Abstract
Invasive species have historically disrupted environments by outcompeting, displacing, and extirpating native species, resulting in significant environmental and economic damage. Developing approaches to detect the presence of invasive species, favorable habitats for their establishment, and predicting their potential spread are underutilized management strategies to effectively protect the environment and the economy. Spotted lanternfly (SLF, Lycorma delicatula) is a phloem-feeding planthopper native to China that poses a severe threat to horticultural and forest products in the United States. Tools are being developed to contain the spread and damage caused by SLF; however, methods to rapidly detect novel infestations or low-density populations are lacking. Vegetation spectroscopy is an approach that can represent vegetation health through changes in the reflectance and absorption of radiation based on plant physiochemical status. Here, we hypothesize that SLF infestations change the spectral and chemical characteristics of tree canopies. To test this hypothesis, we used a full range spectroradiometer to sample canopy foliage of silver maple (Acer saccharinum) and red maple (Acer rubrum) trees in a common garden in Berks County, Pennsylvania that were exposed to varying levels of SLF infestation. Foliar spectral profiles separated between SLF infestation levels, and the magnitude of separation was greater for the zero-SLF control compared with higher infestation levels. We found the red-edge and portions of the NIR and SWIR regions were most strongly related to SLF infestation densities and that corresponding changes in vegetation indexes related to levels of chlorophyll were influenced by SLF infestations, although we found no change in foliar levels of chlorophyll. We found no influence of SLF densities on levels of primary metabolites (i.e., pigments, nonstructural carbohydrates, carbon, and nitrogen), but did find an increase in the phenolic compound ferulic acid in response to increasing SLF infestations; this response was only in red maple, suggesting a possible species-specific response related to SLF feeding. By identifying changes in spectral and chemical properties of canopy leaves in response to SLF infestation, we can link them together to potentially better understand how trees respond to SLF feeding pressure and more rapidly identify SLF infestations.
1. Introduction
Invasive species outcompete, displace, and extirpate native species when encroaching on novel environments, resulting in significant negative environmental and economic consequences [1,2,3,4,5]. It is estimated that more than 40% of forest biomass in the United States is at risk of invasions from already established non-native species [6]. It is further projected that the United States could experience severe economic losses from further invasions, through disruption of agriculture and natural environments [7]. Understanding how invasive species move within novel landscapes and the ability to detect and identify their movement is imperative to potentially mitigate losses caused by future invasions.
An emerging invasive species that has gained recent notoriety is the spotted lanternfly (SLF, Lycorma delicatula [White]). SLF is a phloem-feeding planthopper native to China that was introduced to the United States, via a South Korean population [8], and first detected in Pennsylvania in 2014 and poses a severe threat to vineyards, orchards, and forests in the United States [9,10,11]. SLF is highly polyphagous, with over 70 different reported host species [10], but it prefers a smaller subset of hosts [12] and switches hosts throughout the growing season [10,13]. Currently, SLF has been found in 17 states [14], but climate models predict that it can survive throughout large portions the United States, specifically in hardwood forest regions in the Midwest and areas where the wine industry is prevalent [15,16]. The annual impact on SLF feeding on forest trees in Pennsylvania alone is estimated to be USD 152.6 million statewide, with worst-case scenarios projected to cost USD 219.6 million statewide [17]; thus, developing novel technological approaches to remotely detect current SLF infestations and identify future susceptible habitat favorable for SLF infestation could increase management effectiveness and help limit economic damage.
Vegetation spectroscopy has emerged as a novel technological approach that has shown considerable promise for remotely and non-destructively assessing plant health at different spatial scales, including UAV and satellite platforms. Spectral data have been shown to have direct relationships with plant physiochemical status, and this approach has been used to detect plant responses caused by a wide variety of abiotic and biotic stressors that reflect changes in chemical, physiological, and anatomical responses of vegetation associated with stress events [18,19,20,21,22,23,24,25]. Moreover, vegetation spectroscopy can be scalable to aerial vehicles and satellites, suggesting the possibility of monitoring vegetation responses to stress at large spatial scales [25,26,27]. Relatively modest densities of SLF can have a negative influence on host photosynthetic activity, foliar nitrogen levels, stem and wood soluble carbohydrates, and tree growth [28,29], and at the landscape level, it has been suggested that SLF may be attracted to higher nutritional quality hosts [30]. Little is known, however, about the extent to which SLF influences host foliar chemical composition, and thus the ability of spectral data to detect trees infested with SLF.
Here, we examine the influence of SLF on the spectral and chemical properties of foliage from two congeneric tree species exposed to different SLF densities. Specifically, we hypothesize that foliar spectral properties will be influenced by SLF infestation levels, and the responses will be more pronounced as infestation levels increase. We also hypothesize that foliar chemical profiles will be altered by SLF infestation levels, and predict decreases in primary metabolites (e.g., pigments, nonstructural carbohydrates, carbon, and nitrogen) and increases in secondary metabolites (e.g., phenolic compounds) as infestation levels increase.
2. Materials and Methods
2.1. Experimental Design
Data used in this study were collected at a two-acre common garden (40°27′08.7″N, 75°52′34.8″W) in Blandon, Berks County, Pennsylvania operated by Penn State University. The experimental design of the common garden consisted of four different tree species [black walnut (Juglans nigra), silver maple (Acer saccharinum), red maple (Acer rubrum), and tree of heaven (Ailanthus altissima)] planted in three blocks and with twelve subplots for each of four tree species within each block (n = 48 subplots total per block; 12 per tree species) in a randomized design. A complete description of the site experimental design can be found in Lavely [28]. The tree species focused on in this study were silver and red maple. Maple is known to be an important host species for SLF during multiple life stages, with red maple acting as a host for SLF during most life stages [31], and SLF has been reported to affect maple chemical and physiological parameters [28,29].
Individual trees were caged with mesh bags and secured at the top and on the base with zip ties to prevent hatched SLF from moving to other trees. To keep the mesh enclosure from falling over during heavy summer rains, a steel rod was hammered into the ground with a polyvinylchloride pipe and a T-cross was attached at the top of the pipe to provide more space for the branches (SI Figure S1). For both red and silver maple, two trees within each subplot were randomly selected, caged, and initially assigned to one of four SLF infestation treatment densities. Because of logistical and resource constraints associated with caging the trees, only one of the three blocks had cages installed.
Trees were initially randomly assigned to one of five SLF infestation densities in each subplot: 0, 100, 150, 200, and 300. Initial SLF densities were chosen based on Lavely [28] but expanded to increase the maximum infestation densities. This initial number corresponded with the number of adult SLF placed on each tree on 20 August 2021, four weeks prior to the collection of spectral measurements and the termination of this study on 16 September 2021. Densities of SLF were monitored daily and if mortality occurred, SLF were added to maintain the density approximate to the initial treatment listed above. The number of replacement SLF did not vary among treatments or replacements (all ANOVAs: p > 0.60). Because SLF mortality necessitated the need for replacement of individuals during the course of the experiment, SLF treatments were adjusted to represent cumulative SLF pressure applied throughout the experiment by summing SLF densities over all count periods prior to replacement, and reclassifying SLF treatments using a quantile distribution of the sum of SLF counted into the following levels: none (zero), low density (285.2 ± 94.2), medium density (507.0 ± 56.9), and high density (858.9 ± 163.5; Figure 1).
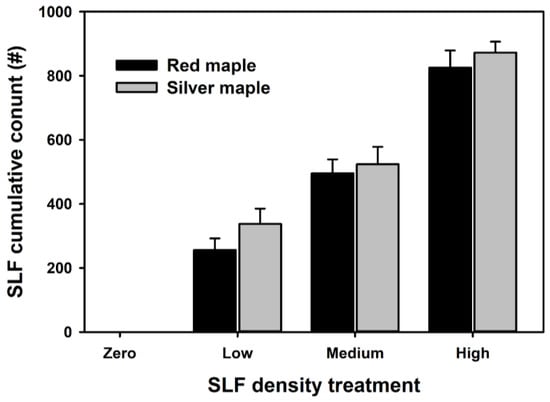
Figure 1.
Cumulative SLF count for density treatments over the course of study for each density category for red (black bars) and silver (gray bars) maple trees used in this study. Error bars represent standard error.
The total number of biological replicates used in the current study was 48 (1 block × 12 subplots × 2 tree species × 2 trees per subplot = 48 trees). The perimeter of the entire common garden was lined with a single row of buffer trees to prevent edge effects. The species of the buffer trees corresponded to the neighboring tree species. Experimental trees were fertilized and pruned prior to bud break. At the time of measurements, maples trees were approximately three meters tall.
2.2. Leaf Spectral and Tissue Collections
Leaf spectral collections occurred on 16 September 2021. Leaf spectra were collected with an SVC-1024i spectroradiometer (Spectral Vista Inc., Poughkeepsie, NY, USA) using a fiberoptic cable attached to a plant probe fitted with a leaf clip. Scan time was set to two seconds and full-range (350–2500 nm) reflectance profiles of randomly selected leaves from the lower canopy were collected. Three sunlit leaves per tree were collected from relatively comparable mid-canopy levels across all trees. Each leaf was measured once, totaling three scanned leaves per tree collected. White reference measurements were taken every 30 scans. Spectral data were examined for errors and measurements with low reflectance and deformations of spectral profiles due to the leaf clip not being completely closed or detector jumps that needed to be removed.
Immediately after the leaf spectra were collected, leaves were wrapped in aluminum foil, flash frozen in liquid nitrogen, and stored in a portable field freezer until lyophilization. Once the leaves were freeze-dried, leaf tissue was ball milled and stored until chemical analyses.
2.3. Spectral Indexes Calculated from Leaf Spectral Data
Hundreds of spectral indexes exist to indirectly infer plant physiochemical status. Three indexes commonly used to identify changes in plant health that are familiar to the spectral and remote sensing communities were calculated to determine if there were relationships between the indexes and SLF densities. The normalized difference red-edge (NDRE) index, an estimation of plant chlorophyll and health, was calculated as (R790 − R720)/(R790 + R720) following Colovic [32], the modified chlorophyll absorption ratio index (MCARI) following Daughtry [33], and the photochemical reflectance index, an estimation of photosynthetic efficiency, was calculated as (R531 − R570)/(R531 + R570) following Gamon [34] and scaled as (PRI + 1)/2 to avoid negative values following Letts [35].
2.4. Chemical Quantification of Leaf Tissue
Ground maple leaves were assayed for concentrations of phenolic acids (gallic acid, ferulic acid, salicylic acid, synaptic acid, ellagic acid, and trans-cinnamic acid), flavonoids (rutin and myricetin), sugars (glucose, fructose, and sucrose), starch, pigments (neoxanthin, lutein, zeaxanthin, chlorophyll b, chlorophyll a, and beta carotene), carbon, and nitrogen.
Phenolic acids and flavonoids were quantified using high profile liquid chromatography (HPLC; Shimadzu Prominence, Shimadzu, Kyoto, Japan) with gradient elution and diode-array detection following a slightly modified protocol by Nour [36]. Briefly, approximately 20 mg of dried and ground leaf tissue were weighed into a 2 mL tube and 1 mL methanol with 1% BHT (butylated hydroxytoluene) was added. Samples were sonicated in an ice bath for 40 min then centrifuged at 15,000 RPM for 10 min and filtered through a 0.2 µm syringe filter (13 mm diameter PTFE, Fisherbrand, Fisher Scientific, Hampton, NH, USA) into an amber HPLC vial and stored at −20 °C until analysis via HPLC. A Hypersil Gold C18 column was used to perform chromatographic separation and three different wavelengths (254, 278, and 300 nm) were used for detection. Methanol and 1% aqueous acetic acid were used as the mobile phase for the elution gradient, flow rate was 1 mL/min, and the injection volume was 5 µL. Purified phenolic acids and flavonoids (Sigma Aldrich, St. Louis, MO, USA) were used as standards and concentrations were calculated using an external standard curve generated via serial dilution of individual compounds in methanol.
Sugar concentrations were quantified following Pellegrini [37] using a refractive index detector (Shimadzu RIC-20A, Shimadzu, Chicago, IL, USA). Briefly, 60 mg of dried and ground leaf tissue were weighed into a 2 mL tube and 1 mL of HPLC grade water was added. Samples were heated in a 60 °C water bath for 1 h and centrifuged for 20 min at 5000 RPM. The supernatant was removed and filtered through a 0.2 µm syringe filter (13 mm diameter PES, Fisherbrand, Fisher Scientific, Hampton, NH, USA) into an amber HPLC vial before being analyzed on the HPLC. A Phenomenex Rezex RCM-Monosaccharide Ca+2 300 × 7.8 mm column (Phenomenex, Torrence, CA, USA) fitted with a guard column was used to perform chromatographic separations and three separate retention times (9.6, 11.5, and 14.6 min) were used for detection. HPLC-grade water was used as the mobile phase, flow rate was 0.6 mL/min, and the injection volume was 5 µL. Purified sucrose, glucose, and fructose (Sigma Aldrich, St. Louis, MO, USA) were used as standards and concentrations were calculated using an external standard curve generated via serial dilution of individual compounds in HPLC-grade water.
Starch concentrations were quantified following protocols by Lopez-Hernandez [38] Smith and Zeeman [39], and Vu [40] over a two-day period. Roughly 100 mg of dried and ground leaf tissue were weighed into 15 mL plastic tubes and 5 mL of 80% ethanol was added before incubating for 3 min in a boiling water bath. Samples were then centrifuged for 10 min at 4000 RPM and the supernatant was poured off. This was repeated three times and then the resulting pellet was dried overnight at 60 °C. The next day, 1 mL of 0.2 M KOH was added to the pellet and vortexed before incubating in a boiling water bath for 30 min. After samples were vortexed and cooled to room temperature, 0.2 mL of 1 M acetic acid, 0.5 mL of 200 mM sodium acetate buffer (pH 5.5), and 6 U of amyloglucosidase were added and vortexed. Samples were then incubated for 1 h at 55 °C, vortexed, and centrifuged for 10 min at 4000 RPM. The supernatant was then collected and diluted with HPLC grade water at a 3:1 ratio before being filtered through a 0.2 µm syringe filter (13 mm diameter PES, Fisherbrand, Fisher Scientific, Hampton, NH, USA) into an amber HPLC vial before being analyzed on the HPLC. The same column, mobile phase, and flow rate that were used in sugar quantification were used for starch. Purified glucose was used as a standard and concentrations were calculated using an external standard curve generated via serial dilution of glucose in HPLC-grade water.
Pigment concentrations were determined following Cotrozzi [41]. Briefly, 50 mg of dried and ground leaf tissue were weighed into a 2 mL amber microtube and 1 mL of HPLC-grade methanol was added. Samples were incubated overnight at 4 °C in the dark. The following day, samples were centrifuged for 15 min at 15,000 RPM. The supernatant was then filtered through a 0.2 µm syringe filter (13 mm diameter PTFE, Fisherbrand, Fisher Scientific, Hampton, NH, USA) into a 2 mL amber HPLC vial and placed in a cooled autosampler for analysis. A C18, 5 µm particle size, 4.6 mm ID × 150 mm length with guard column was used to perform chromatic separations at 7 different retention times (3.7, 4.3, 7.5, 8.1, 16.8, 18.3, and 23.2 min) for analysis. Mobile phase A consisted of 75:25% acetonitrile/methanol mix and mobile phase B consisted of a 68:32 methanol/ethylacetate mix; flow rate was set to 1 mL/min and injection volume was 5 µL. The mobile phase gradient was as follows: 0–15.5 min 100:0 A/B; 15.5–30 min 0:100 A/B; 30–35 min 100:0 A/B. Pigments were detected by absorbance at 445 nm and purified neoxanthin, lutein, zeaxanthin, chlorophyll b, chlorophyll a, and beta-carotene (Sigma Aldrich, St. Louis, MO, USA) were used as standards to relate peak area to concentration via serial dilution of individual compounds in methanol.
Carbon and nitrogen were determined using combustion analysis with a Flash 1112 Elemental Analyzer (San Jose, CA, USA) and analyzed as a ratio of carbon to nitrogen (C/N). Atropine (CE Elantech, Lakewood, NJ, USA) was used as a standard.
2.5. Statistical Analysis
To determine if there were differences in full-range spectral profiles between cumulative SLF pressure levels (i.e., none, low, medium, and high) and tree species, a permutational multivariate analysis of variance (perMANOVA), employing Euclidean measurements of distance and 10,000 permutations, was used. To determine regions of the spectrum related with cumulative SLF pressure levels, a normalized differential spectral index (NDSI) was calculated and related with SLF pressure classes using a Spearman rank order correlation coefficient. Next, differences among cumulative SLF pressure levels were visualized using principal coordinate analysis (PCoA), also employing Euclidean measurements of distance. Lastly, a one-factor ANOVA was performed separately on the first two PCoA axis extracted from spectral data to identify statistical differences in cumulative SLF pressure levels. If a PCoA axis was found to be statistically significant, then a least significant difference post-hoc test was used to determine statistically significant differences among cumulative SLF pressure levels.
A different statistical analysis workflow was used for analysis of spectral indexes and foliar chemical profiles. First, a one factor ANOVA was used to determine the influence of SLF pressure levels on each individual chemical compound or spectral index. Relationships between cumulative SLF pressure levels with chemical compounds and spectral indexes found to be statistically significant to SLF densities were further assessed using a linear mixed model with cumulative SLF count as a continuous variable and tree species as a categorical variable. Assessment of residuals for data used in ANOVA and linear mixed model analyses revealed data met assumptions of normality and homogeneity of variance. All statistical analyses were performed in either R using the vegan package (R v4.1.2, www.R-project.org, accessed 1 January 2022) or JMP v16.1.0 (SAS Institute Inc., Cary, NC, USA, 2021).
3. Results
3.1. Foliar Spectral Responses to Different SLF Infestation Levels and Tree Species
Average reflectance of each SLF treatment across the full wavelength range showed that the zero control and low-density treatments appeared more similar and that spectral profiles of medium- and high-density treatments appeared more similar with each other but were different from the zero control and low-density treatment spectral profiles (Figure 2A). NDSI revealed areas of high correlation with SLF densities in the VNIR, and some SWIR regions, with 500–1300, 1600–1800, and 2200–2400 nm having stronger correlations with infestation levels (Figure 2B), while the VIS region showed the most dramatic difference in relative change (Figure 2C). There was variation in correlation coefficients within each of the ranges. In the 500–1300 range, we found 26 wavelengths with a correlation coefficient > 0.4 (658, 659, 660, 661, 662, 663, 664, 665, 666, 667, 668, 669, 670, 671, 704, 705, 706, 707, 708, 709, 710, 711, 762, 1212, and 1253 nm). Between 1600 and 1800 nm, we found two wavelengths with a correlation coefficient > 0.3 (1794 and 1795 nm), and between 2200 and 2400 nm, we found three wavelengths with a correlation coefficient > 0.3 (2352, 2353, and 2381 nm). Canopy foliar spectral profiles separated when trees were infested with SLF and between tree species, but the SLF response was consistent for both species (no SLF x spp. interaction, Figure 2D). Analysis of individual PCoA dissimilarity axes found that only the first PCoA axis was found to be statistically significant for SLF treatments (ANOVA: F = 3.6; df = 3; P = 0.010). Post-hoc analyses of PCoA coordinates revealed that spectral profiles from high SLF densities were significantly different from spectral profiles from zero-SLF densities and spectral profiles from medium SLF densities were significantly different from spectral profiles of zero and low SLF densities (Figure 2D). There was no significant difference between spectral profiles of zero and low SLF densities, low and high SLF densities, and medium and high SLF densities (Figure 2D).
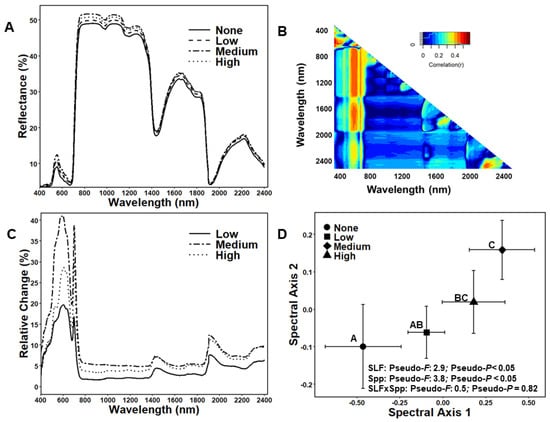
Figure 2.
(A) Average silver maple (Acer saccharinum) and red maple (Acer rubrum) leaf reflectance for each treatment. (B) Normalized differential spectral index (NDSI) shows the correlation of all dual wavelengths’ combination. (C) Relative change in reflectance of leaves at different treatment levels. (D) Principal coordinate analysis (PCoA) visualization of variation in composite spectral profiles in response to SLF treatments. Treatments not connected by the same letter are significantly different. Error bars represent ± 1 SE.
3.2. Foliar Spectral Index and Chemical Responses to SLF Infestation Levels
We found that NDRE decreased and MCARI increased in response to SLF, and responses were similar for both host species (no significant SLF × tree spp. interaction, SI Tables S1 and S2). NDRE decreased 13, 20, and 19% for low, medium, and high infestation levels relative to the control, respectively, while MCARI increased 30, 71, and 86% for low, medium, and high infestation levels relative to the control, respectively (SI Table S2). PRI did not respond to either SLF level or tree species (SI Tables S1 and S2).
We found no influence of SLF density on levels of foliar pigments, carbon, nitrogen, or nonstructural carbohydrates (SI Tables S3 and S4). We also found little influence of SLF densities on phenolic acids and flavonoids, except for ferulic acid, which increased as SLF density increased (SI Tables S3 and S4). Overall, ferulic acid increased 42, 69, and 63% in the low, medium, and high SLF treatments, respectively, relative to the zero-SLF density control (SI Table S4). The response magnitude was much larger, however, in red maple compared with silver maple (significant SLF treatment × tree spp. interaction, SI Table S3; red maple: 68, 125, and 143% increase for low, med, and high SLF treatments, respectively, relative to the zero-SLF density control; silver maple: 22, 31, and 9% increase for low, med, and high SLF treatments, respectively, relative to the zero-SLF density control, SI Table S4).
3.3. Relationships of Spectral Indexes and Foliar Chemistry with SLF Cumulative Densities
We found a negative relationship of NDRE, and a positive relationship of MCARI, with SLF cumulative densities (Figure 3A and Figure 3B, respectively). For both indexes the responses were consistent across species (Figure 3A,B, no significant SLF × tree spp. interactions). We also found a positive relationship between cumulative SLF densities and ferulic acid for red maple, but not for silver maple (Figure 3C, no significant SLF × tree spp. interactions).
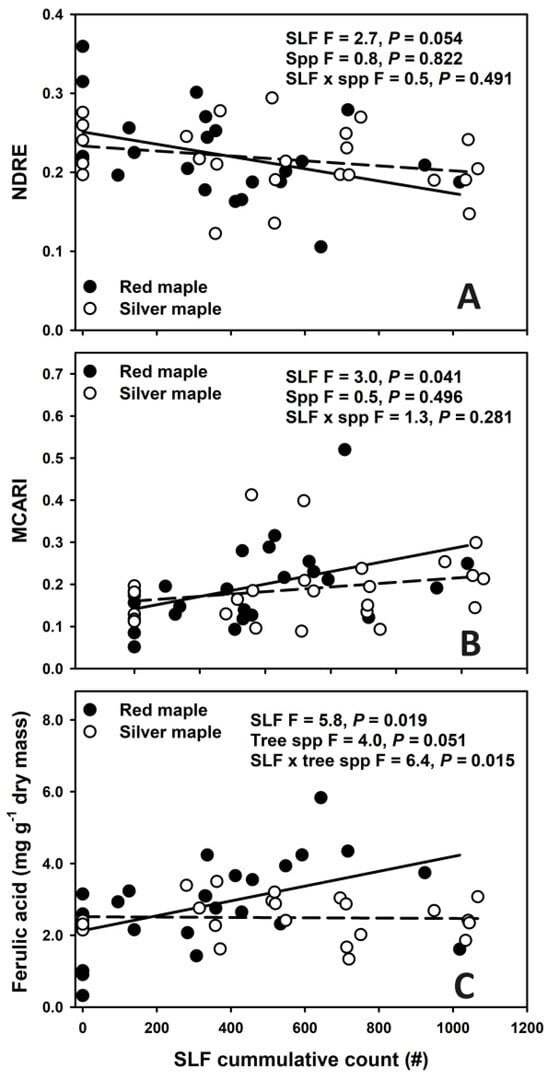
Figure 3.
Relationship between cumulative SLF counts and normalized red edge (NDRE) index (A), modified chlorophyll absorption ratio index (MCARI, B), and ferulic acid (C) for red maple (closed circles, solid line) and silver maple (open circles, dashed line).
4. Discussion
In this study, we examined the influence of different infestation levels of an invasive insect herbivore on the spectral and foliar chemical properties of two congeneric host trees. We found that SLF infestation levels altered spectral profiles of both red and silver maple relative to a zero-SLF control, but only at medium and high SLF densities. We also found that spectral indexes related to chlorophyll were altered by SLF densities. Overall, while we found little influence of SLF densities on the primary and secondary metabolites measured, we did find an increase in ferulic acid in SLF-infested trees, and the response generally increased with increased SLF pressure; the response, however, was greater in red maple compared with silver maple, and only red maple showed a statistically significant positive relationship between SLF cumulative densities and ferulic acid. These findings suggest that the optical properties of canopy foliar vegetation may be used to identify infestations of a trunk or lateral stem-feeding, invasive insect, but may be limited to certain infestation levels, and highlight potential chemical mechanisms for shifts in spectral profiles based on foliar chemical characteristics.
Supporting our first hypothesis, we were able to detect changes in canopy spectral profiles in trees infested with SLF. While the spectral responses of red and silver maple were different, the response to SLF densities was consistent for both species. The magnitude of separation, however, depended on SLF density. We found relationships among spectral data and SLF densities in VNIR, red-edge, and portions of the SWIR region. While we observed the greatest variation in relative change in the VIS region, specifically involving chlorophyll absorption regions and the red-edge region, we did not find any change in pigment profiles in response to SLF densities. We also found strong relationships between SLF infestation levels and the red-edge region and shifts in the red edge have been related with changes in chlorophyll content and can be generally used as an indicator of plant stress [25,26,42,43] and we did find relationships with spectral indexes (e.g., NDRE, MCARI) that exploit the red edge and are putatively associated with chlorophyll content. Both short and long-term feeding by higher densities of SLF negatively impact photosynthesis of multiple woody plant species and can do so in a density-dependent manner [28,29,44]. Previous research has reported a four-to-twenty-fold reduction in carbon assimilation within a few days of exposure to higher, compared to zero control, adult SLF densities caged on branches of red and silver maple [28]. In the same study, however, photosynthetic suppression was not as pronounced when SLF were given access to the entire tree [28], and similar studies have reported no effects or increases in levels of photosynthesis in silver maple in response to SLF feeding [29]. We suggest that the negative relationship of the red edge-related spectral indexes potentially reveals indirect, index-based indicators of changes in photosynthesis caused by SLF pressure and ultimately captured SLF-induced stress responses in trees in both maple species.
Somewhat consistent with our second hypothesis, we observed a change in tree foliar chemical composition, with ferulic acid levels increasing as SLF cumulative pressure increased. Phenolic acids are generally associated with plant responses to stress [45] and feeding by other phloem-feeding insects can elicit systemic responses in plants away from the site of feeding, including an upregulation of phenolic compounds [46,47,48,49]. Ferulic acid responds to and negatively influences plant herbivore and pathogen performance, and the increase in ferulic acid levels we found are consistent with previous studies [50,51,52,53,54]. Moreover, shifts in phenolic compounds have been associated with variation in specific regions of spectral profiles and we noticed strong relationships with spectral data and SLF feeding pressure in the 1600–1800 and 2200–2400 nm wavelength regions, which have been reported to be associated with absorption features of phenolic compounds [20,55,56,57]. The combination of these outcomes supports the idea that if whole-plant, systemic responses to stress of sufficient magnitude occur, they can cause shifts in canopy foliar spectral profiles that allow for the identification of stress events happening away from the direct site of stress occurrence [58,59,60,61,62,63,64].
Inconsistent with our second hypothesis, we found no change in foliar levels of pigments, nitrogen, and nonstructural carbohydrates in response to SLF infestation. We anticipated decreases in these compounds, as SLF feeding can result in a loss of free sugars and starch and a reduction in foliar nitrogen levels [11,28,29,44]. The inconsistency of our results with those found previously might be related with plant responses being species specific, SLF density-dependent, or the temporal nature of plant responses to SLF feeding [28,44]. One caveat of this study is that while spectral separation was found over one sampling period, collections over multiple times points that capture the physiochemical properties that drive separation in spectral data might be necessary to detect changes in infestation dynamics. For example, the two tree species used in the current study have demonstrated differential temporal variation in changes in foliar nutrient and carbohydrate pools in response to SLF feeding [28,29], suggesting a single sampling period might not accurately capture complex tree physiochemical tree responses to SLF infestations.
In conclusion, we found shifts in spectral profiles and relationships of spectral indexes, many using chlorophyll absorption features and the red-ed region, related to SLF feeding pressure. In addition, we found some evidence of changes in canopy foliar secondary metabolite composition related with stress responses and biotic resistance that have known absorption features in spectral regions, specifically 1600–1800 and 2200–2400 nm, which changed in response to SLF feeding. Exploiting spectral variation has emerged as a powerful tool to understand and interpret plant responses to biotic and abiotic stress [18,21,22,23,24,25,26,27,65,66,67]. While some insecticide control options exist for SLF [68,69], remote detection of SLF infestations can provide more efficient efforts for SLF control. Given the scalable nature of spectral data from individual plants to landscapes using aerial platforms, this work highlights the potential to track SLF movement and infestations and to identify drivers of invasive species movement at landscape scales.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/rs16152706/s1, Figure S1: Visual example of cages used in the current study depicting frame and t-cross for supporting mesh enclosure for control of spotted lanternfly densities; Table S1: Summary of F and p values for the effects of spotted lanternfly density level, tree species, and their interactions on spectral indexes. P values less than 0.05 are bolded. NDRE: normalized differential red edge; MCARI: modified chlorophyll absorption in reflectance index; PRI: photochemical reflectance index. df: degrees of freedom; Table S2: Values of spectral indexes calculated in this study. NDRE: normalized differential red edge; MCARI: modified chlorophyll absorption in reflectance index; PRI: photochemical reflectance index. SE: standard error; Table S3: Summary of F and p values for the effects of spotted lanternfly density level, tree species, and their interactions on foliar chemical parameters. p values less than 0.05 are bolded. C/N: ratio of carbon to nitrogen. df: degrees of freedom; Table S4: Values of chemical compounds quantified in this study from red and silver maple at different spotted lanternfly density levels. C/N: ratio of carbon to nitrogen. RM: red maple; SM: silver maple. SE: standard error.
Author Contributions
Conceptualization, M.D.G., K.H. and J.J.C.; methodology, J.J.C.; formal analysis, E.G.J. and J.J.C.; data curation, J.J.C.; writing—original draft preparation, E.G.J.; writing—review and editing, E.G.J., M.D.G., K.H. and J.J.C.; funding acquisition, M.D.G., K.H. and J.J.C. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by USDA NIFA AFRI grant 00097118 to JJC, MDG, and KH. This work was also funded by the Hardwood Tree Improvement and Regeneration Center (HTIRC) and the Institute for Digital Forestry at Purdue University. Additional support was provided by USDA NIFA Hatch awards IND011490 to JJC, the Northeast Multistate Chemical Ecology Project NE2001: Harnessing Chemical Ecology to Address Agricultural Pests and USDA Hatch Project Accession number 1021211 and Project Number PEN04728 to KH.
Data Availability Statement
Spectral data from this project are available at www.ecosis.org.
Acknowledgments
We would like to thank Anne Johnson, Elizabeth Wagner, Adam Scherr, Kelsey Tobin, Scott Gula, Sylvia Park, and Daniel Edwards for assistance with data collection in the field and site maintenance. We would also like to thank Madison Kresse for assistance with sample processing and Megan Haas for assistance with chemical analyses.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Davidson, C.B.; Gottschalk, K.W.; Johnson, J.E. Tree mortality following defoliation by the European gypsy moth (Lymantria dispar L.) in the United States: A review. For. Sci. 1999, 45, 74–84. [Google Scholar] [CrossRef]
- McManus, M.; Csóka, G. History and impact of gypsy moth in North America and comparison to the recent outbreaks in Europe. Acta Silv. Lignaria Hung. 2007, 3, 47–64. [Google Scholar] [CrossRef]
- Bradshaw, C.J.; Leroy, B.; Bellard, C.; Roiz, D.; Albert, C.; Fournier, A.; Barbet-Massin, M.; Salles, J.-M.; Simard, F.; Courchamp, F. Massive yet grossly underestimated global costs of invasive insects. Nat. Commun. 2016, 7, 12986. [Google Scholar] [CrossRef] [PubMed]
- Ward, S.F.; Liebhold, A.M.; Morin, R.S.; Fei, S. Population dynamics of ash across the eastern USA following invasion by emerald ash borer. For. Ecol. Manag. 2021, 479, 118574. [Google Scholar] [CrossRef]
- Fantle-Lepczyk, J.E.; Haubrock, P.J.; Kramer, A.M.; Cuthbert, R.N.; Turbelin, A.J.; Crystal-Ornelas, R.; Diagne, C.; Courchamp, F. Economic costs of biological invasions in the United States. Sci. Total Environ. 2022, 806, 151318. [Google Scholar] [CrossRef]
- Fei, S.; Morin, R.S.; Oswalt, C.M.; Liebhold, A.M. Biomass losses resulting from insect and disease invasions in US forests. Proc. Natl. Acad. Sci. USA 2019, 116, 17371–17376. [Google Scholar] [CrossRef] [PubMed]
- Paini, D.R.; Sheppard, A.W.; Cook, D.C.; De Barro, P.J.; Worner, S.P.; Thomas, M.B. Global threat to agriculture from invasive species. Proc. Natl. Acad. Sci. USA 2016, 113, 7575–7579. [Google Scholar] [CrossRef]
- Du, Z.; Wu, Y.; Zhou, C.; Cao, L.; Ishikawa, T.; Kamitani, S.; Sota, T.; Song, F.; Tian, L.; Cai, W.; et al. Lobal phylogeny and invasion history of the spotted lanternfly revealed by mitochondrial phylogenomics. Evol. App. 2020, 14, 915–930. [Google Scholar] [CrossRef]
- Dara, S.K.; Barringer, L.; Arthurs, S.P. Lycorma delicatula (Hemiptera: Fulgoridae): A New Invasive Pest in the United States. J. Integr. Pest Manag. 2015, 6, 20. [Google Scholar] [CrossRef]
- Barringer, L.; Ciafré, C.M. Worldwide feeding host plants of spotted lanternfly, with significant additions from North America. Environ. Entomol. 2020, 49, 999–1011. [Google Scholar] [CrossRef]
- Urban, J.M. Perspective: Shedding light on spotted lanternfly impacts in the USA. Pest Manag. Sci. 2020, 76, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Murman, K.; Setliff, G.P.; Pugh, C.V.; Toolan, M.J.; Canlas, I.; Cannon, S.; Abreu, L.; Fetchen, M.; Zhang, L.; Warden, M.L.; et al. Distribution, survival, and development of spotted lanternfly on host plants found in North America. Environ. Entomol. 2020, 49, 1270–1281. [Google Scholar] [CrossRef] [PubMed]
- Liu, H. Oviposition substrate selection, egg mass characteristics, host preference, and life history of the spotted lanternfly (Hemiptera: Fulgoridae) in North America. Environ. Entomol. 2019, 48, 1452–1468. [Google Scholar] [CrossRef] [PubMed]
- Spotted Lanternfly. Available online: https://www.aphis.usda.gov/aphis/resources/pests-diseases/hungry-pests/the-threat/spotted-lanternfly/spotted-lanternfly (accessed on 20 February 2024).
- Jung, J.; Jung, S.; Byeon, D.; Lee, W. Model-based prediction of potential distribution of the invasive insect pest, spotted lanternfly Lycorma delicatula (Hemiptera: Fulgoridae), by using CLIMEX. J. Asia-Pac. Biodivers. 2017, 10, 532–538. [Google Scholar] [CrossRef]
- Wakie, T.T.; Neven, L.G.; Yee, W.L.; Lu, Z. The establishment risk of Lycorma delicatula (Hemiptera: Fulgoridae) in the United States and globally. J. Econ. Entomol. 2019, 113, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Harper, J.K.; Stone, W.; Kelsey, T.W.; Kime, L.F. Potential Economic Impact of the Spotted Lanternfly on Agriculture and Forestry in Pennsylvania; Center for Rural Pennsylvania: Harrisburg, PA, USA, 2019; pp. 1–84. [Google Scholar]
- Mahlein, A.K.; Steiner, U.; Hillnhütter, C.; Dehne, H.W.; Oerke, E.C. Hyperspectral imaging for small-scale analysis of symptoms caused by different sugar beet diseases. Plant Methods 2012, 8, 3. [Google Scholar] [CrossRef] [PubMed]
- Couture, J.J.; Serbin, S.P.; Townsend, P.A. Spectroscopic sensitivity of real-time, rapidly induced phytochemical change in response to damage. New Phytol. 2013, 198, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Couture, J.J.; Singh, A.; Rubert-Nason, K.F.; Serbin, S.P.; Lindroth, R.L.; Townsend, P.A. Spectroscopic determination of ecologically relevant plant secondary metabolites. Methods Ecol. Evol. 2016, 7, 1402–1412. [Google Scholar] [CrossRef]
- Mutka, A.M.; Bart, R.S. Image-based phenotyping of plant disease symptoms. Front. Plant Sci. 2015, 5, 734. [Google Scholar] [CrossRef] [PubMed]
- Zarco-Tejada, P.J.; Camino, C.; Beck, P.S.A.; Calderon, R.; Hornero, A.; Hernández-Clemente, R.; Kattenborn, T.; Montes-Borrego, M.; Susca, L.; Morelli, M.; et al. Previsual symptoms of Xylella fastidiosa infection revealed in spectral plant-trait alterations. Nat. Plants 2018, 4, 432–439. [Google Scholar] [CrossRef] [PubMed]
- Cotrozzi, L.; Couture, J.J. Hyperspectral assessment of plant responses to multi-stress environments: Prospects for managing protected agrosystems. Plants People Planet 2020, 2, 244–258. [Google Scholar] [CrossRef]
- Gold, K.M.; Townsend, P.A.; Chlus, A.; Herrmann, I.; Couture, J.J.; Larson, E.R.; Gevens, A.J. Hyperspectral measurements enable pre-symptomatic detection and differentiation of contrasting physiological effects of late blight and early blight in potato. Remote Sens. 2020, 12, 286. [Google Scholar] [CrossRef]
- Cotrozzi, L. Spectroscopic detection of forest diseases: A review (1970–2020). J. For. Res. 2022, 33, 21–38. [Google Scholar] [CrossRef]
- Cotrozzi, L.; Townsend, P.A.; Pellegrini, E.; Nali, C.; Couture, J.J. Reflectance spectroscopy: A novel approach to better understand and monitor the impact of air pollution on Mediterranean plants. Environ. Sci. Pollut. Res. 2018, 25, 8249–8267. [Google Scholar] [CrossRef] [PubMed]
- Nay, J.; Burchfield, E.; Gilligan, J. A machine-learning approach to forecasting remotely sensed vegetation health. Int. J. Remote Sens. 2018, 39, 1800–1816. [Google Scholar] [CrossRef]
- Lavely, E.; Iavorivska, L.; Uyi, O.; Eissenstat, D.M.; Walsh, B.; Primka, E.J.; Harper, J.; Hoover, K. Impacts of short-term feeding by spotted lanternfly (Lycorma delicatula) on ecophysiology of young hardwood trees in a common garden. Front. Insect Sci. 2022, 2, 1080124. [Google Scholar] [CrossRef]
- Hoover, K.; Iavorivska, L.; Lavely, E.K.; Uyi, O.; Walsh, B.; Swackhamer, E.; Johnson, A.; Eissenstat, D.M. Effects of long-term feeding by spotted lanternfly (Hemiptera: Fulgoridae) on ecophysiology of common hardwood host trees. Environ. Entomol. 2023, 52, 888–899. [Google Scholar] [CrossRef] [PubMed]
- Mason, C.J.; Walsh, B.; Keller, J.; Couture, J.J.; Calvin, D.; Urban, J.M. Fidelity and timing of spotted lanternfly (Hemiptera: Fulgoridae) attack patterns on ornamental trees in the suburban landscape. Environ. Entomol. 2020, 49, 1427–1436. [Google Scholar] [CrossRef] [PubMed]
- Calvin, D.D.; Rost, J.; Keller, J.; Crawford, S.; Walsh, B.; Bosold, M.; Urban, J. Seasonal activity of spotted lanternfly (Hemiptera: Fulgoridae) in Southeast Pennsylvania. Environ. Entomol. 2023, 52, 1108–1125. [Google Scholar] [CrossRef]
- Colovic, M.; Yu, K.; Todorovic, M.; Cantore, V.; Hamze, M.; Albrizio, R.; Stellacci, A.M. Hyperspectral vegetation indices to assess water and nitrogen status of sweet maize crop. Agronomy 2022, 12, 2181. [Google Scholar] [CrossRef]
- Daughtry, C.S.; Walthall, C.L.; Kim, M.S.; De Colstoun, E.B.; McMurtrey, J.E., III. Estimating corn leaf chlorophyll concentration from leaf and canopy reflectance. Remote Sens. Environ. 2000, 74, 229–239. [Google Scholar] [CrossRef]
- Gamon, J.A.; Serrano, L.; Surfus, J.S. The photochemical reflectance index: An optical indicator of photosynthetic radiation use efficiency across species, functional types, and nutrient levels. Oecologia 1997, 112, 492–501. [Google Scholar] [CrossRef]
- Letts, M.G.; Phelan, C.A.; Johnson, D.R.E.; Rood, S.B. Seasonal photosynthetic gas exchange and leaf reflectance characteristics of male and female cottonwoods in a riparian woodland. Tree Physiol. 2008, 28, 1037–1048. [Google Scholar] [CrossRef] [PubMed]
- Nour, V.; Trandafir, I.; Cosmulescu, S. HPLC determination of phenolic acids, flavonoids and juglone in walnut leaves. J. Chromatogr. Sci. 2013, 51, 883–890. [Google Scholar] [CrossRef] [PubMed]
- Pellegrini, E.; Campanella, A.; Paolocci, M.; Trivellini, A.; Gennai, C.; Muganu, M.; Nali, C.; Lorenzini, G. Functional leaf traits and diurnal dynamics of photosynthetic parameters predict the behavior of grapevine varieties towards ozone. PLoS ONE 2015, 10, e0135056. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Hernandez, J.; Gonzalez-Castro, M.J.; Vazquez-Blanco, M.E.; Vazquez-Oderiz, M.L.; Simal-Lozano, J. HPLC determination of sugars and starch in green beans. J. Food Sci. 1994, 59, 1048–1049. [Google Scholar] [CrossRef]
- Smith, A.M.; Zeeman, S.C. Quantification of starch in plant tissues. Nat. Protoc. 2006, 1, 1342–1345. [Google Scholar] [CrossRef] [PubMed]
- Vu, J.C.V.; Gesch, R.W.; Pennanen, A.H.; Allen, L.H.; Boote, K.J.; Bowes, G. Soybean photosynthesis, Rubisco, and carbohydrate enzymes function at supraoptimal temperatures in elevated CO2. J. Plant Physiol. 2001, 158, 295–307. [Google Scholar] [CrossRef]
- Cotrozzi, L.; Remorini, D.; Pellegrini, E.; Landi, M.; Massai, R.; Nali, C.; Guidi, L.; Lorenzini, G. Variations in physiological and biochemical traits of oak seedlings grown under drought and ozone stress. Physiol. Plant. 2016, 157, 69–84. [Google Scholar] [CrossRef] [PubMed]
- Rock, B.N.; Hoshizaki, T.; Miller, J.R. Comparison of In Situ and Airborne Spectral Measurements of the Blue Shift Associated with Forest Decline. Remote Sens. Environ. 1988, 24, 109–127. [Google Scholar] [CrossRef]
- Filella, I.; Peñuelas, J. The red edge position and shape as indicators of plant chlorophyll content, biomass and hydric status. Int. J. Remote Sens. 1994, 15, 1459–1470. [Google Scholar] [CrossRef]
- Harner, A.D.; Leach, H.L.; Briggs, L.; Centinari, M. Prolonged phloem feeding by the spotted lanternfly, an invasive planthopper, alters resource allocation and inhibits gas exchange in grapevines. Plant Direct 2022, 6, e452. [Google Scholar] [CrossRef] [PubMed]
- Rehman, F.; Khan, F.A.; Badruddin, S.M.A. Role of Phenolics in Plant Defense Against Insect Herbivory. In Chemistry of Phytopotentials: Health, Energy and Environmental Perspectives; Khemani, L., Srivastava, M., Srivastava, S., Eds.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 309–313. [Google Scholar] [CrossRef]
- Walling, L.L. The myriad plant responses to herbivores. J. Plant Growth Regul. 2000, 19, 195–216. [Google Scholar] [CrossRef] [PubMed]
- Thompson, G.A.; Goggin, F.L. Transcriptomics and functional genomics of plant defence induction by phloem-feeding insects. J. Exp. Bot. 2006, 57, 755–766. [Google Scholar] [CrossRef] [PubMed]
- Kerchev, P.I.; Fenton, B.; Foyer, C.H.; Hancock, R.D. Plant responses to insect herbivory: Interactions between photosynthesis, reactive oxygen species and hormonal signaling pathways. Plant Cell Environ. 2012, 35, 441–453. [Google Scholar] [CrossRef] [PubMed]
- Pincebourde, S.; Ngao, J. The impact of phloem feeding insects on leaf ecophysiology varies with leaf age. Front. Plant Sci. 2021, 12, 625689. [Google Scholar] [CrossRef]
- Wang, Y.; Cai, Q.N.; Zhang, Q.W.; Han, Y. Effect of the secondary substances from wheat on the growth and digestive physiology of cotton bollworm Helicoverpa armigera (Lepidoptera: Noctuidae). Eur. J. Entomol. 2006, 103, 255–258. [Google Scholar] [CrossRef]
- Czerniewicz, P.; Leszczynski, B.; Chrzanowski, G.; Sempruch, C.; Sytykiewicz, H. Effects of host plant phenolics on spring migration of bird cherry-oat aphid (Rhopalosiphum padi L.). Allelopath. J. 2011, 27, 309–316. [Google Scholar]
- Zhang, M.; Fang, T.; Pu, G.; Sun, X.; Zhou, X.; Cai, Q. Xenobiotic metabolism of plant secondary compounds in the English grain aphid, Sitobion avenae (F.) (Hemiptera: Aphididae). Pestic. Biochem. Physiol. 2013, 107, 44–49. [Google Scholar] [CrossRef]
- Abuley, I.K.; Pedersen, H.A.; Lekfeldt, J.D.S.; Fomsgaard, I.S.; Ravnskov, S. Metabolite profiling of Solanum tuberosum reveals a differential response to Phytophthora infestans dependent on host resistance and pathogen isolate. Plant Pathol. 2023, 72, 924–932. [Google Scholar] [CrossRef]
- Gao, P.; Qi, Y.; Li, L.; Yang, S.; Liu, J.; Wei, H.; Huang, F.; Yu, L. Amorphophallus muelleri activates ferulic acid and phenylpropane biosynthesis pathways to defend against Fusarium solani infection. Front. Plant Sci. 2023, 14, 1207970. [Google Scholar] [CrossRef] [PubMed]
- Kokaly, R.F.; Asner, G.P.; Ollinger, S.V.; Martin, M.E.; Wessman, C.A. Characterizing canopy biochemistry from imaging spectroscopy and its application to ecosystem studies. Remote Sens. Environ. 2009, 113, S78–S91. [Google Scholar] [CrossRef]
- Kokaly, R.F.; Skidmore, A.K. Plant phenolics and absorption features in vegetation reflectance spectra near 1.66 μm. Int. J. Appl. Earth Obs. Geoinf. 2015, 43, 55–83. [Google Scholar] [CrossRef]
- Fine, P.V.A.; Salazar, D.; Martin, R.E.; Metz, M.R.; Misiewicz, T.M.; Asner, G.P. Exploring the links between secondary metabolites and leaf spectral reflectance in a diverse genus of Amazonian trees. Ecosphere 2021, 12, e03362. [Google Scholar] [CrossRef]
- Pozdnyakova, L.; Oudemans, P.V.; Hughes, M.G.; Giménez, D. Estimation of spatial and spectral properties of phytophthora root rot and its effects on cranberry yield. Comput. Electron. Agric. 2002, 37, 57–70. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, M.; Zhu, J.; Geng, S. Spectral prediction of Phytophthora infestans infection on tomatoes using artificial neural network (ANN). Int. J. Remote Sens. 2008, 29, 1693–1706. [Google Scholar] [CrossRef]
- Reynolds, G.J.; Windels, C.E.; MacRae, I.V.; Laguette, S. Remote sensing for assessing rhizoctonia crown and root rot severity in sugar beet. Plant Dis. 2012, 96, 497–505. [Google Scholar] [CrossRef] [PubMed]
- Omer, M.; Locke, J.C.; Frantz, J.M. Using leaf temperature as a nondestructive procedure to detect root rot stress in geranium. HortTechnology 2015, 17, 532–536. [Google Scholar] [CrossRef]
- Calamita, F.; Imran, H.A.; Vescovo, L.; Mekhalfi, M.L.; La Porta, N. Early identification of root rot disease by using hyperspectral reflectance: The case of pathosystem grapevine/armillaria. Remote Sens. 2021, 13, 2436. [Google Scholar] [CrossRef]
- Weksler, S.; Rozenstein, O.; Haish, N.; Moshelion, M.; Wallach, R.; Ben-Dor, E. Pepper plants leaf spectral reflectance changes as a result of root rot damage. Remote Sens. 2021, 13, 980. [Google Scholar] [CrossRef]
- Peron-Danaher, R.; Cotrozzi, L.; Masjedi, A.; Enders, L.S.; Krupke, C.H.; Mickelbart, M.V.; Couture, J.J. Drought stress affects spectral separation of maize infested by western corn rootworm. Agronomy 2023, 13, 2562. [Google Scholar] [CrossRef]
- Lowe, A.; Harrison, N.; French, A.P. Hyperspectral image analysis techniques for the detection and classification of the early onset of plant disease and stress. Plant Methods 2017, 13, 80. [Google Scholar] [CrossRef] [PubMed]
- Susič, N.; Žibrat, U.; Širca, S.; Strajnar, P.; Razinger, J.; Knapič, M.; Vončina, A.; Urek, G.; Stare, B.G. Discrimination between abiotic and biotic drought stress in tomatoes using hyperspectral imaging. Sens. Actuators B Chem. 2018, 273, 842–852. [Google Scholar] [CrossRef]
- Williams, L.J.; Cavender-Bares, J.; Townsend, P.A.; Couture, J.J.; Wang, Z.; Stefanski, A.; Messier, C.; Reich, P.B. Remote spectral detection of biodiversity effects on forest biomass. Nat. Ecol. Evol. 2021, 5, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Leach, H.; Walsh, B.; Urban, J. Evaluation of insecticides for control of spotted lanternfly in ornamental nursery crop, 2019. Arthropod Manag. Tests 2021, 46, tsab043. [Google Scholar] [CrossRef]
- Leach, H.; Urban, J. Management and control of the spotted lanternfly, Lycorma delicatula (Hemiptera: Fulgoridae), in the United States. Annu. Rev. Entomol. 2023, 68, 151–167. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).