Early Detection of Pine Wilt Disease by Combining Pigment and Moisture Content Indices Using UAV-Based Hyperspectral Imagery
Abstract
1. Introduction
2. Materials and Methods
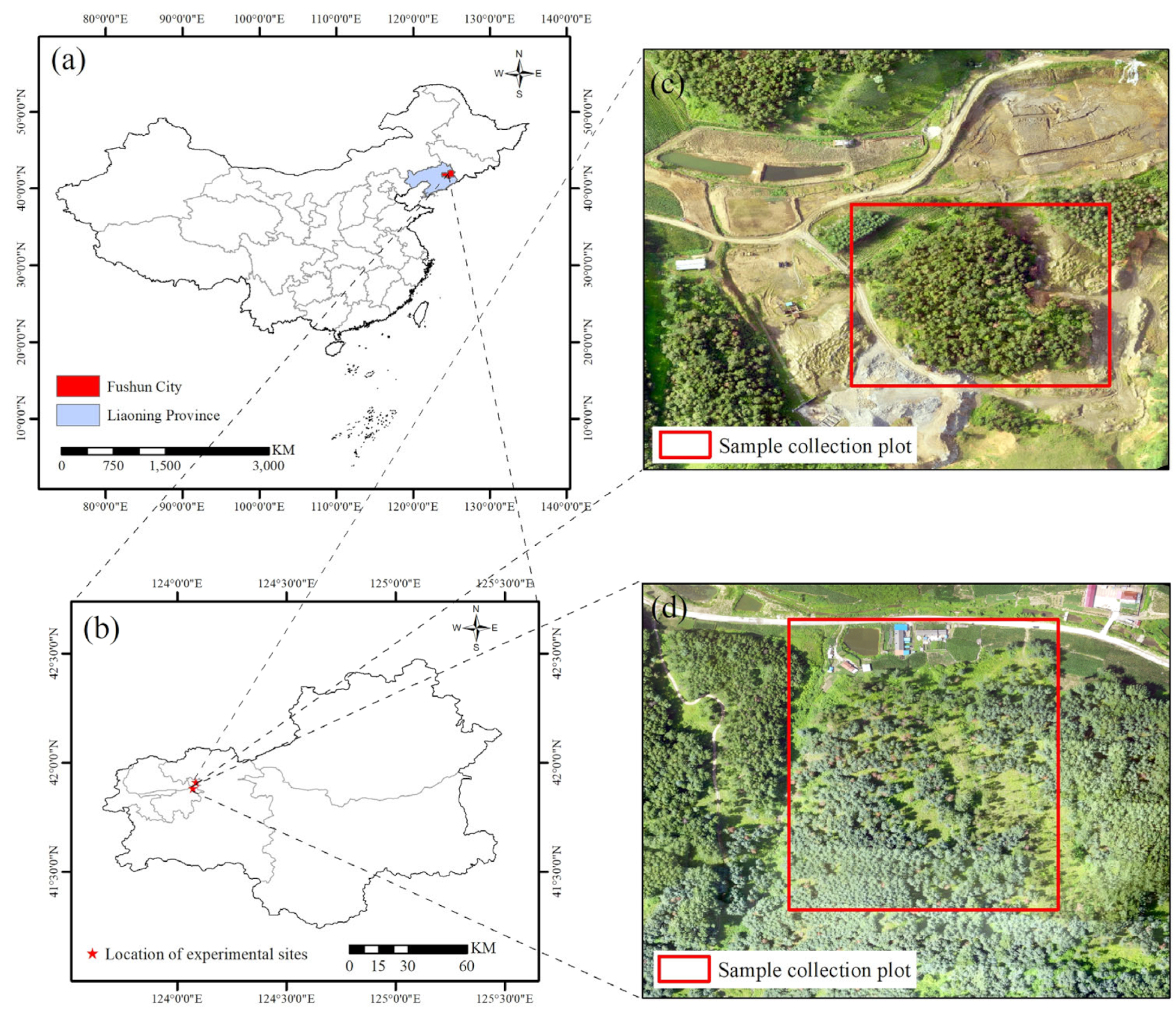
2.1. Study Site
2.2. Data Acquisition and Preprocessing
2.2.1. Ground Data Collection
2.2.2. Ground ASD Spectral Data Acquisition and Processing
2.2.3. Physiological Parameter Data Acquisition and Processing
2.2.4. UAV Hyperspectral Remote Sensing Imagery Data Acquisition
2.2.5. UAV Hyperspectral Remote Sensing Imagery Data Processing
2.3. Methods
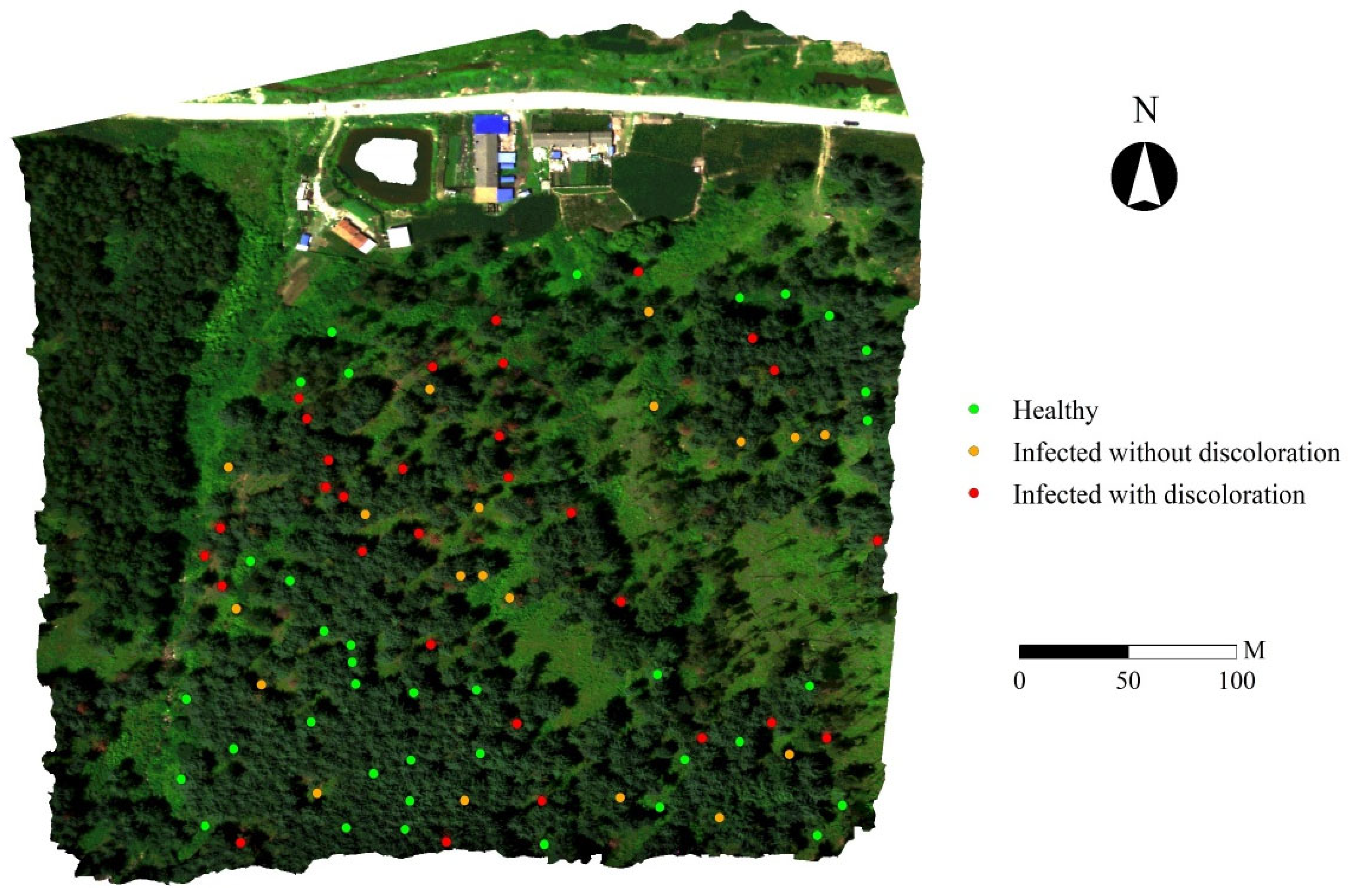
2.3.1. Selection of Representative Pixels from UAV Hyperspectral Imagery
- (1)
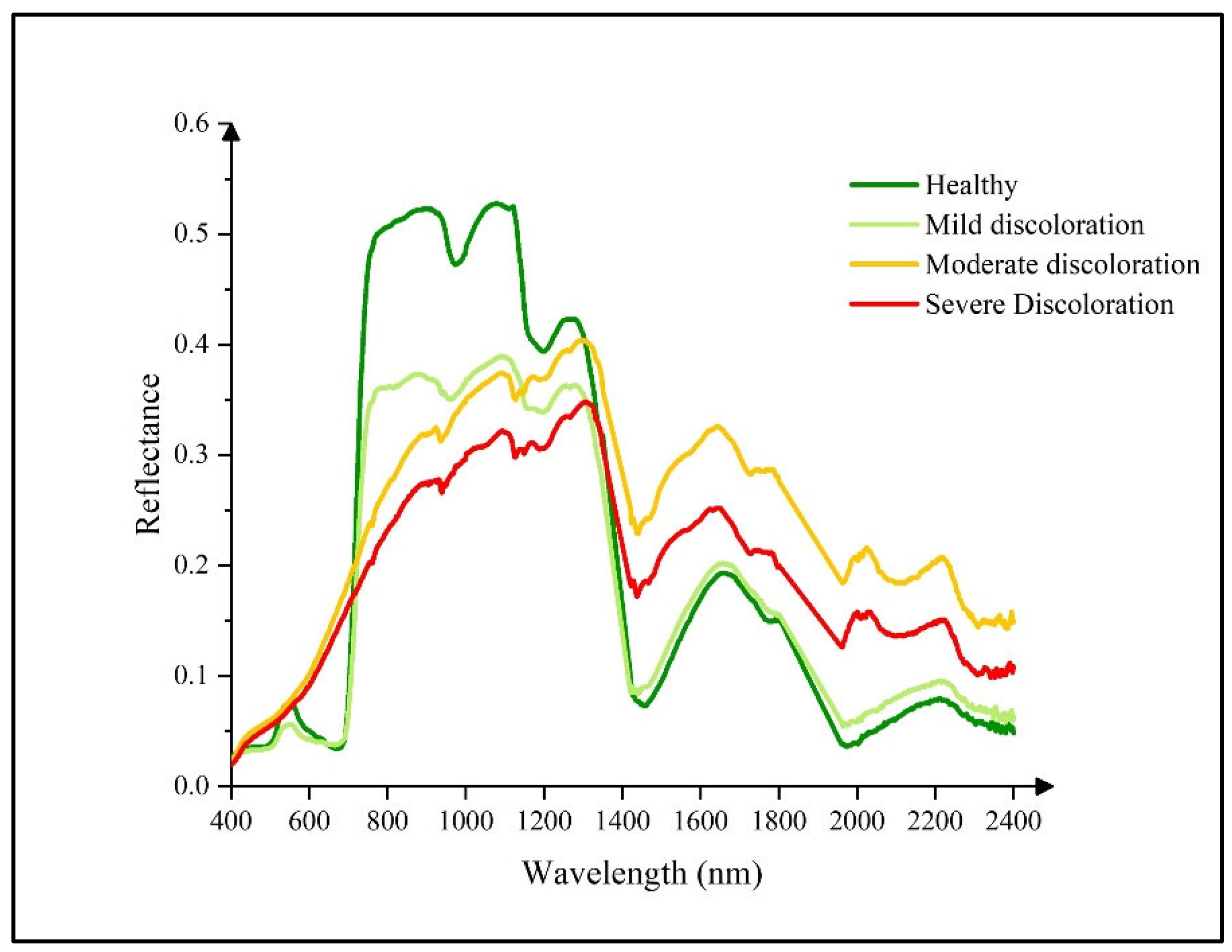
- Healthy: Based on field observations, regions of interest (ROIs) corresponding to healthy pine trees were identified in the July hyperspectral imagery. These ROIs exclusively comprised green pixels with no signs of discoloration. Furthermore, to ensure temporal consistency, only trees that exhibited no visible discoloration across all available temporal image sequences were included in this category.
- (2)
- Infected without discoloration: ROIs in this category were retrospectively selected by integrating field survey data with multi-temporal UAV imagery. These ROIs consisted entirely of green, non-discolored pixels and met at least one of the following criteria: field observations indicated that discoloration had initiated in the lower canopy, while the upper canopy remained green or trees appeared green in the July imagery but exhibited visible discoloration (red or yellow-green tones) in later imagery (e.g., from August or October). Pixel sets were extracted from the earlier time points—prior to the onset of visible symptoms—for analysis as “infected without discoloration”.
- (3)
- Infected with discoloration: This category included ROIs representing trees with visible signs of infection as confirmed by field observations. In the July hyperspectral imagery, these ROIs were composed entirely of red or yellow-green pixels, indicative of advanced disease progression.
2.3.2. Construction of an Indicator Set for Identifying Disease Stages in Infected Trees
2.3.3. Optimal Indicator Analysis and Disease Stage Monitoring Model Construction
- (1)
- The optimal classification threshold was determined for each individual indicator. A scatter plot of the indicators in a two-dimensional plane was generated to analyze the distribution pattern of the samples. The one-dimensional feature threshold segmentation method was employed to search for the classification threshold α within the indicator range corresponding to the two categories. The step size for searching α was adaptively adjusted according to the indicator accuracy. The goal was to minimize the intra-group distance and maximize the inter-group distance. The discrimination index was then constructed, as follows [61]:
- (2)
- The optimal decision boundary was obtained based on combined indicators. A scatter plot of the indicators and the 95% confidence ellipses for each class of samples were plotted in the two-dimensional plane. The sample distribution was analyzed, and Linear Discriminant Analysis (LDA) was applied to reduce dimensionality and to classify the data [63]. To maximize the differences between different classes of samples, the discriminant index (generalized Rayleigh quotient) was calculated, as follows [62]:
- (3)
- The single-indicator and composite-indicator approaches were applied to classify pine trees at different infection stages. Using the optimally validated segmentation thresholds derived previously, each pixel in the UAV hyperspectral imagery was assigned an infection-stage label. To improve classification reliability, the following spatial consistency check was then applied: if all neighboring pixels around a target pixel were assigned a different class than the target, then the target pixel was flagged as anomalous and its label reassigned to the majority class of its neighbors; otherwise, the original classification was retained.
2.3.4. Accuracy Evaluation
3. Results
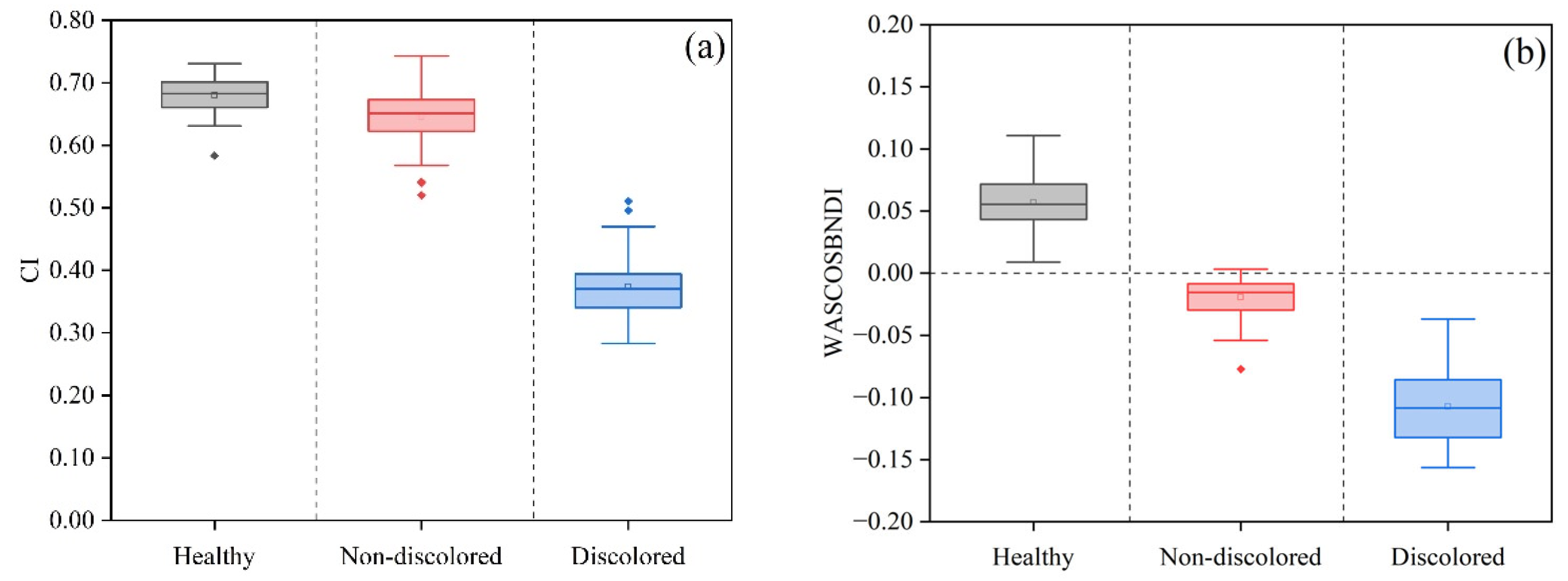
3.1. Construction of Disease Stage Identification Indicator Set and Selection of Optimal Indicators
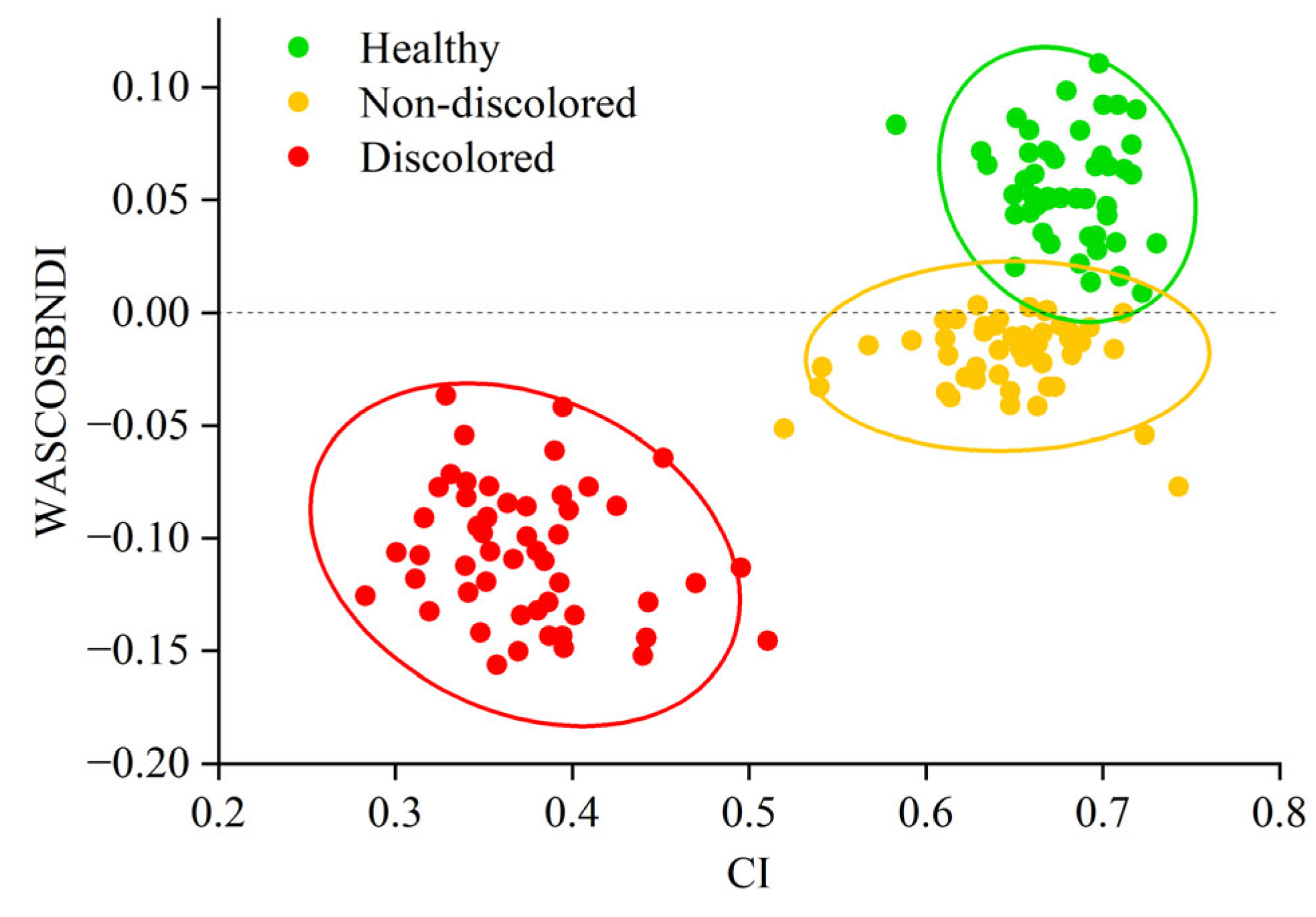
3.2. Construction of PWD Stage Identification Model for Infected Trees
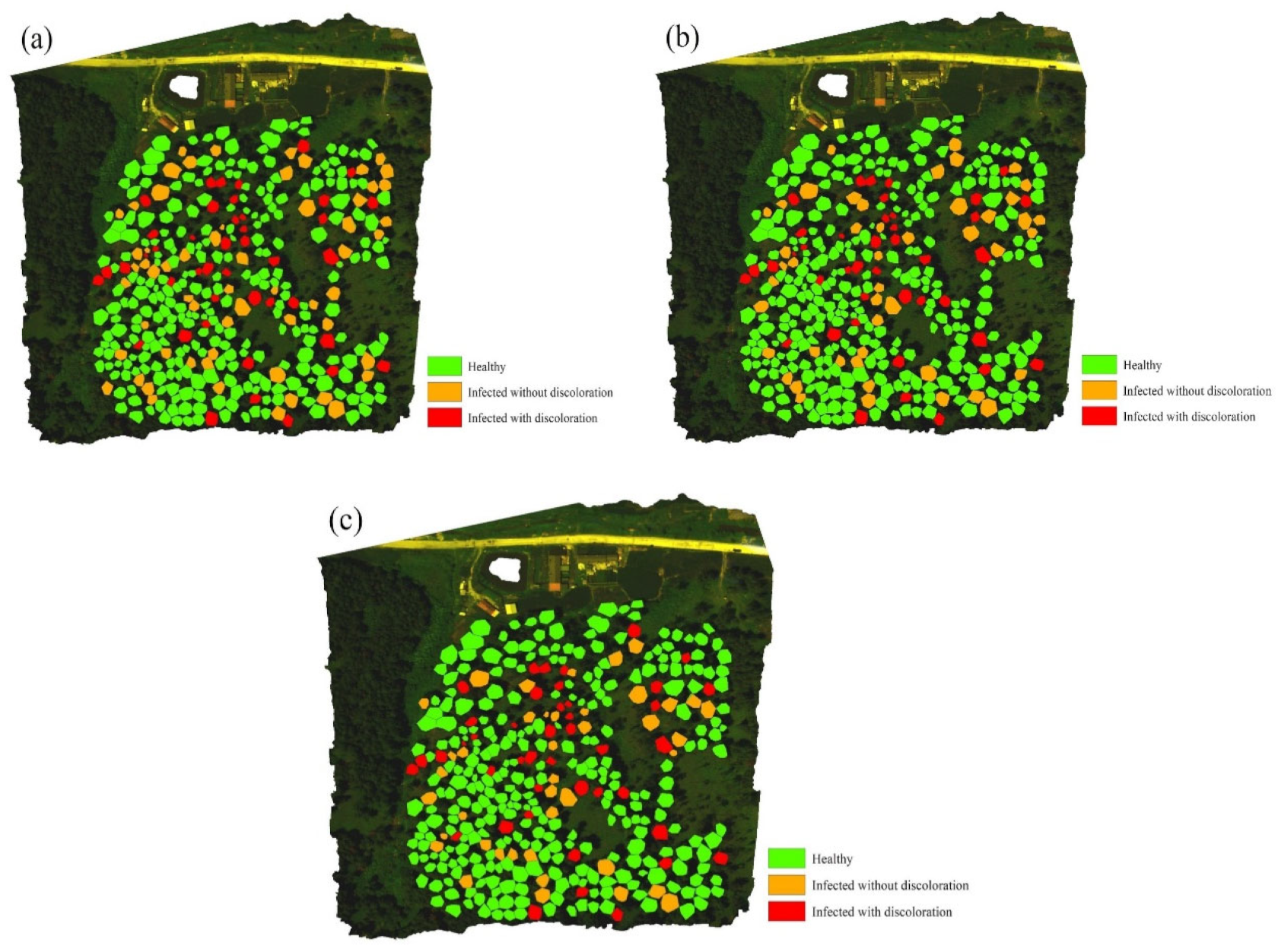
3.3. Early Detection and Accuracy Evaluation of PWD Infected Trees
4. Discussion
4.1. Optimal Indicator Selection for Early Recognition of Infected Trees
4.2. Analysis of Collaborative Recognition Effect Based on Canopy Pigment-Water Content Indicators
4.3. Analysis of Early Recognition Model and Its Relationship with Disease Severity and Onset Time
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vicente, C.; Espada, M.; Vieira, P.; Mota, M. Erratum to: Pine wilt disease: A threat to European forestry. Eur. J. Plant Pathol. 2012, 133, 497. [Google Scholar] [CrossRef]
- Douda, O.; Zouhar, M.; Maňasová, M.; Dlouhý, M.; Lišková, J.; Ryšánek, P. Hydrogen cyanide for treating wood against pine wood nematode (Bursaphelenchus xylophilus): Results of a model study. J. Wood Sci. 2015, 61, 204–210. [Google Scholar] [CrossRef]
- Tao, H.; Li, C.; Zhao, D.; Deng, S.; Hu, H.; Xu, X.; Jing, W. Deep learning-based dead pine trees detection from unmanned aerial vehicle images. Int. J. Remote Sens. 2020, 41, 8238–8255. [Google Scholar] [CrossRef]
- Syifa, M.; Park, S.-J.; Lee, C.-W. Detection of the pine wilt disease tree candidates for drone remote sensing using artificial intelligence techniques. Engineering 2020, 6, 919–926. [Google Scholar] [CrossRef]
- Liu, W.; Chang, Q.; Guo, M.; Xing, D.; Yuan, Y. Monitoring of leaf nitrogen content in summer corn with first derivative of spectrum based on modified red edge. J. Northwest A F Univ. 2010, 38, 91–98. [Google Scholar] [CrossRef]
- Pan, L.; Li, Y.; Liu, Z.; Meng, F.; Chen, J.; Zhang, X. Isolation and identification of pine wood nematode in Pinus koraiensis in Fengcheng, Liaoning Province. Forest Pest Dis. 2019, 38, 1–4. [Google Scholar] [CrossRef]
- Hyun, M.W.; Kim, J.H.; Suh, D.Y.; Lee, S.K.; Kim, S.H. Fungi isolated from pine wood nematode, its vector Japanese pine sawyer, and the nematode-infected Japanese black pine wood in Korea. Mycobiology 2007, 35, 159–161. [Google Scholar] [CrossRef]
- Firtha, F. Development of data reduction function for hyperspectral imaging. Prog. Agric. Eng. Sci. 2007, 3, 67–88. [Google Scholar] [CrossRef]
- Näsi, R.; Honkavaara, E.; Lyytikäinen-Saarenmaa, P.; Blomqvist, M.; Litkey, P.; Hakala, T.; Viljanen, N.; Kantola, T.; Tanhuanpää, T.; Holopainen, M. Using UAV-based photogrammetry and hyperspectral imaging for mapping bark beetle damage at tree-level. Remote Sens. 2015, 7, 15467–15493. [Google Scholar] [CrossRef]
- Du, H.; Ge, H.; Fan, Y.; Jin, W.; Li, J. Application of fractal theory in hyperspectral detecting the early stage of pine wood nematode disease (Bursaphelenchus xylophilus) of Pinus massoniana with hyperspectrum. Sci. Silv. Sin. 2009, 45, 68–76. [Google Scholar] [CrossRef]
- Shen, P.; Shao, Y. UAV + hyperspectral brings new opportunities for forest pest and disease remote sensing monitoring. Chin. J. Geod. Geodyn. 2022, 2022, 76–77. [Google Scholar]
- Zhao, H.; Shu, Q.; Wang, K.; Yuan, Z.; Tan, D. Application of Hyperspectral Remote Sensing Technology in Forest Pest and Disease Monitoring. Green Sci. Technol. 2020, 19, 145–148. [Google Scholar] [CrossRef]
- Huang, H.; Ma, X.; Hu, L.; Huang, Y.; Huan, H. Preliminary application of Fast R-CNN deep learning combined with UAV remote sensing in the monitoring of pine wilt disease. J. Environ. Entomol. 2021, 43, 1295–1303. [Google Scholar]
- Li, F.; Shen, W.; Wu, J.; Sun, F.; Xu, L.; Liu, Z.; Lan, P. Research on pine wilt disease tree detection based on YOLOv3-CIoU. J. Shandong Agric. Univ. (Nat. Sci. Ed.) 2021, 52, 224–233. [Google Scholar]
- Wang, C.; Zhang, H.; Le, J.; Zhao, S. Pine wilt disease detection using deep learning and remote sensing imagery. J. Nanjing Norm. Univ. (Nat. Sci. Ed.) 2021, 44, 84–89. [Google Scholar]
- Zhang, P.; Wang, Z.; Rao, Y.; Zheng, J.; Zhang, N.; Wang, D.; Zhu, J.; Fang, Y.; Gao, X. Identification of pine wilt disease infected wood using UAV RGB imagery and improved YOLOv5 models integrated with attention mechanisms. Forests 2023, 14, 588. [Google Scholar] [CrossRef]
- Jie, Y.; Ruirui, Z.; Joonwhoan, L. A deep learning-based generalized system for detecting pine wilt disease using RGB-based UAV images. Remote Sens. 2021, 14, 150. [Google Scholar] [CrossRef]
- Han, Z.; Hu, W.; Peng, S.; Lin, H.; Zhang, J.; Zhou, J.; Wang, P.; Dian, Y. Detection of standing dead trees after pine wilt disease outbreak with airborne remote sensing imagery by multi-scale spatial attention deep learning and Gaussian kernel approach. Remote Sens. 2022, 14, 3075. [Google Scholar] [CrossRef]
- Choi, I.W.; Park, Y. Monitoring, assessment, and management of forest insect pests and diseases. Forests 2019, 10, 865. [Google Scholar] [CrossRef]
- Duarte, A.; Borralho, N.; Cabral, P.; Caetano, M. Recent advances in forest insect pests and diseases monitoring using UAV-based data: A systematic review. Forests 2022, 13, 911. [Google Scholar] [CrossRef]
- Bai, X.; Zhang, X.; Zhang, N.; Ma, Y. Hyperspectral remote sensing monitoring model for the degree of damage by the pine caterpillar. J. Beijing For. Univ. 2016, 38, 16–22. [Google Scholar] [CrossRef]
- Chen, P.; Xie, Y.; Liu, S.; Zhang, L. Exploration of Real-Time Monitoring Technology for Forest Pests and Diseases Using UAV Remote Sensing. Sci. Technol. Innov. 2020, 34, 60–61. [Google Scholar]
- Dong, Y.; Yan, H.; Pan, J.; Zhang, Y.; Guan, Z.; Yan, J. Control strategies for pine wood nematode disease in China. Chin. J. For. Pest Dis. 2022, 41, 1–8. [Google Scholar] [CrossRef]
- Hu, L.; Wu, X. Research progress on the mechanisms of pine tree resistance to pine wood nematode disease. Life Sci. 2018, 30, 659–666. [Google Scholar] [CrossRef]
- Xu, H.; Luo, Y.; Zhang, Q. Changes in water content, pigments, and antioxidant enzyme activities in pine needles of Pinus thunbergii and Pinus massoniana affected by pine wood nematode. Sci. Silvae Sin. 2012, 11, 140–143. [Google Scholar]
- Li, X.; Huang, H.; Huang, Y.; Fan, J.; Wu, H.; Wu, J. Study on the changes in disease process characteristics of pine wood nematode. Guangdong For. Sci. Technol. 2010, 26, 92–96. [Google Scholar]
- Xiang, R.; Yuan, L.; Qin, L.; Song, Q.; Zhang, J.; Qu, Y.; Zhou, J. Correlation analysis between spectral characteristic parameters and chlorophyll content of pine needles. Sci. Technol. Innov. 2018, 35, 5–7. [Google Scholar]
- Hu, H. UAV Remote Sensing Monitoring Technology: Building a New Line of Defense for Pest and Disease Control with Intelligent Technology—Taking the Application of UAV Remote Sensing Monitoring Technology in the Detection and Survey of Pine Wilt Disease as an Example. China For. Ind. 2023, 6, 24–25. [Google Scholar]
- Wang, Z.; Zhang, X.; An, S. Spectral characteristics of pine trees in Pinus massoniana forests affected by pine wood nematode disease. Remote Sens. Technol. Appl. 2007, 3, 367–370. [Google Scholar]
- Huang, M.; Gong, J.; Li, S.; Zhang, B.; Hao, Q. Study on hyperspectral time series and sensitive features for pine wood nematode disease. Remote Sens. Technol. Appl. 2012, 27, 954–960. [Google Scholar] [CrossRef]
- Pan, J.; Xie, T.; You, C.; Xia, X. Dynamic Analysis of Early Stage Pine Wilt Disease in Pinus massoniana Using Ground-level Hyperspectral Imaging. Forest Sci. 2023, 69, 529–537. [Google Scholar] [CrossRef]
- Carter, G.A.; Miller, R.L. Early detection of plant stress by digital imaging within narrow stress-sensitive wavebands. Remote Sens. Environ. 1994, 50, 295–302. [Google Scholar] [CrossRef]
- Carter, G.A.; Cibula, W.G.; Miller, R.L. Narrow-band reflectance imagery compared with thermal imagery for early detection of plant stress. J. Plant Physiol. 1996, 148, 515–522. [Google Scholar] [CrossRef]
- Jung, K.Y.; Park, J.K. Analysis of vegetation infection information using unmanned aerial vehicle with optical sensor. Sensor Mater. 2019, 31, 3319–3326. [Google Scholar] [CrossRef]
- Huang, B. Monitoring Bursaphelenchus xylophilus with multispectrum camera in UAV. Guangxi For. Sci. 2020, 49, 380–384. [Google Scholar] [CrossRef]
- Iordache, M.-D.; Mantas, V.; Baltazar, E.; Pauly, K.; Lewyckyj, N. A machine learning approach to detecting pine wilt disease using airborne spectral imagery. Remote Sens. 2020, 12, 2280. [Google Scholar] [CrossRef]
- Liu, W.; Xie, Z.; Du, J.; Li, Y.; Long, Y.; Lan, Y.; Liu, T.; Sun, S.; Zhao, J. Early Detection of Pine Wilt Disease Based on UAV Reconstructed Hyperspectral Image. Front. Plant Sci. 2024, 15, 1453761. [Google Scholar] [CrossRef]
- Zeng, Q.; Pu, Y.; Xiao, Y.; Yang, S.; Yang, Y.; Wang, X.; Xie, T.; Man, J.; Jia, Y. Early monitoring of southern Sichuan epidemic wood forest area based on UAV hyperspectral. Sichuan For. Sci. Technol. 2024, 45, 115–122. [Google Scholar]
- Li, H.; Chen, L.; Yao, Z.; Li, N.; Long, L.; Zhang, X. Intelligent Identification of Pine Wilt Disease Infected Individual Trees Using UAV-Based Hyperspectral Imagery. Remote Sens. 2023, 15, 3295. [Google Scholar] [CrossRef]
- Wu, W.; Zhang, Z.; Zheng, L.; Han, C.; Wang, X.; Xu, J.; Wang, X. Research progress on the early monitoring of pine wilt disease using hyperspectral techniques. Sensors 2020, 20, 3729. [Google Scholar] [CrossRef]
- Duan, G. Derivation of the Arnon formula for chlorophyll content determination. Plant Physiol. Commun. 1992, 3, 221. [Google Scholar] [CrossRef]
- Cubert-GmbH Hyperspectral Firefleye X20P. Available online: http://cubert-gmbh.de/ (accessed on 15 October 2020).
- Wang, Y.; Wang, Y. Application Analysis of UAV Remote Sensing Technology in Forestry Resource Survey and Monitoring. Agric. Technol. 2018, 38, 167. [Google Scholar]
- Yu, R.; Huo, L.; Huang, H.; Yuan, Y.; Gao, B.; Liu, Y.; Yu, L.; Li, H.; Yang, L.; Ren, L.; et al. Early Detection of Pine Wilt Disease Tree Candidates Using Time-Series of Spectral Signatures. Front. Plant Sci. 2022, 13, 1000093. [Google Scholar] [CrossRef]
- Li, K.; Yang, S. Image smoothing and denoising based on the Savitzky–Golay algorithm. Data Acquis. Process. 2010, 25, 72–74. [Google Scholar] [CrossRef]
- Kim, S.-R.; Lee, W.-K.; Lim, C.-H.; Kim, M.; Kafatos, M.C.; Lee, S.-H.; Lee, S.-S. Hyperspectral analysis of pine wilt disease to determine an optimal detection index. Forests 2018, 9, 115. [Google Scholar] [CrossRef]
- Li, N.; Huo, L.; Zhang, X. Classification of pine wilt disease at different infection stages by diagnostic hyperspectral bands. Ecol. Indic. 2022, 142, 109198. [Google Scholar] [CrossRef]
- Datt, B. Visible/near infrared reflectance and chlorophyll content in Eucalyptus leaves. Int. J. Remote Sens. 1999, 20, 2741–2759. [Google Scholar] [CrossRef]
- Blackburn, G.A. Spectral indices for estimating photosynthetic pigment concentrations: A test using senescent tree leaves. Int. J. Remote Sens. 1998, 19, 657–675. [Google Scholar] [CrossRef]
- Hunt, E.R., Jr.; Rock, B.N. Detection of changes in leaf water content using near- and middle-infrared reflectances. Remote Sens. Environ. 1989, 30, 43–54. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Merzlyak, M.N.; Chivkunova, O.B. Optical properties and nondestructive estimation of anthocyanin content in plant leaves. Photochem. Photobiol. 2001, 74, 38–45. [Google Scholar] [CrossRef]
- Ashourloo, D.; Mobasheri, M.R.; Huete, A. Developing two spectral disease indices for detection of wheat leaf rust (Puccinia triticina). Remote Sens. 2014, 6, 4723–4740. [Google Scholar] [CrossRef]
- Gamon, J.; Penuelas, J.; Field, C. A narrow-waveband spectral index that tracks diurnal changes in photosynthetic efficiency. Remote Sens. Environ. 1992, 41, 35–44. [Google Scholar] [CrossRef]
- Penuelas, J.; Pinol, J.; Ogaya, R.; Filella, I. Estimation of plant water concentration by the reflectance water index WI (R900/R970). Int. J. Remote Sens. 1997, 18, 2869–2875. [Google Scholar] [CrossRef]
- Hardisky, M.A.; Smart, R.M.; Klemas, V. Seasonal spectral characteristics and aboveground biomass of the tidal marsh plant, Spartina alterniflora. Photogramm. Eng. Remote Sens. 1983, 49, 85–92. [Google Scholar]
- Raj, R.; Walker, J.P.; Vinod, V.; Pingale, R.; Naik, B.; Jagarlapudi, A. Leaf water content estimation using top-of-canopy airborne hyperspectral data. Int. J. Appl. Earth Observ. Geoinf. 2021, 102, 104024. [Google Scholar] [CrossRef]
- Liu, Q.; Qu, Z.; Hu, X.; Bai, Y.; Yang, W.; Yang, Y.; Bian, J.; Zhang, D.; Shi, L. Combining UAV remote sensing data to estimate daily-scale crop water stress index: Enhancing diagnostic temporal representativeness. Agric. Water Manag. 2024, 305, 109130. [Google Scholar] [CrossRef]
- Liu, S.; Wang, Q.; Wei, W.; Tang, Q.; Liu, L.; He, H.; Lu, J. Recognition of pine wilt disease trees in high-resolution images based on multi-feature analysis. Green Sci. Technol. 2021, 23, 237–240. [Google Scholar] [CrossRef]
- Li, H.; Yu, L.; Zhan, Z.; Chi, S.; Ren, L.; Luo, Y. Effects of stand and landscape-level factors on the occurrence of pine wilt disease: A case study of pine forests in Weihai, Shandong. J. Environ. Entomol. 2024, 46, 616–624. [Google Scholar]
- Luo, J.; Yang, Y.; Shi, B. Two-dimensional Otsu multi-threshold image segmentation based on improved adaptive differential evolution algorithm. Electron. Info Tech. 2019, 41, 2017–2024. [Google Scholar] [CrossRef]
- Mohamed, A.; Reda, M.; Mohamed, A. A new fusion of whale optimizer algorithm with Kapur’s entropy for multi-threshold image segmentation: Analysis and validations. Artif. Intell. Rev. 2022, 55, 71. [Google Scholar] [CrossRef]
- Xu, M.; Chen, S.; Gao, X.; Ye, Q.; Ke, Y.; Huo, C.; Liu, X. Research on fast multi-threshold image segmentation technique using histogram analysis. Electronics 2023, 12, 3785. [Google Scholar] [CrossRef]
- Ding, G.; Huang, S. Image retrieval combining multi-feature and linear discriminant analysis. Comput. Appl. Softw. 2024, 41, 212–218. [Google Scholar]
- Xie, C.; Qin, Y.; Zhang, K.; Wang, X. Pseudo 3D residual network based on minimum intra-class variance. J. Comput. Appl. Res. 2021, 38, 3801–3807. [Google Scholar] [CrossRef]
- Li, J.; Wang, X.; Zhao, H.; Hu, X.; Zhong, Y. Detecting Pine Wilt Disease at the Pixel Level from High Spatial and Spectral Resolution UAV-Borne Imagery in Complex Forest Landscapes Using Deep One-Class Classification. Int. J. Appl. Earth Obs. Geoinf. 2022, 112, 102947. [Google Scholar] [CrossRef]
- Xie, W.; Wang, H.; Liu, W.; Zang, H. Early-Stage Pine Wilt Disease Detection via Multi-Feature Fusion in UAV Imagery. Forests 2024, 15, 171. [Google Scholar] [CrossRef]
- Xia, L.; Zhang, R.; Chen, L.; Li, L.; Yi, T.; Wen, Y.; Ding, C.; Xie, C. Evaluation of deep learning segmentation models for detection of pine wilt disease in unmanned aerial vehicle images. Remote Sens. 2021, 13, 3594. [Google Scholar] [CrossRef]
- Tao, H.; Li, C.; Cheng, C.; Jiang, L.; Hu, H. Research progress on remote sensing monitoring of pine wilt disease–discolored pine trees. J. Forest Sci. Res. 2020, 33, 172–183. [Google Scholar] [CrossRef]
- Li, N.; Huo, L.; Zhang, X. Using Only the Red-Edge Bands Is Sufficient to Detect Tree Stress: A Case Study on the Early Detection of PWD Using Hyperspectral Drone Images. Comput. Electron. Agric. 2024, 217, 108665. [Google Scholar] [CrossRef]
- Zhang, X. Spectral feature enhancement-based hyperspectral image object recognition. Infrared Technol. 2010, 32, 717–722. [Google Scholar]
- Zhang, K. Hyperspectral Image Classification Based on Separable Features. Ph.D. Thesis, Hefei University of Technology, Hefei, China, 2022. [Google Scholar]


| Mean | Standard Deviation | Maximum | Minimum | Coefficient of Variation | |
|---|---|---|---|---|---|
| Cab (mg/g) | 0.329 | 0.264 | 1.115 | 0.006 | 0.803 |
| WC (%) | 35.664 | 21.613 | 62.276 | 4.951 | 0.606 |
| Variable | Description | Formula | Source |
|---|---|---|---|
| NDVI | Normalized difference vegetation index | [34] | |
| CI | Chlorophyll index | [48] | |
| PSND | Pigment-specific normalized difference | [27] | |
| PSSR | Pigment specific simple ratio | [49] | |
| RVSI | Ratio vegetation stress index | [50] | |
| PSI | Plant stress index | [38] | |
| TCARI | Transformed chlorophyll absorption Ratio index | [35] | |
| ARI | Anthocyanin reflectance index | [51] | |
| GI | Greenness index | [52] | |
| SIPI | Structural independent pigment index | [53] |
| Variable | Description | Formula | Source |
|---|---|---|---|
| WI1 | Water index 1 | [54] | |
| WI2 | Water index 2 | [55] | |
| WASCOSBNDI | Water absorption shoulder due to combined overtone of stretching bands—normalized difference index | [56] | |
| COSBNDI | Combined overtone of stretching bands—normalized difference index | [57] | |
| SAPSBNDI | Small absorption peak of stretching bands—normalized difference index | [57] |
| Moisture Content Index | Healthy—Infected but Non-Discolored | Infected but Non-Discolored—Infected and Discolored |
|---|---|---|
| WASCOSBNDI | −133.73% ** | 459.02% ** |
| SAPSBNDI | −15.57% | 362.49% ** |
| WI1 | −12.17% | 184.76%** |
| Pigment Index | Healthy—Infected but Non-Discolored | Infected but Non-Discolored— Infected and Discolored |
|---|---|---|
| CI | −4.97% | −43.22% ** |
| NDVI | −1.52% | −45.84% ** |
| PSND | −1.66% | −44.37% ** |
| CI | WASCOSBNDI | CI-WASCOSBDI | |
|---|---|---|---|
| Healthy—Infected but Non-Discolored | 0.52 | 0.015 | 0.126CI + WASCOSBNDI – 0.101 = 0 |
| Infected but Non-Discolored— Infected and Discolored | 0.67 | −0.048 | 1.103CI + WASCOSBNDI – 0.522 = 0 |
| Stage | Healthy | Infected but Non-Discolored | Infected and Discolored | Total | PA (%) |
|---|---|---|---|---|---|
| Healthy | 246 | 48 | 0 | 294 | 83.67% |
| Infected but Non-Discolored | 27 | 9 | 0 | 36 | 25% |
| Infected and Discolored | 0 | 2 | 42 | 44 | 95.45% |
| Total | 273 | 59 | 42 | 374 | |
| UA (%) | 90.11% | 15.25% | 100% | OA (%) | 81.82% |
| Stage | Healthy | Infected but Non-Discolored | Infected and Discolored | Total | PA (%) |
|---|---|---|---|---|---|
| Healthy | 271 | 23 | 0 | 294 | 92.18% |
| Infected but Non-Discolored | 11 | 24 | 1 | 36 | 66.67% |
| Infected and Discolored | 0 | 5 | 39 | 44 | 88.64% |
| Total | 282 | 52 | 40 | 374 | |
| UA (%) | 96.1% | 46.15% | 97.5% | OA (%) | 89.31% |
| Stage | Healthy | Infected but Non-Discolored | Infected and Discolored | Total | PA (%) |
|---|---|---|---|---|---|
| Healthy | 279 | 15 | 0 | 294 | 94.9% |
| Infected but Non-Discolored | 10 | 26 | 0 | 36 | 72.22% |
| Infected and Discolored | 0 | 2 | 42 | 44 | 95.45% |
| Total | 289 | 43 | 42 | 374 | |
| UA (%) | 96.54% | 60.47% | 100% | OA (%) | 92.78% |
| Stage | Healthy | Infected but Non-Discolored | Infected and Discolored | PA (%) |
|---|---|---|---|---|
| Discoloration by August | 5 | 25 | 0 | 83.3% |
| Discoloration by October | 5 | 1 | 0 | 16.7% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hou, R.; Zhang, B.; Fang, G.; Yang, S.; Guo, L.; Huang, W.; Yao, J.; Jiao, Q.; Sun, H.; Yan, J. Early Detection of Pine Wilt Disease by Combining Pigment and Moisture Content Indices Using UAV-Based Hyperspectral Imagery. Remote Sens. 2025, 17, 1833. https://doi.org/10.3390/rs17111833
Hou R, Zhang B, Fang G, Yang S, Guo L, Huang W, Yao J, Jiao Q, Sun H, Yan J. Early Detection of Pine Wilt Disease by Combining Pigment and Moisture Content Indices Using UAV-Based Hyperspectral Imagery. Remote Sensing. 2025; 17(11):1833. https://doi.org/10.3390/rs17111833
Chicago/Turabian StyleHou, Rui, Biyao Zhang, Guofei Fang, Sihan Yang, Lei Guo, Wenjiang Huang, Jing Yao, Quanjun Jiao, Hong Sun, and Jiayu Yan. 2025. "Early Detection of Pine Wilt Disease by Combining Pigment and Moisture Content Indices Using UAV-Based Hyperspectral Imagery" Remote Sensing 17, no. 11: 1833. https://doi.org/10.3390/rs17111833
APA StyleHou, R., Zhang, B., Fang, G., Yang, S., Guo, L., Huang, W., Yao, J., Jiao, Q., Sun, H., & Yan, J. (2025). Early Detection of Pine Wilt Disease by Combining Pigment and Moisture Content Indices Using UAV-Based Hyperspectral Imagery. Remote Sensing, 17(11), 1833. https://doi.org/10.3390/rs17111833









