Winter–Spring Phytoplankton Phenology Associated with the Kuroshio Extension Instability
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Region
2.2. Satellite and Reanalyzed Data
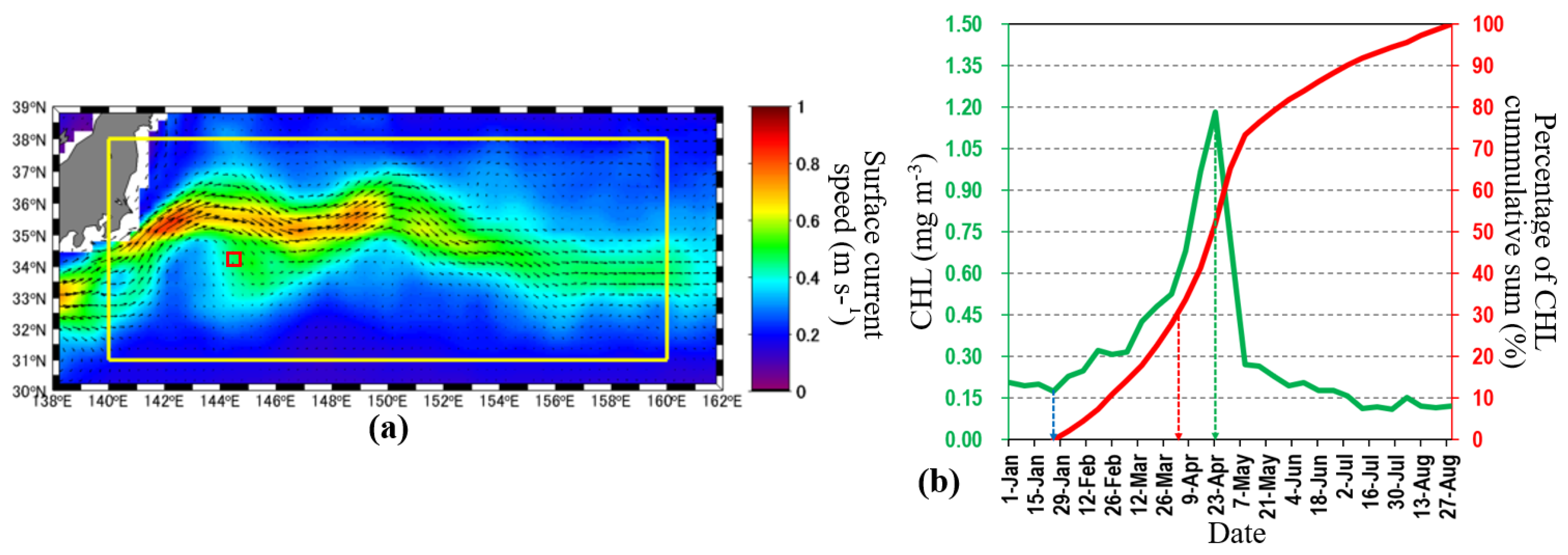
2.3. Defining the Spring Bloom Onset
3. Results
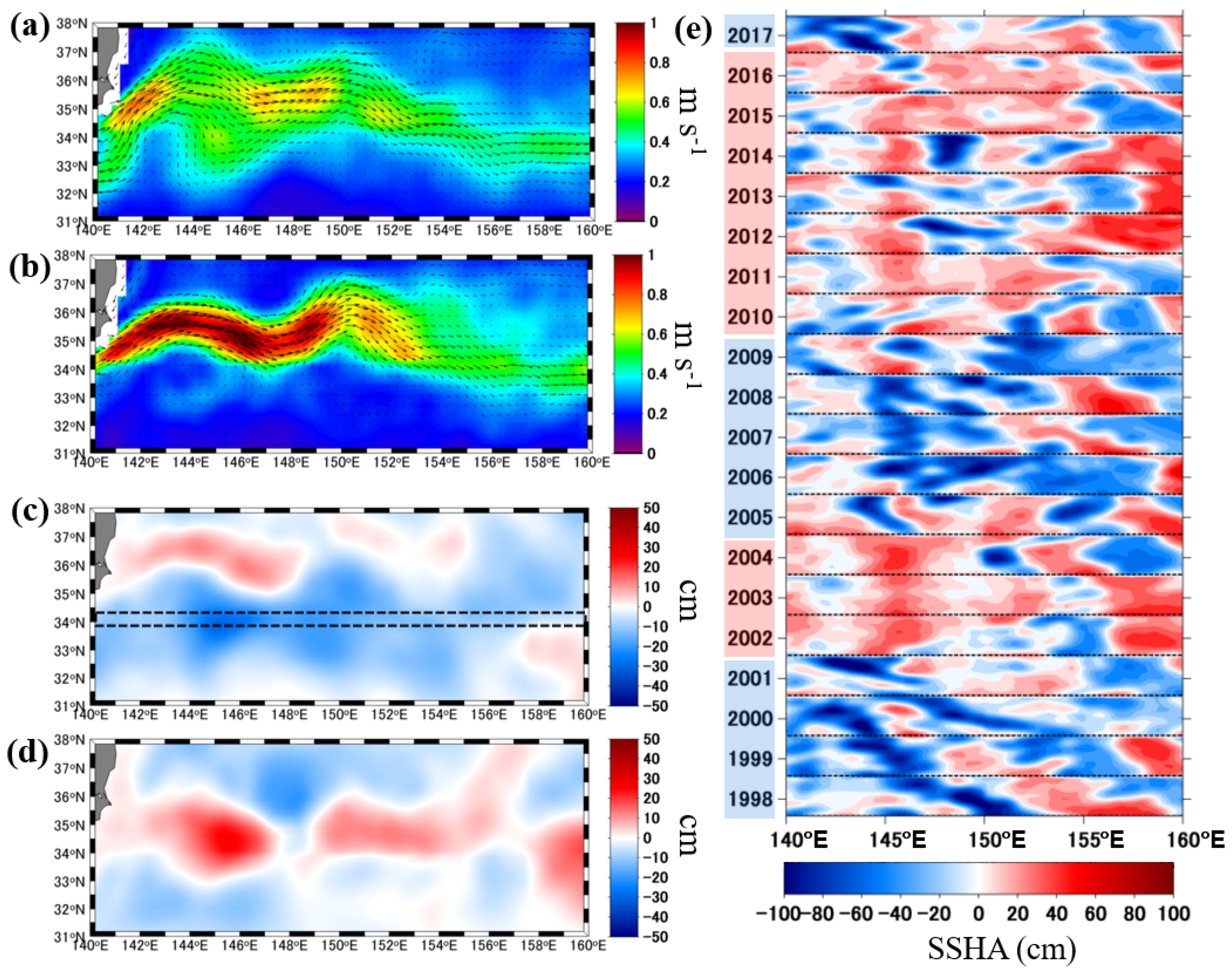
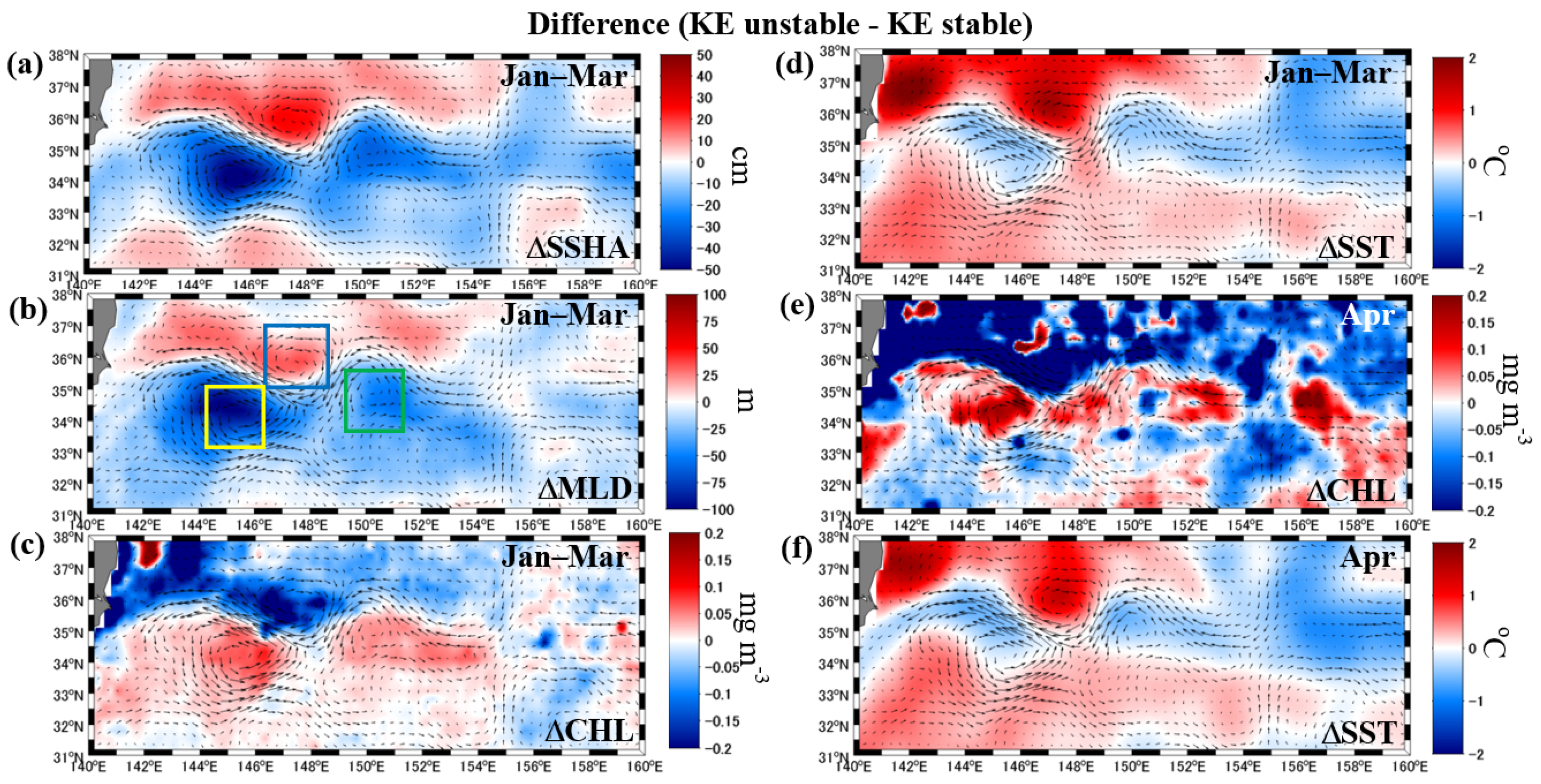
3.1. Biogeophysical Properties during the Periods of Unstable and Stable KEs
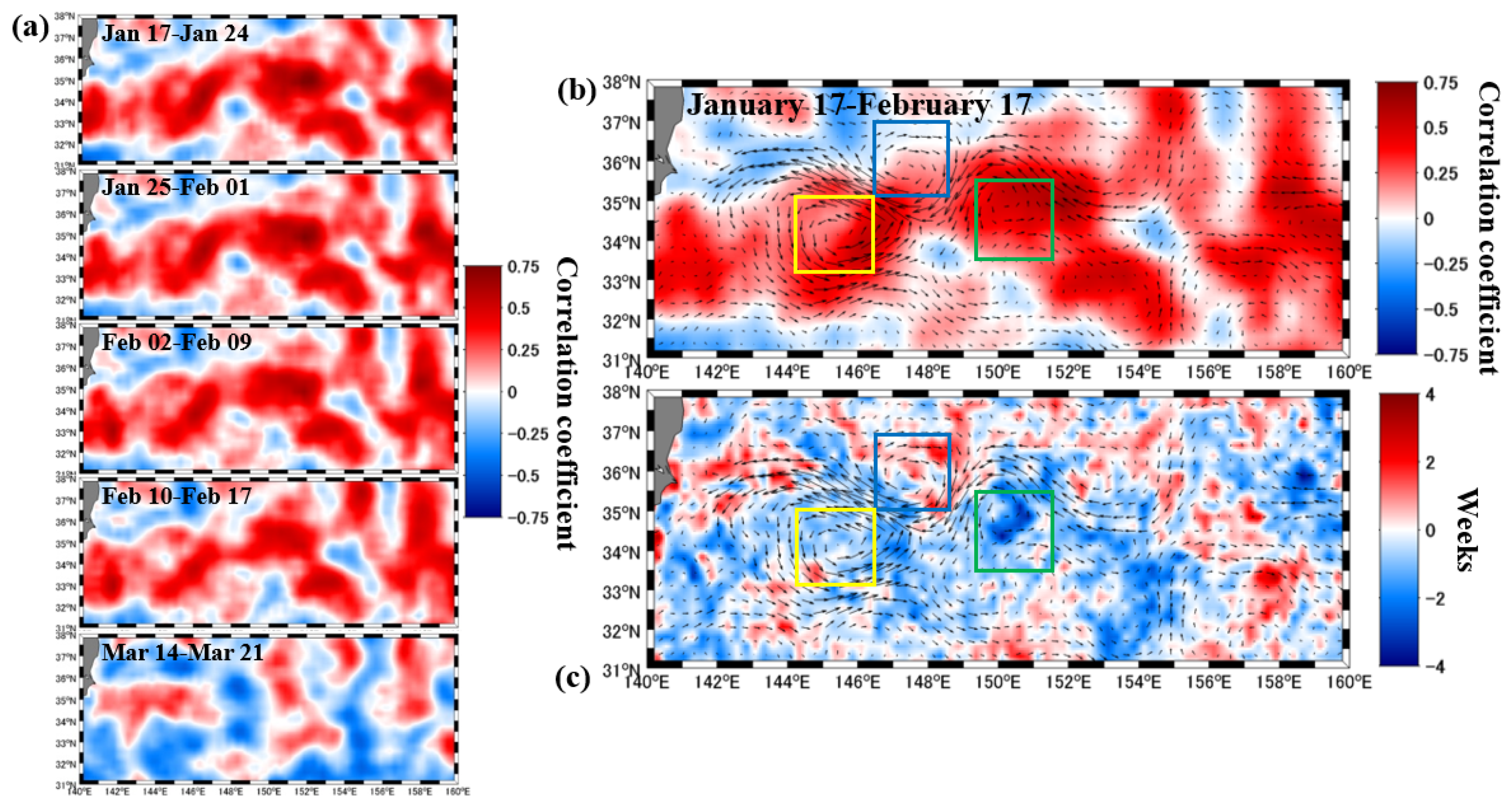
3.2. Relationship between SSHA and Phytoplankton Spring Bloom Onset
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Qiu, B. Kuroshio Extension variability and forcing of the Pacific decadal oscillations: Responses and potential feedback. J. Phys. Oceanogr. 2003, 33, 2465–2482. [Google Scholar] [CrossRef]
- Qiu, B.; Chen, S. Variability of the Kuroshio Extension jet, recirculation gyre, and mesoscale eddies on decadal time scales. J. Phys. Oceanogr. 2005, 35, 2090–2102. [Google Scholar] [CrossRef]
- Taguchi, B.; Xie, S.-P.; Schneider, N.; Nonaka, M.; Sasaki, H.; Sasai, Y. Decadal variability of the Kuroshio Extension: Observations and Eddy-resolving model hindcast. J. Clim. 2007, 20, 2357–2377. [Google Scholar] [CrossRef] [Green Version]
- Mantua, N.J.; Hare, S.R.; Zhang, Y.; Wallace, J.M.; Francis, R.C. A Pacific interdecadal climate oscillation with impacts on salmon production. Bull. Am. Meteorol. Soc. 1997, 78, 1069–1080. [Google Scholar] [CrossRef]
- Lin, P.; Ma, J.; Chai, F.; Xiu, P.; Liu, H. Decadal variability of nutrients and biomass in the southern region of Kuroshio Extension. Prog. Oceanogr. 2020, 188, 102441. [Google Scholar] [CrossRef]
- Di Lorenzo, E.; Schneider, N.; Cobb, K.; Franks, P.; Chhak, K.; Miller, A.; Mcwilliams, J.; Bograd, S.; Arango, H.; Curchitser, E.; et al. North Pacific Gyre Oscillation links ocean climate and ecosystem change. Geophys. Res. Lett. 2008, 35, L08607. [Google Scholar] [CrossRef] [Green Version]
- Oka, E.; Qiu, B.; Kouketsu, S.; Uehara, K.; Suga, T. Decadal seesaw of the Central and Subtropical Mode Water formation associated with the Kuroshio Extension variability. J. Oceanogr. 2012, 68, 355–360. [Google Scholar] [CrossRef]
- Yang, H.; Qiu, B.; Chang, P.; Wu, L.; Wang, S.; Chen, Z.; Yang, Y. Decadal variability of eddy characteristics and energetics in the Kuroshio Extension: Unstable versus stable states. J. Geophys. Res. Oceans 2018, 123, 6653–6669. [Google Scholar] [CrossRef]
- Kouketsu, S.; Kaneko, H.; Okunishi, T.; Sasaoka, K.; Itoh, S.; Inoue, R.; Ueno, H. Mesoscale eddy effects on temporal variability of surface chlorophyll a in the Kuroshio Extension. J. Oceanogr. 2016, 72, 439–451. [Google Scholar] [CrossRef]
- Lin, P.; Chai, F.; Xue, H.; Xiu, P. Modulation of decadal oscillation on surface chlorophyll in the Kuroshio Extension. J. Geophys. Res. Oceans 2014, 119, 187–199. [Google Scholar] [CrossRef]
- Nishikawa, H.; Yasuda, I.; Komatsu, K.; Sasaki, H.; Sasai, Y.; Setou, T.; Shimizu, M. Winter mixed layer depth and spring bloom along the Kuroshio front: Implications for the Japanese sardine stock. Mar. Ecol. Prog. Ser. 2013, 487, 217–229. [Google Scholar] [CrossRef] [Green Version]
- Kodama, T.; Wagawa, T.; Ohshimo, S.; Morimoto, H.; Iguchi, N.; Fukudome, K.; Goto, T.; Takahashi, M.; Yasuda, T. Improvement in recruitment of Japanese sardine with delays of the spring phytoplankton bloom in the Sea of Japan. Fish. Oceanogr. 2018, 27, 289–301. [Google Scholar] [CrossRef]
- Nishikawa, H.; Yasuda, I. Japanese sardine (Sardinops melanostictus) mortality in relation to the winter mixed layer depth in the Kuroshio Extension region. Fish. Oceanogr. 2008, 17, 411–420. [Google Scholar] [CrossRef]
- Siswanto, E.; Matsumoto, K.; Honda, M.C.; Fujiki, T.; Sasaoka, K.; Saino, T. Reappraisal of meridional differences of factors controlling phytoplankton biomass and initial increase preceding seasonal bloom in the northwestern Pacific Ocean. Remote Sens. Environ. 2015, 159, 44–56. [Google Scholar] [CrossRef] [Green Version]
- Matsumoto, K.; Sasai, Y.; Sasaoka, K.; Siswanto, E.; Honda, M.C. The formation of subtropical phytoplankton blooms is dictated by water column stability during winter and spring in the oligotrophic Northwestern North Pacific. J. Geophys. Res. Oceans 2021, 126, e2020JC016864. [Google Scholar] [CrossRef]
- Mahadevan, A.; D’Asaro, E.; Lee, C.; Perry, M.J. Eddy-driven stratification initiates North Atlantic spring phytoplankton blooms. Science 2016, 337, 54–58. [Google Scholar] [CrossRef]
- Maúre, E.R.; Ishizaka, J.; Sukigara, C.; Mino, Y.; Aiki, H.; Matsuno, T.; Tomita, H.; Goes, J.I.; Gomes, H.R. Mesoscale eddies control the timing of spring phytoplankton blooms: A case study in the Japan Sea. J. Geophys. Res. Oceans 2017, 44, 11115–11124. [Google Scholar] [CrossRef]
- Matsumoto, K.; Honda, M.C.; Sasaoka, K.; Wakita, M.; Kawakami, H.; Watanabe, S. Seasonal variability of primary production and phytoplankton biomass in the western Pacific subarctic gyre: Control by light availability within the mixed layer. J. Geophys. Res. Oceans 2014, 119, 6523–6534. [Google Scholar] [CrossRef]
- Alvera-Azcárate, A.; Barth, A.; Rixen, M.; Beckers, J.-M. Reconstruction of incomplete oceanographic data sets using Empirical Orthogonal Functions. Application to the Adriatic Sea. Ocean Model. 2005, 9, 325–346. [Google Scholar] [CrossRef] [Green Version]
- Shiozaki, T.; Ito, S.I.; Takahashi, K.; Saito, H.; Nagata, T.; Furuya, K. Regional variability of factors controlling the onset timing and magnitude of spring algal blooms in the northwestern North Pacific. J. Geophys. Res. Oceans 2014, 119, 253–265. [Google Scholar] [CrossRef]
- Brody, S.R.; Lozier, M.S.; Dunne, J.P. A comparison of methods to determine phytoplankton bloom initiation. J. Geophys. Res. Oceans 2013, 118, 2345–2357. [Google Scholar] [CrossRef]
- Chiba, S.; Batten, S.; Sasaoka, K.; Sasai, Y.; Sugisaki, H. Influence of the Pacific Decadal Oscillation on phytoplankton phenology and community structure in the western North Pacific. Geophys. Res. Lett. 2012, 39, L15603. [Google Scholar] [CrossRef]
- Shi, J.; Liu, Y.; Mao, X.; Guo, X.; Wei, H.; Gao, H. Interannual variation of spring phytoplankton bloom and response to turbulent energy generated by atmospheric forcing in the central Southern Yellow Sea of China: Satellite observations and numerical model study. Cont. Shelf Res. 2017, 143, 257–270. [Google Scholar] [CrossRef]
- Goes, J.I.; Gomes, H.R.; Limsakul, A.; Saino, T. The influence of large-scale environmental changes on carbon export in the North Pacific Ocean using satellite and shipboard data. Deep-Sea Res. II 2004, 51, 247–279. [Google Scholar] [CrossRef]
- Siswanto, E.; Honda, M.C.; Matsumoto, K.; Sasai, Y.; Fujiki, T.; Sasaoka, K.; Saino, T. Sixteen-year phytoplankton biomass trends in the northwestern Pacific Ocean by the SeaWiFS and MODIS ocean color sensors. J. Oceanogr. 2016, 71, 479–489. [Google Scholar] [CrossRef]
- Honda, M.C.; Sasai, Y.; Siswanto, E.; Kuwano-Yoshida, A.; Aiki, H.; Cronin, M.F. Impact of cyclonic eddies and typhoons on biogeochemistry in the oligotrophic ocean based on biogeochemical/physical/meteorological time-series at station KEO. Prog. Earth Planet. Sci. 2018, 5, 42. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siswanto, E.; Sasai, Y.; Matsumoto, K.; Honda, M.C. Winter–Spring Phytoplankton Phenology Associated with the Kuroshio Extension Instability. Remote Sens. 2022, 14, 1186. https://doi.org/10.3390/rs14051186
Siswanto E, Sasai Y, Matsumoto K, Honda MC. Winter–Spring Phytoplankton Phenology Associated with the Kuroshio Extension Instability. Remote Sensing. 2022; 14(5):1186. https://doi.org/10.3390/rs14051186
Chicago/Turabian StyleSiswanto, Eko, Yoshikazu Sasai, Kazuhiko Matsumoto, and Makio C. Honda. 2022. "Winter–Spring Phytoplankton Phenology Associated with the Kuroshio Extension Instability" Remote Sensing 14, no. 5: 1186. https://doi.org/10.3390/rs14051186
APA StyleSiswanto, E., Sasai, Y., Matsumoto, K., & Honda, M. C. (2022). Winter–Spring Phytoplankton Phenology Associated with the Kuroshio Extension Instability. Remote Sensing, 14(5), 1186. https://doi.org/10.3390/rs14051186





