High-Throughput Remote Sensing of Vertical Green Living Walls (VGWs) in Workplaces
Abstract
:1. Vertical Green Living Walls (VGWs) as an Urban Nature-Based Solution (NBS)
2. Using Remote-Sensing-Based Precision Agriculture Tools in VGWs
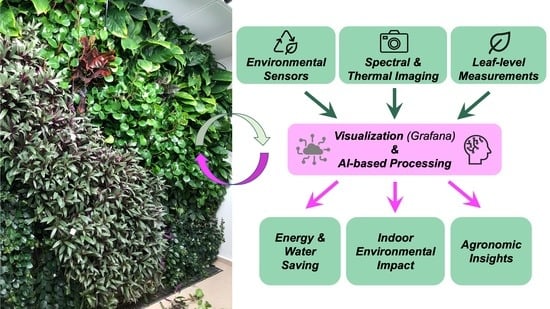
3. Description and First Results of the High-Throughput VGW Monitoring System
3.1. The VGW System in the Modeling and Monitoring Vegetation Systems Lab
3.1.1. Peperomia obtusifolia
3.1.2. Tradescantia spathacea
3.1.3. Chlorophytum comosum
3.1.4. Spathiphyllum wallisii
3.1.5. Aeschynanthus radicans
3.1.6. Philodendron hederaceum
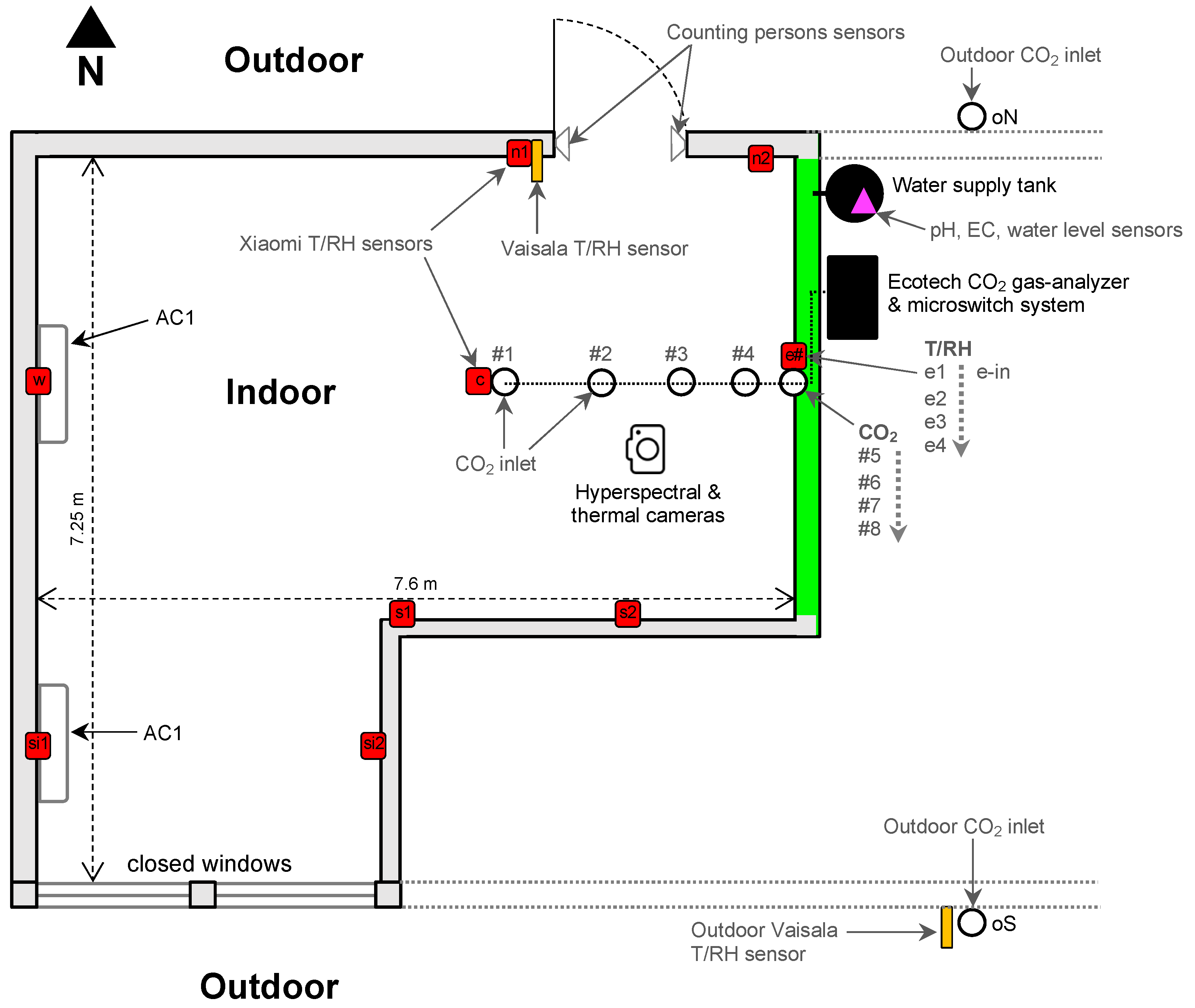
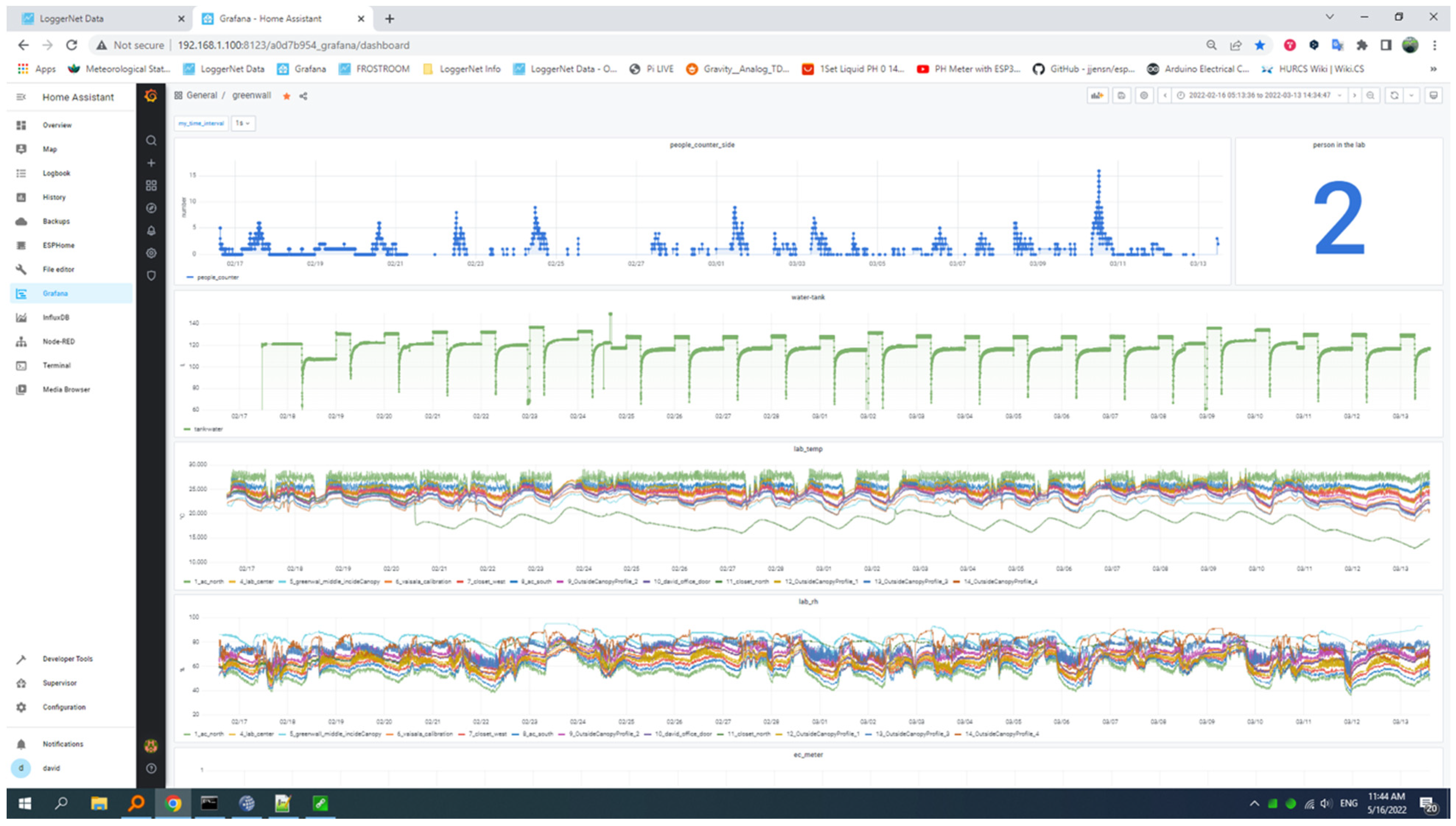
3.2. The Working Space and Its Indoor Environmental Monitoring System
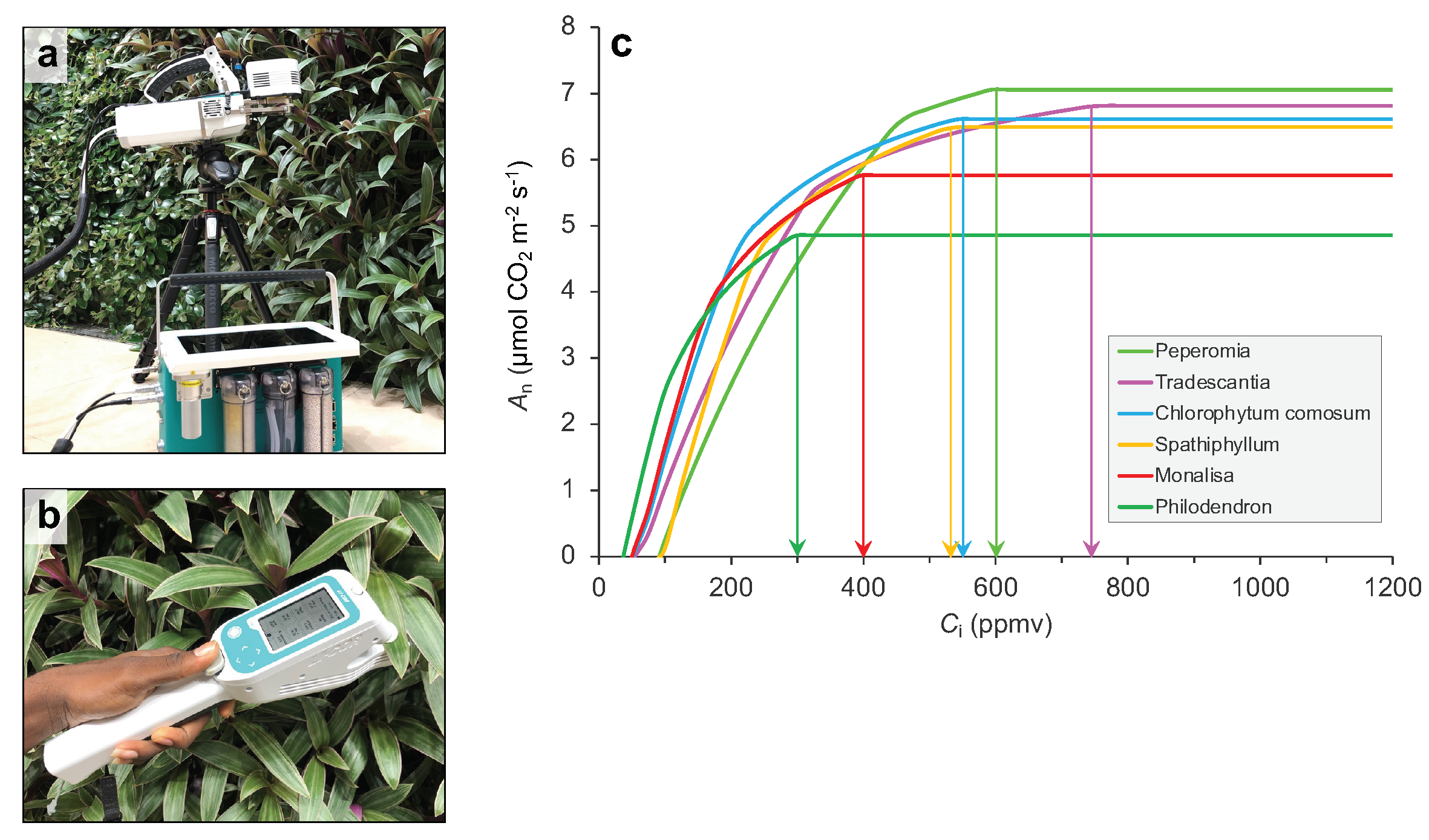
3.3. Leaf Level Gas Exchange Measurements
4. Remote Sensing and Artificial Intelligence for VGWs
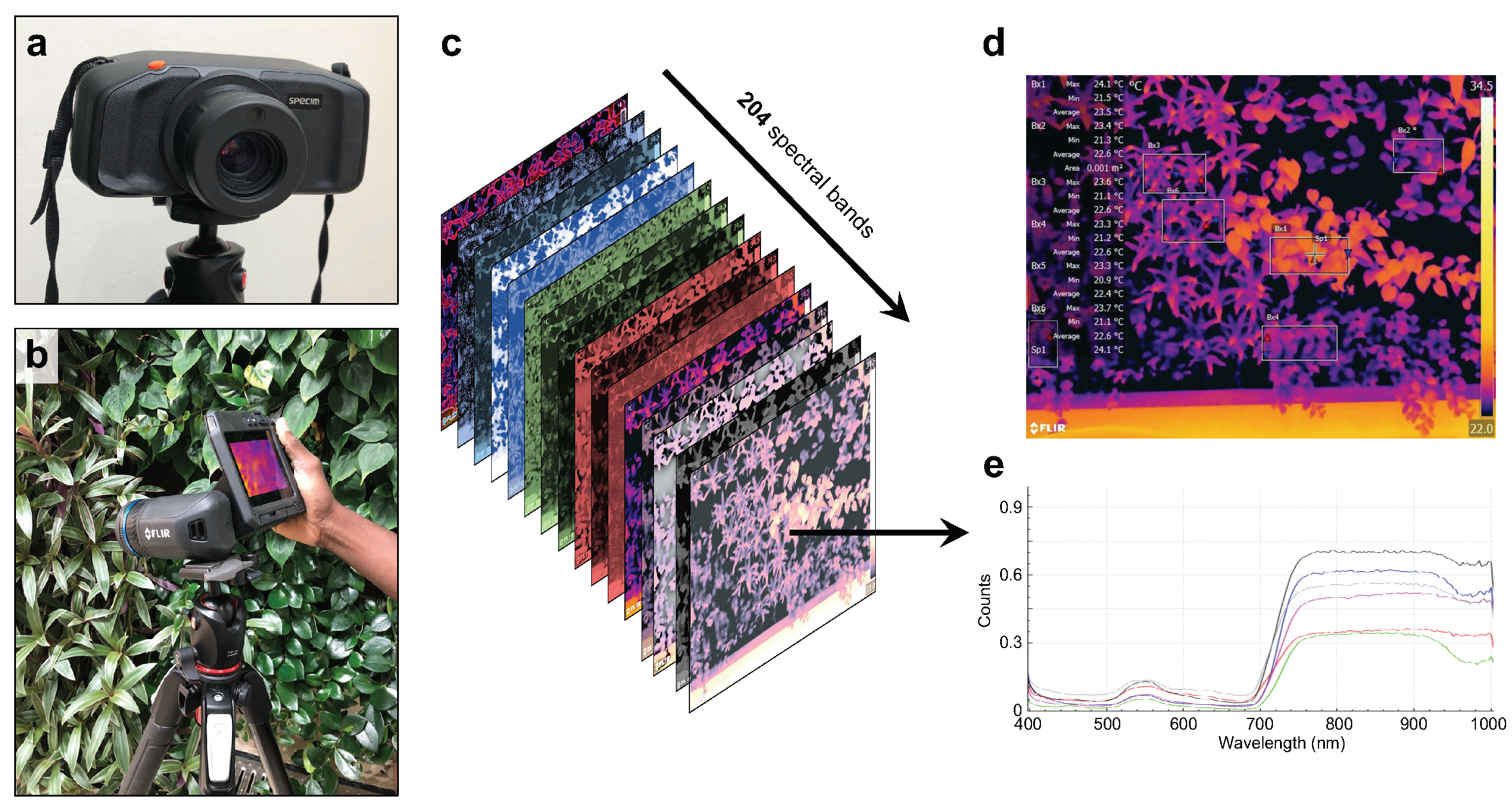
4.1. Creating the Spectral and Thermal Data Collections
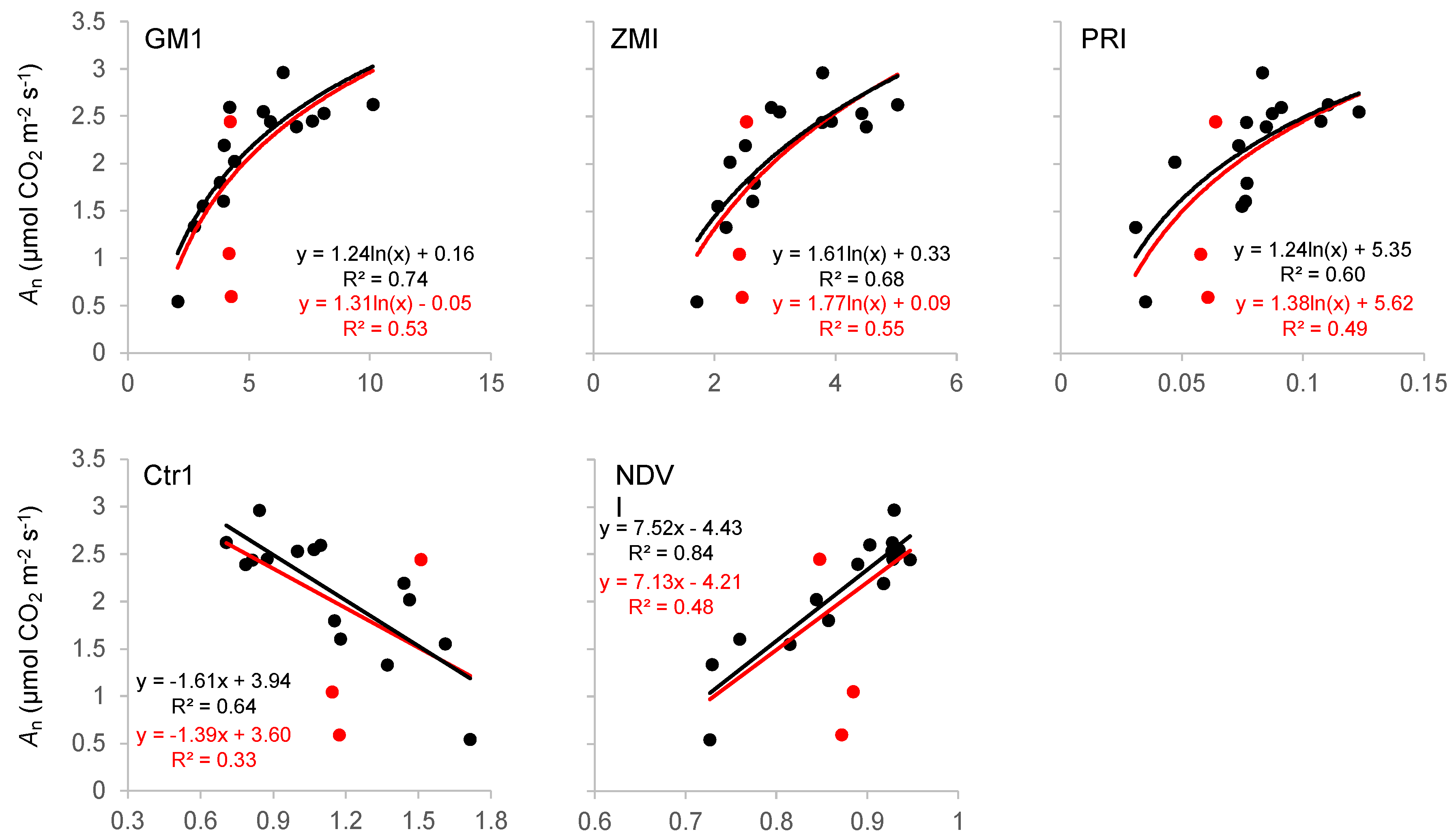
4.2. Remote Sensing of Gas-Exchange Parameters
4.3. AI and Machine Learning Supervised Classification for Tracking Vegetation Dynamics
5. Future Work and Implications for NBS and Urban Farming
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kabisch, N.; Frantzeskaki, N.; Pauleit, S.; Naumann, S.; Davis, M.; Artmann, M.; Haase, D.; Knapp, S.; Korn, H.; Stadler, J.; et al. Nature-based solutions to climate change mitigation and adaptation in urban areas. Ecol. Soc. 2016, 21, 15. [Google Scholar] [CrossRef] [Green Version]
- Seto, K.C.; Golden, J.S.; Alberti, M.; Turner, B.L. Sustainability in an urbanizing planet. Proc. Natl. Acad. Sci. USA 2017, 114, 8935–8938. [Google Scholar] [CrossRef] [Green Version]
- Sanchez Rodriguez, R.; Ürge-Vorsatz, D.; Barau, A.S. Sustainable Development Goals and climate change adaptation in cities. Nat. Clim. Chang. 2018, 8, 181–183. [Google Scholar] [CrossRef]
- European Commission. Towards an EU Research and Innovation Policy Agenda for Nature-Based Solutions and Re-Naturing Cities. Final Report of the Horizon 2020 Expert Group on “NatureBased Solutions and Re-Naturing Cities”; Publications Office of the European Union: Brussels, Belgium, 2015. [Google Scholar]
- Nan, X.; Yan, H.; Wu, R.; Shi, Y.; Bao, Z. Assessing the thermal performance of living wall systems in wet and cold climates during the winter. Energy Build. 2020, 208, 109680. [Google Scholar] [CrossRef]
- De Jesus, M.P.; Lourenço, J.M.; Arce, R.M.; Macias, M. Green façades and in situ measurements of outdoor building thermal behaviour. Build. Environ. 2017, 119, 11–19. [Google Scholar] [CrossRef] [Green Version]
- Gunawardena, K.; Steemers, K. Living walls in indoor environments. Build. Environ. 2019, 148, 478–487. [Google Scholar] [CrossRef]
- Rotem-Mindali, O.; Michael, Y.; Helman, D.; Lensky, I.M. The role of local land-use on the urban heat island effect of Tel Aviv as assessed from satellite remote sensing. Appl. Geogr. 2015, 56, 145–153. [Google Scholar] [CrossRef]
- Pérez-Urrestarazu, L.; Fernández-Cañero, R.; Franco, A.; Egea, G. Influence of an active living wall on indoor temperature and humidity conditions. Ecol. Eng. 2016, 90, 120–124. [Google Scholar] [CrossRef]
- Abdo, P.; Huynh, B.P. Effect of Passive Green Wall Modules on Air Temperature and Humidity; American Society of Mechanical Engineers: New York, NY, USA, 2018. [Google Scholar]
- Irga, P.J.; Paull, N.J.; Abdo, P.; Torpy, F.R. An assessment of the atmospheric particle removal efficiency of an in-room botanical biofilter system. Build. Environ. 2017, 115, 281–290. [Google Scholar] [CrossRef]
- Torpy, F.; Zavattaro, M. Bench-study of green-wall plants for indoor air pollution reduction. J. Living Archit. 2018, 5, 1–15. [Google Scholar] [CrossRef]
- Pettit, T.; Irga, P.J.; Torpy, F.R. The in situ pilot-scale phytoremediation of airborne VOCs and particulate matter with an active green wall. Air Qual. Atmos. Health 2019, 12, 33–44. [Google Scholar] [CrossRef]
- Torpy, F.; Zavattaro, M.; Irga, P. Green wall technology for the phytoremediation of indoor air: A system for the reduction of high CO2 concentrations. Air Qual. Atmos. Health 2017, 10, 575–585. [Google Scholar] [CrossRef]
- Torpy, F.; Clements, N.; Pollinger, M.; Dengel, A.; Mulvihill, I.; He, C.; Irga, P. Testing the single-pass VOC removal efficiency of an active green wall using methyl ethyl ketone (MEK). Air Qual. Atmos. Health 2018, 11, 163–170. [Google Scholar] [CrossRef] [Green Version]
- Gawrońska, H.; Bakera, B. Phytoremediation of particulate matter from indoor air by Chlorophytum comosum L. plants. Air Qual. Atmos. Health 2015, 8, 265–272. [Google Scholar] [CrossRef] [Green Version]
- Charoenkit, S.; Yiemwattana, S. Living walls and their contribution to improved thermal comfort and carbon emission reduction: A review. Build. Environ. 2016, 105, 82–94. [Google Scholar] [CrossRef]
- Soreanu, G. 12—Biotechnologies for Improving Indoor Air Quality; Pacheco-Torgal, F., Rasmussen, E., Granqvist, C.-G., Ivanov, V., Kaklauskas, A., Makonin, S.B.T.-S.-U.C., Eds.; Woodhead Publishing: Thorston, UK, 2016; pp. 301–328. ISBN 978-0-08-100546-0. [Google Scholar]
- Irga, P.J.; Pettit, T.J.; Torpy, F.R. The phytoremediation of indoor air pollution: A review on the technology development from the potted plant through to functional green wall biofilters. Rev. Environ. Sci. Biotechnol. 2018, 17, 395–415. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, J.S. Characterization and performance evaluation of a full-scale activated carbon-based dynamic botanical air filtration system for improving indoor air quality. Build. Environ. 2011, 46, 758–768. [Google Scholar] [CrossRef]
- Allen, J.G.; MacNaughton, P.; Satish, U.; Santanam, S.; Vallarino, J.; Spengler, J.D. Associations of cognitive function scores with carbon dioxide, ventilation, and volatile organic compound exposures in office workers: A controlled exposure study of green and conventional office environments. Environ. Health Perspect. 2016, 124, 805–812. [Google Scholar] [CrossRef] [Green Version]
- Yuan, X.; Laakso, K.; Davis, C.D.; Guzmán Q., J.A.; Meng, Q.; Sanchez-Azofeifa, A. Monitoring the Water Stress of an Indoor Living Wall System Using the “Triangle Method”. Sensors 2020, 20, 3261. [Google Scholar] [CrossRef]
- Peñuelas, J.; Marino, G.; LLusia, J.; Morfopoulos, C.; Farré-Armengol, G.; Filella, I. Photochemical reflectance index as an indirect estimator of foliar isoprenoid emissions at the ecosystem level. Nat. Commun. 2013, 4, 2604. [Google Scholar] [CrossRef] [Green Version]
- Peñuelas, J.; Garbulsky, M.F.; Filella, I. Photochemical reflectance index (PRI) and remote sensing of plant CO2 uptake. New Phytol. 2011, 191, 596–599. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Filella, I.; Garbulsky, F.M.; Peñuelas, J. Affecting Factors and Recent Improvements of the Photochemical Reflectance Index (PRI) for Remotely Sensing Foliar, Canopy and Ecosystemic Radiation-Use Efficiencies. Remote Sens. 2016, 8, 677. [Google Scholar] [CrossRef] [Green Version]
- Helman, D.; Lensky, I.M.; Osem, Y.; Rohatyn, S.; Rotenberg, E.; Yakir, D. A biophysical approach using water deficit factor for daily estimations of evapotranspiration and CO2 uptake in Mediterranean environments. Biogeosciences 2017, 14, 3909–3926. [Google Scholar] [CrossRef] [Green Version]
- Helman, D.; Bonfil, D.J.; Lensky, I.M. Crop RS-Met: A biophysical evapotranspiration and root-zone soil water content model for crops based on proximal sensing and meteorological data. Agric. Water Manag. 2019, 211, 210–219. [Google Scholar] [CrossRef]
- Helman, D.; Lensky, I.M.; Bonfil, D.J. Early prediction of wheat grain yield production from root-zone soil water content at heading using Crop RS-Met. Field Crops Res. 2019, 232, 11–23. [Google Scholar] [CrossRef]
- Lapidot, O.; Ignat, T.; Rud, R.; Rog, I.; Alchanatis, V.; Klein, T. Use of thermal imaging to detect evaporative cooling in coniferous and broadleaved tree species of the Mediterranean maquis. Agric. For. Meteorol. 2019, 271, 285–294. [Google Scholar] [CrossRef]
- Cohen, Y.; Alchanatis, V.; Saranga, Y.; Rosenberg, O.; Sela, E.; Bosak, A. Mapping water status based on aerial thermal imagery: Comparison of methodologies for upscaling from a single leaf to commercial fields. Precis. Agric. 2017, 18, 801–822. [Google Scholar] [CrossRef]
- Mulero, G.; Jiang, D.; Bonfil, D.J.; Helman, D. Use of thermal imaging and the Photochemical Reflectance Index (PRI) to detect wheat response to elevated CO2 and drought. Under Rev. 2022. [Google Scholar]
- Blanc, P. The Vertical Garden: From Nature to the City, 2nd ed.; W.W. Norton: New York, NY, USA, 2012. [Google Scholar]
- Johnstone, G. How to Grow Peperomia Obtusifolia (Baby Rubber Plants). Available online: https://www.thespruce.com/peperomia-obtusifolia-growing-guide-5271088 (accessed on 1 June 2022).
- ASPCA Blunt Leaf Peperomia. Available online: https://www.aspca.org/pet-care/animal-poison-control/toxic-and-non-toxic-plants/blunt-leaf-peperomia (accessed on 1 June 2022).
- Richard, A.; Ramey, V. Invasive and Nonnative Plants You Should Know–Recognition Cards; University of Florida-IFAS Publication # SP: Gainesville, FL, USA, 2007. [Google Scholar]
- Langeland, K.A.; Burks, K.C. Identification & Biology of Non-Native Plants in Florida’s Natural Areas 1998; University of Florida: Gainesville, FL, USA, 2013; pp. 138–157. [Google Scholar]
- Extension Gardener Tradescantia Spathacea. Available online: https://plants.ces.ncsu.edu/plants/tradescantia-spathacea/ (accessed on 1 June 2022).
- Rzhepakovsky, I.V.; Areshidze, D.A.; Avanesyan, S.S.; Grimm, W.D.; Filatova, N.V.; Kalinin, A.V.; Kochergin, S.G.; Kozlova, M.A.; Kurchenko, V.P.; Sizonenko, M.N.; et al. Phytochemical Characterization, Antioxidant Activity, and Cytotoxicity of Methanolic Leaf Extract of Chlorophytum Comosum (Green Type) (Thunb.) Jacq. Molecules 2022, 27, 762. [Google Scholar] [CrossRef]
- Wolverton, B. Plants and soil microorganisms: Removal of formaldehyde, xylene, and ammonia from the indoor environment. J. Miss. Acad. Sci. 1993, 38, 11. [Google Scholar]
- Turner, C. Ten Poisonous Indoor Plants Your Children and Pets Should Avoid. Available online: https://blog.mytastefulspace.com/2020/03/04/poisonous-indoor-plants/ (accessed on 1 June 2022).
- USDA Taxon: Philodendron Hederaceum (Jacq.) Schott. Available online: https://npgsweb.ars-grin.gov/gringlobal/taxon/taxonomydetail?id=102625 (accessed on 1 June 2022).
- Wong, S.C.; Cowan, I.R.; Farquhar, G.D. Stomatal conductance correlates with photosynthetic capacity. Nature 1979, 282, 424–426. [Google Scholar] [CrossRef]
- Behmann, J.; Acebron, K.; Emin, D.; Bennertz, S.; Matsubara, S.; Thomas, S.; Bohnenkamp, D.; Kuska, M.T.; Jussila, J.; Salo, H.; et al. Specim IQ: Evaluation of a New, Miniaturized Handheld Hyperspectral Camera and Its Application for Plant Phenotyping and Disease Detection. Sensors 2018, 18, 441. [Google Scholar] [CrossRef] [Green Version]
- Ben-Gal, A.; Agam, N.; Alchanatis, V.; Cohen, Y.; Yermiyahu, U.; Zipori, I.; Presnov, E.; Sprintsin, M.; Dag, A. Evaluating water stress in irrigated olives: Correlation of soil water status, tree water status, and thermal imagery. Irrig. Sci. 2009, 27, 367–376. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, Y.; Pu, R.; Gonzalez-Moreno, P.; Yuan, L.; Wu, K.; Huang, W. Monitoring plant diseases and pests through remote sensing technology: A review. Comput. Electron. Agric. 2019, 165, 104943. [Google Scholar] [CrossRef]
- Helman, D. Land surface phenology: What do we really ‘see’ from space? Sci. Total Environ. 2018, 618, 665–673. [Google Scholar] [CrossRef]
- Helman, D.; Mussery, A. Using Landsat satellites to assess the impact of check dams built across erosive gullies on vegetation rehabilitation. Sci. Total Environ. 2020, 730, 138873. [Google Scholar] [CrossRef]
- Manfreda, S.; McCabe, M.F.; Miller, P.E.; Lucas, R.; Madrigal, V.P.; Mallinis, G.; Ben Dor, E.; Helman, D.; Estes, L.; Ciraolo, G.; et al. On the use of unmanned aerial systems for environmental monitoring. Remote Sens. 2018, 10, 641. [Google Scholar] [CrossRef] [Green Version]
- Gitelson, A.A.; Merzlyak, M.N. Remote estimation of chlorophyll content in higher plant leaves. Int. J. Remote Sens. 1997, 18, 2691–2697. [Google Scholar] [CrossRef]
- Zarco-Tejada, P.J.; Miller, J.R.; Noland, T.L.; Mohammed, G.H.; Sampson, P.H. Scaling-up and model inversion methods with narrowband optical indices for chlorophyll content estimation in closed forest canopies with hyperspectral data. IEEE Trans. Geosci. Remote Sens. 2001, 39, 1491–1507. [Google Scholar] [CrossRef] [Green Version]
- Gamon, J.A.; Penuelas, J.; Field, C.B. A Narrow-Waveband Spectral Index That Tracks Diurnal Changes in Photosynthetic Efficiency. Remote Sens. Environ. 1992, 41, 35–44. [Google Scholar] [CrossRef]
- Carter, G.A. Ratios of leaf reflectances in narrow wavebands as indicators of plant stress. Int. J. Remote Sens. 1994, 15, 517–520. [Google Scholar] [CrossRef]
- Rouse, J.W.; Haas, R.H.; Schell, J.A.; Deering, D.W.; Harlan, J.C. Monitoring the Vernal Advancement and Retrogradation of Natural Vegetation, O F Natural; Texas A&M Univ.: College Station, TX, USA, 1974. [Google Scholar]
- Garbulsky, M.F.; Peñuelas, J.; Gamon, J.; Inoue, Y.; Filella, I. The photochemical reflectance index (PRI) and the remote sensing of leaf, canopy and ecosystem radiation use efficiencies: A review and meta-analysis. Remote Sens. Environ. 2011, 115, 281–297. [Google Scholar] [CrossRef]
- Filella, I.; Peñuelas, J.; Llorens, L.; Estiarte, M. Reflectance assessment of seasonal and annual changes in biomass and CO2 uptake of a Mediterranean shrubland submitted to experimental warming and drought. Remote Sens. Environ. 2004, 90, 308–318. [Google Scholar] [CrossRef]
- Gamon, J.A.; Serrano, L.; Surfus, J.S. The photochemical reflectance index: An optical indicator of photosynthetic radiation use efficiency across species, functional types, and nutrient levels. Oecologia 1997, 112, 492–501. [Google Scholar] [CrossRef]
- Amthor, J.S. Scaling CO2-photosynthesis relationships from the leaf to the canopy. Photosynth. Res. 1994, 39, 321–350. [Google Scholar] [CrossRef]
- Magney, T.S.; Vierling, L.A.; Eitel, J.U.H.; Huggins, D.R.; Garrity, S.R. Response of high frequency Photochemical Reflectance Index (PRI) measurements to environmental conditions in wheat. Remote Sens. Environ. 2016, 173, 84–97. [Google Scholar] [CrossRef]
- Cortes, C.; Vapnik, V. Support-vector networks. Mach. Learn. 1995, 20, 273–297. [Google Scholar] [CrossRef]
- ESRI ArcGIS PRO, Version 2.9. Redlands, California. 2019. Available online: https://www.esri.com/en-us/about/about-esri/company (accessed on 1 July 2022).
- Liu, Y.; Hassan, K.A.; Karlsson, M.; Weister, O.; Gong, S. Active Plant Wall for Green Indoor Climate Based on Cloud and Internet of Things. IEEE Access 2018, 6, 33631–33644. [Google Scholar] [CrossRef]
- Helman, D.; Bahat, I.; Netzer, Y.; Ben-Gal, A.; Alchanatis, V.; Peeters, A.; Cohen, Y. Using time series of high-resolution planet satellite images to monitor grapevine stem water potential in commercial vineyards. Remote Sens. 2018, 10, 1615. [Google Scholar] [CrossRef] [Green Version]
- Troncoso-Pastoriza, F.; Martínez-Comesaña, M.; Ogando-Martínez, A.; López-Gómez, J.; Eguía-Oller, P.; Febrero-Garrido, L. IoT-based platform for automated IEQ spatio-temporal analysis in buildings using machine learning techniques. Autom. Constr. 2022, 139, 104261. [Google Scholar] [CrossRef]
- Saleem, M.H.; Potgieter, J.; Arif, K.M. Plant Disease Detection and Classification by Deep Learning. Plants 2019, 8, 468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Pang, Z.; Karlsson, M.; Gong, S. Anomaly detection based on machine learning in IoT-based vertical plant wall for indoor climate control. Build. Environ. 2020, 183, 107212. [Google Scholar] [CrossRef]
- Kpienbaareh, D.; Kansanga, M.; Luginaah, I. Examining the potential of open source remote sensing for building effective decision support systems for precision agriculture in resource-poor settings. GeoJournal 2019, 84, 1481–1497. [Google Scholar] [CrossRef]
- Ennouri, K.; Smaoui, S.; Gharbi, Y.; Cheffi, M.; Ben Braiek, O.; Ennouri, M.; Triki, M.A. Usage of Artificial Intelligence and Remote Sensing as Efficient Devices to Increase Agricultural System Yields. J. Food Qual. 2021, 2021, 6242288. [Google Scholar] [CrossRef]
- Jie, Q. Precision and intelligent agricultural decision support system based on big data analysis. Acta Agric. Scand. Sect. B—Soil Plant Sci. 2022, 72, 401–414. [Google Scholar] [CrossRef]



| Scientific Name | Common Name | C-Pathway | Native Area | Optimal Growth Temperatures (°C) |
|---|---|---|---|---|
| Peperomia obtusifolia | Baby rubber plant | C3 | Mexico, South America, and West Indies | 16–26 |
| Tradescantia spathacea | Moses in the cradle or wondering jew | C3 | Southern Mexico, Belize, Guatemala | 14–27 |
| Chlorophytum comosum | Spider plant | C3 | South Africa | 15–30 |
| Spathiphyllum wallisii Regel | Peace lily | C3 | Central America | 15–30 |
| Aeschynanthus radicans “Monalisa” | Monalisa or lipstick plant | C3 | Malaysia | 15–30 |
| Philodendron hederaceum | Philodendron | C3 | North and South America | 15–26 |
| Index | Full Name | Formula | Main Characteristics and Uses |
|---|---|---|---|
| GM1 [49] | Gitelson and Merzlyak index 1 | The GM1 was developed based on the sensitivity of the 550 nm band to a wide range of chlorophyll variations. It is a useful index for monitoring plant chlorophyll content and photosynthetic capacity. | |
| ZMI [50] | Zarco-Tejada and Miller index | The ZMI, based on the red-edge band, was developed to assess changes in available pigment content in leaves and over canopies. | |
| PRI [51] | Photochemical reflectance index | The PRI uses the 531 nm band, which is sensitive to variations in the dissipation of light energy via xanthophyll de-epoxidation. It is related to the fast transition in the xanthophyll cycle, making it a good proxy of the plant light use efficiency, an important factor in the photosynthetic process. | |
| Ctr1 [52] | Carter index 1 | The Ctr1 features the 695 and 420 nm bands, which are sensitive to changes in total chlorophyll concentrations, especially under stress. It has been used for the early detection of stresses in plants. | |
| NDVI [53] | Normalized difference vegetation index | The NDVI is the most commonly used vegetation index in proximal and remote sensing [46]. It has been used to measure the state of plant health as well as its phenology and leaf area index. It is also a useful index for estimating vegetation biomass and productivity [47]. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Helman, D.; Yungstein, Y.; Mulero, G.; Michael, Y. High-Throughput Remote Sensing of Vertical Green Living Walls (VGWs) in Workplaces. Remote Sens. 2022, 14, 3485. https://doi.org/10.3390/rs14143485
Helman D, Yungstein Y, Mulero G, Michael Y. High-Throughput Remote Sensing of Vertical Green Living Walls (VGWs) in Workplaces. Remote Sensing. 2022; 14(14):3485. https://doi.org/10.3390/rs14143485
Chicago/Turabian StyleHelman, David, Yehuda Yungstein, Gabriel Mulero, and Yaron Michael. 2022. "High-Throughput Remote Sensing of Vertical Green Living Walls (VGWs) in Workplaces" Remote Sensing 14, no. 14: 3485. https://doi.org/10.3390/rs14143485
APA StyleHelman, D., Yungstein, Y., Mulero, G., & Michael, Y. (2022). High-Throughput Remote Sensing of Vertical Green Living Walls (VGWs) in Workplaces. Remote Sensing, 14(14), 3485. https://doi.org/10.3390/rs14143485