Leveraging Phenology to Assess Seasonal Variations of Plant Communities for Mapping Dynamic Ecosystems
Abstract
:1. Introduction
2. Methods and Materials
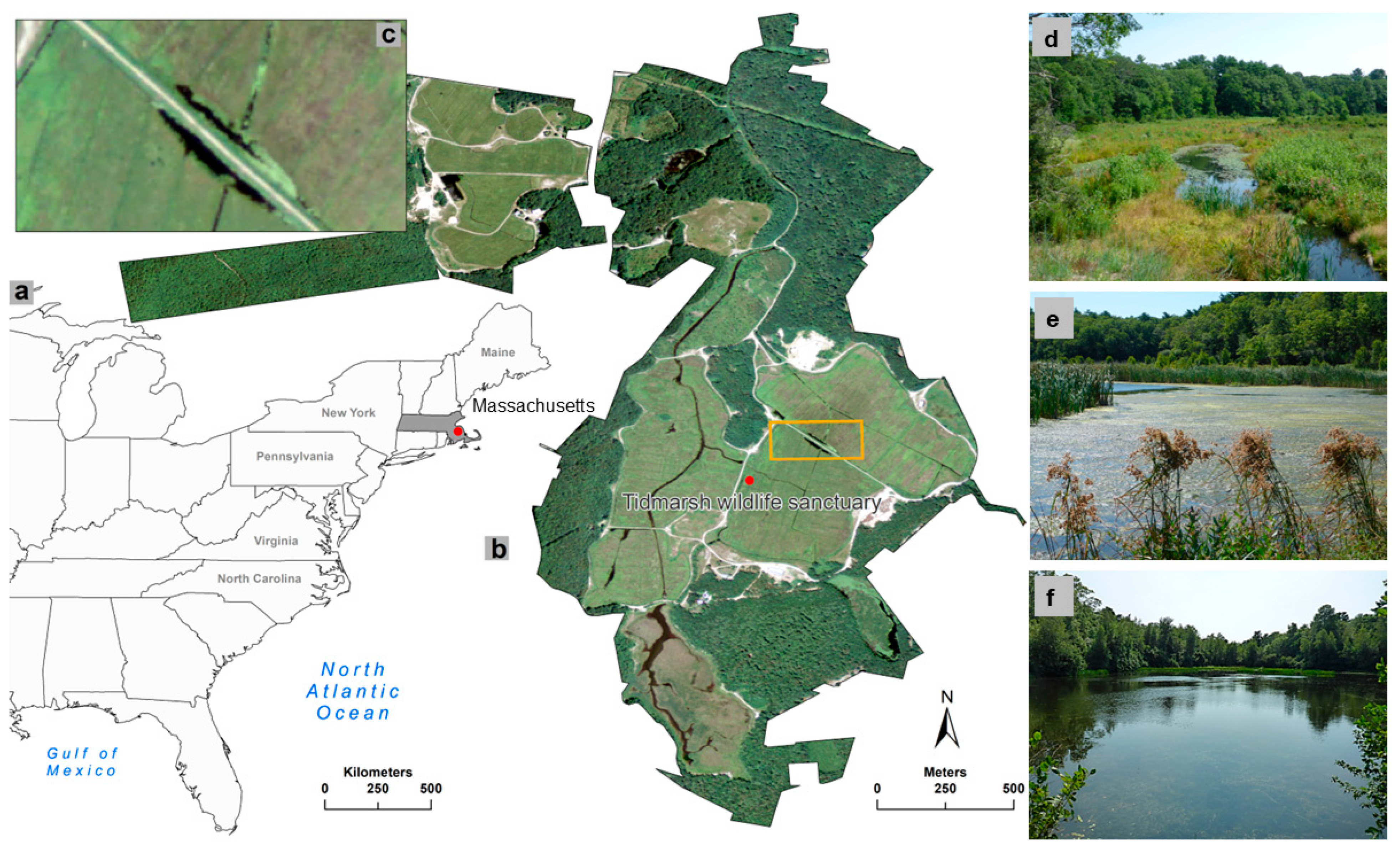
2.1. Temperate Freshwater Wetlands as a Case Study
2.2. Wetland Ecosystem Classification Scheme
2.3. Field Data Collection
2.4. Remote Sensing Data Acquisition and Processing
2.5. Phenology for Identifying Time Periods for Imagery Acquisition and Modifying Wetland Ecosystem Classification Scheme
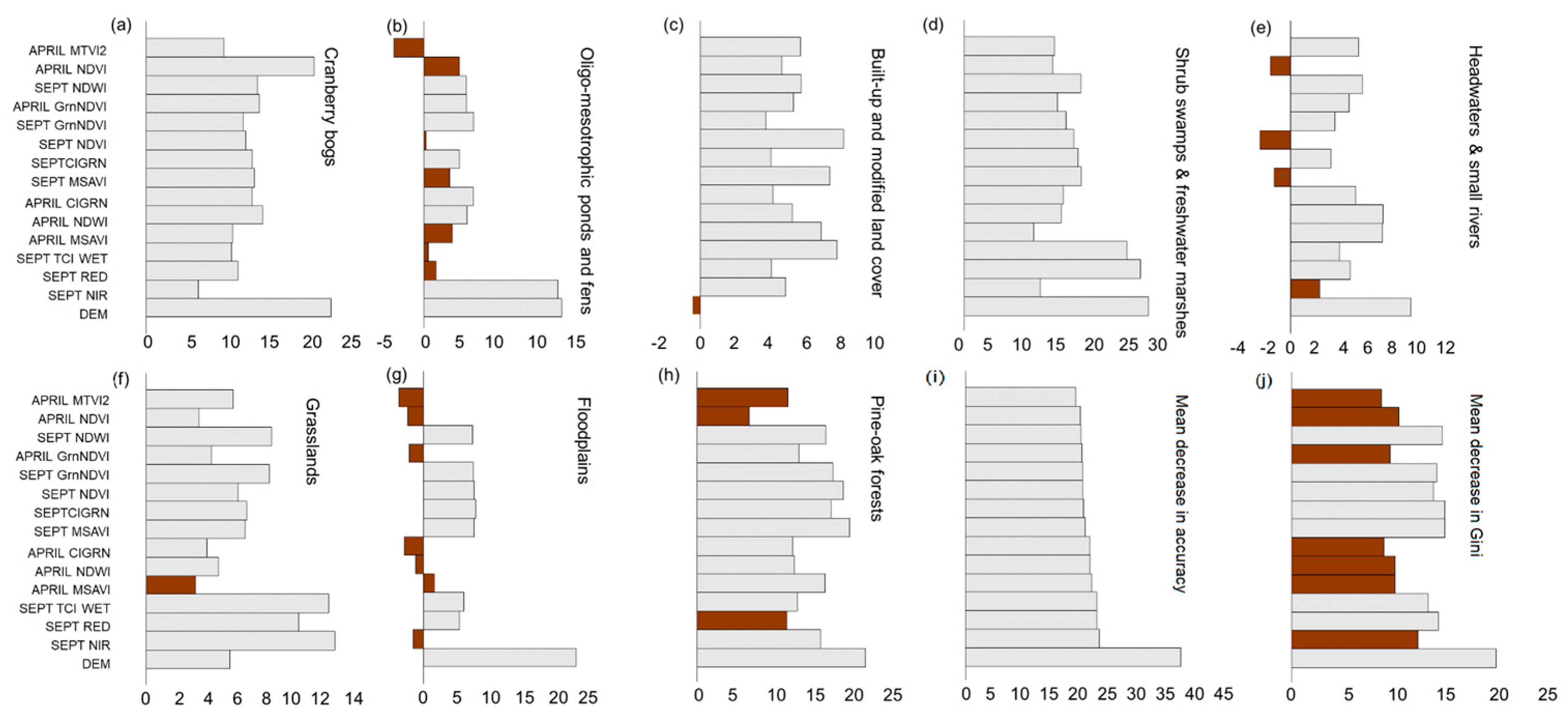
2.6. Classification Algorithm and Evaluation of Performance
3. Results
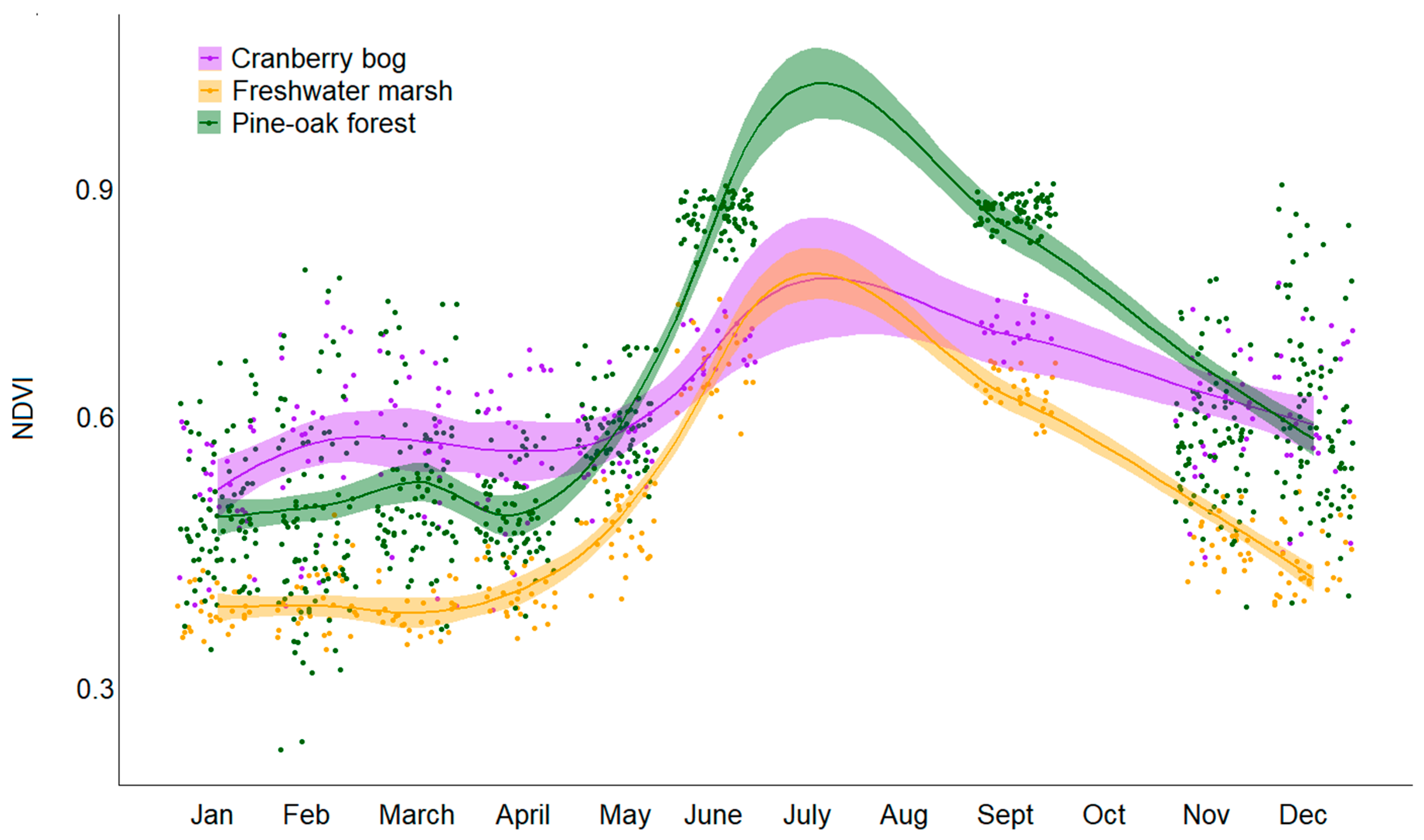
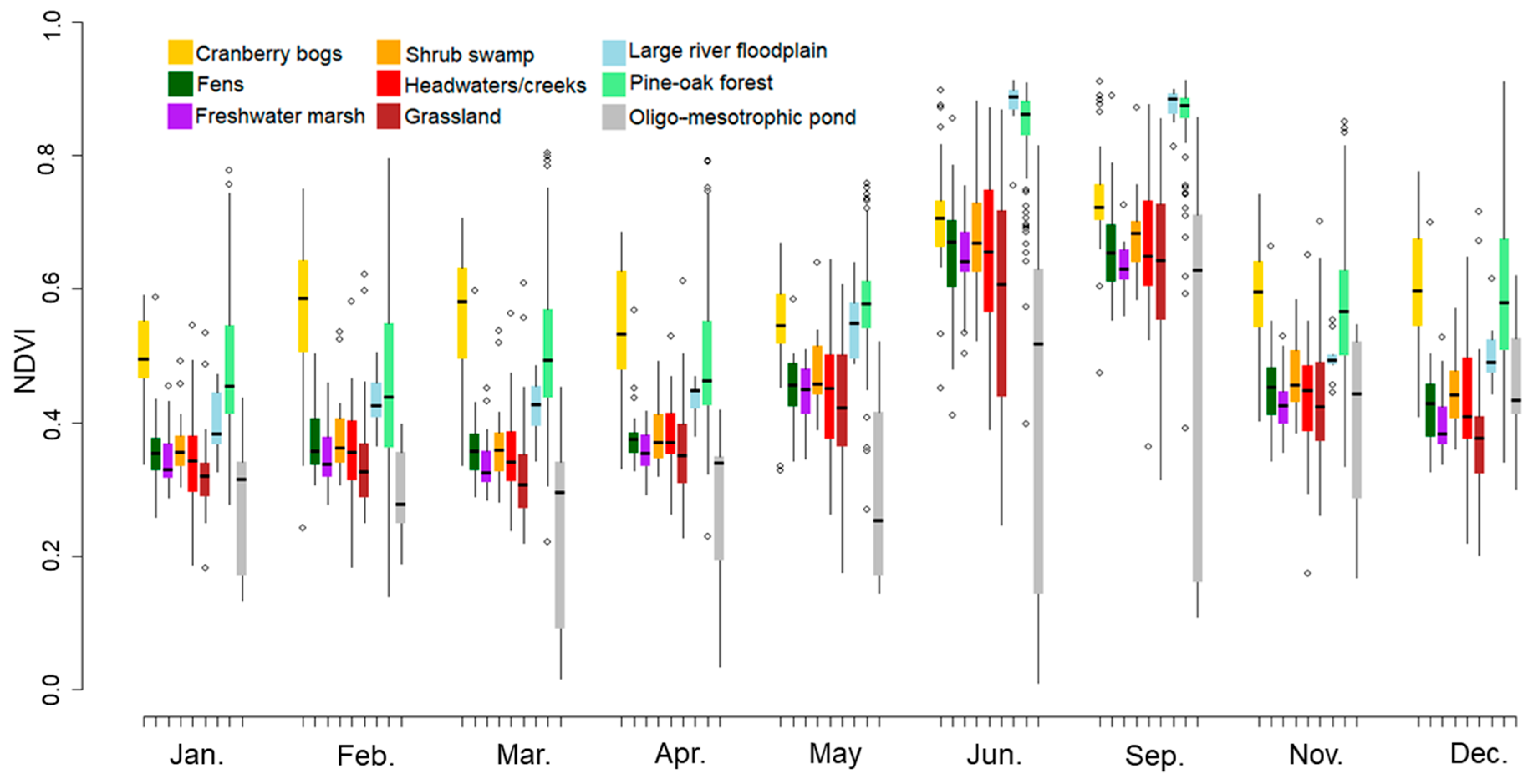
3.1. Phenological Variations in Plant Communities
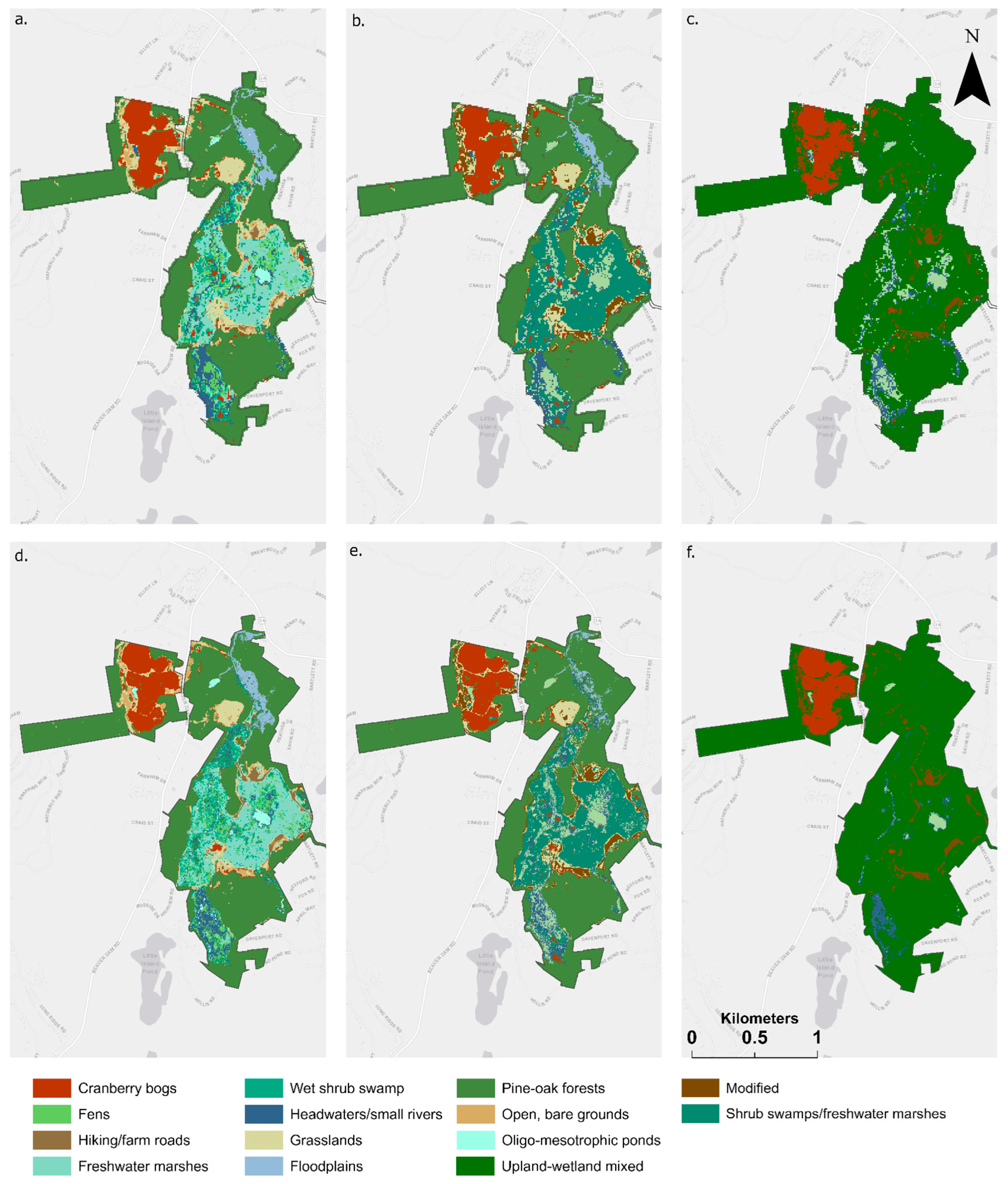
3.2. Modification of Wetland Ecosystem Classification Schemes
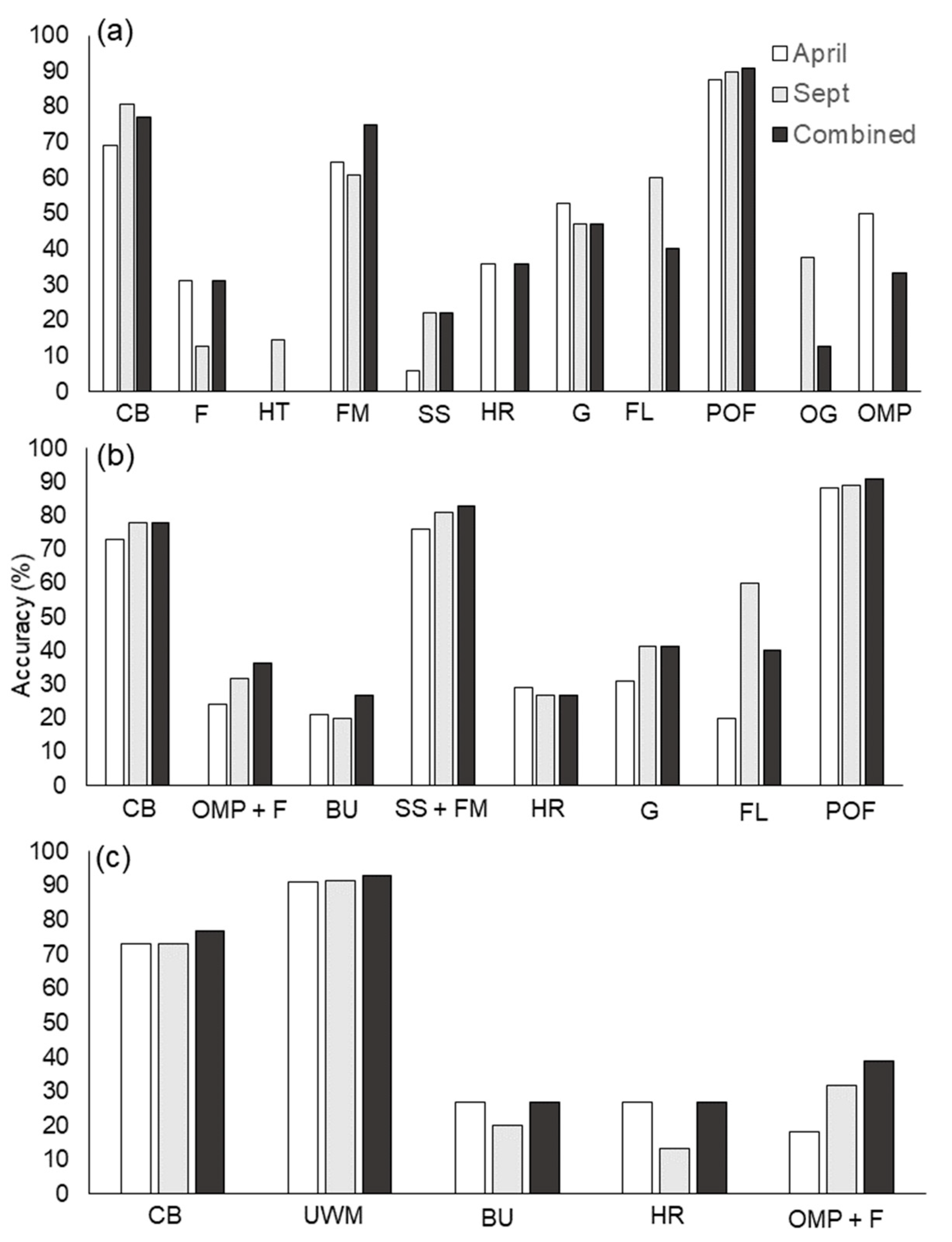
3.3. Performance of Classification Schemes and High-Resolution, Multi-Season Imagery in Mapping
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ibarrola-Ulzurrun, E.; Marcello, J.; Gonzalo-Martín, C.; Martín-Esquivel, J.L. Temporal dynamic analysis of a mountain ecosystem based on multi-source and multi-scale remote sensing data. Ecosphere 2019, 10, e02708. [Google Scholar] [CrossRef]
- Beamish, A.; Raynolds, M.K.; Epstein, H.; Frost, G.V.; Macander, M.J.; Bergstedt, H.; Bartsch, A.; Kruse, S.; Miles, V.; Tanis, C.M.; et al. Recent trends and remaining challenges for optical remote sensing of Arctic tundra vegetation: A review and outlook. Remote Sens. Environ. 2020, 246, 111872. [Google Scholar] [CrossRef]
- Dronova, I.; Gong, P.; Wang, L.; Zhong, L. Mapping dynamic cover types in a large seasonally flooded wetland using extended principal component analysis and object-based classification. Remote Sens. Environ. 2015, 158, 193–206. [Google Scholar] [CrossRef]
- Mialon, A.; Royer, A.; Fily, M. Wetland seasonal dynamics and interannual variability over northern high latitudes, derived from microwave satellite data. J. Geophys. Res. Atmos. 2005, 110. [Google Scholar] [CrossRef]
- Zhao, Y.; Feng, D.; Yu, L.; Wang, X.; Chen, Y.; Bai, Y.; Hernández, H.J.; Galleguillos, M.; Estades, C.; Biging, G.S.; et al. Detailed dynamic land cover mapping of Chile: Accuracy improvement by integrating multi-temporal data. Remote Sens. Environ. 2016, 183, 170–185. [Google Scholar] [CrossRef]
- Diao, C.; Wang, L. Incorporating plant phenological trajectory in exotic saltcedar detection with monthly time series of Landsat imagery. Remote Sens. Environ. 2016, 182, 60–71. [Google Scholar] [CrossRef]
- Tuanmu, M.-N.; Viña, A.; Bearer, S.; Xu, W.; Ouyang, Z.; Zhang, H.; Liu, J. Mapping understory vegetation using phenological characteristics derived from remotely sensed data. Remote Sens. Environ. 2010, 114, 1833–1844. [Google Scholar] [CrossRef]
- Running, S.W.; Loveland, T.R.; Pierce, L.L.; Nemani, R.R.; Hunt, E.R. A remote sensing based vegetation classification logic for global land cover analysis. Remote Sens. Environ. 1995, 51, 39–48. [Google Scholar] [CrossRef]
- Higginbottom, T.P.; Symeonakis, E.; Meyer, H.; van der Linden, S. Mapping fractional woody cover in semi-arid savannahs using multi-seasonal composites from Landsat data. ISPRS J. Photogramm. Remote Sens. 2018, 139, 88–102. [Google Scholar] [CrossRef]
- Pu, R.; Landry, S.; Yu, Q. Assessing the potential of multi-seasonal high resolution Pléiades satellite imagery for mapping urban tree species. Int. J. Appl. Earth Obs. Geoinf. 2018, 71, 144–158. [Google Scholar]
- Jia, K.; Liang, S.; Wei, X.; Yao, Y.; Su, Y.; Jiang, B.; Wang, X. Land cover classification of Landsat data with phenological features extracted from time series MODIS NDVI data. Remote Sens. 2014, 6, 11518–11532. [Google Scholar] [CrossRef]
- Bellón, B.; Bégué, A.; Lo Seen, D.; De Almeida, C.A.; Simões, M. A remote sensing approach for regional-scale mapping of agricultural land-use systems based on NDVI time series. Remote Sens. 2017, 9, 600. [Google Scholar] [CrossRef]
- Foerster, S.; Kaden, K.; Foerster, M.; Itzerott, S. Crop type mapping using spectral–temporal profiles and phenological information. Comput. Electron. Agric. 2012, 89, 30–40. [Google Scholar] [CrossRef]
- Hunter, F.D.L.; Mitchard, E.T.A.; Tyrrell, P.; Russell, S. Inter-Seasonal Time Series Imagery Enhances Classification Accuracy of Grazing Resource and Land Degradation Maps in a Savanna Ecosystem. Remote Sens. 2020, 12, 198. [Google Scholar] [CrossRef]
- Richardson, A.D.; Braswell, B.H.; Hollinger, D.Y.; Jenkins, J.P.; Ollinger, S.V. Near-surface remote sensing of spatial and temporal variation in canopy phenology. Ecol. Appl. 2009, 19, 1417–1428. [Google Scholar] [CrossRef]
- Cerrejón, C.; Valeria, O.; Marchand, P.; Caners, R.T.; Fenton, N.J. No place to hide: Rare plant detection through remote sensing. Divers. Distrib. 2021, 27, 948–961. [Google Scholar] [CrossRef]
- CaraDonna, P.J.; Iler, A.M.; Inouye, D.W. Shifts in flowering phenology reshape a subalpine plant community. Proc. Natl. Acad. Sci. USA 2014, 111, 4916–4921. [Google Scholar] [CrossRef]
- Oetter, D.R.; Cohen, W.B.; Berterretche, M.; Maiersperger, T.K.; Kennedy, R.E. Land cover mapping in an agricultural setting using multiseasonal Thematic Mapper data. Remote Sens. Environ. 2001, 76, 139–155. [Google Scholar] [CrossRef]
- Pu, R.; Landry, S. Evaluating seasonal effect on forest leaf area index mapping using multi-seasonal high resolution satellite pléiades imagery. Int. J. Appl. Earth Obs. Geoinf. 2019, 80, 268–279. [Google Scholar] [CrossRef]
- Hill, A.C.; Vázquez-Lule, A.; Vargas, R. Linking vegetation spectral reflectance with ecosystem carbon phenology in a temperate salt marsh. Agric. For. Meteorol. 2021, 307, 108481. [Google Scholar] [CrossRef]
- Smith, M.O.; Ustin, S.L.; Adams, J.B.; Gillespie, A.R. Vegetation in deserts: I. A regional measure of abundance from multispectral images. Remote Sens. Environ. 1990, 31, 1–26. [Google Scholar] [CrossRef]
- Koldasbayeva, D.; Tregubova, P.; Gasanov, M.; Zaytsev, A.; Petrovskaia, A.; Burnaev, E. Challenges in data-driven geospatial modeling for environmental research and practice. Nat. Commun. 2024, 15, 10700. [Google Scholar] [CrossRef] [PubMed]
- Mehner, H.; Cutler, M.; Fairbairn, D.; Thompson, G. Remote sensing of upland vegetation: The potential of high spatial resolution satellite sensors. Glob. Ecol. Biogeogr. 2004, 13, 359–369. [Google Scholar] [CrossRef]
- Qi, J.; Chehbouni, A.; Huete, A.R.; Kerr, Y.H.; Sorooshian, S. A modified soil adjusted vegetation index. Remote Sens. Environ. 1994, 48, 119–126. [Google Scholar] [CrossRef]
- Esch, T.; Metz, A.; Marconcini, M.; Keil, M. Combined use of multi-seasonal high and medium resolution satellite imagery for parcel-related mapping of cropland and grassland. Int. J. Appl. Earth Obs. Geoinf. 2014, 28, 230–237. [Google Scholar] [CrossRef]
- Nagendra, H. Using remote sensing to assess biodiversity. Int. J. Remote Sens. 2001, 22, 2377–2400. [Google Scholar] [CrossRef]
- Nagendra, H.; Lucas, R.; Honrado, J.P.; Jongman, R.H.G.; Tarantino, C.; Adamo, M.; Mairota, P. Remote sensing for conservation monitoring: Assessing protected areas, habitat extent, habitat condition, species diversity, and threats. Ecol. Indic. 2013, 33, 45–59. [Google Scholar] [CrossRef]
- Dash, J.; Ogutu, B.O. Recent advances in space-borne optical remote sensing systems for monitoring global terrestrial ecosystems. Prog. Phys. Geogr. 2016, 40, 322–351. [Google Scholar] [CrossRef]
- Pettorelli, N. The Normalized Difference Vegetation Index; Oxford University Press: Oxford, UK, 2013. [Google Scholar]
- Brando, P.M.; Goetz, S.J.; Baccini, A.; Nepstad, D.C.; Beck, P.S.A.; Christman, M.C. Seasonal and interannual variability of climate and vegetation indices across the Amazon. Proc. Natl. Acad. Sci. USA 2010, 107, 14685–14690. [Google Scholar] [CrossRef]
- Jakubauskas, M.E.; Legates, D.R.; Kastens, J.H. Crop identification using harmonic analysis of time-series AVHRR NDVI data. Comput. Electron. Agric. 2002, 37, 127–139. [Google Scholar] [CrossRef]
- Pei, F.; Zhou, Y.; Xia, Y. Application of Normalized Difference Vegetation Index (NDVI) for the Detection of Extreme Precipitation Change. Forests 2021, 12, 594. [Google Scholar] [CrossRef]
- Ashourloo, D.; Nematollahi, H.; Huete, A.; Aghighi, H.; Azadbakht, M.; Shahrabi, H.S.; Goodarzdashti, S. A new phenology-based method for mapping wheat and barley using time-series of Sentinel-2 images. Remote Sens. Environ. 2022, 280, 113206. [Google Scholar] [CrossRef]
- d’Andrimont, R.; Taymans, M.; Lemoine, G.; Ceglar, A.; Yordanov, M.; van der Velde, M. Detecting flowering phenology in oil seed rape parcels with Sentinel-1 and-2 time series. Remote Sens. Environ. 2020, 239, 111660. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.K.; Davis, A.J.; Meentemeyer, R.K. Detecting understory plant invasion in urban forests using LiDAR. Int. J. Appl. Earth Obs. Geoinf. 2015, 38, 267–279. [Google Scholar] [CrossRef]
- Dengler, J.; Bergmeier, E.; Willner, W.; Chytrý, M. Towards a consistent classification of European grasslands. Appl. Veg. Sci 2013, 16, 518–520. [Google Scholar] [CrossRef]
- Ivanova, N.; Fomin, V.; Kusbach, A. Experience of Forest Ecological Classification in Assessment of Vegetation Dynamics. Sustainability 2022, 14, 3384. [Google Scholar] [CrossRef]
- De Cáceres, M.; Chytrý, M.; Agrillo, E.; Attorre, F.; Botta-Dukát, Z.; Capelo, J.; Czúcz, B.; Dengler, J.; Ewald, J.; Faber-Langendoen, D.; et al. A comparative framework for broad-scale plot-based vegetation classification. Appl. Veg. Sci 2015, 18, 543–560. [Google Scholar] [CrossRef]
- Pomara, L.Y.; Lee, D.C.; Brooks, B.-G.; Hargrove, W.W. Using land surface phenology and information theory to assess and map complex landscape dynamics. Landsc. Ecol. 2024, 39, 203. [Google Scholar] [CrossRef]
- Räsänen, A.; Virtanen, T. Data and resolution requirements in mapping vegetation in spatially heterogeneous landscapes. Remote Sens. Environ. 2019, 230, 111207. [Google Scholar] [CrossRef]
- Lechner, A.M.; Foody, G.M.; Boyd, D.S. Applications in Remote Sensing to Forest Ecology and Management. One Earth 2020, 2, 405–412. [Google Scholar] [CrossRef]
- Avila-Sanchez, J.S.; Perotto-Baldivieso, H.L.; Massey, L.D.; Ortega-S, J.A.; Brennan, L.A.; Hernández, F. Fine spatial scale assessment of structure and configuration of vegetation cover for northern bobwhites in grazed pastures. Ecol. Process. 2024, 13, 64. [Google Scholar] [CrossRef]
- Beatty, D.S.; Aoki, L.R.; Graham, O.J.; Yang, B. The Future Is Big—And Small: Remote Sensing Enables Cross-Scale Comparisons of Microbiome Dynamics and Ecological Consequences. mSystems 2021, 6, e01106–e01121. [Google Scholar] [CrossRef] [PubMed]
- Chave, J. The problem of pattern and scale in ecology: What have we learned in 20 years? Ecol. Lett. 2013, 16, 4–16. [Google Scholar] [CrossRef]
- Enwright, N.M.; Griffith, K.T.; Osland, M.J. Barriers to and opportunities for landward migration of coastal wetlands with sea-level rise. Front. Ecol. Environ. 2016, 14, 307–316. [Google Scholar] [CrossRef]
- Novitski, R.P.; Smith, R.D.; Fretwell, J.D. Wetland functions, values, and assessment. Natl. Summ. Wetl. Resour. USGS Water Supply Pap. 1996, 2425, 79–86. [Google Scholar]
- Zedler, J.B.; Kercher, S. Wetland resources: Status, trends, ecosystem services, and restorability. Annu. Rev. Environ. Resour. 2005, 30, 39–74. [Google Scholar] [CrossRef]
- Jones, K.; Lanthier, Y.; van der Voet, P.; van Valkengoed, E.; Taylor, D.; Fernández-Prieto, D. Monitoring and assessment of wetlands using Earth Observation: The GlobWetland project. J. Environ. Manag. 2009, 90, 2154–2169. [Google Scholar] [CrossRef]
- Surasinghe, T.D.; Chen, Y.-H.; Singh, K.K. Restored wetlands show rapid vegetation recovery and substantial surface-water expansion. Restor. Ecol. 2025, e70046. [Google Scholar] [CrossRef]
- NatureServe. International Ecological Classification Standard: Terrestrial Ecological Classifications; NatureServe Central Databases: Arlington, VA, USA, 2009. [Google Scholar]
- Anderson, M.G.; Clark, M.; Ferree, C.E.; Jospe, A.; Sheldon, A.O.; Weaver, K.J. Northeast Habitat Guides: A Companion to the Terrestrial and Aquatic Habitat Maps; The Nature Conservancy, Eastern Conservation Science, Eastern Regional Office: Boston, MA, USA. Available online: https://northeastwildlifediversity.org/project/northeast-habitat-guides-companion-terrestrial-and-aquatic-habitat-maps (accessed on 30 April 2025).
- Federal Geographic Data Committee. Classification of Wetlands and Deepwater Habitats of the United States. FGDC-STD-004-2013, 2nd ed.; Wetlands Subcommittee, Federal Geographic Data Committee and U.S. Fish and Wildlife Service: Washington, DC, USA, 2013. [Google Scholar]
- Olivero-Sheldon, A.; Anderson, M.G. Northeast Lake and Pond Classification; The Nature Conservancy, Eastern Conservation Science, Eastern Regional Office: Boston, MA, USA, 2016. [Google Scholar]
- McGarigal, K.; Compton, B.W.; Plunkett, E.B.; DeLuca, W.V.; Grand, J. Designing Sustainable Landscapes: Ecological Systems; Report to the North Atlantic Conservation Cooperative, US Fish and Wildlife Service, Northeast Region, 2017. Available online: https://umassdsl.org/DSLdocs/DSL_documentation_ecological_systems.pdf (accessed on 30 April 2025).
- Ballantine, K.A.; Davenport, G.; Deegan, L.; Gladfelter, E.; Hatch, C.E.; Kennedy, C.; Klionsky, S.; Mayton, B.; Neil, C.; Surasinghe, T.D.; et al. Learning from the Restoration of Wetlands on Cranberry Farmland: Preliminary Benefits Assessment; Massachusetts Division of Ecological Restoration, Cranberry Bog Program: Living Observatory, 2020; Available online: https://view.publitas.com/p222-2239/preliminary-benefits-assessment (accessed on 30 April 2025).
- Comer, P.; Faber-Langendoen, D.; Evans, R.; Gawler, S.; Josse, C.; Kittel, G.; Menard, S.; Pyne, M.; Reid, M.; Schulz, K.; et al. Ecological Systems of the United States: A Working Classification of U.S. Terrestrial Systems; NatureServe: Arlington, VA, USA, 2003. [Google Scholar]
- MA Department of Environmental Protection. Mass DEP Wetlands data, MassDEP Wetland Conservancy Program, MassGIS. Available online: https://maps.massgis.digital.mass.gov/images/dep/omv/wetviewer.htm (accessed on 30 April 2025).
- Gorelick, N.; Hancher, M.; Dixon, M.; Ilyushchenko, S.; Thau, D.; Moore, R. Google Earth Engine: Planetary-scale geospatial analysis for everyone. Remote Sens. Environ. 2017, 202, 18–27. [Google Scholar] [CrossRef]
- Hird, J.N.; DeLancey, E.R.; McDermid, G.J.; Kariyeva, J. Google earth engine, open-access satellite data, and machine learning in support of large-area probabilisticwetland mapping. Remote Sens. 2017, 9, 1315. [Google Scholar] [CrossRef]
- Rouse, J.W.; Haas, R.H.; Schell, J.A.; Deering, D.W. Monitoring vegetation systems in the Great Plains with ERTS. In Proceedings of the Third Earth Resources Technology Satellite-1 Symposium, Washington, DC, USA, 10–14 December 1973; pp. 301–317. [Google Scholar]
- Ju, Y.; Bohrer, G. Classification of Wetland Vegetation Based on NDVI Time Series from the HLS Dataset. Remote Sens. 2022, 14, 2107. [Google Scholar] [CrossRef]
- Cortese, L.; Jensen, D.J.; Simard, M.; Fagherazzi, S. Using Normalize Difference Vegetation Index to Infer Wetlands Salinity and Organic Contribution to Vertical Accretion Rates. J. Geophys. Res. Biogeosci. 2023, 128, e2023JG007631. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Merzlyak, M.N. Remote estimation of chlorophyll content in higher plant leaves. Int. J. Remote Sens. 1997, 18, 2691–2697. [Google Scholar] [CrossRef]
- McFeeters, S.K. The use of the Normalized Difference Water Index (NDWI) in the delineation of open water features. Int. J. Remote Sens. 1996, 17, 1425–1432. [Google Scholar] [CrossRef]
- Jenson, S.K.; Domingue, J.O. Extracting topographic structure from digital elevation data for geographic information system analysis. Photogramm. Eng. Remote Sens. 1988, 54, 1593–1600. [Google Scholar]
- Tarboton, D.G.; Bras, R.L.; Rodriguez-Iturbe, I. On the extraction of channel networks from digital elevation data. Hydrol. Process. 1991, 5, 81–100. [Google Scholar] [CrossRef]
- Kauth, R.J.; Thomas, G.S. The Tasselled Cap—A Graphic Description of the Spectral-Temporal Development of Agricultural Crops as Seen by Landsat; The Laboratory for Applications of Remote Sensing Symposia, Purdue University: West Lafayette, IN, USA, 1976; p. 159. [Google Scholar]
- Haboudane, D.; Miller, J.R.; Tremblay, N.; Zarco-Tejada, P.J.; Dextraze, L. Integrated narrow-band vegetation indices for prediction of crop chlorophyll content for application to precision agriculture. Remote Sens. Environ. 2002, 81, 416–426. [Google Scholar] [CrossRef]
- Iverson, L.R.; Dale, M.E.; Scott, C.T.; Prasad, A. A GIS-derived integrated moisture index to predict forest composition and productivity of Ohio forests (USA). Landsc. Ecol. 1997, 12, 331–348. [Google Scholar] [CrossRef]
- Huete, A.R. A soil-adjusted vegetation index (SAVI). Remote Sens. Environ. 1988, 25, 295–309. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Kaufman, Y.J.; Stark, R.; Rundquist, D. Novel algorithms for remote estimation of vegetation fraction. Remote Sens. Environ. 2002, 80, 76–87. [Google Scholar] [CrossRef]
- Zeng, Y.; Hao, D.; Badgley, G.; Damm, A.; Rascher, U.; Ryu, Y.; Johnson, J.; Krieger, V.; Wu, S.; Qiu, H.; et al. Estimating near-infrared reflectance of vegetation from hyperspectral data. Remote Sens. Environ. 2021, 267, 112723. [Google Scholar] [CrossRef]
- Jin, X.; Jihua, M. Retrieval Of canopy chlorophyll content for spring corn using multispectral remote sensing data. In Proceedings of the Third International Conference on Agro-Geoinformatics, Beijing, China, 11–14 August 2014; pp. 1–5. [Google Scholar]
- McPartland, M.Y.; Kane, E.S.; Falkowski, M.J.; Kolka, R.; Turetsky, M.R.; Palik, B.; Montgomery, R.A. The response of boreal peatland community composition and NDVI to hydrologic change, warming, and elevated carbon dioxide. Glob. Change Biol. 2019, 25, 93–107. [Google Scholar] [CrossRef] [PubMed]
- Hollander, M.; Wolfe, D.A.; Chicken, E. Nonparametric Statistical Methods; John Wiley & Sons: Hoboken, NJ, USA, 2013. [Google Scholar]
- Nemenyi, P.B. Distribution-Free Multiple Comparisons. Princeton University: Princeton, NJ, USA, 1963. [Google Scholar]
- Breiman, L. Random forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef]
- Cutler, D.R.; Edwards Jr, T.C.; Beard, K.H.; Cutler, A.; Hess, K.T.; Gibson, J.; Lawler, J.J. Random forests for classification in ecology. Ecology 2007, 88, 2783–2792. [Google Scholar] [CrossRef]
- Hudak, A.T.; Strand, E.K.; Vierling, L.A.; Byrne, J.C.; Eitel, J.U.H.; Martinuzzi, S.; Falkowski, M.J. Quantifying aboveground forest carbon pools and fluxes from repeat LiDAR surveys. Remote Sens. Environ. 2012, 123, 25–40. [Google Scholar] [CrossRef]
- Dronova, I.; Taddeo, S. Remote sensing of phenology: Towards the comprehensive indicators of plant community dynamics from species to regional scales. J. Ecol. 2022, 110, 1460–1484. [Google Scholar] [CrossRef]
- Zagajewski, B.; Kluczek, M.; Zdunek, K.B.; Holland, D. Sentinel-2 versus PlanetScope Images for Goldenrod Invasive Plant Species Mapping. Remote Sens. 2024, 16, 636. [Google Scholar] [CrossRef]
- van Aardt, J.A. Spectral Separability Among Six Southern Tree Species; Virginia Polytechnic Institute and State University: Blacksburg, VA, USA, 2000. [Google Scholar]
- Nilson, T.; Rautiainen, M.; Pisek, J.; Peterson, U. Seasonal reflectance courses of forests. In New Advances and Contributions to Forestry Research; Oteng-Amoako, A.A., Ed.; IntechOpen: Rijeka, Croatia, 2012; pp. 33–58. [Google Scholar]
- Getzin, S.; Wiegand, T.; Wiegand, K.; He, F. Heterogeneity influences spatial patterns and demographics in forest stands. J. Ecol. 2008, 96, 807–820. [Google Scholar] [CrossRef]
- Soudani, K.; Hmimina, G.; Delpierre, N.; Pontailler, J.Y.; Aubinet, M.; Bonal, D.; Caquet, B.; de Grandcourt, A.; Burban, B.; Flechard, C.; et al. Ground-based Network of NDVI measurements for tracking temporal dynamics of canopy structure and vegetation phenology in different biomes. Remote Sens. Environ. 2012, 123, 234–245. [Google Scholar] [CrossRef]
- Collins, L.; McCarthy, G.; Mellor, A.; Newell, G.; Smith, L. Training data requirements for fire severity mapping using Landsat imagery and random forest. Remote Sens. Environ. 2020, 245, 111839. [Google Scholar] [CrossRef]
- Singh, K.K.; Vogler, J.B.; Shoemaker, D.A.; Meentemeyer, R.K. LiDAR-Landsat data fusion for large-area assessment of urban land cover: Balancing spatial resolution, data volume and mapping accuracy. ISPRS J. Photogramm. Remote Sens. 2012, 74, 110–121. [Google Scholar] [CrossRef]
- Millard, K.; Richardson, M. On the Importance of Training Data Sample Selection in Random Forest Image Classification: A Case Study in Peatland Ecosystem Mapping. Remote Sens. 2015, 7, 8489–8515. [Google Scholar] [CrossRef]
- Macintyre, P.; van Niekerk, A.; Mucina, L. Efficacy of multi-season Sentinel-2 imagery for compositional vegetation classification. Int. J. Appl. Earth Obs. Geoinf. 2020, 85, 101980. [Google Scholar] [CrossRef]
- Boyle, S.A.; Kennedy, C.M.; Torres, J.; Colman, K.; Pérez-Estigarribia, P.E.; de la Sancha, N.U. High-Resolution Satellite Imagery Is an Important yet Underutilized Resource in Conservation Biology. PLoS ONE 2014, 9, e86908. [Google Scholar] [CrossRef]
- Tu, Y.; Lang, W.; Yu, L.; Li, Y.; Jiang, J.; Qin, Y.; Wu, J.; Chen, T.; Xu, B. Improved mapping results of 10 m resolution land cover classification in Guangdong, China using multisource remote sensing data with google Earth engine. IEEE J. Sel. Top. Appl. Earth Obs. Remote Sens. 2020, 13, 5384–5397. [Google Scholar] [CrossRef]
- Yang, C.; Everitt, J.H.; Murden, D. Evaluating high resolution SPOT 5 satellite imagery for crop identification. Comput. Electron. Agric. 2011, 75, 347–354. [Google Scholar] [CrossRef]
- Zhang, J. Multi-source remote sensing data fusion: Status and trends. Int. J. Image Data Fusion 2010, 1, 5–24. [Google Scholar] [CrossRef]
- Chen, B.; Huang, B.; Xu, B. Multi-source remotely sensed data fusion for improving land cover classification. ISPRS J. Photogramm. Remote Sens. 2017, 124, 27–39. [Google Scholar] [CrossRef]
- Farmonov, N.; Khilola, A.; József, S.; Jamol, U.; Zafar, N.; Jakhongir, N.; and Mucsi, L. Combining PlanetScope and Sentinel-2 images with environmental data for improved wheat yield estimation. Int. J. Digit. Earth 2023, 16, 847–867. [Google Scholar] [CrossRef]
- Vuolo, F.; Neuwirth, M.; Immitzer, M.; Atzberger, C.; Ng, W.-T. How much does multi-temporal Sentinel-2 data improve crop type classification? Int. J. Appl. Earth Obs. Geoinf. 2018, 72, 122–130. [Google Scholar] [CrossRef]
- Zhang, X.; Friedl, M.A.; Schaaf, C.B.; Strahler, A.H. Climate controls on vegetation phenological patterns in northern mid- and high latitudes inferred from MODIS data. Glob. Change Biol. 2004, 10, 1133–1145. [Google Scholar] [CrossRef]
- Gašparović, M.; Medak, D.; Pilaš, I.; Jurjević, L.; Balenović, I. Fusion of sentinel-2 and planetscope imagery for vegetation detection and monitoring. Int. Arch. Photogramm. Remote Sens. Spatial Inf. Sci. 2018, XLII-1, 155–160. [Google Scholar] [CrossRef]
- Shendryk, Y.; Rist, Y.; Ticehurst, C.; Thorburn, P. Deep learning for multi-modal classification of cloud, shadow and land cover scenes in PlanetScope and Sentinel-2 imagery. ISPRS J. Photogramm. Remote Sens. 2019, 157, 124–136. [Google Scholar] [CrossRef]
- Turner, W.; Spector, S.; Gardiner, N.; Fladeland, M.; Sterling, E.; Steininger, M. Remote sensing for biodiversity science and conservation. Trends Ecol. Evol. 2003, 18, 306–314. [Google Scholar] [CrossRef]
- Kelly, M.; Tuxen, K.A.; Stralberg, D. Mapping changes to vegetation pattern in a restoring wetland: Finding pattern metrics that are consistent across spatial scale and time. Ecol. Indic. 2011, 11, 263–273. [Google Scholar] [CrossRef]
- Shuman, C.S.; Ambrose, R.F. A Comparison of Remote Sensing and Ground-Based Methods for Monitoring Wetland Restoration Success. Restor. Ecol. 2003, 11, 325–333. [Google Scholar] [CrossRef]
- Camarretta, N.; Harrison, P.A.; Bailey, T.; Potts, B.; Lucieer, A.; Davidson, N.; Hunt, M. Monitoring forest structure to guide adaptive management of forest restoration: A review of remote sensing approaches. New For. 2020, 51, 573–596. [Google Scholar] [CrossRef]
- Zhu, Z.; Piao, S.; Myneni, R.B.; Huang, M.; Zeng, Z.; Canadell, J.G.; Ciais, P.; Sitch, S.; Friedlingstein, P.; Arneth, A.; et al. Greening of the Earth and its drivers. Nat. Clim. Change 2016, 6, 791–795. [Google Scholar] [CrossRef]
- Proy, C.; Tanré, D.; Deschamps, P.Y. Evaluation of topographic effects in remotely sensed data. Remote Sens. Environ. 1989, 30, 21–32. [Google Scholar] [CrossRef]
- Hao, D.; Wen, J.; Xiao, Q.; Wu, S.; Lin, X.; You, D.; Tang, Y. Modeling Anisotropic Reflectance Over Composite Sloping Terrain. IEEE Trans. Geosci. Remote Sens. 2018, 56, 3903–3923. [Google Scholar] [CrossRef]
- Evans, J.S.; Cushman, S.A. Gradient modeling of conifer species using random forests. Landsc. Ecol. 2009, 24, 673–683. [Google Scholar] [CrossRef]
- Bork, E.W.; Su, J.G. Integrating LIDAR data and multispectral imagery for enhanced classification of rangeland vegetation: A meta analysis. Remote Sens. Environ. 2007, 111, 11–24. [Google Scholar] [CrossRef]
- Ferraz, A.; Bretar, F.; Jacquemoud, S.; Gonçalves, G.; Pereira, L.; Tomé, M.; Soares, P. 3-D mapping of a multi-layered Mediterranean forest using ALS data. Remote Sens. Environ. 2012, 121, 210–223. [Google Scholar] [CrossRef]
- Ussyshkin, V.; Theriault, L. Airborne Lidar: Advances in Discrete Return Technology for 3D Vegetation Mapping. Remote Sens. 2011, 3, 416–434. [Google Scholar] [CrossRef]
- Sánchez-Espinosa, A.; Schröder, C. Land use and land cover mapping in wetlands one step closer to the ground: Sentinel-2 versus landsat 8. J. Environ. Manag. 2019, 247, 484–498. [Google Scholar] [CrossRef]
- Yan, E.; Wang, G.; Lin, H.; Xia, C.; Sun, H. Phenology-based classification of vegetation cover types in Northeast China using MODIS NDVI and EVI time series. Int. J. Remote Sens. 2015, 36, 489–512. [Google Scholar] [CrossRef]
- Hesketh, M.; Sánchez-Azofeifa, G.A. The effect of seasonal spectral variation on species classification in the Panamanian tropical forest. Remote Sens. Environ. 2012, 118, 73–82. [Google Scholar] [CrossRef]
- Schmidt, T.; Schuster, C.; Kleinschmit, B.; Förster, M. Evaluating an Intra-Annual Time Series for Grassland Classification—How Many Acquisitions and What Seasonal Origin Are Optimal? IEEE J. Sel. Top. Appl. Earth Obs. Remote Sens. 2014, 7, 3428–3439. [Google Scholar] [CrossRef]
- Rominger, K.; Meyer, S.E. Application of UAV-based methodology for census of an endangered plant species in a fragile habitat. Remote Sens. 2019, 11, 719. [Google Scholar] [CrossRef]
- Zhong, L.; Yu, L.; Li, X.; Hu, L.; Gong, P. Rapid corn and soybean mapping in US Corn Belt and neighboring areas. Sci. Rep. 2016, 6, 36240. [Google Scholar] [CrossRef]
- Fundisi, E.; Tesfamichael, S.G.; Ahmed, F. A bi-seasonal classification of woody plant species using Sentinel-2A and SPOT-6 in a localised species-rich savanna environment. Geocarto Int. 2021, 37, 6272–6293. [Google Scholar] [CrossRef]
- Townsend, P.A.; Walsh, S.J. Remote sensing of forested wetlands: Application of multitemporal and multispectral satellite imagery to determine plant community composition and structure in southeastern USA. Plant Ecol. 2001, 157, 129–149. [Google Scholar] [CrossRef]
- Surasinghe, T.D.; Singh, K.K.; Frazier, A.E. Harnessing the full potential of drones for fieldwork. Bioscience 2025. [Google Scholar] [CrossRef]
| Land-Cover Category | Plant Community Types | Description of Plant Communities | Reference |
|---|---|---|---|
| Terrestrial systems | North Atlantic coastal plain heathlands and grasslands | Vegetation includes annual grasses with a mixture of perennial shrubs and herbaceous plants. The soil is well drained and nutrient poor. | Anderson et al. [51]; NatureServe [50] |
| Laurentian–Acadian northern coastal pine–oak forests | Mixed forests and woodlands dominated by white pine, red oak, and hemlock in varying proportions. Forest stands are characterized by closed-canopy vegetation. Soils have low to moderate moisture levels. | ||
| Open-water lentic systems | Warm-to-cool, oligo-mesotrophic (acidic) ponds (hereafter, oligo-mesotrophic pond) | These are moderate-depth (2.5 m) and -sized (0.17 km2) lakes and ponds where warm-to-cool, moderately oxygenated water is present year-round. Water alkalinity is low, supporting biota-tolerant acidic waters, and these water bodies may support beds of submerged aquatic vegetation. Very acidic water bodies can be highly colored due to high dissolved organic carbon and organic acid content. | Anderson et al. [51]; Federal Geographic Data Committee [52]; Olivero-Sheldon and Anderson [53] |
| Open-water lotic systems—rivers and streams | Low gradient, cool, headwaters, creeks, small streams and small rivers (hereafter headwaters and small rivers) | Slow-moving headwaters, creeks, and small rivers and streams that flow through marshlands. These small streams of moderate to low gradient occur on flat or gentle slopes (<100 km2). The waters may have high turbidity and low oxygen levels. Stream channels are dominated by glide–pool and ripple–dune systems with runs interspersed by pools. Stream substrates consisted of sand and silt. Can have high channel sinuosity. | |
| Wetlands | Laurentian–Acadian wet meadow–shrub swamp (hereafter, shrub swamps) | A shrub-dominated swamp or wet meadow on mineral soils. These wetlands are often found closer to lakes, ponds, or streams, and can be part of a larger wetland complex. A patchwork of shrub and herb dominance, including some soft-stemmed plants and non-woody plants, characterize these wetlands while trees are generally absent or thinly scattered. Standing water may only be limited to certain parts of the year. | Federal Geographic Data Committee [52]; McGarigal et al. [54] |
| Laurentian–Acadian freshwater marsh (hereafter, freshwater marshes) | Dominated by emergent or submergent herbaceous vegetation and associated with flat or shallow basins associated with lakes, ponds, slow-moving streams, and seepage slopes. These species tolerate sustained inundations but do not persist throughout the winter. Trees are absent although scattered shrubs often account for <25% of the wetland surface. The presence of standing water can be relatively longer. | ||
| North-Central Appalachian large river floodplain (hereafter, floodplains) | A mosaic of wetland complexes and upland vegetation that is characteristic of floodplain forests. Usually occurs on broad floodplains of medium to large rivers with a lower channel gradient. Many of the wetland areas are flooded during each spring. | ||
| Fens around ponds, and other lentic systems (hereafter, fens) | Peat-forming wetlands receive an abundant flux of nutrients from sources other than precipitation, such as upslope mineral soil drainages. Soil and water are less acidic compared to other wetlands. The high nutrient levels support diverse plant and animal communities. Often covered by grass, sedges, rushes, and wildflowers. | Federal Geographic Data Committee [52] | |
| Cranberry bogs | Legacy of the historical, commercial scale cranberry farming, cranberry bogs are not actively managed. The dominant vegetation includes cranberry mats with a variety of herbaceous and/or graminoid species that can tolerate water-logged conditions. These bogs underlay peat and sand layers and remain connected to irrigation ditches. | MA Department of Environmental Protection [57] | |
| Modified land-cover types | Hiking trails and farm roads | Includes roads, trails, parking areas, etc. | McGarigal et al. [54] |
| Open, bare grounds | Areas with little to no natural vegetation cover |
| Plant Communities and Land-Cover Types | Number of Ground-Reference Locations |
|---|---|
| Grasslands | 19 |
| Pine–oak forests | 95 |
| Oligo-mesotrophic ponds | 7 |
| Fens | 25 |
| Headwaters and small rivers | 17 |
| Shrub swamps | 19 |
| Freshwater marshes | 30 |
| Floodplains | 9 |
| Cranberry bogs | 27 |
| Hiking trails and farm roads | 10 |
| Open, bare grounds | 10 |
| Total | 268 |
| Utilized Indices | Description of Spectral Indices Used | Reference |
|---|---|---|
| Tasseled cap greenness (TCI GREEN) | The TCI transforms spectral data into three composite indices—brightness, greenness, and wetness—capturing soil reflectance, vegetation vigor, and surface moisture, respectively. | Kauth and Thomas [67] |
| Tasseled cap brightness (TCI BRIGHT) | ||
| Tasseled cap wetness (TCI WET) | ||
| Normalized difference vegetation index (NDVI) | The NDVI assesses vegetation greenness and health and is calculated from red and near-infrared (NIR) reflectance values. | Rouse et al. [60] |
| Green normalized difference vegetation index (GrnNDVI) | The GrnNDVI is an indicator of photosynthetic activity and used to assess plant canopy conditions. | Gitelson et al. [63] |
| Normalized difference water index (NDWI) | The NDWI is effective at delineating open water bodies and suppressing vegetation and soil noise. It enhances the presence of open water bodies by using the difference between NIR and green light reflectance. | McFeeters [64] |
| Modified soil-adjusted vegetation index (MSAVI) | The MSAVI is a vegetation index designed to minimize soil brightness influences in areas with sparse vegetation cover. | Huete [70]; Qi et al. [24] |
| Chlorophyll green (ClGRN) | The CIGRN is a vegetation index that leverages reflectance in the green band to estimate chlorophyll content in plants, indicating plant health, photosynthetic activity, and nitrogen content. | Gitelson and Merzlyak [63] |
| Visual atmospheric resistance index (VARI) | The VARI enhances vegetation detection in the visible spectrum by reducing the impact of atmospheric interference and variable illumination. | Gitelson et al. [71] |
| Modified triangular vegetation index (MTVI2) | The MTVI2 estimates chlorophyll content in plant canopies, with minimal sensitivity to variations in leaf area index. | Haboudane et al. [68] |
| Topographic wetness index (TWI) | The TWI quantifies how local terrain influences the direction and accumulation of surface runoff. | Iverson et al. [69] |
| Flow direction (FlowDir) | This measure determines the direction of water flow across a raster surface. | Jenson and Domingue [65]; Tarboton et al. [66] |
| Flow accumulation (FlowAccum) | FlowAccum shows how much water flows into each cell in a landscape, based on elevation. It adds up how many upstream cells drain into each location, helping identify areas where water collects and forms streams. |
| Wetland Ecosystem Classification Scheme | Modified Classification Scheme | ||
|---|---|---|---|
| Growing Season | Transitional Season | ||
| 1 | Grasslands | Grasslands | Upland–wetland mixed, transient systems (combination of classes 1, 2, and 6–8) |
| 2 | Pine–oak forests | Pine–oak forests | |
| 3 | Oligo-mesotrophic ponds | Oligo-mesotrophic ponds and fens | Oligo-mesotrophic ponds and fens |
| 4 | Fens | ||
| 5 | Headwaters and small rivers | Headwaters and small rivers | Headwaters and small rivers |
| 6 | Shrub swamps | Shrub swamps and freshwater marshes | |
| 7 | Freshwater marshes | ||
| 8 | Floodplains | Floodplains | |
| 9 | Cranberry bogs | Cranberry bogs | Cranberry bogs |
| 10 | Hiking trails and farm roads | Modified | Modified |
| 11 | Open, bare grounds | ||
| Months | Cranberry Bogs | Oligo-Mesotrophic Ponds and Fens | Shrub Swamps and Freshwater Marshes | Headwaters and Small Rivers | Grasslands | Floodplains | |
|---|---|---|---|---|---|---|---|
| April | Oligo-mesotrophic ponds and fens | 9.58 (0.00 ****) | - | - | - | - | - |
| Shrub swamps and freshwater marshes | 10.88 (0.00 ****) | 0.79 (1.00) | - | - | - | - | |
| Headwaters and small rivers | 7.13 (0.00 ****) | 1.37 (0.98) | 0.88 (1.00) | - | - | - | |
| Grasslands | 8.74 (0.00 ****) | 0.37 (1.00) | 0.31 (1.00) | 0.99 (1.00) | - | - | |
| Floodplains | 3.01 (0.40) | 4.15 (0.07) | 4.01 (0.09) | 2.78 (0.50) | 3.75 (0.14) | - | |
| Pine–Oak Forests | 3.70 (0.15) | 7.96 (0.00 ****) | 9.71 (0.00 ****) | 5.23 (0.01 *) | 7.00 (0.00 ****) | 0.94 (1.00) | |
| September | Cranberry bogs | Oligo-mesotrophic ponds and fens | Headwaters and small rivers | Upland–wetland mixed, transient systems | |||
| Oligo-mesotrophic ponds and fens | 2.15 (0.55) | - | - | - | |||
| Headwaters and small rivers | 1.79 (0.71) | 0.80 (1.00) | - | - | |||
| Upland–wetland mixed, transient systems | 1.67 (0.76) | 3.44 (0.11) | 3.85 (0.05 *) | - | |||
| Sentinel Imagery | PlanetScope Imagery | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total plant communities | 11 | 8 | 5 | 11 | 8 | 5 | ||||||
| CA | κ | CA | κ | CA | κ | CA | κ | CA | κ | CA | κ | |
| April | 0.58 | 0.46 | 0.66 | 0.53 | 0.74 | 0.43 | 0.52 | 0.39 | 0.6 | 0.47 | 0.79 | 0.42 |
| September | 0.59 | 0.49 | 0.68 | 0.59 | 0.74 | 0.46 | 0.6 | 0.48 | 0.66 | 0.57 | 0.82 | 0.52 |
| Combined * | 0.64 | 0.54 | 0.70 | 0.6 | 0.77 | 0.51 | 0.58 | 0.47 | 0.68 | 0.57 | 0.8 | 0.457 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Surasinghe, T.D.; Singh, K.K.; Smart, L.S. Leveraging Phenology to Assess Seasonal Variations of Plant Communities for Mapping Dynamic Ecosystems. Remote Sens. 2025, 17, 1778. https://doi.org/10.3390/rs17101778
Surasinghe TD, Singh KK, Smart LS. Leveraging Phenology to Assess Seasonal Variations of Plant Communities for Mapping Dynamic Ecosystems. Remote Sensing. 2025; 17(10):1778. https://doi.org/10.3390/rs17101778
Chicago/Turabian StyleSurasinghe, Thilina D., Kunwar K. Singh, and Lindsey S. Smart. 2025. "Leveraging Phenology to Assess Seasonal Variations of Plant Communities for Mapping Dynamic Ecosystems" Remote Sensing 17, no. 10: 1778. https://doi.org/10.3390/rs17101778
APA StyleSurasinghe, T. D., Singh, K. K., & Smart, L. S. (2025). Leveraging Phenology to Assess Seasonal Variations of Plant Communities for Mapping Dynamic Ecosystems. Remote Sensing, 17(10), 1778. https://doi.org/10.3390/rs17101778








