Abstract
An increase in grassland rodent pests in China has seriously affected grassland ecological environments and the development of husbandry. Here, we used remote sensing data and a species–environmental matching model to predict the potential spatial distribution of the five major rodent pest species (Microtus, Citellus, Myospalax, Meriones, Ochotona) in northern China, and examined how the predicted suitability of the area depends on environmental variables. The results were consistent and significant, better than random, and close to optimal. Meriones and Microtus had the largest areas of High Suitability and Moderate Suitability with regard to environmental conditions. The combination analysis of areas of Moderate Suitability and High Suitability showed that for 66% of the total area, conditions were suitable for just one rodent species, while conditions suitable for two and three kinds of rodents accounted for 31% and 3%, respectively. Altitude, land surface temperature in winter (November, December, February) and summer (May, June, July), vegetation cover in summer (July, August), and precipitation from spring to summer (April, May, June) determined the spatial distribution of grassland rodents. Our findings provide a powerful and useful methodological tool for tracking the five major rodent pest species in northern China and for future management measures to ensure grassland ecological environment security.
1. Introduction
It is well-established that rodents are a principal vector for many infectious diseases [1,2,3], especially zoonotic diseases [1] such as spotted fever group rickettsiae [4], bubonic plague [5], tick-borne relapsing fever [6], cutaneous leishmaniasis [7], plague associated with the bacillus Yersinia pestis [8] and leptospirosis [9]. Therefore, an outbreak of rodents has the risk of transmitting infectious diseases that can threaten human health. In addition, rodents can injure humans directly through bites [10] and outbreaks can damage grassland ecosystems [8].
Recently, many studies have shown that the number and distribution of rodents is affected by various environmental factors. For example, studies have demonstrated strong correlations between landscape composition and fragmentation and the number of rodents [11]. It has been suggested that a detectable relationship between land cover, vegetation index, elevation and rodent abundance could form the basis of rodent monitoring systems [12,13,14]. On the macro scale, climatic conditions and topography have been used as predictive variables [15,16,17,18]. Specific climatic conditions that have a significant impact on the distribution of rodents [19,20] include precipitation [7,21], drought, temperature, and humidity [7]. Studies have confirmed that temperature and precipitation are also be important for the distribution of two rodent species in Mexico [22]. Due to the different living habits of rodent species, the relationship between rodent species and climatic conditions is affected by regionality and heterogeneity [23]. Plant abundance or coverage can also be significant predictors [24], and are often reflected by the vegetation index [25].
Meteorological data, land cover type and topographical variables were used to successfully identify areas containing the fat dormouse in Iran [26]. Annual precipitation, temperature, Normalized Difference Vegetation Index (NDVI) and altitude were used to quantify the impacts on a potential risk zone and the spatial pattern of the Spermophilus dauricus in China [8]. Climatic variables and NDVI values were combined with a Digital Elevation Model (DEM) to investigate environmental factors in relation to four rodents in the Nahuel Huapi National Park [16]. Elevation, bioclimatic variables, NDVI values and enhanced vegetation indices (EVI) were used to define local conditions associated with fourteen sigmodontine rodent species inhabiting the Andean Patagonian Forest region and adjacent areas [27]. Elevation, temperature, precipitation, net primary productivity and potential evapotranspiration were used as the environmental variables to analyze the congruence of spatial patterns for species richness and functional diversity of cricetid rodents in the state of Oaxaca, southern Mexico [28]. Vegetation, biogeographic units, climate, and disturbance indices were suggested as the predictive variables to identify environmental conditions associated with the occurrence and abundance of rodents in French Guiana [23]. Landscape variables (slope and soil composition) and climate variables (temperature, precipitation and climatic water deficit) were used to predict the future habitat suitability for an endangered, keystone rodent (Dipodomys ingens, giant kangaroo rat) in California, USA [29]. In summary, the above studies show that in general, climatic conditions, topography, land cover or vegetation cover conditions are the most frequently investigated environmental factors in the simulation of the potential distribution of many rodent species [26,30,31].
It is worth noting that the availability of environmental data and the development of computer technology have promoted the rapid growth of species’ environmental suitability studies and geographical distribution prediction models. Phillips first used the maximum entropy method (Maxent) to simulate the geographical distribution of species [32]. Maxent is a general machine learning algorithm, and is suitable for species distribution modeling in a variety of species [32]. Maxent is also the best model in the face of positioning error [33]. Maxent has been used in the study of the potential distribution of many species, such as plant species [34,35], primate species [23], bat species [36,37,38], ungulate species [23], wolf [39], aquatic species [40], giant kangaroo rat [41], insect species [42,43], and rodent species [7,8,10,19,20,22,23,24,26,28,30,42,44,45,46,47,48,49,50,51,52].
Remote sensing data have been widely used in the study of species distribution [53,54]. For example, remote sensing data were used to interpret and predict the number and distribution of rodents in agroecosystem habitats [25]. NDVI derived from remote sensing images was used as a predictor variable to predict the number of rodents in small-scale agricultural ecosystems [55]. Remote sensing data, such as NDVI, Leaf Area Index (LAI), the fraction of photosynthetically-active radiation absorbed by vegetation, net photosynthesis, gross primary productivity and land surface temperature were used as breeding season indicators to examine the relationships between the dynamics of rodent populations and environmental conditions [48].
Rodent pests have always been the most significant type of grassland pest in China. In 2019, the area damaged by grassland rodent pests accounted for 56% of the total area of grassland vegetation damaged by disease, invasive plants and all pests. The area damaged by grassland rodent pests was 3.67 million ha in 2019, and 3.45 million ha in 2020 (1.45 million ha was seriously damaged, accounting for 42.25% of the total damage area). The five main grassland rodent species in northern China are from the Microtus, Citellus, Myospalax, Meriones, and Ochotona genera. Grassland rodent pests cause grassland degradation and desertification, further exacerbating the deterioration of grassland environments. However, current rodent science cannot predict the large-scale spatial distribution of the main rodent species in the grasslands of northern China. This is partly due to the insufficient application of remote sensing data coupled with the lack of observed rodent distribution data. The inability to predict the potential distribution of grassland rodents affects the ability to control the damage they cause.
In this study, the Maxent algorithm and remote sensing big data were used to develop species–environmental matching models. These were used to identify the potential distribution of the major five rodents in grassland of northern China and to identify the most relevant environmental factors that can predict the distribution of the five major grassland rodents. Our ultimate objective is to provide early warning of rodent damage to grasslands, and hence guide future management and decision-making.
2. Materials and Methods
2.1. Study Area
The study area covers four provinces of northern China, i.e., Inner Mongolia, Ningxia, Gansu and Xinjiang (Figure 1). These four provinces are located in the north and west of China and account for 40% of China’s grassland. The grassland in Inner Mongolia accounts for 20.1%, Xinjiang’s grassland accounts for 14.6%, Gansu’s grassland accounts for 4.6% and Ningxia’s grassland accounts for 0.8%. Climate types vary within the study area and are mainly of the temperate continental climate and alpine climate types, while only a few areas in the southeast have temperate monsoon climates, characterized by aridity with sparse precipitation and a decreasing precipitation trend from east to west. The main geomorphological types are Loess Plateau, Gobi Desert, and Desert. Vegetation types mainly include desert vegetation, Stipa grass, Leymus chinensis grass and weedy grass. The soil types mainly include meadow soils, Chernozems, Castanozems, cultivated loessial soils, dark loessial soils, sierozems, aeolian sandy soils, brown desert soils, brown earths and gray desert soils.
Figure 1.
Study area: four provinces in northern China. 1—Inner Mongolia. 2—Ningxia. 3—Gansu. 4—Xinjiang. The four provinces include most of the grassland in northern China.
In our study area, the total area damaged by grassland rodent pests accounts for 61.5% of all such damaged grasslands in China. In Xinjiang province, 17.8% of the grassland has been damaged by rodent pests, 13.8% in Inner Mongolia, 12.1% in Gansu province, and 1% in Ningxia province. Grassland rodent pests have seriously limited agricultural production and endangered residents’ health in the four provinces. In 2020, the average mouse hole density for Myospalax in the area exceeded 300/ha, and in some areas, it exceeded 1000/ha. The highest mouse hole density exceeded 2500/ha in Hezuo City in Gansu, which caused serious damage to grassland. The average effective mouse hole density for Meriones in their main occurrence area can reach 200–300/ha. The average mouse hole density for Ochotona in their occurrence area exceeded 200/ha, and in some areas, it exceeded 600/ha with the highest mouse hole density exceeding 2000/ha. The average effective mouse hole density for Microtus in their main occurrence area reached 1000/ha. In order to prevent and control grassland rodent pests, grassland pest control stations are located at county level and are supervised by the National Forestry and Grassland Administration of China’s Central Government. This organization is also responsible for monitoring and reporting the area of grassland damaged by rodent pests.
2.2. Research Framework
This study was performed in three steps (Figure S1). First, the habitat suitability factors were obtained from the meteorological and remote sensing indicators based on the analysis of the main factors affecting the occurrence of rodents, such as topography (altitude, slope), meteorology (land surface temperature, precipitation), vegetation (vegetation type, vegetation index), soil (soil type, soil texture). Then, habitat suitability factors and observational occurrence data were input into the machine learning model (Maxent) to model, validate and extract the areas suitable for rodents at the class level. Finally, the ROC curve, percent variable contribution and response curve were analyzed to identify the impacts of habitat suitability factors on rodents’ occurrence. The key technologies and methods used in our study are described below.
2.3. Rodent Species Occurrence Data
Rodent species occurrence records for 2020 were obtained from the Forest and Grassland Pest Control Station of the National Forestry and Grassland Administration of China (http://www.forestry.gov.cn/, accessed on 21 June 2021). Occurrence records for the five main rodent species (Microtus, Citellus, Myospalax, Meriones, Ochotona) consist of the occurrence area size and location, including four levels of location information: province, city, county and township (Figure S2).
More precise geographic grassland data were obtained from GlobCover (Global Land Cover Map) 2009, which were downloaded from EAS (http://due.esrin.esa.int/globcover/, accessed on 10 October 2020). The original GlobCover data originated from the ENVISAT satellite and were obtained using the MERIS (medium-resolution imaging spectrometer) remote sensor. High-quality images received from 1 January to 31 December of 2009 were selected for image synthesis. To reduce spatial autocorrelations, grassland data were spatially filtered with a radius of 5 km using SDMtoolbox 2.0 (Version 2.4, created by Jason L. Brown, Durham, NC, USA. Python-based GIS toolkit for species distribution model analyses), and the rodent occurrence sites was randomly assigned to the grassland locations in the observed rodent occurrence areas [56].
2.4. Environmental Factors from Remote-Sensing Big Data
We used four types of environmental variables to analyze habitat suitability including topography, meteorology, vegetation and soil (Table S1). In total, 26 environmental variables (some variables included the status of multiple months) were extracted.
Topographic data, including altitude and slope with a spatial resolution of 90 m, were obtained from China’s Geospatial Data Cloud (http://www.gscloud.cn/, accessed on 5 November 2020).
Meteorological data, including land surface temperature with a spatial resolution of 1 km and precipitation with a spatial resolution of 5 km, were obtained from Google Earth Engine (https://earthengine.google.com/, accessed on 20 June 2021). The land surface temperature data used “MOD11A2.006 Terra Land Surface Temperature and Emissivity 8-Day Global 1 km” products (https://lpdaac.usgs.gov/products/mod11a2v006/, accessed on 20 June 2021). Monthly land surface temperature from the period November 2019 to July 2020 were extracted, which covered the overwintering, growth and breeding period of the target rodent species. The precipitation data used “CHIRPS Daily: Climate Hazards Group InfraRed Precipitation with Station Data (Version 2.0 Final)” products (https://chc.ucsb.edu/data/chirps, accessed on 20 June 2021). Data from the period from April 2020 to July 2020 were extracted, which covered the growth and breeding period of the five rodent species.
Vegetation data, including vegetation type and the Normalized Difference Vegetation Index (NDVI) with a spatial resolution of 1 km, were obtained from China’s Resource and Environment Science and Data Center (https://www.resdc.cn/, accessed on 11 July 2019) and Google Earth Engine (https://earthengine.google.com/, accessed on 20 June 2021), respectively. The NDVI data used “MOD13A2.006 Terra Vegetation Indices 16-Day Global 1 km” products (https://lpdaac.usgs.gov/products/mod13a2v006/, accessed on 20 June 2021). Monthly NDVI data from the periods August to October 2019 and April to July 2020 were extracted, which covered the food storage, growth and breeding period of the study rodent species.
Soil factors were considered, including soil type, 0–5 cm sand content and 0–5 cm clay content with a spatial resolution of 1 km. These showed no significant differences between months. The soil data was downloaded from China’s Resource and Environment Science and Data Center (https://www.resdc.cn/, accessed on 1 April 2019).
All environmental variable data used in this study were either at 1 km resolution or were resampled to 1 km resolution using the nearest-neighbor method. To avoid strong collinearity between variables, we retained the variables with Pearson correlation coefficients of less than 0.9. A batch processing code based on Python in ArcGIS was used to pre-process to a unified data coordinate system, spatial resolution and data analysis range, so as to facilitate the subsequent modeling analysis.
2.5. Maxent Modeling and Validation
Maximum entropy species distribution modeling (Maxent) has proven to be effective for predictively modeling species’ niches and distributions, using small or large sample sizes [36,54]. The Maxent model only requires the georeferenced species’ occurrence records and uses a set of environmental (e.g., climatic) grids in the study area. The model is nonparametric, allows for nonlinearities, and can automatically calculate interactions among predictor variables [54]. The output of the model is probabilistic, and the probability of presence can be interpreted in terms of relative potential spatial distribution (0 being the lowest and 1 the highest) [36].
Maxent version 3.4.1 (downloaded from the American Museum of Natural History, https://biodiversityinformatics.amnh.org/open_source/maxent/, accessed on 1 September 2020) was used to model the potential spatial distribution of suitable environmental conditions for the five major rodent species in northern China and study the relevant environmental factors. The Maxent model formula in our analysis was as follows [54]:
where represents each input environmental variable, is the grassland location of the five major rodent species, is the characteristic function, is the weight of the characteristic function, represents the number of datasets, and is the output of the potential spatial distribution for the five major rodent species in northern China.
The ‘subsample’ routine was used for replicate runs of the Maxent model with 50 replicate runs. Seventy percent of the randomly selected rodent species occurrence data were used for model training and 30% for model testing in each run. The convergence threshold was set to 0.5 in each run. The training process ended once the log loss per iteration dropped below the threshold. Predictions from 50 replicate Maxent model runs were averaged to produce the final maps of the potential spatial distribution of the five major rodent species in northern China.
The area under the receiver operating characteristic (ROC) curve (AUC) was used to evaluate the accuracy of the model. In the ROC curves, sensitivity is known as the true positive rate and represents the absence of omission error, while the quantity 1 − specificity is known as the false positive rate and represents the commission error [32,57,58]. The value range of AUC varies from 0.5 to 1 (with 0.5 indicating a random model and close to 1 representing high discrimination) [32,57,58]. The true skill statistic (TSS) was also used to validate the performance of the models. The formula of TSS is ‘sensitivity + specificity – 1’ and the values vary between −1 and 1; a value closer to 1 indicates that the model is better [59,60]. Meanwhile, the ‘Percent variable contributions’ and ‘Jackknife estimation’ routines were selected to evaluate the relative importance of environmental variables to the habitat suitability for the five major rodent species. The ‘Response Curve’ routine was also used to examine the relationships between different environmental predictor variables and the probability of the presence of the five major rodent species [57,58].
3. Results
3.1. The Potential Suitable Distribution of Five Major Rodents in Northern China
Figure S3 shows the ROC curve of all five Maxent models. The final Maxent models for the five rodent species produced highly predictive results with average test AUC values of 0.96 (Microtus), 0.93 (Citellus), 0.96 (Myospalax), 0.92 (Meriones) and 0.99 (Ochotona) [30]. The value of the TSS was 0.86 (Microtus), 0.88 (Citellus), 0.85 (Myospalax), 0.77 (Meriones) and 0.93 (Ochotona) (Table 1). The TSS showed more variation between the five rodent species than the AUC. All the TSS values were larger than 0.7, which indicated that the performance of the five models was fair [60].

Table 1.
The AUC and TSS of all Maxent models for five rodents.
To quantify the potential spatial distribution of Microtus, Citellus, Myospalax, Meriones and Ochotona, the degree of environmental suitability was classified at four levels: High Suitability (HS) (predicted probability > 0.75), Moderate Suitability (MS) (predicted probability ranged from 0.5 to 0.75), Low Suitability (LS) (predicted probability ranged from 0.25 to 0.5), and Unsuitable (US) (predicted probability ranged from <0.25) [3,8]. The prediction results for all five rodent species are shown in Figure 2. The Microtus Maxent model predicted MS and HS environmental conditions for Microtus in parts of the northeastern, central and southwestern grasslands of northern China. For Citellus, MS and HS environmental conditions were predicted in the southeast, for Myospalax in the northeast and southwest, for Meriones in the central grasslands, and for Ochotona in the southwestern grasslands of northern China.
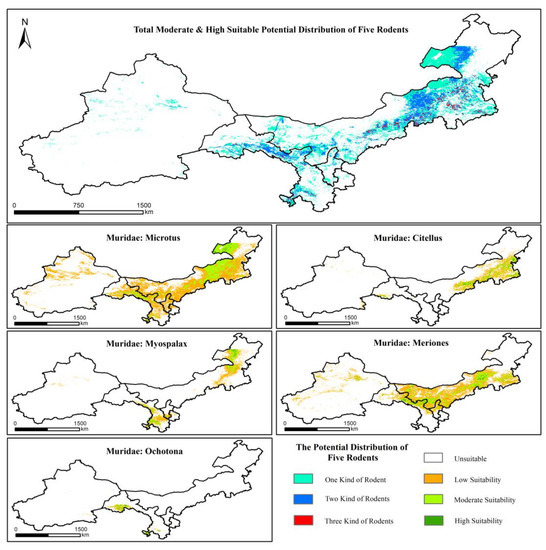
Figure 2.
The potential spatial distributions of suitable environmental conditions for five grassland pest rodent species based on the Maxent modeling.
The proportions of the four suitability categories for the five rodent species Maxent models are shown in Table 2. The area of HS conditions for Meriones was 29,370 km2 (0.71% of study area), for Citellus it was 21,707 km2 (0.53% of study area), for Microtus, 13,216 km2 (0.32% of study area), for Myospalax, 12,568 km2 (0.31% of study area), and for Ochotona, 8112 km2 (0.2% of study area). The area of MS conditions for Microtus was 386,754 km2 (9.39% of study area), for Meriones, 278,004 km2 (6.75% of study area), for Citellus, 111,910 km2 (2.72% of study area), for Myospalax, 96,585 km2 (2.35% of study area), and for Ochotona, 20,616 km2 (0.5% of study area). In summary, Meriones and Microtus had the largest area of MS and HS environments. Myospalax and Citellus had the second largest area of MS and HS environments and Ochotona had the smallest area.

Table 2.
The proportions of four environmental suitability categories based on Maxent modeling for five rodent species.
We also conducted a combination analysis of the total MS and HS potential spatial distributions for all five rodent species in order to identify environmental conditions that were suitable for multiple rodent species. There were, at most, three rodent species with suitable conditions at the same time and in the same area. Quantitatively, environmental suitability for just one species of rodent accounted for 66% of the total MS and HS potential environment distribution, while suitability for two and three species of rodents accounted for 31% and 3%, respectively.
3.2. Environmental Variable Importance Analysis on Habitat Suitability
Estimates of the relative importance and relative contributions of each of the environmental variables showed that average land surface temperature in November 2019, and altitude and average land surface temperature in June 2020 had the most predictive power in the Microtus Maxent model (i.e., a higher permutation importance value, see Table S2A; i.e., higher training gains and test AUC values, see Figure S4A). Average land surface temperature in February 2020, soil type, average land surface temperature in December 2019 and altitude also highly influenced the final Microtus Maxent model, with contributions of 33.2%, 15.5%, 11% and 10%, respectively.
The average land surface temperature in July 2020, average land surface temperature in February 2020 and altitude had the most predictive power in the Citellus Maxent model (see Table S2B; Figure S4B). The average land surface temperature in December 2019, vegetation type, soil type and average precipitation in May 2020 also highly influenced the final Citellus Maxent model, with contributions of 20.9%, 18.1%, 17.6% and 14.2%, respectively.
The altitude had the most predictive power in the Myospalax Maxent model (see Table S2C; Figure S4C). Altitude, average NDVI in August 2019 and vegetation type also highly influenced the final Myospalax Maxent model, with contributions of 23.8%, 12.8% and 10.7%, respectively.
The average land surface temperature in July 2020, average land surface temperature in May 2020 and average NDVI in July 2020 had the most predictive power in the Meriones Maxent model (see Table S2D; Figure S4D). Altitude, vegetation type, average precipitation in April 2020, average land surface temperature in November 2019 and soil type also highly influenced the final Meriones Maxent model, with contributions of 18.7%, 15.9%, 14.9%, 11.2% and 10.7%, respectively.
The average land surface temperature in May 2020 and average precipitation in June 2020 had the most predictive power in the Ochotona Maxent model (see Table S2E; Figure S4E). Altitude, average precipitation in April 2020, soil type, vegetation type and average precipitation in May 2020 also highly influenced the final Ochotona Maxent model, with contributions of 32.7%, 16.1%, 13.2%, 12.7% and 11.7%, respectively.
Overall, the jackknife test showed that vegetation type and soil type were general influential predictors in Maxent modeling for the potential spatial distribution of suitable environments for all five rodent species in northern China.
3.3. Response Curves of the Top Four Environmental Variables
Individual response curves describing the relationships between environmental suitability for all five major rodents and the top predictor variables are shown in Figure S5. The top five environmental predictors with the highest independent training gain in the Microtus Maxent model were altitude, average land surface temperature in June 2020, average land surface temperature in November 2019, average land surface temperature in February 2020 and average land surface temperature in December 2019 (Figure S5A). The response curve for altitude showed a peak probability of suitable Microtus habitat at about 550 m and then had a decreasing probability. The response curve showed that the probability of suitable environments for Microtus increased to the highest value when the average land surface temperature in June 2020 was 26 °C, and when the average land surface temperature in November 2019 was −15 °C, and the average land surface temperature in February 2020 was −24.5 °C, and then declined. The response curve for the average land surface temperature in December 2019 showed a high probability of suitable Microtus conditions when the temperature was lower than −27.5 °C and then a rapidly decreasing probability.
The top five environmental predictors in the Citellus Maxent model were altitude, average land surface temperature in July 2020, average land surface temperature in February 2020, average land surface temperature in December 2019 and average precipitation in May 2020 (Figure S5B). The response curve for altitude showed a peak probability of environmental conditions suitable for Citellus at about 200 m. The probability of suitable Citellus conditions had the highest value when the average land surface temperature in July 2020 was between 22.5 °C and 28.5 °C, and when the average land surface temperature in February 2020 was −5 °C, and when the average land surface temperature in December 2019 was −19 °C and with a high value between −23 °C and −18 °C, and when the average precipitation in May 2020 was between 1 mm/day and 1.8 mm/day.
The top two environmental predictors in the Myospalax Maxent model were altitude and average NDVI in August 2019 (Figure S5C). The response curve for altitude showed two peak probabilities of suitable conditions for Myospalax presence when the altitude was about 720 m and ranged between 3310 m and 3470 m. The response curve for the average NDVI in August 2019 showed that the probability of suitable Myospalax conditions increased to the highest value when the NDVI was 0.74, and then rapidly decreased.
The top six environmental predictors in the Meriones Maxent model were average NDVI in July 2020, average land surface temperature in May 2020, average land surface temperature in July 2020, altitude, average precipitation in April 2020 and average land surface temperature in November 2019 (Figure S5D). The response curve for average NDVI in July 2020 showed that the peak probability of suitable environmental conditions for Meriones appeared between 0.37 and 0.47. The response curve for the average land surface temperature in May 2020 showed that the highest probability presented between 17 °C and 27.5 °C. The response curve for average land surface temperature in July 2020 showed a high probability of suitable Meriones conditions when the temperature was higher than 25 °C, with the highest probability at 39 °C. The response curve for altitude showed the peak probability of conditions suitable for Meriones when the altitude was about 1308 m. The response curve for average precipitation in April 2020 showed a peak probability of environmental conditions suitable for Meriones where evaporation was greater than precipitation. The response curve for the average land surface temperature in November 2019 showed a peak probability of conditions suitable for Meriones when the temperature was about 4 °C and with a high value between −6 °C and 4 °C.
The top five environmental predictors in the Ochotona Maxent model were average precipitation in June 2020, average land surface temperature in May 2020, altitude, average precipitation in April 2020 and average precipitation in May 2020 (Figure S5E). The response curve for average precipitation in June 2020 showed a peak probability of suitable environmental conditions for Ochotona at about 9 mm/day and a second-highest probability at about 1 mm/day. The response curve showed the highest probability of suitable Ochotona conditions when the average land surface temperature in May 2020 was between 2 °C and 12 °C, when the altitude was 3460 m, when the average precipitation in April 2020 was 0.1 mm/day and 1.6 mm/day, and when the average precipitation in May 2020 was 0.6 mm/day.
4. Discussion
4.1. Innovations and Caveats
Grassland rodent pests have always had the most significant impact amongst grassland vegetation diseases, pests and poisonous weeds in China and have extensively endangered agricultural and human health in northern China. However, the large-scale monitoring of the main grassland rodent pests in China and the effects of related environmental factors on their distribution have not been well studied. The vast grassland area in northern China is characterized by a temperate continental climate, temperate continental monsoon climate, and an alpine climate, with spatial differences in temperature and precipitation, and large fluctuations in altitude and vegetation coverage. This has made it difficult to conduct research on the distribution of grassland rodents. In this study, compared with traditional research on rodents’ distribution based on meteorological data [20,45,49,52], we have constructed index systems to predict distribution and environmental suitability and models for five rodent species mainly based on remote sensing data, which was innovative in integrating machine learning, remote sensing and GIS technologies. Besides, this study comprehensively considered 26 environmental variables corresponding to four categories (topography, meteorological, vegetation, and soil) based on a large number of studies, statistical results and the theoretical analysis of the rodents’ life cycle, whereas previous studies have mostly considered a single factor (such as meteorological) or a small number of factors that affect the suitability for grassland rodents [29,49,52].
We have achieved the goal of predicting rodents’ distribution and improved our understanding of the most suitable environmental conditions for rodent species by using an integrated method. Maxent modeling based on remote sensing data provided a good first approximation of the potential distribution of the five main rodents in northern China and the key environmental factors. Remote sensing data provided meaningful and significant contributions to modeling and explaining the potential spatial distributions. The main contributions of remote sensing data were the improvement in the spatial and temporal resolution, expansion of the spatial scale, and provision of directly related environmental data. The tests of the species–environmental matching models showed that the predictions derived from remote sensing data had reasonable distribution patterns, and provided confidence in the modeled potential spatial distributions of rodents. Our research also provided a methodological tool for early warning and the efficient prevention and control of damage by grassland rodents, as well as providing a basis for decision-making for future management measures to ensure national ecological environment security and the sustainable development of husbandry.
The advantage of remote sensing data over other data is that these remote sensing products can be expanded to provide higher accuracy. Furthermore, improved spatial resolution of satellite observations can help with the identification of species’ environmental requirements, and the microwave products and reduced-scale remote sensing data can be added into the model. In the future, remote sensing data will be more effective in supporting the prevention and control of grassland rodent pests, such as, by monitoring the severity of damage caused by grassland rodent pests based on biomass changes in damaged area or by narrowing the scope of control through remote sensing inversion of high probability occurrence area.
However, the species–environmental matching models may be unstable in space and time due to the following error and bias effects in the study: (i) internal error or inaccuracies related to rodent occurrence locations; (ii) misreporting or failure to report rodent occurrence; (iii) resolution of predictor environmental variables; and (iv) error or inaccuracies of predictor environmental variables derived from remote sensing inversion methods. Generally, accuracy assessment was conducted with complete species occurrence data rather than similarly limited (e.g., few or biased) test data [55]. Recent research has suggested that rodent occurrence data reported by citizens can provide reliable predictions and estimates of habitat relationships to advance efforts to predict potential rodent distributions [61].
4.2. Environmental Factors
Our results showed that 12 of the 26 environmental factors considered by the Maxent models were strongly associated with the potential spatial distribution of the five major rodents in northern China (Figure S5), thus supporting the hypothesis that environmental conditions might restrict the distribution of the five major rodents in northern China. In summary, altitude, land surface temperature in winter (November, December, February) and summer (May, June, July), vegetation cover in summer (July, August), precipitation from spring to summer (April, May, June) determine the spatial distribution of grassland rodents and affect the spatial heterogeneity of rodents’ distribution. Moreover, vegetation type and soil type are general influential predictors in the Maxent modeling of the potential spatial distribution for all five rodent species in northern China. The response curves indicated that each kind of rodent has its own unique environmental conditions that are most suitable for growth and survival, such as altitude range, surface temperature, precipitation and vegetation richness over a few months. Our study confirms that meteorological factors have an important effect on the suitability of the habitat for grassland rodents, but the influence of other environmental factors, such as topography, vegetation and soil type, also needed to be considered. The results of our modeling have been shown to outperform other studies, which mostly considered a single factor (such as meteorological) or a small number of factors that impact on the suitability of the environment for grassland rodents, and also other ecological niche modeling (ENM) models such as GARP and BIOCLIM [62].
Compared with other studies [8], this study not only constructed the index system and model for rodents’ environmental suitability, but also analyzed and compared the similarities and differences in the environmental conditions for five main rodent species in northern China. The relative importance and contributions of the environmental variables and the jackknife analysis illustrated that the greatest predictive environmental variables for each rodent in the Maxent model are different. For Microtus, temperature in early-winter and mid-summer had the most predictive power and the greatest impact on its potential spatial distribution. For Citellus, temperature in late-winter and mid-summer had the greatest impacts on its potential spatial distribution. For Myospalax, altitude had the greatest impact on its potential spatial distribution. For Meriones, temperature and the vegetation richness in mid-summer had the most predictive power, and for Ochotona, temperature and precipitation in early-summer had the greatest impacts on its potential spatial distribution.
Microtus and Citellus were more sensitive to temperature, Myospalax was more sensitive to altitude, Meriones was more sensitive to both temperature and vegetation, and Ochotona was more sensitive to temperature and precipitation. A reasonable explanation is that Microtus and Citellus are generally distributed in areas with richer vegetation, so the temperatures in the overwintering and summer breeding period have the greatest impacts on the distribution of these rodents. Meriones generally live in sandy areas, so vegetation and temperature together affect these rodents’ distribution. Ochotona are mainly distributed on the plateau with less precipitation, so their distribution is significantly affected by temperature and precipitation.
A comprehensive understanding of the environmental conditions favored by the five major rodents in northern China will be important for managing the damage caused by the rodents, because management and conservation actions, such as the institution of precautions, can be prioritized to focus on areas where the potential for the presence of rodents is highest. Moreover, clearly defining the environmental conditions will help us determine whether the management actions on rodents’ damage (e.g., the institution of universal precautions) are likely to be inefficient. Furthermore, our findings could be the basis for further research (e.g., assessing losses of grassland ecosystem services and husbandry, evaluating the risk of plague and predicting the impacts of global climate change on the grassland rodents).
5. Conclusions
This study produced a map of the potential presence of rodents with high probability, and also identified the suitable environmental factors for the five major grassland rodent pests (Microtus, Citellus, Myospalax, Meriones, Ochotona) in northern China using the Maxent and remote sensing big data. Our study shows that Meriones and Microtus had the largest areas of High Suitability and Moderate Suitability with regard to environmental conditions within the study region. Myospalax and Citellus had the second largest areas of High Suitability and Moderate Suitability but this was a relatively agglomerate area. Ochotona had the smallest area of High Suitability and Moderate Suitability. The combination analysis of the areas of Moderate Suitability and High Suitability environmental conditions showed that for 66% of the total area, the conditions were suitable for just one rodent species, while conditions suitable for two and three kinds of rodents accounted for 31% and 3%, respectively. This suggests that 97% of the rodents’ potential distribution areas in the grassland of northern China have less than two rodent species present at the same time, due to the different environments. The altitude, land surface temperature in winter (November, December, February) and summer (May, June, July), vegetation covers in summer (July, August), precipitation from spring to summer (April, May, June) mainly determine the spatial distribution of grassland rodents and affect the spatial heterogeneity of rodents’ distribution. Our study confirms that meteorological factors are significant in assessing the suitability of the environment for grassland rodents, but other environmental factors (topography, vegetation and soil) also need to be taken into consideration.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/rs14092168/s1, Figure S1: Flowchart of this study; Figure S2: The observed occurrence of the five main rodent species (Microtus, Citellus, Myospalax, Meriones, Ochotona) in 2020; Figure S3: The receiver operating characteristic (ROC) curve of all Maxent models for five rodents; Figure S4: Environmental variable contributions to (a) training gain and (b) AUC of the Maxent model for Microtus (A), Citellus (B), Myospalax (C), Meriones (D), Ochotona (E); Figure S5: The response curves of the top predictor environmental variables of Maxent modeling for Microtus (A), Citellus (B), Myospalax (C), Meriones (D), Ochotona (E); Table S1: The environmental variables influencing rodent species distribution extracted from remote sensing data; Table S2: Estimates of relative importance and contributions of the environmental variables in the Maxent model for Microtus (A), Citellus (B), Myospalax (C), Meriones (D), Ochotona (E).
Author Contributions
L.L. and Z.S. designed the methodology; L.L., C.N., K.W. and Z.S. processed and analyzed the data; Y.Z. and E.Q. provided the data; W.H. and H.Y. conducted the funding acquisition; L.L. led the writing of the manuscript; W.H. and H.Y. conducted the review and editing. All authors conceived the ideas, and contributed critically to the drafts and the interpretation of the results. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Strategic Priority Research Program of the Chinese Academy of Sciences (Grant No. XDA26010304); Future Star Talent Program of Aerospace Information Research Institute, Chinese Academy of Sciences (Grant No. 2020KTYWLZX08).
Data Availability Statement
The data are not publicly available because the data needs to be used in future work.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Khan, A.S.; Young, J.C. Hantavirus pulmonary syndrome: At the crossroads. Curr. Opin. Infect. Dis. 2001, 14, 205–209. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Guan, Y.; Tian, T.; Wu, W.; Wang, Q.; Huang, Y.; Li, G.; Wang, L. Distribution of the intermediate hosts of Echinococcus multilocularis in Shiqu County, Sichuan, China. Chin. Med. J. 2011, 124, 2834–2837. [Google Scholar] [CrossRef] [PubMed]
- Ocampo-Chavira, P.; Eaton-Gonzalez, R.; Riquelme, M. Of Mice and Fungi: Coccidioides spp. Distribution Models. J. Fungi 2020, 6, 320. [Google Scholar] [CrossRef] [PubMed]
- Lerdthusnee, K.; Nigro, J.; Monkanna, T.; Leepitakrat, W.; Leepitakrat, S.; Insuan, S.; Charoensongsermkit, W.; Khlaimanee, N.; Akkagraisee, W.; Chayapum, K.; et al. Surveys of rodent-borne disease in Thailand with a focus on scrub typhus assessment. Integr. Zool. 2008, 3, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Addink, E.A.; De Jong, S.M.; Davis, S.A.; Dubyanskiy, V.; Burdelov, L.A.; Leirs, H. The use of high-resolution remote sensing for plague surveillance in Kazakhstan. Remote Sens. Environ. 2010, 114, 674–681. [Google Scholar] [CrossRef]
- Sage, K.M.; Johnson, T.L.; Teglas, M.B.; Nieto, N.C.; Schwan, T.G. Ecological niche modeling and distribution of Ornithodoros hermsi associated with tick-borne relapsing fever in western North America. PLoS Negl. Trop. Dis. 2017, 11, e0006047. [Google Scholar] [CrossRef]
- Hamidi, K.; Mohammadi, S.; Eskandarzadeh, N. How will climate change affect the temporal and spatial distributions of a reservoir host, the Indian gerbil (Tatera indica), and the spread of zoonotic diseases that it carries? Evol. Ecol. Res. 2018, 19, 215–226. [Google Scholar]
- Tian, L. Relationship between environmental factors and the spatial distribution of Spermophilus dauricus during 2000–2015 in China. Int. J. Biometeorol. 2018, 62, 1781–1789. [Google Scholar] [CrossRef]
- Biscornet, L.; Revillion, C.; Jego, S.; Lagadec, E.; Gomard, Y.; Le Minter, G.; Rocamora, G.; Guernier-Cambert, V.; Melade, J.; Dellagi, K.; et al. Predicting the Presence of Leptospires in Rodents from Environmental Indicators Opens Up Opportunities for Environmental Monitoring of Human Leptospirosis. Remote Sens. 2021, 13, 325. [Google Scholar] [CrossRef]
- Fatima, S.H.; Zaidi, F.; Adnan, M.; Ali, A.; Jamal, Q.; Khisroon, M. Rat-bites of an epidemic proportion in Peshawar vale; a GIS based approach in risk assessment. Environ. Monit. Assess. 2018, 190, 233. [Google Scholar] [CrossRef]
- Graham, A.J.; Danson, F.M.; Pleydell, D. Remote sensing for disease transmission: Small mammal and vegetation interactions. In Proceedings of the International Goscience and Remote Sensing Symposium (IGARSS), Toulouse, France, 21–25 July 2003; pp. 4546–4548. [Google Scholar] [CrossRef]
- Goodin, D.G.; Koch, D.E.; Owen, R.D.; Chu, Y.K.; Hutchinson, J.M.S.; Jonsson, C.B. Land cover associated with hantavirus presence in Paraguay. Glob. Ecol. Biogeogr. 2006, 15, 519–527. [Google Scholar] [CrossRef]
- Herbreteau, V.; Demoraes, F.; Hugot, J.P.; Kittayapong, P.; Salem, G.; Souris, M.; Gonzalez, J.P. Perspectives on applied spatial analysis to animal health: A case of rodents in Thailand. Ann. N. Y. Acad. Sci. 2006, 1081, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Qian, Q.; Wang, Z.; Glass, G.; Song, S.; Zhang, W.; Li, X.; Yang, H.; Wang, X.; Fang, L.; et al. Using Geographic Information System-based Ecologic Niche Models to Forecast the Risk of Hantavirus Infection in Shandong Province, China. Am. J. Trop. Med. Hyg. 2011, 84, 497–503. [Google Scholar] [CrossRef] [PubMed]
- Lahoz-Monfort, J.J.; Guillera-Arroita, G.; Milner-Gulland, E.J.; Young, R.P.; Nicholson, E. Satellite imagery as a single source of predictor variables for habitat suitability modelling: How Landsat can inform the conservation of a critically endangered lemur. J. Appl. Ecol. 2010, 47, 1094–1102. [Google Scholar] [CrossRef]
- Monjeau, J.A.; Rotela, C.H.; Lamfri, M.; Marquez, J.; Scavuzzo, C.M.; Stanulescu, M.; Nabte, M.J.; Rial, E.G. Estimating habitat suitability for potential hantavirus reservoirs in north-western Patagonia using satellite imagery: Searching for the best predictive tools. Mamm. Biol. 2011, 76, 409–416. [Google Scholar] [CrossRef]
- Mohammadi, S.; Ebrahimi, E.; Shahriari Moghadam, M.; Bosso, L. Modelling current and future potential distributions of two desert jerboas under climate change in Iran. Ecol. Inform. 2019, 52, 7–13. [Google Scholar] [CrossRef]
- Valerio, F.; Ferreira, E.; Godinho, S.; Pita, R.; Mira, A.; Fernandes, N.; Santos, S.M. Predicting Microhabitat Suitability for an Endangered Small Mammal Using Sentinel-2 Data. Remote Sens. 2020, 12, 562. [Google Scholar] [CrossRef]
- Su, J.H.; Aryal, A.; Nan, Z.B.; Ji, W.H. Climate Change-Induced Range Expansion of a Subterranean Rodent: Implications for Rangeland Management in Qinghai-Tibetan Plateau. PLoS ONE 2015, 10, e0138969. [Google Scholar] [CrossRef]
- Hamidi, K.; Matin, M.M.; Kilpatrick, C.W.; Eskandarzadeh, N. Landscape and niche specialisation of two brush-tailed mice species Calomyscus elburzensis and C. hotsoni in Iran: A case of the role of ecological niche modelling in finding area(s) of contact. Ethol. Ecol. Evol. 2019, 31, 435–456. [Google Scholar] [CrossRef]
- Barros, M.I.; Brito, J.C.; Campos, J.C.; Mappes, T.; Qninba, A.; Sousa, F.V.; Boratynski, Z. The effect of rainfall on population dynamics in Sahara-Sahel rodents. Mammal Res. 2018, 63, 485–492. [Google Scholar] [CrossRef]
- Lorenzo, C.; Rioja-Paradela, T.; Carrillo-Reyes, A.; Santiz-Lopez, E.; Bolanos-Citalan, J. Projected impact of global warming on the distribution of two pocket mouse species with implications on the conservation of Heteromys nelsoni (Rodentia: Heteromyidae). Rev. Biol. Trop. 2019, 67, 1210–1219. [Google Scholar] [CrossRef]
- Clement, L.; Catzeflis, F.; Richard-Hansen, C.; Barrioz, S.; de Thoisy, B. Conservation interests of applying spatial distribution modelling to large vagile Neotropical mammals. Trop. Conserv. Sci. 2014, 7, 202–223. [Google Scholar] [CrossRef]
- Simone, I.; Provensal, C.; Polop, J. Habitat use by corn mice (Calomys musculinus) in cropfield borders of agricultural ecosystems in Argentina. Wildlife Res. 2012, 39, 112–122. [Google Scholar] [CrossRef]
- Andreo, V.; Belgiu, M.; Hoyos, D.B.; Osei, F.; Provensal, C.; Stein, A. Rodents and satellites: Predicting mice abundance and distribution with Sentinel-2 data. Ecol. Inform. 2019, 51, 157–167. [Google Scholar] [CrossRef]
- Naderi, M.; Kaboli, M.; Ahmadi, M.; Krystufek, B. Fat Dormouse (Glis glis) distribution modeling in the Hyrcanian relict forests of Northern Iran. Pol. J. Ecol. 2016, 64, 136–142. [Google Scholar] [CrossRef]
- Barlett, T.R.; Martin, G.M.; Laguna, M.F.; Abramson, G.; Monjeau, A. Climatic constraints and the distribution of Patagonian mice. J. Mammal. 2019, 100, 1979–1991. [Google Scholar] [CrossRef]
- Martin-Regalado, C.N.; Briones-Salas, M.; Lavariega, M.C.; Moreno, C.E. Spatial incongruence in the species richness and functional diversity of cricetid rodents. PLoS ONE 2019, 14, e0217154. [Google Scholar] [CrossRef]
- Widick, I.V.; Bean, W.T. Evaluating current and future range limits of an endangered, keystone rodent (Dipodomys ingens). Divers. Distrib. 2019, 25, 1074–1087. [Google Scholar] [CrossRef]
- Walsh, M.; Haseeb, M.A. Modeling the ecologic niche of plague in sylvan and domestic animal hosts to delineate sources of human exposure in the western United States. PeerJ 2015, 3, e1493. [Google Scholar] [CrossRef]
- Xu, L.; Schmid, B.V.; Liu, J.; Si, X.Y.; Stenseth, N.C.; Zhang, Z.B. The trophic responses of two different rodent-vector-plague systems to climate change. Proc. Biol. Sci. 2015, 282, 20141846. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum entropy modeling of species geographic distributions. Ecol. Model. 2006, 190, 231–259. [Google Scholar] [CrossRef]
- Graham, C.H.; Elith, J.; Hijmans, R.J.; Guisan, A.; Townsend Peterson, A.; Loiselle, B.A. The influence of spatial errors in species occurrence data used in distribution models. J. Appl. Ecol. 2008, 45, 239–247. [Google Scholar] [CrossRef]
- Rodríguez-Veiga, P.; Saatchi, S.; Tansey, K.; Balzter, H. Magnitude, spatial distribution and uncertainty of forest biomass stocks in Mexico. Remote Sens. Environ. 2016, 183, 265–281. [Google Scholar] [CrossRef]
- Dai, J.; Roberts, D.A.; Stow, D.A.; An, L.; Hall, S.J.; Yabiku, S.T.; Kyriakidis, P.C. Mapping understory invasive plant species with field and remotely sensed data in Chitwan, Nepal. Remote Sens. Environ. 2020, 250, 112037. [Google Scholar] [CrossRef]
- Flory, A.R.; Kumar, S.; Stohlgren, T.J.; Cryan, P.M. Environmental conditions associated with bat white-nose syndrome mortality in the north-eastern United States. J. Appl. Ecol. 2012, 49, 680–689. [Google Scholar] [CrossRef]
- Ancillotto, L.; Bosso, L.; Smeraldo, S.; Smeraldo, S.; Mori, E.; Mazza, G.; Herkt, M.; Galimberti, A.; Ramazzotti, F.; Russo, D. An African bat in Europe, Plecotus gaisleri: Biogeographic and ecological insights from molecular taxonomy and Species Distribution Models. Ecol. Evol. 2020, 10, 5785–5800. [Google Scholar] [CrossRef]
- Hintze, F.; Machado, R.B.; Bernard, E. Bioacoustics for in situ validation of species distribution modelling: An example with bats in Brazil. PLoS ONE 2021, 16, e0248797. [Google Scholar] [CrossRef]
- Mateo-Tomás, P.; Olea, P.P.; Sánchez-Barbudo, I.S.; Mateo, R. Alleviating human–wildlife conflicts: Identifying the causes and mapping the risk of illegal poisoning of wild fauna. J. Appl. Ecol. 2012, 49, 376–385. [Google Scholar] [CrossRef]
- Gallardo, B.; Aldridge, D.C. The ‘dirty dozen’: Socio-economic factors amplify the invasion potential of 12 high-risk aquatic invasive species in Great Britain and Ireland. J. Appl. Ecol. 2013, 50, 757–766. [Google Scholar] [CrossRef]
- Bean, W.T.; Prugh, L.R.; Stafford, R.; Butterfield, H.S.; Westphal, M.; Brashares, J.S. Species distribution models of an endangered rodent offer conflicting measures of habitat quality at multiple scales. J. Appl. Ecol. 2014, 51, 1116–1125. [Google Scholar] [CrossRef]
- Ørsted, I.V.; Ørsted, M. Species distribution models of the Spotted Wing Drosophila (Drosophila suzukii, Diptera: Drosophilidae) in its native and invasive range reveal an ecological niche shift. J. Appl. Ecol. 2019, 56, 423–435. [Google Scholar] [CrossRef]
- Alaniz, A.J.; Soares, A.O.; Vergara, P.M.; de Azevedo, E.B.; Grez, A.A. The failed invasion of Harmonia axyridis in the Azores, Portugal: Climatic restriction or wrong population origin? Insect Sci. 2021, 28, 238–250. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.P.; Raza, A. The effect of the extent of the study region on GIS models of species geographic distributions and estimates of niche evolution: Preliminary tests with montane rodents (genus Nephelomys) in Venezuela. J. Biogeogr. 2010, 37, 1378–1393. [Google Scholar] [CrossRef]
- Taylor, P.J.; Nengovhela, A.; Linden, J.; Baxter, R.M. Past, present, and future distribution of Afromontane rodents (Muridae: Otomys) reflect climate-change predicted biome changes. Mammalia 2016, 80, 359–375. [Google Scholar] [CrossRef]
- Aghová, T.; Šumbera, R.; Piálek, L.; Mikula, O.; McDonough, M.M.; Lavrenchenko, L.A.; Meheretu, Y.; Mbau, J.S.; Bryja, J. Multilocus phylogeny of East African gerbils (Rodentia, Gerbilliscus) illuminates the history of the Somali-Masai savanna. J. Biogeogr. 2017, 44, 2295–2307. [Google Scholar] [CrossRef]
- Percequillo, A.R.; Dalapicolla, J.; Abreu, E.F.; Roth, P.R.O.; Ferraz, K.; Chiquito, E.A. How many species of mammals are there in Brazil? New records of rare rodents (Rodentia: Cricetidae: Sigmodontinae) from Amazonia raise the current known diversity. PeerJ 2017, 5, e4071. [Google Scholar] [CrossRef]
- Yu, P.; Li, Y.; Xu, B.; Wei, J.; Li, S.; Dong, J.; Qu, J.; Xu, J.; Huang, Z.; Ma, C.; et al. Using Satellite Data for the Characterization of Local Animal Reservoir Populations of Hantaan Virus on the Weihe Plain, China. Remote Sens. 2017, 9, 1076. [Google Scholar] [CrossRef]
- Boria, R.A.; Blois, J.L. The effect of large sample sizes on ecological niche models: Analysis using a North American rodent, Peromyscus maniculatus. Ecol. Model. 2018, 386, 83–88. [Google Scholar] [CrossRef]
- Galante, P.J.; Alade, B.; Muscarella, R.; Jansa, S.A.; Goodman, S.M.; Anderson, R.P. The challenge of modeling niches and distributions for data-poor species: A comprehensive approach to model complexity. Ecography 2018, 41, 726–736. [Google Scholar] [CrossRef]
- Mayamba, A.; Byamungu, R.M.; Vanden Broecke, B.; Leirs, H.; Hieronimo, P.; Nakiyemba, A.; Isabirye, M.; Kifumba, D.; Kimaro, D.; Mdangi, M.; et al. Factors influencing the distribution and abundance of small rodent pest species in agricultural landscapes in Eastern Uganda. J. Vertebr Biol. 2020, 69, 20002.1-17. [Google Scholar] [CrossRef]
- Austrich, A.; Kittlein, M.J.; Mora, M.S.; Mapelli, F.J. Potential distribution models from two highly endemic species of subterranean rodents of Argentina: Which environmental variables have better performance in highly specialized species? Mamm. Biol. 2021, 101, 503–519. [Google Scholar] [CrossRef]
- Chidodo, D.J.; Kimaro, D.N.; Hieronimo, P.; Makundi, R.H.; Isabirye, M.; Leirs, H.; Massawe, A.; Mdangi, M.; Kifumba, D.; Mulungu, L. Application of normalized difference vegetation index (NDVI) to forecast rodent population abundance in smallholder agro-ecosystems in semi-arid areas in Tanzania. Mammalia 2020, 84, 136–143. [Google Scholar] [CrossRef]
- Huang, Y.; Dong, Y.; Huang, W.; Ren, B.; Deng, Q.; Shi, Y.; Bai, J.; Ren, Y.; Geng, Y.; Ma, H. Overwintering Distribution of Fall Armyworm (Spodoptera frugiperda) in Yunnan, China, and Influencing Environmental Factors. Insects 2020, 11, 805. [Google Scholar] [CrossRef] [PubMed]
- Bean, W.T.; Stafford, R.; Brashares, J.S. The effects of small sample size and sample bias on threshold selection and accuracy assessment of species distribution models. Ecography 2012, 35, 250–258. [Google Scholar] [CrossRef]
- Brown, J.L.; Bennett, J.R.; French, C.M. SDMtoolbox 2.0: The next generation Python-based GIS toolkit for landscape genetic, biogeographic and species distribution model analyses. PeerJ 2017, 5, e4095. [Google Scholar] [CrossRef]
- Phillips, S.J.; Dudík, M. Modeling of species distributions with Maxent: New extensions and a comprehensive evaluation. Ecography 2008, 31, 161–175. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Dudík, M.; Schapire, R.E.; Blair, M.E. Opening the black box: An open-source release of Maxent. Ecography 2017, 40, 887–893. [Google Scholar] [CrossRef]
- Allouche, O.; Tsoar, A.; Kadmon, R. Assessing the accuracy of species distribution models: Prevalence, kappa and the true skill statistic (TSS). J. Appl. Ecol. 2006, 43, 1223–1232. [Google Scholar] [CrossRef]
- Frederico, R.G.; De Marco, P.J.; Zuanon, J. Evaluating the use of macroscale variables as proxies for local aquatic variables and to model stream fish distributions. Freshw. Biol. 2014, 59, 2303–2314. [Google Scholar] [CrossRef]
- Tye, C.A.; McCleery, R.A.; Fletcher, R.J., Jr.; Greene, D.U.; Butryn, R.S. Evaluating citizen vs. professional data for modelling distributions of a rare squirrel. J. Appl. Ecol. 2017, 54, 628–637. [Google Scholar] [CrossRef]
- Elith, J.; Graham, C.H.; Anderson, R.P.; Dudík, M.; Ferrier, S.; Guisan, A.; Hijmans, R.J.; Huettmann, F.; Leathwick, J.R.; Lehmann, A.; et al. Novel methods improve prediction of species’ distributions from occurrence data. Ecography 2006, 29, 129–151. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).