Early Detection of Southern Pine Beetle Attack by UAV-Collected Multispectral Imagery
Abstract
1. Introduction
2. Materials and Methods
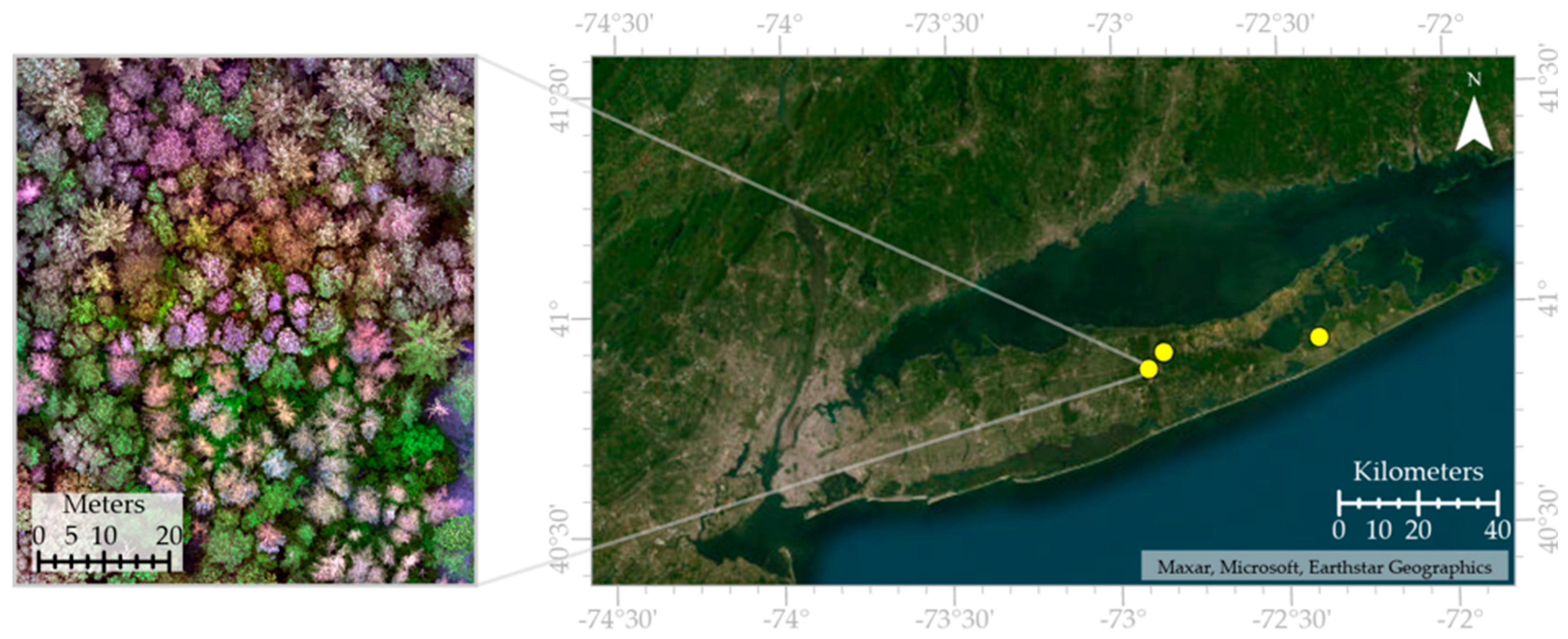
2.1. Sites
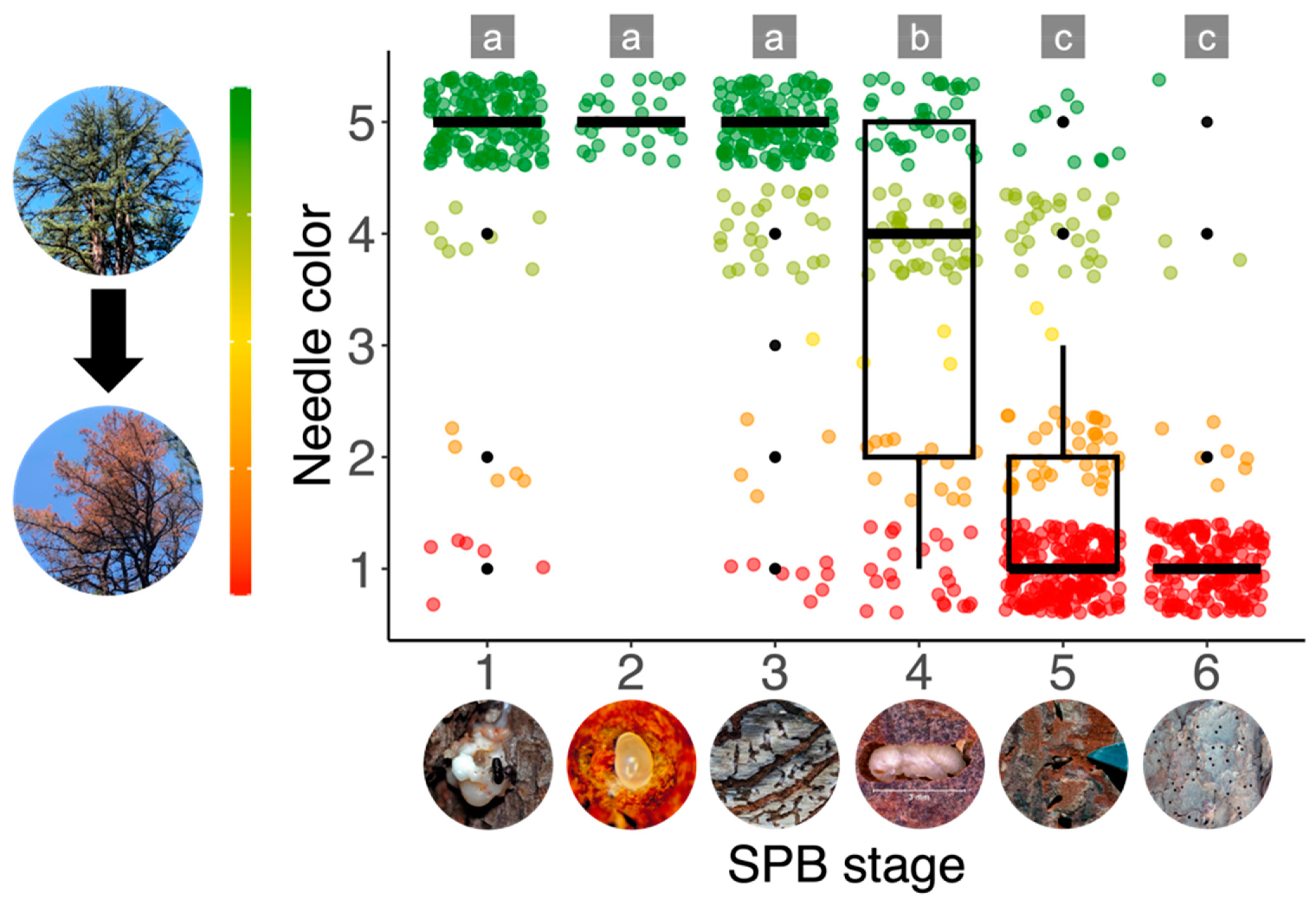
2.2. Ground Data Collection and Analysis
2.3. UAV Imagery Collection
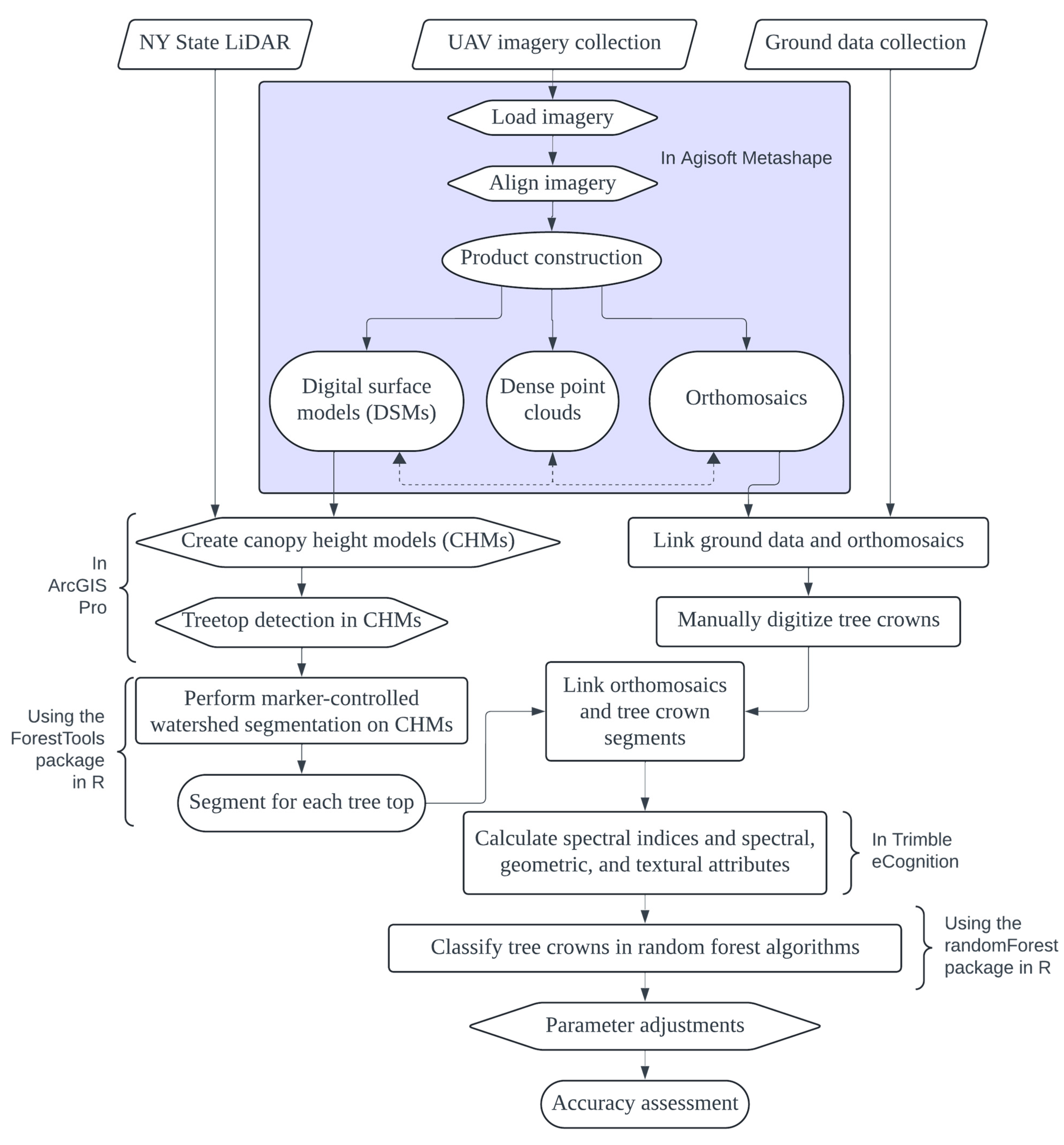
2.4. Image Processing
2.5. Linking Ground Data with UAV Imagery
2.6. Individual Tree Detection and Delineation
2.7. Object-Based Image Analysis, Classification, and Validation
3. Results
3.1. Ground Data
3.2. UAV Data
3.2.1. Individual Tree Detection and Delineation
3.2.2. Classification and Accuracy Assessment
4. Discussion
4.1. Ground Data
4.2. UAV Data
4.3. Data Gaps, Limitations, and Practical Recommendations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Spectral Index | Equation | References | Bark Beetle Detection |
|---|---|---|---|
| ARI (Anthocyanin reflectance index) | [78] | NA | |
| Blue NDVI | [79] | [20,80] | |
| CHLRE (Chlorophyll red edge index) | [81] | NA | |
| DVI (Difference vegetation index) | [82] | [20] | |
| EVI (Enhanced vegetation index) | [83] | NA | |
| GI (Greenness index) | [84] | [12,13] | |
| GLI (Green leaf index) | [85] | [7] | |
| Green NDVI | [86] | [7,12] | |
| LCI (Leaf chlorophyll index) | [87] | NA | |
| LIC (Lichtenthaler index) | [88] | [20,21] | |
| MCARI (Modified chlorophyll absorption in reflectance index) | (RE − Red) − 0.2[(RE − Green) (RE / Red)] | [77] | NA |
| NDVI (Normalized difference vegetation index) | [89,90] | [7,18,30,31,80] | |
| NGRDI (Normalized green–red difference index, or GRVI) | [89] | [7,10,13,18] | |
| PBI (Plant biochemical index) | [11] | [7,11] | |
| PSRI (Plant senescence reflectance index) | [91] | NA | |
| RGI (Red–green ratio) | [92] | [31] | |
| RDVI (Re-normalized difference vegetation index) | SQRT(NDVI DVI) | [93] | NA |
| Red Edge NDVI (Also known as NDRE and RENDWI) | [94] | NA | |
| RVI (Ratio vegetation index) | [82] | [20] | |
| TVI (Triangular vegetation index) | 60 (NIR − Green) − 100 (Red − Green) | [95] | NA |
References
- Coleman, T.W.; Clarke, S.R.; Meeker, J.R.; Rieske, L.K. Forest Composition Following Overstory Mortality from Southern Pine Beetle and Associated Treatments. Can. J. For. Res. 2008, 38, 1406–1418. [Google Scholar] [CrossRef]
- Franceschi, V.R.; Krokene, P.; Christiansen, E.; Krekling, T. Anatomical and Chemical Defenses of Conifer Bark against Bark Beetles and Other Pests. New Phytol. 2005, 167, 353–376. [Google Scholar] [CrossRef] [PubMed]
- Rock, B.N.; Vogelmann, J.E.; Williams, D.L.; Vogelmann, A.F.; Hoshizaki, T. Remote Detection of Forest Damage: Plant Responses to Stress May Have Spectral “Signatures” That Could Be Used to Map, Monitor, and Measure Forest Damage. BioScience 1986, 36, 439–445. [Google Scholar] [CrossRef]
- Ahern, F.J. The Effects of Bark Beetle Stress on the Foliar Spectral Reflectance of Lodgepole Pine. Int. J. Remote Sens. 1988, 9, 1451–1468. [Google Scholar] [CrossRef]
- Curran, P.J. Remote Sensing of Foliar Chemistry. Remote Sens. Environ. 1989, 30, 271–278. [Google Scholar] [CrossRef]
- Horler, D.N.H.; Dockray, M.; Barber, J. The Red Edge of Plant Leaf Reflectance. Int. J. Remote Sens. 1983, 4, 273–288. [Google Scholar] [CrossRef]
- Huo, L.; Lindberg, E.; Bohlin, J.; Persson, H.J. Assessing the Detectability of European Spruce Bark Beetle Green Attack in Multispectral Drone Images with High Spatial- and Temporal Resolutions. Remote Sens. Environ. 2023, 287, 113484. [Google Scholar] [CrossRef]
- Honkavaara, E.; Näsi, R.; Oliveira, R.; Viljanen, N.; Suomalainen, J.; Khoramshahi, E.; Hakala, T.; Nevalainen, O.; Markelin, L.; Vuorinen, M.; et al. Using Multitemporal Hyper- and Multispectral UAV Imaging for Detecting Bark Beetle Infestation on Norway Spruce. Int. Arch. Photogramm. Remote Sens. Spat. Inf. Sci. 2020, XLIII-B3-2020, 429–434. [Google Scholar] [CrossRef]
- Hellwig, F.M.; Stelmaszczuk-Górska, M.A.; Dubois, C.; Wolsza, M.; Truckenbrodt, S.C.; Sagichewski, H.; Chmara, S.; Bannehr, L.; Lausch, A.; Schmullius, C. Mapping European Spruce Bark Beetle Infestation at Its Early Phase Using Gyrocopter-Mounted Hyperspectral Data and Field Measurements. Remote Sens. 2021, 13, 4659. [Google Scholar] [CrossRef]
- Abdullah, H.; Skidmore, A.K.; Darvishzadeh, R.; Heurich, M. Sentinel-2 Accurately Maps Green-Attack Stage of European Spruce Bark Beetle (Ips typographus, L.) Compared with Landsat-8. Remote Sens. Ecol. Conserv. 2019, 5, 87–106. [Google Scholar] [CrossRef]
- Abdullah, H.; Skidmore, A.K.; Darvishzadeh, R.; Heurich, M. Timing of Red-Edge and Shortwave Infrared Reflectance Critical for Early Stress Detection Induced by Bark Beetle (Ips typographus, L.) Attack. Int. J. Appl. Earth Obs. Geoinf. 2019, 82, 101900. [Google Scholar] [CrossRef]
- Bárta, V.; Hanuš, J.; Dobrovolný, L.; Homolová, L. Comparison of Field Survey and Remote Sensing Techniques for Detection of Bark Beetle-Infested Trees. For. Ecol. Manag. 2022, 506, 119984. [Google Scholar] [CrossRef]
- Klouček, T.; Komárek, J.; Surový, P.; Hrach, K.; Janata, P.; Vašíček, B. The Use of UAV Mounted Sensors for Precise Detection of Bark Beetle Infestation. Remote Sens. 2019, 11, 1561. [Google Scholar] [CrossRef]
- Fassnacht, F.E.; Latifi, H.; Ghosh, A.; Joshi, P.K.; Koch, B. Assessing the Potential of Hyperspectral Imagery to Map Bark Beetle-Induced Tree Mortality. Remote Sens. Environ. 2014, 140, 533–548. [Google Scholar] [CrossRef]
- Näsi, R.; Honkavaara, E.; Blomqvist, M.; Lyytikäinen-Saarenmaa, P.; Hakala, T.; Viljanen, N.; Kantola, T.; Holopainen, M. Remote Sensing of Bark Beetle Damage in Urban Forests at Individual Tree Level Using a Novel Hyperspectral Camera from UAV and Aircraft. Urban For. Urban Green. 2018, 30, 72–83. [Google Scholar] [CrossRef]
- Näsi, R.; Honkavaara, E.; Lyytikäinen-Saarenmaa, P.; Blomqvist, M.; Litkey, P.; Hakala, T.; Viljanen, N.; Kantola, T.; Tanhuanpää, T.; Holopainen, M. Using UAV-Based Photogrammetry and Hyperspectral Imaging for Mapping Bark Beetle Damage at Tree-Level. Remote Sens. 2015, 7, 15467–15493. [Google Scholar] [CrossRef]
- Safonova, A.; Hamad, Y.; Alekhina, A.; Kaplun, D. Detection of Norway Spruce Trees (Picea abies) Infested by Bark Beetle in UAV Images Using YOLOs Architectures. IEEE Access 2022, 10, 10384–10392. [Google Scholar] [CrossRef]
- Bárta, V.; Lukeš, P.; Homolová, L. Early Detection of Bark Beetle Infestation in Norway Spruce Forests of Central Europe Using Sentinel-2. Int. J. Appl. Earth Obs. Geoinf. 2021, 100, 102335. [Google Scholar] [CrossRef]
- Kautz, M.; Feurer, J.; Adler, P. Early Detection of Bark Beetle (Ips typographus) Infestations by Remote Sensing—A Critical Review of Recent Research. For. Ecol. Manag. 2024, 556, 121595. [Google Scholar] [CrossRef]
- Gao, B.; Yu, L.; Ren, L.; Zhan, Z.; Luo, Y. Early Detection of Dendroctonus valens Infestation with Machine Learning Algorithms Based on Hyperspectral Reflectance. Remote Sens. 2022, 14, 1373. [Google Scholar] [CrossRef]
- Gao, B.; Yu, L.; Ren, L.; Zhan, Z.; Luo, Y. Early Detection of Dendroctonus valens Infestation at Tree Level with a Hyperspectral UAV Image. Remote Sens. 2023, 15, 407. [Google Scholar] [CrossRef]
- Zhan, Z.; Yu, L.; Li, Z.; Ren, L.; Gao, B.; Wang, L.; Luo, Y. Combining GF-2 and Sentinel-2 Images to Detect Tree Mortality Caused by Red Turpentine Beetle during the Early Outbreak Stage in North China. Forests 2020, 11, 172. [Google Scholar] [CrossRef]
- Mckeeman, T. Thermal Remote Sensing of Mountain Pine Beetle Green Attack. Master’s Thesis, University of Calgary, Calgary, AB, Canada, 2022. [Google Scholar]
- Niemann, K.O.; Quinn, G.; Stephen, R.; Visintini, F.; Parton, D. Hyperspectral Remote Sensing of Mountain Pine Beetle with an Emphasis on Previsual Assessment. Can. J. Remote Sens. 2015, 41, 191–202. [Google Scholar] [CrossRef]
- Runesson, T. Considerations for Early Remote Detection of Mountain Pine Beetle in Green-Foliaged Lodgepole Pine. Ph.D. Thesis, The University of British Columbia, Vancouver, BC, Canada, 1991. [Google Scholar]
- Coops, N.C.; Johnson, M.; Wulder, M.A.; White, J.C. Assessment of QuickBird High Spatial Resolution Imagery to Detect Red Attack Damage Due to Mountain Pine Beetle Infestation. Remote Sens. Environ. 2006, 103, 67–80. [Google Scholar] [CrossRef]
- Lawrence, R.; Labus, M. Early Detection of Douglas-Fir Beetle Infestation with Subcanopy Resolution Hyperspectral Imagery. West. J. Appl. For. 2003, 18, 202–206. [Google Scholar] [CrossRef]
- Foster, A.C.; Walter, J.A.; Shugart, H.H.; Sibold, J.; Negron, J. Spectral Evidence of Early-Stage Spruce Beetle Infestation in Engelmann Spruce. For. Ecol. Manag. 2017, 384, 347–357. [Google Scholar] [CrossRef]
- Ciesla, W.M.; Bell, J.C., Jr.; Curlin, J.W. Color Photos and the Southern Pine Beetle. Photogramm. Eng. 1967, 33, 883–888. [Google Scholar]
- Carter, G.A.; Seal, M.R.; Haley, T. Airborne Detection of Southern Pine Beetle Damage Using Key Spectral Bands. Can. J. For. Res. 1998, 28, 1040–1045. [Google Scholar] [CrossRef]
- Meng, R.; Gao, R.; Zhao, F.; Huang, C.; Sun, R.; Lv, Z.; Huang, Z. Landsat-Based Monitoring of Southern Pine Beetle Infestation Severity and Severity Change in a Temperate Mixed Forest. Remote Sens. Environ. 2022, 269, 112847. [Google Scholar] [CrossRef]
- Crosby, M.K.; McConnell, T.E.; Holderieath, J.J.; Meeker, J.R.; Steiner, C.A.; Strom, B.L.; Johnson, C. The Use of High-Resolution Satellite Imagery to Determine the Status of a Large-Scale Outbreak of Southern Pine Beetle. Remote Sens. 2024, 16, 582. [Google Scholar] [CrossRef]
- Pye, J.M.; Holmes, T.P.; Prestemon, J.P.; Wear, D.N. Economic Impacts of the Southern Pine Beetle. In Southern Pine Beetle II. General Technical Report SRS-140; Coulson, R.N., Klepzig, K.D., Eds.; U.S. Department of Agriculture Forest Service, Southern Research Station: Asheville, NC, USA, 2011; pp. 213–222. [Google Scholar]
- Hain, F.P.; Duehl, A.J.; Gardner, M.J.; Payne, T.L. Natural History of the Southern Pine Beetle. In Southern Pine Beetle II. General Technical Report SRS-140; Coulson, R.N., Klepzig, K.D., Eds.; U.S. Department of Agriculture Forest Service, Southern Research Station: Asheville, NC, USA, 2011; pp. 13–24. [Google Scholar]
- Lombardero, M.J.; Ayres, M.P.; Ayres, B.D.; Reeve, J.D. Cold Tolerance of Four Species of Bark Beetle (Coleoptera: Scolytidae) in North America. Environ. Entomol. 2000, 29, 421–432. [Google Scholar] [CrossRef]
- Tran, J.K.; Ylioja, T.; Billings, R.F.; Régnière, J.; Ayres, M.P. Impact of Minimum Winter Temperatures on the Population Dynamics of Dendroctonus frontalis. Ecol. Appl. 2007, 17, 882–899. [Google Scholar] [CrossRef] [PubMed]
- Dodds, K.J.; Aoki, C.F.; Arango-Velez, A.; Cancelliere, J.; D’Amato, A.W.; DiGirolomo, M.F.; Rabaglia, R.J. Expansion of Southern Pine Beetle into Northeastern Forests: Management and Impact of a Primary Bark Beetle in a New Region. J. For. 2018, 116, 178–191. [Google Scholar] [CrossRef]
- Kanaskie, C.R.; Schmeelk, T.C.; Cancelliere, J.A.; Garnas, J.R. New Records of Southern Pine Beetle (Dendroctonus frontalis Zimmermann; Coleoptera: Curculionidae) in New York, New Hampshire, and Maine, USA Indicate Northward Range Expansion. Coleopt. Bull. 2023, 77, 248–251. [Google Scholar] [CrossRef]
- McGrady, J. Conservation Groups Team Up To Combat Southern Pine Beetle Outbreak. Nantucket Current, 22 August 2023. [Google Scholar]
- Humphrey, T. Pine Beetle Infestation Invades Island Forests. The Vineyard Gazette, 15 August 2023. [Google Scholar]
- Lesk, C.; Coffel, E.; D’Amato, A.W.; Dodds, K.; Horton, R. Threats to North American Forests from Southern Pine Beetle with Warming Winters. Nat. Clim. Change 2017, 7, 713–717. [Google Scholar] [CrossRef]
- Gara, R.I.; Vité, J.P.; Cramer, H.H. Manipulation of Dendroctonus frontalis by Use of a Population Aggregating Pheromone. Contrib. Boyce Thompson Inst. 1965, 23, 55–66. [Google Scholar]
- Coulson, R.N.; McFadden, B.A.; Pulley, P.E.; Lovelady, C.N.; Fitzgerald, J.W.; Jack, S.B. Heterogeneity of Forest Landscapes and the Distribution and Abundance of the Southern Pine Beetle. For. Ecol. Manag. 1999, 114, 471–485. [Google Scholar] [CrossRef]
- Ayres, M.P.; Martinson, S.J.; Friedenberg, N.A. Southern Pine Beetle Ecology: Populations within Stands. In Southern Pine Beetle II. General Technical Report SRS-140; Coulson, R.N., Klepzig, K.D., Eds.; U.S. Department of Agriculture Forest Service, Southern Research Station: Asheville, NC, USA, 2011; pp. 75–90. [Google Scholar]
- Martinson, S.J.; Ylioja, T.; Sullivan, B.T.; Billings, R.F.; Ayres, M.P. Alternate Attractors in the Population Dynamics of a Tree-Killing Bark Beetle. Popul. Ecol. 2013, 55, 95–106. [Google Scholar] [CrossRef]
- Paine, T.D.; Raffa, K.; Harrington, T. Interactions among Scolytid Bark Beetles, Their Associated Fungi, and Live Host Conifers. Annu. Rev. Entomol. 1997, 42, 179–206. [Google Scholar] [CrossRef]
- Gara, R.I.; Coster, J.E. Studies on Attack Behavior of Southern Pine Beetle. III Sequence of Tree Infestation within Stands. Contrib. Boyce Thompson Inst. 1968, 24, 77–85. [Google Scholar]
- Guldin, J.M. Silvicultural Considerations in Managing Southern Pine Stands in the Context of Southern Pine Beetle. In Southern Pine Beetle II. General Technical Report SRS-140; Coulson, R.N., Klepzig, K.D., Eds.; U.S. Department of Agriculture Forest Service, Southern Research Station: Asheville, NC, USA, 2011; pp. 317–352. [Google Scholar]
- Billings, R.F. Mechanical Control of Southern Pine Beetle Infestations. In Southern Pine Beetle II. General Technical Report SRS-140; Coulson, R.N., Klepzig, K.D., Eds.; U.S. Department of Agriculture Forest Service, Southern Research Station: Asheville, NC, USA, 2011; pp. 399–413. [Google Scholar]
- Swain, K.M.; Remion, M.C. Direct Control Methods for the Southern Pine Beetle; U.S. Department of Agriculture: Washington, DC, USA, 1981.
- Cancelliere, J. Effects of Winter Cut-and-Leave Suppression on SPB Overwintering Success; New York Department of Environmental Conservation: Albany, NY, USA, 2018.
- Billings, R.F.; Herbert, A., III. A Field Guide for Ground Checking Southern Pine Beetle Spots; Agriculture Handbook No. 558; United States Department of Agriculture, Combined Forest Pest Research and Development Program: Washington, DC, USA, 1979.
- Gifford, N.A.; Deppen, J.M.; Bried, J.T. Importance of an Urban Pine Barrens for the Conservation of Early-Successional Shrubland Birds. Landsc. Urban Plan. 2010, 94, 54–62. [Google Scholar] [CrossRef]
- Bried, J.T.; Patterson, W.A.; Gifford, N.A. Why Pine Barrens Restoration Should Favor Barrens over Pine. Restor. Ecol. 2014, 22, 442–446. [Google Scholar] [CrossRef]
- Kanaskie, C.R.; Dodds, K.J.; Stephen, F.M.; Garnas, J.R. Southern Pine Beetle (Coleoptera: Curculionidae) and Its Associated Insect Community: Similarities and Key Differences between Northeastern and Southeastern Pine Forests. Environ. Entomol. 2024, 53, 143–156. [Google Scholar] [CrossRef] [PubMed]
- Billings, R.F.; Ward, J.D. How to Conduct a Southern Pine Beetle Aerial Detection Survey; Circular-Texas Forest Service: Lufkin, TX, USA, 1984. [Google Scholar]
- Billings, R.F. Aerial Detection, Ground Evaluation, and Monitoring of the Southern Pine Beetle: State Perspectives. In Southern Pine Beetle II. USDA Forest Service Southern Research Station Gen. Tech Rep. SRS-140; Coulson, R.N., Klepzig, K.D., Eds.; U.S. Department of Agriculture Forest Service, Southern Research Station: Asheville, NC, USA, 2011; pp. 245–261. [Google Scholar]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using Lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Kuznetsova, A.; Brockhoff, P.B.; Christensen, R.H.B. lmerTest Package: Tests in Linear Mixed Effects Models. J. Stat. Softw. 2017, 82, 1–26. [Google Scholar] [CrossRef]
- Hothorn, T.; Bretz, F.; Westfall, P.; Heiberger, R.M.; Schuetzenmeister, A.; Scheibe, S. Multcomp: Simultaneous Inference in General Parametric Models. 2023. Available online: https://cran.r-project.org/web/packages/multcomp/index.html (accessed on 14 July 2024).
- Wickham, H.; Chang, W.; Henry, L.; Pedersen, T.L.; Takahashi, K.; Wilke, C.; Woo, K.; Yutani, H.; Dunnington, D.; van den Brand, T. Ggplot2: Create Elegant Data Visualisations Using the Grammar of Graphics. 2023. Available online: https://cran.r-project.org/web/packages/ggplot2/index.html (accessed on 14 July 2024).
- Fraser, B.T.; Bunyon, C.L.; Reny, S.; Lopez, I.S.; Congalton, R.G. Analysis of Unmanned Aerial System (UAS) Sensor Data for Natural Resource Applications: A Review. Geographies 2022, 2, 303–340. [Google Scholar] [CrossRef]
- Fraser, B.T.; Congalton, R.G. Issues in Unmanned Aerial Systems (UAS) Data Collection of Complex Forest Environments. Remote Sens. 2018, 10, 908. [Google Scholar] [CrossRef]
- Fraser, B.T.; Congalton, R.G. Estimating Primary Forest Attributes and Rare Community Characteristics Using Unmanned Aerial Systems (UAS): An Enrichment of Conventional Forest Inventories. Remote Sens. 2021, 13, 2971. [Google Scholar] [CrossRef]
- OCM Partners. 2014 USGS CMGP Lidar: Post Sandy (Long Island, NY). Available online: https://www.fisheries.noaa.gov/inport/item/49890 (accessed on 14 July 2024).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023. [Google Scholar]
- Plowright, A. Package ForestTools: Tools for Analyzing Remote Sensing Forest Data. 2024. Available online: https://cran.r-project.org/web/packages/ForestTools/index.html (accessed on 14 July 2024).
- Breiman, A.; Cutler, A.; Liaw, A.; Wienner, M. randomForest: Breiman and Cutler’s Random Forests for Classification and Regression. 2022. Available online: https://cran.r-project.org/web/packages/randomForest/index.html (accessed on 14 July 2024).
- Breiman, L. Random Forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef]
- Maxwell, A.E.; Warner, T.A.; Fang, F. Implementation of Machine-Learning Classification in Remote Sensing: An Applied Review. Int. J. Remote Sens. 2018, 39, 2784–2817. [Google Scholar] [CrossRef]
- Tuszynski, J. caTools: Tools: Moving Window Statistics, GIF, Base64, ROC AUC, Etc. 2021. Available online: https://cran.r-project.org/web/packages/caTools/index.html (accessed on 14 July 2024).
- Wickham, H.; François, R.; Henry, L.; Müller, K.; Vaughan, D.; Posit Software, PBC. Dplyr: A Grammar of Data Manipulation. 2023. Available online: https://cran.r-project.org/web/packages/dplyr/index.html (accessed on 14 July 2024).
- Han, H.; Guo, X.; Yu, H. Variable Selection Using Mean Decrease Accuracy and Mean Decrease Gini Based on Random Forest. In Proceedings of the 2016 7th IEEE International Conference on Software Engineering and Service Science (ICSESS), Beijing, China, 26–28 August 2016; pp. 219–224. [Google Scholar]
- Chen, C.; Liaw, A.; Beiman, L. Using Random Forest to Learn Inbalanced Data; University of California Berkeley: Berkeley, CA, USA, 2004; p. 12. [Google Scholar]
- Congalton, R.G. A Review of Assessing the Accuracy of Classifications of Remotely Sensed Data. Remote Sens. Environ. 1991, 37, 35–46. [Google Scholar] [CrossRef]
- Congalton, R.G.; Green, K. Assessing the Accuracy of Remotely Sensed Data: Principles and Practices, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2019; ISBN 978-0-429-62935-8. [Google Scholar]
- Daughtry, C.S.T.; Walthall, C.L.; Kim, M.S.; de Colstoun, E.B.; McMurtrey, J.E., III. Estimating Corn Leaf Chlorophyll Concentration from Leaf and Canopy Reflectance. Remote Sens. Environ. 2000, 74, 229–239. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Merzlyak, M.N.; Chivkunova, O.B. Optical Properties and Nondestructive Estimation of Anthocyanin Content in Plant Leaves. Photochem. Photobiol. 2001, 74, 38–45. [Google Scholar] [CrossRef]
- Blackburn, G.A. Spectral Indices for Estimating Photosynthetic Pigment Concentrations: A Test Using Senescent Tree Leaves. Int. J. Remote Sens. 1998, 19, 657–675. [Google Scholar] [CrossRef]
- Klouček, T.; Modlinger, R.; Zikmundová, M.; Kycko, M.; Komárek, J. Early Detection of Bark Beetle Infestation Using UAV-Borne Multispectral Imagery: A Case Study on the Spruce Forest in the Czech Republic. Front. For. Glob. Change 2024, 7, 1215734. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Keydan, G.P.; Merzlyak, M.N. Three-Band Model for Noninvasive Estimation of Chlorophyll, Carotenoids, and Anthocyanin Contents in Higher Plant Leaves. Geophys. Res. Lett. 2006, 33, L11402. [Google Scholar] [CrossRef]
- Zhu, Y.; Yao, X.; Tian, Y.; Liu, X.; Cao, W. Analysis of Common Canopy Vegetation Indices for Indicating Leaf Nitrogen Accumulations in Wheat and Rice. Int. J. Appl. Earth Obs. Geoinf. 2008, 10, 1–10. [Google Scholar] [CrossRef]
- Liu, H.Q.; Huete, A. A Feedback Based Modification of the NDVI to Minimize Canopy Background and Atmospheric Noise. IEEE Trans. Geosci. Remote Sens. 1995, 33, 457–465. [Google Scholar] [CrossRef]
- Smith, R.C.G.; Adams, J.; Stephens, D.J.; Hick, P.T. Forecasting Wheat Yield in a Mediterranean-Type Environment from the NOAA Satellite. Aust. J. Agric. Res. 1995, 46, 113–125. [Google Scholar] [CrossRef]
- Louhaichi, M.; Borman, M.M.; Johnson, D.E. Spatially Located Platform and Aerial Photography for Documentation of Grazing Impacts on Wheat. Geocarto Int. 2001, 16, 65–70. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Kaufman, Y.J.; Merzlyak, M.N. Use of a Green Channel in Remote Sensing of Global Vegetation from EOS-MODIS. Remote Sens. Environ. 1996, 58, 289–298. [Google Scholar] [CrossRef]
- Yu, R.; Luo, Y.; Zhou, Q.; Zhang, X.; Wu, D.; Ren, L. Early Detection of Pine Wilt Disease Using Deep Learning Algorithms and UAV-Based Multispectral Imagery. For. Ecol. Manag. 2021, 497, 119493. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Lang, M.; Sowinska, M.; Heisel, F.; Miehé, J.A. Detection of Vegetation Stress Via a New High Resolution Fluorescence Imaging System. J. Plant Physiol. 1996, 148, 599–612. [Google Scholar] [CrossRef]
- Tucker, C.J. Red and Photographic Infrared Linear Combinations for Monitoring Vegetation. Remote Sens. Environ. 1979, 8, 127–150. [Google Scholar] [CrossRef]
- Rouse, J.W.; Haas, R.H.; Schell, J.A.; Deering, D.W. Monitoring Vegetation Systems in the Great Plains with ERTS. In Proceedings of the Third ERTS-1 Symposium, Washington, DC, USA, 10–14 December 1973; NASA SP-351. National Aeronautics and Space Administration: Washington, DC, USA, 1974; pp. 309–317. [Google Scholar]
- Merzlyak, M.N.; Gitelson, A.A.; Chivkunova, O.B.; Rakitin, V.Y. Non-Destructive Optical Detection of Pigment Changes during Leaf Senescence and Fruit Ripening. Physiol. Plant. 1999, 106, 135–141. [Google Scholar] [CrossRef]
- de Santos, I.C.L.; dos Santos, A.; Oumar, Z.; Soares, M.A.; Silva, J.C.C.; Zanetti, R.; Zanuncio, J.C. Remote Sensing to Detect Nests of the Leaf-Cutting Ant Atta sexdens (Hymenoptera: Formicidae) in Teak Plantations. Remote Sens. 2019, 11, 1641. [Google Scholar] [CrossRef]
- Roujean, J.-L.; Breon, F.-M. Estimating PAR Absorbed by Vegetation from Bidirectional Reflectance Measurements. Remote Sens. Environ. 1995, 51, 375–384. [Google Scholar] [CrossRef]
- Iordache, M.-D.; Mantas, V.; Baltazar, E.; Pauly, K.; Lewyckyj, N. A Machine Learning Approach to Detecting Pine Wilt Disease Using Airborne Spectral Imagery. Remote Sens. 2020, 12, 2280. [Google Scholar] [CrossRef]
- Broge, N.H.; Leblanc, E. Comparing Prediction Power and Stability of Broadband and Hyperspectral Vegetation Indices for Estimation of Green Leaf Area Index and Canopy Chlorophyll Density. Remote Sens. Environ. 2001, 76, 156–172. [Google Scholar] [CrossRef]


| Geometric Features | Textural Features | Spectral Features |
|---|---|---|
| Area (pixels) Asymmetry Border index Compactness Density Length (pixels) Length/width Radius of long ellipsoid Radius of short ellipsoid Roundness Shape index | GLCM * Contrast GLCM Correlation GLCM Dissimilarity GLCM Entropy GLCM Homogeneity GLCM Mean GLDV † Contrast GLDV Entropy GLDV Mean | Brightness HSI intensity Mean blue Mean green Mean red Mean red edge Mean NIR Standard deviation blue Standard deviation green Standard deviation red Standard deviation red edge Standard deviation NIR |
| Class | Reference Sample Size | Percentage of Reference Samples | Balanced Sample Size | Percentage of Balanced Samples |
|---|---|---|---|---|
| Deciduous | 331 | 34.4% | 62 | 20% |
| Healthy pine | 147 | 15.3% | 62 | 20% |
| SPB green attack | 62 | 6.4% | 62 | 20% |
| SPB visible attack | 102 | 10.6% | 62 | 20% |
| Dead pine | 321 | 33.3% | 62 | 20% |
| Total | 963 | 100.0% | 310 | 100% |
| Tree Class | Mean Producer’s Accuracy | Mean User’s Accuracy |
|---|---|---|
| Deciduous | 89.7 ± 5.6% | 94.9 ± 4.3% |
| Healthy pine | 87.0 ± 8.7% | 82.1 ± 7.4% |
| SPB green attack | 68.3 ± 8.5% | 72.1 ± 8.3% |
| SPB visible attack | 65.9 ± 9.1% | 62.5 ± 9.8% |
| Dead pine | 85.5 ± 6.2% | 83.6 ± 8.6% |
| Reference Data | ||||||||
|---|---|---|---|---|---|---|---|---|
| Deciduous | Healthy Pine | SPB Green Attack | SPB Visible Attack | Dead Pine | Total | User’s Accuracy | ||
| Classified Data | Deciduous | 33 | 0 | 7 | 4 | 0 | 44 | 75.0% |
| Healthy Pine | 0 | 23 | 0 | 1 | 0 | 24 | 95.8% | |
| SPB Green Attack | 0 | 3 | 23 | 3 | 0 | 29 | 79.3% | |
| SPB Visible Attack | 0 | 0 | 7 | 21 | 2 | 30 | 70.0% | |
| Dead Pine | 0 | 2 | 0 | 2 | 24 | 28 | 85.7% | |
| Total | 33 | 28 | 37 | 31 | 26 | 155 | ||
| Producer’s Accuracy | 100% | 82.1% | 62.2% | 67.7% | 92.3% | Overall Accuracy 80.0% | ||
| Reference Data | |||||||
|---|---|---|---|---|---|---|---|
| Healthy Pine | SPB Green Attack | SPB Visible Attack | Dead Pine | Total | User’s Accuracy | ||
| Classified Data | Healthy Pine | 26 | 1 | 2 | 0 | 29 | 89.7% |
| SPB Green Attack | 1 | 25 | 4 | 1 | 31 | 80.6% | |
| SPB Visible Attack | 2 | 4 | 19 | 5 | 30 | 63.3% | |
| Dead Pine | 0 | 0 | 3 | 28 | 31 | 90.3% | |
| Total | 29 | 30 | 28 | 34 | 121 | ||
| Producer’s Accuracy | 89.7% | 83.3% | 67.9% | 82.4% | Overall Accuracy 81.0% | ||
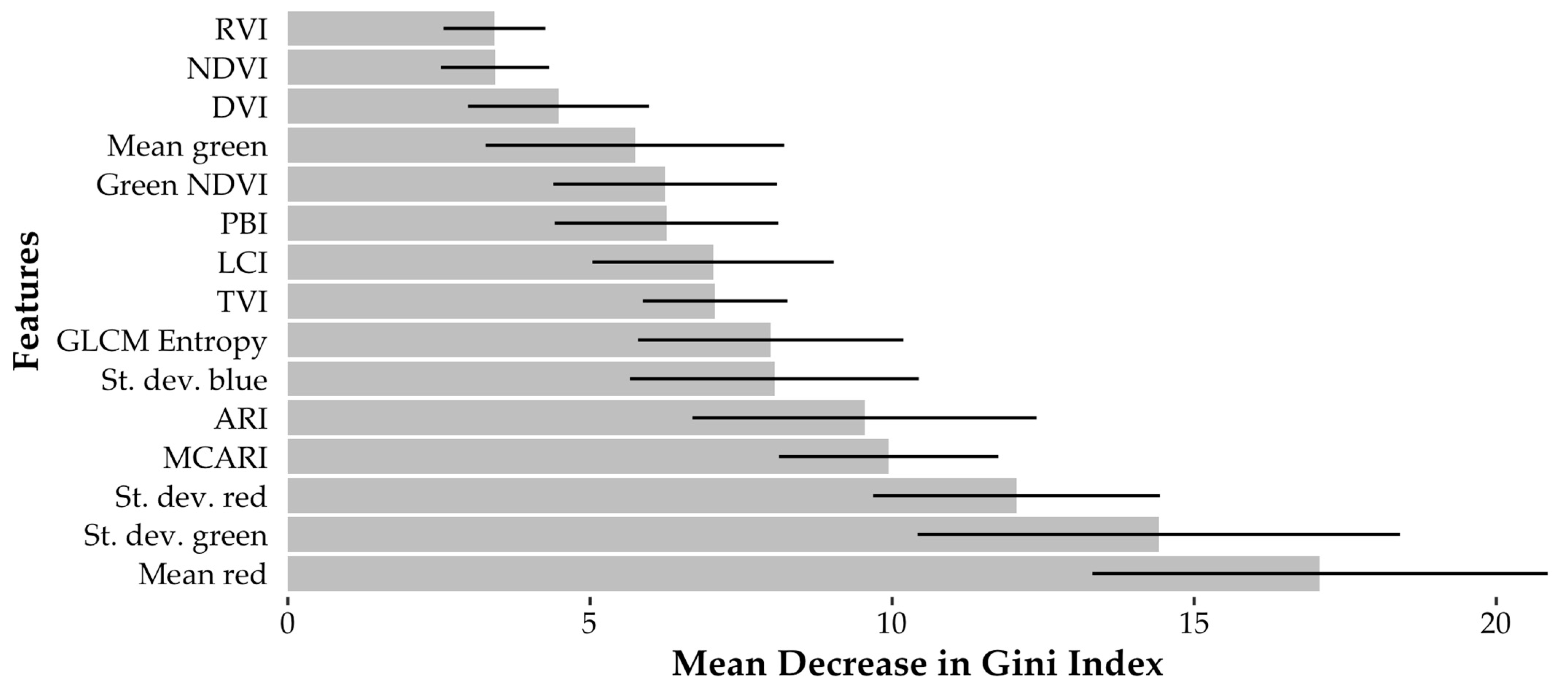
| Feature | Mean Decrease in Accuracy (%) | Mean Decrease in Gini Index | ||||
|---|---|---|---|---|---|---|
| Deciduous | Healthy Pine | SPB Green Attack | SPB Visible Attack | Dead Pine | ||
| Mean red | 36.9± 8.1 | 17.3 ± 4.8 | 8.4 ± 5.7 | 11.1 ± 5.0 | 16.5 ± 4.0 | 17.1± 3.8 |
| St. dev. green | 18.9 ± 4.5 | 16.5 ± 5.4 | 16.5 ± 4.4 | 4.2 ± 4.7 | 25.6 ± 8.7 | 14.4 ± 4.0 |
| St. dev. red | 6.8 ± 3.9 | 17.9 ± 5.4 | 21.9± 5.9 | 23.4± 7.8 | 9.0 ± 5.5 | 12.1 ± 2.4 |
| MCARI * | 10.4 ± 3.5 | 22.3± 4.4 | 21.2 ± 4.0 | 3.2 ± 3.2 | 10.8 ± 3.4 | 9.9 ± 1.8 |
| ARI * | 10.0 ± 2.9 | 13.7 ± 3.9 | 8.4 ± 3.6 | 1.3 ± 4.4 | 25.9± 6.1 | 9.5 ± 2.8 |
| St. dev. blue | 16.6 ± 5.5 | 12.0 ± 4.8 | 8.4 ± 4.0 | 9.2 ± 4.2 | 5.3 ± 3.7 | 8.1 ± 2.4 |
| GLCM entropy | 3.4 ± 2.6 | 14.4 ± 5.3 | 10.7 ± 5.6 | 6.9 ± 5.0 | 11.5 ± 4.9 | 8.0 ± 2.2 |
| TVI * | 9.9 ± 2.7 | 15.7 ± 2.8 | 16.2 ± 3.1 | 4.4 ± 3.3 | 7.1 ± 2.4 | 7.1 ± 1.2 |
| LCI * | 18.9 ± 5.6 | 13.3 ± 3.8 | 2.6 ± 3.6 | 10.7 ± 4.6 | 3.6 ± 2.7 | 7.0 ± 2.0 |
| PBI * | 14.3 ± 3.2 | 12.6 ± 2.6 | 2.6 ± 2.5 | 3.4 ± 3.2 | 13.2 ± 2.8 | 6.3 ± 1.9 |
| Green NDVI * | 14.4 ± 3.2 | 12.5 ± 2.7 | 2.6 ± 2.7 | 3.4 ± 3.3 | 13.2 ± 2.9 | 6.2 ± 1.9 |
| Mean green | 10.9 ± 2.7 | 5.4 ± 4.1 | 6.9 ± 3.7 | 0.7 ± 3.4 | 11.3 ± 5.5 | 5.7 ± 2.5 |
| DVI * | 10.9 ± 3.1 | 9.1 ± 2.7 | 5.7 ± 1.8 | 3.0 ± 2.7 | 8.3 ± 2.5 | 4.5 ± 1.5 |
| NDVI * | 6.0 ± 2.4 | 8.4 ± 2.2 | 8.3 ± 2.2 | 2.3 ± 2.4 | 7.2 ± 2.4 | 3.4 ± 0.9 |
| RVI * | 6.0 ± 2.2 | 8.4 ± 2.2 | 8.6 ± 2.1 | 2.4 ± 2.2 | 7.1 ± 2.2 | 3.4 ± 0.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kanaskie, C.R.; Routhier, M.R.; Fraser, B.T.; Congalton, R.G.; Ayres, M.P.; Garnas, J.R. Early Detection of Southern Pine Beetle Attack by UAV-Collected Multispectral Imagery. Remote Sens. 2024, 16, 2608. https://doi.org/10.3390/rs16142608
Kanaskie CR, Routhier MR, Fraser BT, Congalton RG, Ayres MP, Garnas JR. Early Detection of Southern Pine Beetle Attack by UAV-Collected Multispectral Imagery. Remote Sensing. 2024; 16(14):2608. https://doi.org/10.3390/rs16142608
Chicago/Turabian StyleKanaskie, Caroline R., Michael R. Routhier, Benjamin T. Fraser, Russell G. Congalton, Matthew P. Ayres, and Jeff R. Garnas. 2024. "Early Detection of Southern Pine Beetle Attack by UAV-Collected Multispectral Imagery" Remote Sensing 16, no. 14: 2608. https://doi.org/10.3390/rs16142608
APA StyleKanaskie, C. R., Routhier, M. R., Fraser, B. T., Congalton, R. G., Ayres, M. P., & Garnas, J. R. (2024). Early Detection of Southern Pine Beetle Attack by UAV-Collected Multispectral Imagery. Remote Sensing, 16(14), 2608. https://doi.org/10.3390/rs16142608









