A Novel UAV- and AI-Based Remote Sensing Approach for Quantitative Monitoring of Jellyfish Populations: A Case Study of Acromitus flagellatus in Qinglan Port
Abstract
1. Introduction
2. Materials and Methods
2.1. Studied Jellyfish Species
2.2. Experimental Sea Area
2.3. Aerial Survey
2.3.1. UAVs
2.3.2. Flight Strategy
- (1)
- Flight routes: The monitoring area boundaries and flight schemes were planned using the UAV’s manufacturer-provided mission planning software (UAV Manager Pro Edition). The UAV autonomously executed the predefined missions using RTK positioning, typically initiating from open coastal areas and subsequently following a bidirectional S-shaped flight pattern (Figure 3).
- (2)
- Flight altitudes: To identify the optimal altitude for jellyfish detection while ensuring equipment safety, flight altitudes were set at 100 m, 120 m, 140 m, 160 m, 180 m, and 200 m. This gradient allowed us to compare the minimum detectable jellyfish size and coverage area at different altitudes.
- (3)
- Route overlaps: Route overlaps include both along-track overlap and across-track overlap. Along-track overlap refers to the extent of image overlap between two adjacent images on the same flight path, while across-track overlap refers to the image overlap between adjacent images from neighboring flight paths. For the UAV used in this study, route overlap can be autonomously set during route planning, reducing the potential for manual errors. The extent of overlap was determined based on the monitoring requirements. According to previous research, it is recommended to set overlap percentages between 10% and 15% [28,29,30].
2.4. Jellyfish Identification
2.4.1. Automated Identification
2.4.2. Comparison of Manual and Automated Jellyfish Detection Methods
2.5. Standardization of Jellyfish Bell Diameter Measurement
2.6. Jellyfish Abundance Calculation
3. Results
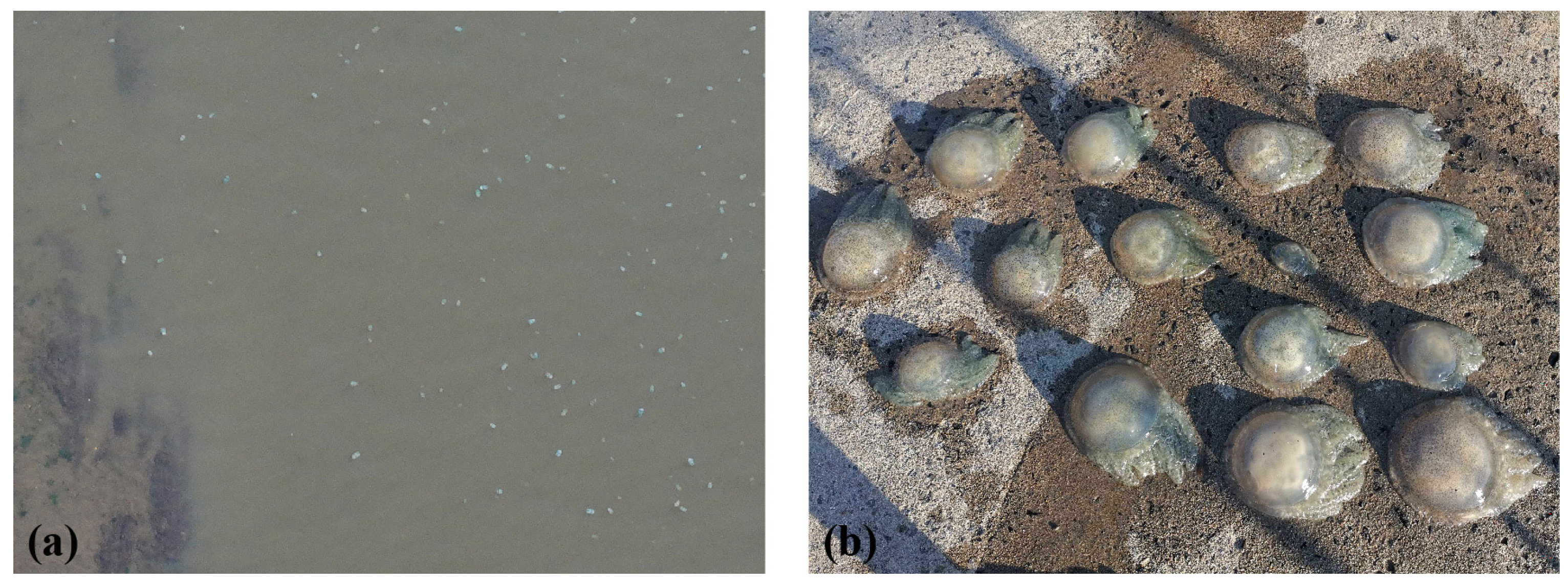
3.1. Minimum Detection Size and Efficiency
3.2. Jellyfish Bell Diameter Estimation Model
3.3. Automated Jellyfish Detection and Enumeration
3.4. Case Study: Population Dynamics of A. flagellatus in Qinglan Port Waters
4. Discussion
4.1. UAV Monitoring Method
4.2. Population Dynamics of A. flagellatus in Qinglan Port
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A

References
- Lee, S.H.; Tseng, L.C.; Yoon, Y.H.; Ramirez-Romero, E.; Hwang, J.S.; Molinero, J.C. The global spread of jellyfish hazards mirrors the pace of human imprint in the marine environment. Environ. Int. 2023, 171, 107699. [Google Scholar] [CrossRef]
- Sanz-Martín, M.; Pitt, K.A.; Condon, R.H.; Lucas, C.H.; Novaes de Santana, C.; Duarte, C.M. Flawed citation practices facilitate the unsubstantiated perception of a global trend toward increased jellyfish blooms. Glob. Ecol. Biogeogr. 2016, 25, 1039–1049. [Google Scholar] [CrossRef]
- Brodeur, R.D.; Link, J.S.; Smith, B.E.; Ford, M.; Kobayashi, D.; Jones, T.T. Ecological and economic consequences of ignoring jellyfish: A plea for increased monitoring of ecosystems. Fisheries 2016, 41, 630–637. [Google Scholar] [CrossRef]
- Purcell, J.E. Successes and challenges in jellyfish ecology: Examples from Aequorea spp. Mar. Ecol. Prog. Ser. 2018, 591, 7–27. [Google Scholar] [CrossRef]
- Fuentes, V.L.; Purcell, J.E.; Condon, R.H.; Lombard, F.; Lucas, C.H. Jellyfish blooms: Advances and challenges. Mar. Ecol. Prog. Ser. 2018, 591, 3–5. [Google Scholar] [CrossRef]
- Purcell, J.E.; Uye, S.I.; Lo, W.T. Anthropogenic causes of jellyfish blooms and their direct consequences for humans: A review. Mar. Ecol. Prog. Ser. 2007, 350, 153–174. [Google Scholar] [CrossRef]
- Sun, S.; Zhang, F.; Li, C.L.; Wang, S.W.; Wang, M.X.; Tao, Z.C.; Wang, Y.T.; Zhang, G.T.; Sun, X.X. Breeding places, population dynamics, and distribution of the giant jellyfish Nemopilema nomurai (Scyphozoa: Rhizostomeae) in the Yellow Sea and the East China Sea. Hydrobiologia 2015, 754, 59–74. [Google Scholar] [CrossRef]
- Martin-Abadal, M.; Ruiz-Frau, A.; Hinz, H.; Gonzalez-Cid, Y. Jellytoring: Real-time jellyfish monitoring based on deep learning object detection. Sensors 2020, 20, 1708. [Google Scholar] [CrossRef]
- Oh, S.; Kim, K.Y.; Oh, H.J.; Park, G.; Oh, W.; Lee, K. Spatio-Temporal Distribution of Giant Jellyfish (Nemopilema nomurai). Water 2022, 14, 2883. [Google Scholar] [CrossRef]
- Shahrestani, S.; Bi, H.; Liang, D.; Lankowicz, K.; Fan, C. Multi-scale spatial dynamics of the Chesapeake Bay nettle, Chrysaora chesapeakei. Ecosphere 2020, 11, e03128. [Google Scholar] [CrossRef]
- Bastian, T.; Haberlin, D.; Purcell, J.E.; Hays, G.C.; Davenport, J.; McAllen, R.; Doyle, T.K. Large-scale sampling reveals the spatio-temporal distributions of the jellyfish Aurelia aurita and Cyanea capillata in the Irish Sea. Mar. Biol. 2011, 158, 2639–2652. [Google Scholar] [CrossRef]
- Zavolokin, A. Distribution and abundance dynamics of jellyfish in the Sea of Okhotsk. Russ. J. Mar. Biol. 2010, 36, 157–166. [Google Scholar] [CrossRef]
- Brierley, A.S.; Boyer, D.C.; Axelsen, B.E.; Lynam, C.P.; Sparks, C.A.; Boyer, H.J.; Gibbons, M.J. Towards the acoustic estimation of jellyfish abundance. Mar. Ecol. Prog. Ser. 2005, 295, 105–111. [Google Scholar] [CrossRef]
- Hoving, H.J.; Christiansen, S.; Fabrizius, E.; Hauss, H.; Kiko, R.; Linke, P.; Neitzel, P.; Piatkowski, U.; Körtzinger, A. The Pelagic In situ Observation System (PELAGIOS) to reveal biodiversity, behavior, and ecology of elusive oceanic fauna. Ocean Sci. 2019, 15, 1327–1340. [Google Scholar] [CrossRef]
- Lynam, C.P.; Hay, S.J.; Brierley, A.S. Interannual variability in abundance of North Sea jellyfish and links to the North Atlantic Oscillation. Limnol. Oceanogr. 2004, 49, 637–643. [Google Scholar] [CrossRef]
- Qi, L.; Hu, C.; Mikelsons, K.; Wang, M.; Lance, V.; Sun, S.; Barnes, B.B.; Zhao, J.; Van der Zande, D. In search of floating algae and other organisms in global oceans and lakes. Remote Sens. Environ. 2020, 239, 111659. [Google Scholar] [CrossRef]
- Hamel, H.; Lhoumeau, S.; Wahlberg, M.; Javidpour, J. Using drones to measure jellyfish density in shallow estuaries. J. Mar. Sci. Eng. 2021, 9, 659. [Google Scholar] [CrossRef]
- Schaub, J.; Hunt, B.P.; Pakhomov, E.A.; Holmes, K.; Lu, Y.; Quayle, L. Using unmanned aerial vehicles (UAVs) to measure jellyfish aggregations. Mar. Ecol. Prog. Ser. 2018, 591, 29–36. [Google Scholar] [CrossRef]
- Choi, S.Y.; Kim, H.J.; Seo, M.H.; Soh, H.Y. Density estimation of Nemopilema nomurai (Scyphozoa, Rhizostomeae) using a drone. J. Indian Soc. Remote Sens. 2021, 49, 1727–1732. [Google Scholar] [CrossRef]
- Raoult, V.; Gaston, T. Rapid biomass and size-frequency estimates of edible jellyfish populations using drones. Fish. Res. 2018, 207, 160–164. [Google Scholar] [CrossRef]
- Rowley, O.C.; Courtney, R.L.; Browning, S.A.; Seymour, J.E. Bay watch: Using unmanned aerial vehicles (UAV’s) to survey the box jellyfish Chironex fleckeri. PLoS ONE 2020, 15, e0241410. [Google Scholar] [CrossRef]
- Rizman-Idid, M.; Farrah-Azwa, A.B.; Chong, V.C. Preliminary taxonomic survey and molecular documentation of jellyfish species (Cnidaria: Scyphozoa and Cubozoa) in Malaysia. Zool. Stud. 2016, 55, e35. [Google Scholar] [CrossRef]
- Siddique, A.; Bhowal, A.; Purushothaman, J.; Athira, A.; Azeez, A. First record of Acromitus flagellatus (Maas, 1903) (Cnidaria: Scyphozoa) swarm from the world’s largest deltaic ecosystem, the Sundarbans, India. Reg. Stud. Mar. Sci. 2022, 55, 102555. [Google Scholar] [CrossRef]
- Syazwan, W.M.; Rizman-Idid, M.; Low, L.B.; Then, A.Y.H.; Chong, V.C. Assessment of scyphozoan diversity, distribution and blooms: Implications of jellyfish outbreaks to the environment and human welfare in Malaysia. Reg. Stud. Mar. Sci. 2020, 39, 101444. [Google Scholar] [CrossRef]
- Hemavathi, M. Studies on the Isolation, Purification, Characterization and Biomedical Applications of Nematocyst Venom Protein from the Jellyfish Acromitus flagellatus. Ph.D. Thesis, University of Madras, Chennai, India, 2017. [Google Scholar]
- Kogovšek, T.; Bogunović, B.; Malej, A. Recurrence of bloom-forming scyphomedusae: Wavelet analysis of a 200-year time series. Hydrobiologia 2010, 645, 81–96. [Google Scholar] [CrossRef]
- Lin, J.N.; Feng, S.; Wang, L.J.; Qiu, Y.H. Complete mitochondrial genome sequence of Acromitus flagellatus and its phylogenetic relationship with related jellyfish species. Mitochondrial DNA Part B 2022, 7, 1823–1824. [Google Scholar] [CrossRef] [PubMed]
- Xiang, H.T.; Tian, L. Development of a low-cost agricultural remote sensing system based on an autonomous unmanned aerial vehicle (UAV). Biosyst. Eng. 2011, 108, 174–190. [Google Scholar] [CrossRef]
- Bao, Z.C.; Sha, J.M.; Li, X.M.; Hanchiso, T.; Shifaw, E. Monitoring of beach litter by automatic interpretation of unmanned aerial vehicle images using the segmentation threshold method. Mar. Pollut. Bull. 2018, 137, 388–398. [Google Scholar] [CrossRef]
- Yao, P.; Xie, Z.; Ren, P. Optimal UAV route planning for coverage search of stationary target in river. IEEE Trans. Control Syst. Technol. 2017, 27, 822–829. [Google Scholar] [CrossRef]
- Igathinathane, C.; Pordesimo, L.; Columbus, E.; Batchelor, W.; Methuku, S. Shape identification and particles size distribution from basic shape parameters using ImageJ. Comput. Electron. Agric. 2008, 63, 168–182. [Google Scholar] [CrossRef]
- Gerst, R.; Cseresnyés, Z.; Figge, M.T. JIPipe: Visual batch processing for ImageJ. Nat. Methods 2023, 20, 168–169. [Google Scholar] [CrossRef]
- Kay, S.; Hedley, J.D.; Lavender, S. Sun glint correction of high and low spatial resolution images of aquatic scenes: A review of methods for visible and near-infrared wavelengths. Remote Sens. 2009, 1, 697–730. [Google Scholar] [CrossRef]
- Hedley, J.; Harborne, A.; Mumby, P. Simple and robust removal of sun glint for mapping shallow-water benthos. Int. J. Remote Sens. 2005, 26, 2107–2112. [Google Scholar] [CrossRef]
- Bi, H.; Cheng, Y.; Cheng, X.; Benfield, M.C.; Kimmel, D.G.; Zheng, H.; Groves, S.; Ying, K. Taming the data deluge: A novel end-to-end deep learning system for classifying marine biological and environmental images. Limnol. Oceanogr. Methods 2024, 22, 47–64. [Google Scholar] [CrossRef]
- He, K.; Zhang, X.; Ren, S.; Sun, J. Deep residual learning for image recognition. In Proceedings of the IEEE Conference on Computer Vision and Pattern Recognition, Las Vegas, NV, USA, 27–30 June 2016; pp. 770–778. [Google Scholar] [CrossRef]
- Wada, K. Labelme: Image Polygonal Annotation with Python. 2016. Available online: https://github.com/mpitid/pylabelme (accessed on 15 August 2023).
- Joyce, K.E.; Duce, S.; Leahy, S.M.; Leon, J.; Maier, S. Principles and practice of acquiring drone-based image data in marine environments. Mar. Freshw. Res. 2018, 70, 952–963. [Google Scholar] [CrossRef]
- Fallati, L.; Polidori, A.; Salvatore, C.; Saponari, L.; Savini, A.; Galli, P. Anthropogenic Marine Debris assessment with Unmanned Aerial Vehicle imagery and deep learning: A case study along the beaches of the Republic of Maldives. Sci. Total Environ. 2019, 693, 133581. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Sung, S. Evaluating spatial resolution for quality assurance of UAV images. Spat. Inf. Res. 2016, 24, 141–154. [Google Scholar] [CrossRef]
- Gauci, A.; Deidun, A.; Abela, J. Automating jellyfish species recognition through faster region-based convolution neural networks. Appl. Sci. 2020, 10, 8257. [Google Scholar] [CrossRef]
- Han, Y.; Chang, Q.; Ding, S.; Gao, M.; Zhang, B.; Li, S. Research on multiple jellyfish classification and detection based on deep learning. Multimed. Tools Appl. 2022, 81, 19429–19444. [Google Scholar] [CrossRef]
- Zhang, S.; Yang, X.T.; Wang, Y.Z.; Zhao, Z.X.; Liu, J.T.; Liu, Y.; Sun, C.H.; Zhou, C. Automatic fish population counting by machine vision and a hybrid deep neural network model. Animals 2020, 10, 364. [Google Scholar] [CrossRef]
- Gao, M.; Li, S.; Wang, K.; Bai, Y.; Ding, Y.; Zhang, B.; Guan, N.; Wang, P. Real-time jellyfish classification and detection algorithm based on improved YOLOv4-tiny and improved underwater image enhancement algorithm. Sci. Rep. 2023, 13, 12989. [Google Scholar] [CrossRef]
- Shkurti, F.; Xu, A.; Meghjani, M.; Higuera, J.C.G.; Girdhar, Y.; Giguere, P.; Dey, B.B.; Li, J.; Kalmbach, A.; Prahacs, C. Multi-domain monitoring of marine environments using a heterogeneous robot team. In Proceedings of the 2012 IEEE/RSJ International Conference on Intelligent Robots and Systems, Vilamoura-Algarve, Portugal, 7–12 October 2012; pp. 1747–1753. [Google Scholar] [CrossRef]
- Båmstedt, U.; Kaartvedt, S.; Youngbluth, M. An evaluation of acoustic and video methods to estimate the abundance and vertical distribution of jellyfish. J. Plankton Res. 2003, 25, 1307–1318. [Google Scholar] [CrossRef][Green Version]
- Banha, T.N.; Morandini, A.C.; Rosário, R.P.; Martinelli Filho, J.E. Scyphozoan jellyfish (Cnidaria, Medusozoa) from Amazon coast: Distribution, temporal variation and length–weight relationship. J. Plankton Res. 2020, 42, 767–778. [Google Scholar] [CrossRef]
- Lee, J.S.; Jo, Y.H. Jellyfish patch detecting using low latitude remote sensing system. In AGU Fall Meeting Abstracts; American Geophysical Union: Washington, DC, USA, 2015; Volume 2015, p. OS31A-1964. [Google Scholar]
- Wakabayashi, K.; Nagai, S.; Tanaka, Y. The complete larval development of Ibacus ciliatus from hatching to the nisto and juvenile stages using jellyfish as the sole diet. Aquaculture 2016, 450, 102–107. [Google Scholar] [CrossRef]
- Bhowal, A.; Siddique, A.; Prasad, H.; Purushothaman, J.; Banerjee, D. Comparison of microzooplankton community structure before and during Acromitus flagellatus swarm in the estuarine waters of northern Bay of Bengal. Reg. Stud. Mar. Sci. 2023, 61, 102864. [Google Scholar] [CrossRef]
- Resgalla Jr, C.; Kruger, K.C.; Costa, M.A.L.M.; Sarraff, T.E.S.; da Silva, A.L. Urticating macromedusae and stinging bathers on the South Atlantic coast: Oceanographic and climatological conditions of Olindias sambaquiensis (Müller, 1861) outbreaks. Cont. Shelf Res. 2023, 269, 105128. [Google Scholar] [CrossRef]
- Kaneshiro-Pineiro, M.Y.; Kimmel, D.G. Local wind dynamics influence the distribution and abundance of Chrysaora quinquecirrha in North Carolina, USA. Estuaries Coasts 2015, 38, 1965–1975. [Google Scholar] [CrossRef]
- Vineetha, G.; Kripa, V.; Karati, K.K.; Madhu, N.; Anil, P.; Nair, M.V. Surge in the jellyfish population of a tropical monsoonal estuary: A boon or bane to its plankton community dynamics? Mar. Pollut. Bull. 2022, 182, 113951. [Google Scholar] [CrossRef]
- Thuesen, E.V.; Rutherford, L.D., Jr.; Brommer, P.L.; Garrison, K.; Gutowska, M.A.; Towanda, T. Intragel oxygen promotes hypoxia tolerance of scyphomedusae. J. Exp. Biol. 2005, 208, 2475–2482. [Google Scholar] [CrossRef]















| Site | Source of Error | Sum of Squares | df | Mean Square | F Value | p Value |
|---|---|---|---|---|---|---|
| intergroup | 0.867 | 1 | 0.867 | 0.109 | 0.742 | |
| S2 | intra-group | 974.367 | 122 | 7.987 | ||
| Total | 975.234 | 123 | ||||
| intergroup | 0.004 | 1 | 0.004 | 0.002 | 0.961 | |
| S3 | intra-group | 44.234 | 28 | 1.580 | ||
| Total | 44.238 | 29 |
| Planned Project | Design Index | Principles |
|---|---|---|
| Operation Dates | 2021.04.13/2021.04.30/2021.05.11/2023.05.26 | Make sure there are no bad weather conditions, like rain or strong winds, on the day of operation. |
| place of departure | 19.548°N, 110.832°E | The ground is flat and open, and the sky is free of foreign bodies. Distance from surrounding high-rise buildings >50m. No source of signal interference. |
| aerial photography time | 6:00–9:00 | Eliminate the interference of ground reflection and sun glare. |
| relative flight height | 100 m | Ensure good spatial resolution, while avoiding the safety threat caused by too low flight altitude. |
| flight speed | 6.0 m/s | Flight safety and energy saving. |
| degree of heading overlap | 10% | All the monitoring areas of the stone were effectively covered, and excessive duplicate data were avoided. |
| degree of lateral overlap | 10% | |
| camera exposure time | 1/1250 s | Avoid over-exposure to ensure image data quality |
| ISO value | 800 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, F.; Wang, S.; Qiu, Y.; Wang, N.; Sun, S.; Bi, H. A Novel UAV- and AI-Based Remote Sensing Approach for Quantitative Monitoring of Jellyfish Populations: A Case Study of Acromitus flagellatus in Qinglan Port. Remote Sens. 2025, 17, 3020. https://doi.org/10.3390/rs17173020
Zhang F, Wang S, Qiu Y, Wang N, Sun S, Bi H. A Novel UAV- and AI-Based Remote Sensing Approach for Quantitative Monitoring of Jellyfish Populations: A Case Study of Acromitus flagellatus in Qinglan Port. Remote Sensing. 2025; 17(17):3020. https://doi.org/10.3390/rs17173020
Chicago/Turabian StyleZhang, Fang, Shuo Wang, Yanhao Qiu, Nan Wang, Song Sun, and Hongsheng Bi. 2025. "A Novel UAV- and AI-Based Remote Sensing Approach for Quantitative Monitoring of Jellyfish Populations: A Case Study of Acromitus flagellatus in Qinglan Port" Remote Sensing 17, no. 17: 3020. https://doi.org/10.3390/rs17173020
APA StyleZhang, F., Wang, S., Qiu, Y., Wang, N., Sun, S., & Bi, H. (2025). A Novel UAV- and AI-Based Remote Sensing Approach for Quantitative Monitoring of Jellyfish Populations: A Case Study of Acromitus flagellatus in Qinglan Port. Remote Sensing, 17(17), 3020. https://doi.org/10.3390/rs17173020






