Spatiotemporal Changes in China’s Mangroves and Their Possible Impacts on Coastal Water Quality from 1998 to 2018
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Data Source and Preprocessing
2.3. Methods
2.3.1. Trend Analysis
2.3.2. Partial Correlation Analysis
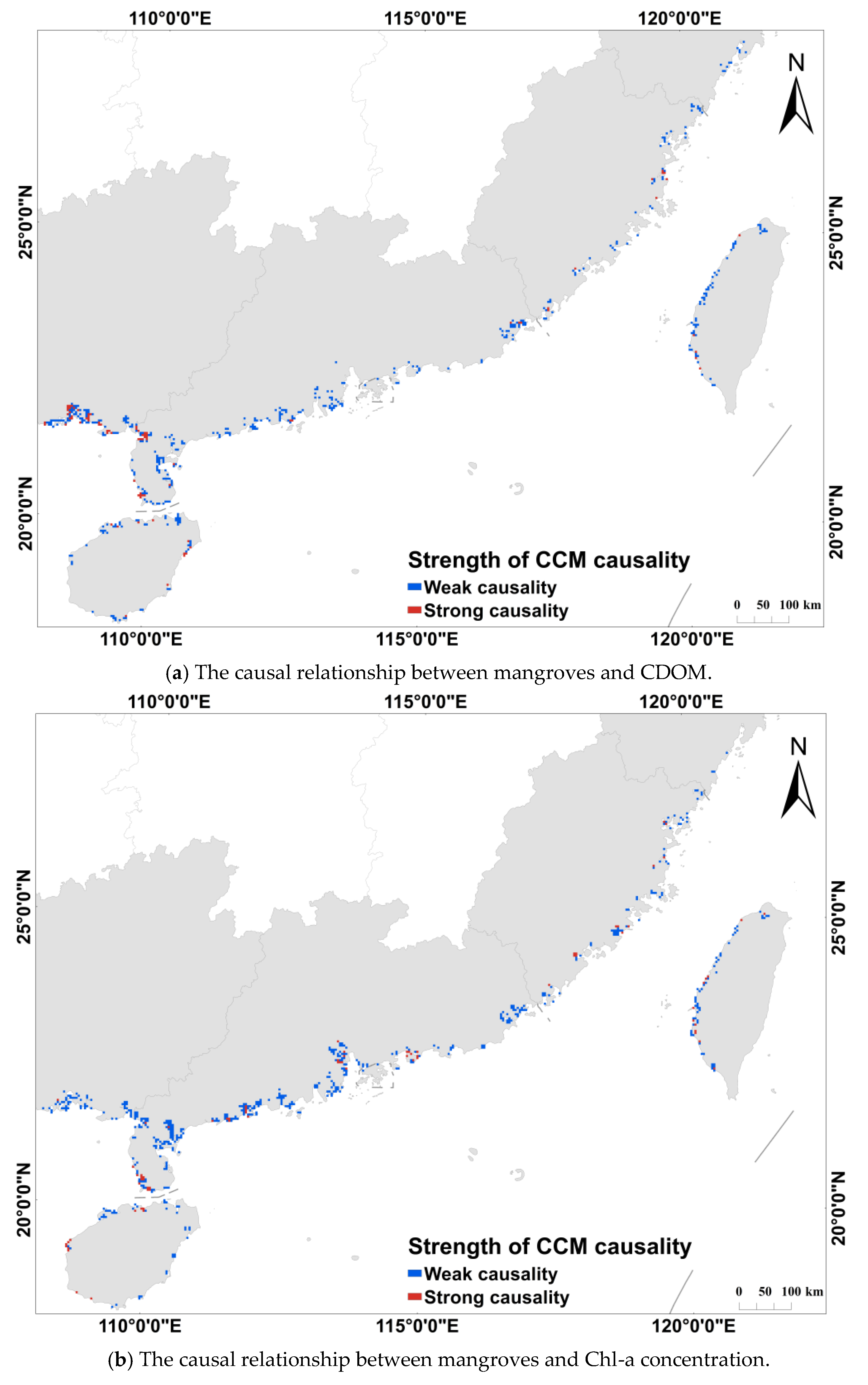
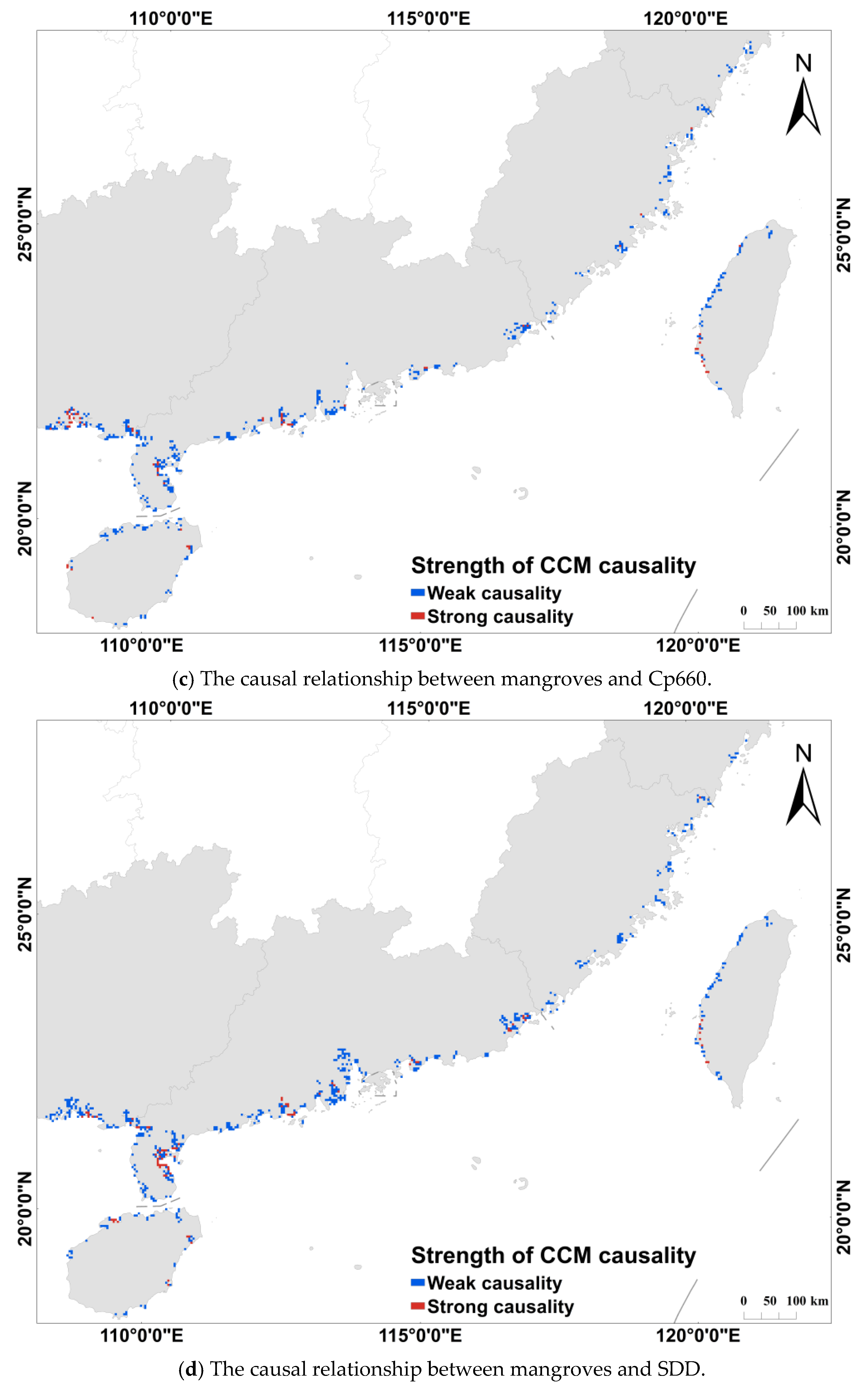
2.3.3. Convergent Cross-Mapping Method
2.3.4. Geodetector
3. Results
3.1. Dynamics of Mangroves in China from 1998 to 2018
3.2. Dynamics of Water Quality Parameters in Coastal from 1998 to 2018
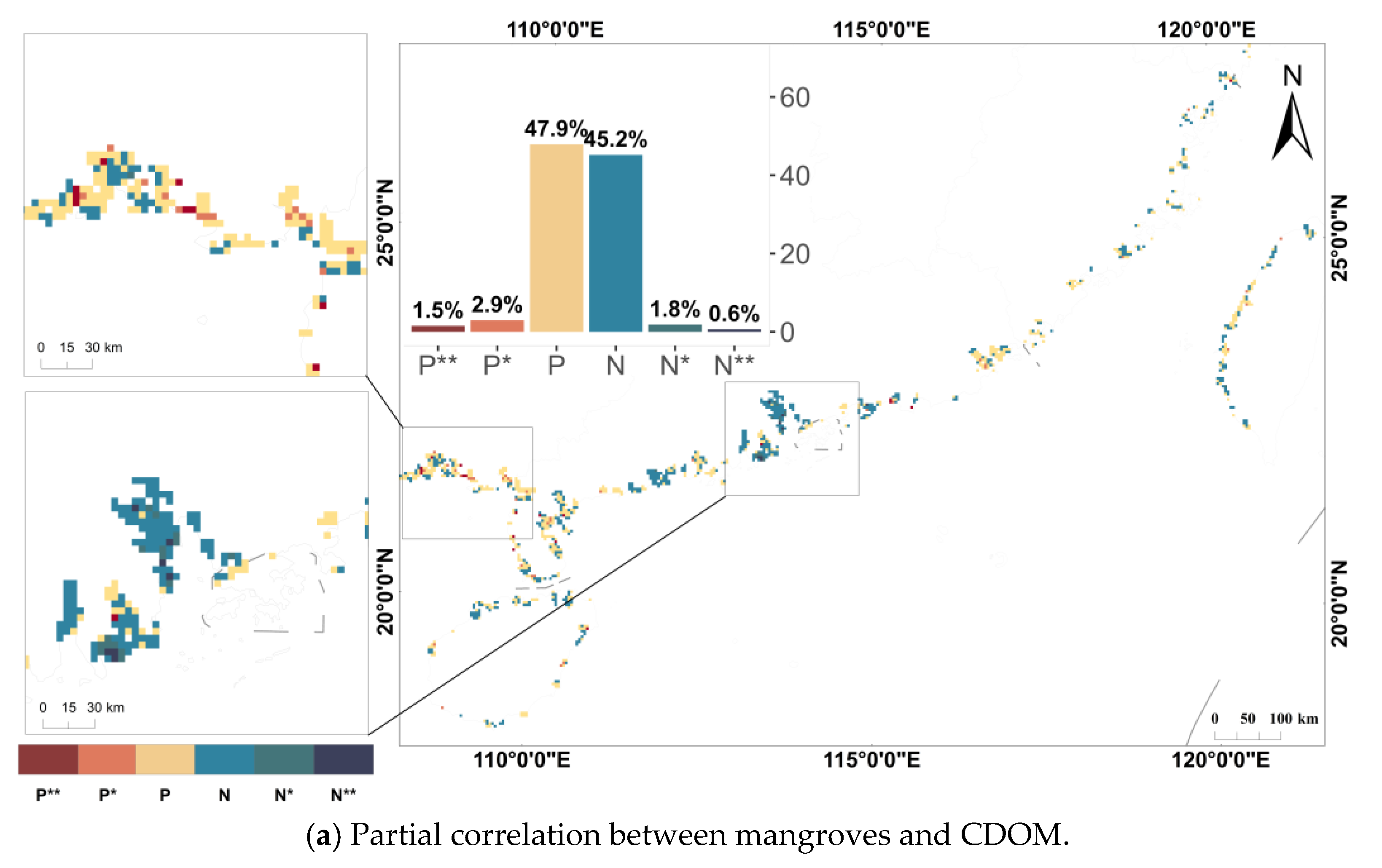
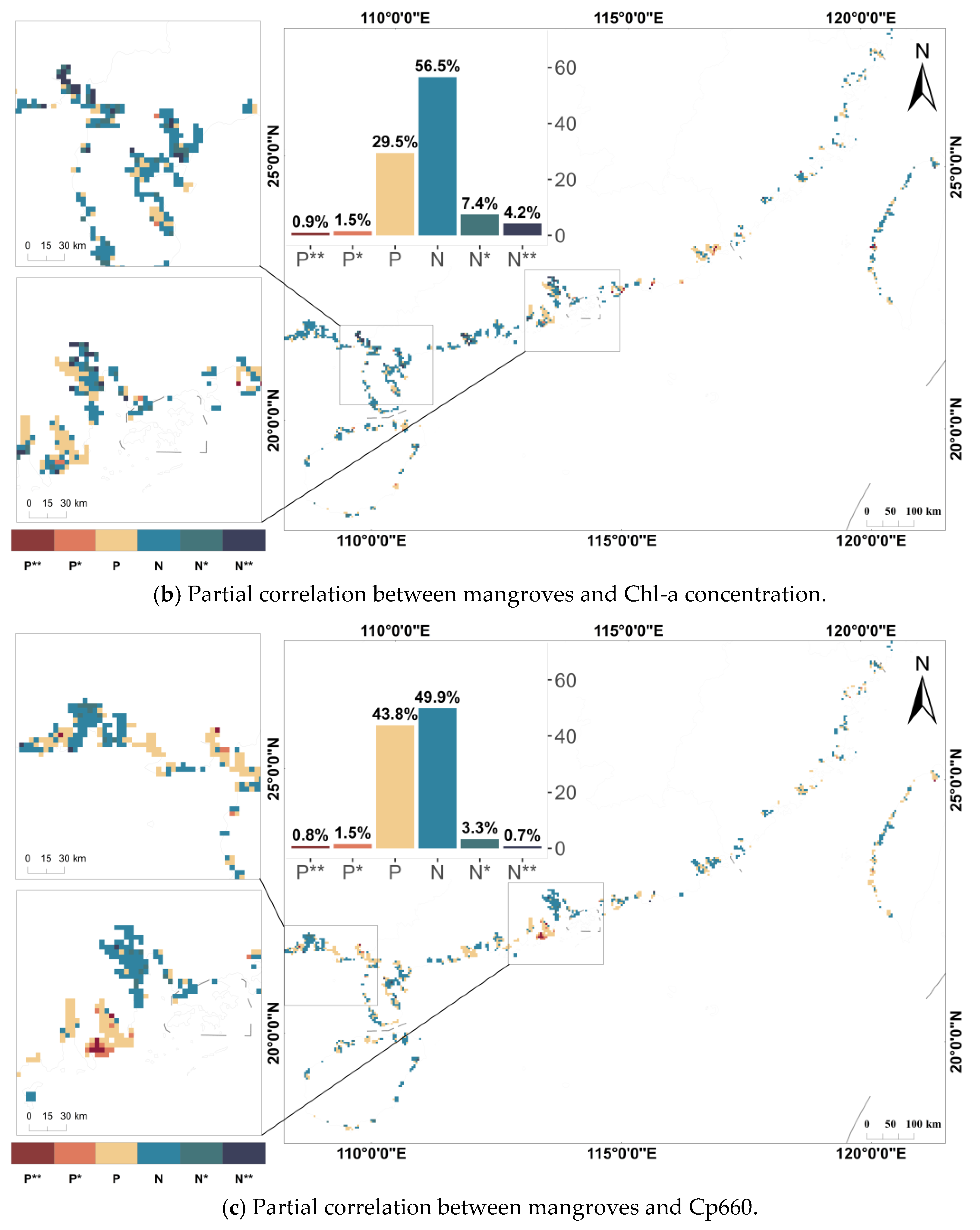
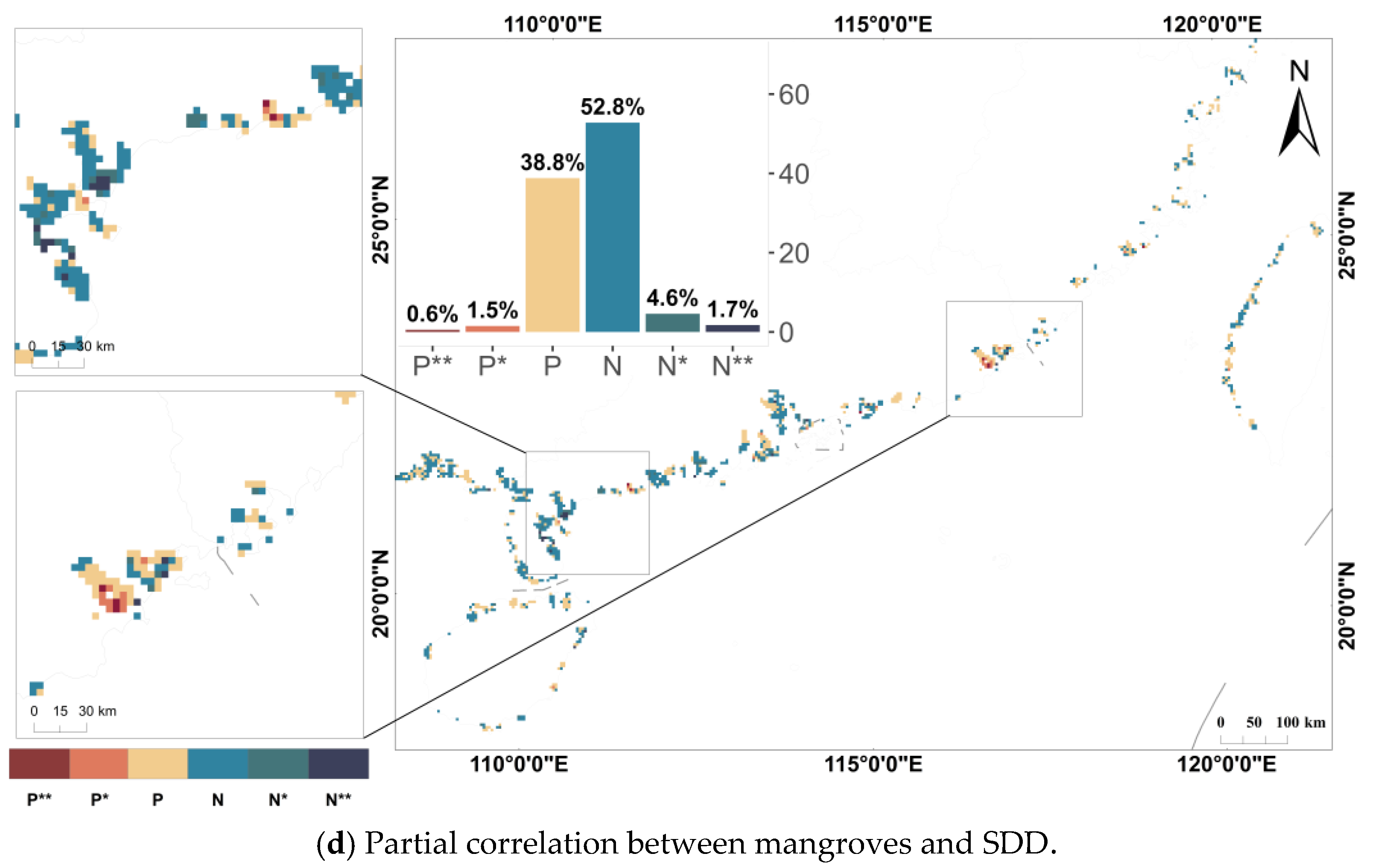
3.3. Relationship Between Mangroves and Water Quality Parameters
4. Discussion
4.1. Causes of Spatiotemporal Changes
4.2. Mangrove Impacts on Water Quality Parameters
4.3. Differences in Impact Results
4.4. Limitations and Future Research Directions
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, N.; Ma, Z. Ecological restoration of coastal wetlands in China: Current status and suggestions. Biol. Conserv. 2024, 291, 110513. [Google Scholar] [CrossRef]
- Alongi, D.M. Carbon cycling and storage in mangrove forests. Annu. Rev. Mar. Sci. 2014, 6, 195–219. [Google Scholar] [CrossRef] [PubMed]
- Twilley, R.R.; Rovai, A.S.; Riul, P. Coastal morphology explains global blue carbon distributions. Front. Ecol. Environ. 2018, 16, 503–508. [Google Scholar] [CrossRef]
- Gopal, B.; Chauhan, M. Biodiversity and its conservation in the Sundarban mangrove ecosystem. Aquat. Sci. 2006, 68, 338–354. [Google Scholar] [CrossRef]
- Shanker, K. Biodiversity of mangrove ecosystems. Conserv. Soc. 2005, 3, 537–539. [Google Scholar]
- Kathiresan, K.; Rajendran, N. Coastal mangrove forests mitigated tsunami. Estuar. Coast. Shelf Sci. 2005, 65, 601–606. [Google Scholar] [CrossRef]
- McIvor, A.; Möller, I.; Spencer, T.; Spalding, M. Reduction of Wind and Swell Waves by Mangroves; The Nature Conservancy: Arlington, WV, USA; Wetlands International: Ede, The Netherlands, 2012. [Google Scholar]
- Nagelkerken, I.; Van der Velde, G.; Gorissen, M.; Meijer, G.; Van’t Hof, T.; Den Hartog, C. Importance of mangroves, seagrass beds and the shallow coral reef as a nursery for important coral reef fishes, using a visual census technique. Estuar. Coast. Shelf Sci. 2000, 51, 31–44. [Google Scholar] [CrossRef]
- Pelegri, S.; Twilley, R. Heterotrophic nitrogen fixation (acetylene reduction) during leaf-litter decomposition of two mangrove species from South Florida, USA. Mar. Biol. 1998, 131, 53–61. [Google Scholar] [CrossRef]
- Mazda, Y.; Wolanski, E.; Ridd, P. The Role of Physical Processes in Mangrove Environments: Manual for the Preservation and Utilization of Mangrove Ecosystems; Terra Pub.: Drobeta-Turnu Severin, Romania, 2007. [Google Scholar]
- Gissi, E.; Manea, E.; Mazaris, A.D.; Fraschetti, S.; Almpanidou, V.; Bevilacqua, S.; Coll, M.; Guarnieri, G.; Lloret-Lloret, E.; Pascual, M. A review of the combined effects of climate change and other local human stressors on the marine environment. Sci. Total Environ. 2021, 755, 142564. [Google Scholar] [CrossRef]
- Zhai, T.; Wang, J.; Fang, Y.; Qin, Y.; Huang, L.; Chen, Y. Assessing ecological risks caused by human activities in rapid urbanization coastal areas: Towards an integrated approach to determining key areas of terrestrial-oceanic ecosystems preservation and restoration. Sci. Total Environ. 2020, 708, 135153. [Google Scholar] [CrossRef]
- Gao, Y.; Hao, P.; Wei, Z.; Li, S.; Song, J.; Yu, C. Dynamic causes contribute to the increasing trend of red tides in the east China sea during 2020–2022. Mar. Environ. Res. 2024, 198, 106521. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Sun, Y.; Xu, G.; Liu, D. Simulation of red tide drift-diffusion process in the Pearl River Estuary and its response to the environment. Front. Mar. Sci. 2023, 10, 1096896. [Google Scholar] [CrossRef]
- Li, H.-M.; Tang, H.-J.; Shi, X.-Y.; Zhang, C.-S.; Wang, X.-L. Increased nutrient loads from the Changjiang (Yangtze) River have led to increased harmful algal blooms. Harmful Algae 2014, 39, 92–101. [Google Scholar] [CrossRef]
- Cavanaugh, K.C.; Kellner, J.R.; Forde, A.J.; Gruner, D.S.; Parker, J.D.; Rodriguez, W.; Feller, I.C. Poleward expansion of mangroves is a threshold response to decreased frequency of extreme cold events. Proc. Natl. Acad. Sci. USA 2014, 111, 723–727. [Google Scholar] [CrossRef] [PubMed]
- Osland, M.J.; Day, R.H.; Hall, C.T.; Brumfield, M.D.; Dugas, J.L.; Jones, W.R. Mangrove expansion and contraction at a poleward range limit: Climate extremes and land-ocean temperature gradients. Ecology 2017, 98, 125–137. [Google Scholar] [CrossRef]
- Wang, Y.; Dong, P.; Hu, W.; Chen, G.; Zhang, D.; Chen, B.; Lei, G. Modeling the Climate Suitability of Northernmost Mangroves in China under Climate Change Scenarios. Forests 2022, 13, 64. [Google Scholar] [CrossRef]
- Krauss, K.W.; McKee, K.L.; Lovelock, C.E.; Cahoon, D.R.; Saintilan, N.; Reef, R.; Chen, L. How mangrove forests adjust to rising sea level. New Phytol. 2014, 202, 19–34. [Google Scholar] [CrossRef]
- Manju, M.; Resmi, P.; Gireesh, K.T.; Ratheesh, K.C.; Rahul, R.; Joseph, M.; Chandramohanakumar, N. Assessment of water quality parameters in mangrove ecosystems along Kerala coast: A statistical approach. Int. J. Environ. Res. 2012, 6, 893–902. [Google Scholar]
- Samara, F.; Solovieva, N.; Ghalayini, T.; Nasrallah, Z.A.; Saburova, M. Assessment of the environmental status of the mangrove ecosystem in the United Arab Emirates. Water 2020, 12, 1623. [Google Scholar] [CrossRef]
- Whelan, K.R.; Smith, T.J.; Cahoon, D.R.; Lynch, J.C.; Anderson, G.H. Groundwater control of mangrove surface elevation: Shrink and swell varies with soil depth. Estuaries 2005, 28, 833–843. [Google Scholar] [CrossRef]
- Kristensen, E.; Bouillon, S.; Dittmar, T.; Marchand, C. Organic carbon dynamics in mangrove ecosystems: A review. Aquat. Bot. 2008, 89, 201–219. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Z.; Feng, L.; Ma, Y.; Du, Q. A new attention-based CNN approach for crop mapping using time series Sentinel-2 images. Comput. Electron. Agric. 2021, 184, 106090. [Google Scholar] [CrossRef]
- Ini, I.A.; Yueyue, Z.; Youshao, W. Characteristics of water quality and their eutrophication assessment on the mangrove ecosystems along the Guangdong coast. J. Trop. Oceanogr. 2022, 41, 1–11. [Google Scholar]
- Luo, J.; Huang, Q.; Zhang, H.; Xu, Y.; Zu, X.; Song, B. Impact of Conservation in the Futian Mangrove National Nature Reserve on Water Quality in the Last Twenty Years. Forests 2024, 15, 1246. [Google Scholar] [CrossRef]
- Satheeshkumar, P.; Khan, A.B. Identification of mangrove water quality by multivariate statistical analysis methods in Pondicherry coast, India. Environ. Monit. Assess. 2012, 184, 3761–3774. [Google Scholar] [CrossRef]
- Sari, N.; Patria, M.P.; Soesilo, T.E.B.; Tejakusuma, I.G. The structure of mangrove communities in response to water quality in Jakarta Bay, Indonesia. Biodiversitas J. Biol. Divers. 2019, 20, 1873–1879. [Google Scholar] [CrossRef]
- Lotfinasabasl, S.; Gunale, V.; Khosroshahi, M. Applying geographic information systems and remote sensing for water quality assessment of mangrove forest. Acta Ecol. Sin. 2018, 38, 135–143. [Google Scholar] [CrossRef]
- Jia, M.; Wang, Z.; Zhang, Y.; Mao, D.; Wang, C. Monitoring loss and recovery of mangrove forests during 42 years: The achievements of mangrove conservation in China. Int. J. Appl. Earth Obs. Geoinf. 2018, 73, 535–545. [Google Scholar] [CrossRef]
- Xu, M.; Wang, Z.; Liang, Y.; Mo, Z.; Zhang, Q. Analysis of spatiotemporal evolution characteristics and recovery patterns of mangrove forests in China since 1978. Ecol. Indic. 2024, 169, 112882. [Google Scholar] [CrossRef]
- Luojia, H.; Wenyu, L.; Bing, X. Monitoring mangrove forest change in China from 1990 to 2015 using Landsat-derived spectral-temporal variability metrics. Int. J. Appl. Earth Obs. Geoinf. 2018, 73, 88–98. [Google Scholar]
- Yang, G.; Huang, K.; Sun, W.; Wang, L.; Chen, B. Mangrove Dynamics in China (MDC): Accurate and Annual Distribution Maps from 1990 to 2020. Science Data Bank. 2024. [Google Scholar] [CrossRef]
- Li, G.; Cheng, L.; Pan, Y.; Wang, G.; Liu, H.; Zhu, J.; Zhang, B.; Ren, H.; Wang, X. A global gridded ocean salinity dataset with 0.5° horizontal resolution since 1960 for the upper 2000 m. Front. Mar. Sci. 2023, 10, 1108919. [Google Scholar] [CrossRef]
- Zhong, G.; Li, X.; Song, J.; Qu, B.; Wang, F.; Wang, Y.; Zhang, B.; Sun, X.; Zhang, W.; Wang, Z. Reconstruction of global surface ocean pCO2 using region-specific predictors based on a stepwise FFNN regression algorithm. Biogeosciences 2022, 19, 845–859. [Google Scholar] [CrossRef]
- Li, L.; Fu, Y.; Xue, C.; Cui, J.; Zhang, Y.; Xu, Y. Development of monthly-seasonal-annual dataset of sea surface chlorophyll-a concentration for 21 years, (1998–2018). J. Glob. Change Data Discov. 2021, 2, 219–225. [Google Scholar]
- Hersbach, H.; Bell, B.; Berrisford, P.; Biavati, G.; Horányi, A.; Muñoz Sabater, J.; Nicolas, J.; Peubey, C.; Radu, R.; Rozum, I. ERA5 monthly averaged data on single levels from 1940 to present. Copernic. Clim. Change Serv. Clim. Data Store 2019, 10, 252–266. [Google Scholar]
- Tucker, C.J.; Newcomb, W.W.; Los, S.O.; Prince, S.D. Mean and inter-year variation of growing-season normalized difference vegetation index for the Sahel 1981-1989. Int. J. Remote Sens. 1991, 12, 1133–1135. [Google Scholar] [CrossRef]
- Lunetta, R.S.; Knight, J.F.; Ediriwickrema, J.; Lyon, J.G.; Worthy, L.D. Land-cover characterization and change detection using multitemporal MODIS NDVI data. Remote Sens. Environ. 2009, 105, 142–154. [Google Scholar] [CrossRef]
- Theil, H. A Rank-Invariant Method of Linear and Polynomial Regression Analysis; Springer: Dordrecht, The Netherlands, 1992. [Google Scholar]
- Alongi, D.M. Present state and future of the world’s mangrove forests. Environ. Conserv. 2002, 29, 331–349. [Google Scholar] [CrossRef]
- Burn, D.H.; Hag Elnur, M.A. Detection of hydrologic trends and variability. J. Hydrol. 2002, 255, 107–122. [Google Scholar] [CrossRef]
- Lipsitz, S.R.; Leong, T.; Ibrahim, J.; Lipshultz, S. A partial correlation coefficient and coefficient of determination for multivariate normal repeated measures data. J. R. Stat. Soc. Ser. D Stat. 2001, 50, 87–95. [Google Scholar] [CrossRef]
- Sugihara, G.; May, R.; Ye, H.; Hsieh, C.-h.; Deyle, E.; Fogarty, M.; Munch, S. Detecting causality in complex ecosystems. Science 2012, 338, 496–500. [Google Scholar] [CrossRef] [PubMed]
- Deyle, E.R.; May, R.M.; Munch, S.B.; Sugihara, G. Tracking and forecasting ecosystem interactions in real time. Proc. R. Soc. B Biol. Sci. 2016, 283, 20152258. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Wang, J.; Ge, Y.; Xu, C. An optimal parameters-based geographical detector model enhances geographic characteristics of explanatory variables for spatial heterogeneity analysis: Cases with different types of spatial data. GIScience Remote Sens. 2020, 57, 593–610. [Google Scholar] [CrossRef]
- Wang, J.; Xu, C. Geodetector: Principle and prospective. Acta Geogr. Sin. 2017, 72, 116–134. [Google Scholar]
- Zhai, Y.; Zhai, G.; Chen, Y.; Liu, J. Research on regional terrestrial carbon storage based on the pattern-process-function. Ecol. Inform. 2024, 80, 102523. [Google Scholar] [CrossRef]
- Chen, L.; Zheng, W.; Yang, S.; Wang, W.; Zhang, Y. Research Progresses of Mangrove Cold-tolerant Classes and Seral Classes, and Their Responses to Climate Change. J. Xiamen Univ. 2017, 56, 305–313. [Google Scholar]
- Wang, W.; Li, X.; Wang, M. Propagule dispersal determines mangrove zonation at intertidal and estuarine scales. Forests 2019, 10, 245. [Google Scholar] [CrossRef]
- Wang, Y.-S.; Gu, J.-D. Ecological responses, adaptation and mechanisms of mangrove wetland ecosystem to global climate change and anthropogenic activities. Int. Biodeterior. Biodegrad. 2021, 162, 105248. [Google Scholar] [CrossRef]
- Zhang, K.; Zhao, X.; Xue, J.; Mo, D.; Zhang, D.; Xiao, Z.; Yang, W.; Wu, Y.; Chen, Y. The temporal and spatial variation of chlorophyll a concentration in the China Seas and its impact on marine fisheries. Front. Mar. Sci. 2023, 10, 1212992. [Google Scholar] [CrossRef]
- Shell, K.M.; Frouin, R.; Nakamoto, S.; Somerville, R.C.J. Atmospheric response to solar radiation absorbed by phytoplankton. J. Geophys. Res. 2003, 108, 4445. [Google Scholar] [CrossRef]
- Parida, A.K.; Jha, B. Salt tolerance mechanisms in mangroves: A review. Trees 2010, 24, 199–217. [Google Scholar] [CrossRef]
- Chang, D.; Li, S.; Lai, Z. Effects of extreme precipitation intensity and duration on the runoff and nutrient yields. J. Hydrol. 2023, 626, 130281. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, X.; Osburn, C.L.; Wang, M.; Qin, B.; Zhou, Y. Photobleaching Response of Different Sources of Chromophoric Dissolved Organic Matter Exposed to Natural Solar Radiation Using Absorption and Excitation–Emission Matrix Spectra. PLoS ONE 2013, 8, e77515. [Google Scholar] [CrossRef] [PubMed]
- Aiello, G. Sedimentary Processes: Examples from Asia, Turkey and Nigeria; BoD–Books on Demand: Norderstedt, Germany, 2020. [Google Scholar]
- Hilaluddin, F.; Yusoff, F.; Natrah, F.; Lim, P. Disturbance of mangrove forests causes alterations in estuarine phytoplankton community structure in Malaysian Matang mangrove forests. Mar. Environ. Res. 2020, 158, 104935. [Google Scholar] [CrossRef]
- Chen, C.; Gao, Y.; Lin, P. Progress in the studies of the diatoms in mangrove environment. Mar. Sci. 2002, 26, 17–19. [Google Scholar]
- Pogson, M.; Smith, P. Effect of spatial data resolution on uncertainty. Environ. Model. Softw. 2015, 63, 87–96. [Google Scholar] [CrossRef]

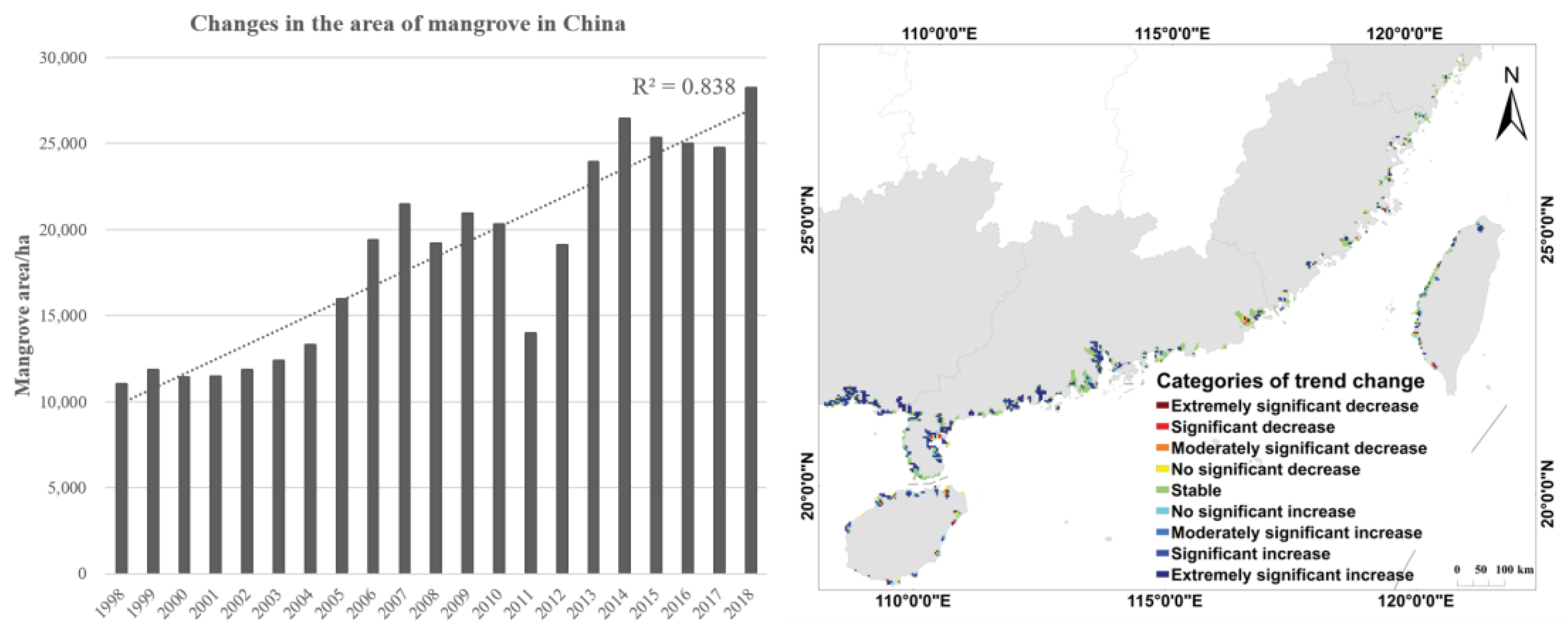
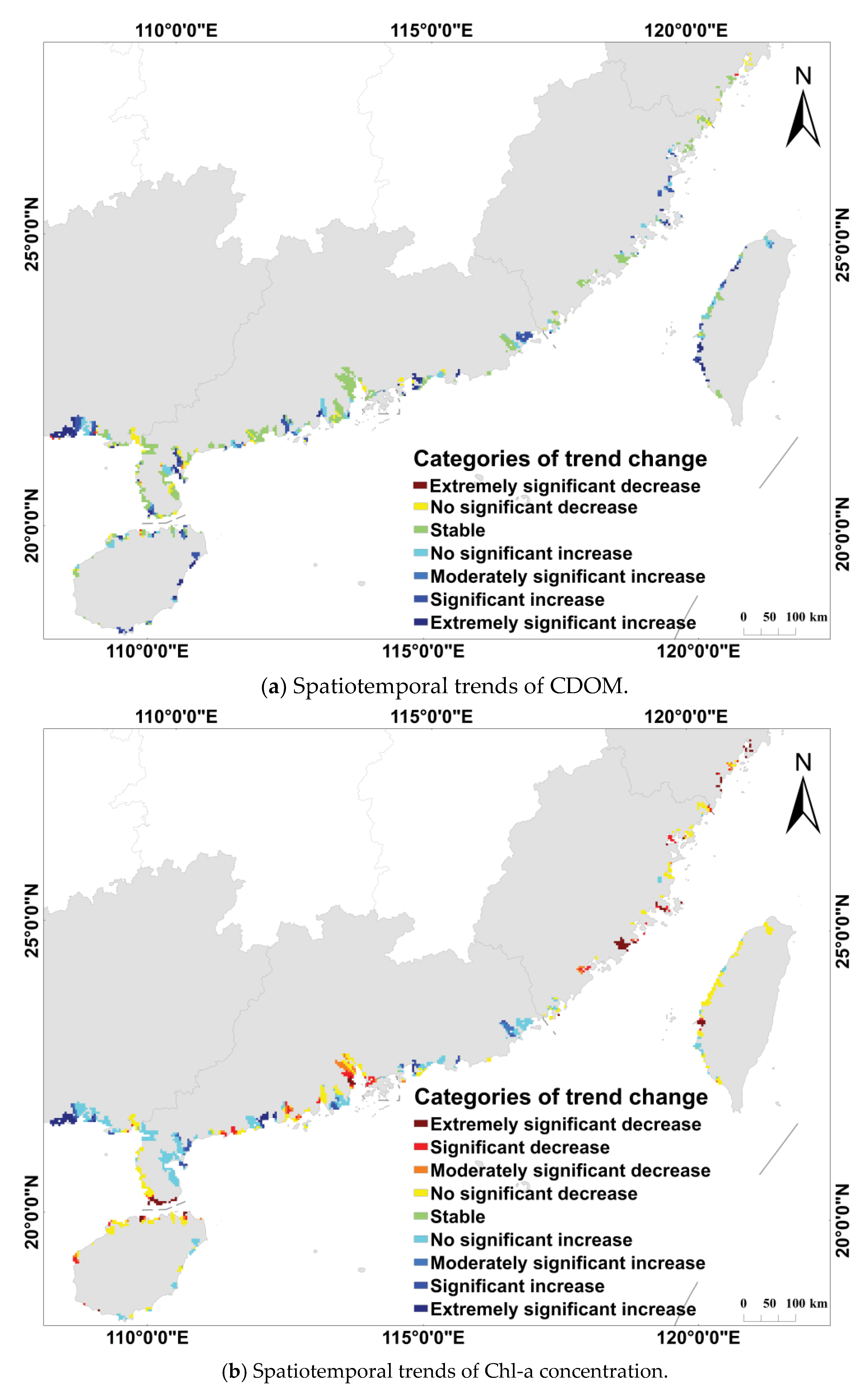
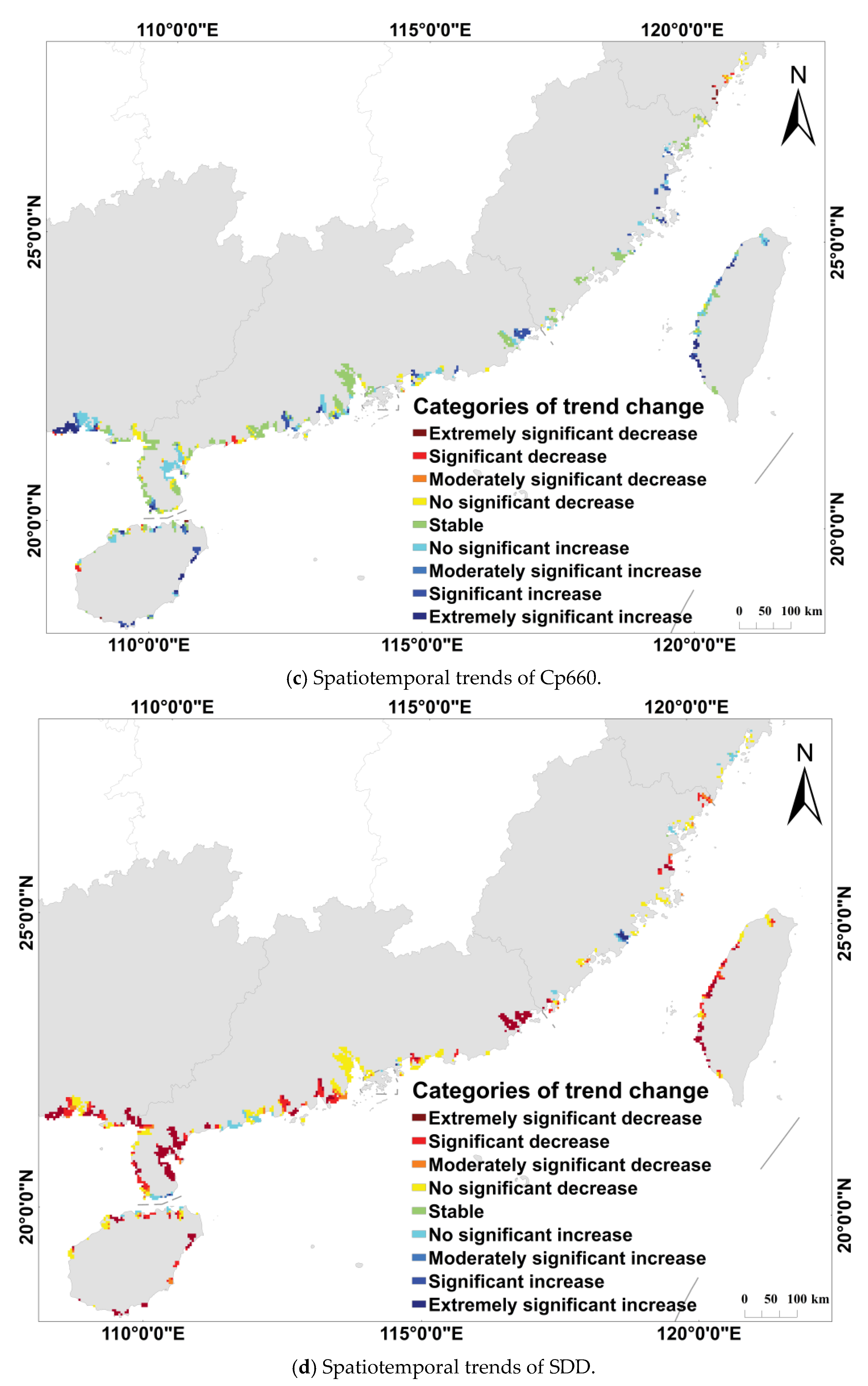

| Categories of Trend Changes | ||
|---|---|---|
| Extremely significant increase | ||
| Significant increase | ||
| Moderately significant increase | ||
| Non-significant increase | ||
| Stable | ||
| Non-significant decrease | ||
| Moderately significant decrease | ||
| Significant decrease | ||
| Extremely significant decrease |
| Class | Denotes Symbols | ||
|---|---|---|---|
| Highly significant negative partial correlation | N ** | ||
| Significant negative partial correlation | N * | ||
| Non-significant negative partial correlation | N | ||
| Non-significant positive partial correlation | P | ||
| Significant positive partial correlation | P * | ||
| Highly significant positive partial correlation | P ** |
| CDOM | 29.70 | 0.35 |
| CHLA | 34.90 | 0.34 |
| CP660 | 28.40 | 0.33 |
| SDD | 36.77 | 0.38 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, J.; Yang, G.; Sun, W.; Huang, K.; Lu, C.; Yu, W.; Zhang, X.; Chen, B.; Liu, W.; Feng, T. Spatiotemporal Changes in China’s Mangroves and Their Possible Impacts on Coastal Water Quality from 1998 to 2018. Remote Sens. 2025, 17, 1640. https://doi.org/10.3390/rs17091640
Ren J, Yang G, Sun W, Huang K, Lu C, Yu W, Zhang X, Chen B, Liu W, Feng T. Spatiotemporal Changes in China’s Mangroves and Their Possible Impacts on Coastal Water Quality from 1998 to 2018. Remote Sensing. 2025; 17(9):1640. https://doi.org/10.3390/rs17091640
Chicago/Turabian StyleRen, Jingwen, Gang Yang, Weiwei Sun, Ke Huang, Chengqi Lu, Wenrui Yu, Xinyi Zhang, Binjie Chen, Weiwei Liu, and Tian Feng. 2025. "Spatiotemporal Changes in China’s Mangroves and Their Possible Impacts on Coastal Water Quality from 1998 to 2018" Remote Sensing 17, no. 9: 1640. https://doi.org/10.3390/rs17091640
APA StyleRen, J., Yang, G., Sun, W., Huang, K., Lu, C., Yu, W., Zhang, X., Chen, B., Liu, W., & Feng, T. (2025). Spatiotemporal Changes in China’s Mangroves and Their Possible Impacts on Coastal Water Quality from 1998 to 2018. Remote Sensing, 17(9), 1640. https://doi.org/10.3390/rs17091640







