Key Environmental Drivers of Summer Phytoplankton Size Class Variability and Decadal Trends in the Northern East China Sea
Abstract
1. Introduction
2. Materials and Methods
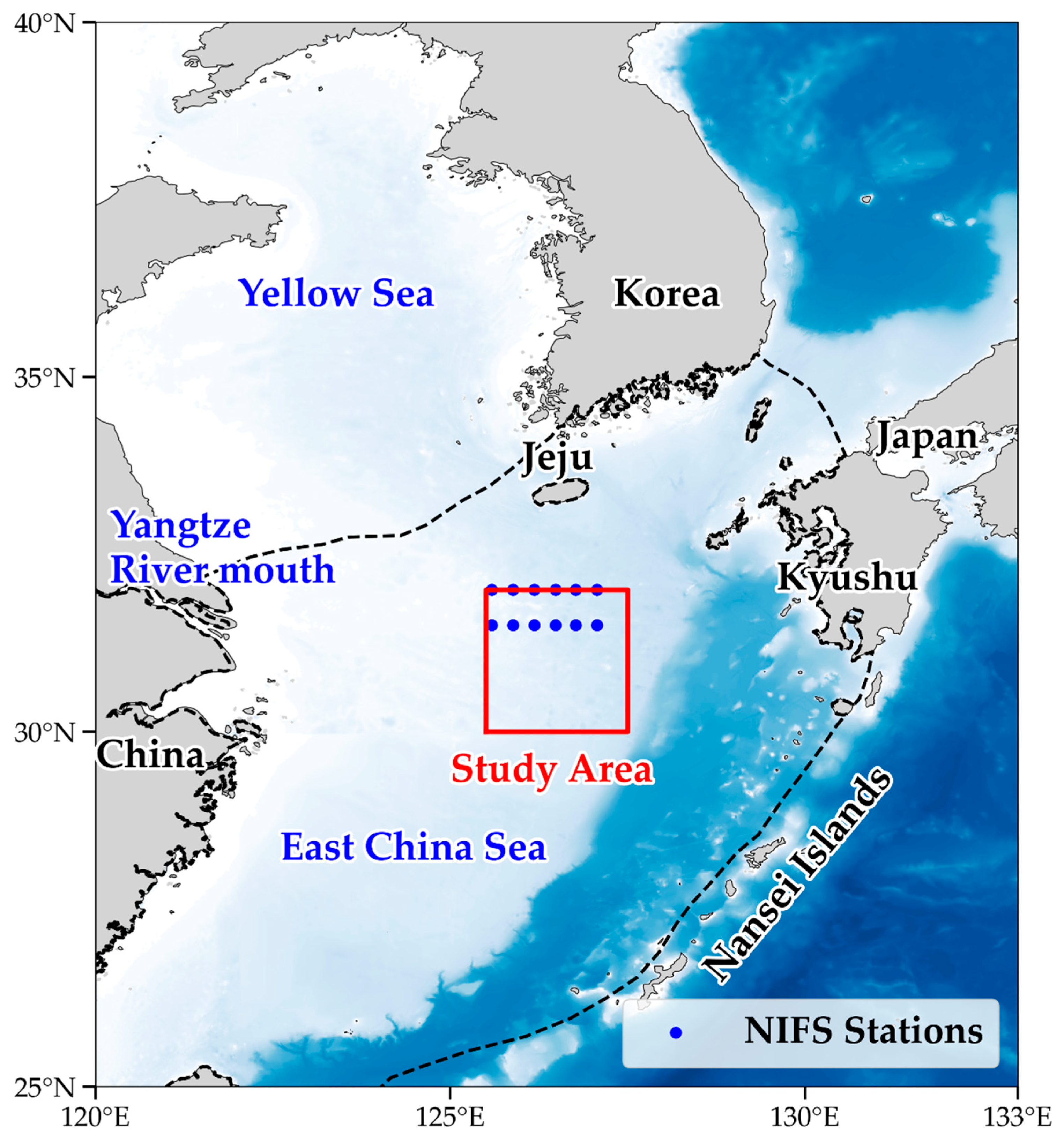
2.1. Study Area and In Situ Measurement
2.2. Satellite Data and PSC Validation
- For aph443 < 0.023 m−1, picophytoplankton dominance;
- For aph443 between 0.023 and 0.069 m−1, nanophytoplankton dominance;
- For aph443 > 0.069 m−1, microphytoplankton dominance.
2.3. Environmental Factors
2.4. Statistical Analysis
2.4.1. Random Forest Model for PSC Analysis
2.4.2. Multiple Linear Regression Analysis for PSCs
2.5. MHW Definition and Detection
3. Results
3.1. Validation of the PSC Models
3.2. Phytoplankton Dynamics During the Summer Season
3.3. Fluctuation of Environmental Variables During the Summer Season
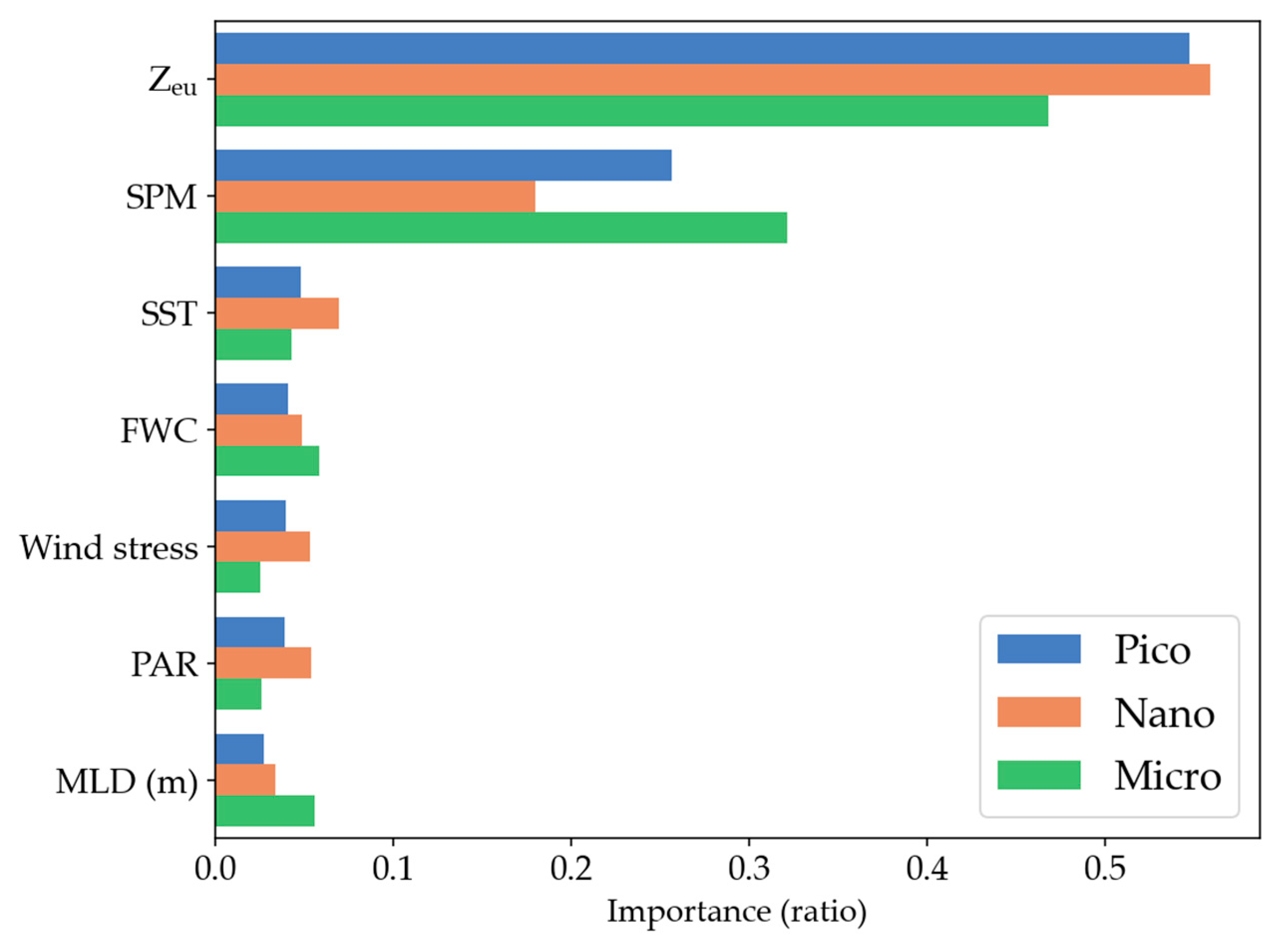
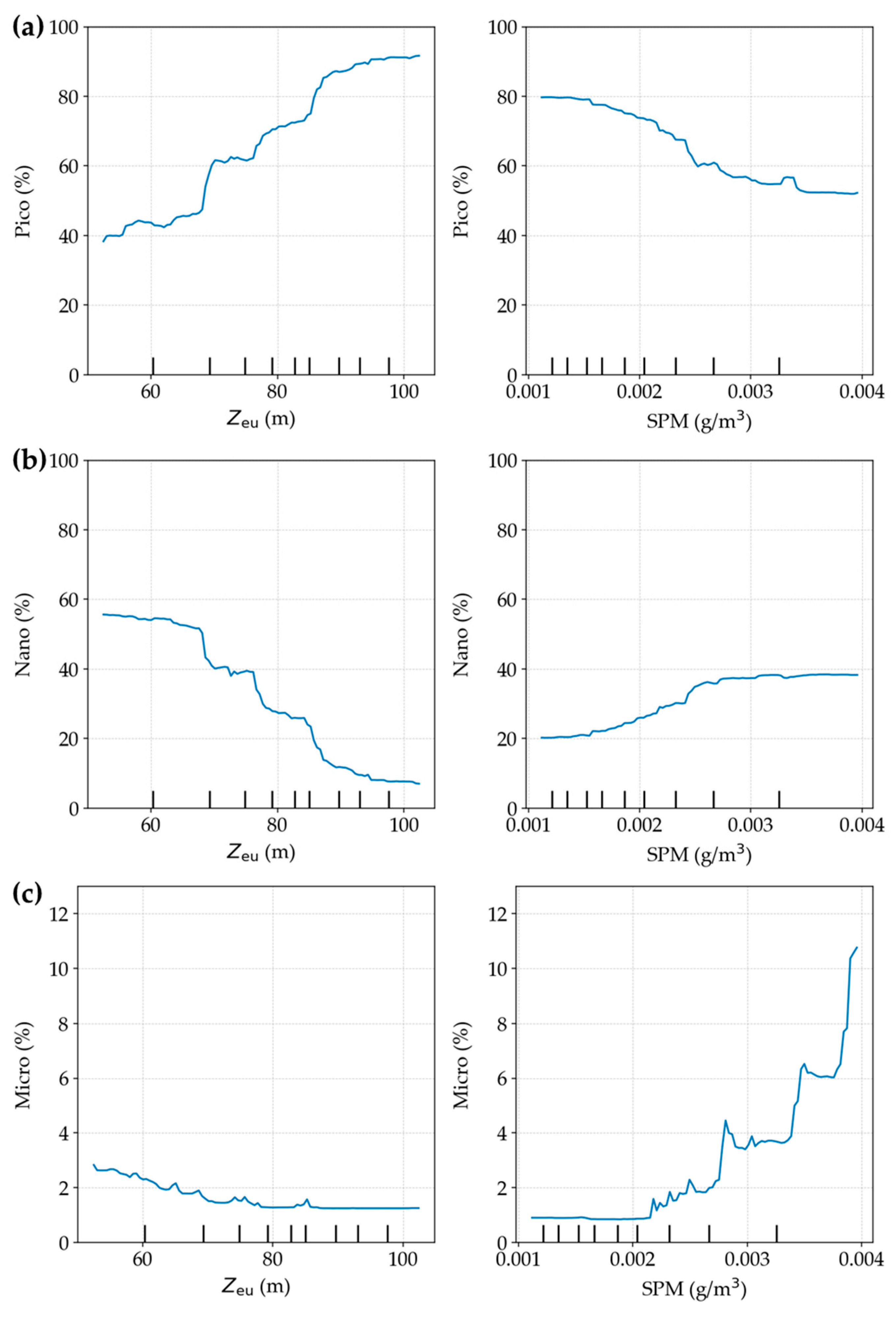
3.4. Major Driving Factors of PSC Variability
Comparison of Random Forest and Multiple Linear Regression Results
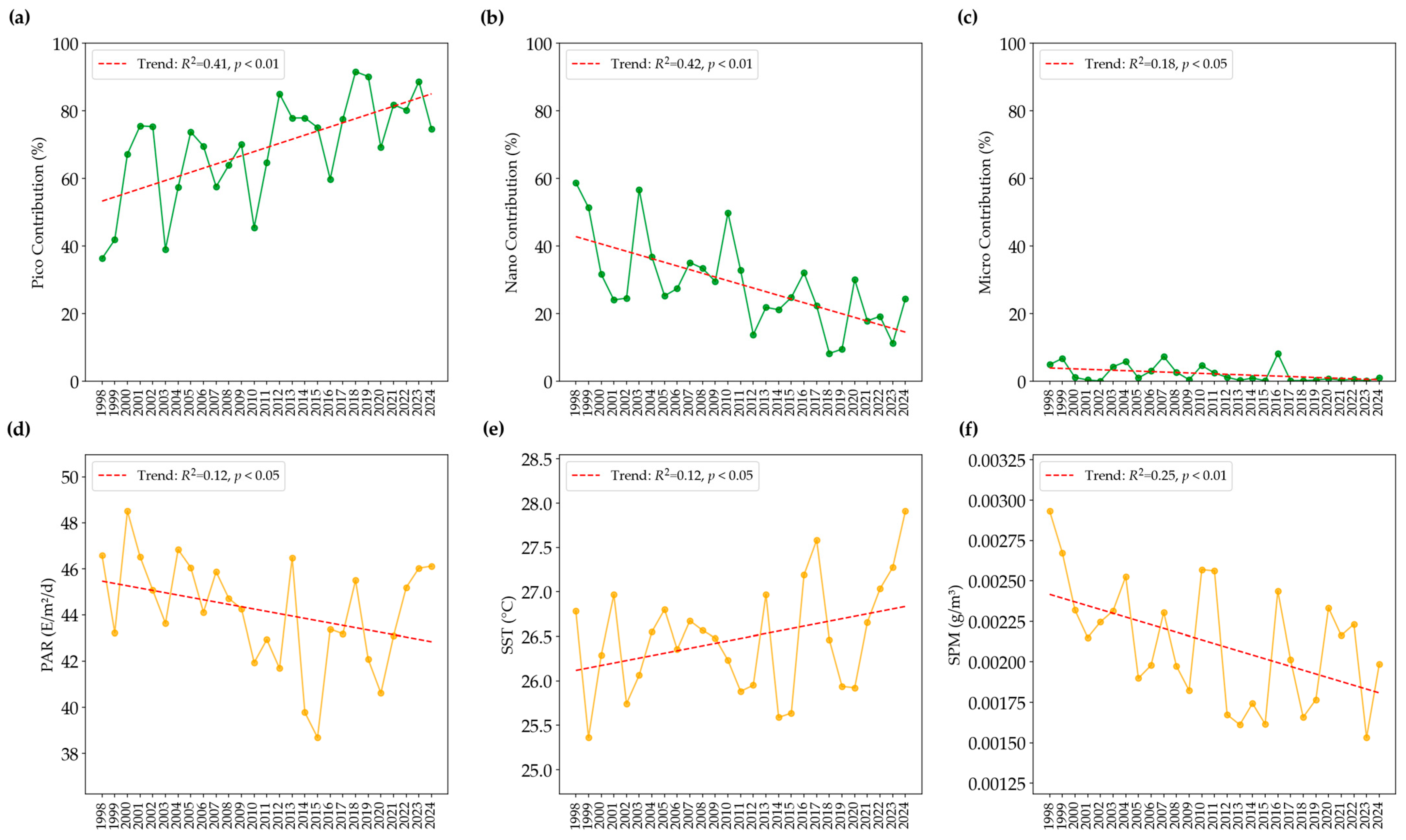
3.5. Decadal Changes in PSCs and Key Environmental Factors in the Summer
3.6. Biological Responses to MHWs and Environmental Differences
4. Discussion
4.1. PSC Variability and Key Environmental Drivers
4.2. Long-Term Trends in PSC Dynamics in the NECS During Summer and Their Implications
4.3. Study Limitations and Future Directions
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PSC | phytoplankton size class |
| NECS | northern East China Sea |
| ECS | East China Sea |
| Zeu | euphotic depth |
| SPM | suspended particulate matter |
| Chl-a | Chlorophyll-a |
| SST | sea surface temperature |
| FWC | freshwater content |
| MLD | mixed-layer depth |
| PAR | photosynthetically active radiation |
| MODIS | Moderate Resolution Imaging Spectroradiometer |
| MHW | marine heat wave |
| OC-CCI | Ocean Colour Climate Change Initiative |
| NIFS | National Institute of Fisheries Science |
| aph443 | phytoplankton absorption coefficient at 443 nm |
| Rrs665 | remote sensing reflectance at 665 nm |
| Kd(490) | diffuse attenuation coefficient at 490 nm |
| OSTIA | Global Ocean Operational SST and Sea Ice Analysis |
| CMEMS | Copernicus Marine Environment Monitoring Service |
| GlobColour | Global Merged Ocean Colour Project |
| PDP | partial dependence plot |
References
- Field, C.B.; Behrenfeld, M.J.; Randerson, J.T.; Falkowski, P. Primary production of the biosphere: Integrating terrestrial and oceanic components. Science 1998, 281, 237–240. [Google Scholar] [CrossRef]
- Agawin, N.S.R.; Duarte, C.M.; Agustí, S. Nutrient and temperature control of the contribution of picoplankton to phytoplankton biomass and production (Errata). Limnol. Oceanogr. 2000, 45, 591–600. [Google Scholar] [CrossRef]
- Platt, T.; Jassby, A.D. The Relationship between Photosynthesis and Light for Natural Assemblages of Coastal Marine Phytoplankton. J. Phycol. 1976, 12, 421–430. [Google Scholar] [CrossRef]
- Sigman, D.M.; Hain, M.P. The biological productivity of the ocean. Nat. Educ. Knowl. 2012, 3, 21. [Google Scholar]
- Bar-On, Y.M.; Phillips, R.; Milo, R. The biomass distribution on earth. Proc. Natl. Acad. Sci. USA 2018, 115, 6506–6511. [Google Scholar] [CrossRef]
- Behrenfeld, M.J.; O’Malley, R.T.; Siegel, D.A.; McClain, C.R.; Sarmiento, J.L.; Feldman, G.C.; Milligan, A.J.; Falkowski, P.G.; Letelier, R.M.; Boss, E.S. Climate-driven trends in contemporary ocean productivity. Nature 2006, 444, 752–755. [Google Scholar] [CrossRef] [PubMed]
- Huot, Y.; Babin, M.; Bruyant, F.; Grob, C.; Twardowski, M.S.; Claustre, H. Does chlorophyll a provide the best index of phytoplankton biomass for primary productivity studies? Biogeosci. Discuss. 2007, 4, 707–745. [Google Scholar] [CrossRef]
- Sieburth, J.M.; Smetacek, V.; Lenz, J. Pelagic ecosystem structure: Heterotrophic compartments of the plankton and their relationship to plankton size fractions. Limnol. Oceanogr. 1978, 23, 1256–1263. [Google Scholar] [CrossRef]
- Ciotti, Á.M.; Lewis, M.R.; Cullen, J.J. Assessment of the Relationships between Dominant Cell Size in Natural Phytoplankton Communities and the Spectral Shape of the Absorption Coefficient. Limnol. Oceanogr. 2002, 47, 404–417. [Google Scholar] [CrossRef]
- Guidi, L.; Stemmann, L.; Jackson, G.A.; Ibanez, F.; Claustre, H.; Legendre, L.; Picheral, M.; Gorskya, G. Effects of phytoplankton community on production, size, and export of large aggregates: A world-ocean analysis. Limnol. Oceanogr. 2009, 54, 1951–1963. [Google Scholar] [CrossRef]
- Zhou, Z.-X.; Yu, R.-C.; Sun, C.; Feng, M.; Zhou, M.-J. Impacts of Changjiang River Discharge and Kuroshio Intrusion on the Diatom and Dinoflagellate Blooms in the East China Sea. J. Geophys. Res. Oceans 2019, 124, 5244–5257. [Google Scholar] [CrossRef]
- Jacob, B.G.; Astudillo, O.; Dewitte, B.; Valladares, M.; Alvarez Vergara, G.; Medel, C.; Crawford, D.W.; Uribe, E.; Yanicelli, B. Abundance and Diversity of Diatoms and Dinoflagellates in an Embayment off Central Chile (30°S): Evidence of an Optimal Environmental Window Driven by Low and High Frequency Winds. Front. Mar. Sci. 2024, 11, 1434007. [Google Scholar] [CrossRef]
- Ward, B.A.; Dutkiewicz, S.; Jahn, O.; Follows, M.J. A Size-Structured Food-Web Model for the Global Ocean. Limnol. Oceanogr. 2012, 57, 1877–1891. [Google Scholar] [CrossRef]
- Balch, W.M.; Drapeau, D.T.; Bowler, B.C.; Lyczskowski, E.; Booth, E.S.; Alley, D. The Contribution of Coccolithophores to the Optical and Inorganic Carbon Budgets during the Southern Ocean Gas Exchange Experiment: New Evidence in Support of the “Great Calcite Belt” Hypothesis. J. Geophys. Res. Oceans 2011, 116, C00F06. [Google Scholar] [CrossRef]
- Flombaum, P.; Gallegos, J.L.; Gordillo, R.A.; Rincón, J.; Zabala, L.L.; Jiao, N.; Karl, D.M.; Li, W.K.W.; Lomas, M.W.; Veneziano, D.; et al. Present and Future Global Distributions of the Marine Cyanobacteria Prochlorococcus and Synechococcus. Proc. Natl. Acad. Sci. USA 2013, 110, 9824–9829. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Choi, J.K.; Youn, S.; Roh, S. Impact of Temperature and Stratification, Modulated by Warming Tsushima Warm Current, on Picoplankton Distribution in the Northern East China Sea. J. Geophys. Res. Oceans 2024, 129, e2024JC021649. [Google Scholar] [CrossRef]
- Azam, F.; Fenchel, T.; Field, J.G.; Gray, J.S.; Meyer-Reil, L.A.; Thingstad, F. The ecological role of water-column microbes in the sea. Mar. Ecol. Prog. Ser. 1983, 10, 257–263. [Google Scholar] [CrossRef]
- Chen, C.T.A. Chemical and physical fronts in the Bohai, yellow and East China seas. J. Mar. Syst. 2009, 78, 394–410. [Google Scholar] [CrossRef]
- Gong, G.-C.; Chen, Y.-L.L.; Liu, K.-K. Chemical Hydrography and Chlorophyll a Distribution in the East China Sea in Summer: Implications in Nutrient Dynamics. Cont. Shelf Res. 1996, 16, 1561–1590. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, S.M.; Ren, J.L.; Wu, Y.; Zhang, G.L. Nutrient Gradients from the Eutrophic Changjiang (Yangtze River) Estuary to the Oligotrophic Kuroshio Waters and Re-Evaluation of Budgets for the East China Sea Shelf. Prog. Oceanogr. 2007, 74, 449–478. [Google Scholar] [CrossRef]
- Guo, S.; Feng, Y.; Wang, L.; Dai, M.; Liu, Z.; Bai, Y.; Sun, J. Seasonal Variation in the Phytoplankton Community of a Continental-Shelf Sea: The East China Sea. Mar. Ecol. Prog. Ser. 2014, 516, 103–126. [Google Scholar] [CrossRef]
- Kim, Y.; Son, S.; Park, J. Spatiotemporal Variation in Phytoplankton Community Driven by Environmental Factors in the Northern East China Sea. Water 2020, 12, 2695. [Google Scholar] [CrossRef]
- Wu, J.; Li, L.; Wang, X.; Chen, J.; Lin, X.; Chen, Z. Monsoon-Driven Dynamics of Environmental Factors and Phytoplankton in Tropical Sanya Bay, South China Sea. Oceanol. Hydrobiol. Stud. 2011, 41, 57–66. [Google Scholar] [CrossRef]
- Behrenfeld, M.J.; Falkowski, P.G. Photosynthetic rates derived from satellite-based chlorophyll concentration. Limnol. Oceanogr. 1997, 42, 1–20. [Google Scholar] [CrossRef]
- Marañón, E.; Holligan, P.M.; Barciela, R.; González, N.; Mouriño, B.; Pazó, M.J.; Varela, M. Patterns of Phytoplankton Size Structure and Productivity in Contrasting Open-Ocean Environments. Mar. Ecol. Prog. Ser. 2001, 216, 43–56. [Google Scholar] [CrossRef]
- Sverdrup, H.U. On conditions for the vernal blooming of phytoplankton. ICES J. Mar. Sci. 1953, 18, 287–295. [Google Scholar] [CrossRef]
- Morel, A.; Huot, Y.; Gentili, B.; Werdell, P.J.; Hooker, S.B.; Franz, B.A. Examining the consistency of products derived from various ocean color sensors in open ocean (Case 1) waters in the perspective of a multi-sensor approach. Remote Sens. Environ. 2007, 111, 69–88. [Google Scholar] [CrossRef]
- Blondeau-Patissier, D.; Gower, J.F.R.; Dekker, A.G.; Phinn, S.R.; Brando, V.E. A Review of Ocean Color Remote Sensing Methods and Statistical Techniques for the Detection, Mapping and Analysis of Phytoplankton Blooms in Coastal and Open Oceans. Prog. Oceanogr. 2014, 122, 123–144. [Google Scholar] [CrossRef]
- Jiang, Z.; Chen, J.; Zhou, F.; Shou, L.; Chen, Q.; Tao, B.; Wang, K.; Yan, X.; Zeng, J.; Huang, W. Controlling factors of summer phytoplankton community in the Changjiang (Yangtze River) estuary and adjacent East China Sea shelf. Cont. Shelf Res. 2015, 101, 71–84. [Google Scholar] [CrossRef]
- Ning, X.; Chai, F.; Xue, H.; Shi, M.; Liu, Z.; Hao, Q.; Liu, C. Physical-biological oceanographic coupling influencing phytoplankton and primary production in the East China Sea. J. Geophys. Res. Oceans 2010, 115, C10009. [Google Scholar]
- Zhang, H.; Wang, S.; Qiu, Z.; Sun, D.; Ishizaka, J.; Sun, S.; He, Y. Phytoplankton size class in the East China Sea derived from MODIS satellite data. Biogeosciences 2018, 15, 4271–4289. [Google Scholar] [CrossRef]
- Gong, G.C.; Chang, J.; Chiang, K.P.; Hsiung, T.M.; Hung, C.C.; Duan, S.W.; Codispoti, L.A. Reduction of primary production and changing of nutrient ratio in the East China sea: Effect of the three gorges Dam? Geophys. Res. Lett. 2006, 33, L07610. [Google Scholar] [CrossRef]
- Wang, L.; Chen, Q.; Han, R.; Wang, B.; Tang, X. Responses of the phytoplankton community in the Yangtze River estuary and adjacent sea areas to the impoundment of the Three Gorges Reservoir. Ann. Limnol. Int. J. Limnol. 2017, 53, 1–10. [Google Scholar] [CrossRef]
- Mouw, C.B.; Barnett, A.; McKinley, G.A.; Gloege, L.; Pilcher, D. Phytoplankton size impact on export flux in the global ocean. Global Biogeochem. Cycles 2016, 30, 1542–1562. [Google Scholar] [CrossRef]
- Brewin, R.J.W.; Sathyendranath, S.; Hirata, T.; Lavender, S.J.; Barciela, R.M.; Hardman-Mountford, N.J. A three-component model of phytoplankton size class for the Atlantic Ocean. Ecol. Modell. 2010, 221, 1472–1483. [Google Scholar] [CrossRef]
- Cao, L.; Tang, R.; Huang, W.; Wang, Y. Seasonal variability and dynamics of coastal sea surface temperature fronts in the East China Sea. Ocean Dyn. 2021, 71, 237–249. [Google Scholar] [CrossRef]
- Polovina, J.J.; Howell, E.A.; Abecassis, M. Ocean’s least productive waters are expanding. Geophys. Res. Lett. 2008, 35, L03618. [Google Scholar] [CrossRef]
- Isobe, A. Recent advances in ocean-circulation research related to the Kuroshio and material transport in the East China Sea. J. Oceanogr. 2008, 64, 569–584. [Google Scholar] [CrossRef]
- Hobday, A.J.; Alexander, L.V.; Perkins, S.E.; Smale, D.A.; Straub, S.C.; Oliver, E.C.J.; Benthuysen, J.A.; Burrows, M.T.; Donat, M.G.; Feng, M.; et al. A hierarchical approach to defining marine heatwaves. Prog. Oceanogr. 2016, 141, 227–238. [Google Scholar] [CrossRef]
- Oliver, E.C.J.; Donat, M.G.; Burrows, M.T.; Moore, P.J.; Smale, D.A.; Alexander, L.V.; Benthuysen, J.A.; Feng, M.; Sen Gupta, A.S.; Hobday, A.J.; et al. Longer and more frequent marine heatwaves over the past century. Nat. Commun. 2018, 9, 1324. [Google Scholar] [CrossRef]
- Smale, D.A.; Wernberg, T.; Oliver, E.C.J.; Thomsen, M.; Harvey, B.P.; Straub, S.C.; Burrows, M.T.; Alexander, L.V.; Benthuysen, J.A.; Donat, M.G.; et al. Marine heatwaves threaten global biodiversity and the provision of ecosystem services. Nat. Clim. Change 2019, 9, 306–312. [Google Scholar] [CrossRef]
- Liu, X.; Xiao, W.; Landry, M.R.; Chiang, K.-P.; Chen, B.; Liu, H. Responses of Phytoplankton Communities to Environmental Variability in the East China Sea. Ecosystems 2016, 19, 832–849. [Google Scholar] [CrossRef]
- Sathyendranath, S.; Jackson, T.; Brockmann, C.; Brotas, V.; Calton, B.; Chuprin, A.; Clements, O.; Cipollini, P.; Danne, O.; Dingle, J.; et al. Colour Climate Change Initiative (Ocean_Colour_cci): Monthly Climatology of Global Ocean Colour Data Products at 4km Resolution, Version 6.0. Available online: https://catalogue.ceda.ac.uk/uuid/690fdf8f229c4d04a2aa68de67beb733/ (accessed on 20 November 2024).
- Falkowski, P.G.; Barber, R.T.; Smetacek, V. Biogeochemical controls and feedbacks on ocean primary production. Science 1998, 281, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Hirata, T.; Aiken, J.; Hardman-Mountford, N.; Smyth, T.J.; Barlow, R.G. An absorption model to determine phytoplankton size classes from satellite ocean colour. Remote Sens. Environ. 2008, 112, 3153–3159. [Google Scholar] [CrossRef]
- Breiman, L. Random Forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef]
- James, G.; Witten, D.; Hastie, T.; Tibshirani, R. An Introduction to Statistical Learning: With Applications in R, 1st ed.; Springer: New York, NY, USA, 2013. [Google Scholar]
- Good, S.; Fiedler, E.; Mao, C.; Martin, M.J.; Maycock, A.; Reid, R.; Roberts-Jones, J.; Searle, T.; Waters, J.; While, J.; et al. The current configuration of the OSTIA system for operational production of foundation sea surface temperature and ice concentration analyses. Remote Sens. 2020, 12, 720. [Google Scholar] [CrossRef]
- Maritorena, S.; d’Andon, O.H.F.; Mangin, A.; Siegel, D.A. Merged satellite ocean color data products using a bio-optical model: Characteristics, benefits and issues. Remote Sens. Environ. 2010, 114, 1791–1804. [Google Scholar] [CrossRef]
- Hersbach, H.; Bell, B.; Berrisford, P.; Hirahara, S.; Horányi, A.; Muñoz-Sabater, J.; Nicolas, J.; Peubey, C.; Radu, R.; Schepers, D.; et al. The ERA5 global reanalysis. Q. J. R. Meteorol. Soc. 2020, 146, 1999–2049. [Google Scholar] [CrossRef]
- Large, W.G.; Pond, S. Open ocean momentum flux measurements in moderate to strong winds. J. Phys. Oceanogr. 1981, 11, 324–336. [Google Scholar] [CrossRef]
- Aagaard, K.; Carmack, E.C. The role of sea Ice and other fresh water in the arctic circulation. J. Geophys. Res. 1989, 94, 14485–14498. [Google Scholar] [CrossRef]
- Nechad, B.; Ruddick, K.G.; Park, Y. Calibration and validation of a generic multisensor algorithm for mapping of total suspended matter in turbid waters. Remote Sens. Environ. 2010, 114, 854–866. [Google Scholar] [CrossRef]
- Finkel, Z.V.; Beardall, J.; Flynn, K.J.; Quigg, A.; Rees, T.A.V.; Raven, J.A. Phytoplankton in a Changing World: Cell Size and Elemental Stoichiometry. J. Plankton Res. 2010, 32, 119–137. [Google Scholar] [CrossRef]
- Yamaguchi, H.; Kim, H.-C.; Son, Y.B.; Kim, S.W.; Okamura, K.; Kiyomoto, Y.; Ishizaka, J. Seasonal and Summer Interannual Variations of SeaWiFS Chlorophyll a in the Yellow Sea and East China Sea. Prog. Oceanogr. 2012, 105, 22–29. [Google Scholar] [CrossRef]
- Furuya, K.; Hayashi, M.; Yabushita, Y.; Ishikawa, A. Phytoplankton Dynamics in the East China Sea in Spring and Summer as Revealed by HPLC-Derived Pigment Signatures. Deep Sea Res. Part II Top. Stud. Oceanogr. 2003, 50, 367–387. [Google Scholar] [CrossRef]
- Hastie, T.; Tibshirani, R.; Friedman, J. The Elements of Statistical Learning: Data Mining, Inference, and Prediction, 2nd ed.; Springer: New York, NY, USA, 2009; ISBN 978-0-387-84857-0. [Google Scholar]
- Kirk, J.T.O. Light and Photosynthesis in Aquatic Ecosystems, 3rd ed.; Cambridge University Press: Cambridge, UK, 2011; ISBN 9780521151757. [Google Scholar]
- Guo, J.; Liu, J.; Jing, Z.; Zhou, L.; Ke, Z.; Long, A.; Wang, J.; Ding, X.; Tan, Y. Monsoon-Driven Phytoplankton Community Succession in the Southern South China Sea. J. Geophys. Res. Oceans 2025, 130, e2024JC021698. [Google Scholar] [CrossRef]
- Cloern, J.E. Turbidity as a control on phytoplankton biomass and productivity in estuaries. Contin. Shelf Res. 1987, 7, 1367–1381. [Google Scholar] [CrossRef]
- Hirata, T.; Hardman-Mountford, N.J.; Brewin, R.J.W.; Aiken, J.; Barlow, R.; Suzuki, K.; Isada, T.; Howell, E.; Hashioka, T.; Noguchi-Aita, M.; et al. Synoptic relationships between surface Chlorophyll-a and diagnostic pigments specific to phytoplankton functional types. Biogeosciences 2011, 8, 311–327. [Google Scholar] [CrossRef]
- Uitz, J.; Claustre, H.; Morel, A.; Hooker, S.B. Vertical distribution of phytoplankton communities in open ocean: An assessment based on surface chlorophyll. J. Geophys. Res. Oceans 2006, 111, C08005. [Google Scholar] [CrossRef]
- Aller, R.C. Bioturbation and remineralization of sedimentary organic matter: Effects of redox oscillation. Chem. Geol. 1994, 114, 331–345. [Google Scholar] [CrossRef]
- Liu, S.M.; Zhang, J.; Li, D.J.; Wang, H. Nutrient budgets for large Chinese estuaries. Biogeosciences 2009, 6, 2245–2263. [Google Scholar] [CrossRef]
- Chisholm, S.W. Phytoplankton size. In Primary Productivity and Biogeochemical Cycles in the Sea; Falkowski, P.G., Woodhead, A.D., Eds.; Springer: Boston, MA, USA, 1992; pp. 213–237. ISBN 978-1-4899-0762-2. [Google Scholar]
- Liu, Q.; Kandasamy, S.; Lin, B.; Wang, H.; Chen, C.-T.A. Biogeochemical characteristics of suspended particulate matter in deep chlorophyll maximum layers in the southern East China Sea. Biogeosciences 2018, 15, 2091–2109. [Google Scholar] [CrossRef]
- Reynolds, C.S. The Ecology of Phytoplankton; Cambridge University Press: Cambridge, UK, 2006; ISBN 978-0-521-84413-0. [Google Scholar] [CrossRef]
- Finkel, Z.V.; Vaillancourt, C.J.; Irwin, A.J.; Reavie, E.D.; Smol, J.P. Environmental control of diatom community size structure varies across aquatic ecosystems. Proc. R. Soc. B 2009, 276, 1627–1634. [Google Scholar] [CrossRef] [PubMed]
- Boyce, D.G.; Lewis, M.R.; Worm, B. Global phytoplankton decline over the past century. Nature 2010, 466, 591–596. [Google Scholar] [CrossRef] [PubMed]
- IPCC. Climate Change 2023 Synthesis Report; Contribution of Working Groups I, II and III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; IPCC: Geneva, Switzerland, 2023; ISBN 978-92-9169-164-7. [Google Scholar]
- FAO. The State of Food and Agriculture 2020. Overcoming Water Challenges in Agriculture; FAO: Rome, Italy, 2020; ISBN 978-92-5-133441-6. [Google Scholar]
- Tréguer, P.J.; De La Rocha, C.L. The world ocean silica cycle. Ann. Rev. Mar. Sci. 2013, 5, 477–501. [Google Scholar] [CrossRef]
- Smayda, T.J. Harmful algal blooms: Their ecophysiology and general relevance to phytoplankton blooms in the sea. Limnol. Oceanogr. 1997, 42, 1137–1153. [Google Scholar] [CrossRef]
- Lee, M.; Kim, H.; Kim, S.; Kang, Y.; Yoo, S. Contrasting responses of phytoplankton productivity between coastal and offshore waters in the Yellow Sea. Front. Mar. Sci. 2020, 7, 572891. [Google Scholar]
- Sathyendranath, S.; Brewin, R.J.W.; Brockmann, C.; Brotas, V.; Calton, B.; Chuprin, A.; Cipollini, P.; Couto, A.B.; Dingle, J.; Doerffer, R.; et al. An ocean-colour time series for use in climate studies: The experience of the ocean-colour climate change initiative (OC-CCI). Sensors 2019, 19, 4285. [Google Scholar] [CrossRef]


| Date (yyyy-mm-dd) | Latitude | Longitude | In Situ Dominant PSC | Satellite Dominant PSC |
|---|---|---|---|---|
| 2018-05-04 | 32.000 | 126.505 | Pico | Pico |
| 2018-05-04 | 31.989 | 126.516 | Pico | Pico |
| 2018-05-04 | 32.003 | 125.889 | Nano | Pico |
| 2019-05-10 | 31.499 | 125.906 | Pico | Pico |
| 2019-05-10 | 31.493 | 125.879 | Nano | Pico |
| 2019-05-09 | 31.998 | 127.068 | Pico | Pico |
| 2019-05-08 | 32.000 | 126.486 | Micro | Pico |
| 2019-05-08 | 31.998 | 126.484 | Pico | Pico |
| 2019-05-08 | 31.498 | 126.492 | Pico | Pico |
| 2022-05-18 | 31.498 | 125.893 | Pico | Nano |
| 2022-05-18 | 32.000 | 127.072 | Pico | Pico |
| 2022-05-16 | 32.009 | 126.456 | Micro | Pico |
| 2022-05-16 | 31.997 | 125.885 | Micro | Pico |
| 2022-05-17 | 31.498 | 126.492 | Pico | Pico |
| 2023-05-02 | 32.012 | 125.887 | Pico | Nano |
| 2023-05-10 | 31.500 | 126.485 | Pico | Pico |
| 2023-05-02 | 31.500 | 126.495 | Pico | Pico |
| 2023-05-02 | 31.998 | 126.484 | Pico | Pico |
| 2018-08-06 | 32.007 | 125.895 | Pico | Nano |
| 2018-08-06 | 31.994 | 125.884 | Nano | Nano |
| 2018-08-05 | 31.502 | 127.072 | Pico | Pico |
| 2018-08-04 | 31.500 | 126.494 | Pico | Pico |
| 2018-08-04 | 31.501 | 126.492 | Pico | Pico |
| 2020-08-14 | 31.508 | 126.510 | Pico | Nano |
| 2020-08-14 | 31.485 | 126.489 | Pico | Pico |
| 2020-08-14 | 31.501 | 125.888 | Pico | Pico |
| 2020-08-15 | 32.000 | 127.071 | Pico | Pico |
| 2020-08-15 | 32.000 | 127.070 | Pico | Pico |
| 2020-08-15 | 31.504 | 127.070 | Pico | Pico |
| 2021-08-28 | 32.000 | 125.888 | Micro | Nano |
| 2021-08-28 | 31.970 | 125.909 | Pico | Nano |
| 2021-08-28 | 31.502 | 127.014 | Pico | Pico |
| 2021-08-31 | 31.998 | 126.484 | Nano | Nano |
| 2022-08-22 | 32.003 | 127.076 | Pico | Pico |
| 2022-08-22 | 32.000 | 127.082 | Pico | Pico |
| 2022-08-23 | 31.498 | 126.492 | Micro | Pico |
| 2022-08-23 | 31.500 | 125.892 | Pico | Pico |
| 2023-08-28 | 31.505 | 127.077 | Pico | Pico |
| 2023-08-27 | 31.499 | 126.494 | Pico | Pico |
| 2023-08-27 | 31.501 | 125.885 | Nano | Pico |
| 2023-08-26 | 31.500 | 125.892 | Pico | Pico |
| 2023-08-26 | 31.997 | 125.885 | Pico | Pico |
| 2023-08-26 | 31.504 | 127.070 | Pico | Pico |
| 30/43 (69.8%) of matched pixels | ||||
| Pico | Coef. (βi) | Std. Err. | t | p > |t| |
| Const. | 0.0000 | 0.0233 | 0.0000 | 1.0000 |
| SST | 0.0837 | 0.0319 | 2.6264 | 0.0089 |
| FWC | 0.1924 | 0.0348 | 5.5252 | 0.0000 |
| MLD | 0.0941 | 0.0319 | 2.9442 | 0.0034 |
| Wind stress | −0.0955 | 0.0245 | −3.9065 | 0.0001 |
| PAR | −0.0763 | 0.0290 | −2.6292 | 0.0088 |
| Zeu | 0.6494 | 0.0326 | 19.8920 | 0.0000 |
| SPM | −0.1985 | 0.0336 | −5.9006 | 0.0000 |
| Nano | Coef. (βi) | Std. Err. | t | p > |t| |
| Const. | 0.0000 | 0.0272 | 0.0000 | 1.0000 |
| SST | −0.1010 | 0.0372 | −2.7181 | 0.0068 |
| FWC | −0.1743 | 0.0406 | −4.2915 | 0.0000 |
| MLD | −0.0236 | 0.0373 | −0.6336 | 0.5266 |
| Wind stress | 0.0849 | 0.0285 | 2.9782 | 0.0030 |
| PAR | 0.0985 | 0.0338 | 2.9115 | 0.0037 |
| Zeu | −0.6891 | 0.0381 | −18.1003 | 0.0000 |
| SPM | 0.0613 | 0.0392 | 1.5625 | 0.1187 |
| Micro | Coef. (βi) | Std. Err. | t | p > |t| |
| Const. | 0.0000 | 0.0308 | 0.0000 | 1.0000 |
| SST | 0.0242 | 0.0421 | 0.5739 | 0.5663 |
| FWC | −0.1388 | 0.0460 | −3.0151 | 0.0027 |
| MLD | −0.2744 | 0.0422 | −6.5001 | 0.0000 |
| Wind stress | 0.0742 | 0.0323 | 2.2978 | 0.0219 |
| PAR | −0.0437 | 0.0384 | −1.1386 | 0.2554 |
| Zeu | −0.1299 | 0.0431 | −3.0116 | 0.0027 |
| SPM | 0.5407 | 0.0445 | 12.1608 | 0.0000 |
| Variable | Slope | Significance (p-Value) | Annual Change (%) | Trend |
|---|---|---|---|---|
| Pico (%) | 0.0032 | <0.01 | +2.4 | Increasing |
| Nano (%) | −0.0029 | <0.01 | −2.2 | Decreasing |
| Micro (%) | −0.0004 | <0.01 | −0.4 | Decreasing |
| Chl-a (mg/m3) | 0.0000 | ≥0.05 | ||
| PAR (E/m2/d) | −0.0003 | <0.05 | −0.3 | Decreasing |
| SST (°C) | 0.0001 | <0.05 | +0.1 | Increasing |
| Zeu (m) | 0.0004 | ≥0.05 | ||
| FWC (m) | 0.0000 | ≥0.05 | ||
| MLD (m) | 0.0001 | ≥0.05 | ||
| Wind stress (N/m2) | 0.0000 | ≥0.05 | ||
| SPM (g/m3) | 0.0000 | <0.01 | −0.3 | Decreasing |
| Variables | Non-MHW Periods | MHW Periods | Relative Increase (%) | Significance |
|---|---|---|---|---|
| Pico | 65.0 ± 27.2% | 91.7 ± 15.2% | 41.2% | p < 0.01 |
| Nano | 31.7 ± 23.3% | 7.8 ± 14.1% | −75.2% | p < 0.01 |
| Micro | 3.4 ± 8.6% | 0.4 ± 1.4% | −87.5% | p < 0.01 |
| Chl-a | 0.60 ± 0.33 mg/m3 | 0.32 ± 0.13 mg/m3 | −46.5% | p < 0.01 |
| PAR | 42.78 ± 8.95 E/m2/d | 43.39 ± 6.0 E/m2/d | 1.4% | p < 0.01 |
| SST | 27.89 ± 1.34 °C | 29.20 ± 0.71 °C | 4.7% | p < 0.01 |
| Zeu | 77.74 ± 15.67 m | 98.26 ± 11.88 m | 26.4% | p < 0.01 |
| FWC | 1.65 ± 0.4 m | 1.59 ± 0.23 m | −3.9% | n.s. |
| MLD | 18.04 ± 6.94 m | 19.4 ± 3.87 m | 7.6% | n.s. |
| Wind stress | 0.0076 ± 0.0036 N/m2 | 0.0072 ± 0.0034 N/m2 | −4.8% | n.s. |
| SPM | 0.0024 ± 0.0012 g/m3 | 0.0016 ± 0.0001 g/m3 | −35.4% | n.s. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, J.-W.; Joo, H.; Jang, H.K.; Kang, J.J.; Lee, J.-S.; Kim, C. Key Environmental Drivers of Summer Phytoplankton Size Class Variability and Decadal Trends in the Northern East China Sea. Remote Sens. 2025, 17, 1954. https://doi.org/10.3390/rs17111954
Park J-W, Joo H, Jang HK, Kang JJ, Lee J-S, Kim C. Key Environmental Drivers of Summer Phytoplankton Size Class Variability and Decadal Trends in the Northern East China Sea. Remote Sensing. 2025; 17(11):1954. https://doi.org/10.3390/rs17111954
Chicago/Turabian StylePark, Jung-Woo, Huitae Joo, Hyo Keun Jang, Jae Joong Kang, Joon-Soo Lee, and Changsin Kim. 2025. "Key Environmental Drivers of Summer Phytoplankton Size Class Variability and Decadal Trends in the Northern East China Sea" Remote Sensing 17, no. 11: 1954. https://doi.org/10.3390/rs17111954
APA StylePark, J.-W., Joo, H., Jang, H. K., Kang, J. J., Lee, J.-S., & Kim, C. (2025). Key Environmental Drivers of Summer Phytoplankton Size Class Variability and Decadal Trends in the Northern East China Sea. Remote Sensing, 17(11), 1954. https://doi.org/10.3390/rs17111954






