Evaluating Threatened Bird Occurrence in the Tropics by Using L-Band SAR Remote Sensing Data
Abstract
:1. Introduction
2. Materials and Methods
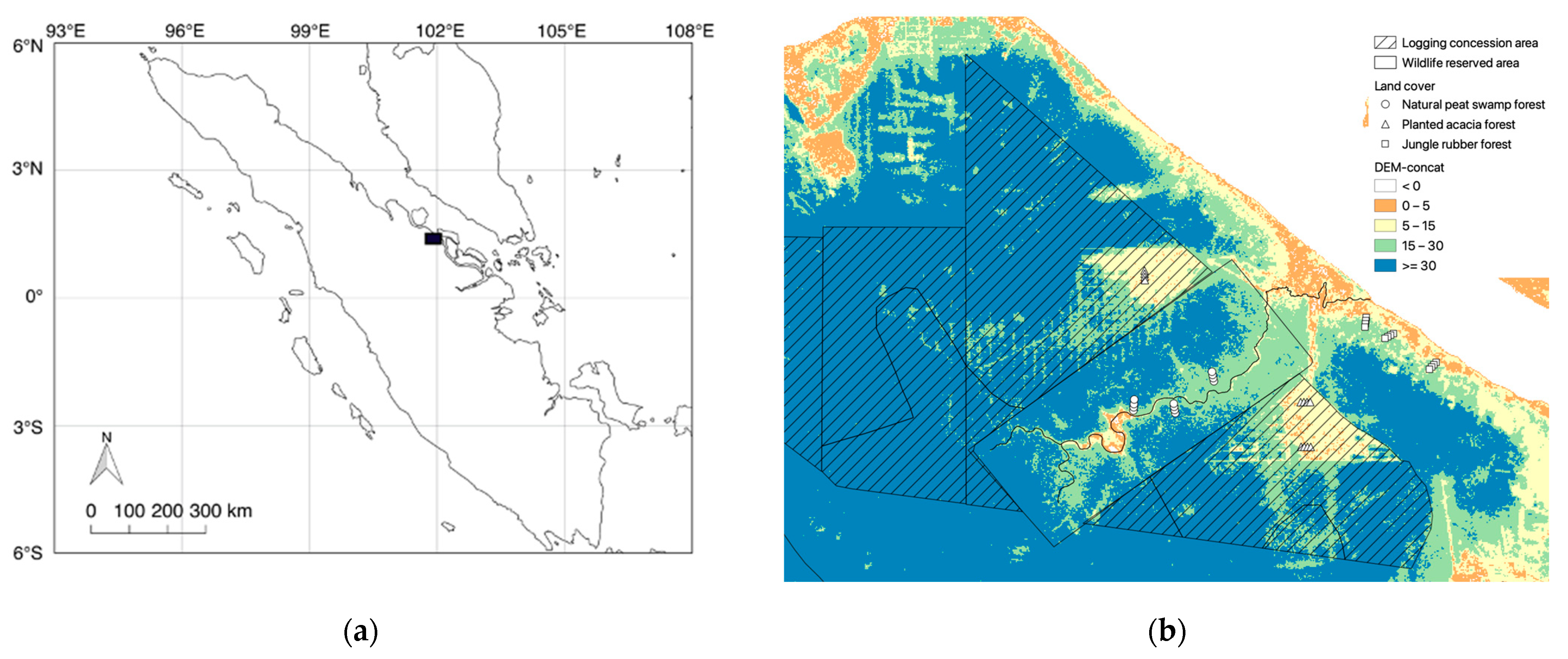
2.1. Study Area
2.2. Bird Census
2.3. Field Survey of Forest Layer Structure
2.4. Preprocessing of Microwave Satellite Remote Sensing Data
3. Data Analysis
3.1. Polarimetric Parameters Obtained from L-Band SAR Data
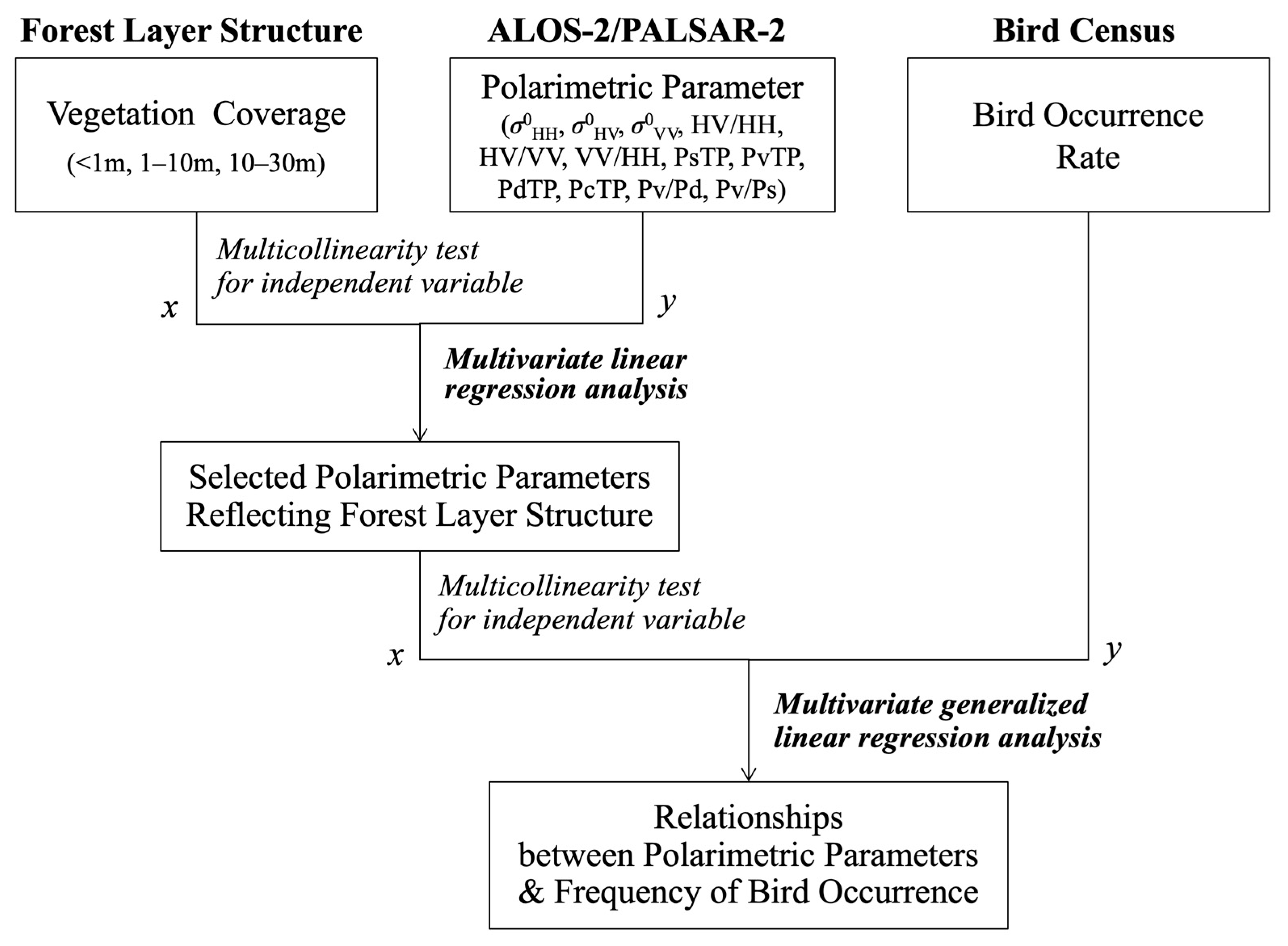
3.2. Statistical Analysis: Polarimetric Parameter Selection and Correlation with Bird Occurrence
4. Results
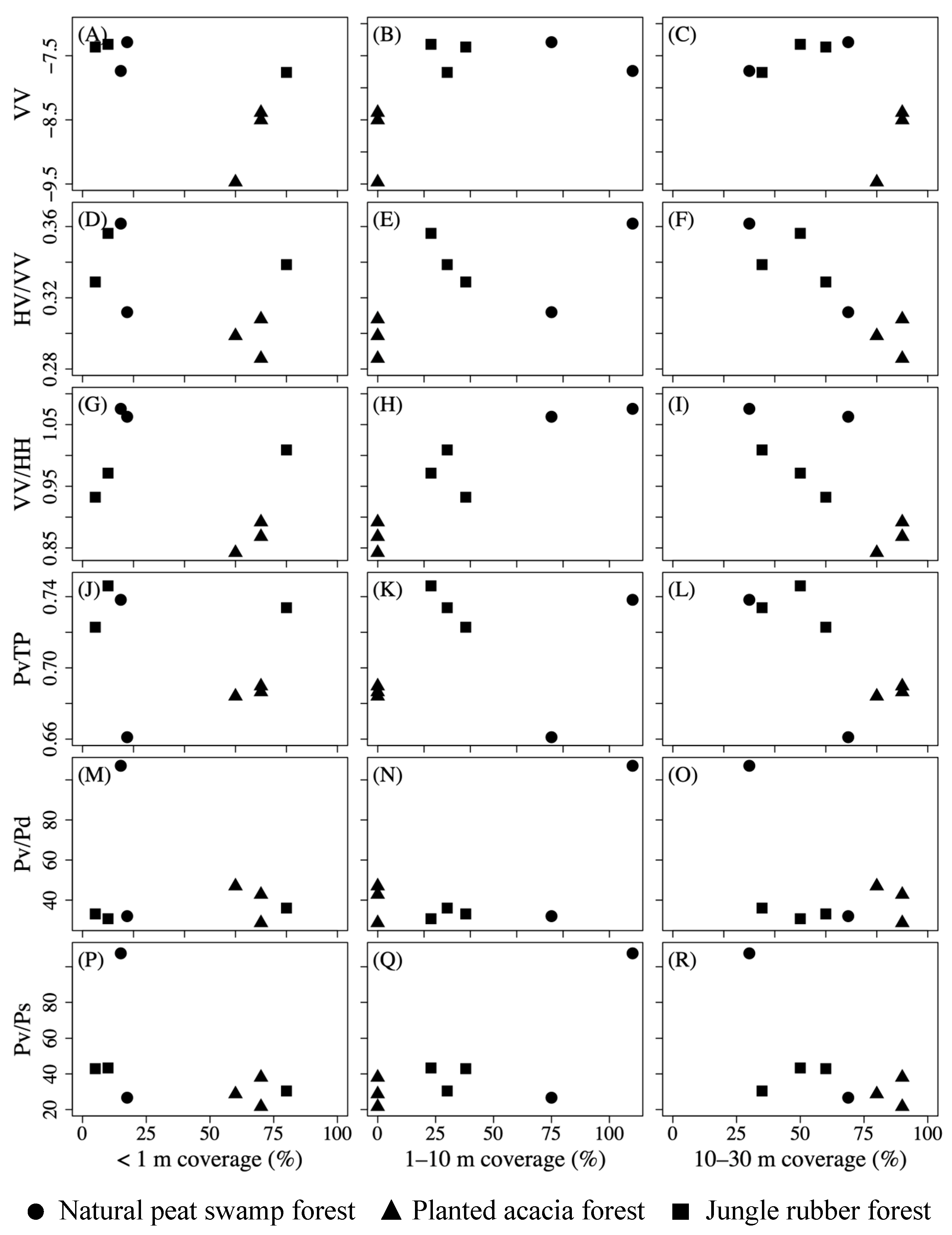
4.1. Polarimetric Parameters Reflecting Forest Layer Structure
4.2. Polarimetric Parameters Correlated with Bird Occurrence
5. Discussion
5.1. Mechanisms of L-Band Backscattering against Forest Layer Structure
5.2. Bird Occurrence Explained by Polarimetric Parameters from L-Band SAR
5.3. Feasibility of Bird Diversity Monitoring by Microwave SAR
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sodhi, N.S.; Brook, B.W. Southeast Asian Biodiversity in Crisis; Cambridge University Press: Cambridge, UK, 2006. [Google Scholar]
- Sodhi, N.S.; Koh, L.P.; Brook, B.W.; Ng, P.K. Southeast Asian biodiversity: An impending disaster. Trends Ecol. Evol. 2004, 19, 654–660. [Google Scholar] [PubMed]
- Sodhi, N.S.; Lee, T.M.; Koh, L.P.; Brook, B.W. A meta-analysis of the impact of anthropogenic forest disturbance on Southeast Asia’s biotas. Biotropica 2009, 41, 103–109. [Google Scholar]
- Andriesse, J. Nature and Management of Tropical Peat Soils; Food & Agriculture Org.: Rome, Italy, 1988. [Google Scholar]
- Page, S.; Rieley, J.; Shotyk, Ø.; Weiss, D. Interdependence of peat and vegetation in a tropical peat swamp forest. In Changes and Disturbance in Tropical Rainforest in South-East Asia; World Scientific: Singapore, 1999; pp. 161–173. [Google Scholar]
- Cheyne, S.M.; Macdonald, D.W. Wild felid diversity and activity patterns in Sabangau peat-swamp forest, Indonesian Borneo. Oryx 2011, 45, 119–124. [Google Scholar]
- Posa, M.R.C. Peat swamp forest avifauna of Central Kalimantan, Indonesia: Effects of habitat loss and degradation. Biol. Conserv. 2011, 144, 2548–2556. [Google Scholar]
- Giam, X.; Clements, G.R.; Aziz, S.A.; Chong, K.Y.; Miettinen, J. Rethinking the ‘back to wilderness’ concept for Sundaland’s forests. Biol. Conserv. 2011, 144, 3149–3152. [Google Scholar] [CrossRef]
- Corlett, R.T. The Ecology of Tropical East Asia; Oxford University Press: Oxford, UK, 2014. [Google Scholar]
- Uryu, Y.; Mott, C.; Foead, N.; Yulianto, K.; Budiman, A.; Setiabudi; Takakai, F.; Nursamsu; Sunarto; Purastuti, E.; et al. Deforestation, Forest Degradation, Biodiversity Loss and CO2 Emissions in Riau, Sumatra, Indonesia; WWF Indonesia Technical Report: Jakarta, Indonesia, 2008. [Google Scholar]
- Proença, V.; Martin, L.J.; Pereira, H.M.; Fernandez, M.; McRae, L.; Belnap, J.; Böhm, M.; Brummitt, N.; García-Moreno, J.; Gregory, R.D. Global biodiversity monitoring: From data sources to essential biodiversity variables. Biol. Conserv. 2017, 213, 256–263. [Google Scholar]
- Nagendra, H.; Lucas, R.; Honrado, J.P.; Jongman, R.H.G.; Tarantino, C.; Adamo, M.; Mairota, P. Remote sensing for conservation monitoring: Assessing protected areas, habitat extent, habitat condition, species diversity, and threats. Ecol. Indic. 2013, 33, 45–59. [Google Scholar] [CrossRef]
- Petrou, Z.I.; Manakos, I.; Stathaki, T. Remote sensing for biodiversity monitoring: A review of methods for biodiversity indicator extraction and assessment of progress towards international targets. Biodivers. Conserv. 2015, 24, 2333–2363. [Google Scholar] [CrossRef]
- Mulatu, K.A.; Mora, B.; Kooistra, L.; Herold, M. Biodiversity monitoring in changing tropical forests: A review of approaches and new opportunities. Remote Sens. 2017, 9, 1059. [Google Scholar] [CrossRef]
- Munro, N.T.; Fischer, J.; Barrett, G.; Wood, J.; Leavesley, A.; Lindenmayer, D.B. Bird’s Response to Revegetation of Different Structure and Floristics-Are “Restoration Plantings” Restoring Bird Communities? Restor. Ecol. 2011, 19, 223–235. [Google Scholar] [CrossRef]
- Wallis, C.I.B.; Paulsch, D.; Zeilinger, J.; Silva, B.; Fernandez, G.F.C.; Brandl, R.; Farwig, N.; Bendix, J. Contrasting performance of Lidar and optical texture models in predicting avian diversity in a tropical mountain forest (vol 174, pg 223, 2016). Remote Sens. Environ. 2016, 178, 223. [Google Scholar] [CrossRef]
- Weisberg, P.J.; Dilts, T.E.; Becker, M.E.; Young, J.S.; Wong-Kone, D.C.; Newton, W.E.; Ammon, E.M. Guild-specific responses of avian species richness to LiDAR-derived habitat heterogeneity. Acta Oecol. 2014, 59, 72–83. [Google Scholar] [CrossRef]
- Erdelen, M. Bird communities and vegetation structure: I. Correlations and comparisons of simple and diversity indices. Oecologia 1984, 61, 277–284. [Google Scholar] [PubMed]
- Karr, J.R.; Roth, R.R. Vegetation structure and avian diversity in several New World areas. Am. Nat. 1971, 105, 423–435. [Google Scholar] [CrossRef]
- Díaz, I.A.; Armesto, J.J.; Reid, S.; Sieving, K.E.; Willson, M.F. Linking forest structure and composition: Avian diversity in successional forests of Chiloé Island, Chile. Biol. Conserv. 2005, 123, 91–101. [Google Scholar]
- Zellweger, F.; Baltensweiler, A.; Ginzler, C.; Roth, T.; Braunisch, V.; Bugmann, H.; Bollmann, K. Environmental predictors of species richness in forest landscapes: Abiotic factors versus vegetation structure. J. Biogeogr. 2016, 43, 1080–1090. [Google Scholar] [CrossRef]
- El Moussawi, I.; Ho Tong Minh, D.; Baghdadi, N.; Abdallah, C.; Jomaah, J.; Strauss, O.; Lavalle, M.; Ngo, Y.-N. Monitoring tropical forest structure using SAR tomography at L-and P-band. Remote Sens. 2019, 11, 1934. [Google Scholar] [CrossRef]
- Joshi, N.; Mitchard, E.; Schumacher, J.; Johannsen, V.; Saatchi, S.; Fensholt, R. L-Band SAR Backscatter Related to Forest Cover, Height and Aboveground Biomass at Multiple Spatial Scales across Denmark. Remote Sens. 2015, 7, 4442–4472. [Google Scholar] [CrossRef]
- Lucas, R.M.; Mitchell, A.L.; Rosenqvist, A.; Proisy, C.; Melius, A.; Ticehurst, C. The potential of L-band SAR for quantifying mangrove characteristics and change: Case studies from the tropics. Aquat. Conserv. 2007, 17, 245–264. [Google Scholar] [CrossRef]
- Robinson, C.; Saatchi, S.; Neumann, M.; Gillespie, T. Impacts of Spatial Variability on Aboveground Biomass Estimation from L-Band Radar in a Temperate Forest. Remote Sens. 2013, 5, 1001–1023. [Google Scholar] [CrossRef]
- Carreiras, J.M.B.; Vasconcelos, M.J.; Lucas, R.M. Understanding the relationship between aboveground biomass and ALOS PALSAR data in the forests of Guinea-Bissau (West Africa). Remote Sens. Environ. 2012, 121, 426–442. [Google Scholar] [CrossRef]
- Cartus, O.; Santoro, M.; Kellndorfer, J. Mapping forest aboveground biomass in the Northeastern United States with ALOS PALSAR dual-polarization L-band. Remote Sens. Environ. 2012, 124, 466–478. [Google Scholar] [CrossRef]
- Imhoff, M.L.; Sisk, T.D.; Milne, A.; Morgan, G.; Orr, T. Remotely sensed indicators of habitat heterogeneity: Use of synthetic aperture radar in mapping vegetation structure and bird habitat. Remote Sens. Environ. 1997, 60, 217–227. [Google Scholar] [CrossRef]
- Betbeder, J.; Hubert-Moy, L.; Burel, F.; Corgne, S.; Baudry, J. Assessing ecological habitat structure from local to landscape scales using synthetic aperture radar. Ecol. Indic. 2015, 52, 545–557. [Google Scholar] [CrossRef]
- Kobayashi, S.; Omura, Y.; Sanga-Ngoie, K.; Yamaguchi, Y.; Widyorini, R.; Fujita, M.S.; Supriadi, B.; Kawai, S. Yearly Variation of Acacia Plantation Forests Obtained by Polarimetric Analysis of ALOS PALSAR Data. IEEE J. Sel. Top. Appl. Earth Obs. Remote Sens. 2015, 8, 5294–5304. [Google Scholar] [CrossRef]
- Mizuno, K.; Fujita, M.S.; Kawai, S. Catastrophe and Regeneration in Indonesia’s Peatlands: Ecology, Economy and Society; NUS Press: Singapore, 2016; Volume 15. [Google Scholar]
- Fujita, M.S.; Prawiradilaga, D.M.; Yoshimura, T. Roles of fragmented and logged forests for bird communities in industrial Acacia mangium plantations in Indonesia. Ecol. Res. 2014, 29, 741–755. [Google Scholar]
- Barlow, J.; Gardner, T.A.; Araujo, I.S.; Avila-Pires, T.C.; Bonaldo, A.B.; Costa, J.E.; Esposito, M.C.; Ferreira, L.V.; Hawes, J.; Hernandez, M.M.; et al. Quantifying the biodiversity value of tropical primary, secondary, and plantation forests. Proc. Natl. Acad. Sci. USA 2007, 104, 18555–18560. [Google Scholar] [CrossRef] [PubMed]
- Fujita, M.S.; Samejima, H.; Haryadi, D.S.; Muhammad, A.; Irham, M.; Shiodera, S. Low conservation value of converted habitat for avifauna in tropical peatland on Sumatra, Indonesia. Ecol. Res. 2016, 31, 275–285. [Google Scholar] [CrossRef]
- Edwards, D.P.; Larsen, T.H.; Docherty, T.D.; Ansell, F.A.; Hsu, W.W.; Derhé, M.A.; Hamer, K.C.; Wilcove, D.S. Degraded lands worth protecting: The biological importance of Southeast Asia’s repeatedly logged forests. Proc. R. Soc. Lond. B Biol. Sci. 2011, 278, 82–90. [Google Scholar]
- Ralph, C.J. Handbook of Field Methods for Monitoring Landbirds; Pacific Southwest Research Station: Albany, CA, USA, 1993; Volume 144. [Google Scholar]
- Morrison, L.W. Observer error in vegetation surveys: A review. J. Plant Ecol. 2016, 9, 367–379. [Google Scholar]
- MacKinnon, J.R.; Phillipps, K. A Field Guide to the Birds of Borneo, Sumatra, Java, and Bali, the Greater Sunda Islands; Oxford University Press: Oxford, UK, 1993. [Google Scholar]
- IUCN. The IUCN Red List of Threatened Species. Version 2015-4. Available online: http://www.iucnredlist.org (accessed on 28 November 2015).
- Wikum, D.A.; Shanholtzer, G.F. Application of the Braun-Blanquet cover-abundance scale for vegetation analysis in land development studies. Environ. Manag. 1978, 2, 323–329. [Google Scholar] [CrossRef]
- Richards, P.W.; Tansley, A.G.; Watt, A.S. The Recording of Structure, Life Form and Flora of Tropical Forest Communities as a Basis for Their Classification. J. Ecol. 1940, 28, 224–239. [Google Scholar] [CrossRef]
- Small, D. Flattening Gamma: Radiometric Terrain Correction for SAR Imagery. IEEE Trans. Geosci. Remote Sens. 2011, 49, 3081–3093. [Google Scholar] [CrossRef]
- Shimada, M.; Isoguchi, O.; Tadono, T.; Isono, K. PALSAR Radiometric and Geometric Calibration. IEEE Trans. Geosci. Remote Sens. 2009, 47, 3915–3932. [Google Scholar] [CrossRef]
- Richards, J.A. Remote Sensing with Imaging Radar; Springer: Berlin/Heidelberg, Germany, 2009. [Google Scholar]
- Freeman, A.; Durden, S.L. A three-component scattering model for polarimetric SAR data. IEEE Trans. Geosci. Remote Sens. 1998, 36, 963–973. [Google Scholar] [CrossRef]
- Yamaguchi, Y.; Moriyama, T.; Ishido, M.; Yamada, H. Four-component scattering model for polarimetric SAR image decomposition. IEEE Trans. Geosci. Remote Sens. 2005, 43, 1699–1706. [Google Scholar] [CrossRef]
- Yamaguchi, Y.; Sato, A.; Boerner, W.M.; Sato, R.; Yamada, H. Four-Component Scattering Power Decomposition With Rotation of Coherency Matrix. IEEE Trans. Geosci. Remote Sens. 2011, 49, 2251–2258. [Google Scholar] [CrossRef]
- Singh, G.; Yamaguchi, Y.; Park, S.E. General Four-Component Scattering Power Decomposition With Unitary Transformation of Coherency Matrix. IEEE Trans. Geosci. Remote Sens. 2013, 51, 3014–3022. [Google Scholar] [CrossRef]
- Austin, P.C.; Steyerberg, E.W. The number of subjects per variable required in linear regression analyses. J. Clin. Epidemiol. 2015, 68, 627–636. [Google Scholar] [CrossRef]
- Montgomery, D.C.; Peck, E.A.; Vining, G.G. Introduction to Linear Regression Analysis; John Wiley & Sons: Hoboken, NJ, USA, 2012; Volume 821. [Google Scholar]
- Hardin, J.W.; Hardin, J.W.; Hilbe, J.M.; Hilbe, J. Generalized Linear Models and Extensions, 2nd ed.; Taylor & Francis: Abingdon, UK, 2007. [Google Scholar]
- Ottinger, M.; Kuenzer, C. Spaceborne L-Band Synthetic Aperture Radar Data for Geoscientific Analyses in Coastal Land Applications: A Review. Remote Sens. 2020, 12, 2228. [Google Scholar] [CrossRef]
- Henderson, F.M.; Ryerson, R.A.; Lewis, A.J.; Photogrammetry, A.S.f.; Sensing, R. Principles and Applications of Imaging Radar; Wiley: Hoboken, NJ, USA, 1998. [Google Scholar]
- Flores-Anderson, A.I.; Herndon, K.E.; Thapa, R.B.; Cherrington, E. The SAR Handbook: Comprehensive Methodologies for Forest Monitoring and Biomass Estimation; SERVIR Global Science. National Space Science and Technology Center: Huntsville, AL, USA, 2019. [Google Scholar]
- Pope, K.O.; Rey-Benayas, J.M.; Paris, J.F. Radar remote sensing of forest and wetland ecosystems in the Central American tropics. Remote Sens. Environ. 1994, 48, 205–219. [Google Scholar]
- Singh, G.; Venkataraman, G.; Yamaguchi, Y.; Park, S.-E. Capability assessment of fully polarimetric ALOS–PALSAR data for discriminating wet snow from other scattering types in mountainous regions. IEEE Trans. Geosci. Remote Sens. 2013, 52, 1177–1196. [Google Scholar] [CrossRef]
- Márquez, A.L.; Real, R.; Vargas, J.M. Dependence of broad-scale geographical variation in fleshy-fruited plant species richness on disperser bird species richness. Glob. Ecol. Biogeogr. 2004, 13, 295–304. [Google Scholar]
- Boelman, N.T.; Holbrook, J.D.; Greaves, H.E.; Krause, J.S.; Chmura, H.E.; Magney, T.S.; Perez, J.H.; Eitel, J.U.H.; Gough, L.; Vierling, K.T.; et al. Airborne laser scanning and spectral remote sensing give a bird’s eye perspective on arctic tundra breeding habitat at multiple spatial scales. Remote Sens. Environ. 2016, 184, 337–349. [Google Scholar] [CrossRef]
- Goetz, S.; Steinberg, D.; Dubayah, R.; Blair, B. Laser remote sensing of canopy habitat heterogeneity as a predictor of bird species richness in an eastern temperate forest, USA. Remote Sens. Environ. 2007, 108, 254–263. [Google Scholar] [CrossRef]
- Vogeler, J.C.; Hudak, A.T.; Vierling, L.A.; Evans, J.; Green, P.; Vierling, K.I.T. Terrain and vegetation structural influences on local avian species richness in two mixed-conifer forests. Remote Sens. Environ. 2014, 147, 13–22. [Google Scholar] [CrossRef]
- Bergen, K.M.; Goetz, S.J.; Dubayah, R.O.; Henebry, G.M.; Hunsaker, C.T.; Imhoff, M.L.; Nelson, R.F.; Parker, G.G.; Radeloff, V.C. Remote sensing of vegetation 3-D structure for biodiversity and habitat: Review and implications for lidar and radar spaceborne missions. J. Geophys. Res.-Biogeosci. 2009, 114. [Google Scholar] [CrossRef]
- Singh, M.; Friess, D.A.; Vilela, B.; Alban, J.D.T.D.; Monzon, A.K.V.; Veridiano, R.K.A.; Tumaneng, R.D. Spatial relationships between above-ground biomass and bird species biodiversity in Palawan, Philippines. PLoS ONE 2017, 12, e0186742. [Google Scholar]
- Singh, M.; Tokola, T.; Hou, Z.; Notarnicola, C. Remote sensing-based landscape indicators for the evaluation of threatened-bird habitats in a tropical forest. Ecol. Evol. 2017, 7, 4552–4567. [Google Scholar]
- Shimada, M. Imaging from Spaceborne and Airborne SARs, Calibration, and Applications; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar]
- Tello, M.; Cazcarra-Bes, V.; Pardini, M.; Papathanassiou, K. Forest structure characterization from SAR tomography at L-band. IEEE J. Sel. Top. Appl. Earth Obs. Remote Sens. 2018, 11, 3402–3414. [Google Scholar] [CrossRef]
- Wu, J.; Chen, B.; Reynolds, G.; Xie, J.; Liang, S.; O’Brien, M.J.; Hector, A. Monitoring tropical forest degradation and restoration with satellite remote sensing: A test using Sabah Biodiversity Experiment. In Advances in Ecological Research; Elsevier: Amsterdam, The Netherlands, 2020; Volume 62, pp. 117–146. [Google Scholar]
- Ribeiro, I.; Proença, V.; Serra, P.; Palma, J.; Domingo-Marimon, C.; Pons, X.; Domingos, T. Remotely sensed indicators and open-access biodiversity data to assess bird diversity patterns in Mediterranean rural landscapes. Sci. Rep. 2019, 9, 6826. [Google Scholar] [PubMed]
- Suttidate, N.; Hobi, M.L.; Pidgeon, A.M.; Round, P.D.; Coops, N.C.; Helmers, D.P.; Keuler, N.S.; Dubinin, M.; Bateman, B.L.; Radeloff, V.C. Tropical bird species richness is strongly associated with patterns of primary productivity captured by the Dynamic Habitat Indices. Remote Sens. Environ. 2019, 232, 111306. [Google Scholar] [CrossRef]
- Wallis, C.I.; Brehm, G.; Donoso, D.A.; Fiedler, K.; Homeier, J.; Paulsch, D.; Süßenbach, D.; Tiede, Y.; Brandl, R.; Farwig, N. Remote sensing improves prediction of tropical montane species diversity but performance differs among taxa. Ecol. Indic. 2017, 83, 538–549. [Google Scholar] [CrossRef]




| English Name | Scientific Name | Author | IUCN (2015) | Occurrence/Census | ||
|---|---|---|---|---|---|---|
| NPF | PAF | JRF | ||||
| Rufous Piculet | Sasia abnormis | (Temminck, CJ 1825) | LC | 0.024 | 0.000 | 0.000 |
| Red-throated Barbet | Megalaima mystacophanos | (Temminck, CJ 1824) | NT | 0.000 | 0.000 | 0.021 |
| Black Hornbill | Anthracoceros malayanus | (Raffles, TS 1822) | NT | 0.000 | 0.000 | 0.083 |
| Helmeted Hornbill | Buceros vigil | (Pennant, T 1781) | CR | 0.000 | 0.042 | 0.000 |
| Diard’s Trogon | Harpactes diardii | (Temminck, CJ 1832) | NT | 0.024 | 0.000 | 0.000 |
| Drongo-cuckoo | Surniculus lugubris | (Horsfield, T 1821) | LC | 0.024 | 0.000 | 0.000 |
| Blue-crowned Hanging-parrot | Loriculus galgulus | (Linnaeus, C 1758) | LC | 0.071 | 0.000 | 0.000 |
| Thick-billed Green-pigeon | Treron curvirostra | (Gmelin, JF 1789) | LC | 0.000 | 0.000 | 0.104 |
| Black-and-yellow Broadbill | Eurylaimus ochromalus | (Raffles, TS 1822) | NT | 0.095 | 0.000 | 0.000 |
| Asian Fairy-bluebird | Irena puella | (Latham, J 1790) | LC | 0.024 | 0.000 | 0.000 |
| Greater Green Leafbird | Chloropsis sonnerati | (Jardine, W; Selby, PJ 1827) | LC | 0.024 | 0.000 | 0.000 |
| Lesser Green Leafbird | Chloropsis cyanopogon | (Temminck, CJ 1830) | NT | 0.143 | 0.000 | 0.000 |
| Black-winged Flycatcher-shrike | Hemipus hirundinaceus | (Temminck, CJ 1822) | LC | 0.000 | 0.000 | 0.417 |
| Greater Racket-tailed Drongo | Dicrurus paradiseus | (Linnaeus, C 1766) | LC | 0.048 | 0.000 | 0.000 |
| Black-naped Monarch | Hypothymis azurea | (Boddaert, P 1783) | LC | 0.024 | 0.000 | 0.000 |
| Indian Paradise-flycatcher | Terpsiphone paradisi | (Linnaeus, C 1758) | LC | 0.024 | 0.000 | 0.000 |
| Rufous-winged Philentoma | Philentoma pyrhopterum | (Temminck, CJ 1836) | LC | 0.024 | 0.000 | 0.000 |
| Grey-chested Jungle-flycatcher | Rhinomyias umbratilis | (Strickland, HE 1849) | NT | 0.167 | 0.000 | 0.000 |
| White-rumped Shama | Copsychus malabaricus | (Scopoli, GA 1786) | LC | 0.000 | 0.000 | 0.021 |
| Common Hill Myna | Gracula religiosa | (Linnaeus, C 1758) | LC | 0.000 | 0.000 | 0.083 |
| Spectacled Bulbul | Pycnonotus erythropthalmos | (Hume, AO 1878) | LC | 0.452 | 0.000 | 0.000 |
| Streaked Bulbul | Ixos malaccensis | (Blyth, E 1845) | NT | 0.024 | 0.000 | 0.000 |
| Ferruginous Babbler | Trichastoma bicolor | (Lesson, RP 1839) | LC | 0.024 | 0.000 | 0.000 |
| Black-throated Babbler | Stachyris nigricollis | (Temminck, CJ 1836) | NT | 0.071 | 0.000 | 0.104 |
| Chestnut-rumped Babbler | Stachyris maculata | (Temminck, CJ 1836) | NT | 0.095 | 0.000 | 0.000 |
| Fluffy-backed Tit-babbler | Macronous ptilosus | (Jardine, W; Selby, PJ 1835) | NT | 0.119 | 0.000 | 0.000 |
| Scarlet-breasted Flowerpecker | Prionochilus thoracicus | (Temminck, CJ 1836) | NT | 0.095 | 0.000 | 0.000 |
| Transect | Number of Census | Bird Occurrence Rate (%) | Vegetation Coverage (%) | ||||
|---|---|---|---|---|---|---|---|
| Forest Dependent | Threatened | <1 m | 1–10 m | 10–30 m | |||
| NPF | 1 | 18 | 41.6 | 27.3 | 15 | 110 | 30 |
| 2 | 16 | 52.8 | 32.1 | 18 | 75 | 69 | |
| 3 | 8 | 35.0 | 30.0 | N/A | N/A | N/A | |
| PAF | 1 | 16 | 2.6 | 6.4 | 70 | 0 | 90 |
| 2 | 16 | 0.0 | 0.0 | 70 | 0 | 90 | |
| 3 | 16 | 0.0 | 0.0 | 60 | 0 | 80 | |
| JRF | 1 | 16 | 1.7 | 8.6 | 5 | 38 | 60 |
| 2 | 16 | 11.0 | 16.5 | 10 | 23 | 50 | |
| 3 | 16 | 13.8 | 14.4 | 80 | 30 | 35 | |
| σ0HH | σ0HV | σ0VV | HV/ HH | HV/ VV | VV/ HH | PsTP | PvTP | PdTP | PcTP | Pv/Pd | Pv/Ps | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NPF | 1 | −7.917 | −12.584 | −7.737 | 0.362 | 0.342 | 1.076 | 0.095 | 0.738 | 0.089 | 0.078 | 107.0 | 107.5 |
| 2 | −7.384 | −12.654 | −7.288 | 0.312 | 0.303 | 1.063 | 0.153 | 0.661 | 0.111 | 0.075 | 32.00 | 26.66 | |
| 3 | −7.392 | −12.474 | −7.780 | 0.320 | 0.352 | 0.936 | 0.116 | 0.708 | 0.097 | 0.079 | 41.15 | 46.20 | |
| PAF | 1 | −7.784 | −13.027 | −8.386 | 0.308 | 0.354 | 0.892 | 0.115 | 0.690 | 0.123 | 0.072 | 28.65 | 38.06 |
| 2 | −7.760 | −13.324 | −8.502 | 0.286 | 0.342 | 0.868 | 0.147 | 0.686 | 0.096 | 0.070 | 42.81 | 21.60 | |
| 3 | −8.492 | −14.022 | −9.471 | 0.299 | 0.370 | 0.842 | 0.141 | 0.684 | 0.106 | 0.070 | 47.00 | 28.72 | |
| JRF | 1 | −6.872 | −11.858 | −7.364 | 0.329 | 0.366 | 0.932 | 0.082 | 0.723 | 0.118 | 0.076 | 33.12 | 42.96 |
| 2 | −7.098 | −11.711 | −7.321 | 0.356 | 0.373 | 0.971 | 0.082 | 0.746 | 0.095 | 0.076 | 30.72 | 43.30 | |
| 3 | −7.658 | −12.536 | −7.760 | 0.339 | 0.341 | 1.009 | 0.105 | 0.734 | 0.088 | 0.073 | 36.03 | 30.44 |
| Response Variable (y) | Estimates and p-Value (in Parentheses) of Selected Explanatory Variables (x) | Model Fitting | |||
|---|---|---|---|---|---|
| Polarimetric Parameter | <1 m | 1–10 m | 10–30 m | Adj. R2 (p-Value) | |
| σ0HH | −0.962 (0.112) | - | - | 0.259 (0.112) | |
| σ0HV | −1.753 (0.116) | −0.990 (0.323) | −2.090 (0.174) | 0.422 (0.180) | |
| ○ | σ0VV | −1.587 (0.074 •) | - | - | 0.343 (0.074 •) |
| ○ | HV/HH | −0.020 (0.236) | - | −0.095 (0.005 **) | 0.819 (0.006 **) |
| HV/VV | −0.041 (0.205) | −0.073 (0.053 •) | −0.060 (0.197) | 0.387 (0.201) | |
| ○ | VV/HH | 0.199 (0.002 **) | 0.778 (0.002 **) | ||
| PsTP | 0.052 (0.177) | 0.058 (0.144) | 0.118 (0.063 •) | 0.476 (0.150) | |
| ○ | PvTP | −0.029 (0.322) | −0.059 (0.085 •) | −0.158 (0.014 *) | 0.708 (0.049 *) |
| PdTP | −0.017 (0.282) | - | 0.045 (0.060 •) | 0.632 (0.140) | |
| PcTP | −0.007 (0.007 **) | - | −0.007 (0.024 *) | 0.870 (0.002 **) | |
| Pv/Pd | - | 44.17 (0.065 •) | - | 0.367 (0.065 •) | |
| Pv/Ps | - | 51.12 (0.035 *) | - | 0.474 (0.035 •) | |
| Response Variable (y) | Estimates and p-Value (in Parentheses) of Selected Explanatory Variables (x) | ||
|---|---|---|---|
| Bird Occurrence Rate | σ0VV | VV/HH | PvTP |
| Forest-dependent | 0.318 (0.087 •) | 0.885 (<0.001 ***) | −0.116 (0.092 •) |
| Threatened | 0.600 (<0.001 ***) | 0.460 (<0.001 ***) | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kobayashi, S.; Fujita, M.S.; Omura, Y.; Haryadi, D.S.; Muhammad, A.; Irham, M.; Shiodera, S. Evaluating Threatened Bird Occurrence in the Tropics by Using L-Band SAR Remote Sensing Data. Remote Sens. 2023, 15, 947. https://doi.org/10.3390/rs15040947
Kobayashi S, Fujita MS, Omura Y, Haryadi DS, Muhammad A, Irham M, Shiodera S. Evaluating Threatened Bird Occurrence in the Tropics by Using L-Band SAR Remote Sensing Data. Remote Sensing. 2023; 15(4):947. https://doi.org/10.3390/rs15040947
Chicago/Turabian StyleKobayashi, Shoko, Motoko S. Fujita, Yoshiharu Omura, Dendy S. Haryadi, Ahmad Muhammad, Mohammad Irham, and Satomi Shiodera. 2023. "Evaluating Threatened Bird Occurrence in the Tropics by Using L-Band SAR Remote Sensing Data" Remote Sensing 15, no. 4: 947. https://doi.org/10.3390/rs15040947
APA StyleKobayashi, S., Fujita, M. S., Omura, Y., Haryadi, D. S., Muhammad, A., Irham, M., & Shiodera, S. (2023). Evaluating Threatened Bird Occurrence in the Tropics by Using L-Band SAR Remote Sensing Data. Remote Sensing, 15(4), 947. https://doi.org/10.3390/rs15040947








