Abstract
Algal cell abundance weakly depends on inherent optical properties and chlorophyll-a concentration in the Bohai Sea, so it is very hard to derive algal cell abundance (ACA) from ocean color data using a simple bio-optical model. To obtain ACA for biological communication at large scale, a neural network model has been developed and then applied for investigating the changing monthly trend of ACA, intracellular chlorophyll-a concentration, and cell size in the Bohai Sea using MODIS data from 2002 to 2015. The results showed that the neural network model could provide an accurate log-transformed value of algal cell abundance (LACA) from ocean color images whose retrieval uncertainty did not exceed 9%. Furthermore, when the model was applied to map the monthly mean LACA and then further convert it to cell size in the Bohai Sea, the results showed that the satellite-derived monthly mean cell size varied from 4.81 to 15.29 μm. The decreasing monthly mean algal cell abundance and increasing monthly mean chlorophyll-a concentration imply that the monthly mean intracellular chlorophyll-a concentration from 2002 to 2015 increased, which indicates that the waters in the Bohai Sea became more eutrophic over those 14 years. Moreover, due to seasonal variations in vertical mixing or other physical forcing factors, the ACA and cell size exhibited significant seasonal variations. Although further tests are required to validate the model’s robustness, these preliminary results indicate that the neural network model is an encouraging approach to exploiting more novel biological parameters such as the LACA from ocean color satellites for oceanic communication.
1. Introduction
Algal cells are relevant to marine food webs because algal cell division accompanies phytoplankton population growth, meaning that the presence of algal cells can indicate the onset of the spring bloom in aquatic ecosystems [1,2]. At the beginning of a spring bloom event, algal cell abundance (ACA; in this study, cell size > 2 μm) increases significantly [3] once light and nutrient concentration are high enough. In some cases, the growth rate can reach up to 10% per day [4]. As the spring bloom develops, theoretically, the ACA depends on the balance between the growth and loss of algal cells. The former is associated with the rate of cell division, which is proportional to the net specific phytoplankton growth (NPG) rate [5]. This division rate is very important for improving the prediction accuracy of climate-ecology coupling models at basin or global scales [6]. The loss of algal cells is associated with mortality, sinking, and grazing procedures of phytoplankton in the upper oceans. However, using field observations to determine the two specific processes is difficult due to the limited spatial and temporal coverages. It is noteworthy that, because the net specific algal growth rate (NAG) is important to marine ecosystems [4], we could approximate the growth and loss of the algal cells, although first we would need to determine the log-transformed ACA value (LACA).
Initially, ocean color remote sensing concentrated on estimating the chlorophyll-a concentration ([Chl]) in the surface layer on basin and global scales [7] because [Chl] is an indicator of primary productivity. Moreover, the [Chl] data have been widely used to analyze biology-climate coupling dynamics and to trace oceanographic plumes [8]. However, the LACA is not physically equal to [Chl] because intracellular [Chl] varies dynamically with phytoplankton size [9] and with physical factors such as solar irradiance, nutrients, and temperature [10].
To understand algal biological characteristics in global oceans, many reports focus on how remote sensing of ocean color data, such as backscattering coefficients, can determine how phytoplankton abundance or algal particle size [11,12,13,14,15,16] increases as the algal particle size decreases for the same phytoplankton abundance [11]. However, as the algal cells do not belong to the traditional ocean color variable, few reports exist that describe how to directly determine the LACA from remote sensing data, particularly for turbid coastal waters such as in the Bohai Sea. For the estimation of the LACA, which is important for studies regarding algal biological features, a model which is capable of providing maps at large scale remains desired.
The neural network approach is a data-driven empirical method which has been widely used to remotely sense marine biogeochemical variables from global oceans [17,18]. Currently, there are few reports about the bio-optical properties of algal cells. When the bio-optical characteristics for remote sensing the LACA are not clear, neural networks can obtain the algal cell information from satellite images. The main objective of our study was to exploit a neural network model to estimate the LACA in the coastal zone of the Bohai Sea. First, we developed a novel neural network-based LACA retrieval algorithm (NNL) to derive ACA from the Bohai Sea. Then, we calculated the NAG rate and cell size from the NNL model-derived LACA using approaches, respectively, proposed by Banse [4] and Roy et al. [19]. Finally, we mapped the satellite-derived distributions to illuminate the spatial and temporal changes of the LACA in the Bohai Sea.
2. Data and Methods
2.1. Study Area
The Bohai Sea is in the western Pacific Ocean, located between 117°38′E and 122°12′E and 37°10′N and 40°51′N. The sea is a semi-enclosed shelf sea with an area of 7.8 × 104 km2, is surrounded by land on three sides, and is divided into four sub-regions: Liaodong Bay, Bohai Bay, Laizhou Bay, and the Central Bohai Sea (Figure 1). The Bohai Sea is shallow, with an average water depth of about 18 m. The deepest (~80 m) area is around the Bohai Strait, which links the Bohai Sea to the Yellow Sea. More than 17 rivers, including the Yellow River, annually contribute 8.0 × 1010 m3 of freshwater to the Bohai Sea; additionally, these rivers annually discharge more than 1.1 × 109 tons of suspended sediment and 6.1 × 109 mol of nitrogen into the sea [20]. These factors make the optical properties of the Bohai Sea complicated and increase the difficulty of satellite remote sensing of the LACA.
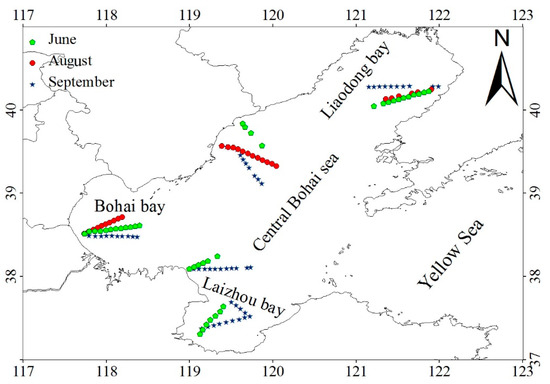
Figure 1.
Study area and field measurements: The training dataset, containing 99 samples, was from Bohai Sea measurements taken in June and August 2005 (green and red circles) and the testing dataset, containing 37 samples, was from Bohai Sea measurements taken in September 2005 (blue stars).
2.2. Field Measurements
To train and assess how well our NNL model retrieved the LACA, we used two independent datasets (Table 1) that consisted of measured remote sensing reflectance (Rrs) and LACA values. The training dataset contained 94 samples measured in the Bohai Sea in June and August 2005 and the testing dataset contained 49 samples measured in the Bohai Sea in September 2005. To evaluate the accuracy of the satellite-derived LACA values, we collected 35 samples of synchronous LACA measurements and satellite-observed Rrs from the Bohai Sea from four research cruises. We used the procedure proposed by Bailey and Werdell [21] to generate the LACA data for a match-up analysis.

Table 1.
Descriptive statistics of the logarithmic value of the measured LACA (unit = log10 (cells/L)) and [Chl] (unit = μg/L): a(443) (unit = m−1), total absorption at 443 nm; and STD, standard deviation.
To initialize and test the performance of the [Chl] retrieval model, we used a global evaluation dataset consisting of measured Rrs and [Chl] that we classified into two sub-datasets: the training dataset and the testing dataset. The training dataset contained 1333 samples from the Yellow Sea, the East China Sea, the Sea-view Wide Field-of-view Sensor (SeaWiFS), and the Bio-optical Archive and Storage System (SeaBASS). The testing dataset contained 371 samples from the Bohai Sea and the West Florida Shelf.
We used field analytical spectral devices to determine the above-surface Rrs and we used the Wetlabs AC-9 photometer and HydroScat-6 to measure the non-water total absorption and particles’ backscattering coefficients, respectively. We measured these data following rigorous and community-defined protocols for deploying experiments and collecting data [22].
We measured the water samples and the radiance simultaneously, conserving the water samples in bottles at low temperature and sending them to the laboratory for analysis on the same day. We determined biochemical properties, such as [Chl], from the water samples in the laboratory within 24 h of sampling. Generally, phytoplankton sizes vary from 0.2 to 2000 μm [9], so we could not count all the phytoplankton together. Instead, we measured the total suspended sediment (TSM) using a weighing method following Mueller et al. [22]. ACA is typically defined as cells whose size is >2 μm (micro-size), consisting mostly of diatoms, dinoflagellates, cyanobacteria, nanoflagellates, and additional flagellates. We measured the ACA after counting the cells microscopically [23].
2.3. Satellite and Auxiliary Data
We collected the monthly mean Rrs observed by MODIS (July 2002 to February 2015) with a 4 km resolution on cylindrical equidistant grids from the NASA ocean color server. Using the MODIS-derived Rrs as inputs, we quantified the LACA using the NNL model. We retrieved the diffuse attenuation coefficient of photosynthetically active radiation (Kpar) using the model (http://pan.baidu.com/s/1ntzJzSH, accessed on 8 May 2015) proposed by Chen et al. [24] so that we could calculate the depth of the euphotic zone (Zeu) using 4.605/Kpar, as proposed by Mobley [25]. We obtained the monthly mean sea potential temperature on a 0.5° grid from the environmental modeling center of the National Weather Service. We computed the monthly mean mixed layer depth (MLD or Zmld) from the monthly mean potential sea temperature using the threshold-based method proposed by Montégut et al. [26]. To keep the same resolution as the MODIS image, we interpolated the original MLD data to a 4 km resolution using bilinear interpolation.
2.4. Establishing the NNL Model
A single hidden layer with adequate nodes allows us to approximate any function that contains a continuous mapping from one finite space to another, so we developed a neural network model with one hidden layer to estimate the LACA from Rrs data. MODIS has seven visible bands and two near-infrared bands for ocean color remote sensing. The two near-infrared bands are commonly used for atmospheric correction, so we excluded these two bands from the NNL model. MODIS has calibration problems at bands around 412 nm and the atmospheric correction is commonly poor at this wavelength [27], so we did not take the 412 nm band into account. As a result, we used 443, 488, 532, 555, 667, and 678 nm as potential bands to develop the NNL model. For comparison, second-generation satellites, including SeaWiFS, the Medium Resolution Imaging Spectrometer (MERIS), and MODIS, have four “common bands” around 443, 488, 555, and 667 nm. Our practical experiments confirmed that when the NNL model used the 443, 488, 555, and 667 nm bands, the model performed as well as when it used the 443, 488, 532, 555, 667, and 678 nm bands. Moreover, from a statistical perspective, increasing the input nodes increases the degrees of freedom of the model, which impacts the model’s stability and accuracy. Therefore, to ensure that the NNL model can retrieve LACA from all second-generation ocean color satellite sensors in the future, even though it is beyond the scope of this study, we selected Rrs(443), Rrs(488), Rrs(555), and Rrs(667) as the optimal inputs for our NNL model.
2.5. Estimating the NAG Rate
We estimated the net specific growth rate of the algal population from two field-measured or satellite-derived LACA values separated by a period of time [1,4]:
where ra is the algal cell division rate and ACAi (i = 0, 1) is the ACA value at time ti. Therefore, to evaluate the ra values, we only needed to know the LACA value at two separate times.
2.6. Estimating the Algal Cell Size
Marañón et al. [28] demonstrated that the [Chl] concentration in a cell is a function of cell size. From their results, we know that cell size (d), in general, varies with intracellular concentration (Ci) [19,28] as:
where Ci is the ratio of [Chl] to ACA. Thus, as long as [Chl] and ACA are known, Ci and d can be determined using Equation (2).
2.7. [Chl] Retrieval Model
Despite there being many empirical and semi-analytical models available for retrieving [Chl] from ocean color, there is a compelling need for an accurate model for optically complex coastal waters because problems can occur when the existing models are applied to the Bohai Sea. For example, semi-analytical models such as those proposed by Gitelson et al. [29] and Carder et al. [30] significantly underestimate [Chl] in the Bohai Sea, despite the fact that these models work well in other turbid and productive waters [29,30]. The optical properties in the Bohai Sea are extremely complicated. Specifically, the waters in the coastal zones are very turbid, but they are clear around the North Bohai Sea and Bohai Strait. To accurately retrieve [Chl], we developed a combined model to retrieve [Chl] from the turbid Bohai Sea. Following Hu et al. [31], [Chl] is a function of color index (CI) in the open ocean (CI < −0.0005 sr−1). Our practical experiment shows that a CI-based model still estimates [Chl] well, even for CI < −0.0001 sr−1, but significantly underestimates [Chl] for turbid waters (CI > 0.0001 sr−1). To overcome this limitation, we developed a neural network model to estimate [Chl] when CI > 0.0001 sr−1. We combined these two algorithms to generate smooth [Chl] data for clear and turbid waters, as well as waters of an intermediate type (−0.0001 sr−1 ≤ CI ≤ 0.0001 sr−1). We used the weighting function developed by Wang et al. [32] to bridge our two algorithms.
2.8. Statistical Evaluation of the Ocean Color Product
In this study, we used the absolute relative error and mean relative error to assess the accuracy of the LACA retrieval model. These statistics are described by the following equations:
where ARE denotes an absolute relative uncertainty, MRE refers to the mean relative uncertainty, xmod,i is the model-derived value of the ith element, xobs,i is the field-measured value of the ith element, and n is the number of elements. However, if xobs,i is small (such as for Rrs for the open ocean at longer wavelengths and for ΔRrs) and xmod,i has substantial errors, the MRE value will become extremely large. For such situations, we used an UMR to estimate the uncertainty of [Chl].
3. Results
3.1. Spectrum and Compositions
As shown in Figure 2, the Rrs collected from the Bohai Sea was highly variable at the visible and near-infrared bands and was similar in magnitude and shape to the typical Rrs from turbid waters [29,33]. Due to the strong absorption of optically active constituents in the blue region, the Rrs in this region (400 nm to 500 nm) was extremely low. However, there were no pronounced spectral characteristics in the blue region for the broad range of [Chl] at all the sites. The magnitude of Rrs at the green bands (500 nm to 600 nm) was much higher than that at the blue bands. Specifically, Rrs values only varied from 0.0001 to 0.0164 sr−1 in the blue region and changed from 0.0035 sr−1 to 0.415 sr−1 in the green region. The Rrs slowly decreased as the wavelengths in the red and near-infrared region increased, except in the samples with high concentrations of suspended matter (106 g L−1). A pronounced reflectance minimum near 670 nm, which corresponded to the [Chl] absorption peak, only occurred when [Chl] was >4.3 μg L−1.
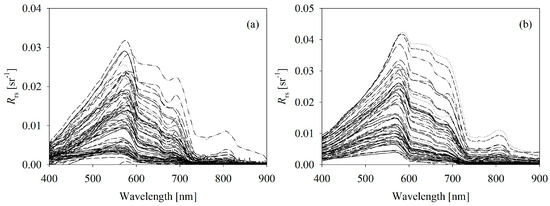
Figure 2.
Spectral curves of remote sensing of the two independent datasets: (a) calibration dataset collected from the Bohai Sea in June and August 2005 (99 samples); (b) validation dataset collected from the Bohai Sea in September 2005 (37 samples).
Many phytoplankton taxonomic groups are found in the Bohai Sea. These include Skeletonema costatum, Prorocentrum minimum, Nitzschia longissima, Nitzschia sp., Diploneis bombus, Rhizosolenia stolterfothii, Pleurosigma sp., Ditylum brightwellii, Coscinodiscus marginatus, Phaeocystis sp., and so on. Among these, the population of Phaeocystis sp. was much larger than the others in our data and accounted for more than 77% of our dataset. These findings imply that most algae in the Bohai Sea can be classified into nano-phytoplankton groups [34,35], but the cell size can vary widely among these algal groups. Marañón et al. [28] showed that the intracellular [Chl] is positively related to phytoplankton size structure, and Carder et al. [30] showed that the absorption coefficient of phytoplankton (aph(λ)) is positively related to [Chl]. Thus, the aph(λ) increases as the cell size increases. However, the wide range of cell sizes complicates the relationship between the LACA and ocean color signals (Figure 3). Specifically, the LACA and inherent optical properties collected from the Bohai Sea indicate that the LACA increases as the backscattering coefficient at 555 nm (bb(555)) or the absorption coefficient of [Chl] at 675 nm (aph(675)) increases, but there is no statistically pronounced relationship between the two variables, even though small particles tend to scatter more [11] in this dataset.
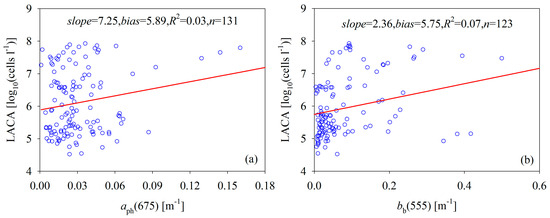
Figure 3.
Scatterplots of the LACA versus inherent optical properties: (a) absorption of chlorophyll-a concentration at 675 nm versus the LACA; (b) backscattering coefficient at 555 nm versus the LACA.
3.2. Evaluating [Chl] Retrieval Using Field Measurements
Using the 1333 samples collected from the Yellow Sea, the East China Sea, and SeaBASS to initialize the model shown in Figure 4a, we used Equation (6) as the optimal model for quantifying [Chl].
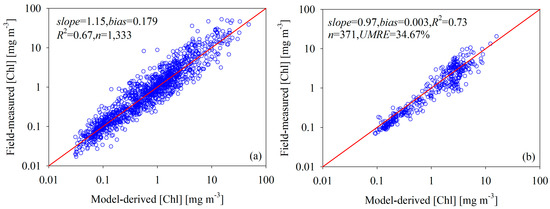
Figure 4.
Scatterplots of field-measured and model-predicted [Chl]: (a) model initialized with data collected from the Yellow Sea, the East China Sea, and SeaBASS; (b) model evaluated using data collected from the Bohai Sea and the West Florida Shelf.
Here, CI = Rrs(555) − 0.5[Rrs(443) + Rrs(667)] and W is a smoothing factor computed from 0.5–5000 CI. [Chl]c and [Chl]t are [Chl] retrieved from clear and turbid water, respectively, and [Chl]m is the [Chl] retrieved from intermediate water.
We found that this model effectively derived [Chl] from the global oceans and coastal waters with a coefficient of determination (R2) of 0.67, and the corresponding slope of the linear relationship between the field-measured and model-derived [Chl] is 1.15. We evaluated the stability and accuracy of the [Chl] retrieval model using the field measurements collected from the Bohai Sea and the West Florida Shelf (Figure 4b). The results indicate that this model did not require further optimization of the site-specific parameterization to accurately retrieve [Chl] in the optically complex coastal waters. Specifically, the slope and R2 of the linear relationship between the field-measured and model-derived [Chl] are 0.97 and 0.73, respectively, while the UMRE value is 34.67%.
3.3. IOP-Based Empirical LACA Model
The absorption of [Chl] and gelbstoff can be analytically separated from the total absorption [36] if the a(λ) values are known for at least two wavelengths. Therefore, if the optical properties of algal cells strongly depend on the optical properties of phytoplankton pigment, the LACA should strongly relate to the band combination of a(λ) at visible bands. However, the results in Figure 5a show that the relationship between the LACA and the band combination of a(λ) was poor, with an R2 of only 0.16. Moreover, to illuminate the effects of algal cells on the backscattering coefficient, we present the relationship between the LACA and the band combination of bb(λ) (Figure 5b). Like the results in Figure 5a, the LACA values do not significantly depend on bb(λ) (R2 = 0.10). The phytoplankton pigment in a cell is subject to perturbations in their size and to environmental conditions. To maintain productivity, the phytoplankton must develop adaptive strategies which fit and compensate for environmental changes [37]. Figure 6 shows that [Chl] increases as the LACA increases according to the slope coefficient of the linear relationship between the two variables, but there is no statistically significant relationship between them (R2 < 0.01, p < 0.001). The weak relationship between [Chl] and LACA is primarily due to phytoplankton size structure, nutrients, solar irradiance, and other physical forces [38]. These results further confirm that it is challenging to accurately and semi-analytically derive the LACA from ocean color products due to the complicated optical behavior of algal cells.
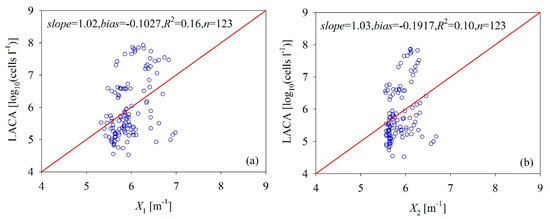
Figure 5.
Scatterplots of inherent optical properties-estimated versus measured LACA from the Bohai Sea, where X1 and X2 represent 0.5935[a(412) + 0.754a(443) + 1.560a(488) − 2.418a(555)]0.114 and 6.626[bb(443) − 0.292bb(488)]. The empirical formulae in the x-axis are regressed to the field measurements.
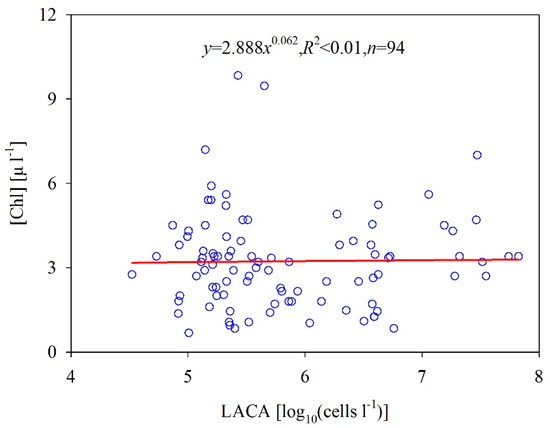
Figure 6.
Co-varying relationship between the chlorophyll-a concentration and the LACA in the Bohai Sea.
3.4. Training and Evaluating the Model
Figure 7a compares the field-measured LACA with the NNL model-predicted LACA values from the training dataset. For the range of LACA values from 4.523 to 7.928 log10 (cells L−1), the NNL model acceptably derived the LACA from the Rrs data with an R2 between the field-measured and model-predicted LACA of 0.61 (n = 99). We evaluated the model by comparing the LACA predicted by the NNL model with the LACA measured analytically from the independent validation dataset (Figure 7b). For the LACA values ranging from 4.732 to 7.826 log10 (cells L−1), the relative uncertainty of this parameter prediction is less than 25.24%, with an average of 8.41%. The slope and bias of the linear relationships between the field-measured and model-predicted LACA are 1.01 and −0.064, respectively, and R2 is 0.74. That means that, for the test data, the NNL model accounted for 74% of the variation in the LACA. These findings imply that the NNL model did not require further retraining to accurately quantify the LACA from the September validation data from the Bohai Sea, even though the water properties of the validation data were different from the summer calibration data (Table 1). Therefore, the neural network model, if properly initialized, could accurately determine the LACA from our testing and training data from the Bohai Sea.
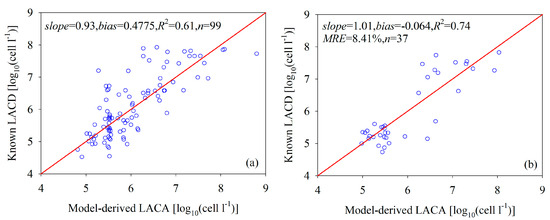
Figure 7.
A comparison of the model-derived LACA with the field-measured LACA: (a) calibration data and (b) validation data, where the NNL model architecture had two hidden layers that had 9 × 11 neurons.
In general, the optical properties of the Bohai Sea depend on phytoplankton and on suspended sediment that does not covary with the phytoplankton [39]. Therefore, it is difficult to separate phytoplankton from mineral particles in these waters. Figure 8 shows the scatterplots of the field-measured TSM concentration versus the model-derived LACA for the calibration and validation data. The figure indicates that the model-derived LACA increases as the TSM concentrations increase, but the correlation is not pronounced (R2 < 0.18), which indicates that the NNL model effectively separated the LACA information from the optically complex coastal waters.
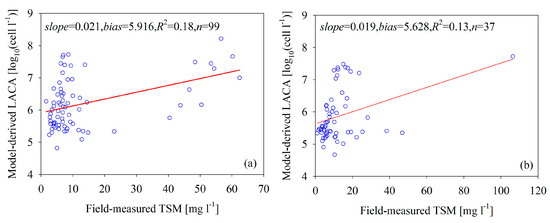
Figure 8.
Scatterplots of the NNL-estimated LACA versus the in situ TSM concentration: (a) calibration data and (b) validation data.
3.5. Accuracy of Satellite-Derived LACA
We quantified the NNL model-derived LACA data from the MODIS data after atmospheric correction using the improved shortwave infrared method. We evaluated the accuracy of our predicted LACA by comparing the satellite products with synchronous field measurements. We used four MODIS images synchronized with in situ measurements for our match-up comparison. Figure 9 shows the satellite-predicted LACA plotted against the synchronous in situ data measured within ± 3 h of the satellite pass over the Bohai Sea. For the LACA values that range from 4.522 to 7.825 log10 (cells L−1), the relative uncertainty of the prediction is lower than 25.32%, with an average of 8.79%. The slope and bias are 1.03 and −0.302, respectively, and the R2 = 0.73. Therefore, the NNL model accurately retrieved the LACA from the satellite data even though the ocean color satellite data were significantly influenced by colored dissolved organic matter (CDOM) and suspended sediments in the turbid coastal waters of the Bohai Sea, which, in turn, influenced the ACA retrievals. We suggest that if an atmospheric correction scheme for MODIS bands is available, the MODIS data could be used for quantitatively detecting the LACA from turbid waters.
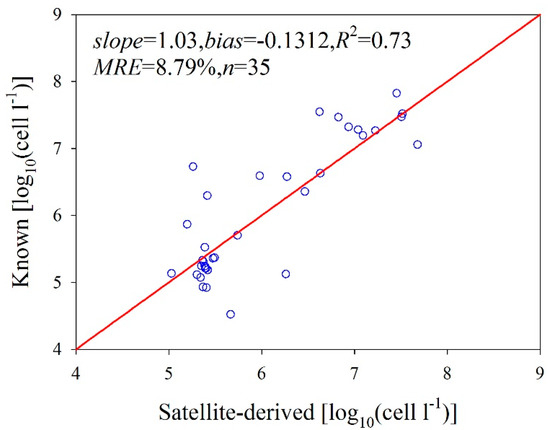
Figure 9.
Scatterplots of the field-measured “Known” and satellite-derived LACA on a logarithmic scale.
4. Applications and Discussion
4.1. The Seasonal Variations of ACA
The NNL model predicted a climatological monthly mean of ACA for the MODIS Rrs data between July 2002 and February 2015. Figure 10 shows that ACA varies largely from 104 to 107.1 cells/L. As expected, the low values were over the outer shelf and the high values were over the coastal zones, which is consistent with the spatial distribution of commonly used optical activity components for this region [40,41]. From the shoreline to the central Bohai Sea, ACA decreased as the offshore distances increased. The highest ACA values occurred in the waters around Bohai Bay, Laizhou Bay, and Liaodong Bay, where there was ACA over 106 cells/L. These high ACA values are due to the strong mixing that carries sufficient nutrients toward the surface and, in turn, produces more phytoplankton cells. In addition, the strong mixing brings significantly high concentrations of suspended particles towards the surface.
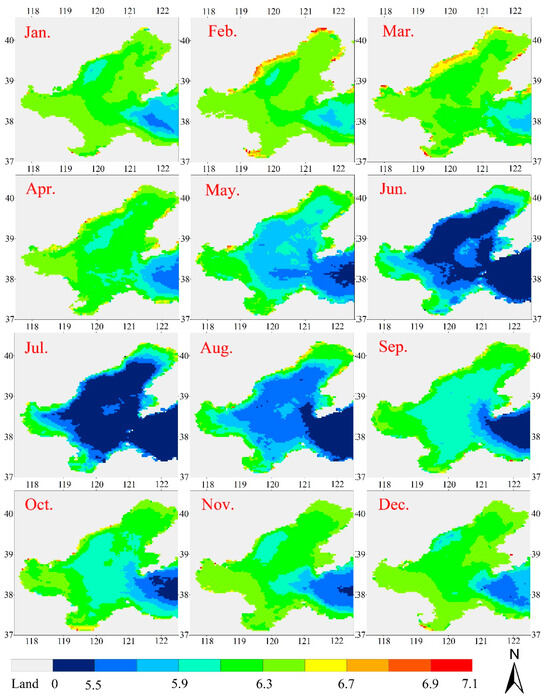
Figure 10.
Seasonal and spatial variations in the LACA in the Bohai Sea. White represents clouds. All data with Rrs > 0 is included in the analysis.
The time series of ACA is shown in Figure 10. Figure 10 shows a clear seasonal pattern in the time series with the mean values decreasing in the order: spring > winter > autumn > summer. This seasonal variation is associated with high nutrient concentration in winter due to dust storm depositions in spring, depositions due to increased fossil fuel burning during the heating period in northern China [42], and nutrients being pumped from the bottom induced by spring mixing due to strong winds and tides [43]. On the other hand, the East Asia Monsoon over China’s eastern coastal zone is northerly during winter–spring and southerly during the summer [44]. The winter–spring monsoon, which lasts from October to March, is much stronger than the summer monsoon, which lasts from May to August. More nutrients are re-suspended and mixed into the ocean surface layer during the winter–spring than during the summer. As a result, the maximum ACA occurs in February and the minimum occurs in July. From summer to winter, ACA gradually increases, and from spring to summer, the LACA slowly decreases. Compared to the time series of the sea surface temperature (SST), which reaches its minimum in February and its maximum in August (not presented), the link between mixing and ACA is displayed clearly in the figure.
Our study provides a novel approach for estimating algal abundance from satellite images, and then applies it to reveal the spatial and temporal variations in algal biological features in the Bohai Sea. This new knowledge on the spatial and temporal variation in ACA is beneficial for understanding algal biological features in the coastal ecological system, which has been minimally reported in the literature. Our approach, as presented here, and in the hands of other researchers and stakeholders, can provide a robust method of impact assessment and assist in future planning, early detection, and ecological efforts. Moreover, the ACA is not a conventional bio-optical variable in ocean color remote sensing, indicating that the ACA would be weakly covarying with the bio-optical properties, so it is very hard to develop analytical or semi-analytical models for ACA estimations. However, when properly parameterized, neural networks are good candidates for ACA estimation even in the optically complicated coastal waters. We note that the ACA model falls clearly within a knowledge-driven empirical framework. We encourage other researchers to evaluate the model with more field and satellite observations. Additionally, we anticipate our research to be a starting point for using remote sensing technology to understand algal biological features, which can also help other researchers to develop more biological indicators for revealing the impacts of global climate changes on coastal ecosystems.
4.2. Decoupling between ACA and [Chl]
In addition to nutrient concentration, light, phytoplankton size structure, and temperature variation are important physical forcing factors for efficient phytoplankton photosynthesis [45], which, in turn, affects the relationship between [Chl] and ACA. The physical forcing factors in the Bohai Sea are seasonal and have spatially changing patterns [46], which make the relationship between [Chl] and ACA very weak (Figure 11a). This fact is similar to the conclusion we draw from the field measurements shown in Figure 6. It is interesting to point out that NAG rates also increase as NPG rates increase (Figure 11b), despite the low correlation between the two rates (R2 = 0.11, n = 152). On the one hand, the physical process of algal cell growth is different from [Chl] growth. Specifically, when one mother cell divides into two daughter cells, the NAG rate changes but [Chl] might remain the same. In the same argument, the changes in [Chl] would not lead to the algal cell division unless the algal cells were big enough and the nutrients were sufficient enough. On the other hand, the intracellular [Chl] always depends on solar irradiance, provided nutrients, phytoplankton structure size, and other factors. Especially for microplankton (size > 20 μm), the size structure of phytoplankton controls the ratio of [Chl] to ACA [9]. Therefore, it is hard to describe the relationship between ACA and [Chl] using a simple mathematical function for a complex aquatic ecosystem such as the Bohai Sea.
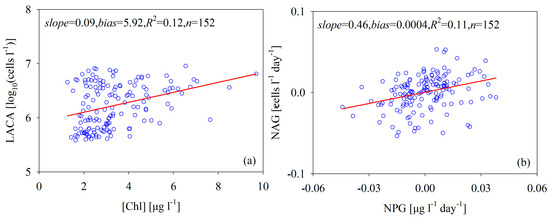
Figure 11.
Scatterplots of (a) the LACA vs. [Chl] and (b) NAG vs. NPG, where the [Chl], NAG, and NPG values were estimated from the monthly mean MODIS data.
Figure 12 displays the changing trends of [Chl], ACA, Ci, and SST from 2002 to 2015, indicating both short-term variations and long-term trends in Ci during that period. The initial 2.0 × 10−10 μg cell−1 per month rise in Ci corresponded to the July 2002 to May 2008 period. Since June 2008, the Ci dropped, on average, 2.0 × 10−10 μg cell−1 per month, with several secondary changing trends that ended in an increase from July 2010 to February 2015. Overall, the Ci slowly increased by, on average, 1.0 × 10−10 μg cell−1 per month, with some regular fluctuations from July 2002 to February 2015 in the Bohai Sea. The Ci maxima occurred around July and the minima occurred around January, representing the seasonal change in the algal community. Large phytoplankton species (i.e., micro-phytoplankton) dominated during summer, and the waters contained a greater number of small phytoplankton species during the winter. It is noteworthy that Ci basically co-varied with ACA during this time, suggesting that the algal species influenced ACA. Comparatively, [Chl] had seasonal variations that were different from ACA, characterized by spring minima and a two-peak pattern from 2009 to 2015. In 2002 and 2003, [Chl] co-varied with ACA, with a trough during the summer and a peak during spring. From 2004 to 2008, the spring peak disappeared and a more durable bloom period occurred from summer to winter. Then, starting from 2009, the seasonality of [Chl] changed again. [Chl] in summer and spring enhanced more, but in autumn [Chl] depressed and had a clear two-peak pattern.
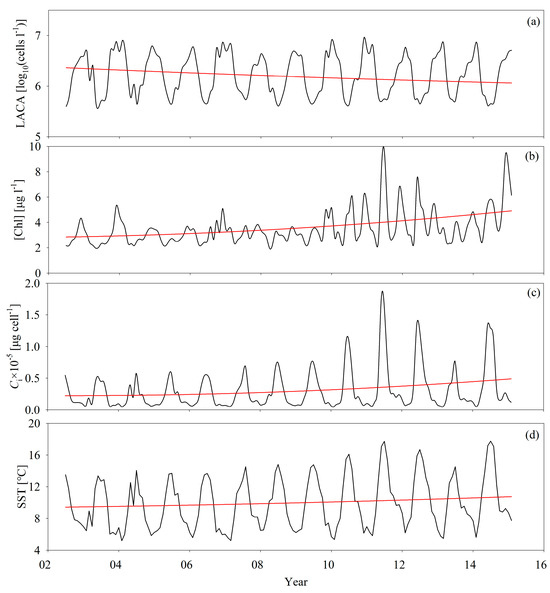
Figure 12.
Time series of (a) ACA, (b) [Chl], (c) Ci, and (d) SST from 2002 to 2015: the black line represents the original data and the red line shows the changing trends extracted using the Hilbert-Huang transform proposed by Huang et al. [47].
A large set of physical and biochemical data is required to get a comprehensive understanding of the mechanisms of [Chl] seasonality and its variation in the Bohai Sea, and research of these mechanisms is beyond the scope of this study. However, the decoupling between [Chl] and ACA suggests that different physical and biological processes in this study area dominate the two biological variables. As we stated before, the high negative correlation between ACA and Ci implies that the temporal rhythms of ACA in the Bohai Sea are due to the seasonal evolution of the algal community, which is regulated by nutrient abundance. In contrast, the seasonal variations of [Chl] are more complicated and are influenced by diverse physical, biological, and physiological processes.
4.3. Inter-Annual Changes of ACA, [Chl], and Ci
We estimated the inter-annual variations of the biological variables for our 14 years of data. According to Figure 12a,b, the ACA decreased by, on average, 2.0 × 10−6 log10 (cells L−1) per month from 2002 to 2015, and the corresponding [Chl] increased by, on average, 4.0 × 10−4 μg L−1 per month. Compared to the results in Figure 12c, the decrease in Ci over the 14 years was due to the comprehensive effects of decreasing ACA and increasing [Chl]. These are new findings that have never been reported before using field measurements due to the lack of sufficient long-term and large-scale field data from the Bohai Sea.
In addition to ACA and [Chl], cell size structure is another important indicator of the trophic status of an aquatic ecosystem. Figure 13 shows the time series of the cell size of the algal population in the Bohai Sea from 2002 to 2015. The figure indicates that the monthly mean cell size varied from 4.81 to 15.29 μm, meaning that nano-phytoplankton (2–20 μm) dominated the algal species in the Bohai Sea. This result is consistent with our practical investigation. Guenther et al. [45] suggests that larger algal cells exhibit lower nutrient absorption efficiency and smaller cells prevail in more oligotrophic conditions. Following the results of Liu and Yin [20], the nutrient concentration during autumn is typically 2 to 6 times lower than during the spring. Figure 12a shows that the ACA that we estimated for the spring was at least ten times larger than in the autumn. Therefore, even though the total nutrient concentration in spring was higher than in the autumn, the exclusive nutrient concentration per cell in the spring was much lower than in the autumn. As a result, the cell size in early autumn was much smaller than in the early spring in the Bohai Sea at that time (Figure 13). The changing trend of cell size that we observed is consistent with that of Ci because the cell size positively relates to the Ci value [26]. Specifically, in our case, the cell size increased by, on average, 0.011 μm per month, with some regular fluctuations since 2002. In general, the cell size structure of algae in aquatic ecosystems varies with the variations in nutrient concentration, solar irradiance, vertical mixing, and turbulence in the upper ocean, as well as with selective grazing by higher trophic organisms. This means that the environmental conditions for algal growth in the Bohai Sea dramatically changed over those 14 years. Following the positively co-varying relationship between algal cell size and nutrient concentration proposed by Geider and MacIntyre [48], an immediate conclusion from our results is that the Bohai Sea has become increasingly eutrophic since 2002. However, we need more biochemical data to support this statement. Obtaining such data is beyond the scope of this study.
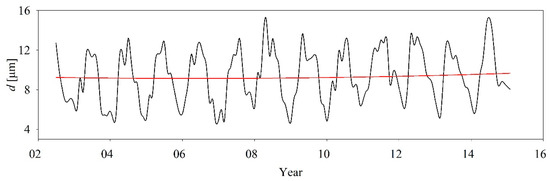
Figure 13.
Time series of algal cell diameter from 2002 to 2015: the black line represents the original data and the red line shows the changing trends extracted using the Hilbert-Huang transform proposed by Huang et al. [47].
Importantly, one disadvantage of the photosynthesis–cell size relationship was that it completely ignores the effects of environmental conditions on the intracellular [Chl] concentration. As a matter of fact, the intracellular [Chl] concentration not only covaried with cell size, but also significantly depended on environmental conditions such as light, nutrient content, temperature, and so on [49,50]. Following Marañón et al. [28], it is at least known that there is certainly a first order size-driven relationship between cell volume and intracellular [Chl] concentrations [19,28]. However, environmental adjustments were well known to appear as variability in intracellular [Chl] concentrations as a second order impact on [Chl] concentrations. This is to say that the seasonal variations in cell size presented in Figure 13 might be polluted by some variations associated with environmental conditions, but more measurements are required to understand this mixed mutual interference. To enhance the claim confidence in the absolute magnitude of seasonal phytoplankton size changes, the variability of photo-physiology independent of cell size should be considered in the future. As Reich et al. [51] remarked, a single, universal scaling rule cannot predict the metabolism–size relationship in all photosynthetic organisms. Thus, to accurately derive cell size from intracellular [Chl] concentrations, a model that can minimize the influence of environmental conditions on intracellular [Chl] concentrations for cell size estimation would still be desired.
4.4. Vertical Mixture-Mediated Dance of the Algal Population in the Bohai Sea
“Surface-down” radiance and “bottom-up” nutrients control the cycle of the algal population in the Bohai Sea. Sverdrup’s critical depth hypothesis proposes that the MLD should be shallower than the critical depth that sustains net primary production [52,53]. The MLD continues to the shoal and the stratification in the surface is well-developed after the breakout of a spring bloom event, which, in turn, blocks eutrophic water pumping from the deeper subsurface layer. Due to the lack of new supplementary nutrients for the surface layer, the waters become increasingly oligotrophic as the residual nutrients are exhausted by the quickly increasing phytoplankton population. However, comparing the phytoplankton population at the beginning of spring bloom, the [Chl] and ACA still increase while the Zmld decreases, but the daily NAG rate might begin to decrease. When Zmld is shallower than the threshold for Zmld/Zeu (Zt), the positive NAG rate translates into a negative value and the phytoplankton population begins to decrease. These statements are supported by the relationship between the ratio of Zmld to Zeu and the LACA displayed in Figure 14. Specifically, the LACA values positively correlated to Zmld/Zeu whenever Zt was <10.124 m, but were negatively correlated to Zmld/Zeu when Zt was <10.124 m. Therefore, Zt could be used to determine when a spring bloom event has terminated due to the “surface-down” radiance and “bottom-up” nutrients physical system.
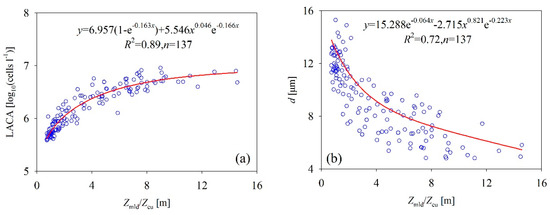
Figure 14.
Empirical relationship of Zmld/Zeu and (a) the LACA and (b) cell size.
5. Summary
A basic goal of oceanic color remote sensing technology is to interpret remote sensing data to provide a better understanding of ocean biology and biogeochemistry. The LACA could identify the health of coastal ecosystems; however, due to the interference originating from phytoplankton size structure and other physical factors, the relationship between inherent optical properties and the LACA is very weak. As a result, it is difficult to use a simple mathematical function to accurately derive the LACA from ocean color measurements. Fortunately, if parameterized and optimized properly, a neural network model can be used to quantify the LACA from turbid coastal waters. We summarize our findings as follows:
- (1)
- The optical properties of algal cells were very complicated, so it was hard to describe the algal cell abundance using IOPs. This was because phytoplankton concentration in the water column depends on the algal cell amount, on the phytoplankton size structure, and other variables.
- (2)
- The neural network model was an effective approach for estimating the LACA from remote sensing reflectance in the Bohai Sea, and produced <9% uncertainty in estimating the LACA from the satellite-derived and/or field-measured Rrs.
- (3)
- Due to increasing algal cell size from 2002 to 2015, the [Chl] inside a cell slowly increased by, on average, 4.0 × 10−4 μg L−1 per month, with some regular fluctuations during that 14-year time span; however, the seasonal variations might be not very accurate due to disturbance associated with the temporal variations of environments. The increase in algal cell size could have primarily been caused by the increasing trophic state in the Bohai Sea, but the mechanisms behind these physical processes are still beyond our knowledge.
- (4)
- The LACA and cell size exhibited seasonal changing patterns due to the seasonal variations in the physical factors of the Bohai Sea. The LACA increased monotonically with Zmld/Zeu, and the trend in the LACA ended in a monotonic drop because Zmld/Zeu < 10.124 m. This phenomenon was primarily caused by the “surface-down” radiance and “bottom-up” nutrient physical mechanisms found in the Bohai Sea.
Although the model was trained and evaluated with limited field measurements, we anticipate our research to be a starting point for using remote sensing technology to detect more biological variables for scientific communication.
Author Contributions
Writing, Conceptualization and Methodology, W.Q.; Writing—review & editing, J.C. All authors have read and agreed to the published version of the manuscript.
Funding
The International Cooperation in Science and Technology Innovation among Governments (2019YFE0127200, Chen), National Natural Science Foundation of China (42022045, Chen), and Shan’xi Key Research and Development Program (2022ZDLSF06-09, Chen) provided financial support for this study.
Data Availability Statement
Not applicable.
Acknowledgments
We would like to thank the two anonymous reviewers for their valuable comments.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Behrenfeld, M.J. Abandoning Sverdrup’s Critical Depth Hypothesis on phytoplankton blooms. Ecology 2010, 91, 977–989. [Google Scholar] [CrossRef] [PubMed]
- Razali, R.M.; Mustapa, N.I.; Yaacob, K.K.K.; Yusof, F.; Teng, S.T.; Hanafiah, A.H.; Hii, K.S.; Mohd-Din, M.; Gu, H.; Leaw, C.P.; et al. Diversity of algal cell and the algal bloom event in the mariculture areas of Johor Strait, Malaysia. Plankton Benthos Res. 2022, 17, 290–300. [Google Scholar] [CrossRef]
- Behrenfeld, M. Climate-mediated dance of the phytoplankton. Nat. Clim. Change 2014, 4, 880–887. [Google Scholar] [CrossRef]
- Banse, K. Rates of phytoplankton cell division in the field and in iron enrichment experiments. Limnol. Oceanogr. 1991, 36, 1886–1898. [Google Scholar] [CrossRef]
- Cullen, J.J.; Davis, R.F. The blank can make a big difference in oceanographic measurements. Limnol. Oceanogr. Bull. 2003, 12, 29–35. [Google Scholar] [CrossRef]
- Edwards, M.; Beaugrand, G.; Reid, P.C.; Rowden, A.A.; Jones, M.B. Ocean climate anomalies and the ecology of the North Sea. Mar. Ecol. Prog. Ser. 2002, 239, 1–10. [Google Scholar] [CrossRef]
- Gordon, H.R.; Clark, D.K.; Mueller, J.L.; Hovis, W.A. Phytoplankton pigments derived from the Nimbus-7 CZCS: Initial comparisons with surface measurements. Science 1980, 210, 63–66. [Google Scholar] [CrossRef]
- O’Reilly, J.E.; Werdell, P.J. Chlorophyll algorithms for ocean color sensors—OC4, OC5 & OC6. Remote Sens. Environ. 2019, 229, 32–47. [Google Scholar]
- Ostos, E.M.; Blanco, J.M.; Agustí, S.; Lubian, L.M.; Rodrigue, Z.; Palomino, R.L.; Liabres, M.; Rodriguez, J. Phytoplankton biovolume is independent from the slope of the size spectrum in the oligotrophic Atlantic ocean. J. Mar. Syst. 2015, 152, 42–50. [Google Scholar] [CrossRef]
- Brewin, R.J.W.; Dall’Olmo, G.; Sathyendranath, S.; Hardman-Mountford, N.J. Particle backscattering as a function of chlorophyll and phytoplankton size structure in the open-ocean. Opt. Express 2012, 20, 17632–17652. [Google Scholar] [CrossRef]
- Kostadinov, T.S.; Siegel, D.A.; Maritorena, S. Retrieval of the particle size distribution from satellite ocean color observations. J. Geophys. Res. Ocean. 2009, 114, C09015. [Google Scholar] [CrossRef]
- Antoine, D.; Siegel, D.A.; Kostadinov, T.; Maritorena, S.; Nelson, N.B.; Gentili, B.; Vellucci, V.; Guillocheau, N. Variability in Optical Particle Backscattering in Contrasting Bio-optical Oceanic Regimes. Limnol. Oceanogr. 2011, 56, 955–973. [Google Scholar] [CrossRef]
- Briggs, N.T.; Slade, W.H.; Boss, E.; Perry, M.J. Method for estimating mean particle size from high-frequency fluctuations in beam attenuation or scattering measurements. Appl. Opt. 2013, 52, 6710–6725. [Google Scholar] [CrossRef] [PubMed]
- Hill, S.C.; Hill, A.C.; Barber, P.W. Light scattering by size/shape distributions of soil particles and spheroids. Appl. Opt. 1984, 23, 1025–1031. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, R.A.; Stramski, D.; Wright, V.M. Particle size distributions of coastal waters measured with an in situ laser diffractometer. In Proceedings of the Ocean Optics XIX, Barga, Italy, 3–4 October 2008. [Google Scholar]
- Uitz, J.; Stramski, D.; Baudoux, A.-C.; Reynolds, R.A.; Wright, V.M.; Dubranna, J.; Azam, F. Variations in the optical properties of a particle suspension associated with viral infection of marine bacteria. Limnol. Oceanogr. 2010, 55, 2317–2330. [Google Scholar] [CrossRef]
- Chen, J.; He, X.; Xing, X.; Xing, Q.; Liu, Z.; Pan, D. An inherent optical properties data processing system for achieving consistent ocean color products from different ocean color satellites. J. Geophys. Res. Ocean. 2020, 125, e2019JC015811. [Google Scholar] [CrossRef]
- Jamet, C.; Loisel, H.; Dessailly, D. Retrieval of the spectral diffuse attenuation coefficient K-d(lambda) in open and coastal ocean waters using a neural network inversion. J. Geophys. Res. Ocean. 2012, 117, C10023. [Google Scholar] [CrossRef]
- Roy, S.; Sathyendranath, S.; Platt, T. Retrieval of phytoplankton size from bio-optical measurements: Theory and applications. J. R. Soc. Interface 2011, 8, 650–660. [Google Scholar] [CrossRef]
- Liu, H.; Yin, B.S. Numerical investigation of nutrient limitations in the Bohai Sea. Mar. Environ. Res. 2010, 70, 308–317. [Google Scholar] [CrossRef]
- Bailey, S.W.; Werdell, P.J. A multi-sensor approach for the on-orbit validation of ocean color satellite data products. Remote Sens. Environ. 2006, 102, 12–23. [Google Scholar] [CrossRef]
- Mueller, J.L.; Fargion, G.S.; McClain, C.R. Ocean Optics Protocols for Satellite Ocean Color Sensor Validation, Revision 4; Goddard Space Flight Center: Greenbelt, MD, USA, 2002.
- Stevenson, R.J.; Bahls, L.L. Rapid Bioassessment Protocols for Use in Wadeable Streams and Rivers: Periphyton, Benthic Macroinvertebrates, and Fish; EPA 841-B-99-002; United States Environmental Protection Agency: Washington, DC, USA, 1999.
- Chen, J.; Ishizaka, J.; Zhu, L.Y.; Cui, T.W. A neural network model for Kd(λ) retrieval and application to global Kpar monitoring. PLoS ONE 2015, 10, e0127514. [Google Scholar]
- Mobley, C.D. Light and Water: Radiative Transfer in Natural Waters; Academic Press: New York, NY, USA, 1994; p. 592. [Google Scholar]
- Montégut, C.B.; Madec, G.; Fisher, A.S.; Lazar, A.; Ludicone, D. Mixed layer depth over the global ocean: An examination of profile data and a profile-based climatology. J. Geophys. Res. 2004, 109, C12003. [Google Scholar] [CrossRef]
- Gordon, H.R.; Voss, K.J. Normalized Water-Leaving Radiance. In MODIS Algorithm Theoretical Basis Document (ATBD-17); Department of Physics, University of Miami: Coral Gables, FL, USA, 1999; p. 33124. [Google Scholar]
- Marañón, E.; Cermeno, P.; Rodriguez, J.; Zubkov, M.V.; Harris, R.P. Scaling of phytoplankton photosynthesis and cell size in the ocean. Limnol. Oceanogr. 2007, 52, 2190–2198. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Dall’Olmo, G.; Moses, W.; Rundquist, D.C.; Barrow, T.; Fisher, T.R.; Gurlin, D.; Holz, J. A Simple Semi-analytical Model for Remote Estimation of Chlorophyll-a in Turbid Waters: Validation. Remote Sens. Environ. 2008, 112, 3582–3593. [Google Scholar] [CrossRef]
- Carder, K.L.; Chen, F.R.; Cannizzaro, J.P.; Campbell, J.W.; Mitchell, B.G. Performance of the MODIS semi-analytical ocean color algorithm for chlorophyll-a. Adv. Space Res. 2004, 33, 1152–1159. [Google Scholar] [CrossRef]
- Hu, C.; Lee, Z.; Franz, B. Chlorophyll algorithms for oligotrophic oceans: A novel approach based on three-band reflectance difference. J. Geophys. Res. Ocean. 2012, 117, C01011. [Google Scholar]
- Wang, M.; Son, S.; Shi, W. Evaluation of MODIS SWIR and NIR-SWIR atmospheric correction algorithm using SeaBASS data. Remote Sens. Environ. 2009, 113, 635–644. [Google Scholar] [CrossRef]
- Chen, J.; He, X.; Quan, W.; Ma, L.; Jia, M.; Pan, D. A statistical analysis of residual errors in satellite remote sensing reflectance data from oligotrophic open oceans. IEEE Trans. Geosci. Remote Sens. 2022, 60, 4203912. [Google Scholar] [CrossRef]
- Grzebyk, D.; Denardou, A.; Berland, B.; Pouchus, Y.F. Evidence of a new toxin in the red-tide dinoflagellate Prorocentrum minimum. J. Plankton Res. 1997, 19, 1111–1124. [Google Scholar] [CrossRef]
- Ryerson, T.A.; Newton, J.A.; Armbrust, E.V. Spring Bloom Development, Genetic Variation, and Population Succession in the Planktonic Diatom Ditylum brightwellii. Limnol. Oceanogr. 2006, 51, 1249–1261. [Google Scholar]
- Werdell, P.J.; McKinna, L.I.W.; Boss, E.; Ackleson, S.G.; Craig, S.E.; Gregg, W.W.; Lee, Z.; Maritorena, S.; Roesler, C.S.; Rousseaux, C.S.; et al. An overview of approaches and challenges for retrieving marine inherent optical properties from ocean color remote sensing. Prog. Oceanogr. 2018, 160, 186–212. [Google Scholar] [PubMed]
- Moore, C.M.; Suggett, D.J.; Hickman, A.E.; Kim, Y.-n.; Tweddle, J.F.; Sharples, J.; Geider, R.J.; Holligan, P.M. Phytoplankton photoacclimation and photoadaptation in response to environmental gradients in a shelf sea. Limnol. Oceanogr. 2006, 51, 936–949. [Google Scholar]
- Agirbas, E.; Martinez-Vicente, V.; Brewin, R.J.W.; Racault, M.-F.; Airs, R.L.; Llewellyn, C.A. Temporal changes in total and size-fractioned chlorophyll-a in surface waters of three provinces in the Atlantic Ocean (September to November) between 2003 and 2010. J. Mar. Syst. 2015, 150, 56–65. [Google Scholar]
- Cui, T.; Zhang, J.; Groom, S.; Sun, L.; Smyth, T.; Sathyendranath, S. Validation of MERIS ocean-color products in the Bohai Sea: A case study for turbid coastal waters. Remote Sens. Environ. 2010, 114, 2326–2336. [Google Scholar]
- Chen, J.; Quan, W.T.; Cui, T.W.; Song, Q.J.; Lin, C.S. Remote sensing of absorption and scattering coefficient using neural network model: Development, validation, and application. Remote Sens. Environ. 2014, 149, 213–226. [Google Scholar]
- Liu, Z.L.; Chen, J.; Cui, T.W.; Tang, J.W.; Wang, L. A combined semi-analytical algorithm for retrieving total suspended sediment concentration from multiple missions: A case study of the China Eastern Coastal Zone. Int. J. Remote Sens. 2021, 40, 8004–8033. [Google Scholar]
- Zhu, L.; Chen, Y.; Guo, L.; Wang, F.J. Estimate of dry deposition fluxes of nutrients over the East China Sea: The implication of aerosol ammonium to non-sea-salt surfate ratio to nutrient deposition of coastal oceans. Atmos. Environ. 2013, 69, 131–138. [Google Scholar]
- Hu, C.H.; Ji, Z.W.; Wang, T. Dynamic characteristics of sea currents and sediment dispersion in the Yellow River Estuary. Int. J. Sediment Res. 1998, 13, 20–30. [Google Scholar]
- Hao, Z.; Sun, D.; Zheng, J. East Asian monsoon signals reflected in temperature and precipitation changes over the past 300 years in the middle and lower reaches of the Yangtze River. PLoS ONE 2015, 10, e0131159. [Google Scholar]
- Guenther, M.; Araújo, M.; Flores-Montes, M.; Gonzalez-Rodriguez, E.; Neumann-Leitão, S. Eutrophication effects on phytoplankton size-fractioned biomass and production at a tropical estuary. Mar. Pollut. Bull. 2015, 91, 537–547. [Google Scholar]
- Sundermann, J.; Feng, S.Z. Analysis and modelling of the Bohai sea ecosystem—A joint German–Chinese study. J. Mar. Syst. 2004, 44, 127–140. [Google Scholar] [CrossRef]
- Huang, N.E. Computer Implemented Empirical Mode Decomposition Method, Apparatus, and Article of Manufacture for Two-Dimensional Signals. U.S. Patent US5983162, 9 November 1999. [Google Scholar]
- Geider, R.; MacIntyre, H.L. A dynamic model of photoadaption in phytoplankton. Limnol. Oceanogr. 1996, 41, 1–15. [Google Scholar] [CrossRef]
- Liu, L.; Fan, M.; Kang, Y. Effect of nutrient supply on cell size evolution of marine phytoplankton. Math. Biosci. Eng. 2023, 20, 4714–4740. [Google Scholar] [CrossRef] [PubMed]
- Lozano, J.; Aranguren-Gassís, M.; García-Martín, E.E.; González, J.; Herrera, J.L.; Hidalgo-Robatto, B.; Mártinez-Castrillón, D.; Pérez-Lorenzo, M.; Varela, R.A.; Serret, P. Seasonality of phytoplankton cell size and the relation between photosynthesis and respiration in the Ría de Vigo (NW Spain). Mar. Ecol. Prog. Ser. 2021, 664, 43–58. [Google Scholar] [CrossRef]
- Reich, P.B.; Tjoelker, M.G.; Machado, J.-L.; Oleksyn, J. Universal scaling of respiratory metabolism, size and nitrogen in plants. Nature 2006, 439, 457–461. [Google Scholar] [CrossRef]
- Sverdrup, H. On conditions for the vernal blooming of phytoplankton. J. Cons. Cons. Perm. Int. Pour L’exploration Mer 1953, 18, 287–295. [Google Scholar] [CrossRef]
- Sathyendranath, S.; Ji, R.B.; Browman, H.I. Revisiting Sverdrup’s critical depth hypothesis. ICES J. Mar. Sci. 2015, 72, 1892–1896. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).