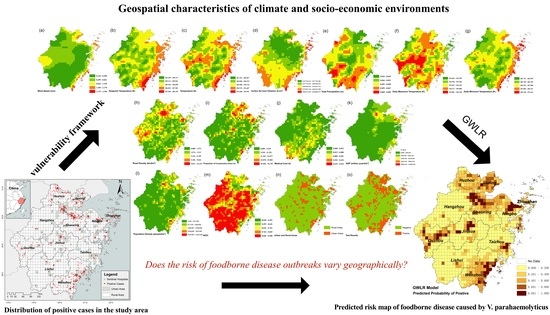
Evaluating the Spatial Risk of Bacterial Foodborne Diseases Using Vulnerability Assessment and Geographically Weighted Logistic Regression
Abstract
1. Introduction
2. Materials and Methods
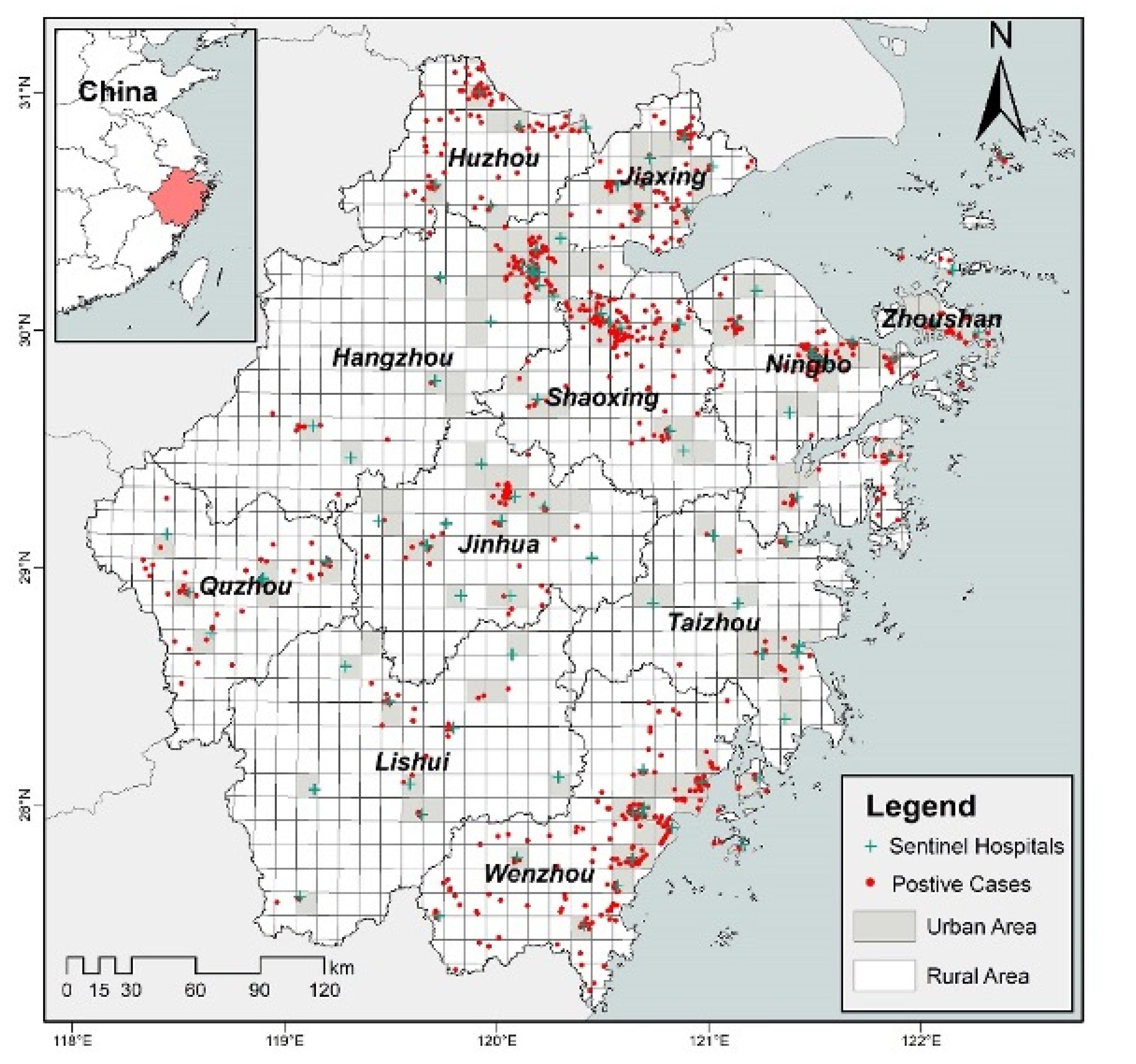
2.1. Study Area
2.2. Data Source
2.3. Foodborne Diseases Vulnerability Assessment Framework
2.4. Global Logistic Regression
2.5. Geographically Weighted Logistic Regression Model
3. Results
3.1. Global Logistic Regression
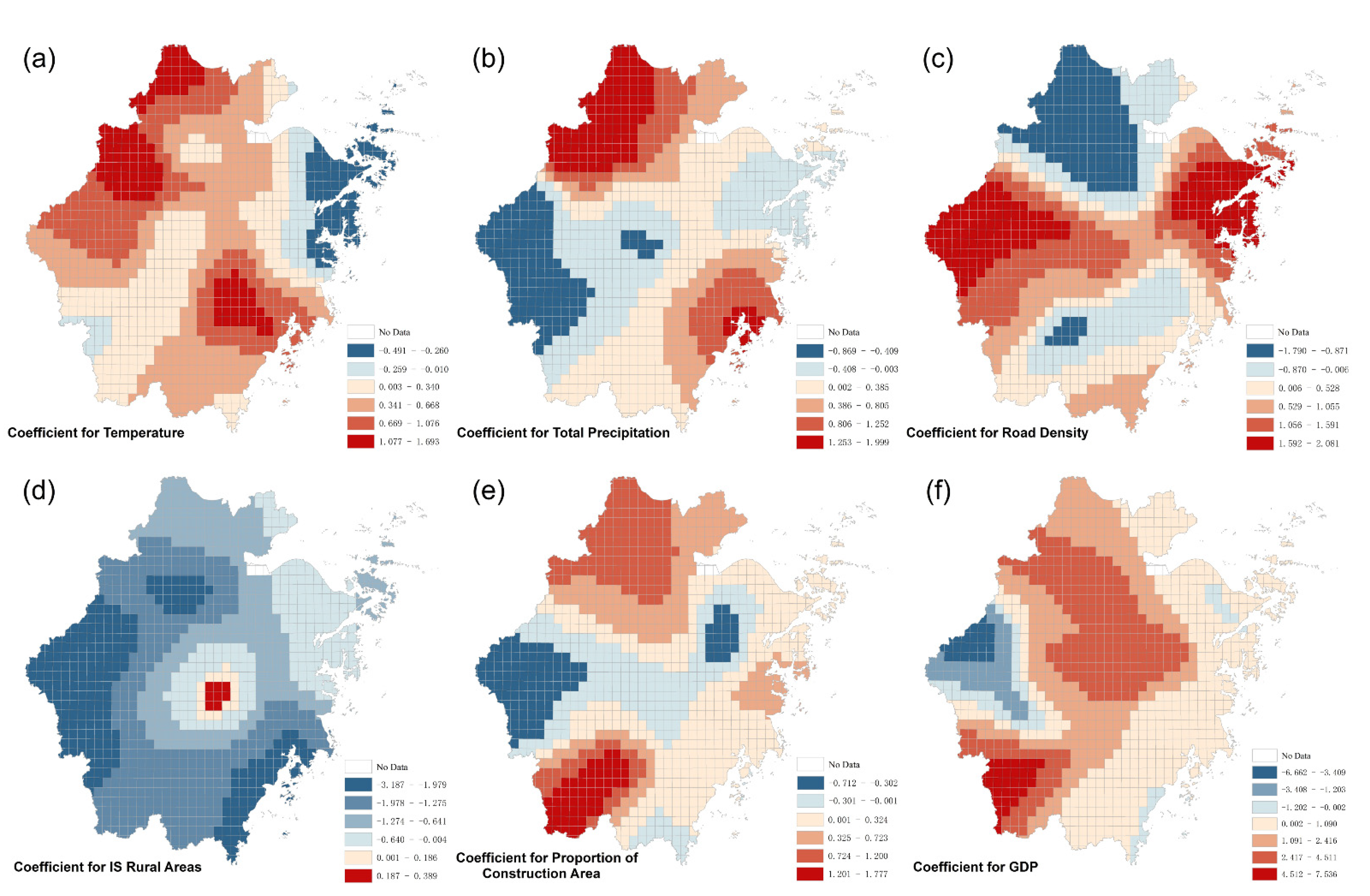
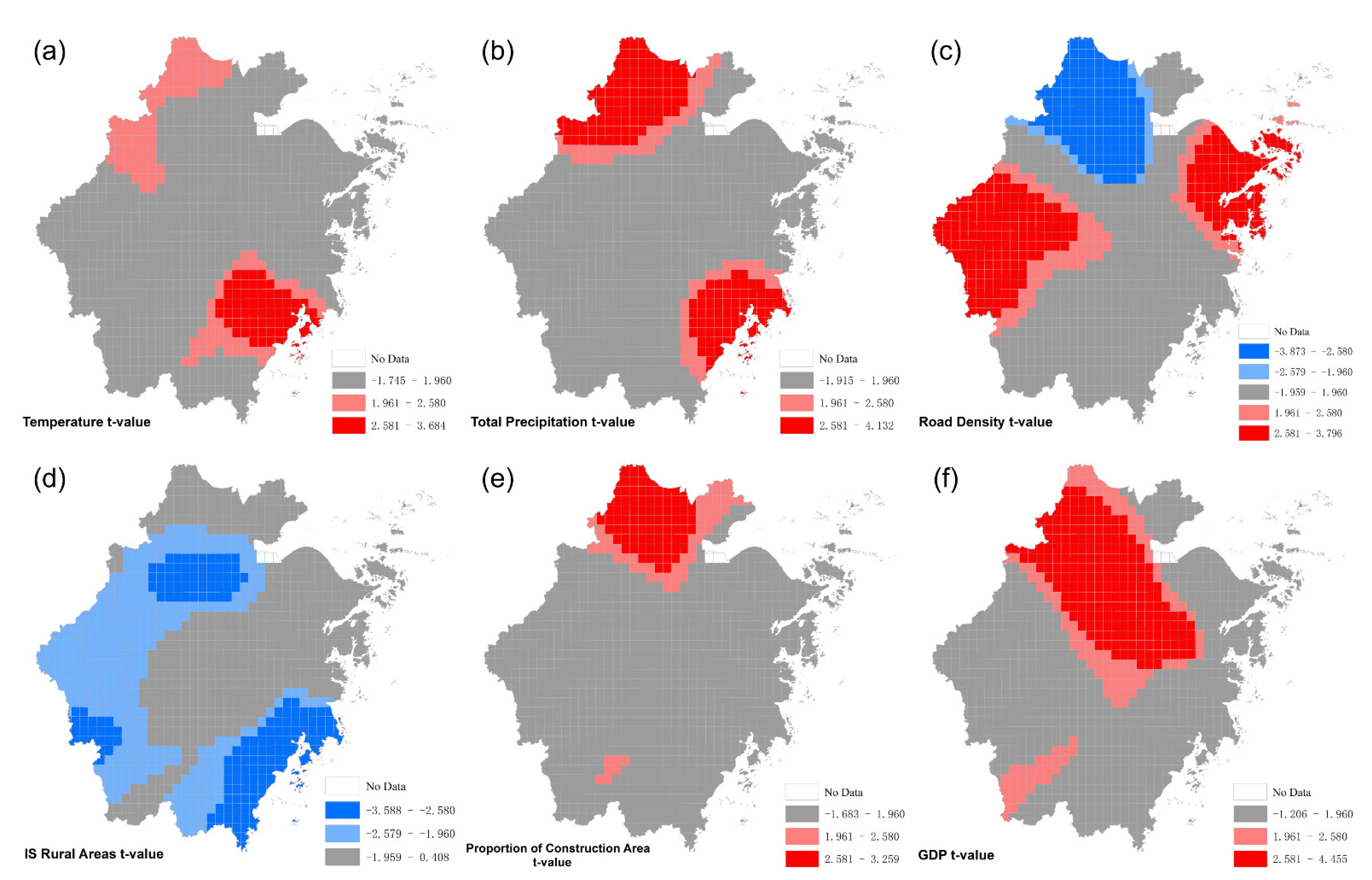
3.2. Geographically Weighted Logistic Regression
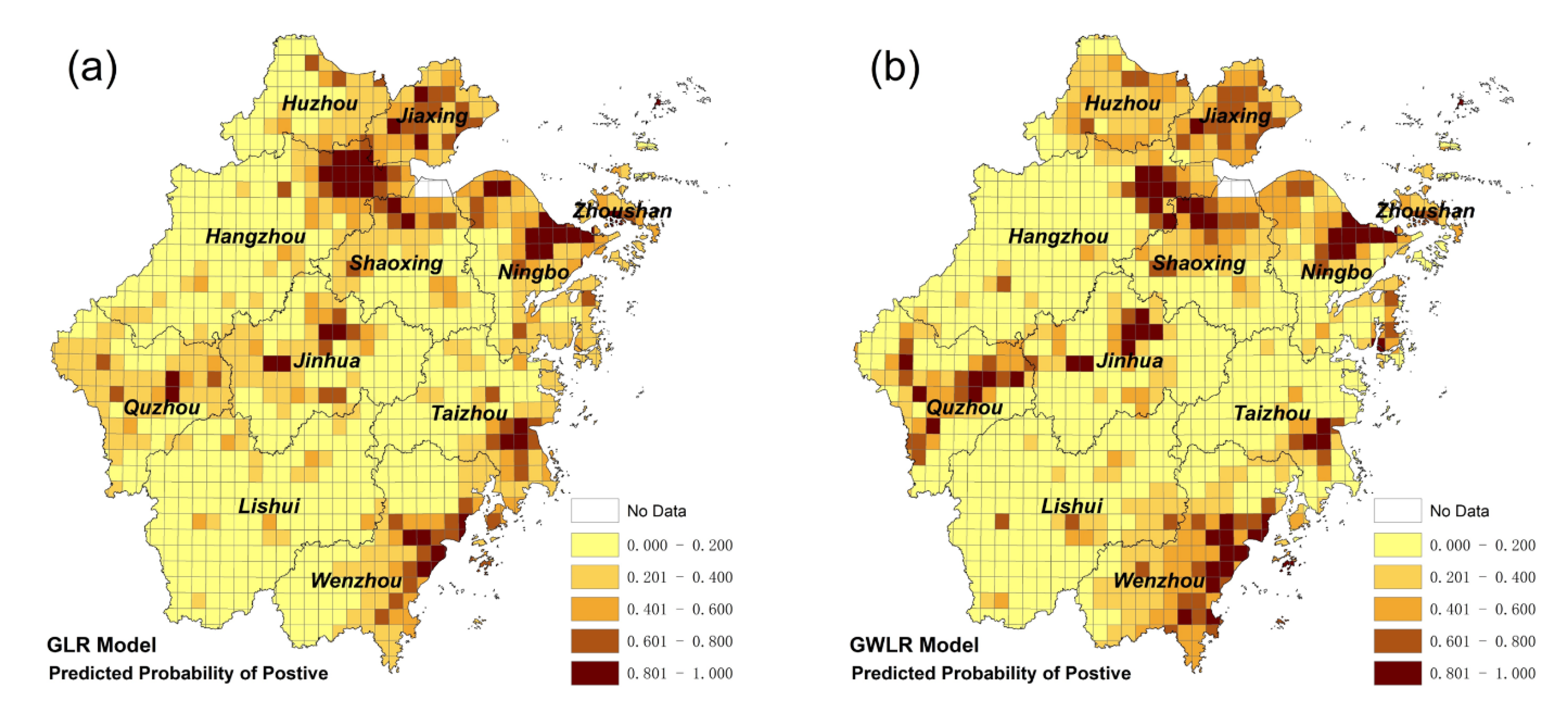
3.3. Mapping the Risk of Foodborne Diseases
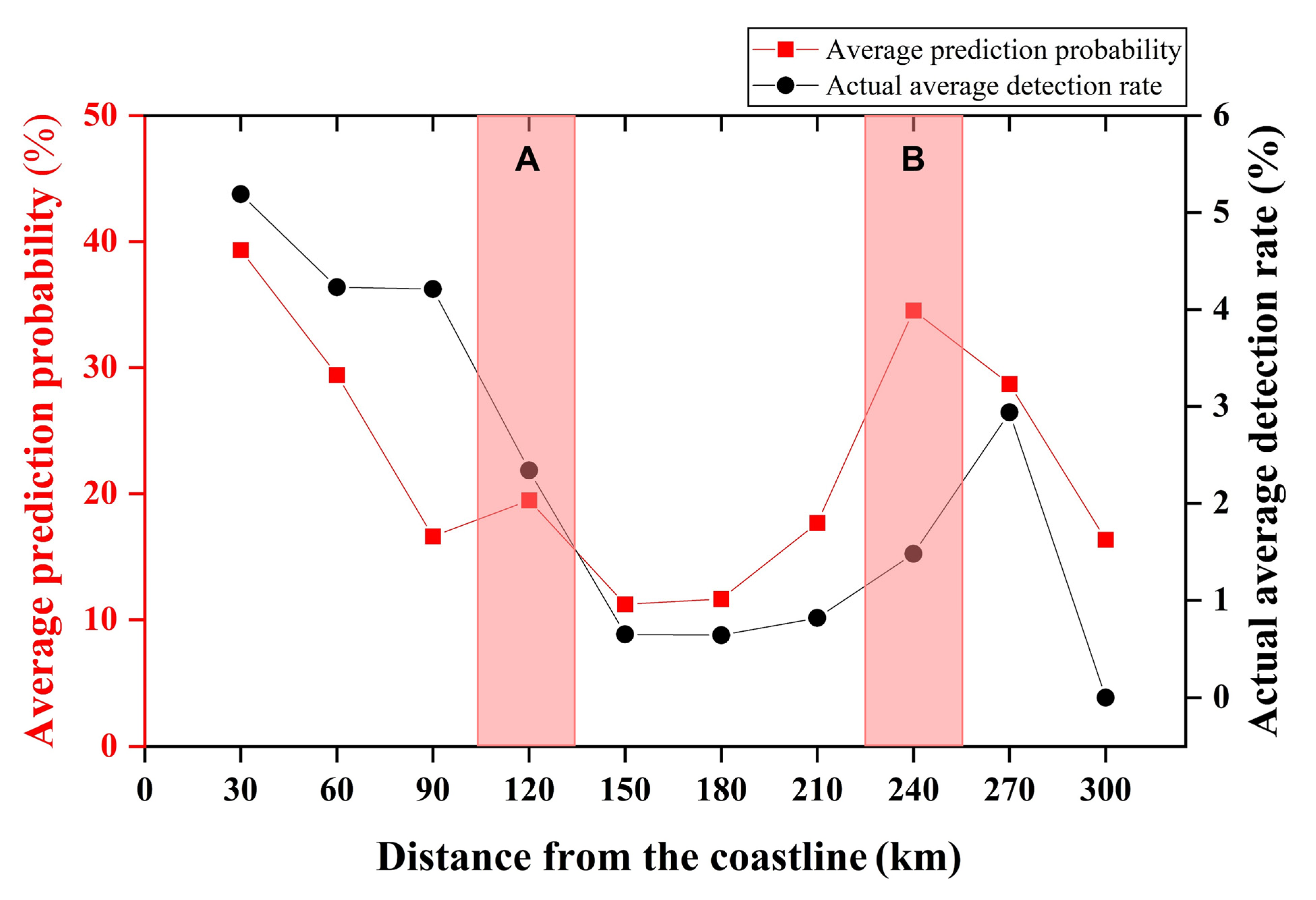
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Foodborne Disease Outbreaks: Guidelines for Investigation and Control; World Health Organization: Geneva, Switzerland, 2008. [Google Scholar]
- World Health Organization. WHO Estimates of the Global Burden of Foodborne Diseases: Foodborne Disease Burden Epidemiology Reference Group 2007–2015; World Health Organization: Geneva, Switzerland, 2015. [Google Scholar]
- Chen, Y.; Yan, W.; Zhou, Y.; Zhen, S.; Zhang, R.; Chen, J.; Liu, Z.; Cheng, H.; Liu, H.; Duan, S.; et al. Burden of Self-reported Acute Gastrointestinal Illness in China: A Population-based Survey. BMC Public Health 2013, 13, 456. [Google Scholar] [CrossRef]
- Li, W.; Wu, S.; Fu, P.; Liu, J.; Han, H.; Bai, L.; Pei, X.; Li, N.; Liu, X.; Guo, Y. National Molecular Tracing Network for Foodborne Disease Surveillance in China. Food Control 2018, 88, 28–32. [Google Scholar] [CrossRef]
- Pang, R.; Li, Y.; Chen, M.; Zeng, H.; Lei, T.; Zhang, J.; Ding, Y.; Wang, J.; Wu, S.; Ye, Q.; et al. A Database for Risk Assessment and Comparative Genomic Analysis of Foodborne Vibrio Parahaemolyticus in China. Sci. Data 2020, 7, 321. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Sun, L.; Zhang, R.; Liao, N.; Qi, X.; Chen, J. Surveillance for Foodborne Disease Outbreaks in Zhejiang Province, China, 2015–2020. BMC Public Health 2022, 22, 135. [Google Scholar] [CrossRef]
- Swoveland, J.L.; Stewart, L.K.; Eckmann, M.K.; Gee, R.; Allen, K.J.; Vandegrift, C.M.; Olson, G.; Kang, M.G.; Tran, M.L.; Melius, E.; et al. Laboratory Review of Foodborne Disease Investigations in Washington State 2007–2017. Foodborne Pathog. Dis. 2019, 16, 513–523. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.K.; Kwak, N.S.; Kim, H.J. Systemic Analysis of Foodborne Disease Outbreak in Korea. Foodborne Pathog. Dis. 2016, 13, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.J.; Wen, Y.F.; Song, J.G.; Chen, B.F.; Ding, S.S.; Ding, L.; Dai, J.J. The Correlation Between Family Food Handling Behaviors and Foodborne Acute Gastroenteritis: A Community-oriented, Population-based Survey in Anhui, China. BMC Public Health 2018, 18, 1290. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Cui, W.; Wang, H.; Du, Y.; Zhou, Y. High-Efficiency Machine Learning Method for Identifying Foodborne Disease Outbreaks and Confounding Factors. Foodborne Pathog. Dis. 2021, 18, 590–598. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.L.; Zhou, M.Q.; Jia, J.Z.; Geng, Z.; Xiao, G.X. A Bayesian Approach to Real-Time Monitoring and Forecasting of Chinese Foodborne Diseases. Int. J. Environ. Res. Public Health 2018, 15, 1740. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Peng, Z.; Zhou, Y.; Zhang, J. Time Series Analysis of Foodborne Diseases During 2012–2018 in Shenzhen, China. J. Consum. Prot. Food Saf. 2021, 17, 83–91. [Google Scholar] [CrossRef]
- Lesiv, M.; Moltchanova, E.; Schepaschenko, D.; See, L.; Shvidenko, A.; Comber, A.; Fritz, S. Comparison of Data Fusion Methods Using Crowdsourced Data in Creating a Hybrid Forest Cover Map. Remote Sens. 2016, 8, 261. [Google Scholar] [CrossRef]
- Yasuo, K.; Nishiura, H. Spatial Epidemiological Determinants of Severe Fever with Thrombocytopenia Syndrome in Miyazaki, Japan: A GWLR Modeling Study. BMC Infect. Dis. 2019, 19, 498. [Google Scholar] [CrossRef] [PubMed]
- Imran, M.; Hamid, Y.; Mazher, A.; Ahmad, S.R. Geo-spatially Modelling Dengue Epidemics in Urban Cities: A Case Study of Lahore, Pakistan. Geocarto. Int. 2021, 36, 197–211. [Google Scholar] [CrossRef]
- Manyangadze, T.; Mavhura, E.; Mudavanhu, C.; Pedzisai, E. An Exploratory Analysis of the Spatial Variation of Malaria Cases and Associated Household Socio-economic Factors in Flood-prone Areas of Mbire district, Zimbabwe. GeoJournal 2021, 1–16. [Google Scholar] [CrossRef]
- Zhou, Y.B.; Wang, Q.X.; Yang, M.X.; Gong, Y.H.; Yang, Y.; Nie, S.J.; Liang, S.; Nan, L.; Coatsworth, A.; Yang, A.H.; et al. Geographical variations of risk factors associated with HCV infection in drug users in southwestern China. Epidemiol. Infect. 2016, 144, 1291–1300. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Birch, E.L. Climate Change 2014: Impacts, Adaptation, and Vulnerability. J. Am. Plann. Assoc. 2014, 80, 184–185. [Google Scholar] [CrossRef]
- Rathi, S.K.; Chakraborty, S.; Mishra, S.K.; Dutta, A.; Nanda, L. A Heat Vulnerability Index: Spatial Patterns of Exposure, Sensitivity and Adaptive Capacity for Urbanites of Four Cities of India. Int. J. Environ. Res. Public Health 2022, 19, 283. [Google Scholar] [CrossRef]
- Udayanga, L.; Gunathilaka, N.; Iqbal, M.C.M.; Abeyewickreme, W. Climate Change Induced Vulnerability and Adaption for Dengue Incidence in Colombo and Kandy Districts: The Detailed Investigation in Sri Lanka. Infect. Dis. Poverty 2020, 9, 102. [Google Scholar] [CrossRef] [PubMed]
- De Andrade, M.M.N.; Szlafsztein, C.F. Vulnerability Assessment Including Tangible and Intangible Components in the Index Composition: An Amazon Case Study of Flooding and Flash Flooding. Sci. Total Environ. 2018, 630, 903–912. [Google Scholar] [CrossRef] [PubMed]
- Mitrica, B.; Mocanu, I.; Grigorescu, I.; Dumitrascu, M.; Pistol, A.; Damian, N.; Serban, P.R. Population Vulnerability to the SARS-CoV-2 Virus Infection. A County-Level Geographical-Methodological Approach in Romania. GeoHealth 2021, 5, e2021GH000461. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, I.; Wang, X.; Waseem, M.; Zaman, M.; Aziz, F.; Khan, R.Z.N.; Ashraf, M. Flood Management, Characterization and Vulnerability Analysis Using an Integrated RS-GIS and 2D Hydrodynamic Modelling Approach: The Case of Deg Nullah, Pakistan. Remote Sens. 2022, 14, 2138. [Google Scholar] [CrossRef]
- Adger, W.N. Vulnerability. Glob. Environ. Change 2006, 16, 268–281. [Google Scholar] [CrossRef]
- Zhang, Y.; Shen, J.; Li, Y. Atmospheric Environment Vulnerability Cause Analysis for the Beijing-Tianjin-Hebei Metropolitan Region. Int. J. Environ. Res. Public Health 2018, 15, 128. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Liu, T.; Ge, Y.; Xia, S.; Yuan, Y.; Li, W.; Xu, H. Examining Social Vulnerability to Flood of Affordable Housing Communities in Nanjing, China: Building Long-term Disaster Resilience of Low-income Communities. Sustain. Cities Soc. 2021, 71, 102939. [Google Scholar] [CrossRef]
- Swami, D.; Parthasarathy, D. Dynamics of Exposure, Sensitivity, Adaptive Capacity and Agricultural Vulnerability at District Scale for Maharashtra, India. Ecol. Indic. 2021, 121, 107206. [Google Scholar] [CrossRef]
- He, C.; Ma, L.; Zhou, L.; Kan, H.; Zhang, Y.; Ma, W.; Chen, B. Exploring the Mechanisms of Heat Wave Vulnerability at the Urban Scale Based on the Application of Big Data and Artificial Societies. Environ. Int. 2019, 127, 573–583. [Google Scholar] [CrossRef]
- Morral-Puigmal, C.; Martinez-Solanas, E.; Villanueva, C.M.; Basagana, X. Weather and Gastrointestinal Disease in Spain: A Retrospective Time Series Regression Study. Environ. Int. 2018, 121, 649–657. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Park, K.H.; Chun, H.S.; Choi, C.; Bahk, G.J. Correlations Between Climatic Conditions and Foodborne Disease. Food Res. Int. 2015, 68, 24–30. [Google Scholar] [CrossRef]
- Bari, L.; Yeasmin, S.; Kawamoto, S. Impact of Climate Change on Foodborne Pathogens and Diseases. J. Jpn. Soc. Food. Sci. 2008, 55, 264–269. [Google Scholar] [CrossRef]
- Strassle, P.D.; Gu, W.; Bruce, B.B.; Gould, L.H. Sex and Age Distributions of Persons in Foodborne Disease Outbreaks and Associations with Food Categories. Epidemiol. Infect. 2019, 147, e200. [Google Scholar] [CrossRef]
- Chen, Y.J.; Wen, Y.F.; Song, J.G.; Chen, B.F.; Wang, L.; Ding, S.S.; Ding, L.; Dai, J.J. Food Handling Behaviors Associated with Reported Acute Gastrointestinal Disease That May Have Been Caused by Food. J. Food Prot. 2019, 82, 494–500. [Google Scholar] [CrossRef] [PubMed]
- Osei-Tutu, B.; Anto, F. Trends of Reported Foodborne Diseases at the Ridge Hospital, Accra, Ghana: A Retrospective Review of Routine Data from 2009–2013. BMC Infect. Dis. 2016, 16, 139. [Google Scholar] [CrossRef][Green Version]
- Czerwinski, M.; Czarkowski, M.P.; Kondej, B. Foodborne Botulism in Poland in 2017. Prz. Epidemiol. 2019, 73, 445–450. [Google Scholar] [CrossRef]
- Xiao, G.X.; Xu, C.D.; Wang, J.F.; Yang, D.Y.; Wang, L. Spatial-temporal Pattern and Risk Factor Analysis of Bacillary Dysentery in the Beijing-Tianjin-Tangshan Urban Region of China. BMC Public Health 2014, 14, 998. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhang, R.; Qi, X.; Zhou, B.; Wang, J.; Chen, Y.; Zhang, H. Epidemiology of Foodborne Disease Outbreaks Caused by Vibrio Parahaemolyticus During 2010–2014 in Zhejiang Province, China. Food Control 2017, 77, 110–115. [Google Scholar] [CrossRef]
- National Bureau of Statistics of China. Provisions on the Statistical Division of Urban and Rural Areas (for Trial Implementation). Available online: http://www.stats.gov.cn/tjsj/pcsj/rkpc/5rp/html/append7.htm (accessed on 22 December 2021).
- Bai, H.M.; Shi, Y.L.; Seong, M.S.; Gao, W.K.; Li, Y.H. Influence of Spatial Resolution on Satellite-Based PM2.5 Estimation: Implications for Health Assessment. Remote Sens. 2022, 14, 2933. [Google Scholar] [CrossRef]
- Hellberg, R.S.; Chu, E. Effects of Climate Change on the Persistence and Dispersal of Foodborne Bacterial Pathogens in the Outdoor Environment: A review. Crit. Rev. Microbiol. 2016, 42, 548–572. [Google Scholar] [CrossRef] [PubMed]
- Prinsen, G.; Benschop, J.; Cleaveland, S.; Crump, J.A.; French, N.P.; Hrynick, T.A.; Mariki, B.; Mmbaga, B.T.; Sharp, J.P.; Swai, E.S.; et al. Meat Safety in Tanzania’s Value Chain: Experiences, Explanations and Expectations in Butcheries and Eateries. Int. J. Environ. Res. Public Health 2020, 17, 82833. [Google Scholar] [CrossRef] [PubMed]
- Cutter, S.L.; Finch, C. Temporal and Spatial Changes in Social Vulnerability to Natural Hazards. Proc. Natl. Acad. Sci. USA 2008, 105, 2301–2306. [Google Scholar] [CrossRef]
- Wu, W.; Zhang, L. Comparison of Spatial and Non-spatial Logistic Regression Models for Modeling the Occurrence of Cloud Cover in North-eastern Puerto Rico. Appl. Geogr. 2013, 37, 52–62. [Google Scholar] [CrossRef]
- Zuur, A.F.; Ieno, E.N.; Elphick, C.S. A Protocol for Data Exploration to Avoid Common Statistical Problems. Methods Ecol. Evol. 2010, 1, 3–14. [Google Scholar] [CrossRef]
- Ye, X.P.; Yu, X.P.; Wang, T.J. Investigating Spatial Non-stationary Environmental Effects on the Distribution of Giant Pandas in the Qinling Mountains, China. Glob. Ecol. Conserv. 2020, 21, e00894. [Google Scholar] [CrossRef]
- Lu, B.; Charlton, M.; Harris, P.; Fotheringham, A.S. Geographically Weighted Regression with a Non-Euclidean Distance Metric: A Case Study Using Hedonic House Price Data. Int. J. Geogr. Inf. Sci. 2014, 28, 660–681. [Google Scholar] [CrossRef]
- Han, H.; Jang, K.-M.; Chung, J.-S. Selecting Suitable Sites for Mountain Ginseng (Panax ginseng) Cultivation by Using Geographically Weighted Logistic Regression. J. Mt. Sci. 2017, 14, 492–500. [Google Scholar] [CrossRef]
- Yang, L.; Yu, K.; Ai, J.; Liu, Y.; Yang, W.; Liu, J. Dominant Factors and Spatial Heterogeneity of Land Surface Temperatures in Urban Areas: A Case Study in Fuzhou, China. Remote Sens. 2022, 14, 1266. [Google Scholar] [CrossRef]
- Hsiao, H.I.; Jan, M.S.; Chi, H.J. Impacts of Climatic Variability on Vibrio Parahaemolyticus Outbreaks in Taiwan. Int. J. Environ. Res. Public Health 2016, 13, 20188. [Google Scholar] [CrossRef]
- Shih, Y.J.; Chen, J.S.; Chen, Y.J.; Yang, P.Y.; Kuo, Y.J.; Chen, T.H.; Hsu, B.M. Impact of Heavy precipitation Events on Pathogen Occurrence in Estuarine Areas of the Puzi River in Taiwan. PLoS ONE 2021, 16, e0256266. [Google Scholar] [CrossRef]
- Yang, L.; Sun, Y.B.; Zhong, Q.; Duan, D.S.; Liu, S.Q.; Zhang, Y. Epidemiological Characteristics and Spatio-temporal Patterns of Foodborne Diseases in Jinan, Northern China. Biomed. Environ. Sci. 2019, 32, 309–313. [Google Scholar] [CrossRef]
- Zhang, L.; Wei, Y.; Meng, R. Spatiotemporal Dynamics and Spatial Determinants of Urban Growth in Suzhou, China. Sustainability 2017, 9, 30393. [Google Scholar] [CrossRef]
- Mayfield, H.J.; Lowry, J.H.; Watson, C.H.; Kama, M.; Nilles, E.J.; Lau, C.L. Use of Geographically Weighted Logistic Regression to Quantify Spatial Variation in the Environmental and Sociodemographic Drivers of Leptospirosis in Fiji: A Modelling Study. Lancet Planet. Health 2018, 2, 223–232. [Google Scholar] [CrossRef]
- Li, H.; Peng, J.; Yanxu, L.; Yi’na, H. Urbanization Impact on Landscape Patterns in Beijing City, China: A Spatial Heterogeneity Perspective. Ecol. Indic. 2017, 82, 50–60. [Google Scholar] [CrossRef]
- Clary, C.; Lewis, D.J.; Flint, E.; Smith, N.R.; Kestens, Y.; Cummins, S. The Local Food Environment and Fruit and Vegetable Intake: A Geographically Weighted Regression Approach in the ORiEL Study. Am. J. Epidemiol. 2016, 184, 837–846. [Google Scholar] [CrossRef] [PubMed]
- Teng, K.T.Y.; Aviles, M.M.; Ugarte-Ruiz, M.; Barcena, C.; de la Torre, A.; Lopez, G.; Moreno, M.A.; Dominguez, L.; Alvarez, J. Spatial Trends in Salmonella Infection in Pigs in Spain. Front. Vet. Sci. 2020, 7, 345. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; You, L.; Wang, X.; Zhang, Z.; Li, F.; Wu, H.; Wu, M.; Zhang, J.; Wu, J.; Chen, C.; et al. Iodine Nutritional Status, the Prevalence of Thyroid Goiter and Nodules in Rural and Urban Residents: A Cross-sectional Study from Guangzhou, China. Endocr. Connect. 2021, 10, 1550–1559. [Google Scholar] [CrossRef] [PubMed]
- Ford, M.W.; Odoi, A.; Majowicz, S.E.; Michel, P.; Middleton, D.; Ciebin, B.; Dore, K.; McEwen, S.A.; Aramini, J.A.; Deeks, S.; et al. A Descriptive Study of Human Salmonella Serotype Typhimurium Infections Reported in Ontario from 1990 to 1998. Can. J. Infect. Dis. Med. Microbiol. 2003, 14, 267–273. [Google Scholar] [CrossRef]
- Hara-Kudo, Y.; Kumagai, S. Impact of Seafood Regulations for Vibrio Parahaemolyticus Infection and Verification by Analyses of Seafood Contamination and Infection. Epidemiol. Infect. 2014, 142, 2237–2247. [Google Scholar] [CrossRef] [PubMed]
- Xie, T.; Xu, X.; Wu, Q.; Zhang, J.; Cheng, J. Prevalence, Molecular Characterization, and Antibiotic Susceptibility of Vibrio Parahaemolyticus from Ready-to-Eat Foods in China. Front. Microbiol. 2016, 7, 17–23. [Google Scholar] [CrossRef] [PubMed]
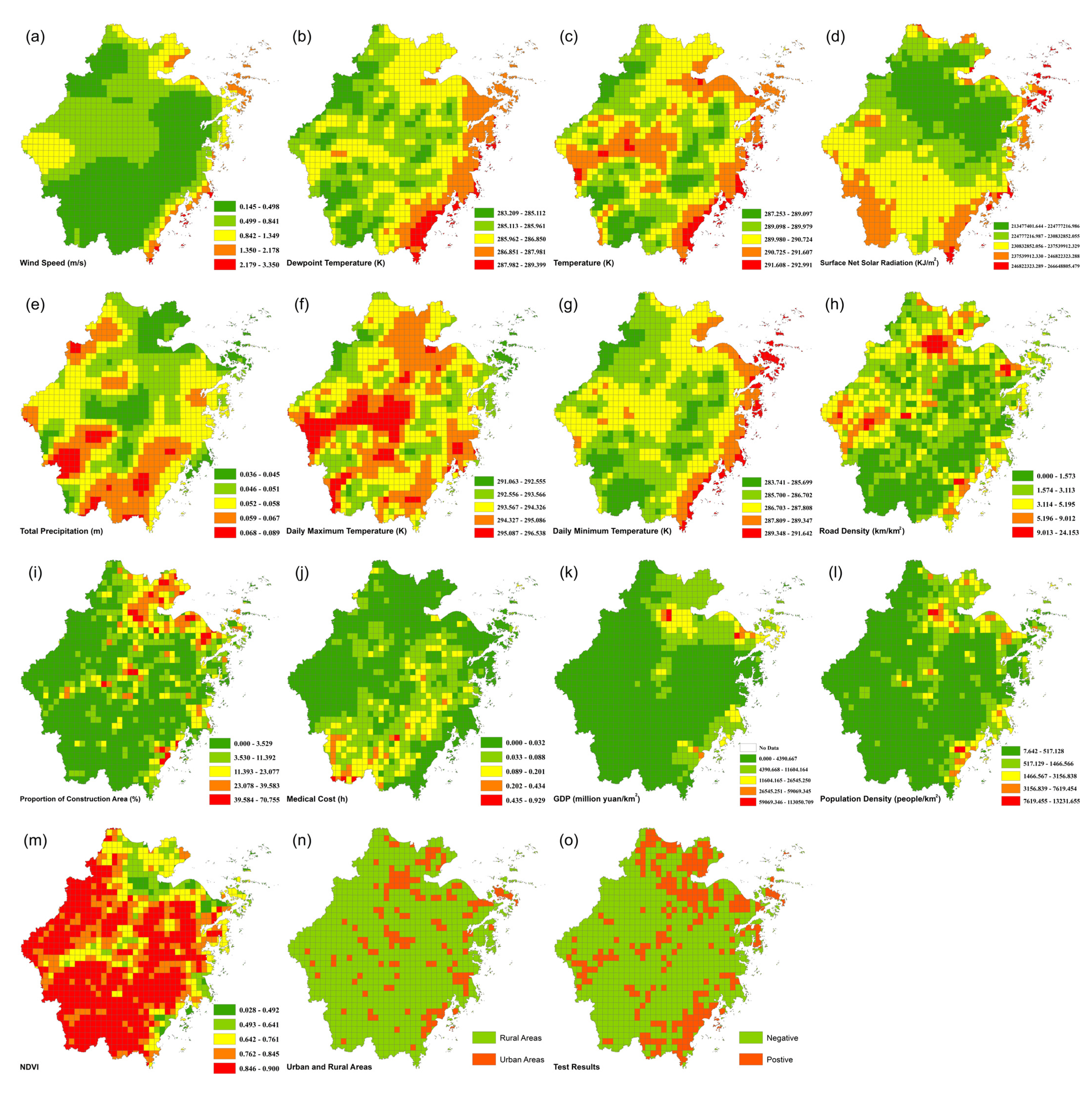
| Criterion | Index | Source | Resolution | Year |
|---|---|---|---|---|
| Exposure | Wind Speed (m/s) | ERA5-Land (https://www.ecmwf.int/ (accessed on 20 October 2021)) | 2018 | |
| Dewpoint Temperature (K) | ||||
| Temperature (K) | ||||
| Surface Net Solar Radiation (KJ/m2) | 0.1° × 0.1° | |||
| Total Precipitation (m) | ||||
| Daily Maximum Temperature (K) | ||||
| Daily Minimum Temperature (K) | ||||
| Sensitivity | Road Density (km/km2) | Road Data (https://www.openstreetmap.org/ (accessed on 23 March 2021)) | Vector | 2021 |
| Proportion of Construction Area (%) | Land Use Data (https://www.resdc.cn/ (accessed on 28 April 2021)) | 1 km | 2015 | |
| Rural Areas | Administrative Division Data (http://www.ngcc.cn/ngcc/ (accessed on 6 November 2021)) | Vector | 2018 | |
| Population Density (people/km2) | Grid Population Density (https://sedac.ciesin.columbia.edu/data/collection/gpw-v4 (accessed on 23 July 2022)) | 1 km | 2020 | |
| NDVI | Grid NDVI (https://www.resdc.cn/ (accessed on 28 April 2021)) | 1 km | 2018 | |
| Adaptability | Medical Cost (h) | POI from Amap (https://restapi.amap.com/v3/place/text (accessed on 15 May 2013)) | Vector | 2012 |
| GDP (million yuan/km2) | Grid GDP (https://www.resdc.cn/ (accessed on 28 April 2021)) | 1 km | 2015 |
| Index | Total | Urban Area | Rural Area |
|---|---|---|---|
| 964 Grids | 155 Grids | 809 Grids | |
| Wind Speed (m/s) | 0.655 (0.405) | 0.767 (0.456) | 0.633 (0.391) |
| Dewpoint Temperature (K) | 286.204 (0.980) | 286.673 (0.792) | 286.114 (0.987) |
| Temperature (K) | 290.307 (0.892) | 290.788 (0.592) | 290.215 (0.911) |
| Surface Net Solar Radiation (KJ/m2) | 231,698.574 (8818.364) | 229,737.638 (9464.728) | 232,074.279 (8644.563) |
| Total Precipitation (m) | 0.052 (0.008) | 0.049 (0.006) | 0.053 (0.008) |
| Daily Maximum Temperature (K) | 294.143 (0.875) | 294.423 (0.830) | 294.089 (0.874) |
| Daily Minimum Temperature (K) | 287.010 (1.316) | 287.614 (1.150) | 286.894 (1.315) |
| Road Density (km/km2) | 3.105 (2.288) | 5.486 (3.544) | 2.649 (1.597) |
| Proportion of Construction Area (%) | 7.946 (11.614) | 22.643 (15.630) | 5.131 (8.052) |
| Population Density (people/km2) | 638.452 (1098.019) | 2048.545 (2105.047) | 407.526 (546.304) |
| NDVI | 0.783 (0.121) | 0.655 (0.130) | 0.807 (0.102) |
| Medical Cost (h) | 0.041 (0.063) | 0.017 (0.038) | 0.046 (0.065) |
| GDP (million yuan/km2) | 4762.486 (9651.622) | 10,687.459 (15866.161) | 3627.293 (7417.539) |
| Variable | β | S.E | z-Value | p | Exp(β) | VIF |
|---|---|---|---|---|---|---|
| Temperature | 0.390 | 0.104 | 3.747 | <0.001 | 1.476 | 1.443 |
| Total Precipitation | 0.262 | 0.099 | 2.652 | 0.008 | 1.300 | 1.412 |
| Road Density | 0.272 | 0.142 | 1.914 | 0.056 | 1.312 | 1.820 |
| Proportion of Construction Area | 0.373 | 0.122 | 3.053 | 0.002 | 1.452 | 2.207 |
| Is Rural Areas | −0.924 | 0.231 | −4.007 | <0.001 | 0.397 | 1.503 |
| GDP | 0.559 | 0.212 | 2.644 | 0.008 | 1.750 | 1.456 |
| Intercept | −1.222 | 0.094 | −13.000 | <0.001 | 0.295 | - |
| AICc | 952.390 | Deviance | 938.390 | |||
| AUC | 0.772 |
| Variable | Mean | STD | Min | Max | % − | % + |
|---|---|---|---|---|---|---|
| Temperature | 0.458 | 0.469 | −0.491 | 1.693 | 16.5% | 83.5% |
| Total Precipitation | 0.297 | 0.637 | −0.830 | 1.982 | 37.4% | 62.6% |
| Road Density | 0.461 | 1.076 | −1.790 | 2.072 | 32.4% | 67.6% |
| Proportion of Construction Area | 0.273 | 0.506 | −0.712 | 1.777 | 29.0% | 71.0% |
| Is Rural Areas | −1.324 | 0.745 | −3.187 | 0.389 | 97.9% | 2.1% |
| GDP | 1.218 | 1.768 | −6.442 | 7.535 | 12.5% | 87.5% |
| Intercept | −0.001 | 1.131 | −3.697 | 4.111 | 51.1% | 48.9% |
| AICc | 874.659 | Deviance | 760.530 | |||
| AUC | 0.871 |
| Grade | Total Area | Urban Area | Rural Area |
|---|---|---|---|
| Very low (0–0.2) | 55.7% | 11.4% | 63.2% |
| Low (0.2–0.4) | 20.1% | 15.8% | 20.8% |
| Middle (0.4–0.6) | 12.3% | 17.1% | 11.5% |
| High (0.6–0.8) | 6.7% | 25.3% | 3.6% |
| Very High (0.8–1.0) | 5.2% | 30.4% | 0.9% |
| Average Prediction probability | 26.0% | 60.6% | 20.1% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bian, W.; Hou, H.; Chen, J.; Zhou, B.; Xia, J.; Xie, S.; Liu, T. Evaluating the Spatial Risk of Bacterial Foodborne Diseases Using Vulnerability Assessment and Geographically Weighted Logistic Regression. Remote Sens. 2022, 14, 3613. https://doi.org/10.3390/rs14153613
Bian W, Hou H, Chen J, Zhou B, Xia J, Xie S, Liu T. Evaluating the Spatial Risk of Bacterial Foodborne Diseases Using Vulnerability Assessment and Geographically Weighted Logistic Regression. Remote Sensing. 2022; 14(15):3613. https://doi.org/10.3390/rs14153613
Chicago/Turabian StyleBian, Wanchao, Hao Hou, Jiang Chen, Bin Zhou, Jianhong Xia, Shanjuan Xie, and Ting Liu. 2022. "Evaluating the Spatial Risk of Bacterial Foodborne Diseases Using Vulnerability Assessment and Geographically Weighted Logistic Regression" Remote Sensing 14, no. 15: 3613. https://doi.org/10.3390/rs14153613
APA StyleBian, W., Hou, H., Chen, J., Zhou, B., Xia, J., Xie, S., & Liu, T. (2022). Evaluating the Spatial Risk of Bacterial Foodborne Diseases Using Vulnerability Assessment and Geographically Weighted Logistic Regression. Remote Sensing, 14(15), 3613. https://doi.org/10.3390/rs14153613