Monitoring Helicoverpa armigera Damage with PRISMA Hyperspectral Imagery: First Experience in Maize and Comparison with Sentinel-2 Imagery
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Maize Field Measurement Methods
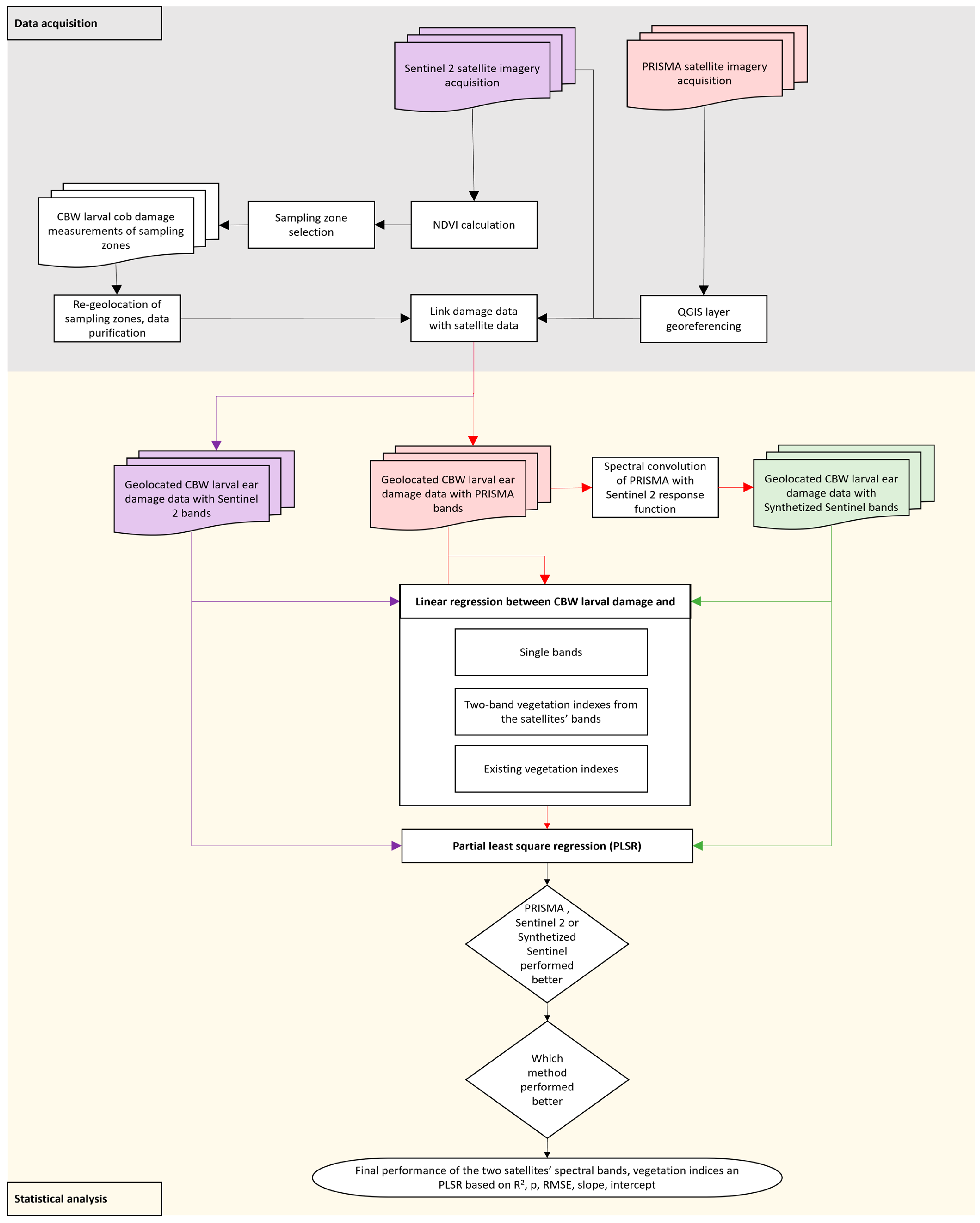
2.3. Satellite Imagery Acquisition and Processing
2.4. Data Analysis
2.4.1. Analysis of Variance
2.4.2. Two-Band Vegetation Indices (TBVIs) and the Selected Vegetation Indices
2.4.3. Partial Least Squares Regression (PLSR)
2.4.4. Model Performance Evaluation
3. Results
3.1. Cotton Bollworm Larval Damage to Maize Ears
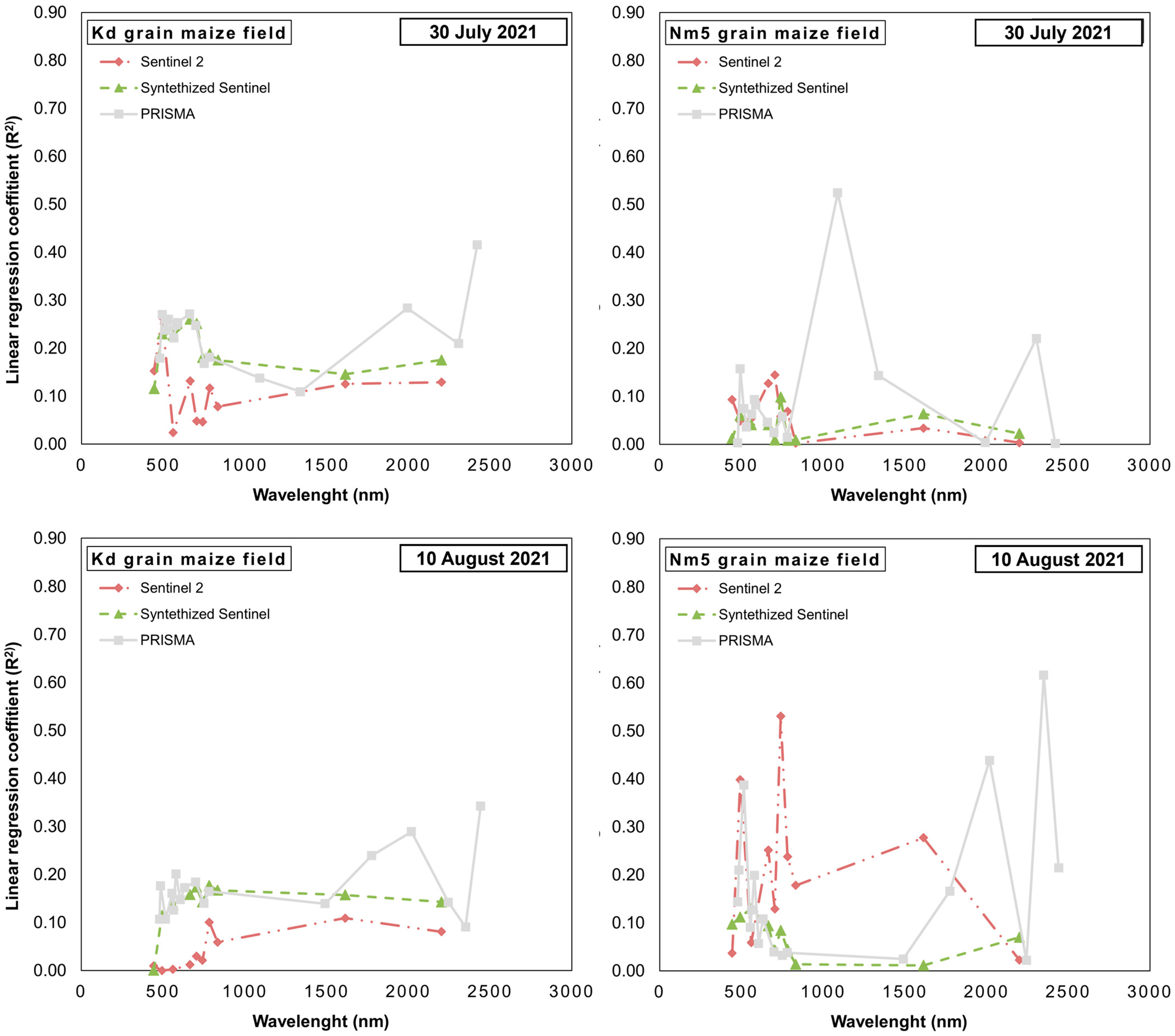
3.2. Cross-Sensor Comparison of Linear Regression of Single Bands with Maize Ear Damage by the CBW
3.3. Assessment of CBW Larval Damage of Maize Ears Using Partial Least Squared Regression
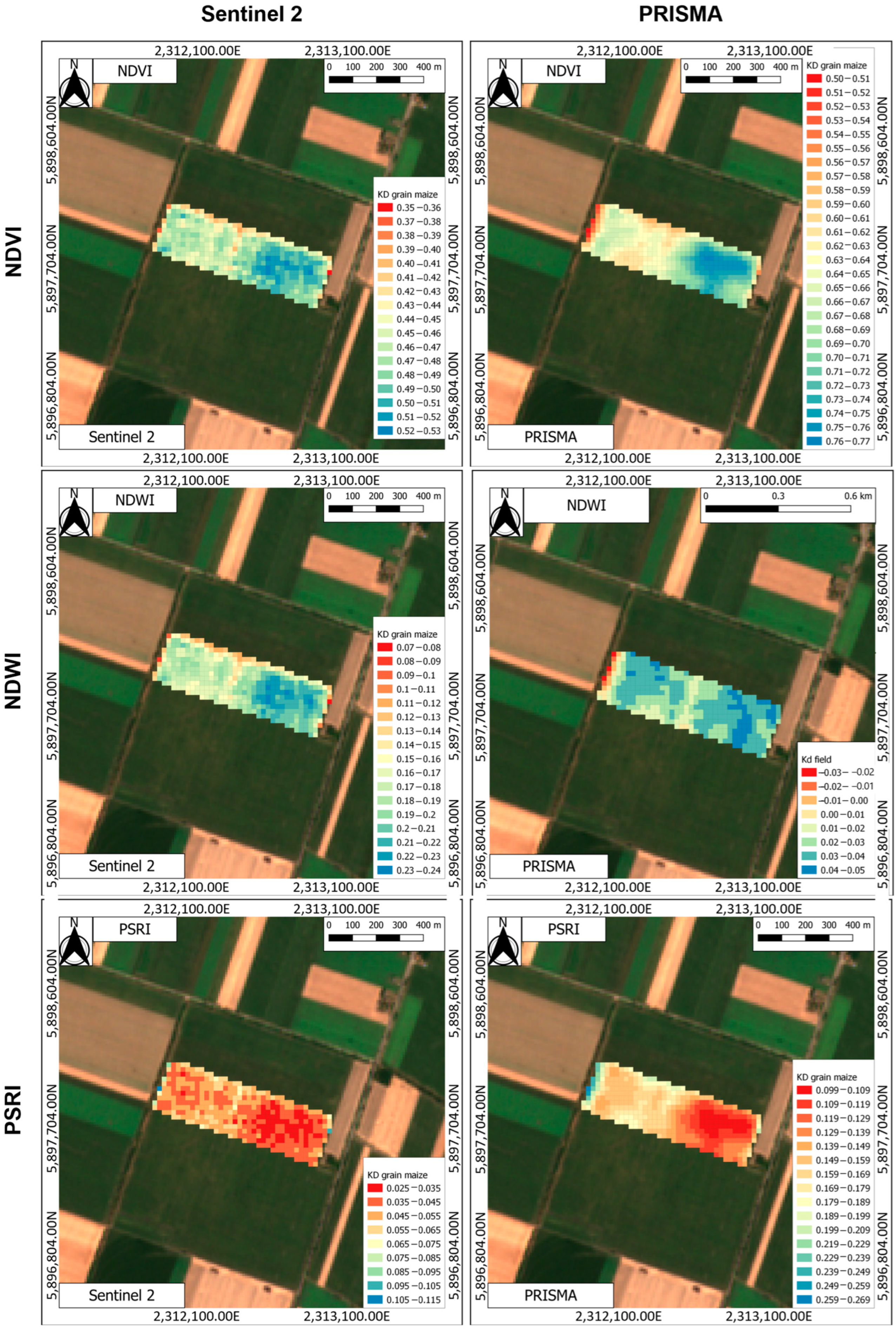
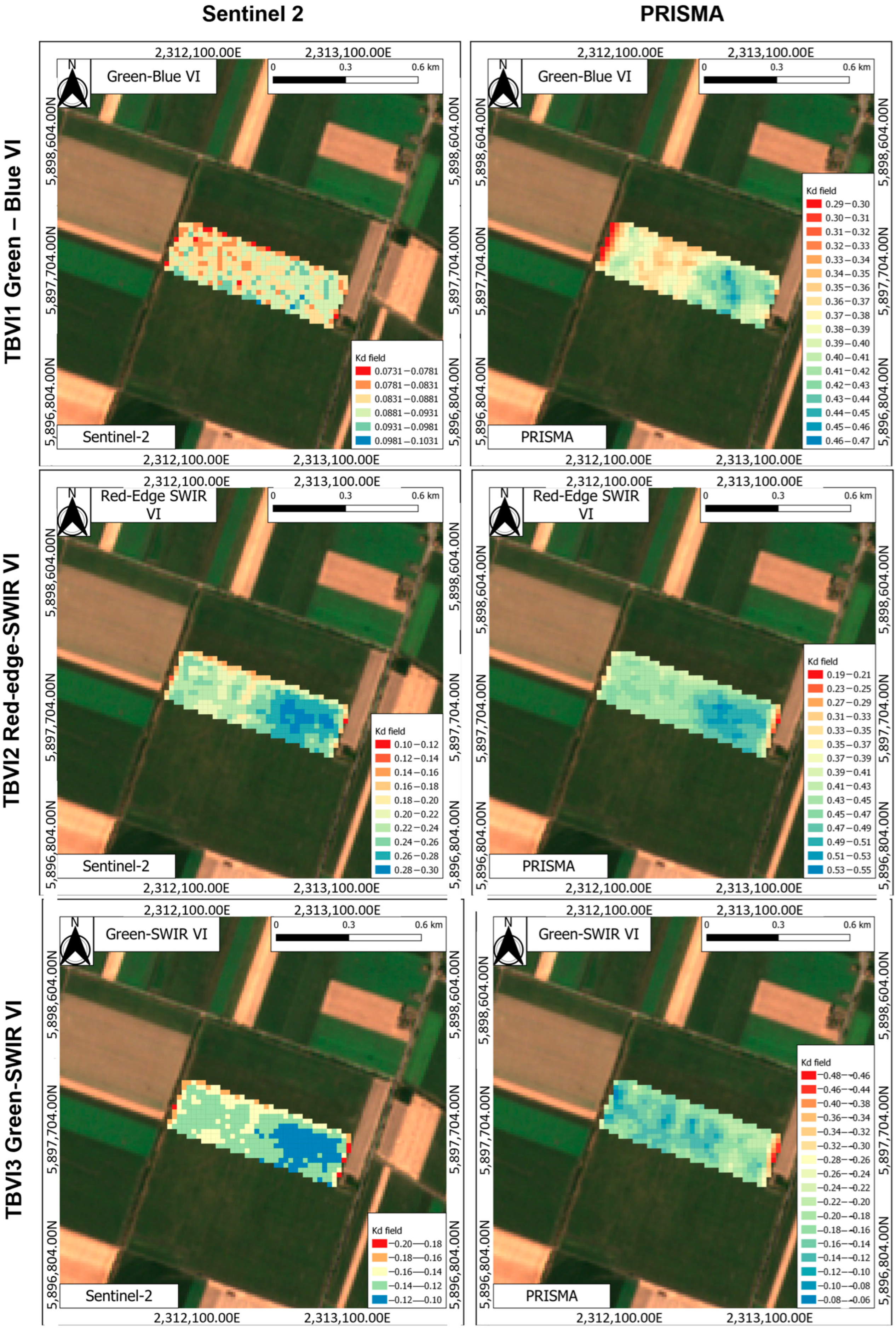
3.4. Assessment of CBW Larval Damage of Maize Ears Using Vegetation Indices
3.4.1. Results Obtained Using Two-Band Vegetation Indices (TBVIs)
Comparison of Selected Vegetation Indices
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Riaz, S.; Johnson, J.B.; Ahmad, M.; Fitt, G.P.; Naiker, M. A Review on Biological Interactions and Management of the Cotton Bollworm, Helicoverpa armigera (Lepidoptera: Noctuidae). J. Appl. Entomol. 2021, 145, 467–498. [Google Scholar] [CrossRef]
- Szőcs, G.; Tóth, M.; Újváry, I.; Szöcs, G.; Tóth, M.; Ujváry, I.; Szarukán, I. Development of a Pheromone Trap for Monitoring the Cotton Bollworm (Helicoverpa armigera Hbn.) an Upcoming Pest in Hungary. Növényvédelem 1995, 31, 261–266. [Google Scholar]
- Yadav, S.P.S.; Lahutiya, V.; Paudel, P. A Review on the Biology, Ecology, and Management Tactics of Helicoverpa armigera (Lepidoptera: Noctuidae). Turk. J. Agric.—Food Sci. Technol. 2022, 10, 2467–2476. [Google Scholar] [CrossRef]
- Kriticos, D.J.; Ota, N.; Hutchison, W.D.; Beddow, J.; Walsh, T.; Tay, W.T.; Borchert, D.M.; Paula-Moreas, S.V.; Czepak, C.; Zalucki, M.P. The Potential Distribution of Invading Helicoverpa armigera in North America: Is It Just a Matter of Time? PLoS ONE 2015, 10, e0119618. [Google Scholar] [CrossRef] [PubMed]
- EPPO Global Database. Available online: https://gd.eppo.int/taxon/HELIAR/distribution (accessed on 12 June 2024).
- Lopes, H.M.; Bastos, C.S.; Boiteux, L.S.; Foresti, J.; Suinaga, F.A. A RAPD-PCR-Based Genetic Diversity Analysis of Helicoverpa armigera and H. zea Populations in Brazil. Genet. Mol. Res. GMR 2017, 16, gmr16038757. [Google Scholar] [CrossRef]
- Sagar, D.; Nebapure, S.M.; Chander, S. Development and Validation of Weather Based Prediction Model for Helicoverpa armigera in Chickpea. J. Agrometeorol. 2017, 19, 328–333. [Google Scholar] [CrossRef]
- Keszthelyi, S.; Nowinszky, L.; Puskás, J. The Growing Abundance of Helicoverpa armigera in Hungary and Its Areal Shift Estimation. Cent. Eur. J. Biol. 2013, 8, 756–764. [Google Scholar] [CrossRef]
- Dömötör, I.; Kiss, J.; Szőcs, G. Coincidence of Silking Time of Corn, Zea Mays and Flight Period of Cotton Bollworm, Helicoverpa armigera Hbn.: How Does It Affect Follow-up Abundancy of Larvae on Cobs and Grain Damage in Various Corn Hybrids? Acta Phytopathol. Entomol. Hung. 2009, 44, 315–326. [Google Scholar] [CrossRef]
- Jin, M.; North, H.L.; Peng, Y.; Liu, H.; Liu, B.; Pan, R.; Zhou, Y.; Zheng, W.; Liu, K.; Yang, B.; et al. Adaptive Evolution to the Natural and Anthropogenic Environment in a Global Invasive Crop Pest, the Cotton Bollworm. Innovation 2023, 4, 100454. [Google Scholar] [CrossRef]
- Whitehouse, M.E.A.; Tann, C.R.; Braunack, M.V. Is ‘Pupae Busting’ or Destroying Overwintering Pupae of Helicoverpa Spp. (Lepidoptera: Noctuidae) Still Relevant Today in Australian Bt Cotton? Austral Entomol. 2023, 62, 392–409. [Google Scholar] [CrossRef]
- Lu, Q.; Li, Y.; Liao, J.; Ni, Z.; Xia, S.; Yang, M.; Li, H.; Guo, J. Histone Acetylation Is Associated with Pupal Diapause in Cotton Bollworm, Helicoverpa Armigera. Pest Manag. Sci. 2024, 80, 1400–1411. [Google Scholar] [CrossRef] [PubMed]
- Huang, J. Effects of Climate Change on Different Geographical Populations of the Cotton Bollworm Helicoverpa armigera (Lepidoptera, Noctuidae). Ecol. Evol. 2021, 11, 18357–18368. [Google Scholar] [CrossRef]
- Bapatla, K.G.; Singh, A.D.; Sengottaiyan, V.; Korada, R.R.; Yeddula, S. Impact of Climate Change on Helicoverpa Armigera Voltinism in Different Agro-Climatic Zones of India. J. Therm. Biol. 2022, 106, 103229. [Google Scholar] [CrossRef]
- Virić Gašparić, H.; Vučemilović Jurić, D.; Bažok, R. Assessment of a possible increase in the harmfulness of the cotton bollworm (Helicoverpa armigera Hubner) in Croatia. Entomol. Croat. 2022, 21, 1–9. [Google Scholar] [CrossRef]
- Srinivasa Rao, M.; Rama Rao, C.A.; Raju, B.M.K.; Subba Rao, A.V.M.; Gayatri, D.L.A.; Islam, A.; Prasad, T.V.; Navya, M.; Srinivas, K.; Pratibha, G.; et al. Pest Scenario of Helicoverpa armigera (Hub.) on Pigeonpea during Future Climate Change Periods under RCP Based Projections in India. Sci. Rep. 2023, 13, 6788. [Google Scholar] [CrossRef]
- Yang, L.; Li, M.; Liu, J.; Zeng, J.; Li, Y. Long-Term Expansion of Cereal Crops Promotes Regional Population Increase of Polyphagous Helicoverpa armigera. Available online: https://www.researchsquare.com (accessed on 17 November 2023).
- El Fakhouri, K.; Boulamtat, R.; Sabraoui, A.; El Bouhssini, M. The Chickpea Pod Borer, Helicoverpa Armigera (Hübner): Yield Loss Estimation and Biorational Insecticide Assessment in Morocco. Agronomy 2022, 12, 3017. [Google Scholar] [CrossRef]
- Fahad, M. Relationship Between Damage by Cotton Bollworm Helicoverpa armigera (Hübner) and Different Plant Characteristics of Bt and Non-Bt Cotton Varieties in Pakistan. PJZ 2023. Available online: https://researcherslinks.com/uploads/articles/1698678764PJZ_MH20230603100625-R1_Fahad%20et%20al.pdf (accessed on 12 June 2024). [CrossRef]
- Duffield, S.J.; Steer, A.P. The Ecology of Helicoverpa Spp. (Lepidoptera: Noctuidae) in the Riverina Region of South-Eastern Australia and the Implications for Tactical and Strategic Management. Bull. Entomol. Res. 2006, 96, 583–596. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, S.; Shirotsuka, K.; Shibao, M. Forecast of Peak Dates of Adult Emergence of Helicoverpa Armigera in Osaka Prefecture by Using a Simulation Program Based on the Total Effective Temperature Provided by JPP-NET. Annu. Rep. Kansai Plant Prot. Soc. 2017, 59, 105–108. [Google Scholar] [CrossRef]
- Ruzmetov, R.; Abdullaev, I.; Gandjaeva, L.; Matyakubov, Z.; Razzakov, K.; Iskandarov, A.; Otaev, O.; Ibragimov, S. Fundamentals of Using Geographical Information Systems in Predicting the Distribution of Helicoverpa armigera (Lepidoptera: Noctuidae). Biodiversitas J. Biol. Divers. 2022, 23, 3251–3256. [Google Scholar] [CrossRef]
- Jung, J.K.; Kim, S.B.; Seo, B.Y.; Kim, J.; Kim, D.-S. Temperature-Dependent Development of Helicoverpa armigera (Lepidoptera: Noctuidae) at Constant Temperatures: Instar Pathways and Stage Transition Models with Semifield Validation. J. Econ. Entomol. 2023, 116, 1689–1705. [Google Scholar] [CrossRef]
- Wu, K.M.; Guo, Y.Y. The Evolution of Cotton Pest Management Practices in China. Annu. Rev. Entomol. 2005, 50, 31–52. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.M.; Papanicolaou, A.; Mironidis, G.K.; Vontas, J.; Yang, Y.; Lim, K.S.; Oakeshott, J.G.; Bass, C.; Chapman, J.W. Genomewide Transcriptional Signatures of Migratory Flight Activity in a Globally Invasive Insect Pest. Mol. Ecol. 2015, 24, 4901–4911. [Google Scholar] [CrossRef] [PubMed]
- Hank, T.B.; Berger, K.; Bach, H.; Clevers, J.G.P.W.; Gitelson, A.; Zarco-Tejada, P.; Mauser, W. Spaceborne Imaging Spectroscopy for Sustainable Agriculture: Contributions and Challenges. Surv. Geophys. 2019, 40, 515–551. [Google Scholar] [CrossRef]
- Xu, C.; Ding, Y.; Zheng, X.; Wang, Y.; Zhang, R.; Zhang, H.; Dai, Z.; Xie, Q. A Comprehensive Comparison of Machine Learning and Feature Selection Methods for Maize Biomass Estimation Using Sentinel-1 SAR, Sentinel-2 Vegetation Indices, and Biophysical Variables. Remote Sens. 2022, 14, 4083. [Google Scholar] [CrossRef]
- Faqe Ibrahim, G.R.; Rasul, A.; Abdullah, H. Sentinel-2 Accurately Estimated Wheat Yield in a Semi-Arid Region Compared with Landsat 8. Int. J. Remote Sens. 2023, 44, 4115–4136. [Google Scholar] [CrossRef]
- Marshall, M.; Belgiu, M.; Boschetti, M.; Pepe, M.; Stein, A.; Nelson, A. Field-Level Crop Yield Estimation with PRISMA and Sentinel-2. ISPRS J. Photogramm. Remote Sens. 2022, 187, 191–210. [Google Scholar] [CrossRef]
- Chang, H.; Cai, J.; Zhang, B.; Wei, Z.; Xu, D. Early Yield Forecasting of Maize by Combining Remote Sensing Images and Field Data with Logistic Models. Remote Sens. 2023, 15, 1025. [Google Scholar] [CrossRef]
- Belgiu, M.; Marshall, M.; Boschetti, M.; Pepe, M.; Stein, A.; Nelson, A. PRISMA and Sentinel-2 Spectral Response to the Nutrient Composition of Grains. Remote Sens. Environ. 2023, 292, 113567. [Google Scholar] [CrossRef]
- Bossung, C.; Schlerf, M.; Machwitz, M. Estimation of Canopy Nitrogen Content in Winter Wheat from Sentinel-2 Images for Operational Agricultural Monitoring. Precis. Agric. 2022, 23, 2229–2252. [Google Scholar] [CrossRef]
- Uribeetxebarria, A.; Castellón, A.; Aizpurua, A. A First Approach to Determine If It Is Possible to Delineate In-Season N Fertilization Maps for Wheat Using NDVI Derived from Sentinel-2. Remote Sens. 2022, 14, 2872. [Google Scholar] [CrossRef]
- Dhau, I.; Dube, T.; Mushore, T.D. Examining the Prospects of Sentinel-2 Multispectral Data in Detecting and Mapping Maize Streak Virus Severity in Smallholder Ofcolaco Farms, South Africa. Geocarto. International. 2021, 36, 1873–1883. [Google Scholar] [CrossRef]
- Ruan, C.; Dong, Y.; Huang, W.; Huang, L.; Ye, H.; Ma, H.; Guo, A.; Ren, Y. Prediction of Wheat Stripe Rust Occurrence with Time Series Sentinel-2 Images. Agriculture 2021, 11, 1079. [Google Scholar] [CrossRef]
- Liu, L.; Dong, Y.; Huang, W.; Du, X.; Ren, B.; Huang, L.; Zheng, Q.; Ma, H. A Disease Index for Efficiently Detecting Wheat Fusarium Head Blight Using Sentinel-2 Multispectral Imagery. IEEE Access 2020, 8, 52181–52191. [Google Scholar] [CrossRef]
- Shi, Y.; Han, L.; González-Moreno, P.; Dancey, D.; Huang, W.; Zhang, Z.; Liu, Y.; Huang, M.; Miao, H.; Dai, M. A Fast Fourier Convolutional Deep Neural Network for Accurate and Explainable Discrimination of Wheat Yellow Rust and Nitrogen Deficiency from Sentinel-2 Time Series Data. Front. Plant Sci. 2023, 14, 1250844. [Google Scholar] [CrossRef]
- Ma, H.; Huang, W.; Jing, Y.; Yang, C.; Han, L.; Dong, Y.; Ye, H.; Shi, Y.; Zheng, Q.; Liu, L.; et al. Integrating Growth and Environmental Parameters to Discriminate Powdery Mildew and Aphid of Winter Wheat Using Bi-Temporal Landsat-8 Imagery. Remote Sens. 2019, 11, 846. [Google Scholar] [CrossRef]
- Fu, H.; Zhao, H.; Song, R.; Yang, Y.; Li, Z.; Zhang, S. Cotton Aphid Infestation Monitoring Using Sentinel-2 MSI Imagery Coupled with Derivative of Ratio Spectroscopy and Random Forest Algorithm. Front. Plant Sci. 2022, 13, 1029529. [Google Scholar] [CrossRef]
- Vilela, E.F.; Ferreira, W.P.M.; de Castro, G.D.M.; de Faria, A.L.R.; Leite, D.H.; Lima, I.A.; de Sousa Machado de Matos, C.; Silva, R.A.; Venzon, M. New Spectral Index and Machine Learning Models for Detecting Coffee Leaf Miner Infestation Using Sentinel-2 Multispectral Imagery. Agriculture 2023, 13, 388. [Google Scholar] [CrossRef]
- Ainunnisa, I.; Haerani, H. The Identification of Pests and Diseases of Rice Plants Using Sentinel-2 Satellite Imagery Data at the End of the Vegetative Stage. IOP Conf. Ser. Earth Environ. Sci. 2023, 1230, 012148. [Google Scholar] [CrossRef]
- Kara, S.; Maden, B.; Ercan, B.S.; Sunar, F.; Aysal, T.; Saglam, O. Assessing the Impact of Beet Webworm Moths on Sunflower Fields Using Multitemporal Sentinel-2 Satellite Imagery and Vegetation Indices. Int. Arch. Photogramm. Remote Sens. Spat. Inf. Sci. 2023, XLVIII-M-1–2023, 521–527. [Google Scholar] [CrossRef]
- Buchaillot, M.L.; Cairns, J.; Hamadziripi, E.; Wilson, K.; Hughes, D.; Chelal, J.; McCloskey, P.; Kehs, A.; Clinton, N.; Araus, J.L.; et al. Regional Monitoring of Fall Armyworm (FAW) Using Early Warning Systems. Remote Sens. 2022, 14, 5003. [Google Scholar] [CrossRef]
- Huang, Y.; Lv, H.; Dong, Y.; Huang, W.; Hu, G.; Liu, Y.; Chen, H.; Geng, Y.; Bai, J.; Guo, P.; et al. Mapping the Spatio-Temporal Distribution of Fall Armyworm in China by Coupling Multi-Factors. Remote Sens. 2022, 14, 4415. [Google Scholar] [CrossRef]
- Prabhakar, M.; Gopinath, K.A.; Kumar, N.R.; Thirupathi, M.; Sravan, U.S.; Kumar, G.S.; Siva, G.S.; Meghalakshmi, G.; Vennila, S. Detecting the Invasive Fall Armyworm Pest Incidence in Farm Fields of Southern India Using Sentinel-2A Satellite Data. Geocarto Int. 2022, 37, 3801–3816. [Google Scholar] [CrossRef]
- Adan, M.; Tonnang, H.E.Z.; Greve, K.; Borgemeister, C.; Goergen, G. Use of Time Series Normalized Difference Vegetation Index (NDVI) to Monitor Fall Armyworm (Spodoptera Frugiperda) Damage on Maize Production Systems in Africa. Geocarto Int. 2023, 38, 2186492. [Google Scholar] [CrossRef]
- Sári-Barnácz, F.E.; Szalai, M.; Kun, M.; Iványi, D.; Chaddadi, M.; Barnácz, F.M.; Kiss, J. Satellite-Based Spectral Indices for Monitoring Helicoverpa armigera Damage in Maize. In Precision Agriculture ’21; Wageningen Academic Publishers: Wageningen, The Netherlands, 2021; pp. 209–216. ISBN 978-90-8686-363-1. [Google Scholar]
- Sári-Barnácz, F.E.; Zalai, M.; Toepfer, S.; Milics, G.; Iványi, D.; Tóthné Kun, M.; Mészáros, J.; Árvai, M.; Kiss, J. Suitability of Satellite Imagery for Surveillance of Maize Ear Damage by Cotton Bollworm (Helicoverpa armigera) Larvae. Remote Sens. 2023, 15, 5602. [Google Scholar] [CrossRef]
- Weiss, M.; Jacob, F.; Duveiller, G. Remote Sensing for Agricultural Applications: A Meta-Review. Remote Sens. Environ. 2020, 236, 111402. [Google Scholar] [CrossRef]
- Wan, L.; Li, H.; Li, C.; Wang, A.; Yang, Y.; Wang, P. Hyperspectral Sensing of Plant Diseases: Principle and Methods. Agronomy 2022, 12, 1451. [Google Scholar] [CrossRef]
- Cheshkova, A.F. A Review of Hyperspectral Image Analysis Techniques for Plant Disease Detection and Identif Ication. Vavilovskii Zhurnal Genet. Sel. 2022, 26, 202–213. [Google Scholar] [CrossRef]
- Liu, C.; Cao, Y.; Wu, E.; Yang, R.; Xu, H.; Qiao, Y. A Discriminative Model for Early Detection of Anthracnose in Strawberry Plants Based on Hyperspectral Imaging Technology. Remote Sens. 2023, 15, 4640. [Google Scholar] [CrossRef]
- Navrozidis, I.; Pantazi, X.E.; Lagopodi, A.; Bochtis, D.; Alexandridis, T.K. Application of Machine Learning for Disease Detection Tasks in Olive Trees Using Hyperspectral Data. Remote Sens. 2023, 15, 5683. [Google Scholar] [CrossRef]
- Ramamoorthy, P.; Samiappan, S.; Wubben, M.J.; Brooks, J.P.; Shrestha, A.; Panda, R.M.; Reddy, K.R.; Bheemanahalli, R. Hyperspectral Reflectance and Machine Learning Approaches for the Detection of Drought and Root–Knot Nematode Infestation in Cotton. Remote Sens. 2022, 14, 4021. [Google Scholar] [CrossRef]
- Pinto, J.; Powell, S.; Peterson, R.; Rosalen, D.; Fernandes, O. Detection of Defoliation Injury in Peanut with Hyperspectral Proximal Remote Sensing. Remote Sens. 2020, 12, 3828. [Google Scholar] [CrossRef]
- Cogliati, S.; Sarti, F.; Chiarantini, L.; Cosi, M.; Lorusso, R.; Lopinto, E.; Miglietta, F.; Genesio, L.; Guanter, L.; Damm, A.; et al. The PRISMA Imaging Spectroscopy Mission: Overview and First Performance Analysis. Remote Sens. Environ. 2021, 262, 112499. [Google Scholar] [CrossRef]
- Rast, M.; Painter, T.H. Earth Observation Imaging Spectroscopy for Terrestrial Systems: An Overview of Its History, Techniques, and Applications of Its Missions. Surv. Geophys. 2019, 40, 303–331. [Google Scholar] [CrossRef]
- Niroumand-Jadidi, M.; Bovolo, F.; Bruzzone, L. Water Quality Retrieval from PRISMA Hyperspectral Images: First Experience in a Turbid Lake and Comparison with Sentinel-2. Remote Sens. 2020, 12, 3984. [Google Scholar] [CrossRef]
- Katlane, R.; Doxaran, D.; ElKilani, B.; Trabelsi, C. Remote Sensing of Turbidity in Optically Shallow Waters Using Sentinel-2 MSI and PRISMA Satellite Data. PFG 2023, 92, 431–447. [Google Scholar] [CrossRef]
- Mzid, N.; Castaldi, F.; Tolomio, M.; Pascucci, S.; Casa, R.; Pignatti, S. Evaluation of Agricultural Bare Soil Properties Retrieval from Landsat 8, Sentinel-2 and PRISMA Satellite Data. Remote Sens. 2022, 14, 714. [Google Scholar] [CrossRef]
- Nico, G.; Masci, O.; Mira, N.C.; Catalão, J.; Mateus, P. Estimating Soil Moisture by Sentinel-1, Sentinel-2 and PRISMA Data: Assessment of Results and Comparison with in-Situ Measurements. In Proceedings of the IGARSS 2023—2023 IEEE International Geoscience and Remote Sensing Symposium, Pasadena, CA, USA, 16–21 July 2023; pp. 2645–2648. [Google Scholar]
- Dutta, A.; Tyagi, R.; Chattopadhyay, A.; Chatterjee, D.; Sarkar, A.; Lall, B.; Sharma, S. Early Detection of Wilt in Cajanus Cajan Using Satellite Hyperspectral Images: Development and Validation of Disease-Specific Spectral Index with Integrated Methodology. Comput. Electron. Agric. 2024, 219, 108784. [Google Scholar] [CrossRef]
- Jallow, M.; Matsumura, M. Influence of Temperature on the Rate of Development of Helicoverpa armigera (Hübner) (Lepidoptera: Noctuidae). Appl. Entomol. Zool. 2001, 36, 427–430. [Google Scholar] [CrossRef][Green Version]
- Mironidis, G.K. Development, Survivorship and Reproduction of Helicoverpa armigera (Lepidoptera: Noctuidae) under Fluctuating Temperatures. Bull. Entomol. Res. 2014, 104, 751–764. [Google Scholar] [CrossRef]
- PRISMA Product Specifications. Available online: https://prisma.asi.it/missionselect/docs/PRISMA%20Product%20Specifications_Is2_3.pdf (accessed on 12 June 2024).
- Laben, C.A.; Brower, B.V. Process for Enhancing the Spatial Resolution of Multispectral Imagery Using Pan-Sharpening. U.S. Patent 6011875, 4 January 2000. [Google Scholar]
- Loncan, L.; de Almeida, L.B.; Bioucas-Dias, J.M.; Briottet, X.; Chanussot, J.; Dobigeon, N.; Fabre, S.; Liao, W.; Licciardi, G.A.; Simões, M.; et al. Hyperspectral Pansharpening: A Review. IEEE Geosci. Remote Sens. Mag. 2015, 3, 27–46. [Google Scholar] [CrossRef]
- Sech, G.; Poggi, G.; Ljubenovic, M.; Fiorucci, M.; Traviglia, A. Pansharpening of PRISMA products for archaeological prospection. arXiv 2024. [Google Scholar] [CrossRef]
- Sola, I.; García-Martín, A.; Sandonís-Pozo, L.; Álvarez-Mozos, J.; Pérez-Cabello, F.; González-Audícana, M.; Montorio Llovería, R. Assessment of Atmospheric Correction Methods for Sentinel-2 Images in Mediterranean Landscapes. Int. J. Appl. Earth Obs. Geoinf. 2018, 73, 63–76. [Google Scholar] [CrossRef]
- Main-Knorn, M.; Pflug, B.; Debaecker, V.; Louis, J. Calibration and validation plan for the l2a processor and products of the sentinel-2 mission. Int. Arch. Photogramm. Remote Sens. Spat. Inf. Sci. 2015, XL-7-W3, 1249–1255. [Google Scholar] [CrossRef]
- RStudio Team. RStudio: Integrated Development Environment for R; RStudio, PBC.: Boston, MA, USA, 2020. [Google Scholar]
- Wickham, H. Reshaping Data with the Reshape Package. J. Stat. Softw. 2007, 21, 1–20. [Google Scholar] [CrossRef]
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016; ISBN 978-3-319-24277-4. [Google Scholar]
- Wickham, H.; François, R.; Henry, L.; Müller, K.; Vaughan, D. Dplyr: A Grammar of Data Manipulation; 2023. Available online: https://dplyr.tidyverse.org (accessed on 17 April 2023).
- Mevik, B.-H.; Wehrens, R. The Pls Package: Principal Component and Partial Least Squares Regression in R. J. Stat. Softw. 2007, 18, 1–23. [Google Scholar] [CrossRef]
- Kuhn, M. Building Predictive Models in R Using the Caret Package. J. Stat. Softw. 2008, 28, 1–26. [Google Scholar] [CrossRef]
- Kucheryavskiy, S. Mdatools—R Package for Chemometrics. Chemom. Intell. Lab. Syst. 2020, 198, 103937. [Google Scholar] [CrossRef]
- Bannari, A.; Morin, D.; Bonn, F.; Huete, A.R. A review of vegetation indices. Remote Sens. Rev. 1995, 13, 95–120. [Google Scholar] [CrossRef]
- Thenkabail, P.S.; Smith, R.B.; De Pauw, E. Hyperspectral Vegetation Indices and Their Relationships with Agricultural Crop Characteristics. Remote Sens. Environ. 2000, 71, 158–182. [Google Scholar] [CrossRef]
- Gao, B. NDWI—A Normalized Difference Water Index for Remote Sensing of Vegetation Liquid Water from Space. Remote Sens. Environ. 1996, 58, 257–266. [Google Scholar] [CrossRef]
- Knipling, E.B. Physical and Physiological Basis for the Reflectance of Visible and Near-Infrared Radiation from Vegetation. Remote Sens. Environ. 1970, 1, 155–159. [Google Scholar] [CrossRef]
- Merzlyak, M.N.; Gitelson, A.A.; Chivkunova, O.B.; Rakitin, V.Y. Non-Destructive Optical Detection of Pigment Changes during Leaf Senescence and Fruit Ripening. Physiol. Plant. 1999, 106, 135–141. [Google Scholar] [CrossRef]
- Abdi, H. Partial Least Squares Regression and Projection on Latent Structure Regression (PLS Regression). WIREs Comput. Stat. 2010, 2, 97–106. [Google Scholar] [CrossRef]
- Montesinos-López, O.A.; Montesinos-López, A.; Crossa, J.; de los Campos, G.; Alvarado, G.; Suchismita, M.; Rutkoski, J.; González-Pérez, L.; Burgueño, J. Predicting Grain Yield Using Canopy Hyperspectral Reflectance in Wheat Breeding Data. Plant Methods 2017, 13, 4. [Google Scholar] [CrossRef]
- Li, B.; Xu, X.; Zhang, L.; Han, J.; Bian, C.; Li, G.; Liu, J.; Jin, L. Above-Ground Biomass Estimation and Yield Prediction in Potato by Using UAV-Based RGB and Hyperspectral Imaging. ISPRS J. Photogramm. Remote Sens. 2020, 162, 161–172. [Google Scholar] [CrossRef]
- Jiang, X.; Zhen, J.; Miao, J.; Zhao, D.; Shen, Z.; Jiang, J.; Gao, C.; Wu, G.; Wang, J. Newly-Developed Three-Band Hyperspectral Vegetation Index for Estimating Leaf Relative Chlorophyll Content of Mangrove under Different Severities of Pest and Disease. Ecol. Indic. 2022, 140, 108978. [Google Scholar] [CrossRef]
- Feng, J.; Sun, Y.; Zhang, K.; Zhao, Y.; Ren, Y.; Chen, Y.; Zhuang, H.; Chen, S. Autonomous Detection of Spodoptera Frugiperda by Feeding Symptoms Directly from UAV RGB Imagery. Appl. Sci. 2022, 12, 2592. [Google Scholar] [CrossRef]
- Fu, J.; Liu, J.; Zhao, R.; Chen, Z.; Qiao, Y.; Li, D. Maize Disease Detection Based on Spectral Recovery from RGB Images. Front. Plant Sci. 2022, 13, 1056842. [Google Scholar] [CrossRef]
- Betti Sorbelli, F.; Palazzetti, L.; Pinotti, C.M. YOLO-Based Detection of Halyomorpha Halys in Orchards Using RGB Cameras and Drones. Comput. Electron. Agric. 2023, 213, 108228. [Google Scholar] [CrossRef]
- Guo, Q.; Wang, C.; Xiao, D.; Huang, Q. Automatic Monitoring of Flying Vegetable Insect Pests Using an RGB Camera and YOLO-SIP Detector. Precis. Agric. 2023, 24, 436–457. [Google Scholar] [CrossRef]
- Hoseny, M.M.E.; Dahi, H.F.; Shafei, A.M.E.; Yones, M.S. Spectroradiometer and Thermal Imaging as Tools from Remote Sensing Used for Early Detection of Spiny Bollworm, Earias insulana (Boisd.) Infestation. Int. J. Trop. Insect Sci. 2023, 43, 245–256. [Google Scholar] [CrossRef]
- Schlemmer, M.; Gitelson, A.; Schepers, J.; Ferguson, R.; Peng, Y.; Shanahan, J.; Rundquist, D. Remote Estimation of Nitrogen and Chlorophyll Contents in Maize at Leaf and Canopy Levels. Int. J. Appl. Earth Obs. Geoinf. 2013, 25, 47–54. [Google Scholar] [CrossRef]
- Casas, A.; Riaño, D.; Ustin, S.L.; Dennison, P.; Salas, J. Estimation of Water-Related Biochemical and Biophysical Vegetation Properties Using Multitemporal Airborne Hyperspectral Data and Its Comparison to MODIS Spectral Response. Remote Sens. Environ. 2014, 148, 28–41. [Google Scholar] [CrossRef]
- Ullah, M.I.; Arshad, M.; Khan, M.I.; Afzal, M.; Khan, A.A.; Zahid, S.M.A.; Saqib, M.; Abdullah, A.; Kousar, S.; Riaz, M. Plant Water Stress Affects the Feeding Performance of American Bollworm, Helicoverpa armigera (Lepidoptera: Noctuidae) on Cotton Plants. Pak. J. Agric. Res. 2019, 32, 629–635. [Google Scholar] [CrossRef]
- Wei, G.; Zhang, Q.; Zhou, M.; Wu, W. Characteristic response of the compound eyes of Helicoverpa armigera to ligh. Kun Chong Xue Bao Acta Entomol. Sin. 2002, 45, 323–328. [Google Scholar]
- Gu, G.; Chen, X.; Han, J.; Ge, H.; Chen, H. Study on Phototaxis Action of Moth of Cotton Bollwor. J. Tianjin Agric. Coll. 2004, 11, 32–36. [Google Scholar]
- Satoh, A.; Kinoshita, M.; Arikawa, K. Innate Preference and Learning of Colour in the Male Cotton Bollworm Moth, Helicoverpa armigera. J. Exp. Biol. 2016, 219, 3857–3860. [Google Scholar] [CrossRef]
- Wang, L.; Wang, J.; Liu, Z.; Zhu, J.; Qin, F. Evaluation of a Deep-Learning Model for Multispectral Remote Sensing of Land Use and Crop Classification. Crop J. 2022, 10, 1435–1451. [Google Scholar] [CrossRef]
- Kurihara, J.; Koo, V.-C.; Guey, C.W.; Lee, Y.P.; Abidin, H. Early Detection of Basal Stem Rot Disease in Oil Palm Tree Using Unmanned Aerial Vehicle-Based Hyperspectral Imaging. Remote Sens. 2022, 14, 799. [Google Scholar] [CrossRef]
- Nguyen, C.; Sagan, V.; Maimaitiyiming, M.; Maimaitijiang, M.; Bhadra, S.; Kwasniewski, M.T. Early Detection of Plant Viral Disease Using Hyperspectral Imaging and Deep Learning. Sensors 2021, 21, 742. [Google Scholar] [CrossRef]
- Tomaszewski, M.; Nalepa, J.; Moliszewska, E.; Ruszczak, B.; Smykała, K. Early Detection of Solanum Lycopersicum Diseases from Temporally-Aggregated Hyperspectral Measurements Using Machine Learning. Sci. Rep. 2023, 13, 7671. [Google Scholar] [CrossRef]
- Bae, S.; Müller, J.; Förster, B.; Hilmers, T.; Hochrein, S.; Jacobs, M.; Leroy, B.M.L.; Pretzsch, H.; Weisser, W.W.; Mitesser, O. Tracking the Temporal Dynamics of Insect Defoliation by High-Resolution Radar Satellite Data. Methods Ecol. Evol. 2022, 13, 121–132. [Google Scholar] [CrossRef]
- Belgiu, M.; Csillik, O. Sentinel-2 Cropland Mapping Using Pixel-Based and Object-Based Time-Weighted Dynamic Time Warping Analysis. Remote Sens. Environ. 2018, 204, 509–523. [Google Scholar] [CrossRef]
- Rustowicz, R.M.; Cheong, R.; Wang, L.; Ermon, S.; Burke, M.; Lobell, D. Semantic Segmentation of Crop Type in Africa: A Novel Dataset and Analysis of Deep Learning Methods. In Proceedings of the IEEE/CVF Conference on Computer Vision and Pattern Recognition (CVPR), Long Beach, CA, USA, 15–20 June 2019; pp. 75–82. [Google Scholar]
- Obasekore, H.; Fanni, M.; Ahmed, S.M.; Parque, V.; Kang, B.-Y. Agricultural Robot-Centered Recognition of Early-Developmental Pest Stage Based on Deep Learning: A Case Study on Fall Armyworm (Spodoptera Frugiperda). Sensors 2023, 23, 3147. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chen, Y.; Huang, J.; Jiang, X.; Wan, K. Leveraging Machine Learning for Advancing Insect Pest Control: A Bibliometric Analysis. J. Appl. Entomol. 2024, 1–16. [Google Scholar] [CrossRef]
- Xiao, Z.; Yin, K.; Geng, L.; Wu, J.; Zhang, F.; Liu, Y. Pest Identification via Hyperspectral Image and Deep Learning. SIViP 2022, 16, 873–880. [Google Scholar] [CrossRef]
- Zhao, N.; Zhou, L.; Huang, T.; Taha, M.F.; He, Y.; Qiu, Z. Development of an Automatic Pest Monitoring System Using a Deep Learning Model of DPeNet. Measurement 2022, 203, 111970. [Google Scholar] [CrossRef]
- Chodey, M.D.; Noorullah Shariff, C. Hybrid Deep Learning Model for In-Field Pest Detection on Real-Time Field Monitoring. J. Plant Dis. Prot. 2022, 129, 635–650. [Google Scholar] [CrossRef]
- Kathole, A.B.; Vhatkar, K.N.; Patil, S.D. IoT-Enabled Pest Identification and Classification with New Meta-Heuristic-Based Deep Learning Framework. Cybern. Syst. 2024, 55, 380–408. [Google Scholar] [CrossRef]
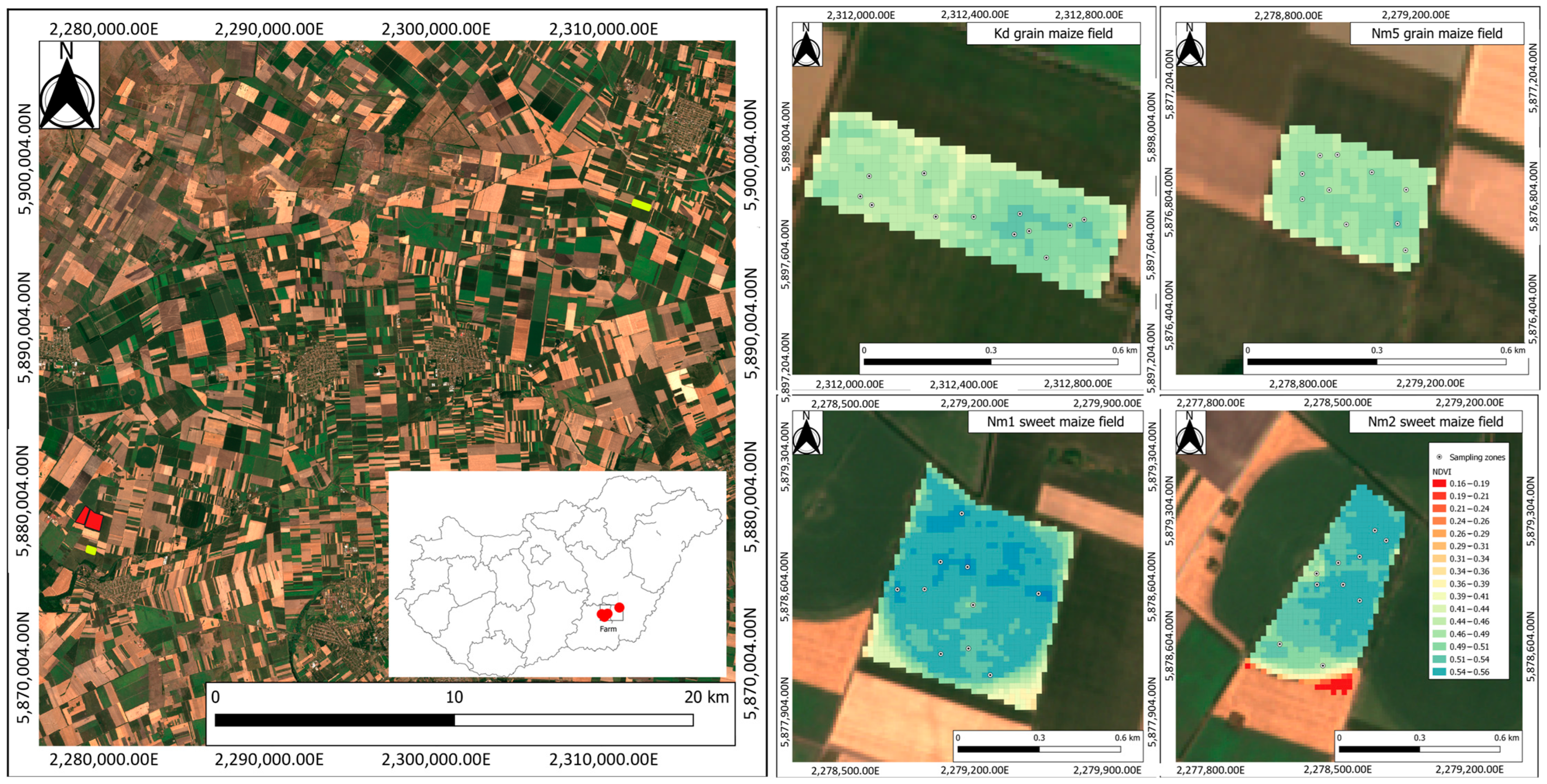


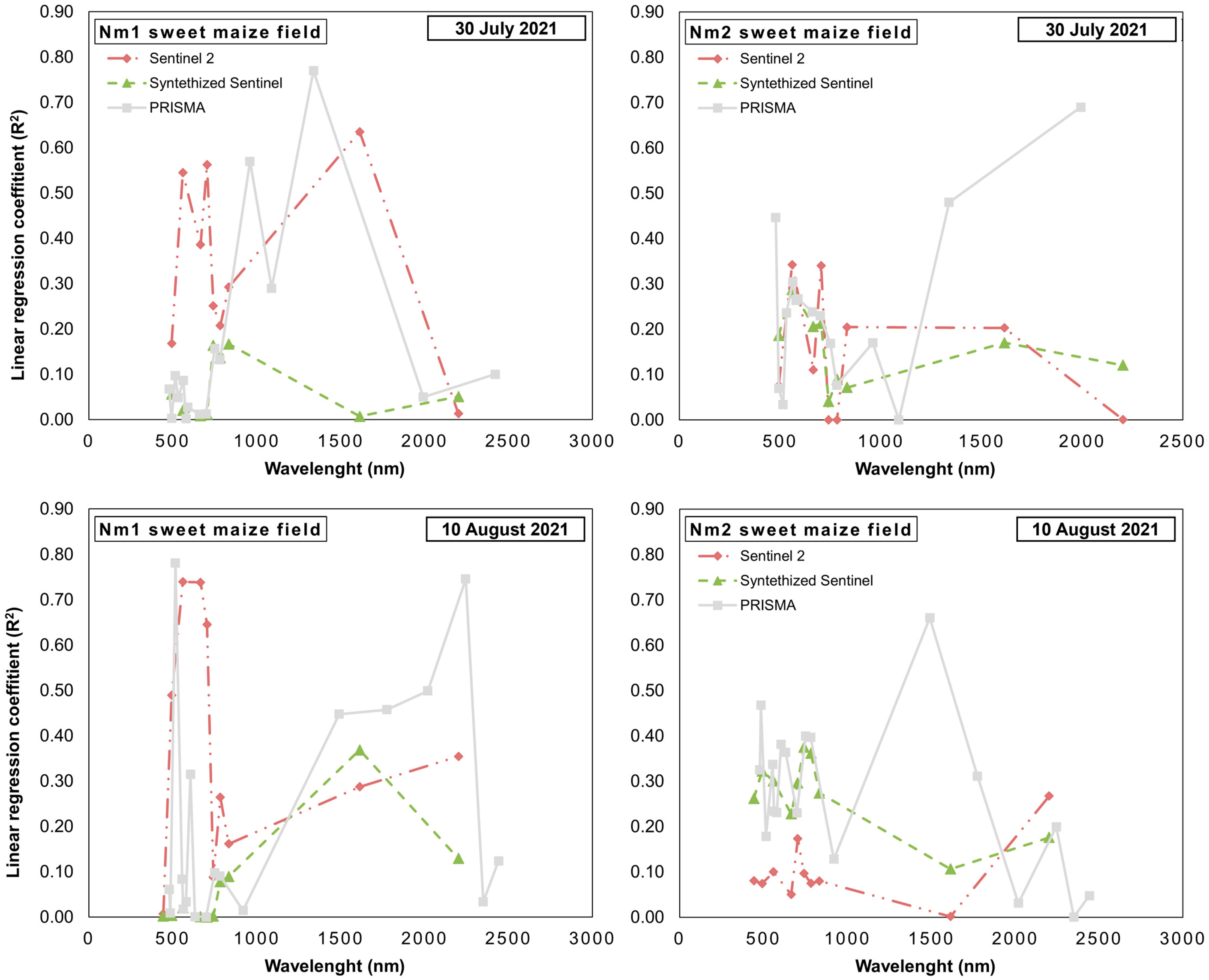
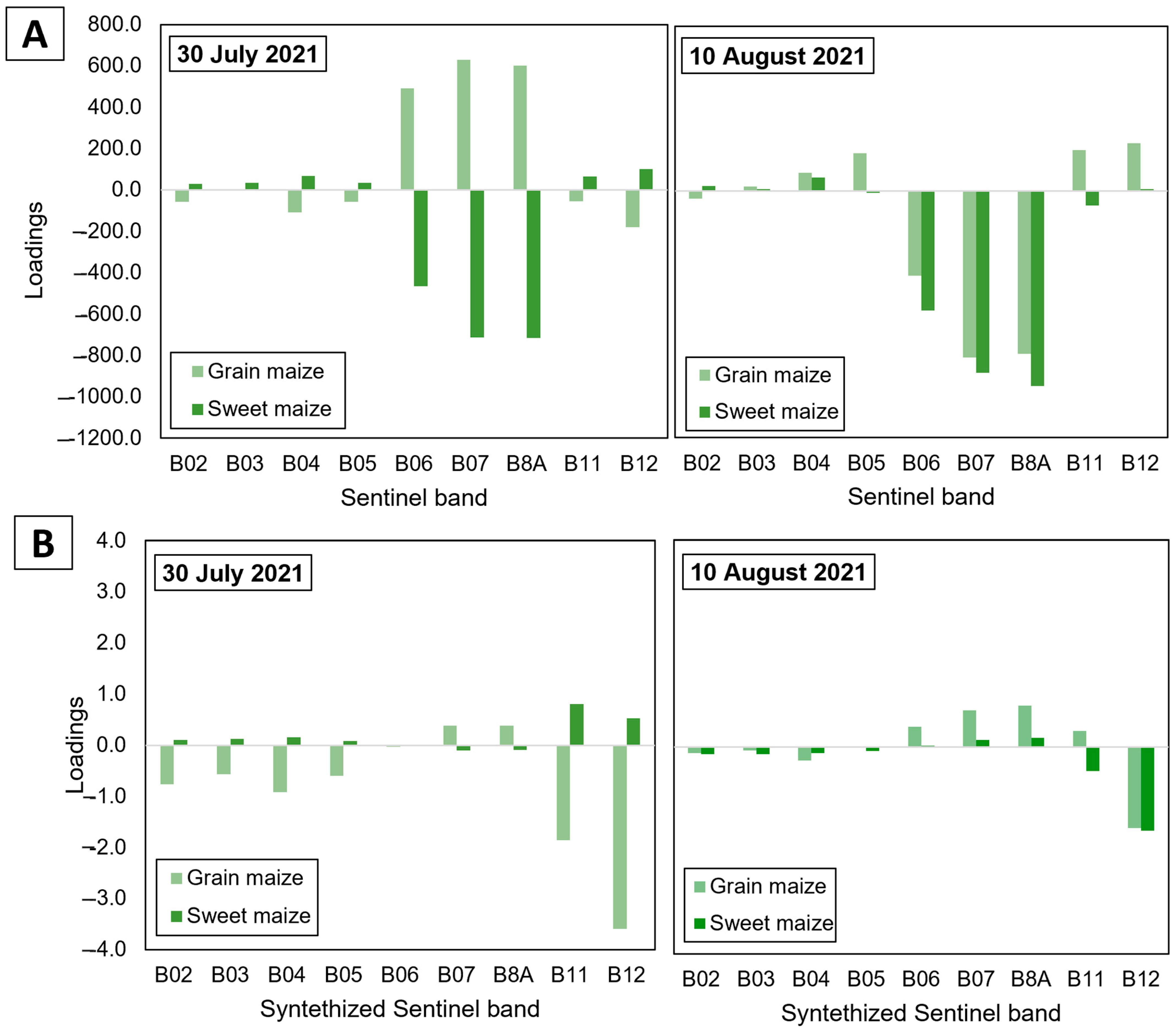
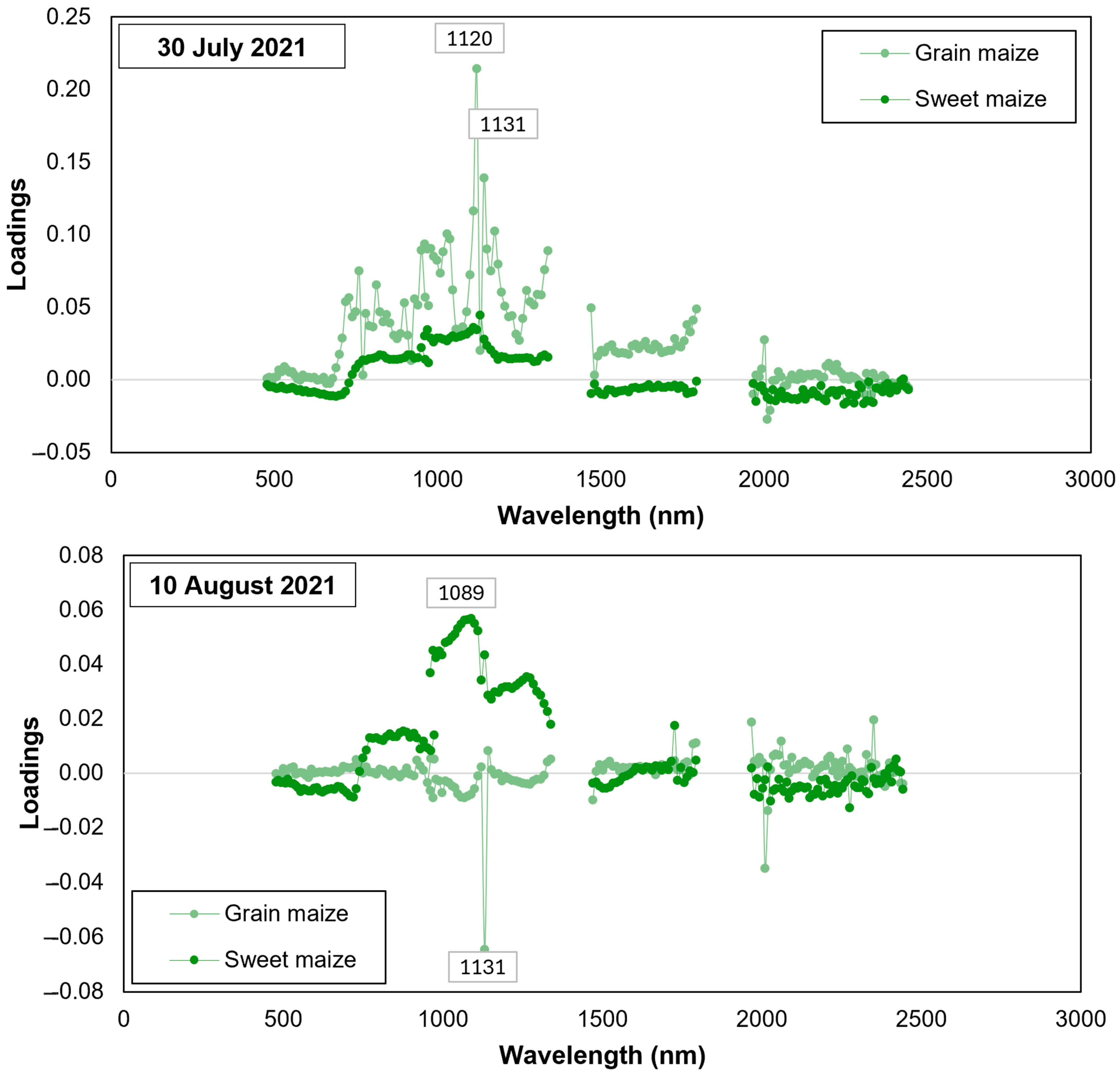
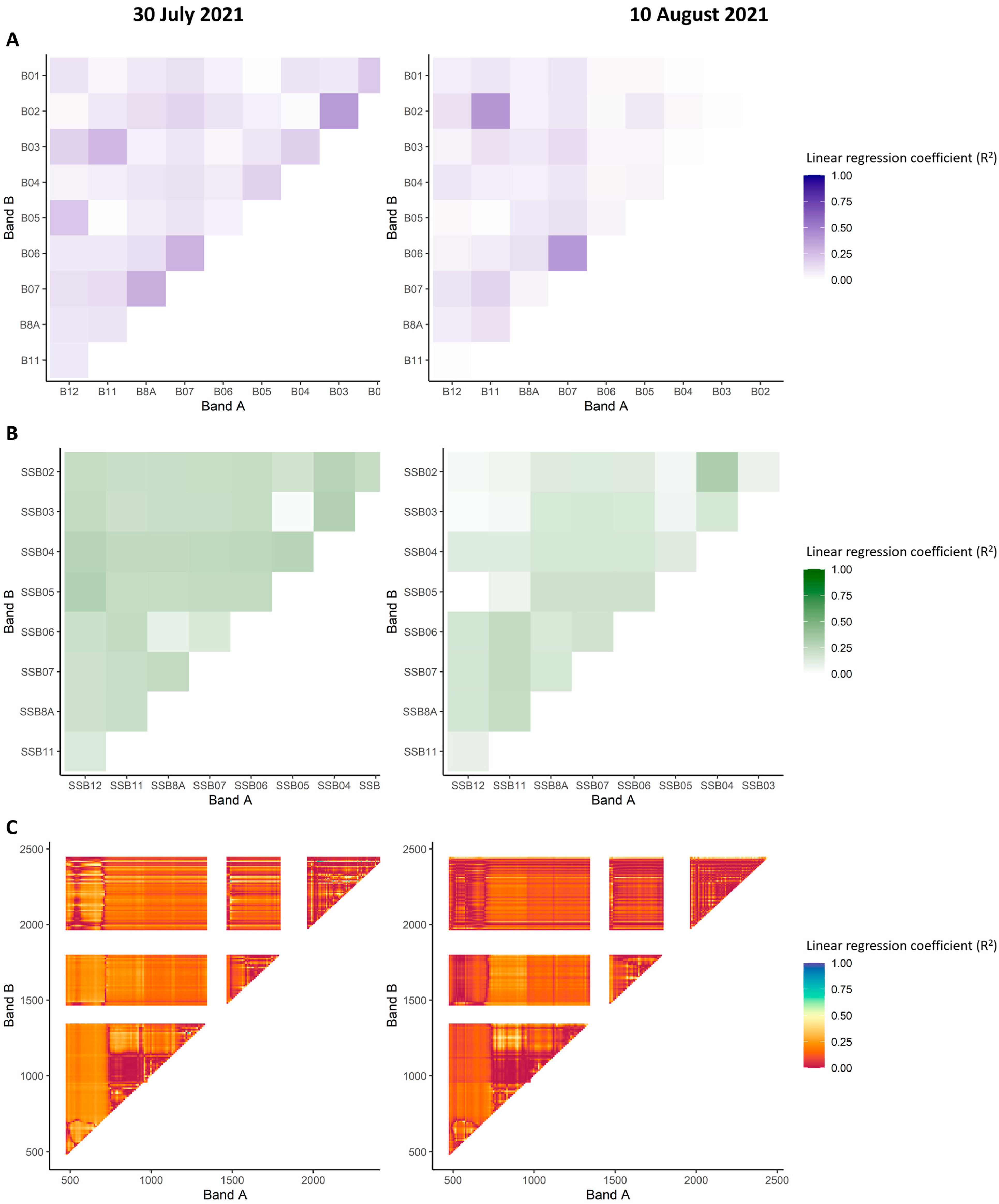

| Field | Cultivation Purpose | Number of Considered Sampling Zones | Number of Sample Plants | Maize Hybrid | Field Size | Date of Field Observations |
|---|---|---|---|---|---|---|
| Kd | grain maize | 12 | 432 | PR37N01 | 16.8 ha | 30 August 2021 |
| Nm5 | grain maize | 9 | 324 | PR37N01 | 9.13 ha | 11 August 2021 |
| Nm1 | sweet maize | 7 | 252 | Kiara | 43.8 ha | 11 August 2021 |
| Nm2 | sweet maize | 7 | 252 | Kiara | 23.3 ha | 11 August 2021 |
| All | 35 | 1260 | 93.03 ha | |||
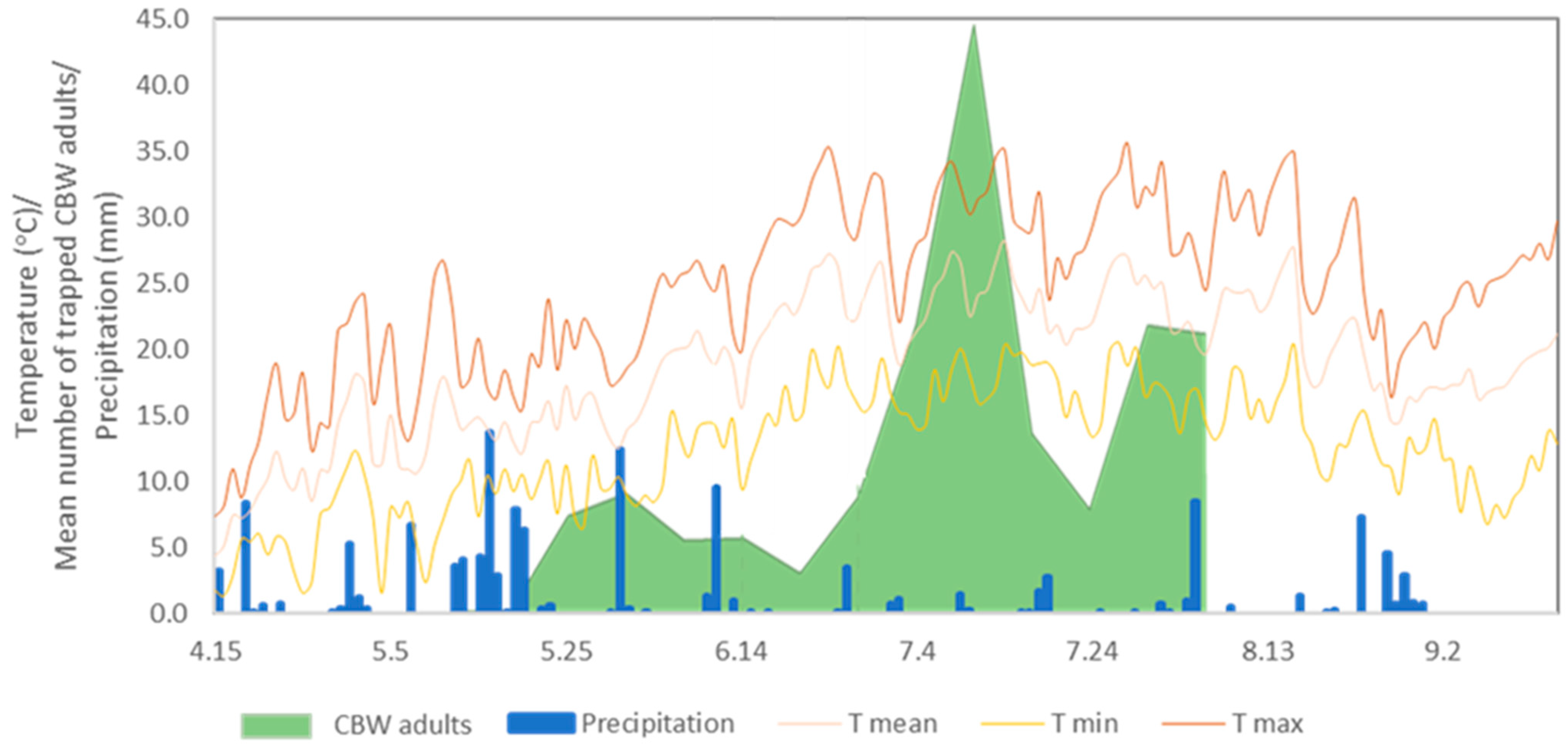
| Date | Developmental Threshold (°C) | GDD | Life Stage | Field Observation |
|---|---|---|---|---|
| 18 July 2021 | - | 0 | The peak of adult appearance | trap |
| 24 July 2021 | 11.5 | 59.5 | Hatching from egg | - |
| 30 July 2021 | 12.5 | 139.2 | Third instar larvae | First satellite image acquisition |
| 10 August 2021 | 12.5 | 239.1 | Fifth instar larvae | Second satellite image acquisition |
| 11 August 2021 | 12.5 | 250.9 | Fifth instar larvae | First field sampling |
| 14 August 2021 | 13.8 | 273.1 | Pupation | - |
| 30 August 2021 | 13.5 | 381.6 | Pupa | Second field sampling |
| PRISMA | Sentinel-2 | |||
|---|---|---|---|---|
| VIS-NIR | SWIR | VIS-NIR | SWIR | |
| Sensor type | Hyperspectral | Multispectral | ||
| Spectral range | 400–1010 nm | 920–2505 nm | 443–885 nm | 1360–2190 nm |
| Bandwidth | ≤12 nm | ≤12 nm | 15–106 nm | 20–175 nm |
| Number of spectral bands | 66 | 171 | 4/7/9 * | 0/2/3 * |
| Swath width | 30 km | 30 km | 290 km | 290 km |
| Spatial resolution | 30 m | 30 m | 10 m/20 m/60 m * | 10 m/20 m/60 m * |
| Revisit period | 29 days | Five days | ||
| Imagery availability | On-demand | Continuous | ||
| Name | Abbr. | Sentinel-2 | PRISMA | Reference | ||
|---|---|---|---|---|---|---|
| Normalized Difference Water Index | NDWI | (3) | (6) | [80] | ||
| Normalized Difference Vegetation Index | NDVI | (4) | (7) | [81] | ||
| Plant Senescence Reflectance Index | PSRI | (5) | (8) | [82] |
| Date | Crop | Satellite | N | R2 | RMSE | n |
|---|---|---|---|---|---|---|
| 30 July 2021 | Grain maize | Sentinel-2 | 1 | 0.11 | 0.077 | 21 |
| Synthetized Sentinel | 2 | 0.21 | 0.072 | |||
| PRISMA | 1 | 0.16 | 0.075 | |||
| Sweet maize | Sentinel-2 | 1 | 0.04 | 0.045 | 14 | |
| Synthetized Sentinel | 2 | 0.34 | 0.037 | |||
| PRISMA | 1 | 0.27 | 0.039 | |||
| 10 August 2021 | Grain maize | Sentinel-2 | 1 | 0.02 | 0.081 | 21 |
| Synthetized Sentinel | 2 | 0.22 | 0.072 | |||
| PRISMA | 3 | 0.55 | 0.055 | |||
| Sweet maize | Sentinel-2 | 1 | 0.01 | 0.046 | 14 | |
| Synthetized Sentinel | 1 | 0.08 | 0.044 | |||
| PRISMA | 1 | 0.15 | 0.042 |
| Satellite | Date | Cultiv. Purpose | Calibr. Field | Band A | Band B | Calibration | Test | Test Field | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| R2 | p | RMSE | R2 | p | RMSE | |||||||||||
| PRISMA | 7.30 | Grain maize | Kd | W571 | W2276 | 0.54 | 0.01 | * | 0.06 | 0.33 | 0.10 | 0.06 | Nm5 | |||
| Nm5 | W898 | W909 | 0.83 | 0.00 | * | 0.03 | 0.00 | 0.84 | 0.08 | Kd | ||||||
| Sweet maize | Nm1 | W866 | W1339 | 0.97 | 0.00 | * | 0.01 | 0.60 | 0.04 | * | 0.02 | Nm2 | ||||
| Nm2 | W750 | W1306 | 0.93 | 0.00 | * | 0.01 | 0.17 | 0.36 | 0.04 | Nm1 | ||||||
| 8.10 | Grain maize | Kd | W571 | W579 | 0.51 | 0.01 | * | 0.06 | 0.11 | 0.39 | 0.06 | Nm5 | ||||
| Nm5 | W719 | W1765 | 0.74 | 0.00 | * | 0.03 | 0.14 | 0.23 | 0.08 | Kd | ||||||
| Sweet maize | Nm1 | W563 | W605 | 0.93 | 0.00 | * | 0.01 | 0.57 | 0.05 | * | 0.02 | Nm2 | ||||
| Nm2 | W546 | W2313 | 0.83 | 0.00 | * | 0.01 | 0.42 | 0.12 | 0.03 | Nm1 | ||||||
| Synthetized Sentinel | 7.30 | Grain maize | Kd | SSB12 | SSB05 | 0.30 | 0.07 | 0.07 | 0.05 | 0.56 | 0.07 | Nm5 | ||||
| Nm5 | SSB05 | SSB02 | 0.21 | 0.22 | 0.06 | 0.18 | 0.17 | 0.08 | Kd | |||||||
| Sweet maize | Nm1 | SSB05 | SSB03 | 0.34 | 0.17 | 0.03 | 0.26 | 0.24 | 0.03 | Nm2 | ||||||
| Nm2 | SSB05 | SSB03 | 0.51 | 0.07 | 0.03 | 0.02 | 0.74 | 0.04 | Nm1 | |||||||
| 8.10 | Grain maize | Kd | SSB04 | SSB02 | 0.32 | 0.06 | 0.07 | 0.21 | 0.22 | 0.06 | Nm5 | |||||
| Nm5 | SSB8A | SSB07 | 0.44 | 0.05 | 0.05 | 0.16 | 0.20 | 0.08 | Kd | |||||||
| Sweet maize | Nm1 | SSB05 | SSB02 | 0.17 | 0.35 | 0.04 | 0.15 | 0.38 | 0.03 | Nm2 | ||||||
| Nm2 | SSB11 | SSB07 | 0.37 | 0.15 | 0.03 | 0.11 | 0.47 | 0.04 | Nm1 | |||||||
| Sentinel | 7.30 | Grain maize | Kd | B03 | B02 | 0.39 | 0.03 | * | 0.07 | 0.01 | 0.95 | 0.07 | Nm5 | |||
| Nm5 | B8A | B07 | 0.53 | 0.03 | * | 0.05 | 0.31 | 0.06 | 0.07 | Kd | ||||||
| Sweet maize | Nm1 | B03 | B02 | 0.72 | 0.02 | * | 0.02 | 0.54 | 0.06 | 0.02 | Nm2 | |||||
| Nm2 | B03 | B02 | 0.54 | 0.06 | 0.02 | 0.72 | 0.02 | * | 0.02 | Nm1 | ||||||
| 8.10 | Grain maize | Kd | B11 | B02 | 0.40 | 0.03 | * | 0.06 | 0.46 | 0.04 | * | 0.05 | Nm5 | |||
| Nm5 | B04 | B03 | 0.57 | 0.02 | * | 0.04 | 0.01 | 0.80 | 0.08 | Kd | ||||||
| Sweet maize | Nm1 | B07 | B06 | 0.81 | 0.01 | * | 0.02 | 0.01 | 0.83 | 0.04 | Nm2 | |||||
| Nm2 | B11 | B05 | 0.27 | 0.23 | 0.03 | 0.35 | 0.16 | 0.03 | Nm1 | |||||||
| NDVI | NDWI | PSRI | Green–Blue VI | Red-Edge–SWIR | Green–SWIR VI | |
|---|---|---|---|---|---|---|
| Nm1 | 37.8% | 11,546.5% | 44.0% | 82.8% | 35.3% | 67.7% |
| Nm2 | 38.7% | 5113.5% | 47.2% | 81.7% | 42.3% | 103.9% |
| Nm5 | 31.4% | 507.0% | 71.1% | 80.2% | 44.2% | 47.9% |
| Kd | 28.4% | 2706.3% | 69.4% | 77.6% | 48.2% | 21.7% |
| Sweet maize | 38.1% | 9309.9% | 45.1% | 82.4% | 37.7% | 80.3% |
| Grain maize | 29.5% | 1936.9% | 70.0% | 78.5% | 46.8% | 30.9% |
| MAPE all fields | 35.7% | 7256.9% | 52.1% | 81.3% | 40.2% | 66.5% |
| Cultivar | Field | Index | Satellite | R2 | p | Slope | Intercept | |
|---|---|---|---|---|---|---|---|---|
| Sweet maize | Nm1 | NDVI | PRISMA | 0.01 | 0.87 | 0.17 | −0.03 | |
| Sentinel-2 | 0.11 | 0.47 | 1.75 | −0.84 | ||||
| NDWI | PRISMA | 0.04 | 0.68 | 0.79 | 0.12 | |||
| Sentinel-2 | 0.20 | 0.31 | 1.84 | −0.36 | ||||
| PSRI | PRISMA | 0.02 | 0.77 | −0.75 | 0.14 | |||
| Sentinel-2 | 0.34 | 0.17 | 13.81 | −0.12 | ||||
| Green–Blue VI | PRISMA | 0.25 | 0.25 | 0.66 | −0.21 | |||
| Sentinel-2 | 0.72 | 0.02 | * | 15.32 | −1.12 | |||
| Green–SWIR VI | PRISMA | 0.03 | 0.69 | −0.12 | 0.08 | |||
| Sentinel-2 | 0.23 | 0.27 | 3.73 | 0.41 | ||||
| Red-edge–SWIR VI | PRISMA | 0.04 | 0.68 | 0.25 | −0.02 | |||
| Sentinel-2 | 0.17 | 0.36 | 1.91 | −0.53 | ||||
| Nm2 | NDVI | PRISMA | 0.19 | 0.32 | 0.51 | −0.40 | ||
| Sentinel-2 | 0.02 | 0.77 | 0.24 | −0.07 | ||||
| NDWI | PRISMA | 0.55 | 0.06 | 4.36 | 0.08 | |||
| Sentinel-2 | 0.02 | 0.79 | 0.18 | 0.01 | ||||
| PSRI | PRISMA | 0.07 | 0.56 | −1.05 | 0.09 | |||
| Sentinel-2 | 0.12 | 0.45 | −2.02 | 0.09 | ||||
| Green–Blue VI | PRISMA | 0.00 | 0.96 | 0.01 | 0.06 | |||
| Sentinel-2 | 0.54 | 0.06 | −8.00 | 0.71 | ||||
| Green–SWIR VI | PRISMA | 0.57 | 0.05 | −0.68 | −0.14 | |||
| Sentinel-2 | 0.05 | 0.63 | 0.66 | 0.12 | ||||
| Red-edge–SWIR VI | PRISMA | 0.35 | 0.16 | 0.75 | −0.33 | |||
| Sentinel-2 | 0.05 | 0.61 | 0.33 | −0.05 | ||||
| Grain maize | Nm5 | NDVI | PRISMA | 0.00 | 0.86 | 0.17 | 0.42 | |
| Sentinel-2 | 0.04 | 0.60 | 1.39 | −0.13 | ||||
| NDWI | PRISMA | 0.11 | 0.38 | −2.89 | 0.66 | |||
| Sentinel-2 | 0.00 | 0.87 | 0.44 | 0.46 | ||||
| PSRI | PRISMA | 0.00 | 0.96 | −0.09 | 0.56 | |||
| Sentinel-2 | 0.17 | 0.28 | −7.81 | 0.80 | ||||
| Green–Blue VI | PRISMA | 0.11 | 0.39 | 0.70 | 0.27 | |||
| Sentinel-2 | 0.00 | 0.95 | −0.60 | 0.59 | ||||
| Green–SWIR VI | PRISMA | 0.33 | 0.11 | −1.34 | 0.29 | |||
| Sentinel-2 | 0.01 | 0.83 | 1.08 | 0.68 | ||||
| Red-edge–SWIR VI | PRISMA | 0.08 | 0.46 | −1.27 | 1.09 | |||
| Sentinel-2 | 0.05 | 0.55 | 1.08 | 0.29 | ||||
| Kd | NDVI | PRISMA | 0.26 | 0.09 | 0.83 | 0.03 | ||
| Sentinel-2 | 0.10 | 0.32 | 1.21 | 0.01 | ||||
| NDWI | PRISMA | 0.26 | 0.09 | 7.07 | 0.38 | |||
| Sentinel-2 | 0.11 | 0.30 | 1.25 | 0.35 | ||||
| PSRI | PRISMA | 0.25 | 0.09 | −1.54 | 0.81 | |||
| Sentinel-2 | 0.03 | 0.60 | −1.97 | 0.68 | ||||
| Green–Blue VI | PRISMA | 0.40 | 0.03 | * | 2.56 | −0.44 | ||
| Sentinel-2 | 0.39 | 0.03 | * | 9.60 | −0.24 | |||
| Green–SWIR VI | PRISMA | 0.34 | 0.04 | * | −1.58 | 0.34 | ||
| Sentinel-2 | 0.17 | 0.18 | 2.99 | 0.97 | ||||
| Red-edge–SWIR VI | PRISMA | 0.20 | 0.15 | 1.20 | 0.02 | |||
| Sentinel-2 | 0.08 | 0.36 | 0.93 | 0.36 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sári-Barnácz, F.E.; Zalai, M.; Milics, G.; Tóthné Kun, M.; Mészáros, J.; Árvai, M.; Kiss, J. Monitoring Helicoverpa armigera Damage with PRISMA Hyperspectral Imagery: First Experience in Maize and Comparison with Sentinel-2 Imagery. Remote Sens. 2024, 16, 3235. https://doi.org/10.3390/rs16173235
Sári-Barnácz FE, Zalai M, Milics G, Tóthné Kun M, Mészáros J, Árvai M, Kiss J. Monitoring Helicoverpa armigera Damage with PRISMA Hyperspectral Imagery: First Experience in Maize and Comparison with Sentinel-2 Imagery. Remote Sensing. 2024; 16(17):3235. https://doi.org/10.3390/rs16173235
Chicago/Turabian StyleSári-Barnácz, Fruzsina Enikő, Mihály Zalai, Gábor Milics, Mariann Tóthné Kun, János Mészáros, Mátyás Árvai, and József Kiss. 2024. "Monitoring Helicoverpa armigera Damage with PRISMA Hyperspectral Imagery: First Experience in Maize and Comparison with Sentinel-2 Imagery" Remote Sensing 16, no. 17: 3235. https://doi.org/10.3390/rs16173235
APA StyleSári-Barnácz, F. E., Zalai, M., Milics, G., Tóthné Kun, M., Mészáros, J., Árvai, M., & Kiss, J. (2024). Monitoring Helicoverpa armigera Damage with PRISMA Hyperspectral Imagery: First Experience in Maize and Comparison with Sentinel-2 Imagery. Remote Sensing, 16(17), 3235. https://doi.org/10.3390/rs16173235







