Discrimination of Multiple Foliar Diseases in Wheat Using Novel Feature Selection and Machine Learning
Abstract
Highlights
- The CWPA-KNN combination achieved a high overall accuracy of 77% in dis-criminating wheat leaves infected with diseases, e.g., powdery mildew, stripe rust, leaf rust.
- Only two key wavelet-based features (668 nm at scale 5 and 894 nm at scale 7) were needed for effective multi-disease differentiation, streamlining the detection process.
- The study provided a systematic and efficient detection method, integrating fea-ture selection and machine learning, and offering direct technical support for pre-cise disease management in the field.
- A comprehensive and severity-classified hyperspectral database was established for wheat foliar disease detection, providing a valuable resource for future re-search.
Abstract
1. Introduction
- (1)
- Building a hyperspectral database of wheat foliar diseases: systematically collect hyperspectral data of wheat leaves from multi-year field trials, with plants affected by powdery mildew, stripe rust, and leaf rust, to build a spatiotemporally representative spectral library.
- (2)
- Extracting and selecting disease spectral features: compare multiple feature selection algorithms (SPA, CWA, CWPA, and Relief-F) to identify disease-related spectral features and reduce redundancy.
- (3)
- Building high-accuracy disease identification models: compare the machine learning algorithms of KNN, naive Bayes (NB), and random forest (RF) for the construction of classification models using the features selected under objective (2).
- (4)
- Evaluating the accuracy of models: test the performance of various algorithms against their classification accuracy and model stability, to provide scientific and technical insight regarding efficient disease monitoring.
2. Materials and Methods
2.1. Experimental Design
2.2. Data Acquisition
2.2.1. Hyperspectral Data Acquisition
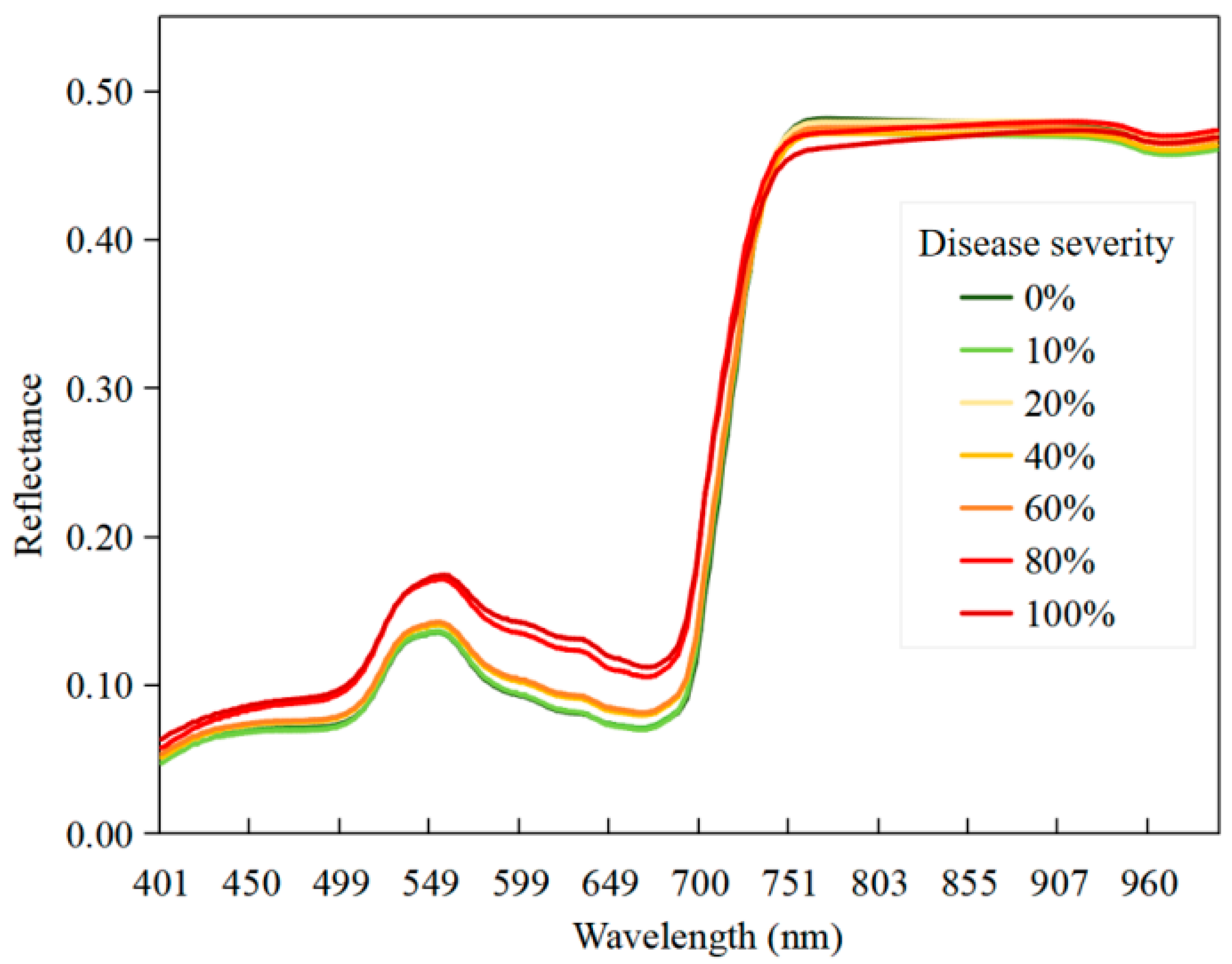
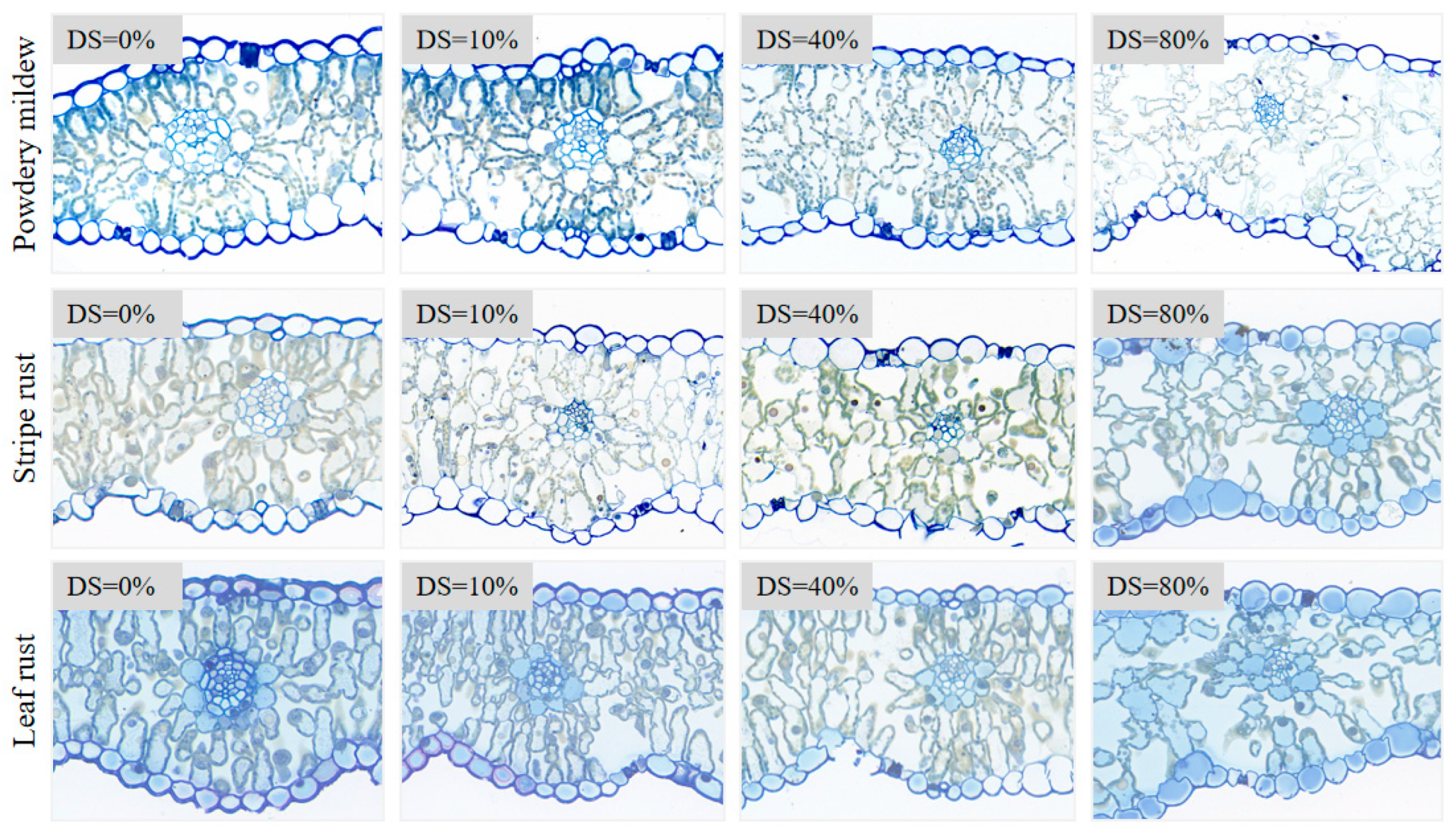
2.2.2. Disease Typing and Severity Assessment
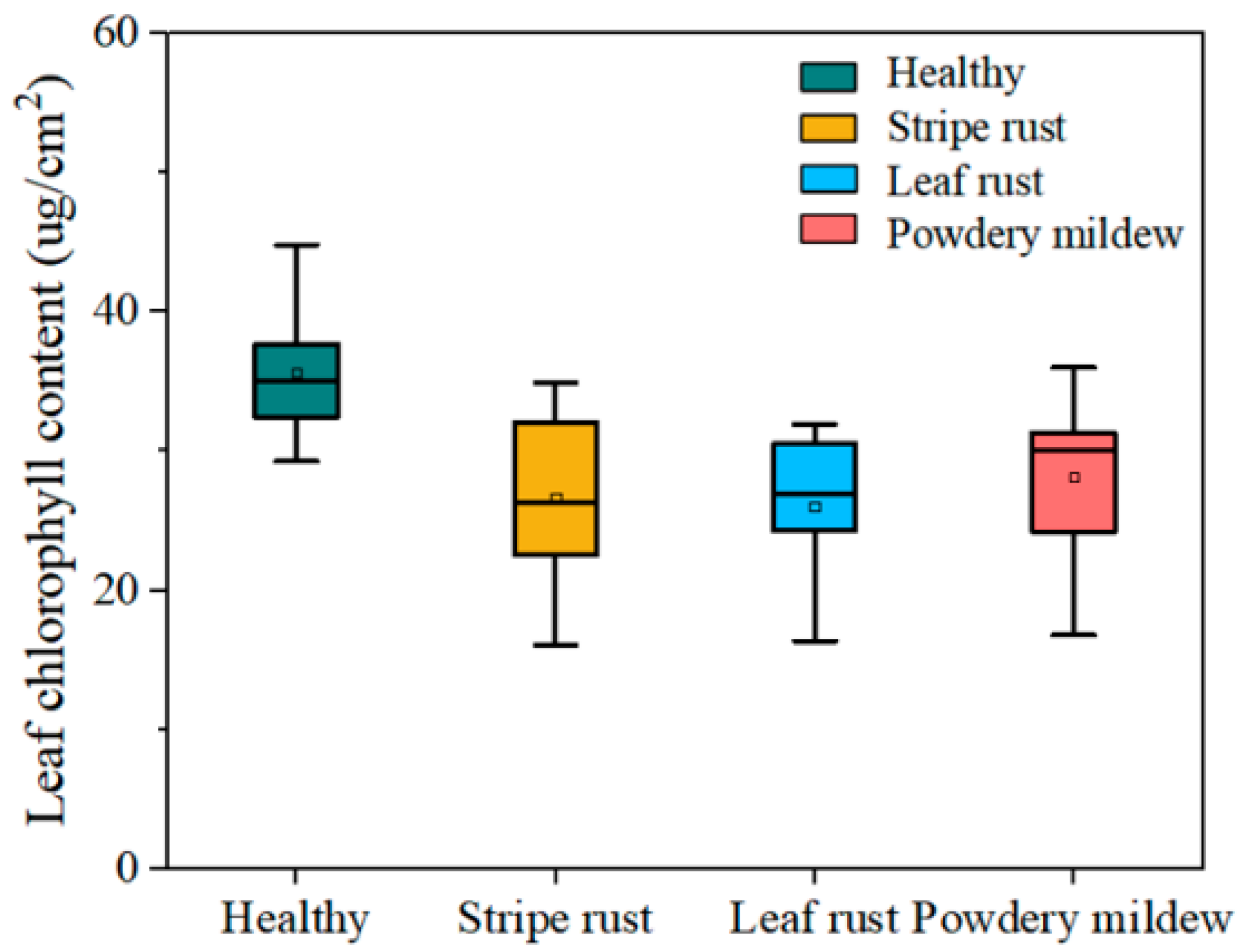
2.2.3. Leaf Chlorophyll Data
2.3. Common Feature Extraction Algorithms for Disease Identification
2.3.1. Continuous Wavelet Analysis (CWA)
2.3.2. Successive Projection Algorithm (SPA)
2.3.3. Continuous Wavelet Projection Algorithm (CWPA)
2.3.4. Relief-F Algorithm
2.4. Disease Classification Model
2.4.1. Random Forest Algorithm (RF)
2.4.2. K-Nearest Neighbors Algorithm (KNN)
2.4.3. Naïve Bayes Algorithm (BAYES)
2.5. Data Analysis
2.6. Model Evaluation Metrics
3. Results
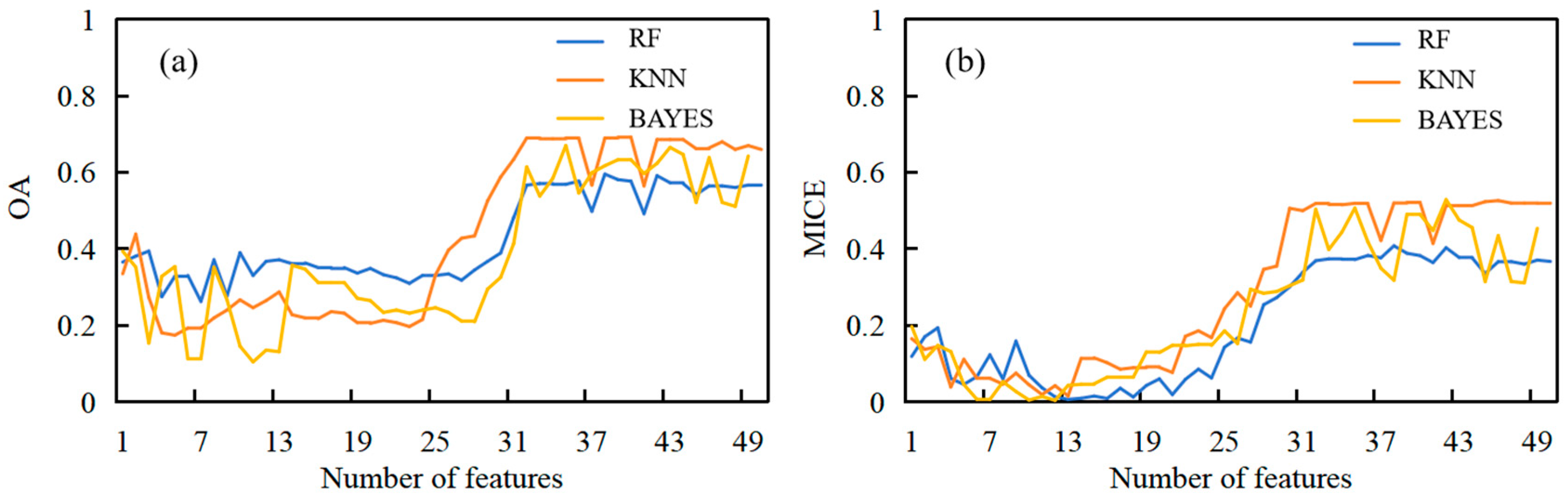
3.1. Wheat Foliar Disease Identification Based on CWA and Machine Learning Models
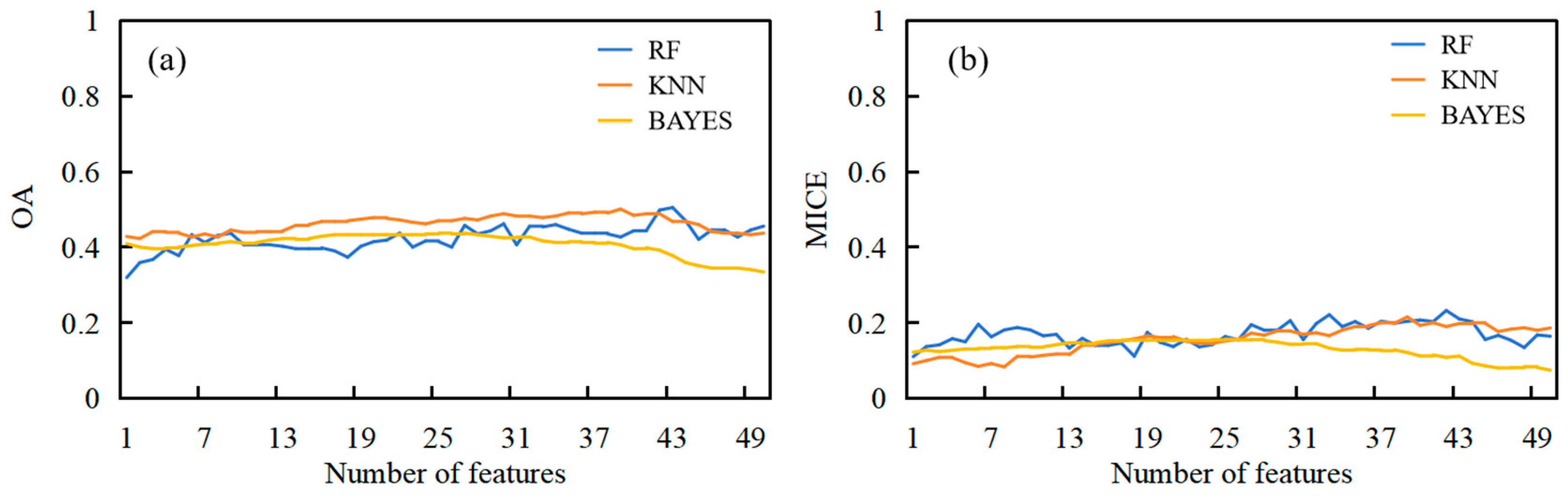
3.2. Wheat Foliar Disease Identification Based on SPA and Machine Learning Models
3.3. Wheat Foliar Disease Identification Based on CWPA and Machine Learning Models
3.4. Wheat Foliar Disease Identification Based on Relief-F and Machine Learning Models
3.5. Summary
4. Discussion
4.1. Challenges in Accurate Identification of Multiple Wheat Foliar Diseases
4.2. Comparison of Advantages and Disadvantages Among Different Feature Selection Algorithms
4.3. Physiological Interpretation and Mechanistic Analysis of the Spectral Features Extracted by CWPA for Disease Identification
4.4. Advantages and Limitations of Coupling Feature Selection with Machine Learning and Its Potential for Early Disease Monitoring
5. Conclusions
- (1)
- The CWPA demonstrated optimal performance in selecting common sensitive features for multiple wheat foliar diseases. CWPA not only effectively extracted sensitive features but also minimized inter-feature redundancy, showing notable advantages in balancing classification accuracy and feature quantity.
- (2)
- The optimal features selected by CWPA were two spectral bands at 668 nm and 894 nm, which, respectively, reflect the spectral response characteristics of pigment dynamics in wheat leaves under disease stress and cellular structure damage.
- (3)
- The CWPA–KNN algorithm developed in this study achieved high-accuracy identification of infected wheat leaves (OA = 77%, MICE = 0.63) using only two spectral features, greatly outperforming other algorithms such as RF and BAYES. This demonstrates the potential of this streamlined approach for efficient and accurate disease monitoring.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zeng, R.; Lin, X.; Welch, S.M.; Yang, S.; Huang, N.; Sassenrath, G.F.; Yao, F. Impact of water deficit and irrigation management on winter wheat yield in China. Agric. Water Manag. 2023, 287, 108431. [Google Scholar] [CrossRef]
- Huang, W.; Shi, Y.; Dong, Y.; Ye, H.; Wu, M.; Cui, B.; Liu, L. Progress and prospects of crop diseases and pests monitoring by remote sensing. Smart Agric. 2019, 1, 1. [Google Scholar]
- Chen, Q.; Conner, R.; Li, H.; Laroche, A.; Graf, R.; Kuzyk, A. Expression of resistance to stripe rust, powdery mildew and the wheat curl mite in Triticum aestivum × Haynaldia villosalines. Can. J. Plant Sci. 2002, 82, 451–456. [Google Scholar] [CrossRef]
- Liu, W.; Sun, C.; Zhao, Y.; Xu, F.; Song, Y.; Fan, J.; Zhou, Y.; Xu, X. Monitoring of wheat powdery mildew under different nitrogen input levels using hyperspectral remote sensing. Remote Sens. 2021, 13, 3753. [Google Scholar] [CrossRef]
- Green, A.J.; Berger, G.; Griffey, C.; Pitman, R.; Thomason, W.; Balota, M. Genetic resistance to and effect of leaf rust and powdery mildew on yield and its components in 50 soft red winter wheat cultivars. Crop Prot. 2014, 64, 177–186. [Google Scholar] [CrossRef]
- Picon, A.; Alvarez-Gila, A.; Seitz, M.; Ortiz-Barredo, A.; Echazarra, J.; Johannes, A. Deep convolutional neural networks for mobile capture device-based crop disease classification in the wild. Comput. Electron. Agric. 2019, 161, 280–290. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, N.; Yuan, L.; Chen, F.; Wu, K. Discrimination of winter wheat disease and insect stresses using continuous wavelet features extracted from foliar spectral measurements. Biosyst. Eng. 2017, 162, 20–29. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, B.; Zhang, X.; Liu, P.; Dong, Y.; Wu, K.; Huang, W. Impact of spectral interval on wavelet features for detecting wheat yellow rust with hyperspectral data. Int. J. Agric. Biol. Eng. 2018, 11, 138–144. [Google Scholar] [CrossRef]
- Genaev, M.A.; Skolotneva, E.S.; Gultyaeva, E.I.; Orlova, E.A.; Bechtold, N.P.; Afonnikov, D.A. Image-based wheat fungi diseases identification by deep learning. Plants 2021, 10, 1500. [Google Scholar] [CrossRef]
- Sosa-Herrera, J.A.; Alvarez-Jarquin, N.; Cid-Garcia, N.M.; López-Araujo, D.J.; Vallejo-Pérez, M.R. Automated health estimation of Capsicum annuum L. crops by means of deep learning and RGB aerial images. Remote Sens. 2022, 14, 4943. [Google Scholar] [CrossRef]
- Mahlein, A.-K.; Rumpf, T.; Welke, P.; Dehne, H.-W.; Plümer, L.; Steiner, U.; Oerke, E.-C. Development of spectral indices for detecting and identifying plant diseases. Remote Sens. Environ. 2013, 128, 21–30. [Google Scholar] [CrossRef]
- Mahlein, A.-K.; Alisaac, E.; Al Masri, A.; Behmann, J.; Dehne, H.-W.; Oerke, E.-C. Comparison and combination of thermal, fluorescence, and hyperspectral imaging for monitoring fusarium head blight of wheat on spikelet scale. Sensors 2019, 19, 2281. [Google Scholar] [CrossRef]
- Tian, L.; Wang, Z.; Xue, B.; Li, D.; Zheng, H.; Yao, X.; Zhu, Y.; Cao, W.; Cheng, T. A disease-specific spectral index tracks Magnaporthe oryzae infection in paddy rice from ground to space. Remote Sens. Environ. 2023, 285, 113384. [Google Scholar] [CrossRef]
- Yuan, L.; Yan, P.; Han, W.; Huang, Y.; Wang, B.; Zhang, J.; Zhang, H.; Bao, Z. Detection of anthracnose in tea plants based on hyperspectral imaging. Comput. Electron. Agric. 2019, 167, 105039. [Google Scholar] [CrossRef]
- Tian, L.; Xue, B.; Wang, Z.; Li, D.; Yao, X.; Cao, Q.; Zhu, Y.; Cao, W.; Cheng, T. Spectroscopic detection of rice leaf blast infection from asymptomatic to mild stages with integrated machine learning and feature selection. Remote Sens. Environ. 2021, 257, 112350. [Google Scholar] [CrossRef]
- Li, H.; Cui, J.; Zhang, X.; Han, Y.; Cao, L. Dimensionality reduction and classification of hyperspectral remote sensing image feature extraction. Remote Sens. 2022, 14, 4579. [Google Scholar] [CrossRef]
- Mustafa, G.; Zheng, H.; Khan, I.H.; Tian, L.; Jia, H.; Li, G.; Cheng, T.; Tian, Y.; Cao, W.; Zhu, Y.; et al. Hyperspectral Reflectance Proxies to Diagnose In-Field Fusarium Head Blight in Wheat with Machine Learning. Remote Sens. 2020, 14, 2784. [Google Scholar] [CrossRef]
- Mustafa, G.; Zheng, H.; Li, W.; Yin, Y.; Wang, Y.; Zhou, M.; Liu, P.; Bilal, M.; Jia, H.; Li, G.; et al. Fusarium head blight monitoring in wheat ears using machine learning and multimodal data from asymptomatic to symptomatic periods. Front. Front. Plant Sci. 2023, 13, 1102341. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, Y.; Xiao, H.; Gu, X.; Pan, L.; Tu, K. Hyperspectral imaging detection of decayed honey peaches based on their chlorophyll content. Food Chem. 2017, 235, 194–202. [Google Scholar] [CrossRef]
- Cheng, T.; Rivard, B.; Sánchez-Azofeifa, G.; Feng, J.; Calvo-Polanco, M. Continuous wavelet analysis for the detection of green attack damage due to mountain pine beetle infestation. Remote Sens. Environ. 2010, 114, 899–910. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, Y.; Pu, R.; Gonzalez-Moreno, P.; Yuan, L.; Wu, K.; Huang, W. Monitoring plant diseases and pests through remote sensing technology: A review. Comput. Electron. Agric. 2019, 165, 104943. [Google Scholar] [CrossRef]
- Zhang, J.; Yuan, L.; Wang, J.; Huang, W.; Chen, L.; Zhang, D. Spectroscopic Leaf Level Detection of Powdery Mildew for Winter Wheat Using Continuous Wavelet Analysis. J. Integr. Agric. 2012, 11, 1474–1484. [Google Scholar] [CrossRef]
- Zhao, J.; Huang, L.; Huang, W.; Zhang, D.; Yuan, L.; Zhang, J.; Liang, D. Hyperspectral measurements of severity of stripe rust on individual wheat leaves. Eur. J. Plant Pathol. 2014, 139, 407–417. [Google Scholar] [CrossRef]
- Zhang, J.; Yuan, L.; Pu, R.; Loraamm, R.W.; Yang, G.; Wang, J. Comparison between wavelet spectral features and conventional spectral features in detecting yellow rust for winter wheat. Comput. Electron. Agric. 2014, 100, 79–87. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, K.; Shi, Y.; Cui, P. A crop disease recognition algorithm based on machine learning. In Proceedings of the 13th EAI International Conference, SIMUtools 2021, Virtual Event, 5–6 November 2021; pp. 513–522. [Google Scholar]
- Shruthi, U.; Nagaveni, V.; Raghavendra, B. A review on machine learning classification techniques for plant disease detection. In Proceedings of the 2019 5th International Conference on Advanced Computing & Communication Systems (ICACCS), Coimbatore, India, 15–16 March 2019; pp. 281–284. [Google Scholar]
- Huang, W.; Lu, J.; Ye, H.; Kong, W.; Mortimer, A.H.; Shi, Y. Quantitative identification of crop disease and nitrogen-water stress in winter wheat using continuous wavelet analysis. Int. J. Agric. Biol. Eng. 2018, 11, 145–152. [Google Scholar] [CrossRef]
- Li, L.; Dong, Y.; Xiao, Y.; Liu, L.; Zhao, X.; Huang, W. Combining disease mechanism and machine learning to predict wheat fusarium head blight. Remote Sens. 2022, 14, 2732. [Google Scholar] [CrossRef]
- Anand, R.; Parray, R.A.; Mani, I.; Khura, T.K.; Kushwaha, H.; Sharma, B.B.; Sarkar, S.; Godara, S. Spectral data driven machine learning classification models for real time leaf spot disease detection in brinjal crops. Eur. J. Agron. 2024, 161, 127384. [Google Scholar] [CrossRef]
- NY/T 613-2002; Ministry of Agriculture of the People’s Republic of China (MARA). Rules for the Investigation and Forecast of Wheat Powdery Mildew [Blumeria graminis (DC.) Speer]. China Agriculture Press: Beijing, China, 2002.
- NY/T 617-2002; Ministry of Agriculture of the People’s Republic of China (MARA). Rules for the Investigation and Forecast of Wheat Leaf Rust (Puccinia recondita Rob.et Desm.). China Agriculture Press: Beijing, China, 2002.
- GB/T 15795-2011; Standardization Administration of China (SAC). Rules of Monitoring and Forecast of the Wheat Stripe Rust (Puccinia striiformis West). China Standard Press: Beijing, China, 2011.
- Zhao, X.; Zhang, J.; Pu, R.; Shu, Z.; He, W.; Wu, K. The continuous wavelet projections algorithm: A practical spectral feature mining approach for crop detection. Crop J. 2022, 10, 1264–1273. [Google Scholar] [CrossRef]
- Cheng, T.; Rivard, B.; Sanchez-Azofeifa, A. Spectroscopic determination of leaf water content using continuous wavelet analysis. Remote Sens. Environ. 2011, 115, 659–670. [Google Scholar] [CrossRef]
- Jia, M.; Li, W.; Wang, K.; Zhou, C.; Cheng, T.; Tian, Y.; Zhu, Y.; Cao, W.; Yao, X. A newly developed method to extract the optimal hyperspectral feature for monitoring leaf biomass in wheat. Comput. Electron. Agric. 2019, 165, 104942. [Google Scholar] [CrossRef]
- Araújo, M.C.U.; Saldanha, T.C.B.; Galvao, R.K.H.; Yoneyama, T.; Chame, H.C.; Visani, V. The successive projections algorithm for variable selection in spectroscopic multicomponent analysis. Chemom. Intell. Lab. Syst. 2001, 57, 65–73. [Google Scholar] [CrossRef]
- Zhang, J.; Li, H.; Tian, Y.; Qiu, H.; Zhou, X.; Ma, H.; Yuan, L. Assessing rice sheath blight disease habitat suitability at a regional scale through Multisource Data Analysis. Remote Sens. 2023, 15, 5530. [Google Scholar] [CrossRef]
- Gu, C.; Wang, D.; Zhang, H.; Zhang, J.; Zhang, D.; Liang, D. Fusion of deep convolution and shallow features to recognize the severity of wheat Fusarium head blight. Front. Plant Sci. 2021, 11, 599886. [Google Scholar] [CrossRef]
- Mustafa, G.; Zheng, H.; Liu, Y.; Yang, S.; Khan, I.H.; Hussain, S.; Liu, J.; Weize, W.; Chen, M.; Cheng, T. Leveraging machine learning to discriminate wheat scab infection levels through hyperspectral reflectance and feature selection methods. Eur. J. Agron. 2024, 161, 127372. [Google Scholar] [CrossRef]
- Belgiu, M.; Drăguţ, L. Random forest in remote sensing: A review of applications and future directions. ISPRS J. Photogramm. Remote Sens. 2016, 114, 24–31. [Google Scholar] [CrossRef]
- Wang, Z.; Zhao, Z.; Yin, C. Fine crop classification based on UAV hyperspectral images and random forest. ISPRS Int. J. Geo-Inf. 2022, 11, 252. [Google Scholar] [CrossRef]
- Li, Z.; Chengjin, Z.; Qingyang, X.; Chunfa, L. Weigted-KNN and its application on UCI. In Proceedings of the 2015 IEEE International Conference on Information and Automation, Lijiang, China, 8–10 August 2015; pp. 1748–1750. [Google Scholar]
- Pérez, A.; Larrañaga, P.; Inza, I. Bayesian classifiers based on kernel density estimation: Flexible classifiers. Int. J. Approx. Reason. 2009, 50, 341–362. [Google Scholar] [CrossRef]
- Tang, L.; Shao, J.; Pang, S.; Wang, Y.; Maxwell, A.; Hu, X.; Gao, Z.; Lan, T.; Shao, G. Bolstering performance evaluation of image segmentation models with efficacy metrics in the absence of a gold standard. IEEE Trans. Geosci. Remote Sens. 2024, 62, 5408012. [Google Scholar] [CrossRef]
- Ma, H.; Jing, Y.; Huang, W.; Shi, Y.; Dong, Y.; Zhang, J.; Liu, L. Integrating early growth information to monitor winter wheat powdery mildew using multi-temporal Landsat-8 imagery. Sensors 2018, 18, 3290. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Dong, Y.; Huang, W.; Du, X.; Luo, J.; Shi, Y.; Ma, H. Enhanced regional monitoring of wheat powdery mildew based on an instance-based transfer learning method. Remote Sens. 2019, 11, 298. [Google Scholar] [CrossRef]
- Yuan, L.; Zhang, J.C.; Deng, Q.; Dong, Y.Y.; Wang, H.L.; Du, X.K. Differentiation of wheat diseases and pests based on hyperspectral imaging technology with a few specific bands. Phyton-Int. J. Exp. Bot. 2023, 92, 611–628. [Google Scholar] [CrossRef]
- Zhang, J.C.; Pu, R.L.; Wang, J.H.; Huang, W.J.; Yuan, L.; Luo, J.H. Detecting powdery mildew of winter wheat using leaf level hyperspectral measurements. Comput. Electron. Agric. 2012, 85, 13–23. [Google Scholar] [CrossRef]
- Zhao, Y.; Jing, X.; Huang, W.; Dong, Y.; Li, C. Comparison of sun-induced chlorophyll fluorescence and reflectance data on estimating severity of wheat stripe rust. Spectrosc. Spectr. Anal. 2019, 39, 2739–2745. [Google Scholar]
- Zhao, J.; Kang, Z. Fighting wheat rusts in China: A look back and into the future. Phytopathol. Res. 2023, 5, 6. [Google Scholar] [CrossRef]
- Yuan, L.; Huang, Y.; Loraamm, R.W.; Nie, C.; Wang, J.; Zhang, J. Spectral analysis of winter wheat leaves for detection and differentiation of diseases and insects. Field Crops Res. 2014, 156, 199–207. [Google Scholar] [CrossRef]
- Bebronne, R.; Carlier, A.; Meurs, R.; Leemans, V.; Vermeulen, P.; Dumont, B.; Mercatoris, B. In-field proximal sensing of septoria tritici blotch, stripe rust and brown rust in winter wheat by means of reflectance and textural features from multispectral imagery. Biosyst. Eng. 2020, 197, 257–269. [Google Scholar] [CrossRef]
- Fernández, C.I.; Leblon, B.; Haddadi, A.; Wang, K.; Wang, J. Potato late blight detection at the leaf and canopy levels based in the red and red-edge spectral regions. Remote Sens. 2020, 12, 1292. [Google Scholar] [CrossRef]
- Zhang, D.; Hou, L.; Lv, L.; Qi, H.; Sun, H.; Zhang, X.; Li, S.; Min, J.; Liu, Y.; Tang, Y. Precision agriculture: Temporal and spatial modeling of wheat canopy spectral characteristics. Agriculture 2025, 15, 326. [Google Scholar] [CrossRef]
- Fahrentrapp, J.; Ria, F.; Geilhausen, M.; Panassiti, B. Detection of gray mold leaf infections prior to visual symptom appearance using a five-band multispectral sensor. Front. Plant Sci. 2019, 10, 628. [Google Scholar] [CrossRef]
- Ren, Y.; Huang, W.; Ye, H.; Zhou, X.; Ma, H.; Dong, Y.; Shi, Y.; Geng, Y.; Huang, Y.; Jiao, Q. Quantitative identification of yellow rust in winter wheat with a new spectral index: Development and validation using simulated and experimental data. Int. J. Appl. Earth Obs. Geoinf. 2021, 102, 102384. [Google Scholar] [CrossRef]
- Faisal, H.M.; Aqib, M.; Rehman, S.U.; Mahmood, K.; Obregon, S.A.; Iglesias, R.C.; Ashraf, I. Detection of cotton crops diseases using customized deep learning model. Sci. Rep. 2025, 15, 10766. [Google Scholar] [CrossRef] [PubMed]
- Shahi, T.B.; Xu, C.Y.; Neupane, A.; Guo, W. Recent advances in crop disease detection using UAV and deep learning techniques. Remote Sens. 2023, 15, 2450. [Google Scholar] [CrossRef]

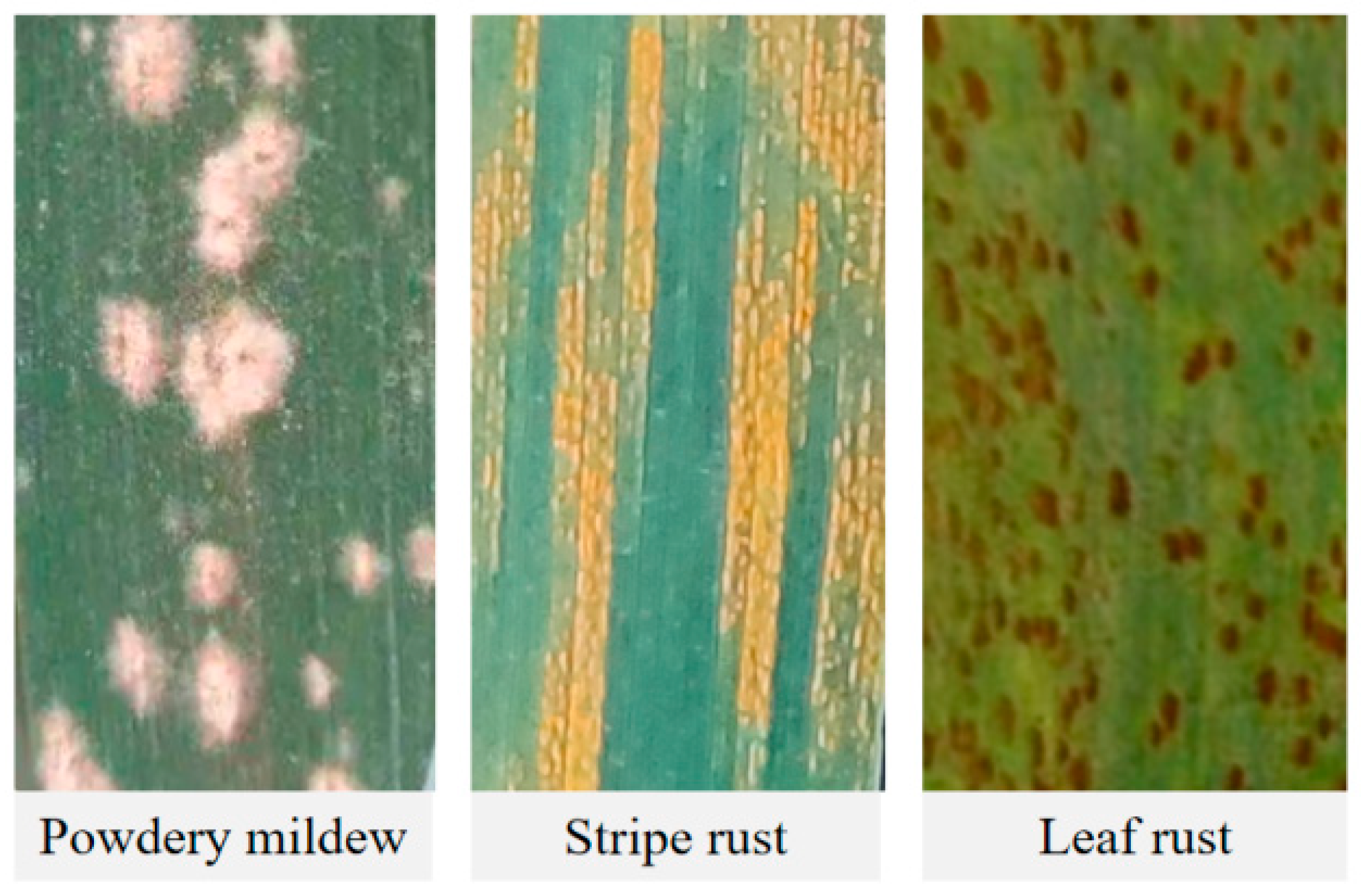
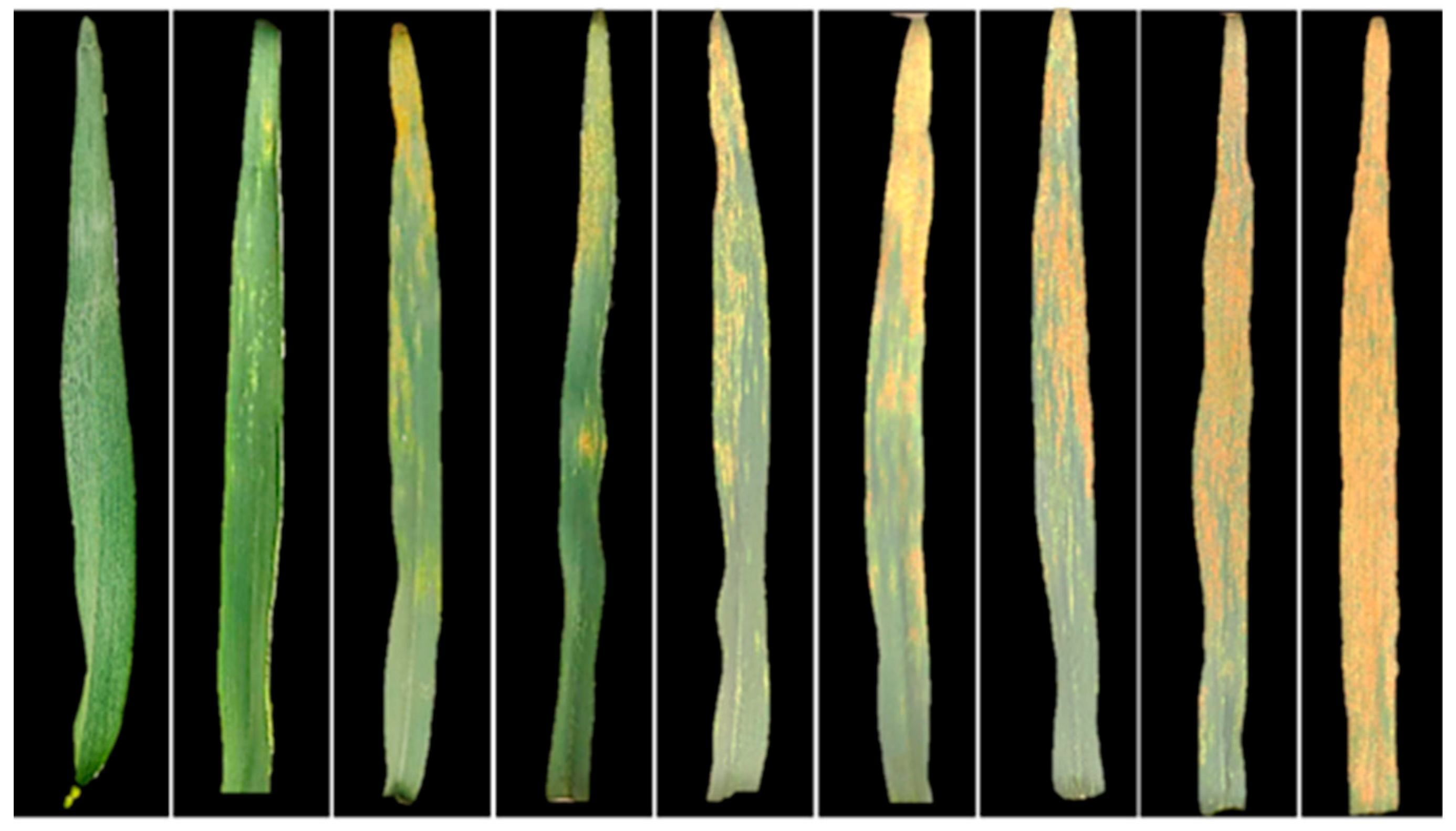
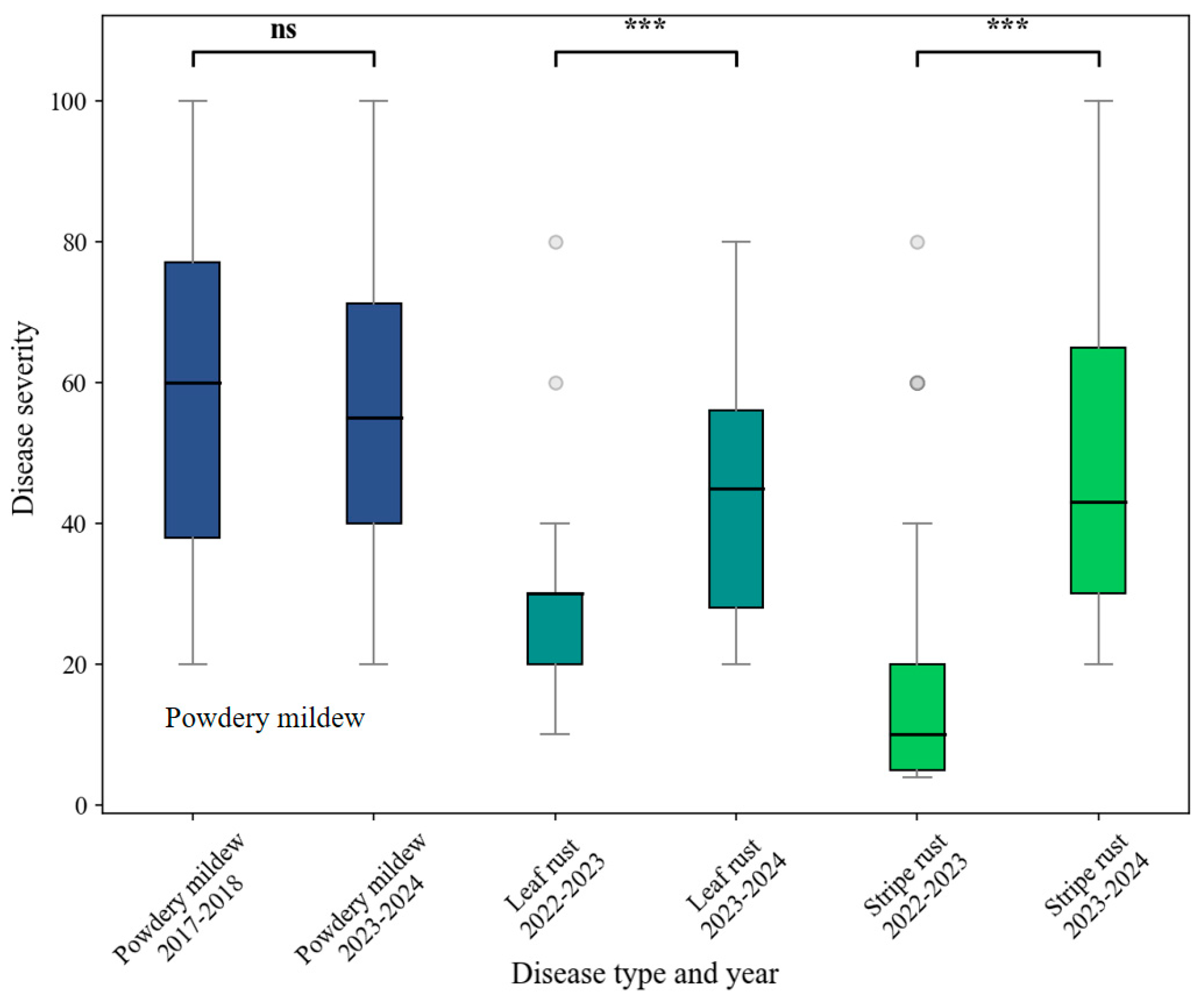
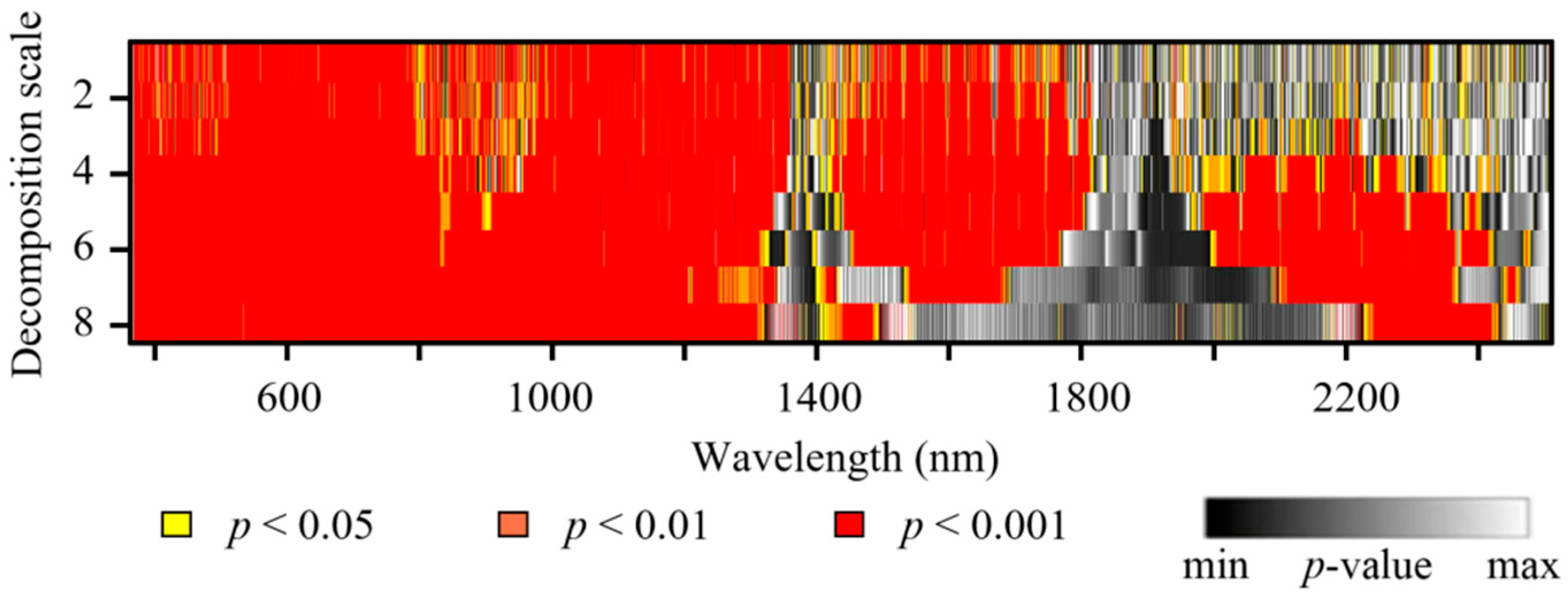


| Disease Type | Experiment | Time | Cultivar | Disease Severity (DS) | Number of Samples | Data Function |
|---|---|---|---|---|---|---|
| Powdery mildew | 1 | 2017–2018 | Nannong-0686 Nannong-9918 | 1–40% | 49 | Validation |
| 40–60% | 29 | |||||
| 60–100% | 63 | |||||
| 4 | 2023–2024 | Nannong-0686 | 1–40% | 60 | Training | |
| 40–60% | 56 | |||||
| 60–100% | 72 | |||||
| Stripe rust | 2 | 2022–2023 | Nannong-0686 Yangfumai-8161 Nannong-92R137 | 1–40% | 62 | Validation |
| 40–60% | 9 | |||||
| 60–100% | 4 | |||||
| 3 | 2023–2024 | Nannong-0686 Yangfumai-8161 Nannong-92R137 | 1–40% | 47 | Training | |
| 40–60% | 29 | |||||
| 60–100% | 28 | |||||
| Leaf rust | 2 | 2022–2023 | Nannong-0686 Yangfumai-8161 Nannong-92R137 | 1–40% | 25 | Validation |
| 40–60% | 4 | |||||
| 60–100% | 3 | |||||
| 3 | 2023–2024 | Nannong-0686 Yangfumai-8161 Nannong-92R137 | 1–40% | 52 | Training | |
| 40–60% | 48 | |||||
| 60–100% | 16 | |||||
| None (healthy leaves only) | 1 | 2017–2018 | Nannong-0686 Nannong-9918 | 0 | 105 | Validation |
| 2 | 2022–2023 | Nannong-0686 Yangfumai-8161 Nannong-92R137 | 0 | 67 | Validation | |
| 3 | 2023–2024 | Nannong-0686 Yangfumai-8161 Nannong-92R137 | 0 | 248 | Training |
| CWA Rank Threshold | Number of Features | Wavelength (nm) (Scale) | RF | KNN | BAYES | |||
|---|---|---|---|---|---|---|---|---|
| OA | MICE | OA | MICE | OA | MICE | |||
| 1% | 29 | See Note 1 | 0.69 | 0.53 | 0.71 | 0.54 | 0.70 | 0.52 |
| 5% | 33 | See Note 2 | 0.64 | 0.46 | 0.68 | 0.50 | 0.68 | 0.52 |
| 10% | 50 | See Note 3 | 0.61 | 0.42 | 0.68 | 0.51 | 0.25 | 0.14 |
| Algorithm | Number of Features | Wavelength (nm) | OA | MICE |
|---|---|---|---|---|
| RF | 38 | See Note 1 | 0.59 | 0.40 |
| KNN | 32 | See Note 2 | 0.69 | 0.51 |
| BAYES | 35 | See Note 3 | 0.67 | 0.50 |
| Algorithm | Number of Features | Wavelength (nm), (Scale) | OA | MICE |
|---|---|---|---|---|
| RF | 2 | 668 (5), 894 (7) | 0.74 | 0.59 |
| KNN | 2 | 668 (5), 894 (7) | 0.77 | 0.63 |
| BAYES | 2 | 668 (5), 894 (7) | 0.76 | 0.62 |
| Algorithm | Number of Features | Wavelength (nm) | OA | MICE |
|---|---|---|---|---|
| RF | 42 | See Note 1 | 0.49 | 0.23 |
| KNN | 39 | See Note 2 | 0.50 | 0.21 |
| BAYES | 25 | See Note 3 | 0.43 | 0.16 |
| RF | KNN | BAYES | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Algorithm | OA | MICE | Number of Features | OA | MICE | Number of Features | OA | MICE | Number of Features |
| CWA | 0.69 | 0.53 | 29 | 0.71 | 0.54 | 29 | 0.70 | 0.52 | 29 |
| SPA | 0.59 | 0.40 | 38 | 0.69 | 0.51 | 32 | 0.67 | 0.50 | 35 |
| CWPA | 0.74 | 0.59 | 2 | 0.77 | 0.63 | 2 | 0.76 | 0.62 | 2 |
| Relief-F | 0.49 | 0.23 | 42 | 0.50 | 0.21 | 39 | 0.43 | 0.16 | 25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhuang, S.; Huang, Y.; Zhu, J.; Yang, Q.; Li, W.; Gu, Y.; Li, T.; Zheng, H.; Jiang, C.; Cheng, T.; et al. Discrimination of Multiple Foliar Diseases in Wheat Using Novel Feature Selection and Machine Learning. Remote Sens. 2025, 17, 3304. https://doi.org/10.3390/rs17193304
Zhuang S, Huang Y, Zhu J, Yang Q, Li W, Gu Y, Li T, Zheng H, Jiang C, Cheng T, et al. Discrimination of Multiple Foliar Diseases in Wheat Using Novel Feature Selection and Machine Learning. Remote Sensing. 2025; 17(19):3304. https://doi.org/10.3390/rs17193304
Chicago/Turabian StyleZhuang, Sen, Yujuan Huang, Jie Zhu, Qingluo Yang, Wei Li, Yangyang Gu, Tongjie Li, Hengbiao Zheng, Chongya Jiang, Tao Cheng, and et al. 2025. "Discrimination of Multiple Foliar Diseases in Wheat Using Novel Feature Selection and Machine Learning" Remote Sensing 17, no. 19: 3304. https://doi.org/10.3390/rs17193304
APA StyleZhuang, S., Huang, Y., Zhu, J., Yang, Q., Li, W., Gu, Y., Li, T., Zheng, H., Jiang, C., Cheng, T., Tian, Y., Zhu, Y., Cao, W., & Yao, X. (2025). Discrimination of Multiple Foliar Diseases in Wheat Using Novel Feature Selection and Machine Learning. Remote Sensing, 17(19), 3304. https://doi.org/10.3390/rs17193304









