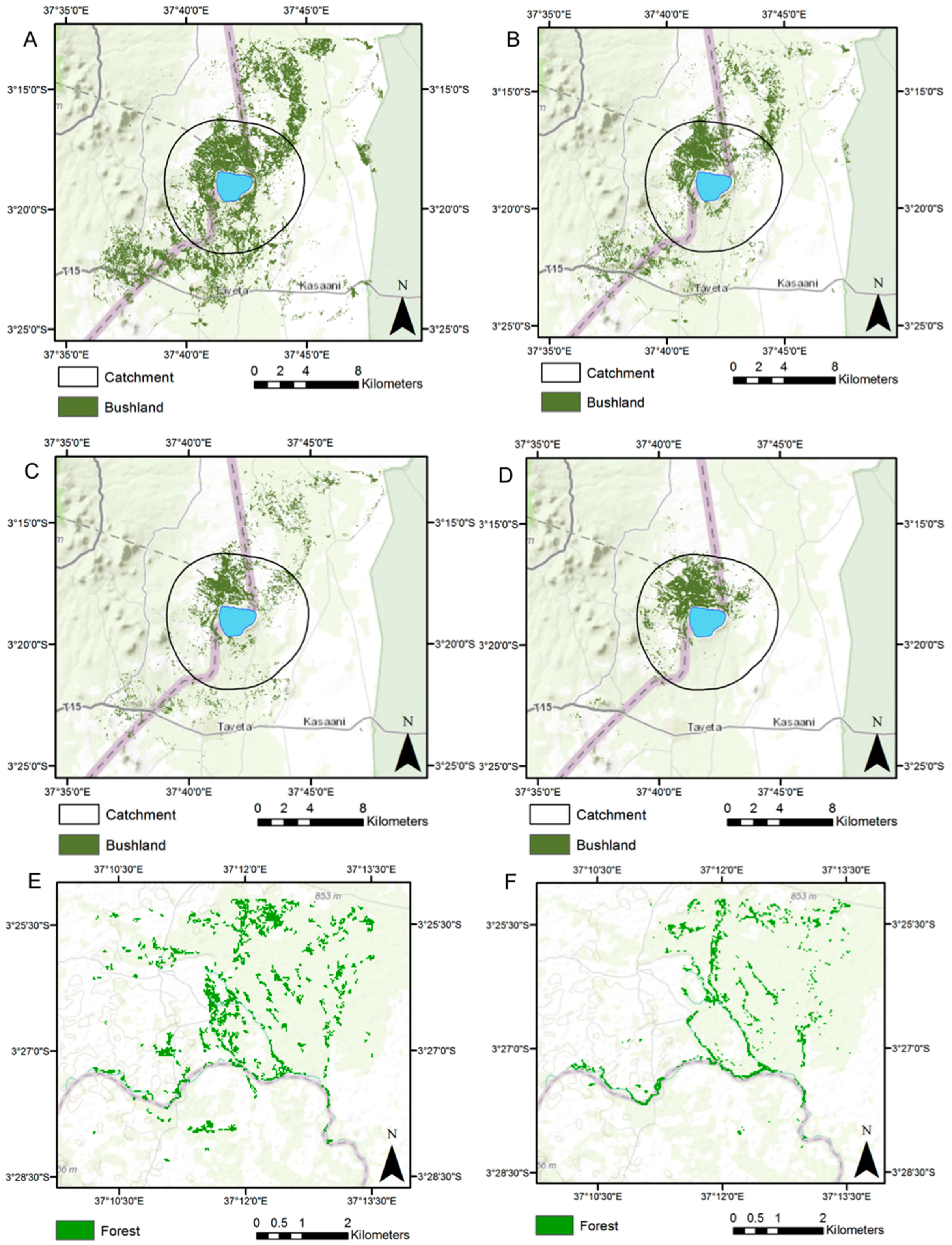
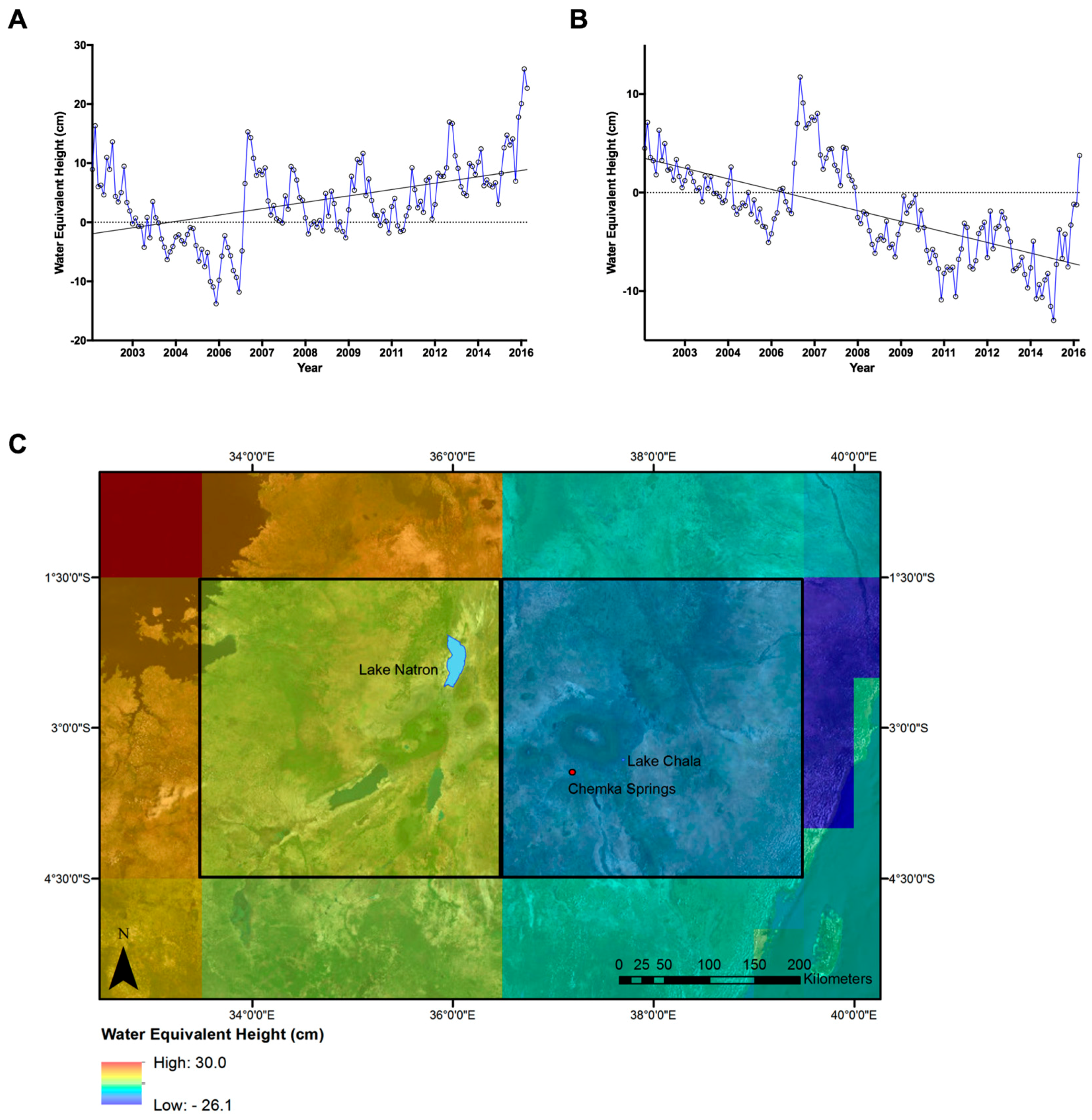
2.1. Study Areas
2.2. Remotely Sensed Data
2.3. Population Growth, Agriculture Expansion and Climate Change Data
2.4. Image Analysis
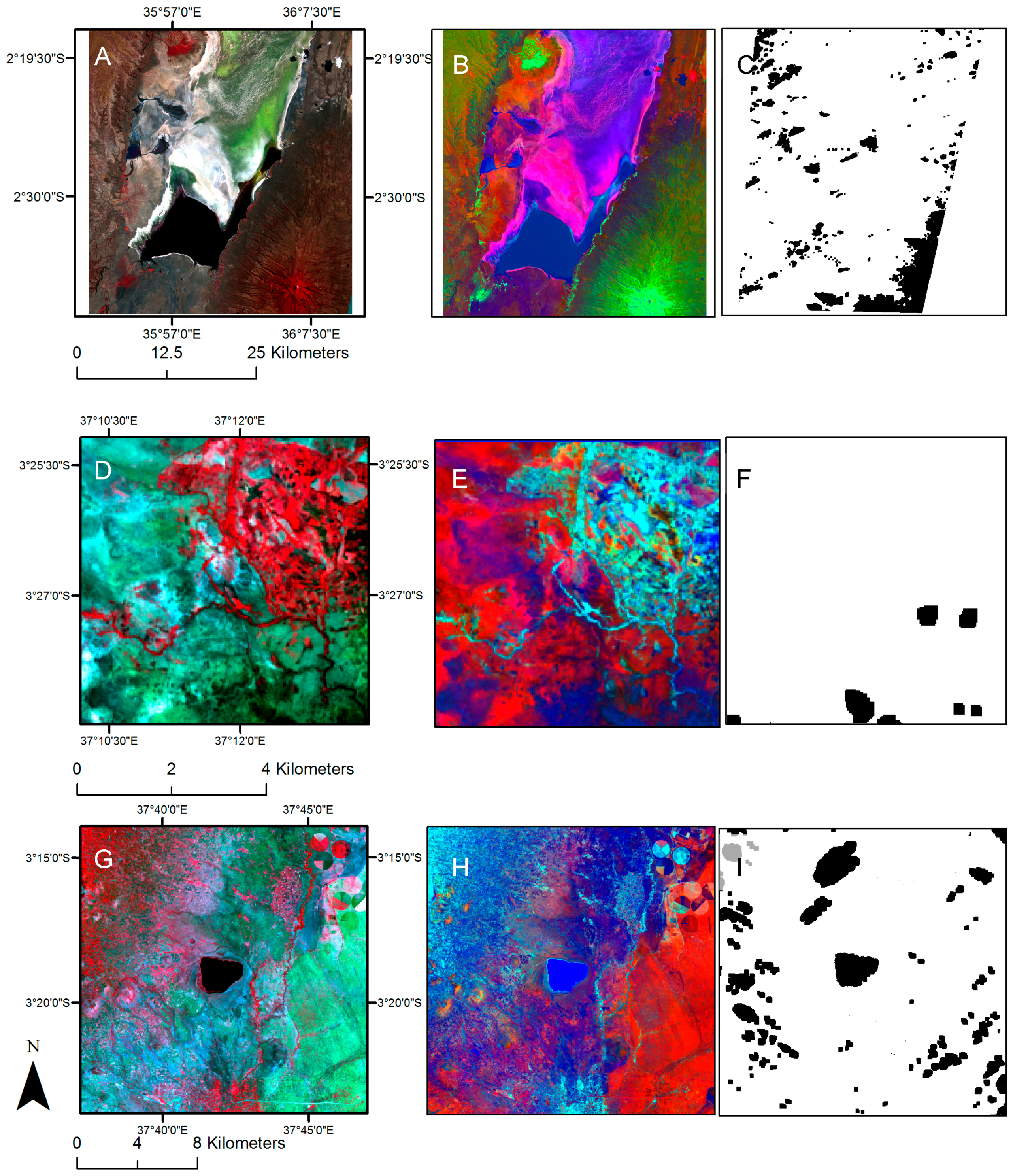
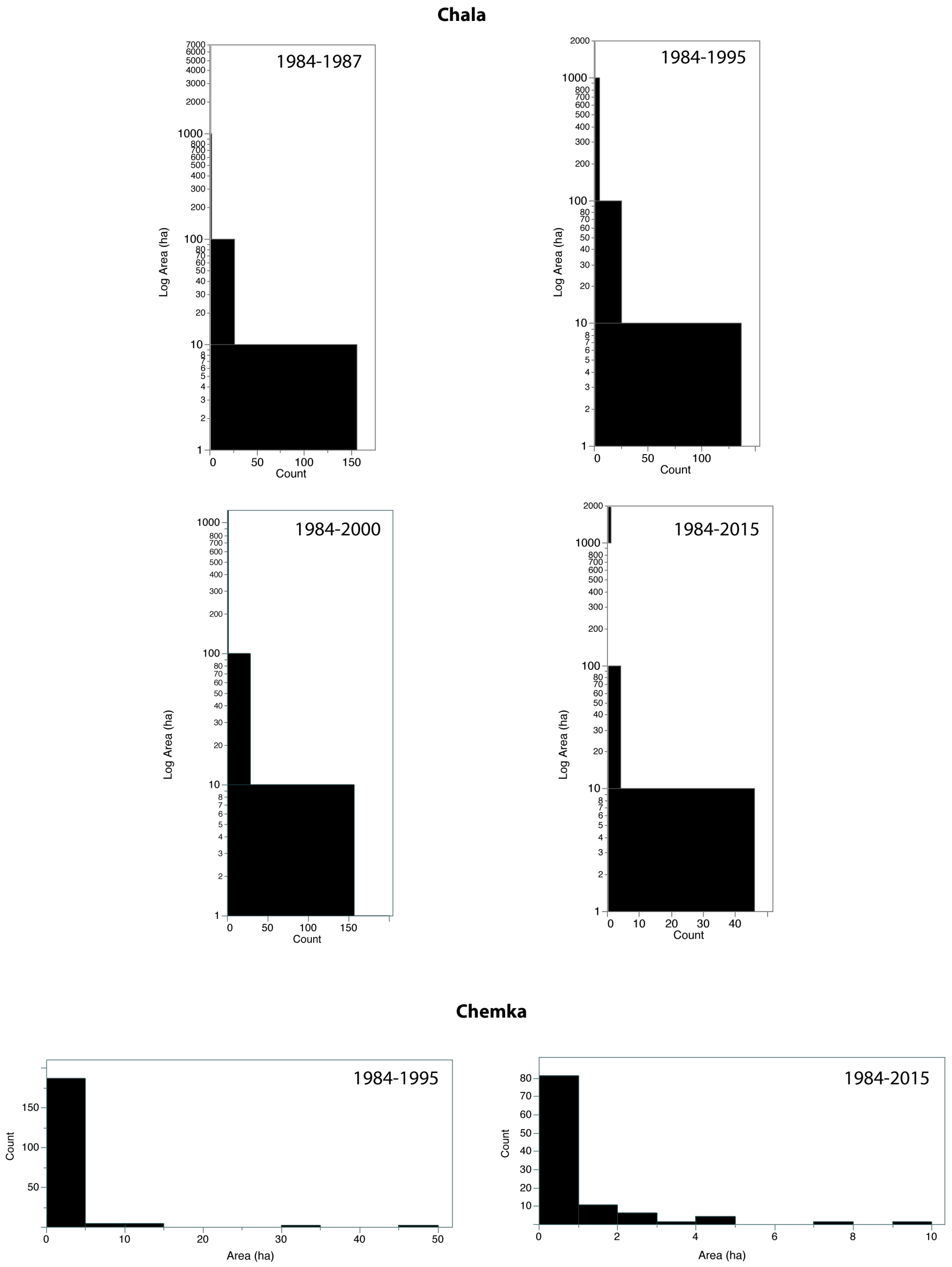
2.5. Fragmentation Analysis
3. Results
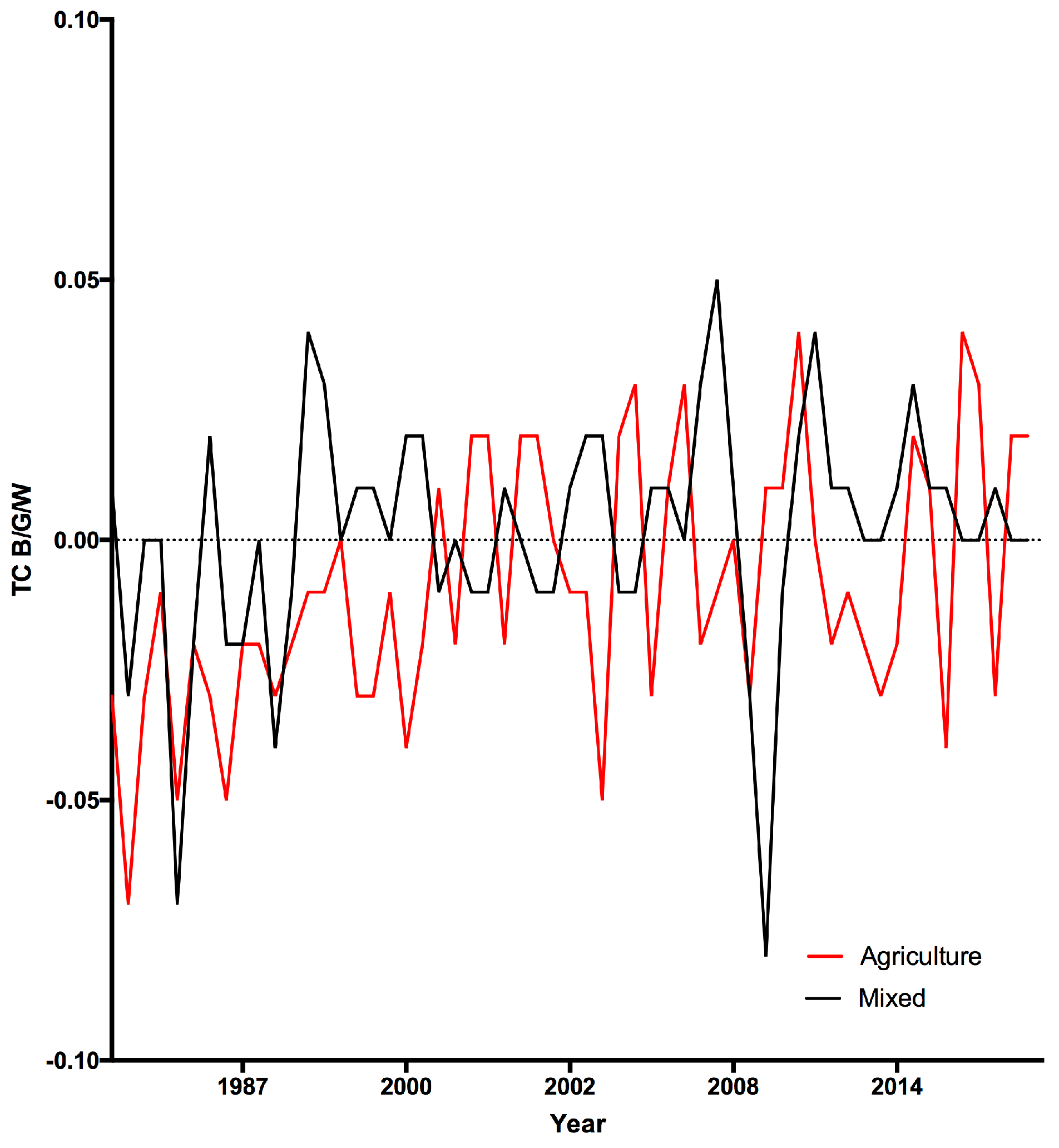
3.1. Land Cover Change
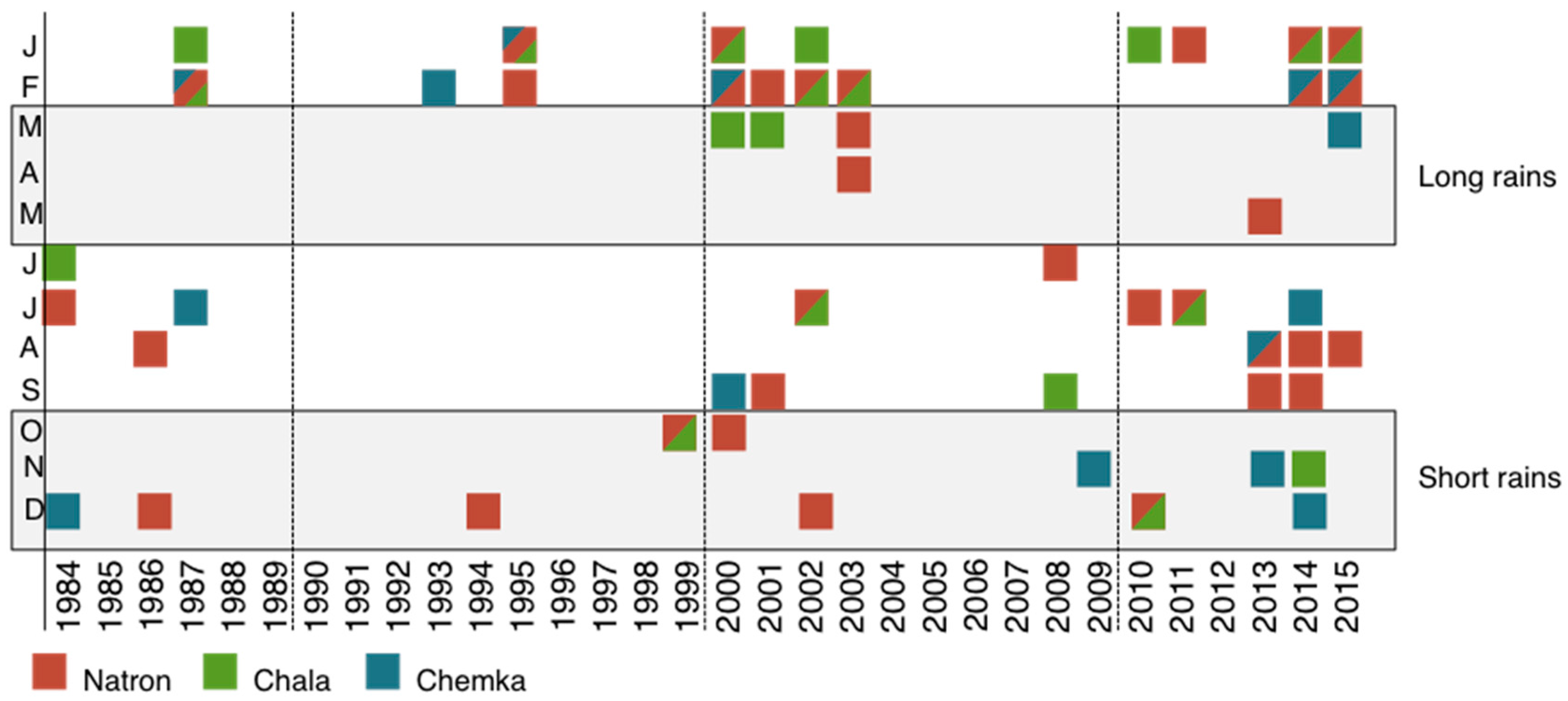
3.2. GRACE Satellite Subsurface Water Data
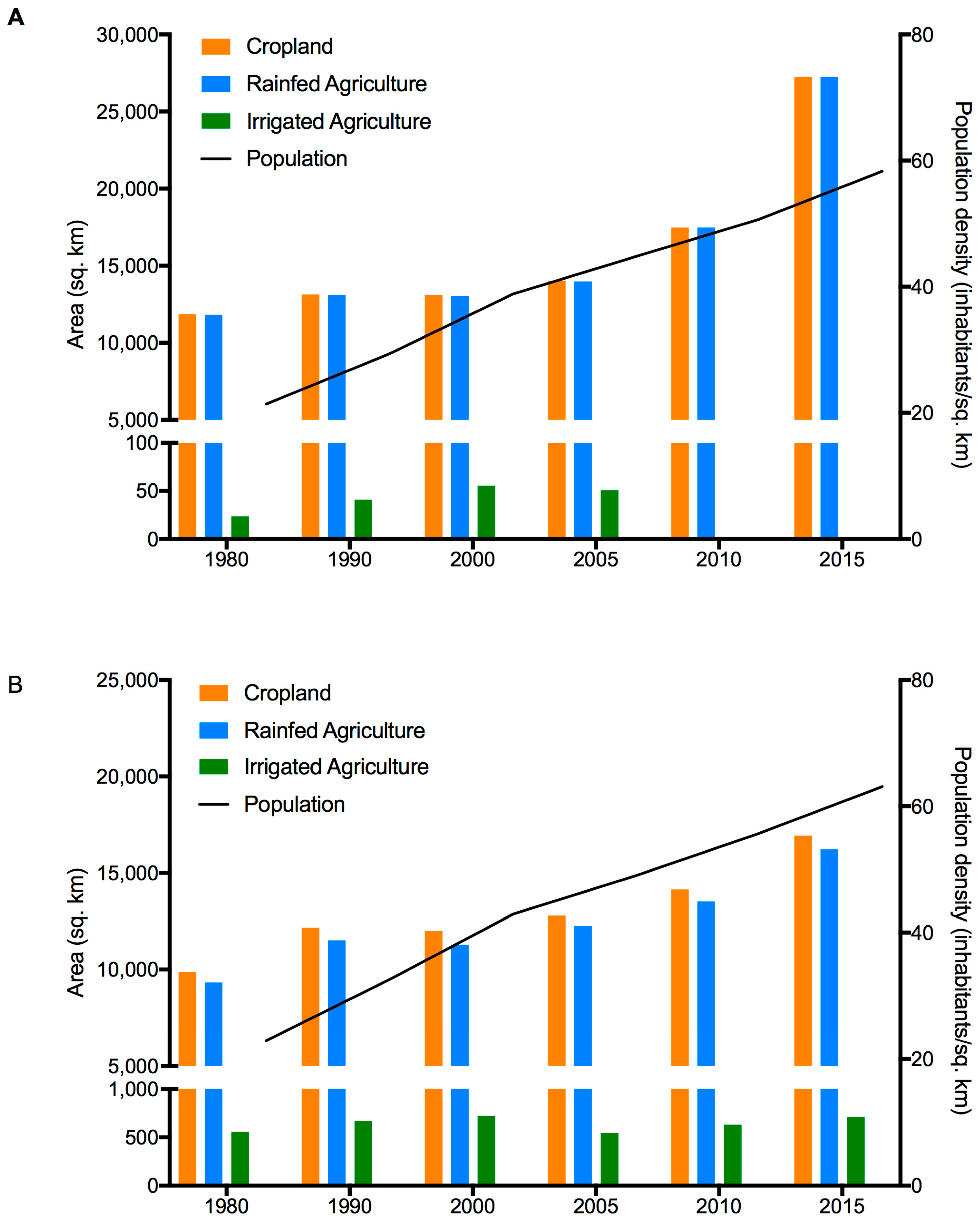
3.3. Population and Climate Model Results
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Geist, J. Integrative freshwater ecology and biodiversity conservation. Ecol. Indic. 2011, 11, 1507–1516. [Google Scholar] [CrossRef]
- Sundermann, A.; Gerhardt, M.; Kappes, H.; Haase, P. Stressor prioritisation in riverine ecosystems: Which environmental factors shape benthic invertebrate assemblage metrics? Ecol. Indic. 2013, 27, 83–96. [Google Scholar] [CrossRef]
- Bootsma, H.A.; Hecky, R.E. A comparative introduction to the biology and limnology of the African Great Lakes. J. Gt. Lakes Res. 2003, 29, 3–18. [Google Scholar] [CrossRef]
- Guse, B.; Kail, J.; Radinger, J.; Schroder, M.; Kiesel, J.; Hering, D.; Wolter, C.; Fohrer, N. Eco-hydrologic model cascades: Simulating land use and climate change impacts on hydrology, hydraulics and habitats for fish and macroinvertebrates. Sci. Total Environ. 2015, 533, 542–556. [Google Scholar] [CrossRef] [PubMed]
- Mantyka-Pringle, C.S.; Martin, T.G.; Moffatt, D.B.; Linke, S.; Rhodes, J.R. Understanding and predicting the combined effects of climate change and land-use change on freshwater macroinvertebrates and fish. J. Appl. Ecol. 2014, 51, 572–581. [Google Scholar] [CrossRef]
- Olden, J.D.; Hogan, Z.S.; Vander Zanden, M.J. Small fish, big fish, red fish, blue fish: Size-biased extinction risk of the world’s freshwater and marine fishes. Glob. Ecol. Biogeogr. 2007, 16, 694–701. [Google Scholar] [CrossRef]
- Reyolds, J.D.; Dulvy, N.K.; Roberta, C.M. Exploitation and other threats to fish conservation. In Handbook of Fish Biology and Fisheries: Volume 2; Hart, P.J.B., Reynolds, J.D., Eds.; Blackwell Publishing: Oxford, UK, 2002; pp. 319–341. [Google Scholar]
- Sodhi, N.S.; Brook, B.W.; Bradshaw, C.A.J. Causes and consequences of species extinctions. In Princeton Guide to Ecology; Levin, S.A., Ed.; Princeton University Press: Princeton, NJ, USA, 2009; pp. 514–520. [Google Scholar]
- Jelks, H.L.; Walsh, S.J.; Burkhead, N.M.; Contreras-Balderas, S.; Diaz-Pardo, E.; Hendrickson, D.A.; Lyons, J.; Mandrak, N.E.; McCormick, F.; Nelson, J.S.; et al. Conservation Status of Imperiled North American Freshwater and Diadromous Fishes. Fisheries 2008, 33, 372–407. [Google Scholar] [CrossRef]
- Harding, J.S.; Benfield, E.F.; Bolstad, P.V.; Helfman, G.S.; Jones, E.B.D. Stream biodiversity: The ghost of land use past. Proc. Natl. Acad. Sci. USA 1998, 95, 14843–14847. [Google Scholar] [CrossRef] [PubMed]
- Downing, A.S.; van Nes, E.H.; Balirwa, J.S.; Beuving, J.; Bwathondi, P.O.J.; Chapman, L.J.; Cornelissen, I.J.M.; Cowx, I.G.; Goudswaard, K.P.C.; Hecky, R.E.; et al. Coupled human and natural system dynamics as key to the sustainability of Lake Victoria’s ecosystem services. Ecol. Soc. 2014, 19. [Google Scholar] [CrossRef]
- Goldewijk, K.K.; Beusen, A.; van Drecht, G.; de Vos, M. The HYDE 3.1 spatially explicit database of human-induced global land-use change over the past 12,000 years. Glob. Ecol. Biogeogr. 2011, 20, 73–86. [Google Scholar] [CrossRef]
- Hijmans, R.J.; Cameron, S.E.; Parra, J.L.; Jones, P.G.; Jarvis, A. Very high resolution interpolated climate surfaces for global land areas. Int. J. Climatol. 2005, 25, 1965–1978. [Google Scholar] [CrossRef]
- Jones, P.D.; Lister, D.H.; Osborn, T.J.; Harpham, C.; Salmon, M.; Morice, C.P. Hemispheric and large-scale land-surface air temperature variations: An extensive revision and an update to 2010. J. Geophys. Res. Atmos. 2012, 117, D05127. [Google Scholar] [CrossRef]
- McKee, J.K.; Sciulli, P.W.; Fooce, C.D.; Waite, T.A. Forecasting global biodiversity threats associated with human population growth. Biol. Conserv. 2004, 115, 161–164. [Google Scholar] [CrossRef]
- Detsch, F.; Otte, I.; Appelhans, T.; Hemp, A.; Nauss, T. Seasonal and long-term vegetation dynamics from 1-km GIMMS-based NDVI time series at Mt. Kilimanjaro, Tanzania. Remote Sens. Environ. 2016, 178, 70–83. [Google Scholar] [CrossRef]
- Kashaigili, J.J.; Majaliwa, A.M. Integrated assessment of land use and cover changes in the Malagarasi river catchment in Tanzania. Phys. Chem. Earth 2010, 35, 730–741. [Google Scholar] [CrossRef]
- Kashaigili, J.J.; Mbilinyi, B.P.; McCartney, M.; Mwanuzi, F.L. Dynamics of Usangu plains wetlands: Use of remote sensing and GIS as management decision tools. Phys. Chem. Earth 2006, 31, 967–975. [Google Scholar] [CrossRef]
- Thieme, M.; Abell, R.; Stiassny, M.L.J.; Skelton, P.; Lehnher, B.; Teugels, G.G.; Dinerstein, E.; Kamden-Toham, A.; Burgess, N.; Olson, D. Freshwater Ecoregions of Arica and Madagascar: A Conservation Assessment; Island Press: Washington, DC, USA, 2005. [Google Scholar]
- Dorn, A.; Musilova, Z.; Platzer, M.; Reichwald, K.; Cellerino, A. The strange case of East African annual fishes: Aridification correlates with diversification for a savannah aquatic group? BMC Evolut. Biol. 2014, 14. [Google Scholar] [CrossRef] [PubMed]
- Friel, J.P.; Vigliotta, T.R. Three new species of African suckermouth catfishes, genus Chiloglanis (Siluriformes: Mochokidae), from the lower Malagarasi and Luiche rivers of western Tanzania. Zootaxa 2011, 3063, 1–21. [Google Scholar]
- Schedel, F.D.B.; Friel, J.P.; Schliewen, U.K. Haplochromis vanheusdeni, a new haplochromine cichlid species from the Great Ruaha River drainage, Rufiji basin, Tanzania (Teleostei, Perciformes, Cichlidae). Spixiana 2014, 37, 135–149. [Google Scholar]
- Schliewen, U.K. Cichlid species flocks in small Cameroonian Lakes. In Freshwater Ecoregions of Africa and Madagascar; Thieme, M., Abell, R., Stiassny, M.L.J., Skelton, P., Lehnher, B., Teugels, G.G., Dinerstein, E., Kamden Tohan, A., Burgess, N., Olson, D., Eds.; Island Press: Washington, DC, USA, 2005; pp. 58–60. [Google Scholar]
- Dudgeon, D.; Arthington, A.H.; Gessner, M.O.; Kawabata, Z.I.; Knowler, D.J.; Leveque, C.; Naiman, R.J.; Prieur-Richard, A.H.; Soto, D.; Stiassny, M.L.J.; et al. Freshwater biodiversity: Importance, threats, status and conservation challenges. Biol. Rev. 2006, 81, 163–182. [Google Scholar] [CrossRef] [PubMed]
- Parry, M.; Canziani, O.; Palutikof, J.; van der Linden, P.; Hanson, C. Climate Change 2007: Impacts, Adaptation and Vulnerability; Working Group II Contribution to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: New York, NY USA, 2007. [Google Scholar]
- McDonnell, L.H.; Chapman, L.J. At the edge of the thermal window: Effects of elevated temperature on the resting metabolism, hypoxia tolerance and upper critical thermal limit of a widespread African cichlid. Conserv. Physiol. 2015, 3, 13. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, A.; Cyrino, J.E.P.; Duarte-Neto, P.J.; Martinelli, L.A. Permeability of riparian forest strips in agricultural, small subtropical watersheds in south-eastern Brazil. Mar. Freshw. Res. 2012, 63, 1272–1282. [Google Scholar] [CrossRef]
- Kyriakeas, S.A.; Watzin, M.C. Effects of adjacent agricultural activities and watershed characteristics on stream macroinvertebrate communities. J. Am. Water Resour. Assoc. 2006, 42, 425–441. [Google Scholar] [CrossRef]
- Kasangaki, A.; Chapman, L.J.; Balirwa, J. Land use and the ecology of benthic macroinvertebrate assemblages of high-altitude rainforest streams in Uganda. Freshw. Biol. 2008, 53, 681–697. [Google Scholar] [CrossRef]
- Iniguez-Armijos, C.; Leiva, A.; Frede, H.G.; Hampel, H.; Breuer, L. Deforestation and Benthic Indicators: How Much Vegetation Cover Is Needed to Sustain Healthy Andean Streams? PLoS ONE 2014, 9, e105869. [Google Scholar] [CrossRef] [PubMed]
- Lorion, C.M.; Kennedy, B.P. Riparian forest buffers mitigate the effects of deforestation on fish assemblages in tropical headwater streams. Ecol. Appl. 2009, 19, 468–479. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, A.; de Paula, F.R.; Ferraz, S.F.D.; Gerhard, P.; Kashiwaqui, E.A.L.; Cyrino, J.E.P.; Martinelli, L.A. Riparian coverage affects diets of characids in neotropical streams. Ecol. Freshw. Fish 2012, 21, 12–22. [Google Scholar] [CrossRef]
- Lorion, C.M.; Kennedy, B.P. Relationships between deforestation, riparian forest buffers and benthic macroinvertebrates in neotropical headwater streams. Freshw. Biol. 2009, 54, 165–180. [Google Scholar] [CrossRef]
- Rodrigues, M.E.; Roque, F.D.; Quintero, J.M.O.; Pena, J.C.D.; de Sousa, D.C.; de Marco, P. Nonlinear responses in damselfly community along a gradient of habitat loss in a savanna landscape. Biol. Conserv. 2016, 194, 113–120. [Google Scholar] [CrossRef]
- Roth, N.E.; Allan, J.D.; Erickson, D.L. Landscape influences on stream biotic integrity assessed at multiple spatial scales. Landsc. Ecol. 1996, 11, 141–156. [Google Scholar] [CrossRef]
- Brown, A.; Abell, R. Southern Eastern Rift. In Freshwater Ecoregions of Arica and Madagascar: A Conservation Assessment; Thieme, M., Abell, R., Stiassny, M.L.J., Skelton, P., Lehnher, B., Teugels, G.G., Dinerstein, E., Kamden Tohan, A., Burgess, N., Olson, D., Eds.; Island Press: Washington, DC, USA, 2005; pp. 176–180. [Google Scholar]
- Ellis, E.C.; Ramankutty, N. Putting people in the map: Anthropogenic biomes of the world. Front. Ecol. Environ. 2008, 6, 439–447. [Google Scholar] [CrossRef]
- Olaka, L.A.; Odada, E.O.; Trauth, M.H.; Olago, D.O. The sensitivity of East African rift lakes to climate fluctuations. J. Paleolimnol. 2010, 44, 629–644. [Google Scholar] [CrossRef]
- Jameson, R.; Wilson, J.; Murray, H. Narrative of Discovery and Adventure in Africa, from the Earliest Ages to the Present Time: With Illustrations of the Geology, Minerology and Zoology; Harper Brothers: New York, NY, USA, 1842. [Google Scholar]
- Ford, A.G.P.; Dasmahapatra, K.K.; Ruber, L.; Gharbi, K.; Cezard, T.; Day, J.J. High levels of interspecific gene flow in an endemic cichlid fish adaptive radiation from an extreme lake environment. Mol. Ecol. 2015, 24, 3421–3440. [Google Scholar] [CrossRef] [PubMed]
- Tebbs, E.J.; Remedios, J.J.; Avery, S.T.; Harper, D.M. Remote sensing the hydrological variability of Tanzania’s Lake Natron, a vital Lesser Flamingo breeding site under threat. Ecohydrol. Hydrobiol. 2013, 13, 148–158. [Google Scholar] [CrossRef]
- IUCN. IUCN Water and Nature Initiative Pangani Basin Water Board. Pangani Basin: A Situation Analysis, 2nd ed.; International Union for Conservation of Nature and Naturals Resources: Nairobi, Kenya, 2009. [Google Scholar]
- Røhr, P.C.; Killingtveit, A. Study of two catchments on the hillside of Mt. Kilimanjaro. In Water Resources Management: The Case of Pangani River Basin Issues and Approaches; Ngana, J.O., Ed.; Dar es Salaam Univesity Press: Dar es Salaam, Tanzania, 2002; pp. 211–231. [Google Scholar]
- Oyugi, D.; Thieme, M. Freshwater Ecoregions of Arica and Madagascar: A Conservation Assessment; Thieme, M., Abell, R., Stiassny, M.L.J., Skelton, P., Lehnher, B., Teugels, G.G., DInerstein, E., Kamden Tohan, A., Burgess, N., Olden, J.D., Eds.; Island Press: Washington, DC, USA, 2005; pp. 316–318. [Google Scholar]
- Van Heusden, H. Ctenochromis pectoralis: A most mysterious Tanzanian river cichlid. Cichlid News 2015, 24, 14–17. [Google Scholar]
- Moernaut, J.; Verschuren, D.; Charlet, F.; Kristen, I.; Fagot, M.; De Batist, M. The seismic-stratigraphic record of lake-level fluctuations in Lake Challa: Hydrological stability and change in equatorial East Africa over the last 140 kyr. Earth Planet. Sci. Lett. 2010, 290, 214–223. [Google Scholar] [CrossRef]
- Mulwa, J.; Mwega, B.; Kigomo, M. Hydrogeochemical analysis and evaluation of water quality in Lake Chala catchment area, Kenya. Glob. Adv. Res. J. Phys. Appl. Sci. 2013, 2, 1–7. [Google Scholar]
- Misana, S.B.; Sokoni, C.; Mbonile, M.J. Land-use/cover changes and their drivers on the slopes of Mount Kilimanjaro, Tanzania. J. Geogr. Reg. Plan. 2012, 5, 151–164. [Google Scholar]
- Wolff, C.; Kristen-Jenny, I.; Schettler, G.; Plessen, B.; Meyer, H.; Dulski, P.; Naumann, R.; Brauer, A.; Verschuren, D.; Haug, G.H. Modern seasonality in Lake Chala (Kenya/Tanzania) and its sedimentary documentation in recent lake sediments. Limnol. Oceanogr. 2014, 59, 1621–1636. [Google Scholar] [CrossRef]
- Payne, B.R. Water balance of Lake Chala and its relation to groundwater from tritium and stable isotope data. J. Hydrol. 1970, 11, 47–58. [Google Scholar] [CrossRef]
- Musyoki, M.M. The Situation Analysis of the Kenyan Part of the Pangani River Basin Catchment: The Lake Jipe Watershed and System; Coastal Development Authority: Mombasa, Kenya, 2003.
- Dieleman, J.; van Bocxlaer, B.; Manntschke, C.; Nyingi, D.W.; Adriaens, D.; Verschuren, D. Tracing functional adaptation in African cichlid fishes through morphometric analysis of fossil teeth: Exploring the methods. Hydrobiologia 2015, 755, 73–88. [Google Scholar] [CrossRef]
- Hermann, C.M.; Sefc, K.M.; Koblmuller, S. Ancient origin and recent divergence of a haplochromine cichlid lineage from isolated water bodies in the East African Rift system. J. Fish Biol. 2011, 79, 1356–1369. [Google Scholar] [CrossRef] [PubMed]
- Trewavas, E. Tilapiine Fishes of the Genera Sarotherodon, Oreochromis and Danakilia; British Museum of Natural History: London, UK, 1983. [Google Scholar]
- Sun, W.; Chen, B.; Messinger, D.W. Nearest-neighbor diffusion-based pan-sharpening algorithm for spectral images. Opt. Eng. 2014, 53, 013107. [Google Scholar] [CrossRef]
- Richey, A.S.; Thomas, B.F.; Lo, M.H.; Reager, J.T.; Famiglietti, J.S.; Voss, K.; Swenson, S.; Rodell, M. Quantifying renewable groundwater stress with GRACE. Water Resour. Res. 2015, 51, 5217–5238. [Google Scholar] [CrossRef] [PubMed]
- Swenson, S.; Wahr, J. Monitoring the water balance of Lake Victoria, East Africa, from space. J. Hydrol. 2009, 370, 163–176. [Google Scholar] [CrossRef]
- Becker, M.; Llovel, W.; Cazenave, A.; Guntner, A.; Cretaux, J.F. Recent hydrological behavior of the East African great lakes region inferred from GRACE, satellite altimetry and rainfall observations. C. R. Geosci. 2010, 342, 223–233. [Google Scholar] [CrossRef]
- Watkins, M.M.; Wiese, D.N.; Yuan, D.N.; Boening, C.; Landerer, F.W. Improved methods for observing Earth’s time variable mass distribution with GRACE using spherical cap mascons. J. Geophys. Res. Solid Earth 2015, 120, 2648–2671. [Google Scholar] [CrossRef]
- Wiese, D.N.; Yuan, D.N.; Boening, C.; Landerer, F.W.; Watkins, M.M. Mascon Ocean, Ice, and Hydrology Equivalent Water Height RL05M.1 CRI Filtered Version 2. Ver. 2. PO.DAAC, CA, USA. 2015. Available online: http://dx.doi.org/10.5067/TEMSC-2LCR5 (accessed on 27 October 2016).
- Rodell, M.; Famiglietti, J.S. Detectability of variations in continental water storage from satellite observations of the time dependent gravity field. Water Resour. Res. 1999, 35, 2705–2723. [Google Scholar] [CrossRef]
- World Bank. Population Estimates and Projections. Available online: http://datatopics.worldbank.org/hnp/popestimates (accessed on 27 October 2016).
- Hansen, J.; Russell, G.; Rind, D.; Stone, P.; Lacis, A.; Lebedeff, S.; Ruedy, R.; Travis, L. Efficient Three-Dimensional Global Models for Climate Studies: Models I and II. Mon. Weather Rev. 1983, 111, 609–662. [Google Scholar] [CrossRef]
- Miller, R.L.; Schmidt, G.A.; Nazarenko, L.S.; Tausnev, N.; Bauer, S.E.; DelGenio, A.D.; Kelley, M.; Lo, K.K.; Ruedy, R.; Shindell, D.T.; et al. CMIP5 historical simulations (1850–2012) with GISS ModelE2. J. Adv. Model. Earth Syst. 2014, 6, 441–477. [Google Scholar] [CrossRef]
- Van Vuuren, D.P.; Edmonds, J.; Kainuma, M.; Riahi, K.; Thomson, A.; Hibbard, K.; Hurtt, G.C.; Kram, T.; Krey, V.; Lamarque, J.-F.; et al. The representative concentration pathways: An overview. Clim. Chang. 2011, 109, 5. [Google Scholar] [CrossRef]
- Sanford, T.; Frumhoff, P.C.; Luers, A.; Gulledge, J. The climate policy narrative for a dangerously warming world. Nat. Clim. Chang. 2014, 4, 164–166. [Google Scholar] [CrossRef]
- Osborn, T.J.; Jones, P.G. The CRUTEM4 land-surface air temperature dataset: Construction, previous versions and dissemination via Google Earth. Earth Syst. Sci. Data 2014, 6, 61–68. [Google Scholar] [CrossRef]
- Zhu, Z.; Woodcock, C.E. Automated cloud, cloud shadow, and snow detection in multitemporal Landsat data: An algorithm designed specifically for monitoring land cover change. Remote Sens. Environ. 2014, 152, 217–234. [Google Scholar] [CrossRef]
- Rufin, P.; Muller, H.; Pflugmacher, D.; Hostert, P. Land use intensity trajectories on Amazonian pastures derived from Landsat time series. Int. J. Appl. Earth Obs. Geoinf. 2015, 41, 1–10. [Google Scholar] [CrossRef]
- Crist, E.P. ATM tasseled cap equivalent transformation for reflectance factor data. Remote Sens. Environ. 1985, 17, 301–306. [Google Scholar] [CrossRef]
- Baig, M.H.A.; Zhang, L.F.; Shuai, T.; Tong, Q.X. Derivation of a tasselled cap transformation based on Landsat 8 at-satellite reflectance. Remote Sens. Lett. 2014, 5, 423–431. [Google Scholar] [CrossRef]
- Kruse, F.A.; Lefkoff, A.B.; Boardman, J.W.; Heidebrecht, K.B.; Shapiro, A.T.; Barloon, P.J.; Goetz, A.F.H. The spectral image-processing system (SIPS)—Interactive visualization and analysis of imaging spectrometer data. Remote Sens. Environ. 1993, 44, 145–163. [Google Scholar] [CrossRef]
- Olofsson, P.; Foody, G.M.; Herold, M.; Stehman, S.V.; Woodcock, C.E.; Wulder, M.A. Good practices for estimating area and assessing accuracy of land change. Remote Sens. Environ. 2014, 148, 42–57. [Google Scholar] [CrossRef]
- McGarigal, K.; Cushman, S.A.; Ene, E. FRAGSTATS v4: Spatial Pattern Analysis Program for Categorical and Continuous Maps. Computer Software Program Produced by the Authors at the University of Massachusetts, Amherst. Available online: http://www.umass.edu/landeco/research/fragstats/fragstats.html2012 (accessed on 14 August 2015).
- Hopkins, R.L.; Whiles, M.R. The importance of land use/land cover data in fish and mussel conservation planning. Ann. Limnol.-Int. J. Limnol. 2011, 47, 199–209. [Google Scholar] [CrossRef]
- Nagy, R.C.; Porder, S.; Neill, C.; Brando, P.; Quintino, R.M.; do Nascimento, S.A. Structure and composition of altered riparian forests in an agricultural Amazonian landscape. Ecol. Appl. 2015, 25, 1725–1738. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.X.; Turner, M.G. Importance of landscape heterogeneity in sustaining hydrologic ecosystem services in an agricultural watershed. Ecosphere 2015, 6, 19. [Google Scholar] [CrossRef]
- Wang, L.Z.; Lyons, J.; Kanehl, P.; Gatti, R. Influences of watershed land use on habitat quality and biotic integrity in Wisconsin streams. Fisheries 1997, 22, 6–12. [Google Scholar] [CrossRef]
- Heller, E.; Rhemtulla, J.M.; Lele, S.; Kalacska, M.; Badiger, S.; Sengupta, R.; Ramankutty, N. Mapping Crop Types, Irrigated Areas, and Cropping Intensities in Heterogeneous Landscapes of Southern India Using Multi-Temporal Medium-Resolution Imagery: Implications for Assessing Water Use in Agriculture. Photogramm. Eng. Remote Sens. 2012, 78, 815–827. [Google Scholar] [CrossRef]
- Hawes, E.; Smith, M. Riparian Buffer Zones: Functions and Recommended Widths; Yale School of Forestry and Environmental Studies: New Haven, CT, USA, 1995. [Google Scholar]
- Hansen, B.; Reich, P.; Lake, P.S.; Cavagnaro, T. Minimum Width Requirements for Riparian Zones to Protect Flowing Waters and to Conserve Biodiversity: A Review and Recommendations; Monash University: Melbourne, Australia, 2010. [Google Scholar]
- Behera, S.K.; Luo, J.J.; Masson, S.; Delecluse, P.; Gualdi, S.; Navarra, A.; Yamagata, T. Paramount impact of the Indian Ocean dipole on the East African short rains: A CGCM study. J. Clim. 2005, 18, 4514–4530. [Google Scholar] [CrossRef]
- Nanteza, J.; de Linage, C.R.; Thomas, B.F.; Famiglietti, J.S. Monitoring groundwater storage changes in complex basement aquifers: An evaluation of the GRACE satellites over East Africa. Water Resour. Res. 2016, 52, 9542–9564. [Google Scholar] [CrossRef]
- Vörösmarty, C.J.; McIntyre, P.B.; Gessner, M.O.; Dudgeon, D.; Prusevich, A.; Green, P.; Glidden, S.; Bunn, S.E.; Sullivan, C.A.; Liermann, C.R.; et al. Global threats to human water security and river biodiversity. Nature 2010, 467, 555–561. [Google Scholar] [CrossRef] [PubMed]
- Watson, J.E.M.; Iwamura, T.; Butt, N. Mapping vulnerability and conservation adaptation strategies under climate change. Nat. Clim. Chang. 2013, 3, 989–994. [Google Scholar] [CrossRef]
- Johnston, I.A.; Eddy, F.B.; Maloiy, G.M.O. The effects of temperature on muscle pH, adenylate and phosphogen concentrations in Oreochromis-alcalicus-grahami, a fish adapted to an alkaline hot-spring. J. Fish Biol. 1983, 23, 717–724. [Google Scholar] [CrossRef]
- Wood, C.M.; Brix, K.V.; de Boeck, G.; Bergman, H.L.; Bianchini, A.; Bianchini, L.F.; Maina, J.N.; Johannsson, O.E.; Kavembe, G.D.; Papah, M.B.; et al. Mammalian metabolic rates in the hottest fish on earth. Sci. Rep. 2016, 6, 26990. [Google Scholar] [CrossRef] [PubMed]
- Stiassny, M.L.J.; de Marchi, G.; Lamboj, A. A new species of Danakilia (Teleostei, Cichlidae) from Lake Abaeded in the Danakil Depression of Eritrea (East Africa). Zootaxa 2010, 2690, 43–52. [Google Scholar]
- Coad, B. A new genus and species of cichlid endemic to southern Iran. Copeia 1982, 1, 28–37. [Google Scholar] [CrossRef]
- McNab, B.K. The Physiological Ecology of Vertebrates: A View from Energetics; Cornell University Press: Ithaca, NY, USA, 2002. [Google Scholar]
- Portner, H.O. Oxygen- and capacity-limitation of thermal tolerance: A matrix for integrating climate-related stressor effects in marine ecosystems. J. Exp. Biol. 2010, 213, 881–893. [Google Scholar] [CrossRef] [PubMed]
- Portner, H.O.; Peck, M.A. Climate change effects on fishes and fisheries: Towards a cause-and-effect understanding. J. Fish Biol. 2010, 77, 1745–1779. [Google Scholar] [CrossRef] [PubMed]
- Tewksbury, J.J.; Huey, R.B.; Deutsch, C.A. Ecology—Putting the heat on tropical animals. Science 2008, 320, 1296–1297. [Google Scholar] [CrossRef] [PubMed]
- Deutsch, C.A.; Tewksbury, J.J.; Huey, R.B.; Sheldon, K.S.; Ghalambor, C.K.; Haak, D.C.; Martin, P.R. Impacts of climate warming on terrestrial ectotherms across latitude. Proc. Natl. Acad. Sci. USA 2008, 105, 6668–6672. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.H. On the relationship between abundance and distribution of species. Am. Nat. 1984, 124, 255–279. [Google Scholar] [CrossRef]
- Lawton, R.J.; Pratchett, M.S. Influence of dietary specialization and resource availability on geographical variation in abundance of butterflyfish. Ecol. Evol. 2012, 2, 1347–1361. [Google Scholar] [CrossRef] [PubMed]
- Canonico, G.C.; Arthington, A.; McCrary, J.K.; Thieme, M.L. The effects of introduced tilapias on native biodiversity. Aquat. Conserv.-Mar. Freshw. Ecosyst. 2005, 15, 463–483. [Google Scholar] [CrossRef]
- Spataru, P. Food and feeding-habits of Tilapia-zillii (gervais) (cichlidae) in Lake Kinneret (Israel). Aquaculture 1978, 14, 327–338. [Google Scholar] [CrossRef]
- Johnston, C.E.; Henderson, A.R.; Hartup, W.W. Precipitous decline and conservation of Slackwater Darter (Etheostoma boschungi) in tributaries of the Tennessee River, Tennessee and Alabama. Biodivers. Conserv. 2013, 22, 3247–3259. [Google Scholar] [CrossRef]
- Kadarusman; Sudarto; Paradis, E.; Pouyaud, L. Description of Melanotaenia fasinensis, a new species of rainbowfish (Melanotaeniidae) from West Papua, Indonesia with comments on the rediscovery of M. ajamaruensis and the endangered status of M. parva. Cybium 2010, 34, 207–215. [Google Scholar]
- Nicol, S.; Haynes, T.B.; Fensham, R.; Kerezsy, A. Quantifying the impact of Gambusia holbrooki on the extinction risk of the critically endangered red-finned blue-eye. Ecosphere 2015, 6, 41. [Google Scholar] [CrossRef]
- Griffiths, D.; McGonigle, C.; Quinn, R. Climate and species richness patterns of freshwater fish in North America and Europe. J. Biogeogr. 2014, 41, 452–463. [Google Scholar] [CrossRef]
- Nilsson, C.; Reidy, C.A.; Dynesius, M.; Revenga, C. Fragmentation and flow regulation of the world’s large river systems. Science 2005, 308, 405–408. [Google Scholar] [CrossRef] [PubMed]
- Ogutuohwayo, R. The decline of the native fishes of Lakes Victoria and Kyoga (East-Africa) and the impact of introduced species, especially the nile perch, Lates-niloticus, and the nile tilapia, Oreochromis-niloticus. Environ. Biol. Fishes 1990, 27, 81–96. [Google Scholar] [CrossRef]
- Raghavan, R.; Das, S.; Nameer, P.O.; Bijukumar, A.; Dahanukar, N. Protected areas and imperilled endemic freshwater biodiversity in the Western Ghats Hotspot. Aquat. Conserv.-Mar. Freshw. Ecosyst. 2016, 26, 78–90. [Google Scholar] [CrossRef]
- Beissinger, S.R. Digging the pupfish out of its hole: Risk analyses to guide harvest of Devils Hole pupfish for captive breeding. PeerJ 2014, 2, e549. [Google Scholar] [CrossRef] [PubMed]
- Hausner, M.B.; Wilson, K.P.; Gaines, D.B.; Suarez, F.; Scoppettone, G.G.; Tyler, S.W. Life in a fishbowl: Prospects for the endangered Devils Hole pupfish (Cyprinodon diabolis) in a changing climate. Water Resour. Res. 2014, 50, 7020–7034. [Google Scholar] [CrossRef]
- Fan, J.L.; Xu, Y.H.; Chen, Z.M.; Xiao, J.; Liu, D.Y.; Luo, J.F.; Bolan, N.; Ding, W.X. Sulfur deposition suppressed nitrogen-induced soil N2O emission from a subtropical forestland in southeastern China. Agric. For. Meteorol. 2017, 233, 163–170. [Google Scholar] [CrossRef]
- Williams, J.J.; Chung, S.H.; Johansen, A.M.; Lamb, B.K.; Vaughan, J.K.; Beutel, M. Evaluation of atmospheric nitrogen deposition model performance in the context of US critical load assessments. Atmos. Environ. 2017, 150, 244–255. [Google Scholar] [CrossRef]
- Sawicka, K.; Rowe, E.C.; Evans, C.D.; Monteith, D.T.; Vanguelova, E.I.; Wade, A.J.; Clark, J.M. Modelling impacts of atmospheric deposition and temperature on long-term DOC trends. Sci. Total Environ. 2017, 578, 323–336. [Google Scholar] [CrossRef] [PubMed]
- Nasr, M.; Arp, P.A. Mercury and Organic Matter Concentrations in Lake and Stream Sediments in relation to One Another and to Atmospheric Mercury Deposition and Climate Variations across Canada. J. Chem. 2017, 2017, 8949502. [Google Scholar] [CrossRef]
- Comte, L.; Buisson, L.; Daufresne, M.; Grenouillet, G. Climate-induced changes in the distribution of freshwater fish: Observed and predicted trends. Freshw. Biol. 2013, 58, 625–639. [Google Scholar] [CrossRef]
- Betancur-R, R.; Broughton, R.E.; Wiley, E.O.; Carpenter, K.; López, J.A.; Li, C.; Holcroft, N.I.; Arcila, D.; Sanciangco, M.; Cureton, I.I.J.C.; et al. The Tree of Life and a New Classification of Bony Fishes. PLoS Curr. 2013. [Google Scholar] [CrossRef] [PubMed]
- Wainwright, P.C.; Smith, W.L.; Price, S.A.; Tang, K.L.; Sparks, J.S.; Ferry, L.A.; Kuhn, K.L.; Eytan, R.I.; Near, T.J. The Evolution of Pharyngognathy: A Phylogenetic and Functional Appraisal of the Pharyngeal Jaw Key Innovation in Labroid Fishes and Beyond. Syst. Biol. 2012, 61, 1001–1027. [Google Scholar] [CrossRef] [PubMed]
- Butchart, S.H.M.; Walpole, M.; Collen, B.; van Strien, A.; Scharlemann, J.P.W.; Almond, R.E.A.; Baillie, J.E.M.; Bomhard, B.; Brown, C.; Bruno, J.; et al. Global Biodiversity: Indicators of Recent Declines. Science 2010, 328, 1164–1168. [Google Scholar] [CrossRef] [PubMed]
- Janse, J.H.; Kuiper, J.J.; Weijters, M.J.; Westerbeek, E.P.; Jeuken, M.; Bakkenes, M.; Alkemade, R.; Mooij, W.M.; Verhoeven, J.T.A. GLOBIO-Aquatic, a global model of human impact on the biodiversity of inland aquatic ecosystems. Environ. Sci. Policy 2015, 48, 99–114. [Google Scholar] [CrossRef]
- Zinkina, J.; Korotayev, A. Explosive population growth in tropical Africa: Crucial omission in development forecasts – emerging risks and way out. World Futures 2014, 70, 120–139. [Google Scholar] [CrossRef]
- Juffe-Bignoli, D.; Harrison, I.; Butchart, S.H.M.; Flitcroft, R.; Hermoso, V.; Jonas, H.; Lukasiewicz, A.; Thieme, M.; Turak, E.; Bingham, H.; et al. Achieving Aichi Biodiversity Target 11 to improve the performance of protected areas and conserve freshwater biodiversity. Aquat. Conserv.-Mar. Freshw. Ecosyst. 2016, 26, 133–151. [Google Scholar] [CrossRef]
- McLaughlin, B.C.; Ackerly, D.D.; Klos, P.Z.; Natali, J.; Dawson, T.E. Hydrologic refugia, plants, and climate change. Glob. Chang. Biol. 2017. [Google Scholar] [CrossRef] [PubMed]

| Site | Species | Brief Description |
|---|---|---|
| Natron Springs | Alcolapia alcalicus. Formerly Oreochromis alcalicus. Species authority: Hilgendorf, 1905. | Physiological adaptions to extreme habitat conditions, including ureotelism, specialized gills, high intracellular pH, trifurcated esophagus, and facultative air-breathing. Generalist, up to 16 cm total length (TL), terminal mouth for feeding on algae and insect larvae. Mouth brooding, breeds in large high walled crater nests. IUCN Red List: Endangered. Potential area of occupancy <10 km2. |
| Natron Springs | Alcolapia ndalalani. Formerly O. ndalalani. Species authority: Seegers & Tichy, 1999. | Physiological adaptions to extreme habitat conditions, including ureotelism, specialized gills, high intracellular pH, trifurcated esophagus and facultative air-breathing. Specialist, up to 8 cm TL, subterminal thin-lipped mouth used to pick algae and insect larvae. Mouth brooding, male defends a single fist sized boulder or elevation. IUCN Red List: Vulnerable. Potential area of occupancy < 10 km2. |
| Natron Springs | Alcolapia latilabris. Formerly O. latilabris. Species authority: Seegers & Tichy, 1999. | Physiological adaptions to extreme habitat conditions, including ureotelism, specialized gills, high intracellular pH, trifurcated esophagus, and facultative air-breathing. Specialist, up to 12 cm TL, mouth brooding, algae grazer with hypertrophied lips, breeds in crater nests consisting of two concentric low walls. IUCN Red List: Vulnerable. Potential area of occupancy <10 km2. |
| Chemka Springs | Ctenochromis sp. (undescribed). | Potential area of occupancy <1 km2. Closely related to C. pectoralis (Pfeffer, 1893), described from the Pangani River further downstream. Up to 12 cm TL, mouth brooding, feeds on snails and micro invertebrates. Dominant males excavate large crater nests (up to 60 cm in diameter) under large boulders. IUCN Red List: Not assessed. |
| Lake Chala | Oreochromis hunter. Species authority: Günther, 1889. | This is the type species of the genus Oreochromis. It is known from only a few specimens, and little is known about its reproduction and breeding habits. Young O. hunteri feed on algae and organic debris [54]. Tooth morphology suggests aufwuchs (small animals, plants and algae that adhere to open surfaces) form a part of their diet [52]. IUCN Red List: Critically endangered. |
| Site/Period | No. Patches | Mean Patch Area (ha) | Patch Standard Deviation (ha) | CA (ha) | PLAND (%) | LPI (%) | LSI | CLUMPY |
|---|---|---|---|---|---|---|---|---|
| Lake Chala, 1987 | 223 | 34.2 | 419.7 | 7633.3 | 15.5 | 12.7 | 24.9 | 0.90 |
| Lake Chala, 1995 | 203 | 18.2 | 112.0 | 3695.3 | 7.5 | 3.1 | 22.2 | 0.89 |
| Lake Chala, 2000 | 526 | 4.9 | 49.3 | 2595.3 | 5.3 | 2.3 | 28.8 | 0.83 |
| Lake Chala, 2015 | 62 | 27.8 | 180.0 | 1726.1 | 3.5 | 2.9 | 11.5 | 0.92 |
| Chemka Springs, 1995 | 194 | 1.5 | 4.3 | 281.8 | 7.4 | 1.2 | 20.6 | 0.61 |
| Chemka Springs, 2015 | 104 | 0.9 | 1.5 | 95.7 | 2.5 | 0.3 | 15.7 | 0.52 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalacska, M.; Arroyo-Mora, J.P.; Lucanus, O.; Kishe-Machumu, M.A. Land Cover, Land Use, and Climate Change Impacts on Endemic Cichlid Habitats in Northern Tanzania. Remote Sens. 2017, 9, 623. https://doi.org/10.3390/rs9060623
Kalacska M, Arroyo-Mora JP, Lucanus O, Kishe-Machumu MA. Land Cover, Land Use, and Climate Change Impacts on Endemic Cichlid Habitats in Northern Tanzania. Remote Sensing. 2017; 9(6):623. https://doi.org/10.3390/rs9060623
Chicago/Turabian StyleKalacska, Margaret, J. Pablo Arroyo-Mora, Oliver Lucanus, and Mary A. Kishe-Machumu. 2017. "Land Cover, Land Use, and Climate Change Impacts on Endemic Cichlid Habitats in Northern Tanzania" Remote Sensing 9, no. 6: 623. https://doi.org/10.3390/rs9060623
APA StyleKalacska, M., Arroyo-Mora, J. P., Lucanus, O., & Kishe-Machumu, M. A. (2017). Land Cover, Land Use, and Climate Change Impacts on Endemic Cichlid Habitats in Northern Tanzania. Remote Sensing, 9(6), 623. https://doi.org/10.3390/rs9060623








