Spectroscopy of Magnesium Sulfate Double Salts and Their Implications for Mars Exploration
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Magnesium Sulfate Double Salts
2.3. Instruments
3. Results
3.1. XRD Pattern
3.2. Raman
3.3. MIR
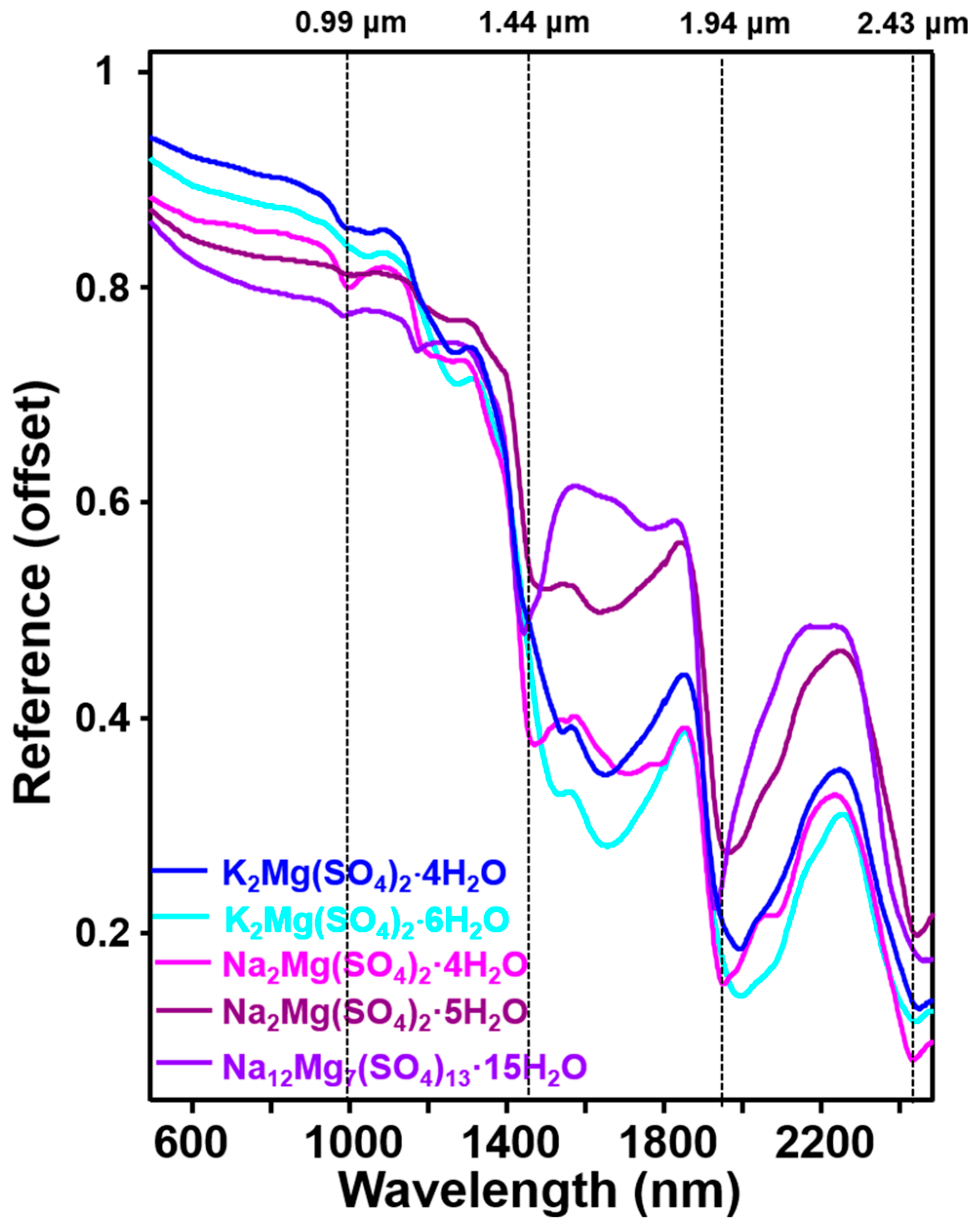
3.4. VNIR
4. Discussion and Implications for Mars
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baird, A.K.; Toulmin, P.; Clark, B.C.; Rose, H.J.; Keil, K.; Christian, R.P.; Gooding, J.L. Mineralogic and Petrologic Implications of Viking Geochemical Results from Mars: Interim Report. Science 1976, 194, 1288–1293. [Google Scholar] [CrossRef]
- Vaniman, D.T.; Bish, D.L.; Chipera, S.J.; Fialips, C.I.; William Carey, J.; Feldman, W.C. Magnesium Sulphate Salts and the History of Water on Mars. Nature 2004, 431, 663–665. [Google Scholar] [CrossRef]
- Di Pietro, I.; Schmidt, G.; Tangari, A.C.; Salese, F.; Silvestro, S.; Fairén, A.G.; Marinangeli, L.; Pondrelli, M. Groundwater-Controlled Deposition of Equatorial Layered Deposits in Central Arabia Terra, Mars. JGR Planets 2023, 128, e2022JE007504. [Google Scholar] [CrossRef]
- Arvidson, R.E.; Poulet, F.; Bibring, J.-P.; Wolff, M.; Gendrin, A.; Morris, R.V.; Freeman, J.J.; Langevin, Y.; Mangold, N.; Bellucci, G. Spectral Reflectance and Morphologic Correlations in Eastern Terra Meridiani, Mars. Science 2005, 307, 1591–1594. [Google Scholar] [CrossRef]
- Gendrin, A.; Mangold, N.; Bibring, J.-P.; Langevin, Y.; Gondet, B.; Poulet, F.; Bonello, G.; Quantin, C.; Mustard, J.; Arvidson, R.; et al. Sulfates in Martian Layered Terrains: The OMEGA/Mars Express View. Science 2005, 307, 1587–1591. [Google Scholar] [CrossRef]
- Peterson, R.C.; Wang, R. Crystal Molds on Mars: Melting of a Possible New Mineral Species to Create Martian Chaotic Terrain. Geology 2006, 34, 957. [Google Scholar] [CrossRef]
- Mangold, N.; Gendrin, A.; Gondet, B.; LeMouelic, S.; Quantin, C.; Ansan, V.; Bibring, J.-P.; Langevin, Y.; Masson, P.; Neukum, G. Spectral and Geological Study of the Sulfate-Rich Region of West Candor Chasma, Mars. Icarus 2008, 194, 519–543. [Google Scholar] [CrossRef]
- Roach, L.H.; Mustard, J.F.; Murchie, S.L.; Bibring, J.-P.; Forget, F.; Lewis, K.W.; Aharonson, O.; Vincendon, M.; Bishop, J.L. Testing Evidence of Recent Hydration State Change in Sulfates on Mars. J. Geophys. Res. 2009, 114, 2008JE003245. [Google Scholar] [CrossRef]
- Wendt, L.; Gross, C.; Kneissl, T.; Sowe, M.; Combe, J.-P.; LeDeit, L.; McGuire, P.C.; Neukum, G. Sulfates and Iron Oxides in Ophir Chasma, Mars, Based on OMEGA and CRISM Observations. Icarus 2011, 213, 86–103. [Google Scholar] [CrossRef]
- Liu, Y.; Goudge, T.A.; Catalano, J.G.; Wang, A. Spectral and Stratigraphic Mapping of Hydrated Minerals Associated with Interior Layered Deposits near the Southern Wall of Melas Chasma, Mars. Icarus 2018, 302, 62–79. [Google Scholar] [CrossRef]
- Sheppard, R.Y.; Thorpe, M.T.; Fraeman, A.A.; Fox, V.K.; Milliken, R.E. Merging Perspectives on Secondary Minerals on Mars: A Review of Ancient Water-Rock Interactions in Gale Crater Inferred from Orbital and In-Situ Observations. Minerals 2021, 11, 986. [Google Scholar] [CrossRef]
- Christensen, P.R.; Wyatt, M.B.; Glotch, T.D.; Rogers, A.D.; Anwar, S.; Arvidson, R.E.; Bandfield, J.L.; Blaney, D.L.; Budney, C.; Calvin, W.M.; et al. Mineralogy at Meridiani Planum from the Mini-TES Experiment on the Opportunity Rover. Science 2004, 306, 1733–1739. [Google Scholar] [CrossRef]
- Arvidson, R.E.; Squyres, S.W.; Anderson, R.C.; Bell, J.F.; Blaney, D.; Brückner, J.; Cabrol, N.A.; Calvin, W.M.; Carr, M.H.; Christensen, P.R.; et al. Overview of the Spirit Mars Exploration Rover Mission to Gusev Crater: Landing Site to Backstay Rock in the Columbia Hills. J. Geophys. Res. 2006, 111, 2005JE002499. [Google Scholar] [CrossRef]
- Rapin, W.; Ehlmann, B.L.; Dromart, G.; Schieber, J.; Thomas, N.H.; Fischer, W.W.; Fox, V.K.; Stein, N.T.; Nachon, M.; Clark, B.C.; et al. An Interval of High Salinity in Ancient Gale Crater Lake on Mars. Nat. Geosci. 2019, 12, 889–895. [Google Scholar] [CrossRef]
- Chipera, S.J.; Vaniman, D.T.; Rampe, E.B.; Bristow, T.F.; Martínez, G.; Tu, V.M.; Peretyazhko, T.S.; Yen, A.S.; Gellert, R.; Berger, J.A.; et al. Mineralogical Investigation of Mg-Sulfate at the Canaima Drill Site, Gale Crater, Mars. JGR Planets 2023, 128, e2023JE008041. [Google Scholar] [CrossRef]
- Vaniman, D.T.; Chipera, S.J. Transformations of Mg- and Ca-Sulfate Hydrates in Mars Regolith. Am. Mineral. 2006, 91, 1628–1642. [Google Scholar] [CrossRef]
- Rapin, W.; Dromart, G.; Clark, B.C.; Schieber, J.; Kite, E.S.; Kah, L.C.; Thompson, L.M.; Gasnault, O.; Lasue, J.; Meslin, P.-Y.; et al. Sustained Wet–Dry Cycling on Early Mars. Nature 2023, 620, 299–302. [Google Scholar] [CrossRef] [PubMed]
- Arvidson, R.E.; Squyres, S.W.; Bell, J.F.; Catalano, J.G.; Clark, B.C.; Crumpler, L.S.; De Souza, P.A.; Fairén, A.G.; Farrand, W.H.; Fox, V.K.; et al. Ancient Aqueous Environments at Endeavour Crater, Mars. Science 2014, 343, 1248097. [Google Scholar] [CrossRef] [PubMed]
- Rapin, W.; Meslin, P.-Y.; Maurice, S.; Vaniman, D.; Nachon, M.; Mangold, N.; Schröder, S.; Gasnault, O.; Forni, O.; Wiens, R.C.; et al. Hydration State of Calcium Sulfates in Gale Crater, Mars: Identification of Bassanite Veins. Earth Planet. Sci. Lett. 2016, 452, 197–205. [Google Scholar] [CrossRef]
- Nachon, M.; Clegg, S.M.; Mangold, N.; Schröder, S.; Kah, L.C.; Dromart, G.; Ollila, A.; Johnson, J.R.; Oehler, D.Z.; Bridges, J.C.; et al. Calcium Sulfate Veins Characterized by ChemCam/Curiosity at Gale Crater, Mars. JGR Planets 2014, 119, 1991–2016. [Google Scholar] [CrossRef]
- Nachon, M.; López-Reyes, G.; Meslin, P.-Y.; Ollila, A.M.; Mandon, L.; Clavé, E.; Forni, O.; Maurice, S.; Wiens, R.C.; Gasnault, O.; et al. Light-Toned Veins and Material in Jezero Crater, Mars, as Seen In-Situ via NASA’s Perseverance Rover (Mars 2020 Mission): Stratigraphic Distribution and Compositional Results. In Proceedings of the LPSC 2024-55th Lunar and Planetary Science Conference, Woodlands, TX, USA, 11–15 March 2023. [Google Scholar]
- Yen, A.S.; Ming, D.W.; Vaniman, D.T.; Gellert, R.; Blake, D.F.; Morris, R.V.; Morrison, S.M.; Bristow, T.F.; Chipera, S.J.; Edgett, K.S.; et al. Multiple Stages of Aqueous Alteration along Fractures in Mudstone and Sandstone Strata in Gale Crater, Mars. Earth Planet. Sci. Lett. 2017, 471, 186–198. [Google Scholar] [CrossRef]
- Clark, B.C.; Morris, R.V.; McLennan, S.M.; Gellert, R.; Jolliff, B.; Knoll, A.H.; Squyres, S.W.; Lowenstein, T.K.; Ming, D.W.; Tosca, N.J.; et al. Chemistry and Mineralogy of Outcrops at Meridiani Planum. Earth Planet. Sci. Lett. 2005, 240, 73–94. [Google Scholar] [CrossRef]
- Banin, A.; Han, F.X.; Kan, I.; Cicelsky, A. Acidic Volatiles and the Mars Soil. J. Geophys. Res. 1997, 102, 13341–13356. [Google Scholar] [CrossRef]
- Catling, D.C. A Chemical Model for Evaporites on Early Mars: Possible Sedimentary Tracers of the Early Climate and Implications for Exploration. J. Geophys. Res. 1999, 104, 16453–16469. [Google Scholar] [CrossRef]
- Newsom, H.E.; Hagerty, J.J.; Goff, F. Mixed Hydrothermal Fluids and the Origin of the Martian Soil. J. Geophys. Res. 1999, 104, 8717–8728. [Google Scholar] [CrossRef]
- King, P.L.; Lescinsky, D.T.; Nesbitt, H.W. The Composition and Evolution of Primordial Solutions on Mars, with Application to Other Planetary Bodies. Geochim. Cosmochim. Acta 2004, 68, 4993–5008. [Google Scholar] [CrossRef]
- Al-Samir, M.; Nabhan, S.; Winkler, A.; Fritz, J.; Greshake, A.; Jaumann, R. Experimental Leaching Processes and the Formation of Sulfates with Sulfuric Acid on Terrestrial Rocks, Martian-Like Rocks and the Martian Meteorite Tissint Related to the Formation of ILD’s on Mars. In Proceedings of the 44th Annual Lunar and Planetary Science Conference, Woodlands, TX, USA, 18–22 March 2013. [Google Scholar]
- Noel, A.; Bishop, J.L.; Al-Samir, M.; Gross, C.; Flahaut, J.; McGuire, P.C.; Weitz, C.M.; Seelos, F.; Murchie, S. Mineralogy, Morphology and Stratigraphy of the Light-Toned Interior Layered Deposits at Juventae Chasma. Icarus 2015, 251, 315–331. [Google Scholar] [CrossRef]
- Kite, E.S.; Melwani Daswani, M. Geochemistry Constrains Global Hydrology on Early Mars. Earth Planet. Sci. Lett. 2019, 524, 115718. [Google Scholar] [CrossRef]
- Gutierrez, A.; Ushak, S.; Mamani, V.; Vargas, P.; Barreneche, C.; Cabeza, L.F.; Grágeda, M. Characterization of Wastes Based on Inorganic Double Salt Hydrates as Potential Thermal Energy Storage Materials. Sol. Energy Mater. Sol. Cells 2017, 170, 149–159. [Google Scholar] [CrossRef]
- Ait Ousaleh, H.; Sair, S.; Zaki, A.; Faik, A.; Mirena Igartua, J.; El Bouari, A. Double Hydrates Salt as Sustainable Thermochemical Energy Storage Materials: Evaluation of Dehydration Behavior and Structural Phase Transition Reversibility. Sol. Energy 2020, 201, 846–856. [Google Scholar] [CrossRef]
- Vargas Jentzsch, P.; Kampe, B.; Rösch, P.; Popp, J. Raman Spectroscopic Study of Crystallization from Solutions Containing MgSO4 and Na2SO4: Raman Spectra of Double Salts. J. Phys. Chem. A 2011, 115, 5540–5546. [Google Scholar] [CrossRef] [PubMed]
- Souamti, A.; Chehimi, D.B.H. Effects of Gd2O3 Doping on the Structure and the Conduction Mechanism of K2Mg2(SO4)3 Langbeinite Ceramics: A Comparative Study. Mater. Sci. Eng. B 2021, 265, 115040. [Google Scholar] [CrossRef]
- Hertweck, B.; Giester, G.; Libowitzky, E. The Crystal Structures of the Low-Temperature Phases of Leonite-Type Compounds, K2Me(SO4)2 4H2O (Me2+ = Mg, Mn, Fe). Am. Miner. 2001, 86, 1282–1292. [Google Scholar] [CrossRef]
- Dhandapani, M.; Thyagu, L.; Prakash, P.A.; Amirthaganesan, G.; Kandhaswamy, M.A.; Srinivasan, V. Synthesis and Characterization of Potassium Magnesium Sulphate Hexahydrate Crystals. Cryst. Res. Technol. 2006, 41, 328–331. [Google Scholar] [CrossRef]
- Gates-Rector, S.; Blanton, T. The Powder Diffraction File: A Quality Materials Characterization Database. Powder Diffr. 2019, 34, 352–360. [Google Scholar] [CrossRef]
- Ling, Z.C.; Wang, A. A Systematic Spectroscopic Study of Eight Hydrous Ferric Sulfates Relevant to Mars. Icarus 2010, 209, 422–433. [Google Scholar] [CrossRef]
- Shi, E.; Wang, A.; Ling, Z. MIR, VNIR, NIR, and Raman Spectra of Magnesium Chlorides with Six Hydration Degrees: Implication for Mars and Europa. J. Raman Spectrosc. 2020, 51, 1589–1602. [Google Scholar] [CrossRef]
- Shi, E.; Zeng, X.; Chen, J.; Ling, Z. Spectroscopic Analysis of Ca-Sr Sulfate Solid Solutions for In-Situ Observations on Mars. LPI Contrib. 2024, 3040, 1794. [Google Scholar]
- Marzougui, H.; Martin, I.R.; Attia-Essaies, S.; Hassen-Chehimi, D.B.; Lalla, E.; Léon-Luis, S.F. Site Selective Luminescence of Eu3+ Ions in K2Mg(SO4)2⋅6H2O Crystal. Opt. Mater. 2015, 46, 339–344. [Google Scholar] [CrossRef]
- Ju, E.; Shi, E.; Xin, Y.; Cao, H.; Liu, C.; Liu, P.; Chen, J.; Fu, X.; Ling, Z. Laboratory Synthesis, Spectroscopic Characteristics, and Conversion Relationships of Five Calcium Sulfate Double Salts Relevant to Mars. Icarus 2023, 401, 115610. [Google Scholar] [CrossRef]
- Cloutis, E.; Hawthorne, F.; Mertzman, S.; Krenn, K.; Craig, M.; Marcino, D.; Methot, M.; Strong, J.; Mustard, J.; Blaney, D. Detection and Discrimination of Sulfate Minerals Using Reflectance Spectroscopy. Icarus 2006, 184, 121–157. [Google Scholar] [CrossRef]
- Mikhailov, M.M.; Yuryev, S.A.; Lapin, A.N. Investigating Changes in the Diffuse Reflectance Spectra of BaSO4 Powders Modified with SiO2 Nanoparticles Exposed to Proton Irradiation. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2023, 539, 62–67. [Google Scholar] [CrossRef]
- De Angelis, S.; Carli, C.; Tosi, F.; Beck, P.; Schmitt, B.; Piccioni, G.; De Sanctis, M.C.; Capaccioni, F.; Di Iorio, T.; Philippe, S. Temperature-Dependent VNIR Spectroscopy of Hydrated Mg-Sulfates. Icarus 2017, 281, 444–458. [Google Scholar] [CrossRef]
- De Waele, J.; Carbone, C.; Sanna, L.; Vattano, M.; Galli, E.; Sauro, F.; Forti, P. Secondary Minerals from Salt Caves in the Atacama Desert (Chile): A Hyperarid and Hypersaline Environment with Potential Analogies to the Martian Subsurface. Int. J. Speleol. 2017, 46, 51–66. [Google Scholar] [CrossRef]
- Chojnacki, M.; Hynek, B.M. Geological Context of Water-altered Minerals in Valles Marineris, Mars. J. Geophys. Res. 2008, 113, 2007JE003070. [Google Scholar] [CrossRef]
- Hughes, E.B.; Gilmore, M.S.; Martin, P.E.; Eleazer, M. Visible to Near-Infrared Reflectance and Raman Spectra of Evaporites from Sulfate-Chloride Mars Analogue Brines. Icarus 2023, 401, 115597. [Google Scholar] [CrossRef]
- Lane, M.D.; Dyar, M.D.; Bishop, J.L. Spectroscopic Evidence for Hydrous Iron Sulfate in the Martian Soil. Geophys. Res. Lett. 2004, 31, 2004GL021231. [Google Scholar] [CrossRef]
- Altheide, T.; Chevrier, V.; Nicholson, C.; Denson, J. Experimental Investigation of the Stability and Evaporation of Sulfate and Chloride Brines on Mars. Earth Planet. Sci. Lett. 2009, 282, 69–78. [Google Scholar] [CrossRef]
- Moore, J.M.; Bullock, M.A.; Newsom, H.; Nelson, M. Laboratory Simulations of Mars Evaporite Geochemistry. J. Geophys. Res. 2010, 115, 2008JE003208. [Google Scholar] [CrossRef]
- Tosca, N.J.; McLennan, S.M.; Dyar, M.D.; Sklute, E.C.; Michel, F.M. Fe Oxidation Processes at Meridiani Planum and Implications for Secondary Fe Mineralogy on Mars. J. Geophys. Res. 2008, 113, 2007JE003019. [Google Scholar] [CrossRef]
- Chou, I.; Seal, R.R. Magnesium and Calcium Sulfate Stabilities and the Water Budget of Mars. J. Geophys. Res. 2007, 112, 2007JE002898. [Google Scholar] [CrossRef]
- García-Florentino, C.; Gomez-Nubla, L.; Huidobro, J.; Torre-Fdez, I.; Ruíz-Galende, P.; Aramendia, J.; Hausrath, E.M.; Castro, K.; Arana, G.; Madariaga, J.M. Interrelationships in the Gypsum–Syngenite–Görgeyite System and Their Possible Formation on Mars. Astrobiology 2021, 21, 332–344. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Shi, E.; Zeng, X.; Ju, E.; Ling, Z. Spectroscopies of Magnesium Sulfate Double Salts and Their Implication for Mars. 2024. Available online: https://figshare.com/articles/dataset/_b_Spectroscopies_of_magnesium_sulfate_double_salts_and_their_implication_for_Mars_b_/24999287 (accessed on 27 April 2024).

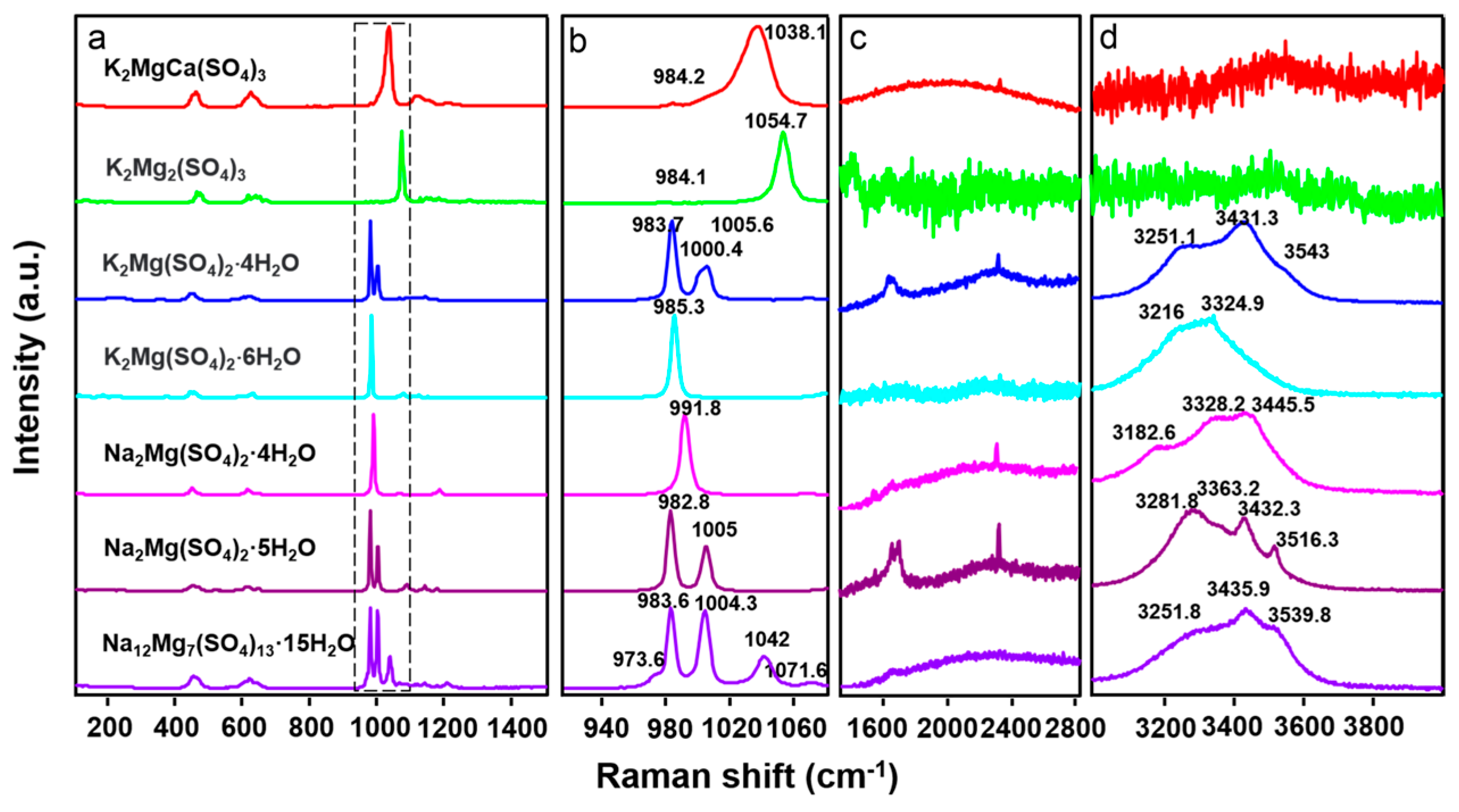

| Sample | H2O Mode | SO4 Vib. Mode | Others | |||||
|---|---|---|---|---|---|---|---|---|
| Stretching | Bending | Bending + Rocking | ν1 | ν2 | ν3 | ν4 | Lattice Vibration | |
| K2MgCa(SO4)3 | 984.2 | 452.9 | 1122.8 | 609.0 | ||||
| 1025.9 | 466.0 | 1154.7 | 625.4 | |||||
| 1038.1 | 1212.4 | 643.5 | ||||||
| 1235.8 | ||||||||
| K2Mg2(SO4)3 | 984.1 | 441.8 | 1109.1 | 606.8 | 128.2 | |||
| 1054.7 | 457.4 | 1124.2 | 618.2 | 139.5 | ||||
| 467.7 | 1136.8 | 628.0 | 183.0 | |||||
| 473.8 | 1151.3 | 640.0 | 202.3 | |||||
| 1162.8 | 658.5 | |||||||
| 1178.1 | ||||||||
| 1197.0 | ||||||||
| 1251.1 | ||||||||
| K2Mg(SO4)2·4H2O | 3251.1 | 1658.8 | 2329.9 | 983.7 | 447.1 | 1068.7 | 582.2 | 105.8 |
| 3431.3 | 1000.4 | 456.5 | 1063.6 | 604.7 | 115.8 | |||
| 3543.0 | 1005.6 | 465.5 | 1116.1 | 627.0 | 123.4 | |||
| 1130.5 | 131.9 | |||||||
| 1145.5 | 138.0 | |||||||
| 1179.7 | 144.0 | |||||||
| 160.5 | ||||||||
| 205.7 | ||||||||
| 220.8 | ||||||||
| 244.7 | ||||||||
| 343.6 | ||||||||
| K2Mg(SO4)2·6H2O | 3216.0 | 985.3 | 448.0 | 1080.3 | 612.0 | 116.8 | ||
| 3324.9 | 460.4 | 1110.7 | 632.7 | 139.2 | ||||
| 1125.8 | 187.2 | |||||||
| 1155.2 | 227.9 | |||||||
| 270.4 | ||||||||
| 303.9 | ||||||||
| 377.4 | ||||||||
| Na2Mg(SO4)2·4H2O | 3182.6 | 2329.0 | 991.8 | 454.9 | 1069.1 | 617.4 | 107.1 | |
| 3328.2 | 470.4 | 1114.6 | 629.2 | 126.5 | ||||
| 3445.5 | 1173.1 | 152.2 | ||||||
| 1188.1 | 181.2 | |||||||
| 197.3 | ||||||||
| 220.9 | ||||||||
| 260.8 | ||||||||
| 299.1 | ||||||||
| 351.6 | ||||||||
| Na2Mg(SO4)2·5H2O | 3281.8 | 2328.9 | 982.8 | 448.8 | 1090.3 | 613.9 | 106.0 | |
| 3363.2 | 1005.0 | 458.7 | 1130.6 | 622.8 | 122.4 | |||
| 3432.3 | 472.4 | 1145.1 | 650.1 | 145.6 | ||||
| 3516.3 | 1180.1 | 170.2 | ||||||
| 194.9 | ||||||||
| 217.8 | ||||||||
| 242.0 | ||||||||
| 254.4 | ||||||||
| Na12Mg7(SO4)13·15H2O | 3251.8 | 1653.3 | 2152.3 | 973.6 | 456.5 | 1071.6 | 608.9 | 119.5 |
| 3435.9 | 983.6 | 463.6 | 1120.5 | 626.2 | 141.0 | |||
| 3539.8 | 1004.3 | 473.3 | 1145.2 | 642.7 | 167.2 | |||
| 1042.0 | 1184.9 | 178.6 | ||||||
| 1212.3 | 205.3 | |||||||
| 219.3 | ||||||||
| 240.6 | ||||||||
| Sample | H2O Mode | SO4 Vib. Mode | |||||
|---|---|---|---|---|---|---|---|
| Stretching | Bending | Vibrational | ν1 | ν2 | ν3 | ν4 | |
| K2MgCa(SO4)3 | 417.9 | 1024.5 | 612.0 | ||||
| 423.3 | 1123.5 | 632.2 | |||||
| 442.1 | 654.4 | ||||||
| K2Mg2(SO4)3 | 1046.5 | 415.6 | 1116.8 | 605.5 | |||
| 426.4 | 1128.5 | 610.5 | |||||
| 437.1 | 1139.0 | 636.4 | |||||
| 448.1 | 1149.8 | 660.2 | |||||
| 1172.2 | 668.0 | ||||||
| K2Mg(SO4)2·4H2O | 3180.9 | 1604.0 | 773.2 | 981.9 | 428.9 | 1090.2 | 568.4 |
| 3365.9 | 1676.5 | 823.1 | 1003.0 | 437.5 | 1114.6 | 623.5 | |
| 3447.6 | 452.3 | 1129.4 | 644.7 | ||||
| 3524.5 | 477.0 | 1149.3 | 668.0 | ||||
| 3558.5 | 515.6 | 1169.3 | 714.7 | ||||
| 1185.3 | |||||||
| K2Mg(SO4)2·6H2O | 3187.5 | 1673.7 | 761.5 | 983.1 | 443.4 | 1077.0 | 584.6 |
| 3497.9 | 875.8 | 444.7 | 1160.0 | 609.1 | |||
| 456.9 | 1209.2 | 632.8 | |||||
| 513.6 | 705.6 | ||||||
| Na2Mg(SO4)2·4H2O | 3185.4 | 1596.5 | 834.9 | 993.3 | 423.5 | 1079.0 | 584.0 |
| 3460.6 | 1631.8 | 881.8 | 441.1 | 1116.9 | 620.4 | ||
| 1675.8 | 459.5 | 1160.5 | 662.3 | ||||
| 466.1 | 1185.9 | 725.8 | |||||
| 474.5 | |||||||
| Na2Mg(SO4)2·5H2O | 3219.8 | 1673.8 | 761.6 | 981.8 | 429.3 | 1080.8 | 573.5 |
| 3443.7 | 825.6 | 1003.5 | 444.6 | 1130.2 | 603.9 | ||
| 3516.5 | 456.1 | 1148.8 | 623.5 | ||||
| 3525.3 | 475.4 | 1186.7 | 644.6 | ||||
| 3562.0 | 480.4 | 668.0 | |||||
| 516.2 | 709.0 | ||||||
| Na12Mg7(SO4)13·15H2O | 3205.4 | 1660.1 | 773.9 | 983.8 | 406.4 | 1085.5 | 589.3 |
| 3425.9 | 997.7 | 418.4 | 1119.7 | 615.7 | |||
| 3574.6 | 451.2 | 1148.1 | 640.0 | ||||
| 1184.6 | 663.9 | ||||||
| K2Mg(SO4)2·4H2O | K2Mg(SO4)2·6H2O | Na2Mg(SO4)2·4H2O | Na2Mg(SO4)2·5H2O | Na12Mg7(SO4)13·15H2O | Assignment |
|---|---|---|---|---|---|
| 990 | 984 | 2 + | |||
| 1051 | 1040 | 1000 | 1000 | 3 or | |
| 1172 | 2 + | ||||
| 1268 | 1273 | 1263 | 1265 | + + | |
| 1541 | 1534 | 1469 | 1491 | 1442 | 2 or 2 |
| 1650 | 1656 | 1550 | 1638 | 2 or 2 | |
| 1709 | 1765 | + (or ) + | |||
| 1951 | 1963 | 1930 | + (or ) | ||
| 1993 | 1994 | 2080 | + (or ) | ||
| 2201 | (or ) + | ||||
| 2450 | 2441 | 2435 | 2445 | 2465 | + + |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, E.; Zhang, R.; Zeng, X.; Xin, Y.; Ju, E.; Ling, Z. Spectroscopy of Magnesium Sulfate Double Salts and Their Implications for Mars Exploration. Remote Sens. 2024, 16, 1592. https://doi.org/10.3390/rs16091592
Shi E, Zhang R, Zeng X, Xin Y, Ju E, Ling Z. Spectroscopy of Magnesium Sulfate Double Salts and Their Implications for Mars Exploration. Remote Sensing. 2024; 16(9):1592. https://doi.org/10.3390/rs16091592
Chicago/Turabian StyleShi, Erbin, Ruize Zhang, Xiaojia Zeng, Yanqing Xin, Enming Ju, and Zongcheng Ling. 2024. "Spectroscopy of Magnesium Sulfate Double Salts and Their Implications for Mars Exploration" Remote Sensing 16, no. 9: 1592. https://doi.org/10.3390/rs16091592
APA StyleShi, E., Zhang, R., Zeng, X., Xin, Y., Ju, E., & Ling, Z. (2024). Spectroscopy of Magnesium Sulfate Double Salts and Their Implications for Mars Exploration. Remote Sensing, 16(9), 1592. https://doi.org/10.3390/rs16091592







