Solar-Induced Fluorescence as Indicator of Downy Oak and the Influence of Some Environmental Variables at the End of the Growing Season
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site
2.1.1. Location
2.1.2. History and Infrastructure
2.1.3. Characteristics of the Oak Forest
2.2. Data Monitoring
Measurements of Environmental Conditions: ICOS Tower and O3HP Site
2.3. Data Filtering
2.4. Data Analysis
3. Results
3.1. Seasonal Dynamics of Photosynthesis and Phenology
3.1.1. Evolution of the Solar-Induced Fluorescence (SIF)
3.1.2. Evolution of Vegetation Indices
3.1.3. Pearson Correlations: SIFs—Environmental Conditions and SIFs—Vegetation Indices
3.2. Diurnal Dynamics of SIFs, Vegetation Indices and Environmental Parameters
3.2.1. Evolution of Solar-Induced Fluorescence
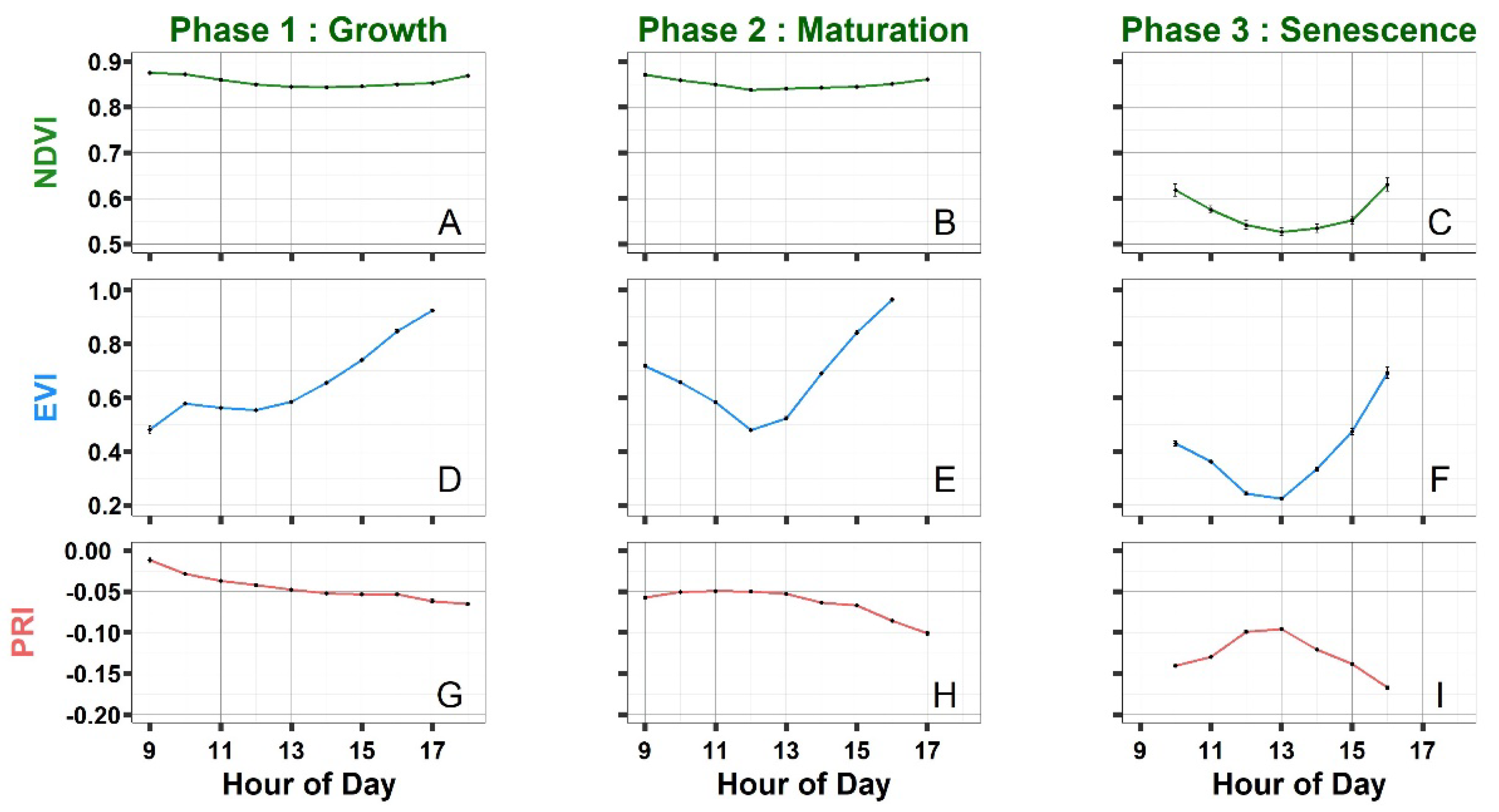
3.2.2. Evolution of Vegetation Indices
3.2.3. Pearson Correlations: SIFs—Environmental Conditions and SIFs—Vegetation Indices
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thomson, A.M.; César Izaurralde, R.; Smith, S.J.; Clarke, L.E. Integrated Estimates of Global Terrestrial Carbon Sequestration. Glob. Environ. Change 2008, 18, 192–203. [Google Scholar] [CrossRef]
- Archibold, O.W. Temperate Forest Ecosystems. In Ecology of World Vegetation; Springer Science + Business Media: Dordrecht, The Netherlands, 1995; pp. 165–203. ISBN 978-94-010-4008-2. [Google Scholar]
- Martin, P.; Nabuurs, G.-J.; Aubinet, M.; Karjalainen, T.; Vine, E.; Kinsman, J.; Heath, L. Carbon Sinks in Temperate Forests1. Annu. Rev. Energy Environ. 2001, 26, 436–465. [Google Scholar] [CrossRef]
- Pan, Y.; Birdsey, R.; Fang, J.; Houghton, R.; Kauppi, P.; Kurz, W.; Phillips, O.; Shvidenko, A.; Lewis, S.; Canadell, J.; et al. A Large and Persistent Carbon Sink in the World’s Forests. Science 2011, 333, 988–993. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Jia, X.; He, G.; Zhou, C.; Yu, H.; Wu, Y.; Bourque, C.P.-A.; Liu, H.; Zha, T. Environmental Control over Seasonal Variation in Carbon Fluxes of an Urban Temperate Forest Ecosystem. Landsc. Urban Plan. 2015, 142, 63–70. [Google Scholar] [CrossRef]
- Allaby, M. Temperate Forests (Biomes of the Earth); AbeBooks: Victoria, BC, Canada, 2006; ISBN 9780816053216. [Google Scholar]
- Luyssaert, S.; Marie, G.; Valade, A.; Chen, Y.-Y.; Njakou Djomo, S.; Ryder, J.; Otto, J.; Naudts, K.; Lansø, A.; Ghattas, J.; et al. Author Correction: Trade-Offs in Using European Forests to Meet Climate Objectives. Nature 2019, 567, E13. [Google Scholar] [CrossRef]
- Vose, J.M.; Peterson, D.L.; Patel-Weynand, T. Effects of Climatic Variability and Change on Forest Ecosystems: A Comprehensive Science Synthesis for the U.S.; General Technical Report PNW-GTR-870; U.S. Department of Agriculture, Forest Service, Pacific Northwest Research Station: Portland, OR, USA, 2012; Volume 870, 265p. [Google Scholar] [CrossRef]
- Kim, J.; Woo, H.R.; Nam, H.G. Toward Systems Understanding of Leaf Senescence: An Integrated Multi-Omics Perspective on Leaf Senescence Research. Mol. Plant 2016, 9, 813–825. [Google Scholar] [CrossRef]
- Li, W.; Wang, L.; He, Z.; Lu, Z.; Cui, J.; Xu, N.; Jin, B.; Wang, L. Physiological and Transcriptomic Changes During Autumn Coloration and Senescence in Ginkgo Biloba Leaves. Hortic. Plant J. 2020, 6, 396–408. [Google Scholar] [CrossRef]
- Wen, C.-H.; Lin, S.-S.; Chu, F.-H. Transcriptome Analysis of a Subtropical Deciduous Tree: Autumn Leaf Senescence Gene Expression Profile of Formosan Gum. Plant Cell Physiol. 2015, 56, 163–174. [Google Scholar] [CrossRef]
- Gao, S.; Zhong, R.; Yan, K.; Ma, X.; Chen, X.; Pu, J.; Gao, S.; Qi, J.; Yin, G.; Myneni, R.B. Evaluating the Saturation Effect of Vegetation Indices in Forests Using 3D Radiative Transfer Simulations and Satellite Observations. Remote Sens. Environ. 2023, 295, 113665. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, L.; Huang, C.; Qiao, N. An NDVI-Based Vegetation Phenology Is Improved to Be More Consistent with Photosynthesis Dynamics through Applying a Light Use Efficiency Model over Boreal High-Latitude Forests. Remote Sens. 2017, 9, 695. [Google Scholar] [CrossRef]
- Frankenberg, C.; Berry, J. 3.10—Solar Induced Chlorophyll Fluorescence: Origins, Relation to Photosynthesis and Retrieval. In Comprehensive Remote Sensing; Liang, S., Ed.; Elsevier: Oxford, UK, 2018; pp. 143–162. ISBN 978-0-12-803221-3. [Google Scholar]
- Zhang, J.; Xiao, J.; Tong, X.; Zhang, J.; Meng, P.; Li, J.; Liu, P.; Yu, P. NIRv and SIF Better Estimate Phenology than NDVI and EVI: Effects of Spring and Autumn Phenology on Ecosystem Production of Planted Forests. Agric. For. Meteorol. 2022, 315, 108819. [Google Scholar] [CrossRef]
- van der Tol, C.; Julitta, T.; Yang, P.; Sabater, N.; Reiter, I.; Tudoroiu, M.; Schuettemeyer, D.; Drusch, M. Retrieval of Chlorophyll Fluorescence from a Large Distance Using Oxygen Absorption Bands. Remote Sens. Environ. 2023, 284, 113304. [Google Scholar] [CrossRef]
- Lelandais, L.; Xueref-Remy, I.; Riandet, A.; Blanc, P.E.; Armengaud, A.; Oppo, S.; Yohia, C.; Ramonet, M.; Delmotte, M. Analysis of 5.5 Years of Atmospheric CO2, CH4, CO Continuous Observations (2014–2020) and Their Correlations, at the Observatoire de Haute Provence, a Station of the ICOS-France National Greenhouse Gases Observation Network. Atmos. Environ. 2022, 277, 119020. [Google Scholar] [CrossRef]
- Xueref-Remy, I.; Milne, M.; Zoghbi, N.; Lelandais, L.; Riandet, A.; Armengaud, A.; Gille, G.; Lanzi, L.; Oppo, S.; Brégonzio-Rozier, L.; et al. Analysis of Atmospheric CO2 Variability in the Marseille City Area and the North-West Mediterranean Basin at Different Time Scales. Atmos. Environ. X 2023, 17, 100208. [Google Scholar] [CrossRef]
- Alonso, L.; Gomez-Chova, L.; Vila-Frances, J.; Amoros-Lopez, J.; Guanter, L.; Calpe, J.; Moreno, J. Improved Fraunhofer Line Discrimination Method for Vegetation Fluorescence Quantification. IEEE Geosci. Remote Sens. Lett. 2008, 5, 620–624. [Google Scholar] [CrossRef]
- Stamford, J.D.; Vialet-Chabrand, S.; Cameron, I.; Lawson, T. Development of an Accurate Low Cost NDVI Imaging System for Assessing Plant Health. Plant Methods 2023, 19, 9. [Google Scholar] [CrossRef]
- Muggeo, V. Segmented: An R Package to Fit Regression Models with Broken-Line Relationships. R News 2008, 8, 20–25. [Google Scholar]
- Campbell, P.K.E.; Huemmrich, K.F.; Middleton, E.M.; Ward, L.A.; Julitta, T.; Daughtry, C.S.T.; Burkart, A.; Russ, A.L.; Kustas, W.P. Diurnal and Seasonal Variations in Chlorophyll Fluorescence Associated with Photosynthesis at Leaf and Canopy Scales. Remote Sens. 2019, 11, 488. [Google Scholar] [CrossRef]
- Wang, F.; Chen, B.; Lin, X.; Zhang, H. Solar-Induced Chlorophyll Fluorescence as an Indicator for Determining the End Date of the Vegetation Growing Season. Ecol. Indic. 2020, 109, 105755. [Google Scholar] [CrossRef]
- Dobrowski, S.Z.; Pushnik, J.C.; Zarco-Tejada, P.J.; Ustin, S.L. Simple Reflectance Indices Track Heat and Water Stress-Induced Changes in Steady-State Chlorophyll Fluorescence at the Canopy Scale. Remote Sens. Environ. 2005, 97, 403–414. [Google Scholar] [CrossRef]
- Garbulsky, M.F.; Filella, I.; Verger, A.; Peñuelas, J. Photosynthetic Light Use Efficiency from Satellite Sensors: From Global to Mediterranean Vegetation. Environ. Exp. Bot. 2014, 103, 3–11. [Google Scholar] [CrossRef]
- Magney, T.S.; Bowling, D.R.; Logan, B.A.; Grossmann, K.; Stutz, J.; Blanken, P.D.; Burns, S.P.; Cheng, R.; Garcia, M.A.; Köhler, P.; et al. Mechanistic Evidence for Tracking the Seasonality of Photosynthesis with Solar-Induced Fluorescence. Proc. Natl. Acad. Sci. USA 2019, 116, 11640–11645. [Google Scholar] [CrossRef] [PubMed]
- Kováč, D.; Novotný, J.; Šigut, L.; Ač, A.; Peñuelas, J.; Grace, J.; Urban, O. Estimation of Photosynthetic Dynamics in Forests from Daily Measured Fluorescence and PRI Data with Adjustment for Canopy Shadow Fraction. Sci. Total Environ. 2023, 898, 166386. [Google Scholar] [CrossRef]
- Chang, C.Y.; Wen, J.; Han, J.; Kira, O.; LeVonne, J.; Melkonian, J.; Riha, S.J.; Skovira, J.; Ng, S.; Gu, L.; et al. Unpacking the Drivers of Diurnal Dynamics of Sun-Induced Chlorophyll Fluorescence (SIF): Canopy Structure, Plant Physiology, Instrument Configuration and Retrieval Methods. Remote Sens. Environ. 2021, 265, 112672. [Google Scholar] [CrossRef]
- Siegmann, B.; Cendrero-Mateo, M.P.; Cogliati, S.; Damm, A.; Gamon, J.; Herrera, D.; Jedmowski, C.; Junker-Frohn, L.V.; Kraska, T.; Muller, O.; et al. Downscaling of Far-Red Solar-Induced Chlorophyll Fluorescence of Different Crops from Canopy to Leaf Level Using a Diurnal Data Set Acquired by the Airborne Imaging Spectrometer HyPlant. Remote Sens. Environ. 2021, 264, 112609. [Google Scholar] [CrossRef]
- Yang, X.; Tang, J.; Mustard, J.F.; Lee, J.-E.; Rossini, M.; Joiner, J.; Munger, J.W.; Kornfeld, A.; Richardson, A.D. Solar-Induced Chlorophyll Fluorescence That Correlates with Canopy Photosynthesis on Diurnal and Seasonal Scales in a Temperate Deciduous Forest: Fluorescence and Photosynthesis. Geophys. Res. Lett. 2015, 42, 2977–2987. [Google Scholar] [CrossRef]
- Magney, T.S.; Frankenberg, C.; Köhler, P.; North, G.; Davis, T.S.; Dold, C.; Dutta, D.; Fisher, J.B.; Grossmann, K.; Harrington, A.; et al. Disentangling Changes in the Spectral Shape of Chlorophyll Fluorescence: Implications for Remote Sensing of Photosynthesis. J. Geophys. Res. Biogeosciences 2019, 124, 1491–1507. [Google Scholar] [CrossRef]
- Wu, L.; Zhang, Y.; Zhang, Z.; Zhang, X.; Wu, Y.; Chen, J.M. Deriving Photosystem-Level Red Chlorophyll Fluorescence Emission by Combining Leaf Chlorophyll Content and Canopy Far-Red Solar-Induced Fluorescence: Possibilities and Challenges. Remote Sens. Environ. 2024, 304, 114043. [Google Scholar] [CrossRef]
- Martínez-Ferri, E.; Balaguer, L.; Valladares, F.; Chico, J.M.; Manrique, E. Energy Dissipation in Drought-Avoiding and Drought-Tolerant Tree Species at Midday during the Mediterranean Summer. Tree Physiol. 2000, 20, 131–138. [Google Scholar] [CrossRef]
- Pons, T.; Welschen, R. Midday Depression of Net Photosynthesis in the Tropical Rainforest Tree Eperua Grandiflora: Contributions of Stomatal and Internal Conductances, Respiration and Rubisco Functioning. Tree Physiol. 2003, 23, 937–947. [Google Scholar] [CrossRef]
- Deng, Z.; Chen, J.; Wang, S.; Li, T.; Huang, K.; Gu, P.; Peng, H.; Chen, Z. Response of Vegetation Photosynthesis to the 2022 Drought in Yangtze River Basin by Diurnal Orbiting Carbon Observatory-2/3 Satellite Observations. J. Remote Sens. 2025, 5, 0445. [Google Scholar] [CrossRef]
- Belviso, S.; Reiter, I.M.; Loubet, B.; Gros, V.; Lathière, J.; Montagne, D.; Delmotte, M.; Ramonet, M.; Kalogridis, C.; Lebegue, B.; et al. A Top-down Approach of Surface Carbonyl Sulfide Exchange by a Mediterranean Oak Forest Ecosystem in Southern France. Atmos. Chem. Phys. 2016, 16, 14909–14923. [Google Scholar] [CrossRef]
- Paul-Limoges, E.; Damm, A.; Hueni, A.; Liebisch, F.; Eugster, W.; Schaepman, M.E.; Buchmann, N. Effect of Environmental Conditions on Sun-Induced Fluorescence in a Mixed Forest and a Cropland. Remote Sens. Environ. 2018, 219, 310–323. [Google Scholar] [CrossRef]
- Biriukova, K.; Celesti, M.; Evdokimov, A.; Pacheco-Labrador, J.; Julitta, T.; Migliavacca, M.; Giardino, C.; Miglietta, F.; Colombo, R.; Panigada, C.; et al. Effects of Varying Solar-View Geometry and Canopy Structure on Solar-Induced Chlorophyll Fluorescence and PRI. Int. J. Appl. Earth Obs. Geoinf. 2020, 89, 102069. [Google Scholar] [CrossRef]
- Liu, L.; Zhao, W.; Wu, J.; Liu, S.; Teng, Y.; Yang, J.; Han, X. The Impacts of Growth and Environmental Parameters on Solar-Induced Chlorophyll Fluorescence at Seasonal and Diurnal Scales. Remote Sens. 2019, 11, 2002. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baulard, A.; Mevy, J.-P.; Xueref-Remy, I.; Reiter, I.M.; Julitta, T.; Miglietta, F. Solar-Induced Fluorescence as Indicator of Downy Oak and the Influence of Some Environmental Variables at the End of the Growing Season. Remote Sens. 2025, 17, 1252. https://doi.org/10.3390/rs17071252
Baulard A, Mevy J-P, Xueref-Remy I, Reiter IM, Julitta T, Miglietta F. Solar-Induced Fluorescence as Indicator of Downy Oak and the Influence of Some Environmental Variables at the End of the Growing Season. Remote Sensing. 2025; 17(7):1252. https://doi.org/10.3390/rs17071252
Chicago/Turabian StyleBaulard, Antoine, Jean-Philippe Mevy, Irène Xueref-Remy, Ilja Marco Reiter, Tommaso Julitta, and Franco Miglietta. 2025. "Solar-Induced Fluorescence as Indicator of Downy Oak and the Influence of Some Environmental Variables at the End of the Growing Season" Remote Sensing 17, no. 7: 1252. https://doi.org/10.3390/rs17071252
APA StyleBaulard, A., Mevy, J.-P., Xueref-Remy, I., Reiter, I. M., Julitta, T., & Miglietta, F. (2025). Solar-Induced Fluorescence as Indicator of Downy Oak and the Influence of Some Environmental Variables at the End of the Growing Season. Remote Sensing, 17(7), 1252. https://doi.org/10.3390/rs17071252






