Phenology-Aware Machine Learning Framework for Chlorophyll Estimation in Cotton Using Hyperspectral Reflectance
Abstract
1. Introduction
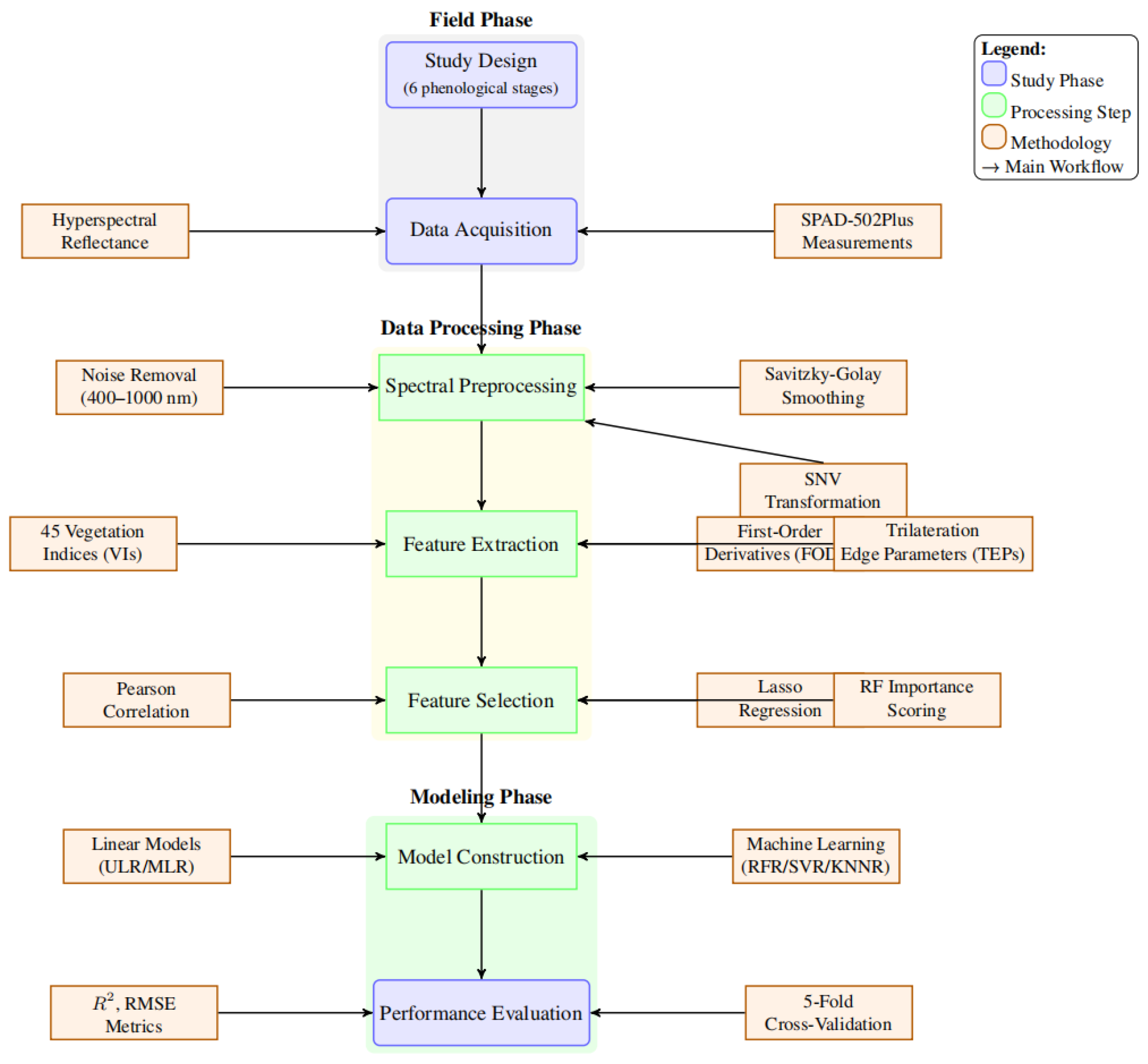
2. Materials and Methods
2.1. Workflow Overview
2.2. Study Area and Experimental Design
2.3. Data Acquisition
2.3.1. Hyperspectral Data Collection
2.3.2. Leaf Chlorophyll Content Measurement
2.4. Data Preprocessing and Feature Extraction
2.4.1. Spectral Preprocessing
2.4.2. Spectral Feature Extraction
2.5. Feature Selection and Dimensionality Reduction
- Pearson correlation () pre-screened candidate features.
- Lasso regression introduced sparsity, identifying key variables.
- Random forest importance scoring quantified predictor relevance.
2.6. Model Construction and Evaluation
2.6.1. Linear Regression Models
2.6.2. Machine Learning Models
- RFR: Ensemble method with ntree and mtry tuning.
- KNNR: Instance-based learning with k tested from 1 to 15.
- SVR: RBF kernel-based model tuned using C and .
2.6.3. Model Evaluation
3. Results
3.1. Statistics of Measured LLC
3.2. Parameter Selection
Biological Significance of Key Features
3.3. Univariate Linear Regression
3.4. Algorithm Implementation
3.5. Multiple Learning Regression
Machine Learning Algorithms
4. Discussion
4.1. Environmental Context of Stage-Specific Variability
4.2. Spectral Predictor Optimization via Linear Regression for Chlorophyll Quantification
4.3. Comparative Efficacy of ML Architectures in Chlorophyll Quantification
4.4. Model Performance Across Key Phenological Stages
4.5. Exploring Future Prospects for Cotton Chlorophyll Research
4.6. Practical Implications of Minimal-Band Modeling
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Peng, Y.; Nguy-Robertson, A.; Arkebauer, T.; Gitelson, A.A. Assessment of canopy chlorophyll content retrieval in maize and soybean: Implications of hysteresis on the development of generic algorithms. Remote Sens. 2017, 9, 226. [Google Scholar] [CrossRef]
- Evans, J.R. Photosynthesis and nitrogen relationships in leaves of C3 plants. Oecologia 1989, 78, 9–19. [Google Scholar] [CrossRef]
- Luo, J.; Zhou, J.J.; Masclaux-Daubresse, C.; Wang, N.; Wang, H.; Zheng, B. Morphological and physiological responses to contrasting nitrogen regimes in Populus cathayana is linked to resources allocation and carbon/nitrogen partition. Environ. Exp. Bot. 2019, 162, 247–255. [Google Scholar] [CrossRef]
- Porcar-Castell, A.; Tyystjärvi, E.; Atherton, J.; Van der Tol, C.; Flexas, J.; Pfündel, E.E.; Moreno, J.; Frankenberg, C.; Berry, J.A. Linking chlorophyll a fluorescence to photosynthesis for remote sensing applications: Mechanisms and challenges. J. Exp. Bot. 2014, 65, 4065–4095. [Google Scholar] [CrossRef]
- Croft, H.; Chen, J.M.; Luo, X.; Bartlett, P.; Chen, B.; Staebler, R.M. Leaf chlorophyll content as a proxy for leaf photosynthetic capacity. Glob. Change Biol. 2017, 23, 3513–3524. [Google Scholar] [CrossRef]
- Sage, R.F.; Pearcy, R.W.; Seemann, J.R. The nitrogen use efficiency of C3 and C4 plants: III. Leaf nitrogen effects on the activity of carboxylating enzymes in Chenopodium album (L.) and Amaranthus retroflexus (L.). Plant Physiol. 1987, 85, 355–359. [Google Scholar] [CrossRef]
- Luo, J.; Zhou, J.; Li, H.; Shi, W.; Polle, A.; Lu, M.; Sun, X.; Luo, Z.B. Global poplar root and leaf transcriptomes reveal links between growth and stress responses under nitrogen starvation and excess. Tree Physiol. 2015, 35, 1283–1302. [Google Scholar] [CrossRef] [PubMed]
- Qiao, L.; Tang, W.; Gao, D.; Zhao, R.; An, L.; Li, M.; Sun, H.; Song, D. UAV-based chlorophyll content estimation by evaluating vegetation index responses under different crop coverages. Comput. Electron. Agric. 2022, 196, 106775. [Google Scholar] [CrossRef]
- Aasen, H.; Honkavaara, E.; Lucieer, A.; Zarco-Tejada, P.J. Quantitative remote sensing at ultra-high resolution with UAV spectroscopy: A review of sensor technology, measurement procedures, and data correction workflows. Remote Sens. 2018, 10, 1091. [Google Scholar] [CrossRef]
- Ta, N.; Chang, Q.; Zhang, Y. Estimation of apple tree leaf chlorophyll content based on machine learning methods. Remote Sens. 2021, 13, 3902. [Google Scholar] [CrossRef]
- Zhou, J.J.; Zhang, Y.H.; Han, Z.M.; Liu, X.Y.; Jian, Y.F.; Hu, C.G.; Dian, Y.Y. Evaluating the performance of hyperspectral leaf reflectance to detect water stress and estimation of photosynthetic capacities. Remote Sens. 2021, 13, 2160. [Google Scholar] [CrossRef]
- Gamon, J.A.; Somers, B.; Malenovskỳ, Z.; Middleton, E.M.; Rascher, U.; Schaepman, M.E. Assessing vegetation function with imaging spectroscopy. Surv. Geophys. 2019, 40, 489–513. [Google Scholar] [CrossRef]
- Zhang, Y.; Hui, J.; Qin, Q.; Sun, Y.; Zhang, T.; Sun, H.; Li, M. Transfer-learning-based approach for leaf chlorophyll content estimation of winter wheat from hyperspectral data. Remote Sens. Environ. 2021, 267, 112724. [Google Scholar] [CrossRef]
- Zarco-Tejada, P.J.; González-Dugo, M.V.; Fereres, E. Seasonal stability of chlorophyll fluorescence quantified from airborne hyperspectral imagery as an indicator of net photosynthesis in the context of precision agriculture. Remote Sens. Environ. 2016, 179, 89–103. [Google Scholar] [CrossRef]
- Datt, B. Remote sensing of chlorophyll a, chlorophyll b, chlorophyll a+ b, and total carotenoid content in eucalyptus leaves. Remote Sens. Environ. 1998, 66, 111–121. [Google Scholar] [CrossRef]
- Yamashita, H.; Sonobe, R.; Hirono, Y.; Morita, A.; Ikka, T. Dissection of hyperspectral reflectance to estimate nitrogen and chlorophyll contents in tea leaves based on machine learning algorithms. Sci. Rep. 2020, 10, 17360. [Google Scholar] [CrossRef]
- Poobalasubramanian, M.; Park, E.S.; Faqeerzada, M.A.; Kim, T.; Kim, M.S.; Baek, I.; Cho, B.K. Identification of early heat and water stress in strawberry plants using chlorophyll-fluorescence indices extracted via hyperspectral images. Sensors 2022, 22, 8706. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.M.; Zhang, T.Q.; Zhang, J.; Chen, X.D.; Shen, X.G. Influence of smooth, 1st derivative and baseline correction on the near-infrared spectrum analysis with PLS. Guang Pu Xue Yu Guang Pu Fen Xi 2004, 24, 1546–1548. [Google Scholar]
- Zhao, T.; Komatsuzaki, M.; Okamoto, H.; Sakai, K. Cover crop nutrient and biomass assessment system using portable hyperspectral camera and laser distance sensor. Eng. Agric. Environ. Food 2010, 3, 105–112. [Google Scholar] [CrossRef]
- Zhao, T.; Nakano, A.; Iwaski, Y.; Umeda, H. Application of hyperspectral imaging for assessment of tomato leaf water status in plant factories. Appl. Sci. 2020, 10, 4665. [Google Scholar] [CrossRef]
- Turpie, K.R. Explaining the spectral red-edge features of inundated marsh vegetation. J. Coast. Res. 2013, 29, 1111–1117. [Google Scholar] [CrossRef]
- Shah, S.H.; Angel, Y.; Houborg, R.; Ali, S.; McCabe, M.F. A random forest machine learning approach for the retrieval of leaf chlorophyll content in wheat. Remote Sens. 2019, 11, 920. [Google Scholar] [CrossRef]
- Cui, B.; Zhao, Q.; Huang, W.; Song, X.; Ye, H.; Zhou, X. A new integrated vegetation index for the estimation of winter wheat leaf chlorophyll content. Remote Sens. 2019, 11, 974. [Google Scholar] [CrossRef]
- Verrelst, J.; Camps-Valls, G.; Muñoz-Marí, J.; Rivera, J.P.; Veroustraete, F.; Clevers, J.G.P.W.; Moreno, J. Optical remote sensing and the retrieval of terrestrial vegetation bio-geophysical properties—A review. Remote Sens. Environ. 2015, 169, 130–147. [Google Scholar] [CrossRef]
- Combal, B.; Baret, F.; Weiss, M.; Trubuil, A.; Macé, D.; Pragnere, A.; Myneni, R.; Knyazikhin, Y.; Wang, L. Retrieval of canopy biophysical variables from bidirectional reflectance: Using prior information to solve the ill-posed inverse problem. Remote Sens. Environ. 2003, 84, 1–15. [Google Scholar] [CrossRef]
- Jacquemoud, S.; Féret, J.-B.; Baret, F.; Weiss, M. PROSPECT + SAIL models: A review of use for vegetation characterization. Remote Sens. Environ. 2009, 113, S56–S66. [Google Scholar] [CrossRef]
- Delegido, J.; Verrelst, J.; Alonso, L.; Moreno, J. Evaluation of Sentinel-2 red-edge bands for empirical estimation of green LAI and chlorophyll content. Remote Sens. 2011, 3, 2874–2898. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Gritz, Y.; Merzlyak, M.N. Relationships between leaf chlorophyll content and spectral reflectance and algorithms for non-destructive chlorophyll assessment in higher plant leaves. J. Plant Physiol. 2003, 160, 271–282. [Google Scholar] [CrossRef]
- Zarco-Tejada, P.J.; González-Dugo, V.; Berni, J.A.J. Fluorescence, temperature and narrow-band indices acquired from a UAV platform for water stress detection using a micro-hyperspectral imager and a thermal camera. Remote Sens. Environ. 2012, 117, 322–337. [Google Scholar] [CrossRef]
- Narmilan, A.; Gonzalez, F.; Salgadoe, A.S.A.; Kumarasiri, U.W.L.M.; Weerasinghe, H.A.S.; Kulasekara, B.R. Predicting canopy chlorophyll content in sugarcane crops using machine learning algorithms and spectral vegetation indices derived from UAV multispectral imagery. Remote Sens. 2022, 14, 1140. [Google Scholar] [CrossRef]
- Weng, H.; Liu, Y.; Captoline, I.; Li, X.; Ye, D.; Wu, R. Citrus Huanglongbing detection based on polyphasic chlorophyll a fluorescence coupled with machine learning and model transfer in two citrus cultivars. Comput. Electron. Agric. 2021, 187, 106289. [Google Scholar] [CrossRef]
- Kong, W.; Huang, W.; Casa, R.; Zhou, X.; Ye, H.; Dong, Y. Off-nadir hyperspectral sensing for estimation of vertical profile of leaf chlorophyll content within wheat canopies. Sensors 2017, 17, 2711. [Google Scholar] [CrossRef]
- Jin, X.; Li, Z.; Feng, H.; Xu, X.; Yang, G. Newly combined spectral indices to improve estimation of total leaf chlorophyll content in cotton. IEEE J. Sel. Top. Appl. Earth Obs. Remote Sens. 2014, 7, 4589–4600. [Google Scholar] [CrossRef]
- Jin, X.; Wang, K.; Xiao, C.; Diao, W.; Wang, F.; Chen, B.; Li, S. Comparison of two methods for estimation of leaf total chlorophyll content using remote sensing in wheat. Field Crop. Res. 2012, 135, 24–29. [Google Scholar] [CrossRef]
- Ali, A.; Imran, M. Remotely sensed real-time quantification of biophysical and biochemical traits of Citrus (Citrus sinensis L.) fruit orchards—A review. Sci. Hortic. 2021, 282, 110024. [Google Scholar] [CrossRef]
- Sari, M.; Sonmez, N.K.; Karaca, M. Relationship between chlorophyll content and canopy reflectance in Washington navel orange trees (Citrus sinensis (L.) Osbeck). Pak. J. Bot. 2006, 38, 1093. [Google Scholar]
- Osco, L.P.; Ramos, A.P.M.; Faita Pinheiro, M.M.; Moriya, É.A.S.; Imai, N.N.; Estrabis, N.; Ianczyk, F.; Araújo, F.F.d.; Liesenberg, V.; Jorge, L.A.d.C.; et al. A machine learning framework to predict nutrient content in valencia-orange leaf hyperspectral measurements. Remote Sens. 2020, 12, 906. [Google Scholar] [CrossRef]
- Gerhards, M.; Schlerf, M.; Mallick, K.; Udelhoven, T. Challenges and future perspectives of multi-/Hyperspectral thermal infrared remote sensing for crop water-stress detection: A review. Remote Sens. 2019, 11, 1240. [Google Scholar] [CrossRef]
- Li, F.; Wang, L.; Liu, J.; Wang, Y.; Chang, Q. Evaluation of leaf N concentration in winter wheat based on discrete wavelet transform analysis. Remote Sens. 2019, 11, 1331. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Merzlyak, M.N.; Chivkunova, O.B. Optical properties and nondestructive estimation of anthocyanin content in plant leaves. Photochem. Photobiol. 2001, 74, 38–45. [Google Scholar] [CrossRef]
- Liang, L.; Qin, Z.; Zhao, S.; Di, L.; Zhang, C.; Deng, M.; Lin, H.; Zhang, L.; Wang, L.; Liu, Z. Estimating crop chlorophyll content with hyperspectral vegetation indices and the hybrid inversion method. Int. J. Remote Sens. 2016, 37, 2923–2949. [Google Scholar] [CrossRef]
- Carter, G.A. Ratios of leaf reflectances in narrow wavebands as indicators of plant stress. Remote Sens. 1994, 15, 697–703. [Google Scholar] [CrossRef]
- Datt, B. A new reflectance index for remote sensing of chlorophyll content in higher plants: Tests using Eucalyptus leaves. J. Plant Physiol. 1999, 154, 30–36. [Google Scholar] [CrossRef]
- Huete, A.; Justice, C.; Liu, H. Development of vegetation and soil indices for MODIS-EOS. Remote Sens. Environ. 1994, 49, 224–234. [Google Scholar] [CrossRef]
- Daughtry, C.S.T.; Walthall, C.L.; Kim, M.S.; De Colstoun, E.B.; McMurtrey Iii, J.E. Estimating corn leaf chlorophyll concentration from leaf and canopy reflectance. Remote Sens. Environ. 2000, 74, 229–239. [Google Scholar] [CrossRef]
- Haboudane, D.; Miller, J.R.; Pattey, E.; Zarco-Tejada, P.J.; Strachan, I.B. Hyperspectral vegetation indices and novel algorithms for predicting green LAI of crop canopies: Modeling and validation in the context of precision agriculture. Remote Sens. Environ. 2004, 90, 337–352. [Google Scholar] [CrossRef]
- Marshak, A.; Knyazikhin, Y.; Davis, A.B.; Wiscombe, W.J.; Pilewskie, P. Cloud-vegetation interaction: Use of normalized difference cloud index for estimation of cloud optical thickness. Geophys. Res. Lett. 2000, 27, 1695–1698. [Google Scholar] [CrossRef]
- Merzlyak, M.N.; Gitelson, A.A.; Chivkunova, O.B.; Rakitin, V.Y. Non-destructive optical detection of pigment changes during leaf senescence and fruit ripening. Physiol. Plant. 1999, 106, 135–141. [Google Scholar] [CrossRef]
- Roujean, J.L.; Breon, F.M. Estimating PAR absorbed by vegetation from bidirectional reflectance measurements. Remote Sens. Environ. 1995, 51, 375–384. [Google Scholar] [CrossRef]
- Clevers, J.G.P.W. Imaging spectrometry in agriculture-plant vitality and yield indicators. In Imaging Spectrometry—A Tool for Environmental Observations; Springer: Berlin, Germany, 1994; pp. 193–219. [Google Scholar]
- Vincini, M.; Frazzi, E.; D’Alessio, P. Angular dependence of maize and sugar beet VIs from directional CHRIS/Proba data. In Proceedings of the 4th ESA CHRIS PROBA Workshop, Frascati, Italy, 19–21 September 2006; pp. 19–21. [Google Scholar]
- Penuelas, J.; Baret, F.; Filella, I. Semi-empirical indices to assess carotenoids/chlorophyll a ratio from leaf spectral reflectance. Photosynthetica 1995, 31, 221–230. [Google Scholar]
- Lichtenthaler, H.K.; Lang, M.; Stober, F.; Sowinska, M.; Heisel, F.; Miehe, J.A. Detection of photosynthetic parameters and vegetation stress via a new high resolution fluorescence imaging-system. In International Colloquium Photosynthesis and Remote Sensing; EARSeL: Strasbourg, France, 1995; pp. 103–112. [Google Scholar]
- McMurtrey Iii, J.E.; Chappelle, E.W.; Kim, M.S.; Meisinger, J.J.; Corp, L.A. Distinguishing nitrogen fertilization levels in field corn (Zea mays L.) with actively induced fluorescence and passive reflectance measurements. Remote Sens. Environ. 1994, 47, 36–44. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Merzlyak, M.N. Remote estimation of chlorophyll content in higher plant leaves. Int. J. Remote Sens. 1997, 18, 2691–2697. [Google Scholar] [CrossRef]
- Zarco-Tejada, P.J.; Miller, J.R. Land cover mapping at BOREAS using red edge spectral parameters from CASI imagery. J. Geophys. Res. Atmos. 1999, 104, 27921–27933. [Google Scholar] [CrossRef]
- Sims, D.A.; Gamon, J.A. Relationships between leaf pigment content and spectral reflectance across a wide range of species, leaf structures and developmental stages. Remote Sens. Environ. 2002, 81, 337–354. [Google Scholar] [CrossRef]
- Haboudane, D.; Miller, J.R.; Tremblay, N.; Zarco-Tejada, P.J.; Dextraze, L. Integrated narrow-band vegetation indices for prediction of crop chlorophyll content for application to precision agriculture. Remote Sens. Environ. 2002, 81, 416–426. [Google Scholar] [CrossRef]
- Rondeaux, G.; Steven, M.; Baret, F. Optimization of soil-adjusted vegetation indices. Remote Sens. Environ. 1996, 55, 95–107. [Google Scholar] [CrossRef]
- Broge, N.H.; Leblanc, E. Comparing prediction power and stability of broadband and hyperspectral vegetation indices for estimation of green leaf area index and canopy chlorophyll density. Remote Sens. Environ. 2001, 76, 156–172. [Google Scholar] [CrossRef]
- Datt, B. Remote sensing of water content in Eucalyptus leaves. Aust. J. Bot. 1999, 47, 909–923. [Google Scholar] [CrossRef]
- Blackburn, G.A. Spectral indices for estimating photosynthetic pigment concentrations: A test using senescent tree leaves. Int. J. Remote Sens. 1998, 19, 657–675. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Viña, A.; Ciganda, V.; Rundquist, D.C.; Arkebauer, T.J. Remote estimation of canopy chlorophyll content in crops. Geophys. Res. Lett. 2005, 32, L08403. [Google Scholar] [CrossRef]
- Fitzgerald, G.; Rodriguez, D.; O’Leary, G. Measuring and predicting canopy nitrogen nutrition in wheat using a spectral index—The canopy chlorophyll content index (CCCI). Field Crop. Res. 2010, 116, 318–324. [Google Scholar] [CrossRef]
- Dash, J.; Curran, P.J. The MERIS terrestrial chlorophyll index. Int. J. Remote Sens. 2004, 25, 5403–5413. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Keydan, G.P.; Merzlyak, M.N. Three-band model for noninvasive estimation of chlorophyll, carotenoids, and anthocyanin contents in higher plant leaves. Geophys. Res. Lett. 2006, 33, L11402. [Google Scholar] [CrossRef]
- Jordan, C.F. Derivation of leaf-area index from quality of light on the forest floor. Ecology 1969, 50, 663–666. [Google Scholar] [CrossRef]
- Li, X.Y.; Liu, G.S.; Yang, Y.F.; Zhao, C.H.; Yu, Q.W.; Song, S.X. Relationship between hyperspectral parameters and physiological and biochemical indexes of flue-cured tobacco leaves. Agric. Sci. China 2007, 6, 665–672. [Google Scholar] [CrossRef]
- Ying, X. An overview of overfitting and its solutions. In Journal of Physics: Conference Series; IOP Publishing: Bristol, UK, 2019; Volume 1168, p. 022022. [Google Scholar]
- Fonti, V.; Belitser, E. Feature selection using lasso. VU Amst. Res. Pap. Bus. Anal. 2017, 30, 1–25. [Google Scholar]
- Gondek, J.S.; Meyer, G.W.; Newman, J.G. Wavelength dependent reflectance functions. In Proceedings of the 21st Annual Conference on Computer Graphics and Interactive Techniques, Orlando, FL, USA, 24–29 July 1994; pp. 213–220. [Google Scholar]
- Dash, J.; Curran, P.J. Evaluation of the MERIS terrestrial chlorophyll index (MTCI). Adv. Space Res. 2007, 39, 100–104. [Google Scholar] [CrossRef]
- Demetriades-Shah, T.H.; Steven, M.D.; Clark, J.A. High resolution derivative spectra in remote sensing. Remote Sens. Environ. 1990, 33, 55–64. [Google Scholar] [CrossRef]
- Zhang, X.; Sun, H.; Qiao, X.; Yan, X.; Feng, M.; Xiao, L.; Song, X.; Zhang, M.; Shafiq, F.; Yang, W.; et al. Hyperspectral estimation of canopy chlorophyll of winter wheat by using the optimized vegetation indices. Comput. Electron. Agric. 2022, 193, 106654. [Google Scholar] [CrossRef]
- Shi, H.; Guo, J.; An, J.; Tang, Z.; Wang, X.; Li, W.; Zhao, X.; Jin, L.; Xiang, Y.; Li, Z.; et al. Estimation of chlorophyll content in soybean crop at different growth stages based on optimal spectral index. Agronomy 2023, 13, 663. [Google Scholar] [CrossRef]
- Li, L.; Ren, T.; Ma, Y.; Wei, Q.; Wang, S.; Li, X.; Cong, R.; Liu, S.; Lu, J. Evaluating chlorophyll density in winter oilseed rape (Brassica napus L.) using canopy hyperspectral red-edge parameters. Comput. Electron. Agric. 2016, 126, 21–31. [Google Scholar] [CrossRef]
- Zarco-Tejada, P.J.; Miller, J.R.; Morales, A.; Berjón, A.; Agüera, J. Hyperspectral indices and model simulation for chlorophyll estimation in open-canopy tree crops. Remote Sens. Environ. 2004, 90, 463–476. [Google Scholar] [CrossRef]
- Bhadra, S.; Sagan, V.; Maimaitijiang, M.; Maimaitiyiming, M.; Newcomb, M.; Shakoor, N.; Mockler, T.C. Quantifying leaf chlorophyll concentration of sorghum from hyperspectral data using derivative calculus and machine learning. Remote Sens. 2020, 12, 2082. [Google Scholar] [CrossRef]
- Angel, Y.; McCabe, M.F. Machine learning strategies for the retrieval of leaf-chlorophyll dynamics: Model choice, sequential versus retraining learning, and hyperspectral predictors. Front. Plant Sci. 2022, 13, 722442. [Google Scholar] [CrossRef] [PubMed]
- An, G.; Xing, M.; He, B.; Liao, C.; Huang, X.; Shang, J.; Kang, H. Using machine learning for estimating rice chlorophyll content from in situ hyperspectral data. Remote Sens. 2020, 12, 3104. [Google Scholar] [CrossRef]
- Maimaitijiang, M.; Sagan, V.; Sidike, P.; Daloye, A.M.; Erkbol, H.; Fritschi, F.B. Crop monitoring using satellite/UAV data fusion and machine learning. Remote Sens. 2020, 12, 1357. [Google Scholar] [CrossRef]








| No. | Name | Formula | Reference |
|---|---|---|---|
| 1 | Anthocyanin Reflectance Index 1 | [30] | |
| 2 | Anthocyanin Reflectance Index 2 | [30] | |
| 3 | Green Normalized Difference Vegetation Index hyper 1 | [31] | |
| 4 | Green Normalized Difference Vegetation Index hyper 2 | [31] | |
| 5 | Modified Normalized Difference Vegetation Index | [31] | |
| 6 | Canopy Chlorophyll Index | [32] | |
| 7 | Vogelmann Index 2 | [33] | |
| 8 | Carter1 | [34] | |
| 9 | Carter2 | [34] | |
| 10 | Carter3 | [34] | |
| 11 | Carter4 | [35] | |
| 12 | Carter5 | [36] | |
| 13 | Datt1 | [37] | |
| 14 | Datt2 | [38] | |
| 15 | Datt3 | [39] | |
| 16 | Enhanced Vegetation Index | [40] | |
| 17 | Modified Chlorophyll Absorption in Reflectance Index | [41] | |
| 18 | Modified Triangular Vegetation Index 1 | [42] | |
| 19 | Normalized Difference Cloud Index | [43] | |
| 20 | Plant Senescence Reflectance Index | [44] | |
| 21 | Renormalized Difference Vegetation Index | [45] | |
| 22 | Red-Edge Position Linear Interpolation | [46] | |
| 23 | Spectral Polygon Vegetation Index 1 | [47] | |
| 24 | Simple Ratio Pigment Index | [48] | |
| 25 | Simple Ratio 440/690 | [49] | |
| 26 | Simple Ratio 700/670 | [50] | |
| 27 | Simple Ratio 750/550 | [51] | |
| 28 | Simple Ratio 750/700 | [52] | |
| 29 | Simple Ratio 750/710 | [53] | |
| 30 | Simple Ratio 752/690 | [54] | |
| 31 | Simple Ratio 800/680 | [55] | |
| 32 | Transformed Chlorophyll Absorption Ratio | [56] | |
| 33 | Optimized Soil Adjusted Vegetation Index | [57] | |
| 34 | Transformed Chlorophyll Absorption in Reflectance Index/Optimized Soil Adjusted Vegetation Index | [58] | |
| 35 | Triangular Vegetation Index | [59] | |
| 36 | Leaf Chlorophyll Index | [60] | |
| 37 | Structure Intensive Pigment Index 1 | [61] | |
| 38 | Structure Intensive Pigment Index 2 | [62] | |
| 39 | Structure Intensive Pigment Index 3 | [63] | |
| 40 | Red-Edge Ratio Vegetation Index | [64] | |
| 41 | Red-Edge Normalized Difference Vegetation Index | [64] | |
| 42 | Green Ratio Vegetation Index | [65] | |
| 43 | MERIS Terrestrial Chlorophyll Index | [66] | |
| 44 | Chlorophyll Index Green | [67] | |
| 45 | Ratio Vegetation Index | [68] | |
| 46 | FODS | First-order differential spectrum | [69] |
| 47 | First-order differential spectral integration in the wavelength range of 680∼760 nm | [69] | |
| 48 | First-order differential spectral integration in the wavelength range of 490∼530 nm | [69] | |
| 49 | Ratio of the red edge area to the blue edge area | [70] | |
| 50 | Normalized value of the red edge area and the blue edge area | [70] |
| Reproductive Stage | Model Equation | RMSE | Best Predictor | |
|---|---|---|---|---|
| Beginning Bud | 0.39 | 8.78 | SR750/710 | |
| Full Bud | 0.62 | 7.85 | MTCI | |
| First, Flower | 0.60 | 8.94 | FODS(752.4) | |
| Full Flower | 0.68 | 9.72 | Datt1 | |
| First, Boll | 0.72 | 8.32 | mNDVI705 | |
| Full Boll | 0.73 | 8.64 | FODS(743) |
| Reproductive Stage | Model Equation | RMSE | Best Predictors | |
|---|---|---|---|---|
| Beginning Bud | 0.59 | 6.87 | SR750/710, Datt1, Carter4 | |
| Full Bud | 0.62 | 7.85 | MTCI, SIPI1, NDVI | |
| First, Flower | 0.60 | 8.94 | FODS, Datt2, RERVI | |
| Full Flower | 0.68 | 9.72 | Datt1, VOG2, Carter5 | |
| First, Boll | 0.72 | 8.32 | mNDVI705 CIgreen, MTCI | |
| Full Boll | 0.77 | 4.37 | FODS(743), MTCI, TCARI |
| Reproductive Stage | Metric | RFR | KNNR | SVR | Key Parameters |
|---|---|---|---|---|---|
| Beginning Bud | 0.85 | 0.80 | 0.72 | RVI, FODS(743) | |
| RMSE | 5.80 | 5.60 | 4.10 | ||
| Full Bud | 0.62 | 0.68 | 0.78 | , RVI | |
| RMSE | 7.70 | 4.07 | 4.30 | ||
| First, Flower | 0.70 | 0.63 | 0.70 | Datt3, mNDVI705 | |
| RMSE | 3.50 | 4.03 | 7.10 | ||
| Full Flower | 0.52 | 0.71 | 0.79 | Datt1, Carter3 | |
| RMSE | 8.80 | 4.25 | 5.90 | ||
| First, Boll | 0.80 | 0.74 | 0.61 | mNDVI705, Carter3 | |
| RMSE | 4.01 | 3.80 | 9.30 | ||
| Full Boll | 0.69 | 0.37 | 0.69 | Datt3, FODS(752.4) | |
| RMSE | 4.07 | 11.53 | 6.30 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, C.; Cheng, Y.; Li, Y.; Peng, L.; Dong, G.; Lai, N.; Geng, Q. Phenology-Aware Machine Learning Framework for Chlorophyll Estimation in Cotton Using Hyperspectral Reflectance. Remote Sens. 2025, 17, 2713. https://doi.org/10.3390/rs17152713
Jiang C, Cheng Y, Li Y, Peng L, Dong G, Lai N, Geng Q. Phenology-Aware Machine Learning Framework for Chlorophyll Estimation in Cotton Using Hyperspectral Reflectance. Remote Sensing. 2025; 17(15):2713. https://doi.org/10.3390/rs17152713
Chicago/Turabian StyleJiang, Chunbo, Yi Cheng, Yongfu Li, Lei Peng, Gangshang Dong, Ning Lai, and Qinglong Geng. 2025. "Phenology-Aware Machine Learning Framework for Chlorophyll Estimation in Cotton Using Hyperspectral Reflectance" Remote Sensing 17, no. 15: 2713. https://doi.org/10.3390/rs17152713
APA StyleJiang, C., Cheng, Y., Li, Y., Peng, L., Dong, G., Lai, N., & Geng, Q. (2025). Phenology-Aware Machine Learning Framework for Chlorophyll Estimation in Cotton Using Hyperspectral Reflectance. Remote Sensing, 17(15), 2713. https://doi.org/10.3390/rs17152713







