Specific Responses to Environmental Factors Cause Discrepancy in the Link Between Solar-Induced Chlorophyll Fluorescence and Transpiration in Three Plantations
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Sites and Measurements
2.1.1. Study Sites
2.1.2. The Observation of Tower-Based SIF
2.1.3. Eddy Covariance Flux and Environment Measurements
2.2. Description of Data Analysis
2.2.1. Acquisition of Vegetation Index
2.2.2. Canopy Transpiration Estimation and Evaluation
3. Results
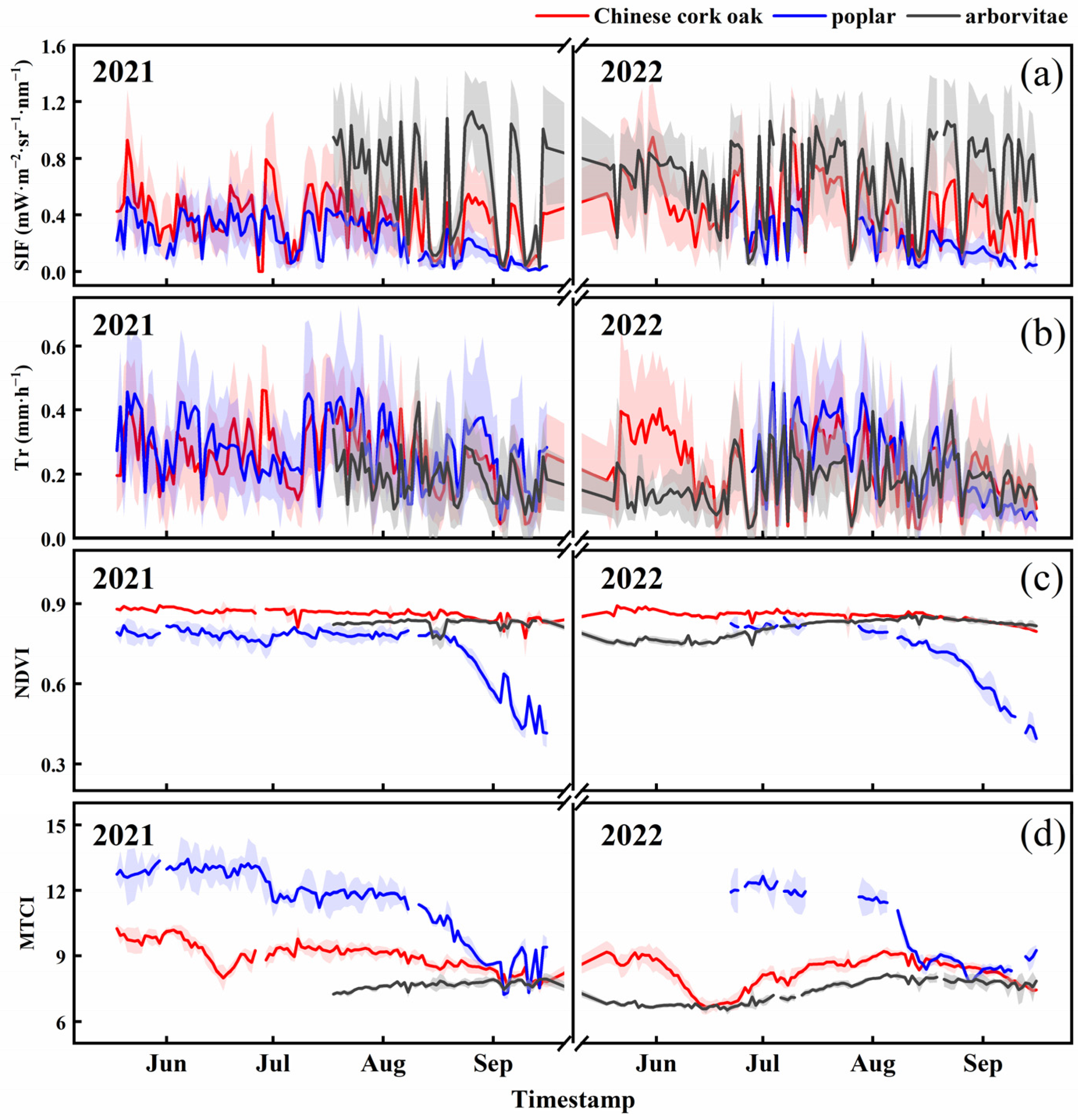
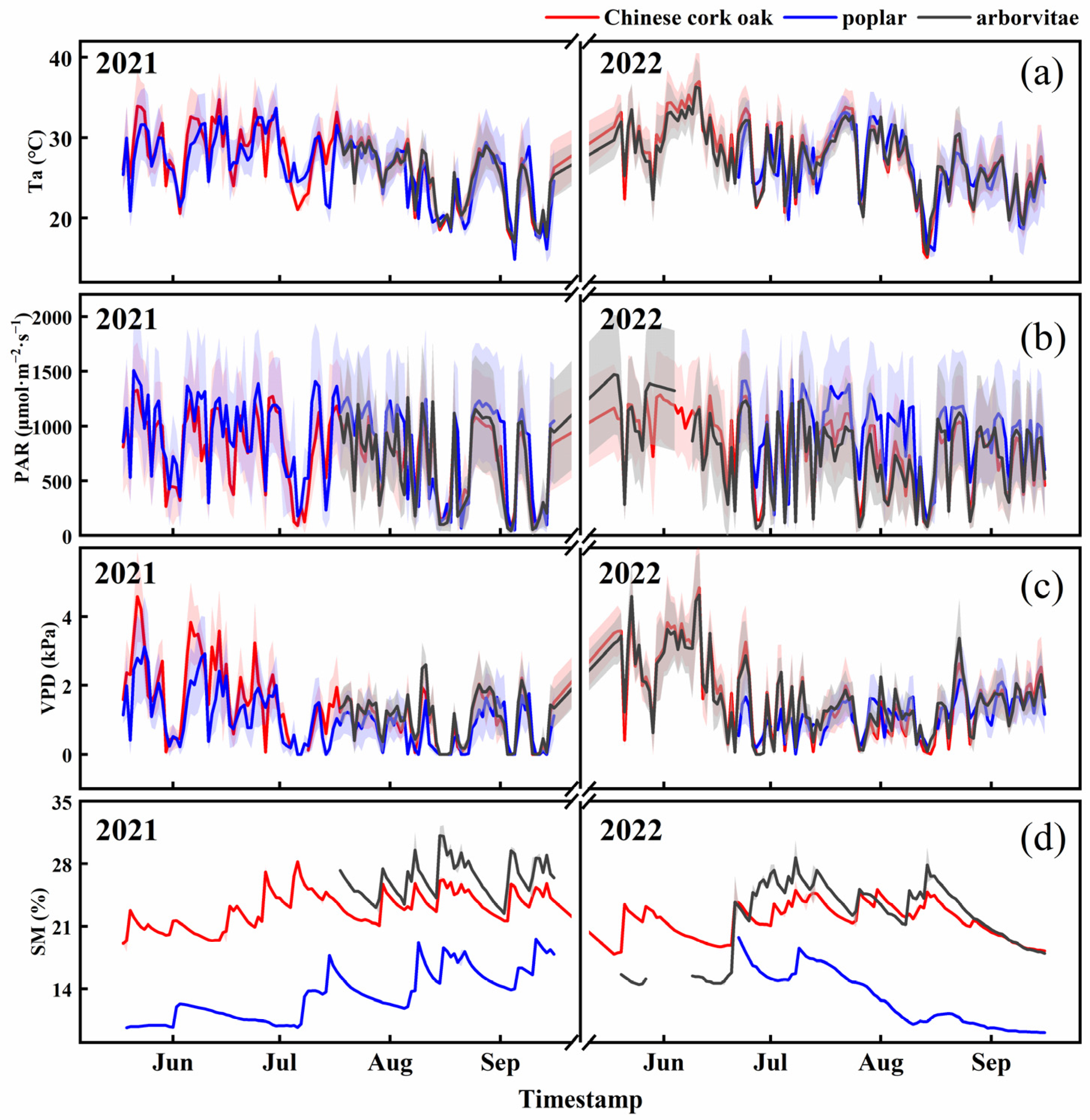
3.1. Temporal Dynamics of SIF, Tr, and Environmental Factors
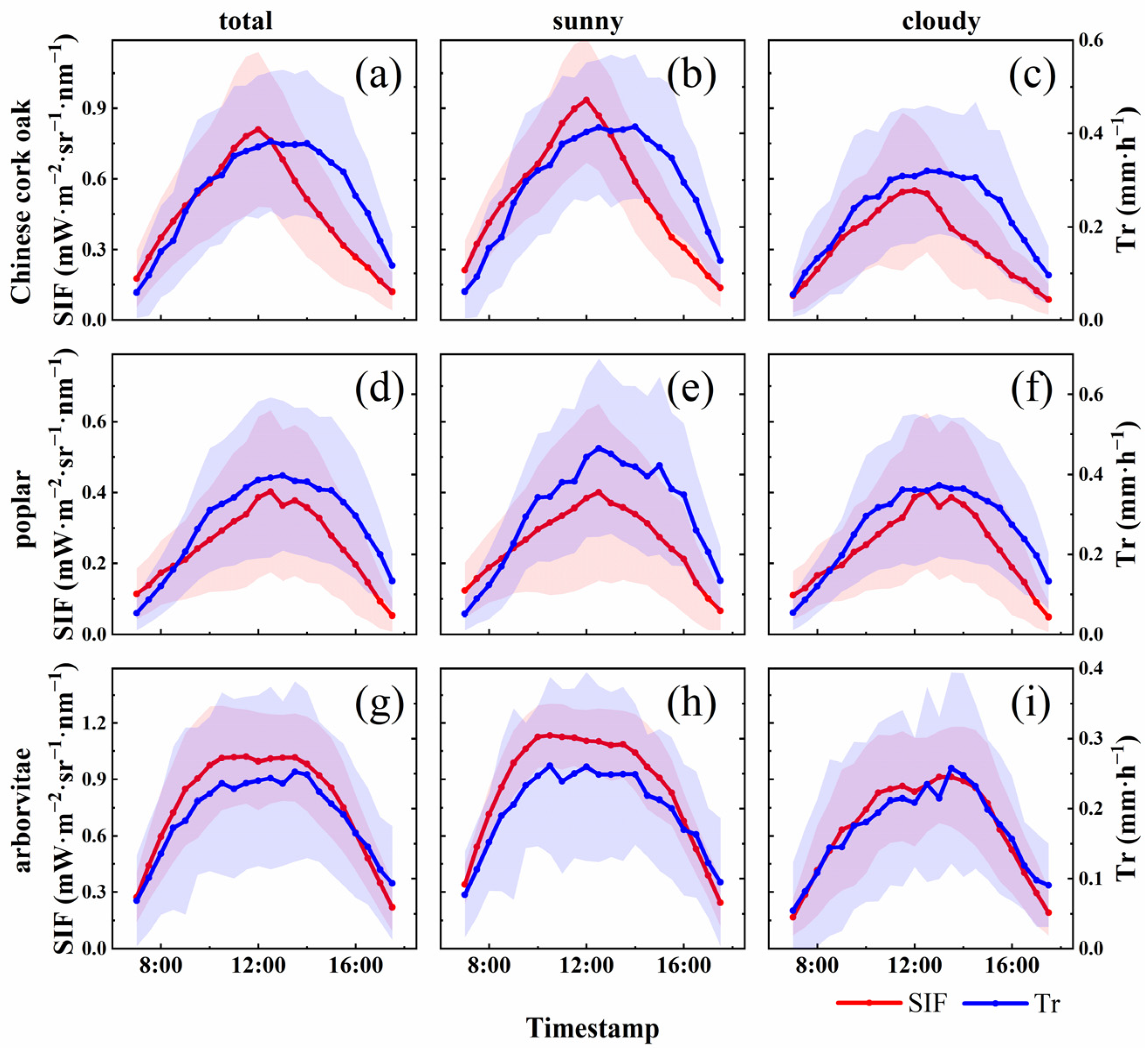
3.2. Relationships Between SIF and Tr
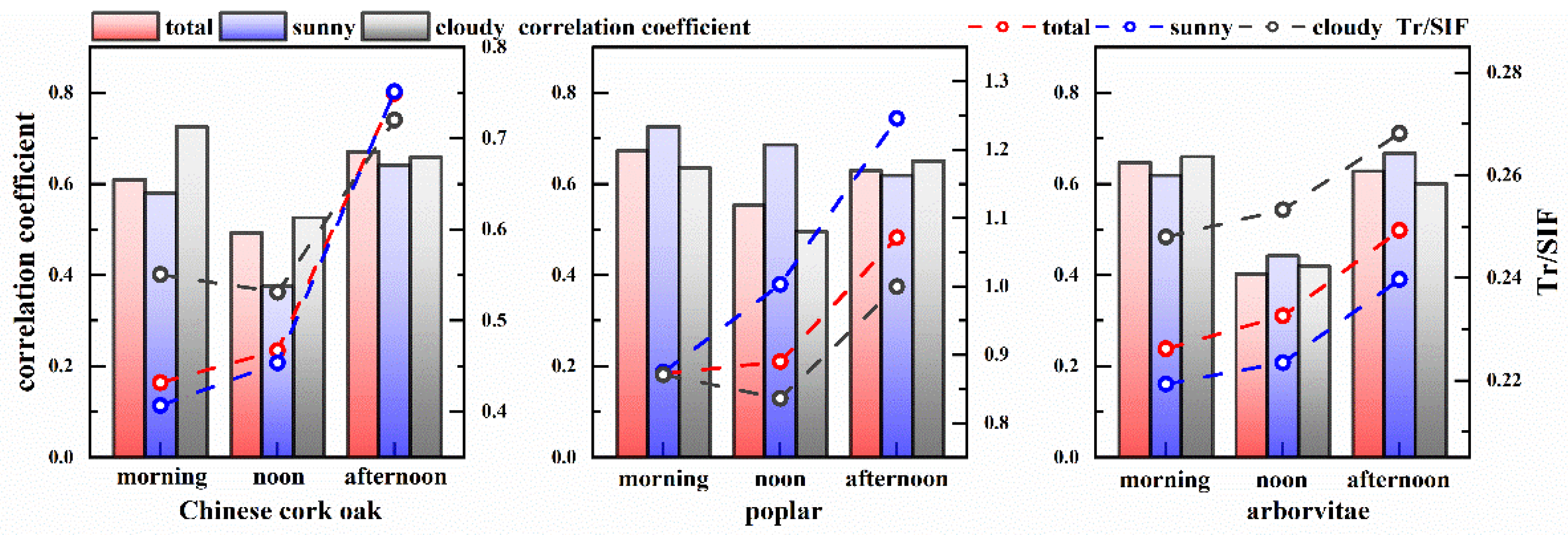
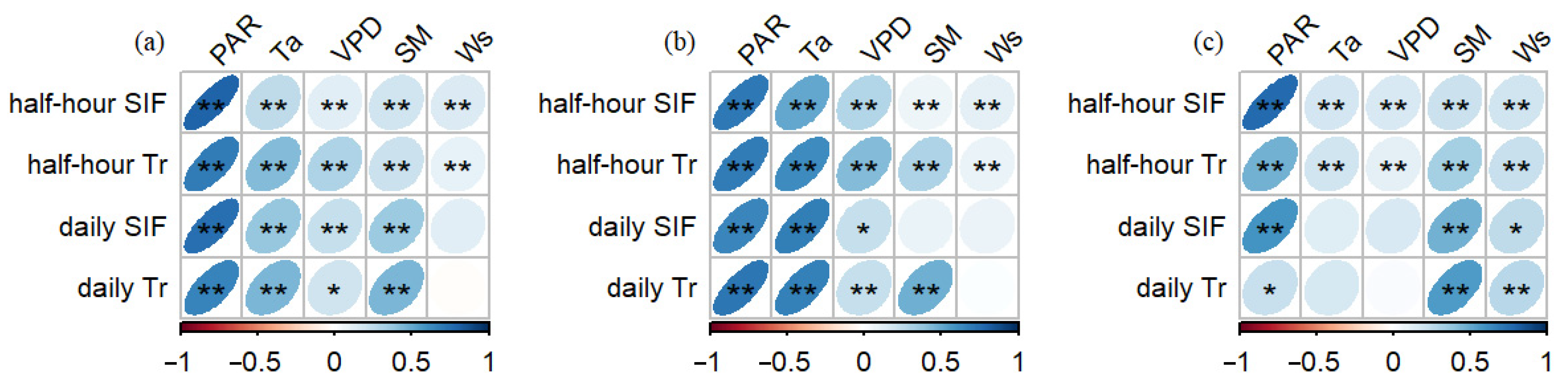
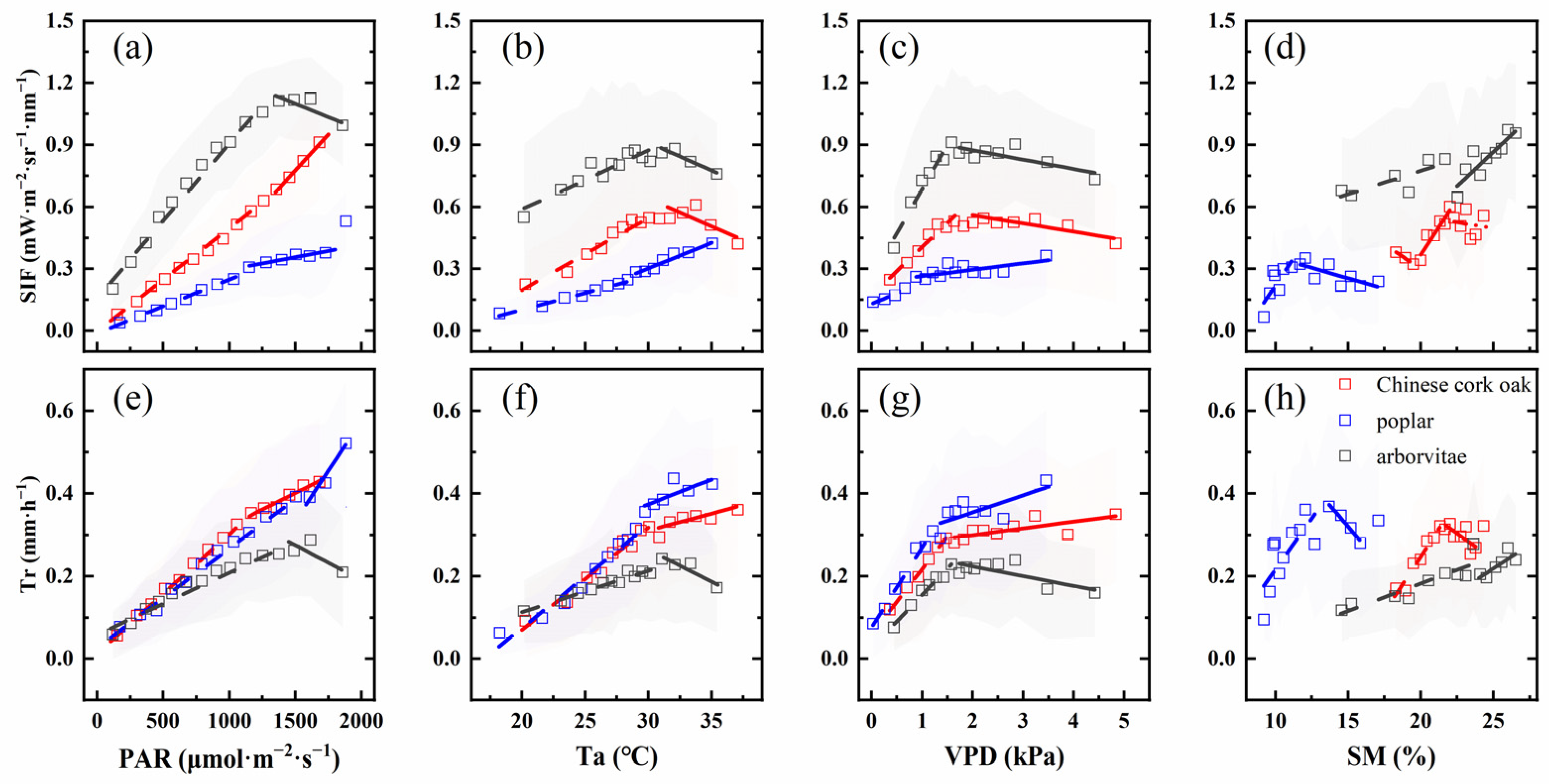
3.3. Impact of Environmental Factors on SIF, Tr, and the SIF–Tr Relationship
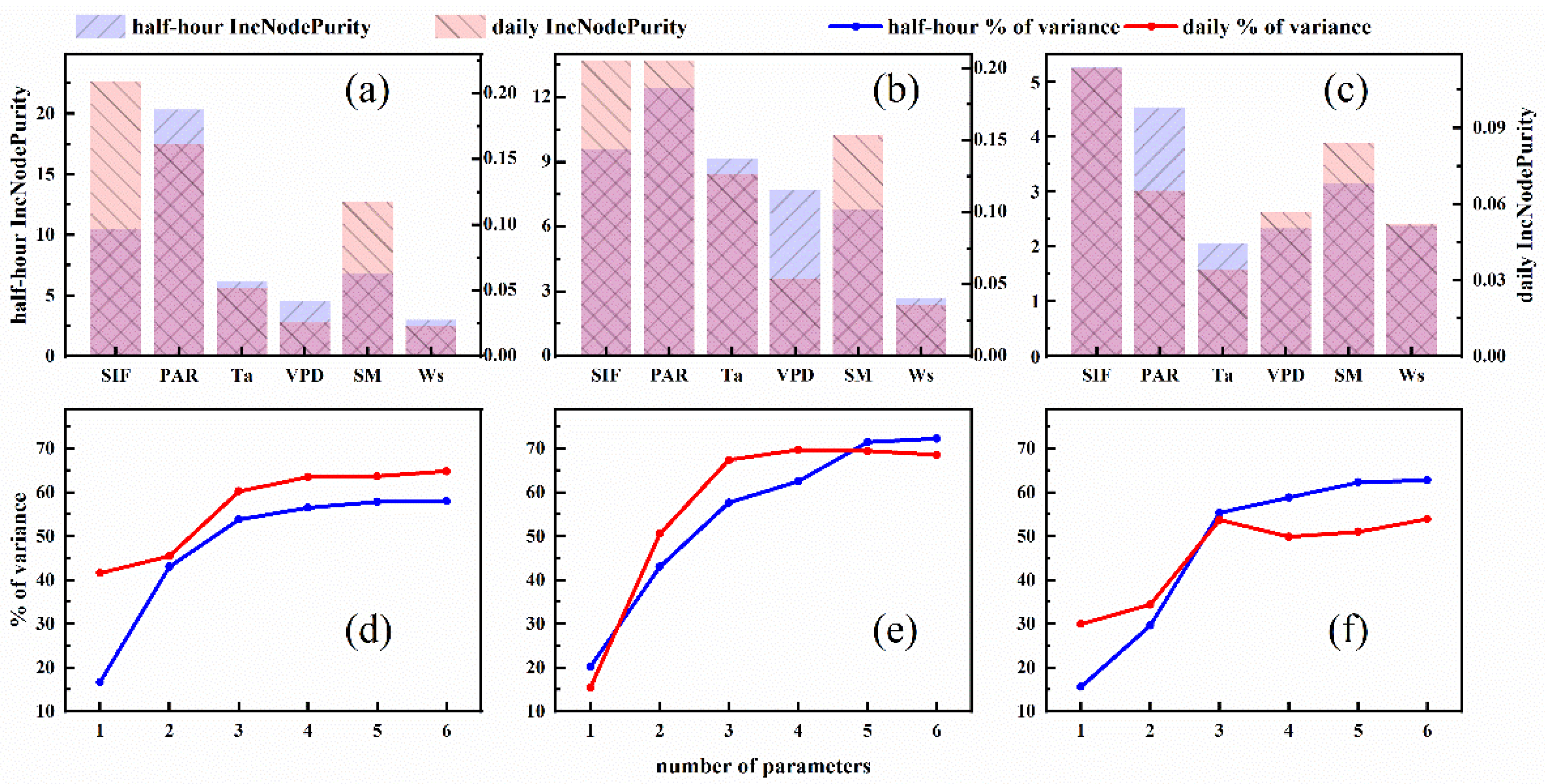
3.4. Assessing the Potential for Estimating Tr from SIF and Environmental Factors
4. Discussion
4.1. The Temporal Dynamics of SIF and Tr in Different Species
4.2. The Relationship Between SIF and Tr and Their Environmental Response
4.3. Evaluation of Estimating Tr from SIF and Environmental Factors
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Monteith, J.L. Evaporation and environment. In Proceedings of the Symposia of the Society for Experimental Biology; Cambridge University Press: Cambridge, UK, 1965; pp. 205–234. [Google Scholar]
- Schlesinger, W.H.; Jasechko, S. Transpiration in the global water cycle. Agric. For. Meteorol. 2014, 189–190, 115–117. [Google Scholar] [CrossRef]
- Fisher, J.B.; Melton, F.; Middleton, E.; Hain, C.; Anderson, M.; Allen, R.; McCabe, M.F.; Hook, S.; Baldocchi, D.; Townsend, P.A. The future of evapotranspiration: Global requirements for ecosystem functioning, carbon and climate feedbacks, agricultural management, and water resources. Water Resour. Res. 2017, 53, 2618–2626. [Google Scholar] [CrossRef]
- Li, X.; Gentine, P.; Lin, C.; Zhou, S.; Sun, Z.; Zheng, Y.; Liu, J.; Zheng, C. A simple and objective method to partition evapotranspiration into transpiration and evaporation at eddy-covariance sites. Agric. For. Meteorol. 2019, 265, 171–182. [Google Scholar] [CrossRef]
- Talsma, C.J.; Good, S.P.; Jimenez, C.; Martens, B.; Fisher, J.B.; Miralles, D.G.; McCabe, M.F.; Purdy, A.J. Partitioning of evapotranspiration in remote sensing-based models. Agric. For. Meteorol. 2018, 260, 131–143. [Google Scholar] [CrossRef]
- Sun, X.; Wilcox, B.P.; Zou, C.B. Evapotranspiration partitioning in dryland ecosystems: A global meta-analysis of in situ studies. J. Hydrol. 2019, 576, 123–136. [Google Scholar] [CrossRef]
- Senay, G.B.; Bohms, S.; Singh, R.K.; Gowda, P.H.; Velpuri, N.M.; Alemu, H.; Verdin, J.P. Operational Evapotranspiration Mapping Using Remote Sensing and Weather Datasets: A New Parameterization for the SSEB Approach. J. Am. Water Resour. Assoc. 2013, 49, 577–591. [Google Scholar] [CrossRef]
- Zeng, Z.; Wang, T.; Zhou, F.; Ciais, P.; Mao, J.; Shi, X.; Piao, S. A worldwide analysis of spatiotemporal changes in water balance-based evapotranspiration from 1982 to 2009. J. Geophys. Res. Atmos. 2014, 119, 1186–1202. [Google Scholar] [CrossRef]
- Mu, Q.; Zhao, M.; Running, S.W. Improvements to a MODIS global terrestrial evapotranspiration algorithm. Remote Sens. Environ. 2011, 115, 1781–1800. [Google Scholar] [CrossRef]
- Jung, M.; Reichstein, M.; Ciais, P.; Seneviratne, S.I.; Sheffield, J.; Goulden, M.L.; Bonan, G.; Cescatti, A.; Chen, J.; De Jeu, R. Recent decline in the global land evapotranspiration trend due to limited moisture supply. Nature 2010, 467, 951–954. [Google Scholar] [CrossRef]
- Porcar-Castell, A.; Tyystjärvi, E.; Atherton, J.; Van der Tol, C.; Flexas, J.; Pfündel, E.E.; Moreno, J.; Frankenberg, C.; Berry, J.A. Linking chlorophyll a fluorescence to photosynthesis for remote sensing applications: Mechanisms and challenges. J. Exp. Bot. 2014, 65, 4065–4095. [Google Scholar] [CrossRef]
- Badgley, G.; Field, C.B.; Berry, J.A. Canopy near-infrared reflectance and terrestrial photosynthesis. Sci. Adv. 2017, 3, e1602244. [Google Scholar] [CrossRef] [PubMed]
- Rossini, M.; Nedbal, L.; Guanter, L.; Ač, A.; Alonso, L.; Burkart, A.; Cogliati, S.; Colombo, R.; Damm, A.; Drusch, M. Red and far red Sun-induced chlorophyll fluorescence as a measure of plant photosynthesis. Geophys. Res. Lett. 2015, 42, 1632–1639. [Google Scholar] [CrossRef]
- Magney, T.S.; Bowling, D.R.; Logan, B.A.; Grossmann, K.; Stutz, J.; Blanken, P.D.; Burns, S.P.; Cheng, R.; Garcia, M.A.; Köhler, P. Mechanistic evidence for tracking the seasonality of photosynthesis with solar-induced fluorescence. Proc. Natl. Acad. Sci. USA 2019, 116, 11640–11645. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Guanter, L.; Berry, J.A.; Joiner, J.; van der Tol, C.; Huete, A.; Gitelson, A.; Voigt, M.; Köhler, P. Estimation of vegetation photosynthetic capacity from space-based measurements of chlorophyll fluorescence for terrestrial biosphere models. Glob. Change Biol. 2014, 20, 3727–3742. [Google Scholar] [CrossRef]
- Maes, W.H.; Pagán, B.R.; Martens, B.; Gentine, P.; Guanter, L.; Steppe, K.; Verhoest, N.E.; Dorigo, W.; Li, X.; Xiao, J. Sun-induced fluorescence closely linked to ecosystem transpiration as evidenced by satellite data and radiative transfer models. Remote Sens. Environ. 2020, 249, 112030. [Google Scholar] [CrossRef]
- Zhou, K.; Zhang, Q.; Xiong, L.; Gentine, P. Estimating evapotranspiration using remotely sensed solar-induced fluorescence measurements. Agric. For. Meteorol. 2022, 314, 108800. [Google Scholar] [CrossRef]
- Lu, X.; Liu, Z.; An, S.; Miralles, D.G.; Maes, W.; Liu, Y.; Tang, J. Potential of solar-induced chlorophyll fluorescence to estimate transpiration in a temperate forest. Agric. For. Meteorol. 2018, 252, 75–87. [Google Scholar] [CrossRef]
- Alemohammad, S.H.; Fang, B.; Konings, A.G.; Aires, F.; Green, J.K.; Kolassa, J.; Miralles, D.; Prigent, C.; Gentine, P. Water, Energy, and Carbon with Artificial Neural Networks (WECANN): A statistically based estimate of global surface turbulent fluxes and gross primary productivity using solar-induced fluorescence. Biogeosciences 2017, 14, 4101–4124. [Google Scholar] [CrossRef]
- Beauclaire, Q.; De Cannière, S.; Jonard, F.; Pezzetti, N.; Delhez, L.; Longdoz, B. Modeling gross primary production and transpiration from sun-induced chlorophyll fluorescence using a mechanistic light-response approach. Remote Sens. Environ. 2024, 307, 114150. [Google Scholar] [CrossRef]
- Damm, A.; Haghighi, E.; Paul-Limoges, E.; van der Tol, C. On the seasonal relation of sun-induced chlorophyll fluorescence and transpiration in a temperate mixed forest. Agric. For. Meteorol. 2021, 304, 108386. [Google Scholar] [CrossRef]
- Pagán, B.R.; Maes, W.H.; Gentine, P.; Martens, B.; Miralles, D.G. Exploring the potential of satellite solar-induced fluorescence to constrain global transpiration estimates. Remote Sens. 2019, 11, 413. [Google Scholar] [CrossRef]
- Song, L.; Ding, Z.; Kustas, W.P.; Hua, W.; Liu, X.; Liu, L.; Liu, S.; Ma, M.; Bai, Y.; Xu, Z. Applications of a Thermal-Based Two-Source Energy Balance Model Coupling the Sun-Induced Chlorophyll Fluorescence Data. IEEE Geosci. Remote Sens. Lett. 2023, 20, 1–5. [Google Scholar] [CrossRef]
- Yang, J.; Liu, Z.; Yu, Q.; Lu, X. Estimation of global transpiration from remotely sensed solar-induced chlorophyll fluorescence. Remote Sens. Environ. 2024, 303, 113998. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Y.; Shan, N.; Zhang, Z.; Wei, Z. Global assessment of partitioning transpiration from evapotranspiration based on satellite solar-induced chlorophyll fluorescence data. J. Hydrol. 2022, 612, 128044. [Google Scholar] [CrossRef]
- Shan, N.; Zhang, Y.; Chen, J.M.; Ju, W.; Goulas, Y. A model for estimating transpiration from remotely sensed solar-induced chlorophyll fluorescence. Remote Sens. Environ. 2021, 252, 112134. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, X.; Zhou, K.; Zhou, Y.; Gentine, P.; Pan, M.; Katul, G.G. Solar-induced chlorophyll fluorescence sheds light on global evapotranspiration. Remote Sens. Environ. 2024, 305, 114061. [Google Scholar] [CrossRef]
- Shang, K.; Yao, Y.; Di, Z.; Jia, K.; Zhang, X.; Fisher, J.B.; Chen, J.; Guo, X.; Yang, J.; Yu, R. Coupling physical constraints with machine learning for satellite-derived evapotranspiration of the Tibetan Plateau. Remote Sens. Environ. 2023, 289, 113519. [Google Scholar] [CrossRef]
- Zheng, C.; Wang, S.; Chen, J.M.; Xiao, J.; Chen, J.; Zhu, K.; Sun, L. Modeling transpiration using solar-induced chlorophyll fluorescence and photochemical reflectance index synergistically in a closed-canopy winter wheat ecosystem. Remote Sens. Environ. 2024, 302, 113981. [Google Scholar] [CrossRef]
- Kira, O.; Sun, Y. Extraction of sub-pixel C3/C4 emissions of solar-induced chlorophyll fluorescence (SIF) using artificial neural network. ISPRS J. Photogramm. Remote Sens. 2020, 161, 135–146. [Google Scholar] [CrossRef]
- Gu, L.; Wood, J.D.; Chang, C.Y.; Sun, Y.; Riggs, J.S. Advancing terrestrial ecosystem science with a novel automated measurement system for sun-induced chlorophyll fluorescence for integration with eddy covariance flux networks. J. Geophys. Res. Biogeosci. 2019, 124, 127–146. [Google Scholar] [CrossRef]
- Shan, N.; Ju, W.; Migliavacca, M.; Martini, D.; Guanter, L.; Chen, J.; Goulas, Y.; Zhang, Y. Modeling canopy conductance and transpiration from solar-induced chlorophyll fluorescence. Agric. For. Meteorol. 2019, 268. [Google Scholar] [CrossRef]
- Martínez-Vilalta, J.; Garcia-Forner, N. Water potential regulation, stomatal behaviour and hydraulic transport under drought: Deconstructing the iso/anisohydric concept. Plant Cell Environ. 2017, 40, 962–976. [Google Scholar] [CrossRef]
- Liu, X.; Liu, L.; Hu, J.; Guo, J.; Du, S. Improving the potential of red SIF for estimating GPP by downscaling from the canopy level to the photosystem level. Agric. For. Meteorol. 2020, 281, 107846. [Google Scholar] [CrossRef]
- Mazzoni, M.; Falorni, P.; Del Bianco, S. Sun-induced leaf fluorescence retrieval in the O2-B atmospheric absorption band. Opt. Express 2008, 16, 7014–7022. [Google Scholar] [CrossRef]
- Hu, M.; Cheng, X.; Zhang, J.; Huang, H.; Zhou, Y.; Wang, X.; Pan, Q.; Guan, C. Temporal Variation in Tower-Based Solar-Induced Chlorophyll Fluorescence and Its Environmental Response in a Chinese Cork Oak Plantation. Remote Sens. 2023, 15, 3568. [Google Scholar] [CrossRef]
- Allen, R.G.; Pereira, L.S.; Raes, D.; Smith, M. Crop Evapotranspiration—Guidelines for Computing Crop Water Requirements—FAO Irrigation and Drainage Paper 56; FAO: Rome, Italy, 1998; Volume 300, p. D05109. [Google Scholar]
- Rouse, J.W.; Haas, R.H.; Schell, J.A.; Deering, D.W. Monitoring vegetation systems in the Great Plains with ERTS. NASA Spec. Publ. 1974, 351, 309. [Google Scholar]
- Viña, A.; Gitelson, A.A.; Nguy-Robertson, A.L.; Peng, Y. Comparison of different vegetation indices for the remote assessment of green leaf area index of crops. Remote Sens. Environ. 2011, 115, 3468–3478. [Google Scholar] [CrossRef]
- Gu, L.; Fuentes, J.D.; Shugart, H.H.; Staebler, R.M.; Black, T.A. Responses of net ecosystem exchanges of carbon dioxide to changes in cloudiness: Results from two North American deciduous forests. J. Geophys. Res. Atmos. 1999, 104, 31421–31434. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, Q.; Li, J.; Yang, X.; Wu, Y.; Zhang, Z.; Wang, S.; Wang, H.; Zhang, Y. Solar-induced chlorophyll fluorescence and its link to canopy photosynthesis in maize from continuous ground measurements. Remote Sens. Environ. 2020, 236, 111420. [Google Scholar] [CrossRef]
- Kumar, R.; Umanand, L. Estimation of global radiation using clearness index model for sizing photovoltaic system. Renew. Energy 2005, 30, 2221–2233. [Google Scholar] [CrossRef]
- Belgiu, M.; Drăguţ, L. Random forest in remote sensing: A review of applications and future directions. ISPRS J. Photogramm. Remote Sens. 2016, 114, 24–31. [Google Scholar] [CrossRef]
- Wang, F.; Chen, B.; Lin, X.; Zhang, H. Solar-induced chlorophyll fluorescence as an indicator for determining the end date of the vegetation growing season. Ecol. Indic. 2020, 109, 105755. [Google Scholar] [CrossRef]
- Biswal, B.; Krupinska, K.; Biswal, U.C. Plastid Development in Leaves During Growth and Senescence; Springer: Dordrecht, The Netherlands, 2013; Volume 36. [Google Scholar]
- Chaves, M.M.; Flexas, J.; Pinheiro, C. Photosynthesis under drought and salt stress: Regulation mechanisms from whole plant to cell. Ann. Bot. 2009, 103, 551–560. [Google Scholar] [CrossRef] [PubMed]
- Wardle, K.; Short, K. Stomatal response of in vitro cultured plantlets. I. Responses in epidermal strips of Chrysanthemum to environmental factors and growth regulators. Biochem. Physiol. Pflanzen 1983, 178, 619–624. [Google Scholar] [CrossRef]
- Cheng, X.; Hu, M.; Zhou, Y.; Wang, F.; Liu, L.; Wang, Y.; Huang, H.; Zhang, J. The divergence of micrometeorology sensitivity leads to changes in GPP/SIF between cork oak and poplar. Agric. For. Meteorol. 2022, 326, 109189. [Google Scholar] [CrossRef]
- Yang, X.; Tang, J.; Mustard, J.F.; Lee, J.E.; Rossini, M.; Joiner, J.; Munger, J.W.; Kornfeld, A.; Richardson, A.D. Solar-induced chlorophyll fluorescence that correlates with canopy photosynthesis on diurnal and seasonal scales in a temperate deciduous forest. Geophys. Res. Lett. 2015, 42, 2977–2987. [Google Scholar] [CrossRef]
- Damm, A.; Guanter, L.; Paul-Limoges, E.; Van der Tol, C.; Hueni, A.; Buchmann, N.; Eugster, W.; Ammann, C.; Schaepman, M.E. Far-red sun-induced chlorophyll fluorescence shows ecosystem-specific relationships to gross primary production: An assessment based on observational and modeling approaches. Remote Sens. Environ. 2015, 166, 91–105. [Google Scholar] [CrossRef]
- Louis, J.; Ounis, A.; Ducruet, J.-M.; Evain, S.; Laurila, T.; Thum, T.; Aurela, M.; Wingsle, G.; Alonso, L.; Pedros, R. Remote sensing of sunlight-induced chlorophyll fluorescence and reflectance of Scots pine in the boreal forest during spring recovery. Remote Sens. Environ. 2005, 96, 37–48. [Google Scholar] [CrossRef]
- Zeng, Y.; Badgley, G.; Dechant, B.; Ryu, Y.; Chen, M.; Berry, J.A. A practical approach for estimating the escape ratio of near-infrared solar-induced chlorophyll fluorescence. Remote Sens. Environ. 2019, 232, 111209. [Google Scholar] [CrossRef]
- Rochdi, N.; Fernandes, R.; Chelle, M. An assessment of needles clumping within shoots when modeling radiative transfer within homogeneous canopies. Remote Sens. Environ. 2006, 102, 116–134. [Google Scholar] [CrossRef]
- Paul-Limoges, E.; Damm, A.; Hueni, A.; Liebisch, F.; Eugster, W.; Schaepman, M.E.; Buchmann, N. Effect of environmental conditions on sun-induced fluorescence in a mixed forest and a cropland. Remote Sens. Environ. 2018, 219, 310–323. [Google Scholar] [CrossRef]
- Zhang, Y.; Xiao, X.; Wolf, S.; Wu, J.; Wu, X.; Gioli, B.; Wohlfahrt, G.; Cescatti, A.; Van der Tol, C.; Zhou, S. Spatio-temporal convergence of maximum daily light-use efficiency based on radiation absorption by canopy chlorophyll. Geophys. Res. Lett. 2018, 45, 3508–3519. [Google Scholar] [CrossRef]
- Baker, N.R. Chlorophyll fluorescence: A probe of photosynthesis in vivo. Annu. Rev. Plant Biol. 2008, 59, 89–113. [Google Scholar] [CrossRef] [PubMed]
- Lawson, T.; Matthews, J. Guard cell metabolism and stomatal function. Annu. Rev. Plant Biol. 2020, 71, 273–302. [Google Scholar] [CrossRef]
- Scafaro, A.P.; Posch, B.C.; Evans, J.R.; Farquhar, G.D.; Atkin, O.K. Rubisco deactivation and chloroplast electron transport rates co-limit photosynthesis above optimal leaf temperature in terrestrial plants. Nat. Commun. 2023, 14, 2820. [Google Scholar] [CrossRef]
- Bachofen, C.; Poyatos, R.; Flo, V.; Martínez-Vilalta, J.; Mencuccini, M.; Granda, V.; Grossiord, C. Stand structure of Central European forests matters more than climate for transpiration sensitivity to VPD. J. Appl. Ecol. 2023, 60, 886–897. [Google Scholar] [CrossRef]
- Maseda, P.H.; Fernández, R.J. Stay wet or else: Three ways in which plants can adjust hydraulically to their environment. J. Exp. Bot. 2006, 57, 3963–3977. [Google Scholar] [CrossRef]
- Broddrick, J.T.; Ware, M.A.; Jallet, D.; Palsson, B.O.; Peers, G. Integration of physiologically relevant photosynthetic energy flows into whole genome models of light-driven metabolism. Plant J. 2022, 112, 603–621. [Google Scholar] [CrossRef]
- Sadok, W.; Lopez, J.R.; Smith, K.P. Transpiration increases under high-temperature stress: Potential mechanisms, trade-offs and prospects for crop resilience in a warming world. Plant Cell Environ. 2021, 44, 2102–2116. [Google Scholar] [CrossRef]
- Kropp, H.; Loranty, M.; Alexander, H.D.; Berner, L.T.; Natali, S.M.; Spawn, S.A. Environmental constraints on transpiration and stomatal conductance in a Siberian Arctic boreal forest. J. Geophys. Res. Biogeosci. 2017, 122, 487–497. [Google Scholar] [CrossRef]
- Liu, L.; Teng, Y.; Wu, J.; Zhao, W.; Liu, S.; Shen, Q. Soil water deficit promotes the effect of atmospheric water deficit on solar-induced chlorophyll fluorescence. Sci. Total Environ. 2020, 720, 137408. [Google Scholar] [CrossRef] [PubMed]
- Jones, H.G. Stomatal control of photosynthesis and transpiration. J. Exp. Bot. 1998, 387–398. [Google Scholar] [CrossRef]
- Nardini, A.; Battistuzzo, M.; Savi, T. Shoot desiccation and hydraulic failure in temperate woody angiosperms during an extreme summer drought. New Phytol. 2013, 200, 322–329. [Google Scholar] [CrossRef]
- Klein, T. The variability of stomatal sensitivity to leaf water potential across tree species indicates a continuum between isohydric and anisohydric behaviours. Funct. Ecol. 2014, 28, 1313–1320. [Google Scholar] [CrossRef]
- Brodribb, T.J.; McAdam, S.A.; Jordan, G.J.; Martins, S.C. Conifer species adapt to low-rainfall climates by following one of two divergent pathways. Proc. Natl. Acad. Sci. USA 2014, 111, 14489–14493. [Google Scholar] [CrossRef] [PubMed]
- Choat, B.; Jansen, S.; Brodribb, T.J.; Cochard, H.; Delzon, S.; Bhaskar, R.; Bucci, S.J.; Feild, T.S.; Gleason, S.M.; Hacke, U.G. Global convergence in the vulnerability of forests to drought. Nature 2012, 491, 752–755. [Google Scholar] [CrossRef]
- Raczka, B.; Porcar-Castell, A.; Magney, T.; Lee, J.; Köhler, P.; Frankenberg, C.; Grossmann, K.; Logan, B.; Stutz, J.; Blanken, P. Sustained nonphotochemical quenching shapes the seasonal pattern of solar-induced fluorescence at a high-elevation evergreen forest. J. Geophys. Res. Biogeosci. 2019, 124, 2005–2020. [Google Scholar] [CrossRef]
- Dodd, A.N.; Salathia, N.; Hall, A.; Kévei, E.; Tóth, R.; Nagy, F.; Hibberd, J.M.; Millar, A.J.; Webb, A.A. Plant circadian clocks increase photosynthesis, growth, survival, and competitive advantage. Science 2005, 309, 630–633. [Google Scholar] [CrossRef]
- Xu, S.; Atherton, J.; Riikonen, A.; Zhang, C.; Oivukkamäki, J.; MacArthur, A.; Honkavaara, E.; Hakala, T.; Koivumäki, N.; Liu, Z. Structural and photosynthetic dynamics mediate the response of SIF to water stress in a potato crop. Remote Sens. Environ. 2021, 263, 112555. [Google Scholar] [CrossRef]
- Damm, A.; Roethlin, S.; Fritsche, L. Towards advanced retrievals of plant transpiration using sun-induced chlorophyll fluorescence: First considerations. In Proceedings of the IGARSS 2018—2018 IEEE International Geoscience and Remote Sensing Symposium, Valencia, Spain, 22–27 July 2018; pp. 5983–5986. [Google Scholar]


| Temporal Scale | Species | Total | Sunny | Cloudy | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pearson Coefficient | R2 | k | Pearson Coefficient | R2 | k | Pearson Coefficient | R2 | k | ||
| half-hour | Chinese cork oak | 0.597 ** | 0.356 | 0.326 | 0.551 ** | 0.303 | 0.306 | 0.650 ** | 0.422 | 0.395 |
| poplar | 0.641 ** | 0.411 | 0.636 | 0.673 ** | 0.453 | 0.715 | 0.629 ** | 0.395 | 0.595 | |
| arborvitae | 0.605 ** | 0.366 | 0.208 | 0.603 ** | 0.364 | 0.223 | 0.615 ** | 0.378 | 0.216 | |
| daily | Chinese cork oak | 0.746 ** | 0.557 | 0.338 | 0.644 ** | 0.415 | 0.325 | 0.793 ** | 0.628 | 0.400 |
| poplar | 0.737 ** | 0.543 | 0.624 | 0.778 ** | 0.605 | 0.665 | 0.724 ** | 0.524 | 0.593 | |
| arborvitae | 0.640 ** | 0.409 | 0.249 | 0.683 ** | 0.466 | 0.354 | 0.669 ** | 0.447 | 0.308 | |
| Temporal Scale | Model | Chinese Cork Oak | Poplar | Arborvitae | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| R2 | RMSE | k0 | R2 | RMSE | k0 | R2 | RMSE | k0 | ||
| half-hour | 1 | 0.26 | 0.1508 | 0.82 | 0.27 | 0.1661 | 0.84 | 0.25 | 0.1102 | 0.92 |
| 2 | 0.41 | 0.1300 | 0.85 | 0.43 | 0.1403 | 0.88 | 0.34 | 0.0986 | 0.94 | |
| 3 | 0.51 | 0.1181 | 0.88 | 0.52 | 0.1273 | 0.90 | 0.40 | 0.0934 | 0.93 | |
| 4 | 0.53 | 0.1151 | 0.87 | 0.53 | 0.1254 | 0.88 | 0.48 | 0.0857 | 0.95 | |
| 5 | 0.54 | 0.1139 | 0.87 | 0.66 | 0.1060 | 0.91 | 0.54 | 0.0791 | 0.94 | |
| 6 | 0.54 | 0.1133 | 0.87 | 0.66 | 0.1063 | 0.91 | 0.53 | 0.0792 | 0.92 | |
| linear | 0.33 | 0.1375 | 0.80 | 0.37 | 0.1433 | 0.83 | 0.39 | 0.0918 | 0.92 | |
| daily | 1 | 0.43 | 0.0594 | 0.96 | 0.49 | 0.0875 | 0.95 | 0.17 | 0.0767 | 0.87 |
| 2 | 0.48 | 0.0569 | 0.95 | 0.58 | 0.0804 | 0.98 | 0.31 | 0.0634 | 0.87 | |
| 3 | 0.67 | 0.0456 | 0.96 | 0.83 | 0.0599 | 1.02 | 0.48 | 0.0543 | 0.86 | |
| 4 | 0.69 | 0.0440 | 0.96 | 0.82 | 0.0612 | 1.02 | 0.41 | 0.0581 | 0.86 | |
| 5 | 0.69 | 0.0436 | 0.96 | 0.84 | 0.0592 | 1.02 | 0.43 | 0.0582 | 0.85 | |
| 6 | 0.68 | 0.0444 | 0.96 | 0.84 | 0.0604 | 1.01 | 0.51 | 0.0540 | 0.85 | |
| linear | 0.56 | 0.0523 | 0.95 | 0.47 | 0.0907 | 0.97 | 0.33 | 0.0607 | 0.87 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, M.; Sun, S.; Cheng, X.; Pan, Q.; Zhang, J.; Wang, X.; Guan, C.; Li, Z.; Gao, X. Specific Responses to Environmental Factors Cause Discrepancy in the Link Between Solar-Induced Chlorophyll Fluorescence and Transpiration in Three Plantations. Remote Sens. 2025, 17, 1625. https://doi.org/10.3390/rs17091625
Hu M, Sun S, Cheng X, Pan Q, Zhang J, Wang X, Guan C, Li Z, Gao X. Specific Responses to Environmental Factors Cause Discrepancy in the Link Between Solar-Induced Chlorophyll Fluorescence and Transpiration in Three Plantations. Remote Sensing. 2025; 17(9):1625. https://doi.org/10.3390/rs17091625
Chicago/Turabian StyleHu, Meijun, Shoujia Sun, Xiangfen Cheng, Qingmei Pan, Jinsong Zhang, Xin Wang, Chongfan Guan, Zhipeng Li, and Xiang Gao. 2025. "Specific Responses to Environmental Factors Cause Discrepancy in the Link Between Solar-Induced Chlorophyll Fluorescence and Transpiration in Three Plantations" Remote Sensing 17, no. 9: 1625. https://doi.org/10.3390/rs17091625
APA StyleHu, M., Sun, S., Cheng, X., Pan, Q., Zhang, J., Wang, X., Guan, C., Li, Z., & Gao, X. (2025). Specific Responses to Environmental Factors Cause Discrepancy in the Link Between Solar-Induced Chlorophyll Fluorescence and Transpiration in Three Plantations. Remote Sensing, 17(9), 1625. https://doi.org/10.3390/rs17091625






