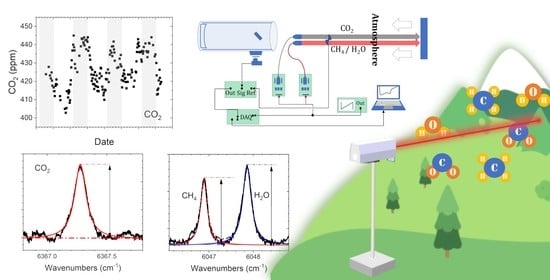
Autonomous Differential Absorption Laser Device for Remote Sensing of Atmospheric Greenhouse Gases
Abstract
:1. Introduction
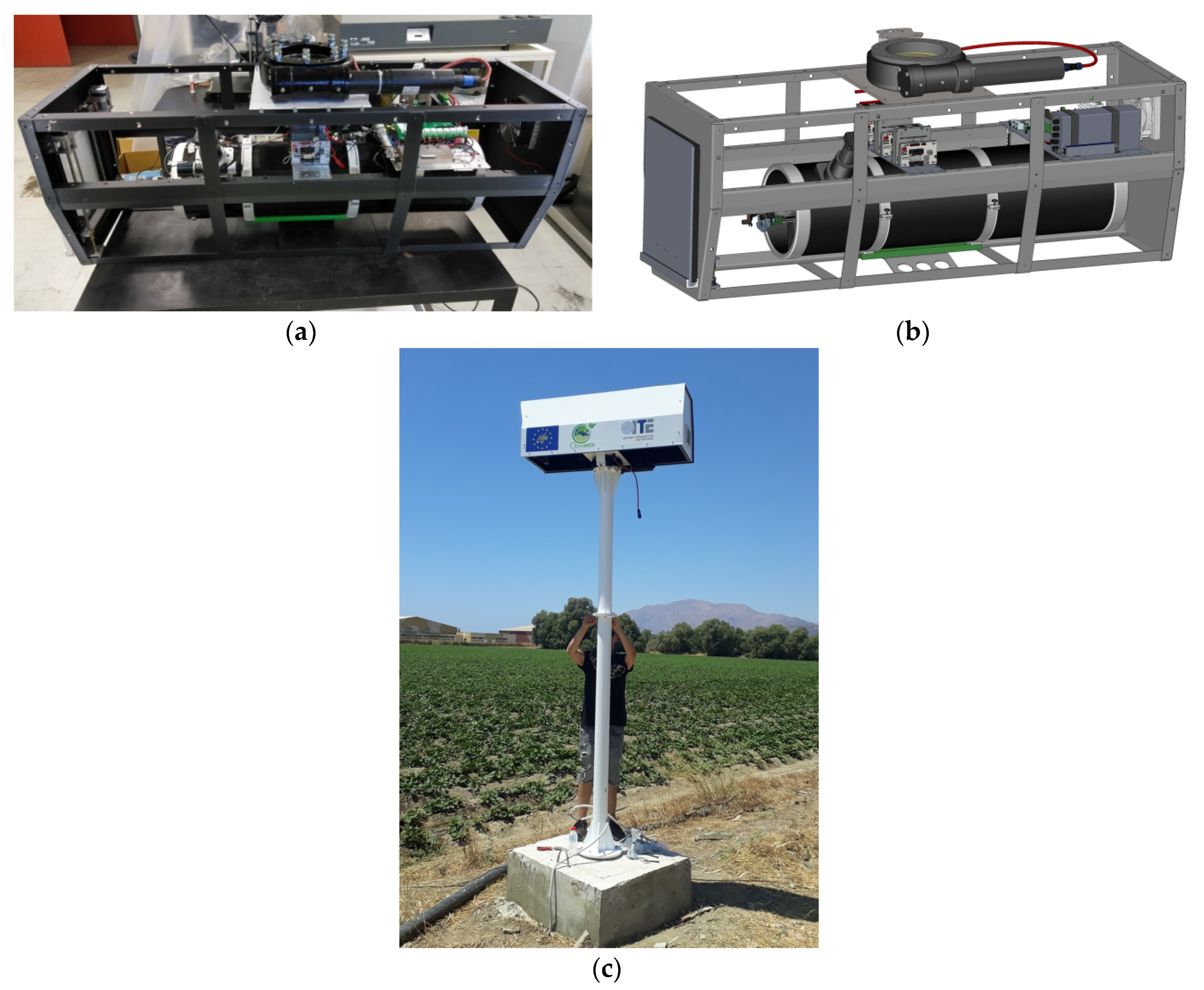
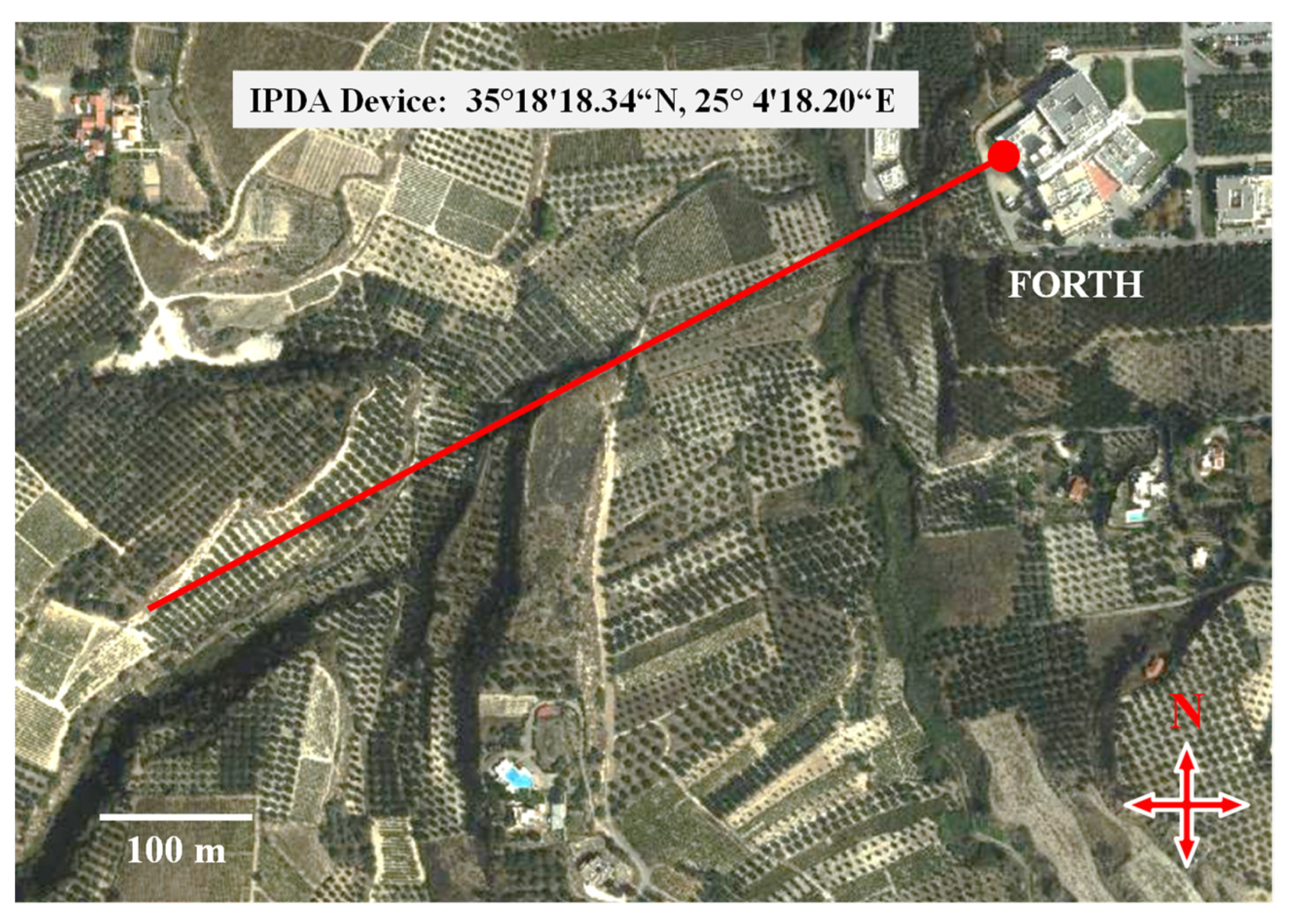
2. Materials and Methods
3. Results and Discussion
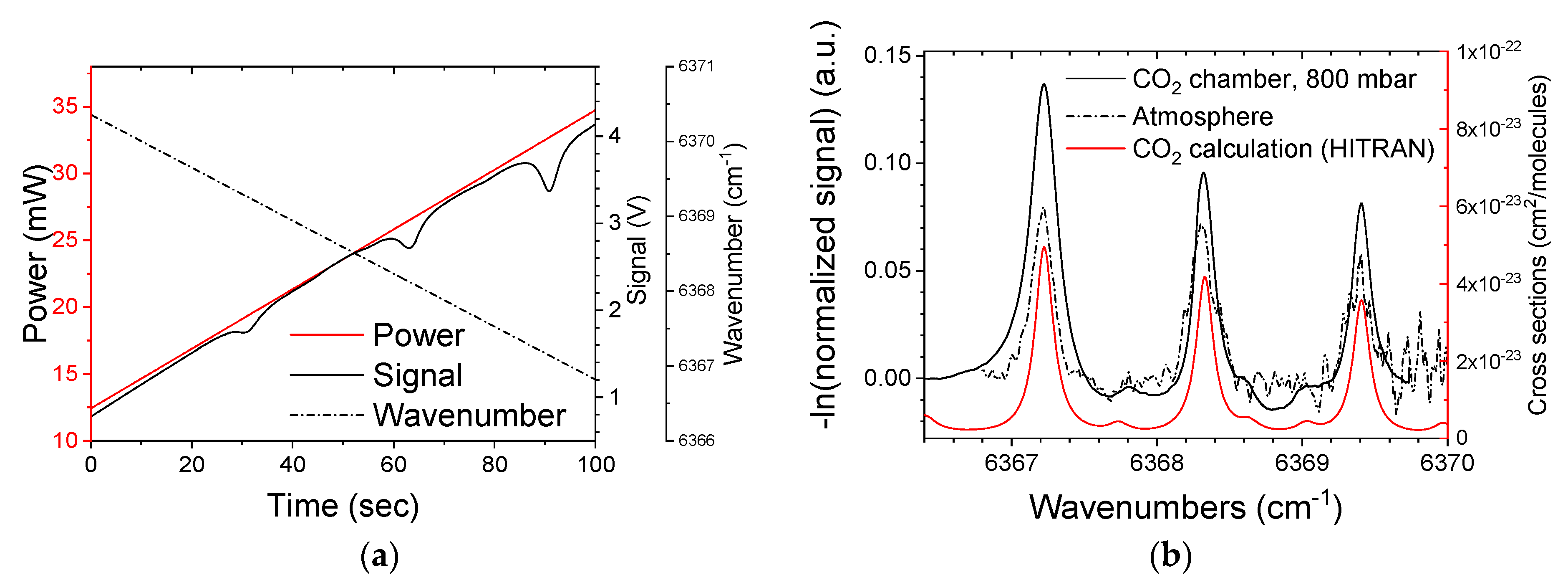
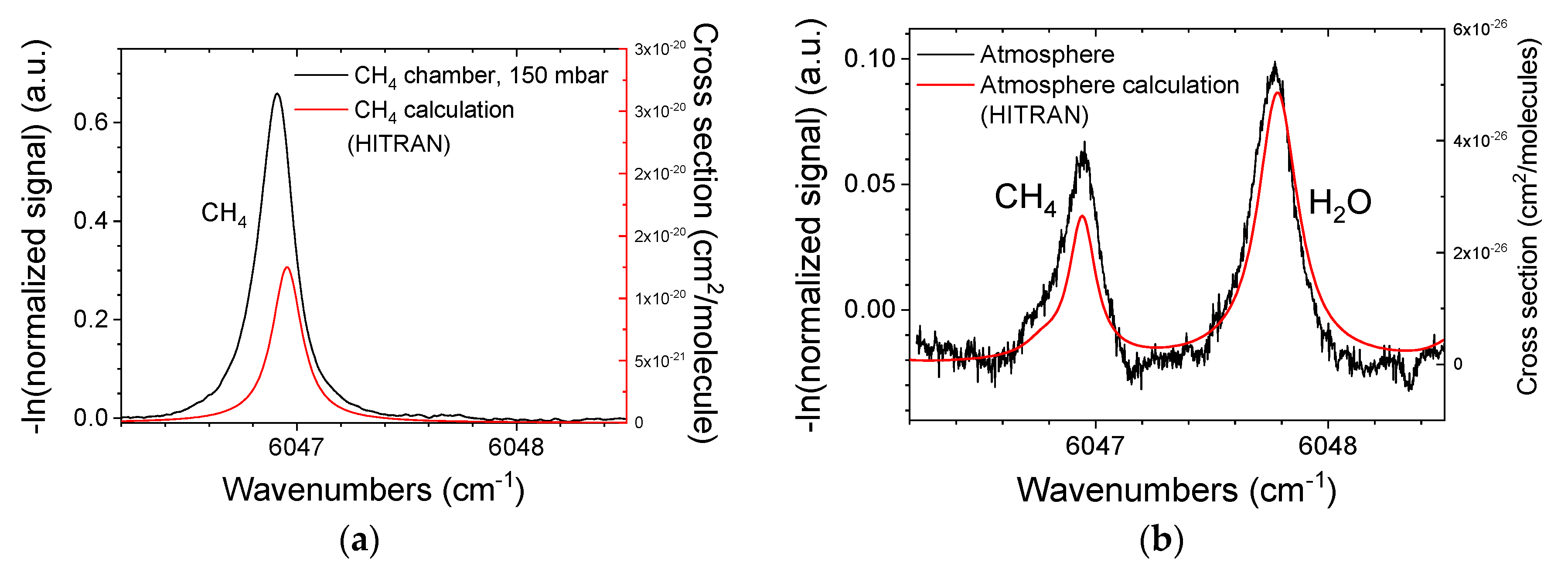
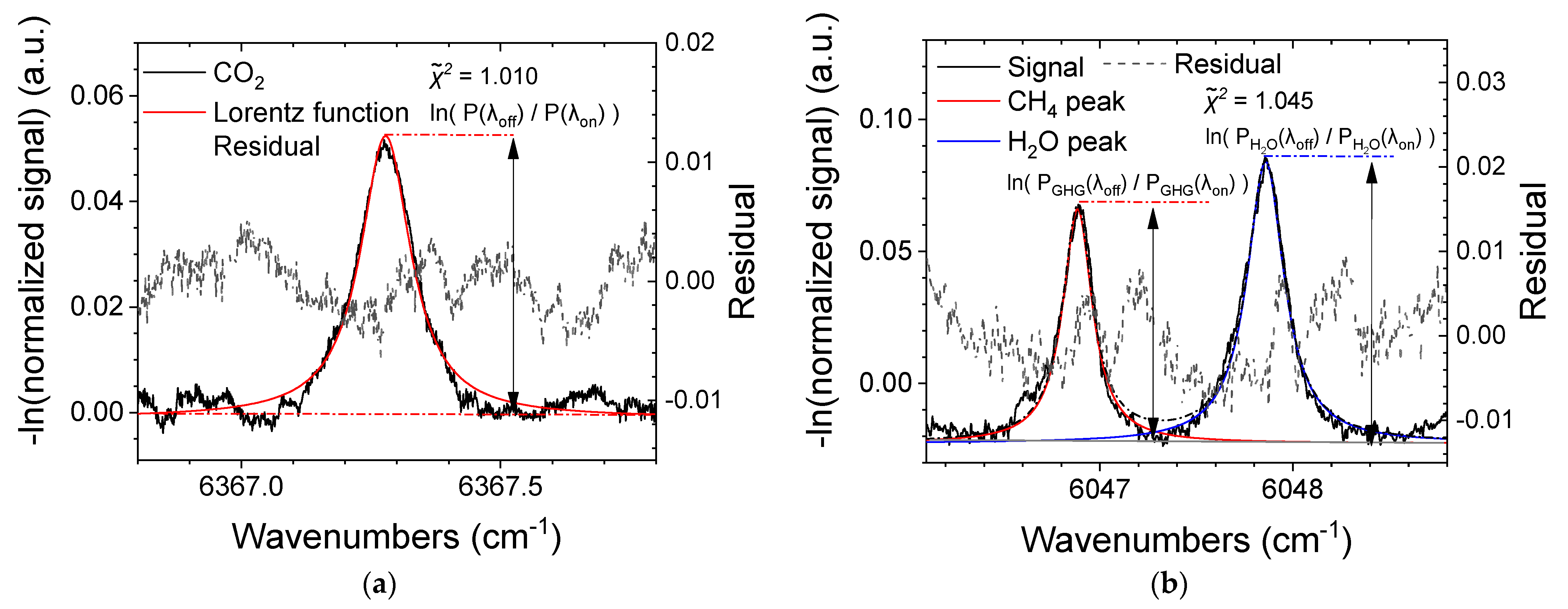
3.1. Gas Chamber Measurements
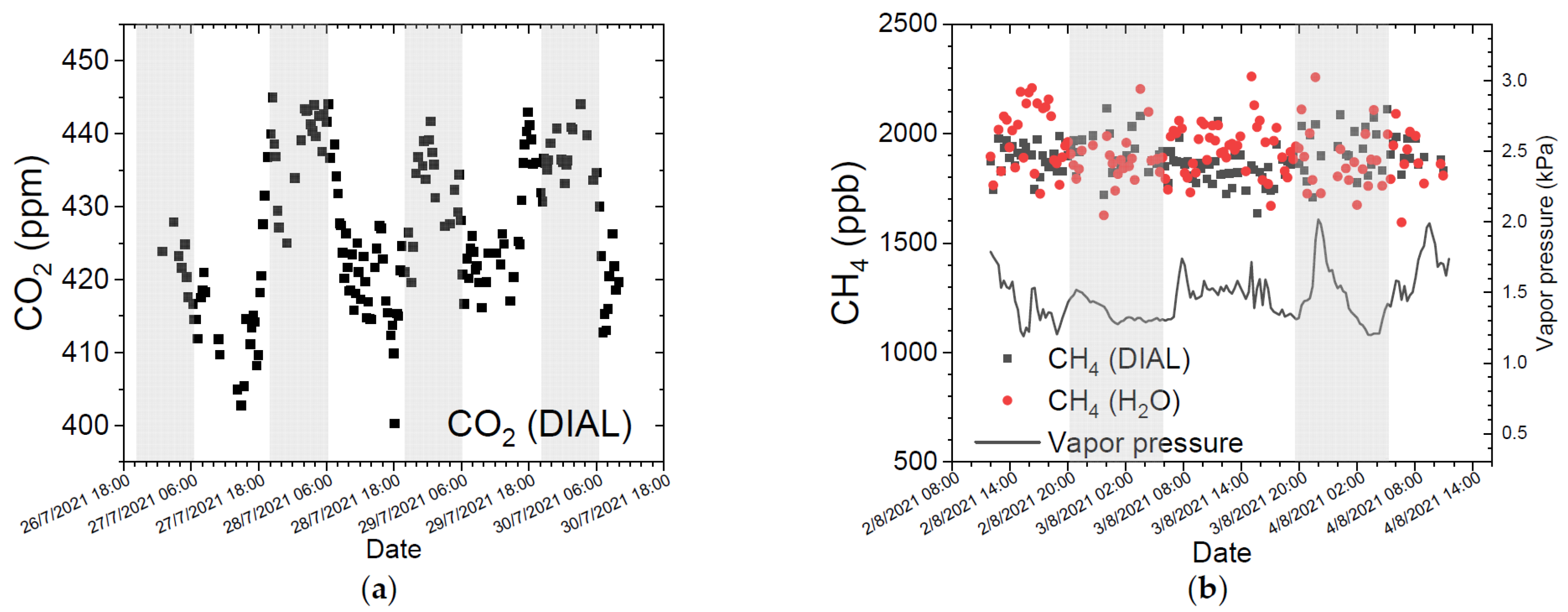
3.2. Atmospheric Measurements
3.3. GHG Concentration Calculation Using the H2O Absorption Line
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- The Global Observing System for Climate: Implementation Needs; World Meteorological Organization: Geneva, Switzerland, 2016.
- Duren, R.M.; Miller, C.E. Measuring the carbon emissions of megacities. Nat. Clim. Chang. 2012, 2, 560–562. [Google Scholar] [CrossRef]
- Velazco, V.A.; Buchwitz, M.; Bovensmann, H.; Reuter, M.; Schneising, O.; Heymann, J.; Krings, T.; Gerilowski, K.; Burrows, J.P. Towards space based verification of CO2 emissions from strong localized sources: Fossil fuel power plant emissions as seen by a CarbonSat constellation. Atmos. Meas. Tech. 2011, 4, 5147–5182. [Google Scholar] [CrossRef] [Green Version]
- Marland, G. Uncertainties in Accounting for CO2 From Fossil Fuels. J. Ind. Ecol. 2008, 12, 136–139. [Google Scholar] [CrossRef]
- Le Quéré, C.; Andrew, R.M.; Friedlingstein, P.; Sitch, S.; Hauck, J.; Pongratz, J.; Pickers, P.A.; Korsbakken, J.I.; Peters, G.P.; Canadell, J.G.; et al. Global Carbon Budget 2018. Earth Syst. Sci. Data 2018, 10, 2141–2194. [Google Scholar] [CrossRef] [Green Version]
- Verifying Greenhouse Gas Emissions: Methods to Support International Climate Agreements; National Research Council (U.S.) (Ed.) National Academies Press: Washington, DC, USA, 2010; p. 63. ISBN 9780309152112. [Google Scholar]
- Zahar, A.; Peel, J.; Godden, L. Australian Climate Law in Global Context; Cambridge University Press: Cambridge UK; New York, NY, USA, 2013; p. 123. ISBN 9780521142106. [Google Scholar]
- Lloyd, C.R.; Rebelo, L.-M.; Max Finlayson, C. Providing low-budget estimations of carbon sequestration and greenhouse gas emissions in agricultural wetlands. Environ. Res. Lett. 2013, 8, 015010. [Google Scholar] [CrossRef] [Green Version]
- Wunch, D.; Toon, G.C.; Blavier, J.-F.L.; Washenfelder, R.A.; Notholt, J.; Connor, B.J.; Griffith, D.W.T.; Sherlock, V.; Wennberg, P.O. The Total Carbon Column Observing Network. Philos. Trans. R. Soc. A 2011, 369, 2087–2112. [Google Scholar] [CrossRef] [Green Version]
- Frey, M.; Sha, M.K.; Hase, F.; Kiel, M.; Blumenstock, T.; Harig, R.; Surawicz, G.; Deutscher, N.M.; Shiomi, K.; Franklin, J.E.; et al. Building the Collaborative Carbon Column Observing Network (COCCON): Long-term stability and ensemble performance of the EM27/SUN Fourier transform spectrometer. Atmos. Meas. Tech. 2019, 12, 1513–1530. [Google Scholar] [CrossRef] [Green Version]
- Mitchell, L.E.; Crosman, E.T.; Jacques, A.A.; Fasoli, B.; Leclair-Marzolf, L.; Horel, J.; Bowling, D.R.; Ehleringer, J.R.; Lin, J.C. Monitoring of greenhouse gases and pollutants across an urban area using a light-rail public transit platform. Atmos. Environ. 2018, 187, 9–23. [Google Scholar] [CrossRef]
- Abdul-Wahab, S.; Al-Rawas, G.; Ali, S.; Fadlallah, S.; Al-Dhamri, H. Atmospheric dispersion modeling of CO2 emissions from a cement plant’s sources. Clean Technol. Environ. Policy 2017, 19, 1621–1638. [Google Scholar] [CrossRef]
- Dimitriou, K.; Bougiatioti, A.; Ramonet, M.; Pierros, F.; Michalopoulos, P.; Liakakou, E.; Solomos, S.; Quehe, P.-Y.; Delmotte, M.; Gerasopoulos, E.; et al. Greenhouse gases (CO2 and CH4) at an urban background site in Athens, Greece: Levels, sources and impact of atmospheric circulation. Atmos. Environ. 2021, 253, 118372. [Google Scholar] [CrossRef]
- Boothroyd, I.M.; Almond, S.; Worrall, F.; Davies, R.K.; Davies, R.J. Assessing fugitive emissions of CH4 from high-pressure gas pipelines in the UK. Sci. Total Environ. 2018, 631–632, 1638–1648. [Google Scholar] [CrossRef]
- Nara, H.; Tanimoto, H.; Tohjima, Y.; Mukai, H.; Nojiri, Y.; Machida, T. Emissions of methane from offshore oil and gas platforms in Southeast Asia. Sci. Rep. 2015, 4, 6503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Machida, T.; Matsueda, H.; Sawa, Y.; Nakagawa, Y.; Hirotani, K.; Kondo, N.; Goto, K.; Nakazawa, T.; Ishikawa, K.; Ogawa, T. Worldwide Measurements of Atmospheric CO2 and Other Trace Gas Species Using Commercial Airlines. J. Atmos. Ocean. Technol. 2008, 25, 1744–1754. [Google Scholar] [CrossRef]
- Sun, W.; Deng, L.; Wu, G.; Wu, L.; Han, P.; Miao, Y.; Yao, B. Atmospheric Monitoring of Methane in Beijing Using a Mobile Observatory. Atmosphere 2019, 10, 554. [Google Scholar] [CrossRef] [Green Version]
- Herman, D.I.; Weerasekara, C.; Hutcherson, L.C.; Giorgetta, F.R.; Cossel, K.C.; Waxman, E.M.; Colacion, G.M.; Newbury, N.R.; Welch, S.M.; DePaola, B.D.; et al. Precise multispecies agricultural gas flux determined using broadband open-path dual-comb spectroscopy. Sci. Adv. 2021, 7, eabe9765. [Google Scholar] [CrossRef]
- Li, J.; Yu, Z.; Du, Z.; Ji, Y.; Liu, C. Standoff Chemical Detection Using Laser Absorption Spectroscopy: A Review. Remote Sens. 2020, 12, 2771. [Google Scholar] [CrossRef]
- Wagner, G.A.; Plusquellic, D.F. Multi-frequency differential absorption LIDAR system for remote sensing of CO2 and H2O near 16 µm. Opt. Express 2018, 26, 19420. [Google Scholar] [CrossRef]
- Innocenti, F.; Robinson, R.; Gardiner, T.; Finlayson, A.; Connor, A. Differential Absorption Lidar (DIAL) Measurements of Landfill Methane Emissions. Remote Sens. 2017, 9, 953. [Google Scholar] [CrossRef] [Green Version]
- Refaat, T.F.; Ismail, S.; Koch, G.J.; Rubio, M.; Mack, T.L.; Notari, A.; Collins, J.E.; Lewis, J.; De Young, R.; Choi, Y.; et al. Backscatter 2μm Lidar Validation for Atmospheric CO2 Differential Absorption Lidar Applications. IEEE Trans. Geosci. Remote Sens. 2011, 49, 572–580. [Google Scholar] [CrossRef]
- Yakovlev, S.; Sadovnikov, S.; Kharchenko, O.; Kravtsova, N. Remote Sensing of Atmospheric Methane with IR OPO Lidar System. Atmosphere 2020, 11, 70. [Google Scholar] [CrossRef] [Green Version]
- Meng, L.; Fix, A.; Wirth, M.; Høgstedt, L.; Tidemand-Lichtenberg, P.; Pedersen, C.; Rodrigo, P.J. Upconversion detector for range-resolved DIAL measurement of atmospheric CH4. Opt. Express 2018, 26, 3850. [Google Scholar] [CrossRef] [Green Version]
- Larsson, J.; Bood, J.; Xu, C.T.; Yang, X.; Lindberg, R.; Laurell, F.; Brydegaard, M. Atmospheric CO2 sensing using Scheimpflug-lidar based on a 157-µm fiber source. Opt. Express 2019, 27, 17348. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Lindberg, R.; Larsson, J.; Bood, J.; Brydegaard, M.; Laurell, F. 1.57 µm fiber source for atmospheric CO2 continuous-wave differential absorption lidar. Opt. Express 2019, 27, 10304. [Google Scholar] [CrossRef] [PubMed]
- Babchenko, S.V.; Matvienko, G.G.; Sukhanov, A.Y. Assessing the possibilities of sensing CH4 and CO2 greenhouse gases above the underlying surface with satellite-based IPDA lidar. Atmos. Ocean. Opt. 2015, 28, 245–253. [Google Scholar] [CrossRef]
- Ehret, G.; Kiemle, C.; Wirth, M.; Amediek, A.; Fix, A.; Houweling, S. Space-borne remote sensing of CO2, CH4, and N2O by integrated path differential absorption lidar: A sensitivity analysis. Appl. Phys. B 2008, 90, 593–608. [Google Scholar] [CrossRef] [Green Version]
- Fix, A.; Büdenbender, C.; Wirth, M.; Quatrevalet, M.; Amediek, A.; Kiemle, C.; Ehret, G. Optical Parametric Oscillators and Amplifiers for Airborne and Spaceborne Active Remote Sensing of CO2 and CH4; Singh, U.N., Pappalardo, G., Eds.; SPIE: Prague, Czech Republic, 2011; p. 818206. [Google Scholar]
- Cezard, N.; Dolfi-Bouteyre, A.; Durécu, A.; Faure, B.; Goular, D.; Gustave, F.; Hébert, P.-J.; Lahyani, J.; Le Gouët, J.; Lemaître, F.; et al. Recent advances on fiber-based laser and Lidar systems for future space-borne monitoring of greenhouse gas. In Proceedings of the International Conference on Space Optics—ICSO 2020, Virtual, 30 March–2 April 2021; p. 67. [Google Scholar]
- Fix, A.; Amediek, A.; Büdenbender, C.; Ehret, G.; Quatrevalet, M.; Wirth, M.; Löhring, J.; Kasemann, R.; Klein, J.; Hoffmann, H.-D.; et al. Development and First Results of a new Near-IR Airborne Greenhouse Gas Lidar. In Proceedings of the Advanced Solid State Lasers 2015, Berlin, Germany, 4–9 October 2015; p. EM3A.3. [Google Scholar]
- Fix, A.; Amediek, A.; Bovensmann, H.; Ehret, G.; Gerbig, C.; Gerilowski, K.; Pfeilsticker, K.; Roiger, A.; Zöger, M. CoMet: An airborne mission to simultaneously measure CO2 and CH4 using lidar, passive remote sensing, and in-situ techniques. EPJ Web Conf. 2018, 176, 02003. [Google Scholar] [CrossRef] [Green Version]
- Wagner, G.A.; Plusquellic, D.F. Ground-based, integrated path differential absorption LIDAR measurement of CO2, CH4, and H2O near 1.6 μm. Appl. Opt. 2016, 55, 6292. [Google Scholar] [CrossRef]
- Barrientos Barria, J.; Dobroc, A.; Coudert-Alteirac, H.; Raybaut, M.; Cézard, N.; Dherbecourt, J.-B.; Schmid, T.; Faure, B.; Souhaité, G.; Pelon, J.; et al. Simultaneous remote monitoring of atmospheric methane and water vapor using an integrated path DIAL instrument based on a widely tunable optical parametric source. Appl. Phys. B 2014, 117, 509–518. [Google Scholar] [CrossRef]
- Numata, K.; Riris, H.; Li, S.; Wu, S.; Kawa, S.R.; Krainak, M.; Abshire, J. Ground demonstration of trace gas lidar based on optical parametric amplifier. J. Appl. Remote Sens. 2012, 6, 063561-1. [Google Scholar] [CrossRef]
- Peng, W.Y.; Goldenstein, C.S.; Mitchell Spearrin, R.; Jeffries, J.B.; Hanson, R.K. Single-ended mid-infrared laser-absorption sensor for simultaneous in situ measurements of H2O, CO2, CO, and temperature in combustion flows. Appl. Opt. 2016, 55, 9347. [Google Scholar] [CrossRef] [PubMed]
- Diaz, A.; Thomas, B.; Castillo, P.; Gross, B.; Moshary, F. Active standoff detection of CH4 and N2O leaks using hard-target backscattered light using an open-path quantum cascade laser sensor. Appl. Phys. B 2016, 122, 121. [Google Scholar] [CrossRef]
- Frish, M.B.; Wainner, R.T.; Laderer, M.C.; Allen, M.G.; Rutherford, J.; Wehnert, P.; Dey, S.; Gilchrist, J.; Corbi, R.; Picciaia, D.; et al. Low-Cost Lightweight Airborne Laser-Based Sensors for Pipeline Leak Detection and Reporting; Druy, M.A., Crocombe, R.A., Eds.; SPIE: Baltimore, MD, USA, 2013; p. 87260C. [Google Scholar]
- Frish, M.B.; Wainner, R.T.; Laderer, M.C.; Green, B.D.; Allen, M.G. Standoff and Miniature Chemical Vapor Detectors Based on Tunable Diode Laser Absorption Spectroscopy. IEEE Sens. J. 2010, 10, 639–646. [Google Scholar] [CrossRef]
- Ismail, S.; Browell, E.V. LIDAR|Differential Absorption Lidar. In Encyclopedia of Atmospheric Sciences; North, G.R., Pyle, J., Zhang, F., Eds.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 277–288. ISBN 9780123822253. [Google Scholar]
- Climate Change 1995: The Science of Climate Change; Houghton, J.T.; Intergovernmental Panel on Climate Change (Eds.) Cambridge University Press: Cambridge, UK, 1996; ISBN 9780521564335. [Google Scholar]
- Royer, S.-J.; Ferrón, S.; Wilson, S.T.; Karl, D.M. Production of methane and ethylene from plastic in the environment. PLoS ONE 2018, 13, e0200574. [Google Scholar] [CrossRef] [PubMed]
- Gordon, I.E.; Rothman, L.S.; Hill, C.; Kochanov, R.V.; Tan, Y.; Bernath, P.F.; Birk, M.; Boudon, V.; Campargue, A.; Chance, K.V.; et al. The HITRAN2016 molecular spectroscopic database. J. Quant. Spectrosc. Radiat. Transf. 2017, 203, 3–69. [Google Scholar] [CrossRef]
- HITRAN on the Web. Available online: https://hitran.iao.ru/home (accessed on 3 August 2021).
- Differential Absorption Lidar Technique (DIAL). In Elastic Lidar: Theory, Practice, and Analysis Methods; Vladimir, A.; Kovalev; Eichinger, W.E. (Eds.) John Wiley: Hoboken, NJ, USA, 2004; ISBN 9780471201717. [Google Scholar]
- Hellenic Land Registry. Available online: http://gis.ktimanet.gr/ (accessed on 1 September 2021).
- Liou, K.-N. An Introduction to Atmospheric Radiation, 2nd ed.; International Geophysics Series; Academic Press: Amsterdam, The Netherlands; Boston, MA, USA, 2002; ISBN 9780124514515. [Google Scholar]
- Mihalopoulos, N.; Stephanou, E.; Kanakidou, M.; Pilitsidis, S.; Bousquet, P. Tropospheric aerosol ionic composition in the Eastern Mediterranean region. Tellus B Chem. Phys. Meteorol. 1997, 49, 314–326. [Google Scholar] [CrossRef]
- El Yazidi, A.; Ramonet, M.; Ciais, P.; Broquet, G.; Pison, I.; Abbaris, A.; Brunner, D.; Conil, S.; Delmotte, M.; Gheusi, F.; et al. Identification of spikes associated with local sources in continuous time series of atmospheric CO, CO2 and CH4. Atmos. Meas. Tech. 2018, 11, 1599–1614. [Google Scholar] [CrossRef] [Green Version]
- Gialesakis, N.; Kouvarakis, G.; Kalivitis, N.; Ramonet, M.; Mihalopoulos, N.; Delmotte, M.; Lett, C.; Legendre, V.; Kanakidou, M. Interannual and seasonal variability of greenhouse gases at Finokalia station in the East Mediterranean. In Proceedings of the 15th International Conference on Meteorology, Climatology and Atmospheric Physics COMECAP 2021, Ioannina, Greece, 26–29 September 2021; Bartzokas, A., Nastos, P., Eds.; 2021; pp. 58–63. [Google Scholar]
- Koroneos, C.; Haritakis, I.; Michaloglou, K.; Moussiopoulos, N. Energy Analysis for Power Plant Alternative Designs, Part I. Energy Sources 2004, 26, 1277–1285. [Google Scholar] [CrossRef]
- Dlugokencky, NOAA/GML. Available online: http://gml.noaa.gov/ccgg/trends_ch4/ (accessed on 1 September 2021).
- Sheng, J.-X.; Jacob, D.J.; Maasakkers, J.D.; Zhang, Y.; Sulprizio, M.P. Comparative analysis of low-Earth orbit (TROPOMI) and geostationary (GeoCARB, GEO-CAPE) satellite instruments for constraining methane emissions on fine regional scales: Application to the Southeast US. Atmos. Meas. Tech. 2018, 11, 6379–6388. [Google Scholar] [CrossRef] [Green Version]
- Hu, H.; Landgraf, J.; Detmers, R.; Borsdorff, T.; Aan de Brugh, J.; Aben, I.; Butz, A.; Hasekamp, O. Toward Global Mapping of Methane With TROPOMI: First Results and Intersatellite Comparison to GOSAT. Geophys. Res. Lett. 2018, 45, 3682–3689. [Google Scholar] [CrossRef]
- Urban Heat and CO2 Fluxes-RSLab. Available online: http://rslab.gr/heraklion_eddy.html (accessed on 1 September 2021).
- Riris, H.; Rodriguez, M.D.; Allan, G.R.; Hasselbrack, W.E.; Stephen, M.A.; Abshire, J.B. Airborne lidar measurements of atmospheric pressure made using the oxygen A-band. In Proceedings of the Laser Applications to Chemical, Security and Environmental Analysis 2012, San Diego, CA, USA, 29 January–1 February 2012; p. LT2B.5. [Google Scholar]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siozos, P.; Psyllakis, G.; Samartzis, P.C.; Velegrakis, M. Autonomous Differential Absorption Laser Device for Remote Sensing of Atmospheric Greenhouse Gases. Remote Sens. 2022, 14, 460. https://doi.org/10.3390/rs14030460
Siozos P, Psyllakis G, Samartzis PC, Velegrakis M. Autonomous Differential Absorption Laser Device for Remote Sensing of Atmospheric Greenhouse Gases. Remote Sensing. 2022; 14(3):460. https://doi.org/10.3390/rs14030460
Chicago/Turabian StyleSiozos, Panagiotis, Giannis Psyllakis, Peter C. Samartzis, and Michalis Velegrakis. 2022. "Autonomous Differential Absorption Laser Device for Remote Sensing of Atmospheric Greenhouse Gases" Remote Sensing 14, no. 3: 460. https://doi.org/10.3390/rs14030460
APA StyleSiozos, P., Psyllakis, G., Samartzis, P. C., & Velegrakis, M. (2022). Autonomous Differential Absorption Laser Device for Remote Sensing of Atmospheric Greenhouse Gases. Remote Sensing, 14(3), 460. https://doi.org/10.3390/rs14030460