Mapping a Knowledge-Based Malaria Hazard Index Related to Landscape Using Remote Sensing: Application to the Cross-Border Area between French Guiana and Brazil
Abstract
:1. Introduction
2. Materials and Methods
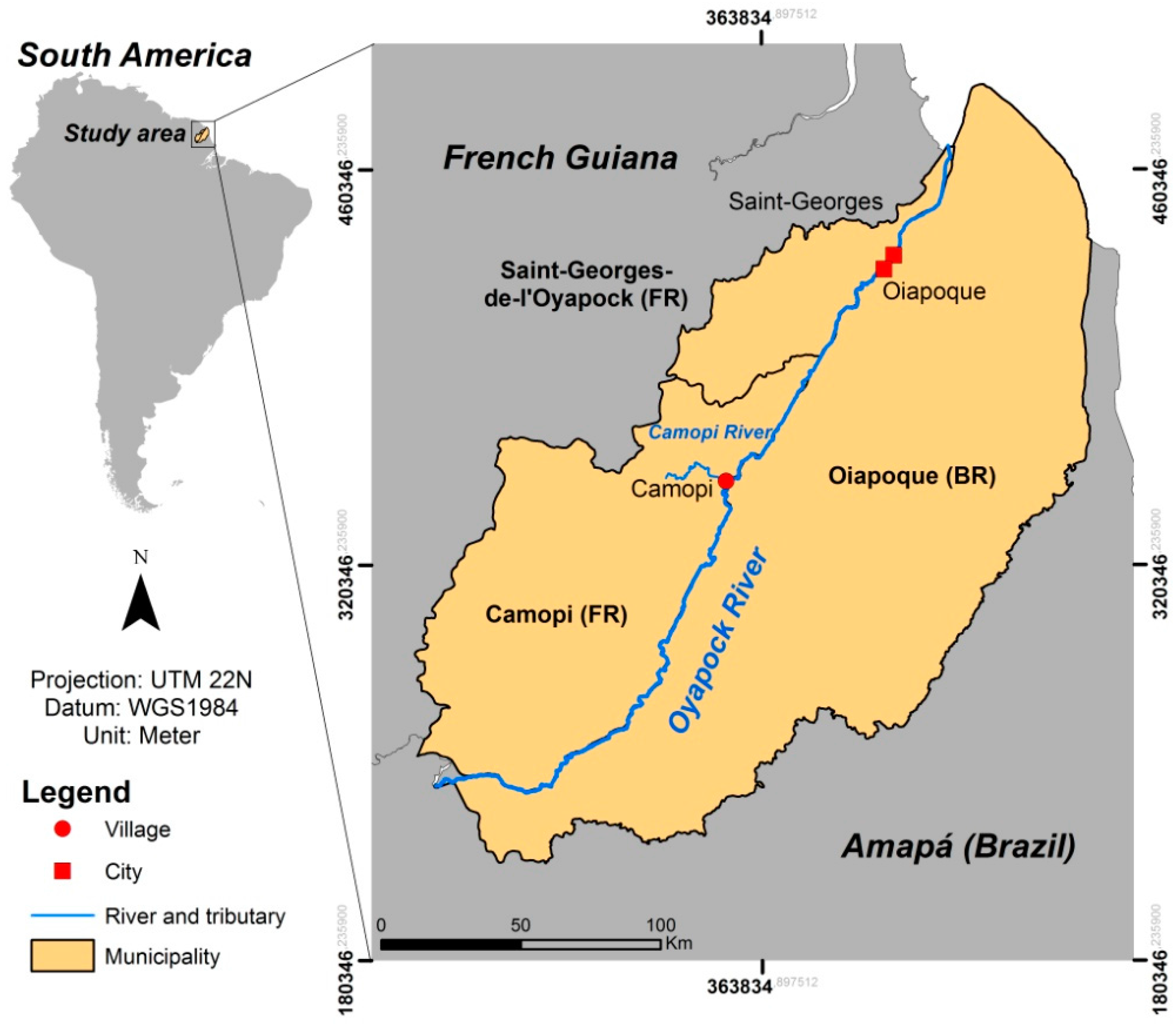
2.1. Study Area
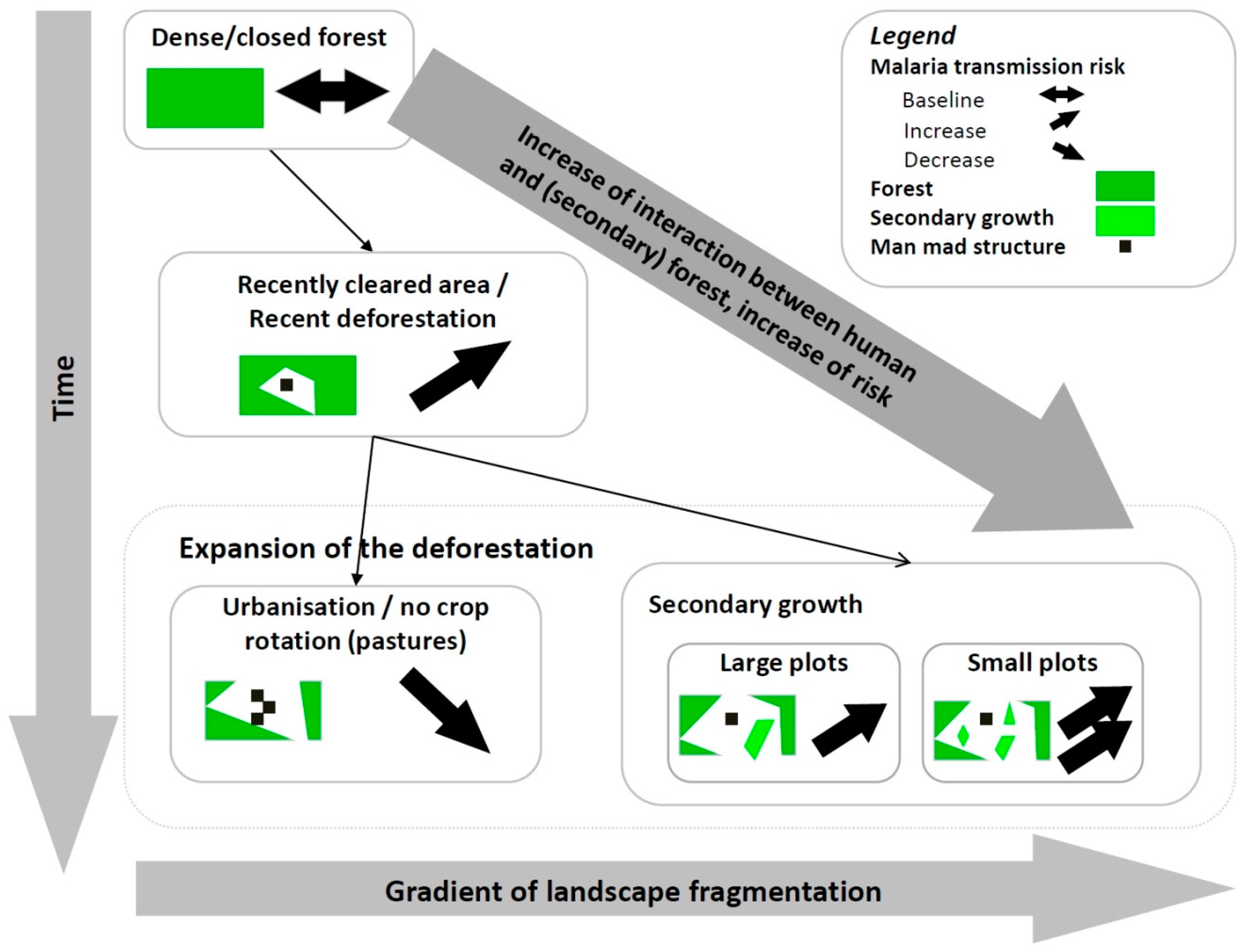
2.2. Knowledge-Based Model
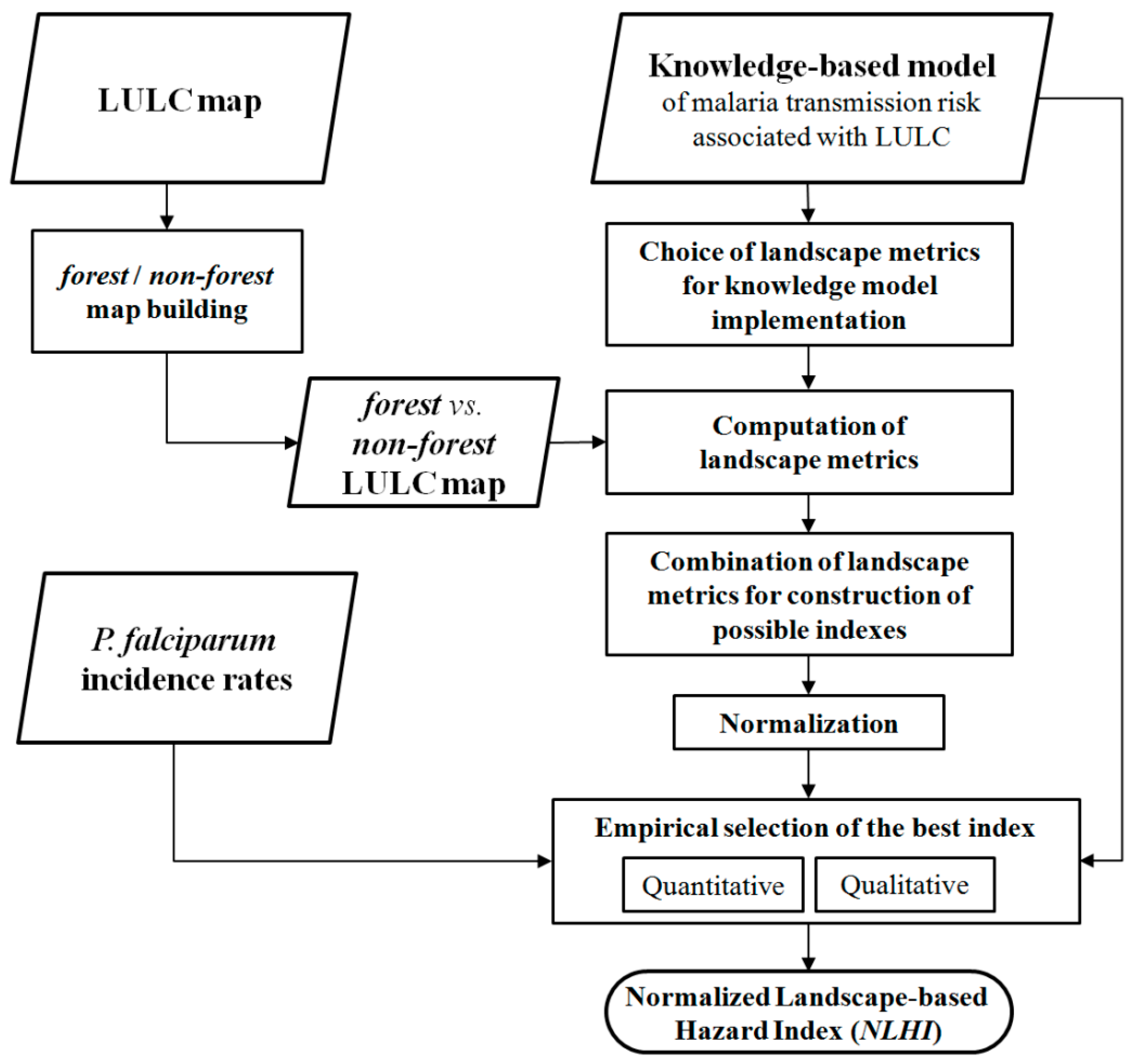
2.3. Methodology of Model Implementation
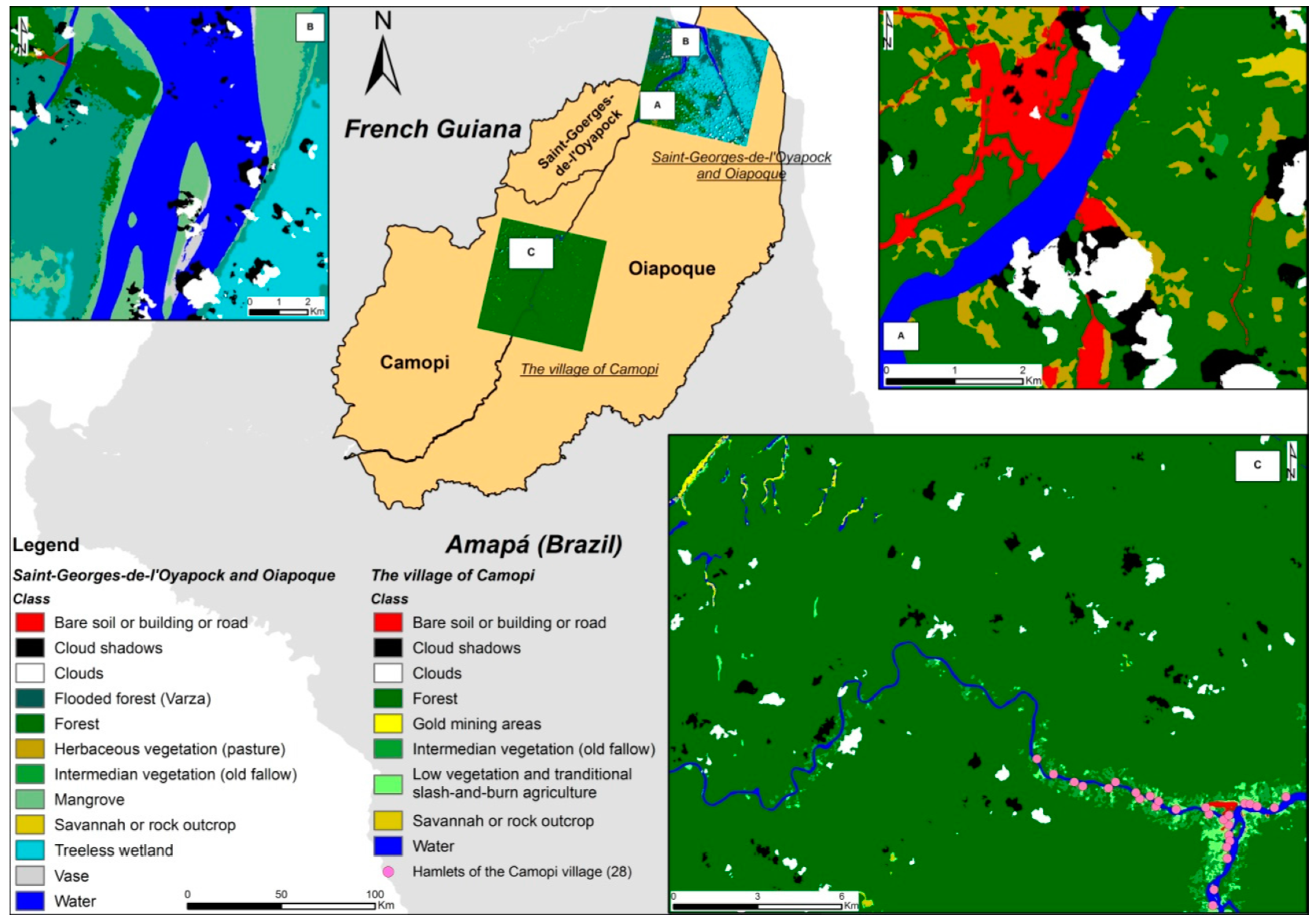
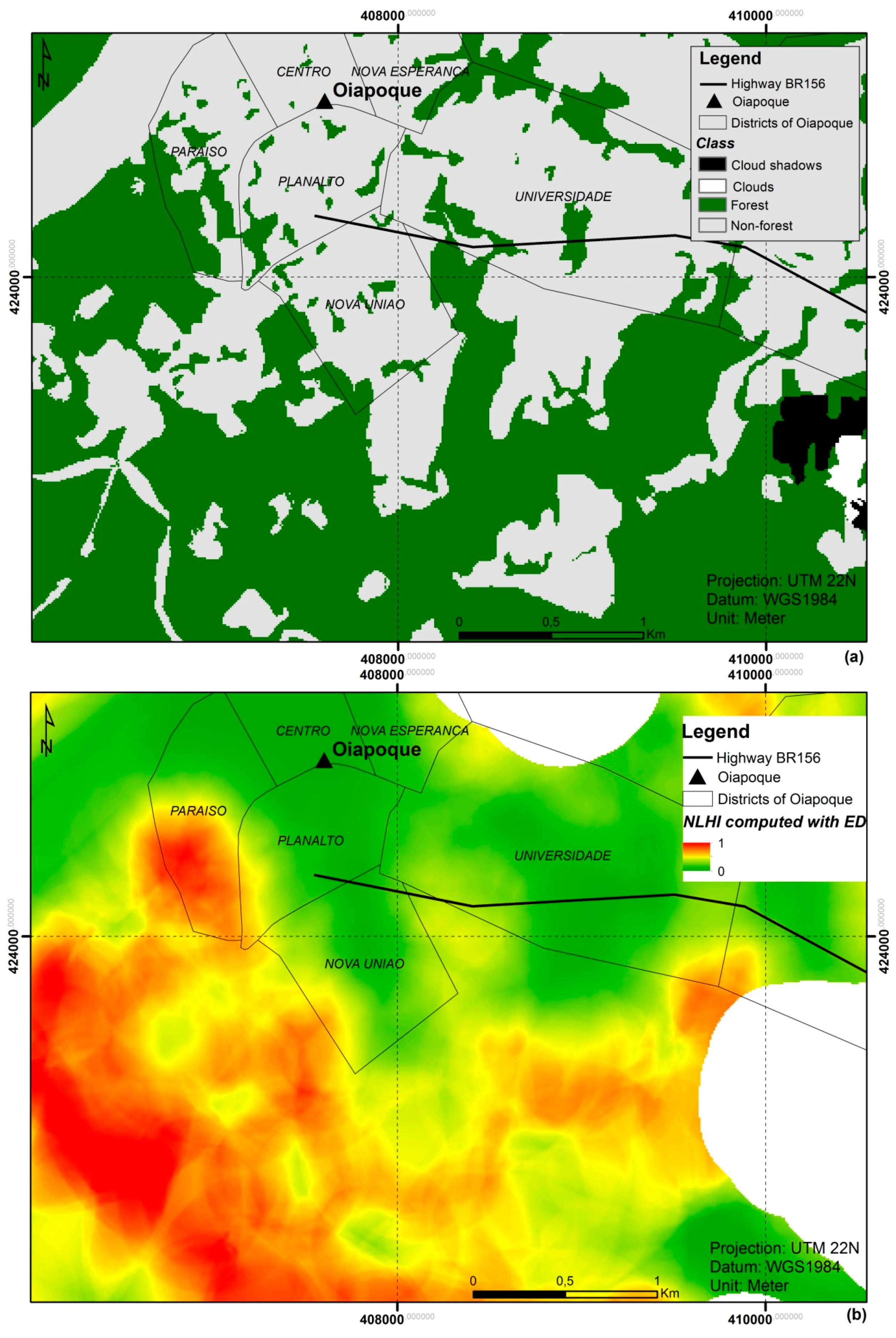
2.3.1. Land Use and Land Cover Mapping
2.3.2. Landscape Metric Computation
2.3.3. Landscape-Based Hazard Index Construction
2.3.4. Qualitative Analysis
2.3.5. Quantitative Evaluation
- Incidence rates
- Computation of domiciliary NLHI
- Statistical relationship between incidence rates and candidate NLHI
3. Results
3.1. Qualitative Analysis: Information Content Analysis of Configurational Metrics
3.2. Qualitative Analysis: Empirical Behavior of Configurational Metrics and Associated NLHI
3.3. Quantitative Evaluation: Relationship between NLHI and Actual Incidence Rates
3.4. Proposed NLHI
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| AM | Area-weighted mean |
| API | Annual Parasite Index |
| ASF | African swine fever |
| CIRCLE | Related Circumscribing Circle |
| CM | Configurational metrics |
| CONTIG | Contiguity Index |
| DIVISION | Landscape Division Index |
| ED | Edge Density |
| FRAC | Fractal Dimension Index |
| GPS | Global Positioning System |
| IBGE | Brazilian Institute of Geography and Statistics |
| IGN | National Institute of Geographic and Forestry Information |
| INSEE | National Institute of Statistics and Economic Studies |
| LULC | Land use and land cover |
| LHI | Landscape-based Hazard Index |
| MN | Mean |
| NLHI | Normalization Landscape-based Hazard Index |
| PARA | Perimeter-area ratio |
| PCA | Principal Component Analysis |
| PD | Patch density |
| pF | Proportion of the forest |
| SAR | Synthetic aperture radar |
| SHAPE | Shape Index |
| SPLIT | Splitting Index |
| SVM | Support Vector Machine |
References
- WHO. World Malaria Report 2015; 9789241565158; WHO: Geneva, Switzerland, 2015. [Google Scholar]
- Peiter, P.C. Living conditions, health status and health services availability along the brazilian border: A geographical approach. Cad Saúde Pública 2007, 23, 237–250. [Google Scholar] [CrossRef]
- Wangdi, K.; Gatton, M.L.; Kelly, G.C.; Clements, A.C. Cross-border malaria: A major obstacle for malaria elimination. Adv. Parasitol. 2015, 89, 79–107. [Google Scholar] [CrossRef] [PubMed]
- Musset, L.; Pelleau, S.; Girod, R.; Ardillon, V.; Carvalho, L.; Dusfour, I.; Gomes, M.S.M.; Djossou, F.; Legrand, E. Malaria on the guiana shield: A review of the situation in French Guiana. Mem. Inst. Oswaldo Cruz 2014, 109, 525–533. [Google Scholar] [CrossRef] [PubMed]
- Sinka, M.E.; Rubio-Palis, Y.; Manguin, S.; Patil, A.P.; Temperley, W.H.; Gething, P.W.; Van Boeckel, T.; Kabaria, C.W.; Harbach, R.E.; Hay, S.I. The dominant anopheles vectors of human malaria in the Americas: Occurrence data, distribution maps and bionomic precis. Parasites Vectors 2010, 3. [Google Scholar] [CrossRef] [PubMed]
- Galardo, A.K.; Arruda, M.; D’Almeida Couto, A.A.; Wirtz, R.; Lounibos, L.P.; Zimmerman, R.H. Malaria vector incrimination in three rural riverine villages in the Brazilian Amazon. Am. J. Trop. Med. Hyg. 2007, 76, 461–469. [Google Scholar] [PubMed]
- Girod, R.; Roux, E.; Berger, F.; Stefani, A.; Gaborit, P.; Carinci, R.; Issaly, J.; Carme, B.; Dusfour, I. Unravelling the relationships between anopheles darlingi (diptera: Culicidae) densities, environmental factors and malaria incidence: Understanding the variable patterns of malarial transmission in French Guiana (South America). Ann. Trop. Med. Parasitol. 2011, 105, 107–122. [Google Scholar] [CrossRef] [PubMed]
- Dusfour, I.; Issaly, J.; Carinci, R.; Gaborit, P.; Girod, R. Incrimination of anopheles (anopheles) intermedius peryassu, an. (Nyssorhynchus) nuneztovari gabaldon, an. (NYS.) oswaldoi peryassu as natural vectors of plasmodium falciparum in French Guiana. Mem. Inst. Oswaldo Cruz 2012, 107, 429–432. [Google Scholar] [CrossRef] [PubMed]
- Hiwat, H.; Bretas, G. Ecology of anopheles darlingi root with respect to vector importance: A review. Parasites Vectors 2011, 4, 177. [Google Scholar] [CrossRef] [PubMed]
- Stefani, A.; Hanf, M.; Nacher, M.; Girod, R.; Carme, B. Environmental, entomological, socioeconomic and behavioural risk factors for malaria attacks in Amerindian children of Camopi, French Guiana. Malar. J. 2011, 10, 246. [Google Scholar] [CrossRef] [PubMed]
- Patz, J.A.; Olson, S.H. Malaria risk and temperature: Influences from global climate change and local land use practices. Proc. Natl. Acad. Sci. USA 2006, 103, 5635–5636. [Google Scholar] [CrossRef] [PubMed]
- Olson, S.H.; Gangnon, R.; Elguero, E.; Durieux, L.; Guegan, J.F.; Foley, J.A.; Patz, J.A. Links between climate, malaria, and wetlands in the Amazon Basin. Emerg. Infect. Dis. 2009, 15, 659–662. [Google Scholar] [CrossRef] [PubMed]
- Kraemer, M.U.; Hay, S.I.; Pigott, D.M.; Smith, D.L.; Wint, G.R.; Golding, N. Progress and challenges in infectious disease cartography. Trends Parasitol. 2016, 32, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Stefani, A.; Dusfour, I.; Correa, A.P.; Cruz, M.C.; Dessay, N.; Galardo, A.K.; Galardo, C.D.; Girod, R.; Gomes, M.S.; Gurgel, H.; et al. Land cover, land use and malaria in the Amazon: A systematic literature review of studies using remotely sensed data. Mala. J. 2013, 12, 192. [Google Scholar] [CrossRef] [PubMed]
- Barbieri, A.F.; Sawyer, I.O.; Soares-Filho, B.S. Population and land use effects on malaria prevalence in the southern Brazilian Amazon. Hum. Ecol. 2005, 33, 847–874. [Google Scholar] [CrossRef]
- Guerra, C.A.; Snow, R.W.; Hay, S.I. A global assessment of closed forests, deforestation and malaria risk. Ann. Trop. Med. Parasitol. 2006, 100, 189–204. [Google Scholar] [CrossRef] [PubMed]
- Vittor, A.Y.; Gilman, R.H.; Tielsch, J.; Glass, G.; Shields, T.; Lozano, W.S.; Pinedo-Cancino, V.; Patz, J.A. The effect of deforestation on the human-biting rate of anopheles darlingi, the primary vector of falciparum malaria in the Peruvian Amazon. Am. J. Trop. Med. Hyg. 2006, 74, 3–11. [Google Scholar] [PubMed]
- Vittor, A.Y.; Pan, W.; Gilman, R.H.; Tielsch, J.; Glass, G.; Shields, T.; Sanchez-Lozano, W.; Pinedo, V.V.; Salas-Cobos, E.; Flores, S.; et al. Linking deforestation to malaria in the Amazon: Characterization of the breeding habitat of the principal malaria vector, anopheles darlingi. Am. J. Trop. Med. Hyg. 2009, 81, 5–12. [Google Scholar] [PubMed]
- Olson, S.H.; Gangnon, R.; Silveira, G.A.; Patz, J.A. Deforestation and malaria in Mancio Lima county, Brazil. Emerg. Infect. Dis. 2010, 16, 1108–1115. [Google Scholar] [CrossRef] [PubMed]
- Fraser, B. Taking on malaria in the Amazon. Lancet 2010, 376, 1133–1134. [Google Scholar] [CrossRef]
- Omumbo, J.A.; Hay, S.I.; Snow, R.W.; Tatem, A.J.; Rogers, D.J. Modelling malaria risk in east africa at high-spatial resolution. Trop. Med. Int. Health 2005, 10, 557–566. [Google Scholar] [CrossRef] [PubMed]
- Zeilhofer, P.; dos Santos, E.S.; Ribeiro, A.L.; Miyazaki, R.D.; dos Santos, M.A. Habitat suitability mapping of anopheles darlingi in the surroundings of the Manso hydropower plant reservoir, Mato Grosso, Central Brazil. Int. J. Health Geogr. 2007, 6, 7. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Maheu-Giroux, M.; Casapia, M.; Soto-Calle, V.E.; Ford, L.B.; Buckeridge, D.L.; Coomes, O.T.; Gyorkos, T.W. Risk of malaria transmission from fish ponds in the peruvian amazon. Acta Trop. 2010, 115, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Patil, A.P.; Gething, P.W.; Piel, F.B.; Hay, S.I. Bayesian geostatistics in health cartography: The perspective of malaria. Trends Parasitol. 2011, 27, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Stuckey, E.M.; Smith, T.A.; Chitnis, N. Estimating malaria transmission through mathematical models. Trends Parasitol. 2013, 29, 477–482. [Google Scholar] [CrossRef] [PubMed]
- Hardy, A.; Mageni, Z.; Dongus, S.; Killeen, G.; Macklin, M.G.; Majambare, S.; Ali, A.; Msellem, M.; Al-Mafazy, A.W.; Smith, M.; et al. Mapping hotspots of malaria transmission from pre-existing hydrology, geology and geomorphology data in the pre-elimination context of Zanzibar, United Republic of Tanzania. Parasites Vectors 2015, 8, 41. [Google Scholar] [CrossRef] [PubMed]
- Clements, A.C.; Pfeiffer, D.U.; Martin, V. Application of knowledge-driven spatial modelling approaches and uncertainty management to a study of rift valley fever in Africa. Int. J. Health Geogr. 2006, 5, 57. [Google Scholar] [CrossRef] [PubMed][Green Version]
- De Glanville, W.A.; Vial, L.; Costard, S.; Wieland, B.; Pfeiffer, D.U. Spatial multi-criteria decision analysis to predict suitability for African swine fever endemicity in Africa. BMC Vet. Res. 2014, 10, 9. [Google Scholar] [CrossRef] [PubMed]
- Stevens, K.B.; Pfeiffer, D.U. Spatial modelling of disease using data- and knowledge-driven approaches. Spat Spatiotemporal Epidemiol. 2011, 2, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Garni, R.; Tran, A.; Guis, H.; Baldet, T.; Benallal, K.; Boubidi, S.; Harrat, Z. Remote sensing, land cover changes, and vector-borne diseases: Use of high spatial resolution satellite imagery to map the risk of occurrence of cutaneous leishmaniasis in Ghardaia, Algeria. Infect. Genet. Evol. 2014, 28, 725–734. [Google Scholar] [CrossRef] [PubMed]
- Alimi, T.O.; Fuller, D.O.; Quinones, M.L.; Xue, R.D.; Herrera, S.V.; Arevalo-Herrera, M.; Ulrich, J.N.; Qualls, W.A.; Beier, J.C. Prospects and recommendations for risk mapping to improve strategies for effective malaria vector control interventions in Latin America. Malaria J. 2015, 14, 519. [Google Scholar] [CrossRef] [PubMed]
- Uuemaa, E.; Mander, Ü.; Marja, R. Trends in the use of landscape spatial metrics as landscape indicators: A review. Ecol. Indic. 2013, 28, 100–106. [Google Scholar] [CrossRef]
- Ostfeld, R.S.; Glass, G.E.; Keesing, F. Spatial epidemiology: An emerging (or re-emerging) discipline. Trends Ecol. Evol. 2005, 20, 328–336. [Google Scholar] [CrossRef] [PubMed]
- Overgaard, H.J.; Ekbom, B.; Suwonkerd, W.; Takagi, M. Effect of landscape structure on anopheline mosquito density and diversity in Northern Thailand: Implications for malaria transmission and control. Landsc. Ecol. 2003, 18, 605–619. [Google Scholar] [CrossRef]
- INSEE. Insee. Saint-Georges (97038). Available online: http://www.insee.fr/fr/themes/comparateur.asp?codgeo=com-97308 (accessed on 11 February 2015).
- INSEE. Insee. Camopi (97356). Available online: http://www.insee.fr/fr/themes/comparateur.asp?codgeo=com-97356 (accessed on 11 February 2015).
- IBGE. Oiapoque. Available online: http://cod.ibge.gov.br/12XVS (accessed on 12 February 2015).
- Boyrie, A.; Laques, A.E.; Gurgel, H.C. Avaliação de políticas públicas para o desenvolvimento a partir de um estudo sobre a fragmentação da floresta na bacia do oiapoque (guiana francesa/amapá). In Proceedings of the 16th Brazilian Symposium of Remote Sensing, Foz do Iguaçu, Brazil, 13–18 April 2013.
- Carme, B.; Ardillon, V.; Girod, R.; Grenier, C.; Joubert, M.; Djossou, F.; Ravachol, F. Update on the epidemiology of malaria in French Guiana. Med. Trop. 2009, 69, 19–25. [Google Scholar]
- Ardillon, V.; Eltges, F.; Chocho, A.; Chantilly, S.; Carvalho, L.; Flamand, C.; Carme, B. Evolution de la Situation Epidémiologique du Paludisme en Guyane de 2005 à 2011; French Institute for Public Health Surveillance: Saint-Maurice, French, 2012. [Google Scholar]
- Ardillon, V.; Carvalho, L.; Prince, C.; Djossou, F. Bilan 2012 de la Situation Epidémiologique du Paludisme en Guyane; French Institute for Public Health Surveillance: Saint-Maurice, French, 2013. [Google Scholar]
- Cenci, D.; Ferreira, A.C.; Santelli, S. Situation Epidémiologique du Paludisme dans L’etat D’amapà et dans la Minicipalité D’oiapoque (Brésil); French Institute for Public Health Surveillance: Saint-Maurice, French, 2012. [Google Scholar]
- Zanini, V.M.; Gomes, M.S.M.; Galardo, A.K.R.; Lima, A.C.S.F.; Correa, A.P.S.A.; Stefani, A.; Roux, E.; Souza, R.T.L.; Póvoa, M.M.; Souto, R.N.P. Potencial de transmissão de malária no município de oiapoque-Amapá-Brasil. In Proceedings of the 50th Congress of the Brazilian Society of Tropical Medicine, Rio Branco, Brazil, 21–24 September 2014.
- Tadei, W.P.; Thatcher, B.D.; Santos, J.M.; Scarpassa, V.M.; Rodrigues, I.B.; Rafael, M.S. Ecologic observations on anopheline vectors of malaria in the brazilian amazon. Am. J. Trop. Med. Hyg. 1998, 59, 325–335. [Google Scholar] [PubMed]
- McGarigal, K.; Cushman, S.; Ene, E. Fragstats v4: Spatial Pattern Analysis Program for Categorical and Continuous Maps. Available online: http://www.umass.edu/landeco/research/fragstats/fragstats.html (accessed on 4 April 2016).
- Stefani, A.; Roux, E.; Fotsing, J.M.; Carme, B. Studying relationships between environment and malaria incidence in Camopi (French Guiana) through the objective selection of buffer-based landscape characterisations. Int. J. Health Geogr. 2011, 10, 65. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, F.M.V.; De Marco, P.; Ferreira, L.G. The cerrado into-pieces: Habitat fragmentation as a function of landscape use in the savannas of central brazil. Biol. Conserv. 2009, 142, 1392–1403. [Google Scholar] [CrossRef]
- Hargis, C.D.; Bissonette, J.A.; David, J.L. The behavior of landscape metrics commonly used in the study of habitat fragmentation. Landsc. Ecol. 1998, 13, 167–186. [Google Scholar] [CrossRef]
- Hustache, S.; Nacher, M.; Djossou, F.; Carme, B. Malaria risk factors in Amerindian children in French Guiana. Am. J. Trop. Med. Hyg. 2007, 76, 619–625. [Google Scholar] [PubMed]
- Hongoh, V.; Hoen, A.G.; Aenishaenslin, C.; Waaub, J.P.; Belanger, D.; Michel, P.; Lyme, M.C. Spatially explicit multi-criteria decision analysis for managing vector-borne diseases. Int. J. Health Geogr. 2011, 10, 70. [Google Scholar] [CrossRef] [PubMed]
- De Thoisy, B.; Richard-Hansen, C.; Goguillon, B.; Joubert, P.; Obstancias, J.; Winterton, P.; Brosse, S. Rapid evaluation of threats to biodiversity: Human footprint score and large vertebrate species responses in French Guiana. Biodivers. Conserv. 2010, 19, 1567–1584. [Google Scholar] [CrossRef]
- Machault, V.; Vignolles, C.; Borchi, F.; Vounatsou, P.; Pages, F.; Briolant, S.; Lacaux, J.P.; Rogier, C. The use of remotely sensed environmental data in the study of malaria. Geospat. Health 2011, 5, 151–168. [Google Scholar] [CrossRef] [PubMed]
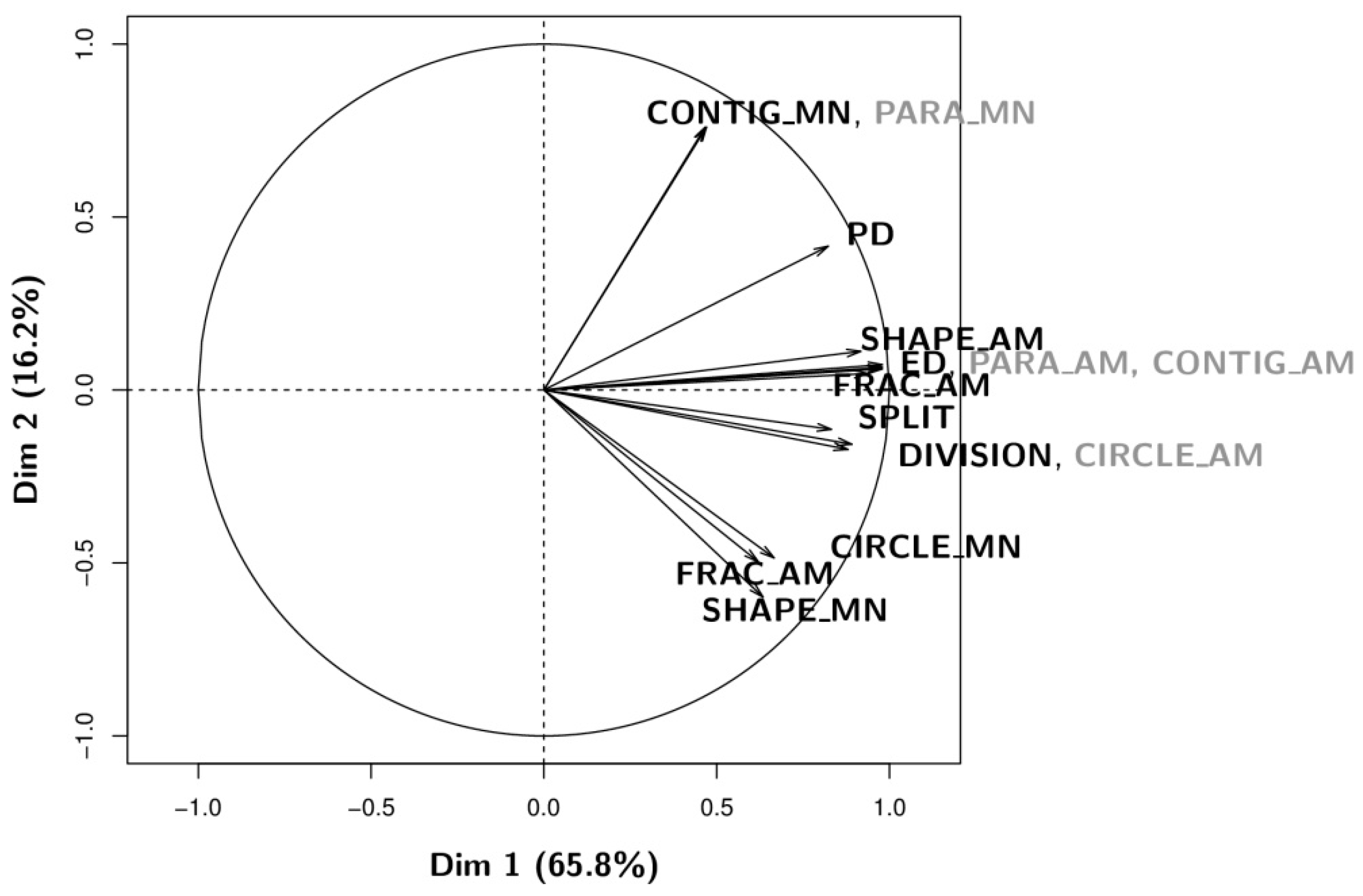
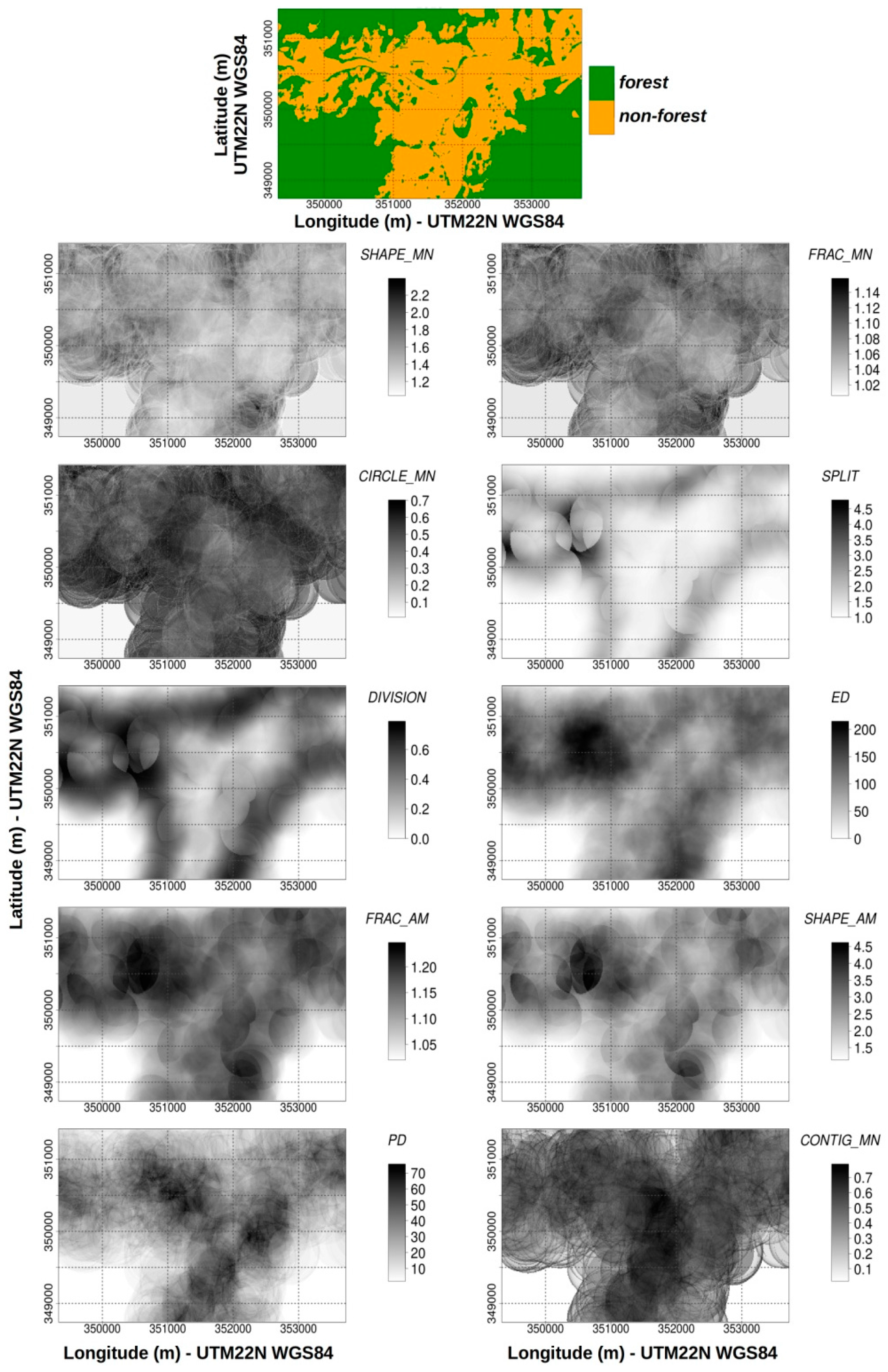

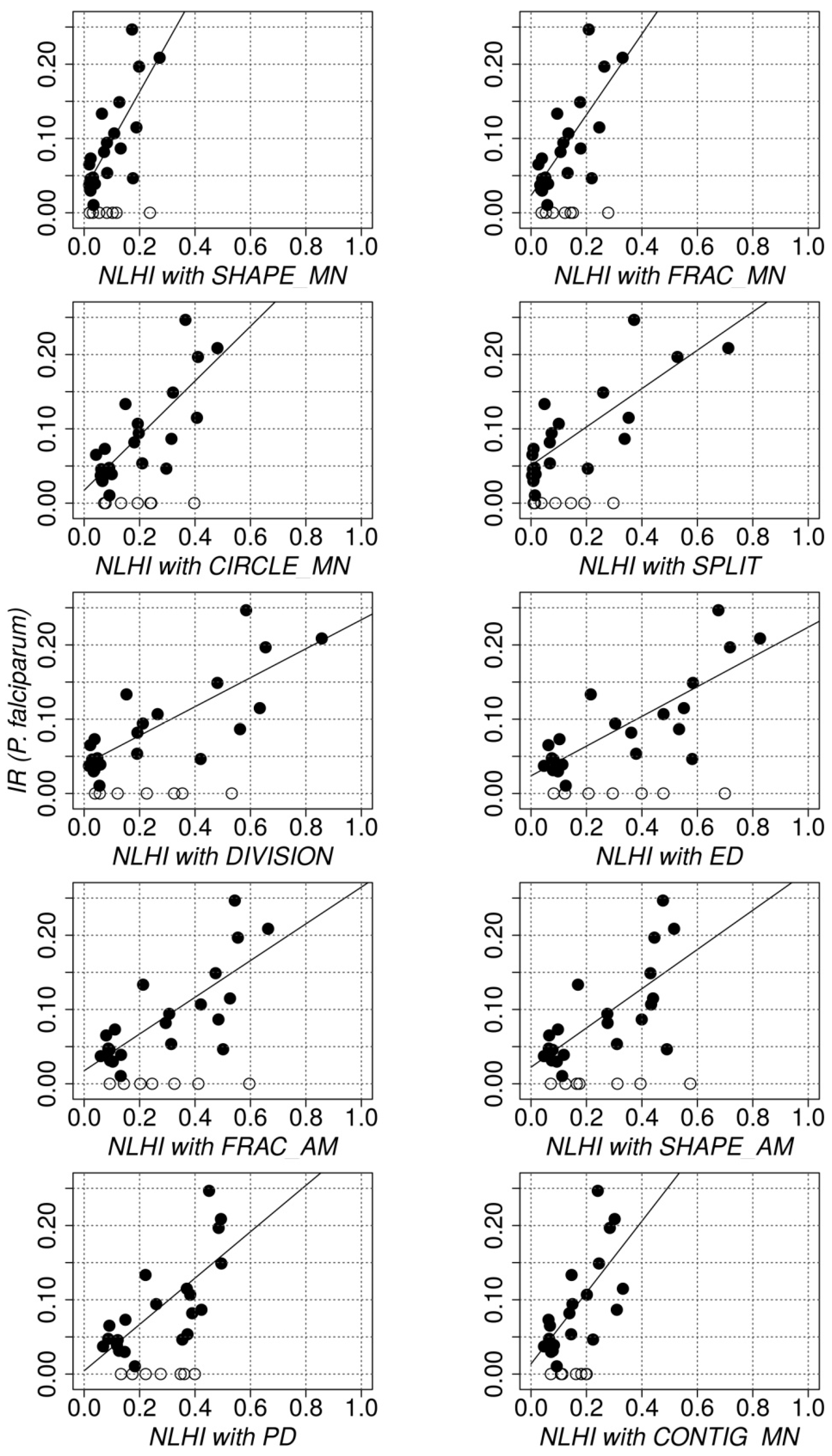
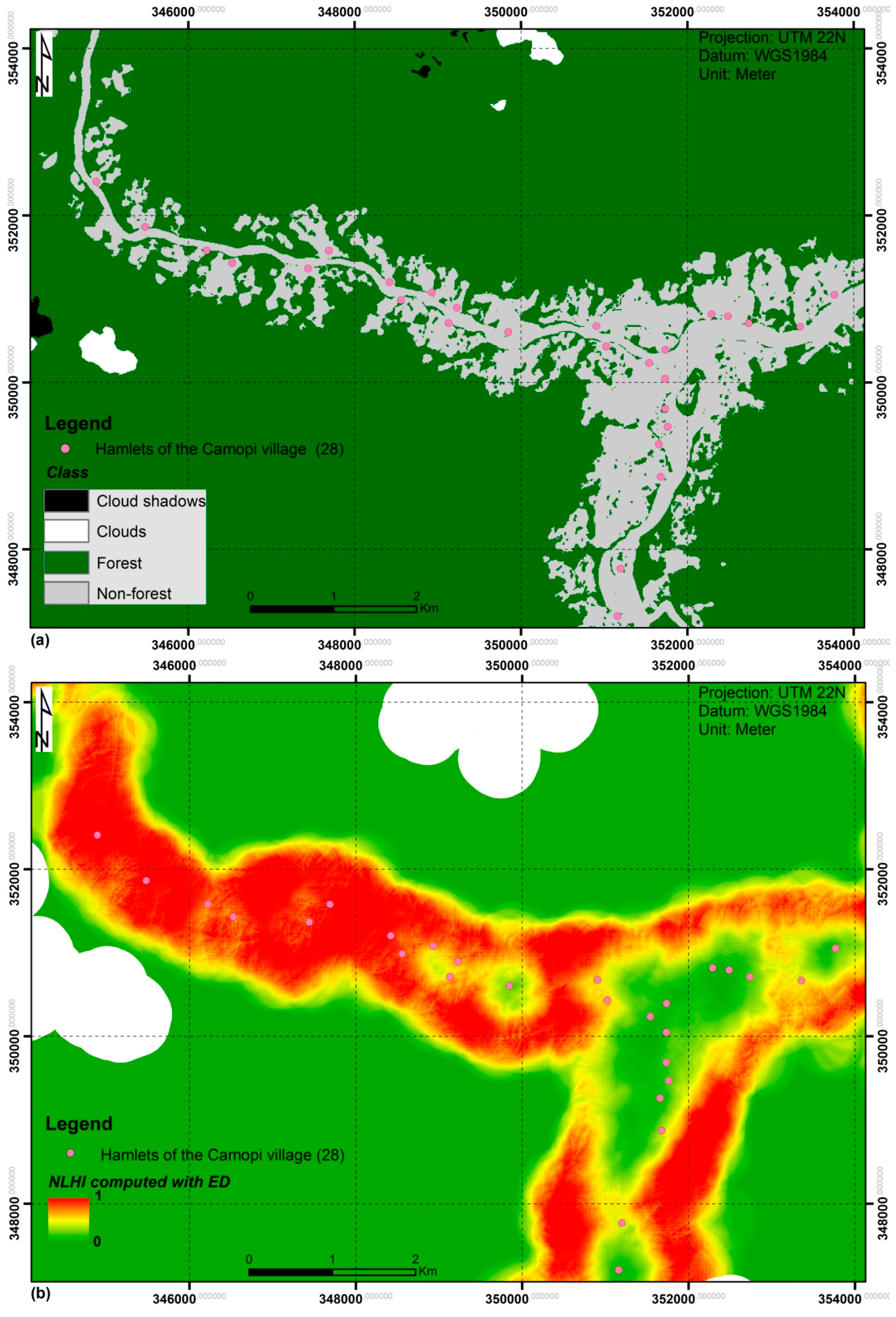
| Metric (Abbreviation) | Description (Adapted from [45]) | Units | Range |
|---|---|---|---|
| Percentage of forest (pF) | Percentage of forest in the computation window (ratio of forest area to area of computation window), multiplied by 100. | Percent | (0, 100) |
| Edge Density (ED) | Total length of forest edges in the computation window, per hectare. | Meters/hectare | ≥0 |
| Patch Density (PD) | Total number of patches of forest and non-forest in the computation window, per square meter. | Number/m2 | ≥1.99 |
| Landscape Division Index (DIVISION) | Probability that two randomly chosen pixels in the computation window are not situated in the same patch. | Proportion | (0, 1) |
| Splitting Index (SPLIT) | Number of patches one gets when dividing the computation window into parts of equal size in such a way that this new configuration leads to the same degree of landscape division as obtained for the observed landscape in the computation window. | No units | (1, number of cells in the landscape area squared) |
| Perimeter-area Ratio (PARA) | Simple ratio of patch perimeter to area for each patch in the computation window | No units | >0 |
| Shape Index (SHAPE) | Normalized ratio of patch perimeter to area in which the complexity of patch shape is compared to a square of the same size, for each patch in the computation window. | No units | ≥1 |
| Fractal Dimension Index (FRAC) | Normalized ratio of patch perimeter to area in which perimeter and area are log transformed. Reflecting shape complexity across a range of spatial scale (patch size), for each patch in the computation window. | No units | (1, 2) |
| Related Circumscribing Circle (CIRCLE) | Assessing shape based on the ratio of patch area to the area of the smallest circumscribing circle, for each patch in the computation window. | No units | (0, 1) |
| Contiguity Index (CONTIG) | Assessing patch shape based on the spatial connectedness, or contiguity, of cells within a patch, for each patch in the computation window. Large contiguous patches (small non-contiguous patches) result in low (high) configurational complexity. Note that this index corresponds to one minus the contiguity index as defined by Fragstats in order to be consistent with other complexity metrics. | No units | (0, 1) |
| Whole Dataset | Non Null Incidence Rates Only | |||
|---|---|---|---|---|
| Metric Alone | Candidate NLHI | Metric Alone | Candidate NLHI | |
| r; rho; R2 | r; rho; R2 | r; rho; R2 | r; rho; R2 | |
| pF | 0.61 **; 0.43; 0.37 ** | 0.78 **; 0.77 **; 0.60 ** | ||
| SHAPE_MN | 0.33; 0.32; 0.11 | 0.54 *; 0.42; 0.30 * | 0.61 *; 0.67 *; 0.37 * | 0.77 **; 0.74 **; 0.59 ** |
| FRAC_MN | 0.11; 0.04; 0.01 | 0.55 *; 0.38; 0.30 * | 0.20; 0.02; 0.04 | 0.76 **; 0.71 **; 0.57 ** |
| CIRCLE_MN | 0.15; 0.14; 0.02 | 0.60 **; 0.43; 0.35 ** | 0.18; 0.18; 0.03 | 0.79 **; 0.76 **; 0.62 ** |
| SPLIT | 0.65 **; 0.40; 0.42 ** | 0.68 **; 0.43; 0.47 ** | 0.83 **; 0.77 **; 0.69 ** | 0.81 **; 0.77 **; 0.65 ** |
| DIVISION | 0.54 *; 0.40; 0.29 * | 0.64 **; 0.43; 0.40 ** | 0.79 **; 0.77 **; 0.62 ** | 0.80 **; 0.78 **; 0.64 ** |
| ED | 0.32; 0.24; 0.10 | 0.59 **; 0.43; 0.35 ** | 0.53; 0.56 *; 0.28 | 0.79 **; 0.75 **; 0.63 ** |
| FRAC_AM | 0.13; 0.11; 0.02 | 0.57 *; 0.42; 0.33 * | 0.27; 0.39; 0.07 | 0.77 **; 0.74 **; 0.59 ** |
| SHAPE_AM | 0.04; 0.04; 0.00 | 0.50 *; 0.39; 0.25 * | 0.13; 0.25; 0.02 | 0.71 **; 0.71 **; 0.51 ** |
| PD | −0.31; −0.26; 0.09 | 0.57 *; 0.47; 0.33 * | −0.40; −0.37; 0.16 | 0.74 **; 0.76 **; 0.55 ** |
| CONTIG_MN | −0.38; −0.34; 0.14 | 0.59 *; 0.44; 0.35 * | −0.65 *; −0.68 **; 0.43 * | 0.71 **; 0.71 **; 0.50 ** |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Roux, E.; Dessay, N.; Girod, R.; Stefani, A.; Nacher, M.; Moiret, A.; Seyler, F. Mapping a Knowledge-Based Malaria Hazard Index Related to Landscape Using Remote Sensing: Application to the Cross-Border Area between French Guiana and Brazil. Remote Sens. 2016, 8, 319. https://doi.org/10.3390/rs8040319
Li Z, Roux E, Dessay N, Girod R, Stefani A, Nacher M, Moiret A, Seyler F. Mapping a Knowledge-Based Malaria Hazard Index Related to Landscape Using Remote Sensing: Application to the Cross-Border Area between French Guiana and Brazil. Remote Sensing. 2016; 8(4):319. https://doi.org/10.3390/rs8040319
Chicago/Turabian StyleLi, Zhichao, Emmanuel Roux, Nadine Dessay, Romain Girod, Aurélia Stefani, Mathieu Nacher, Adrien Moiret, and Frédérique Seyler. 2016. "Mapping a Knowledge-Based Malaria Hazard Index Related to Landscape Using Remote Sensing: Application to the Cross-Border Area between French Guiana and Brazil" Remote Sensing 8, no. 4: 319. https://doi.org/10.3390/rs8040319
APA StyleLi, Z., Roux, E., Dessay, N., Girod, R., Stefani, A., Nacher, M., Moiret, A., & Seyler, F. (2016). Mapping a Knowledge-Based Malaria Hazard Index Related to Landscape Using Remote Sensing: Application to the Cross-Border Area between French Guiana and Brazil. Remote Sensing, 8(4), 319. https://doi.org/10.3390/rs8040319








