Variability in the Spatiotemporal Distribution Patterns of Greater Amberjack in Response to Environmental Factors in the Taiwan Strait Using Remote Sensing Data
Abstract
:1. Introduction
2. Materials and Methods
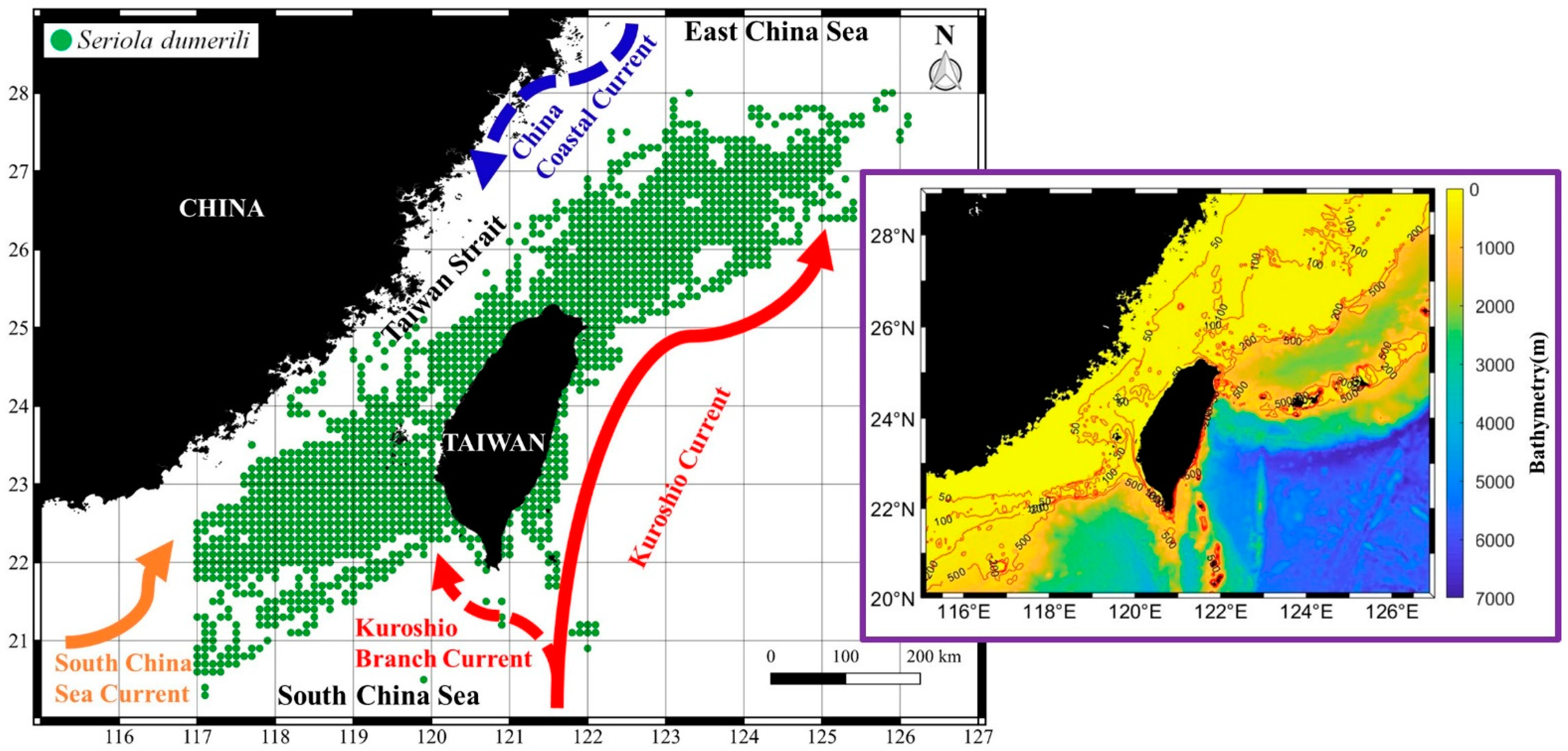
2.1. Greater Amberjack Fisheries Data
2.2. Satellite-Sensed Environmental Data
2.3. Statistical Models for the Spatiotemporal Predictions of Catch Rates
2.4. Predictions of Greater Amberjack Catch Rates
3. Results
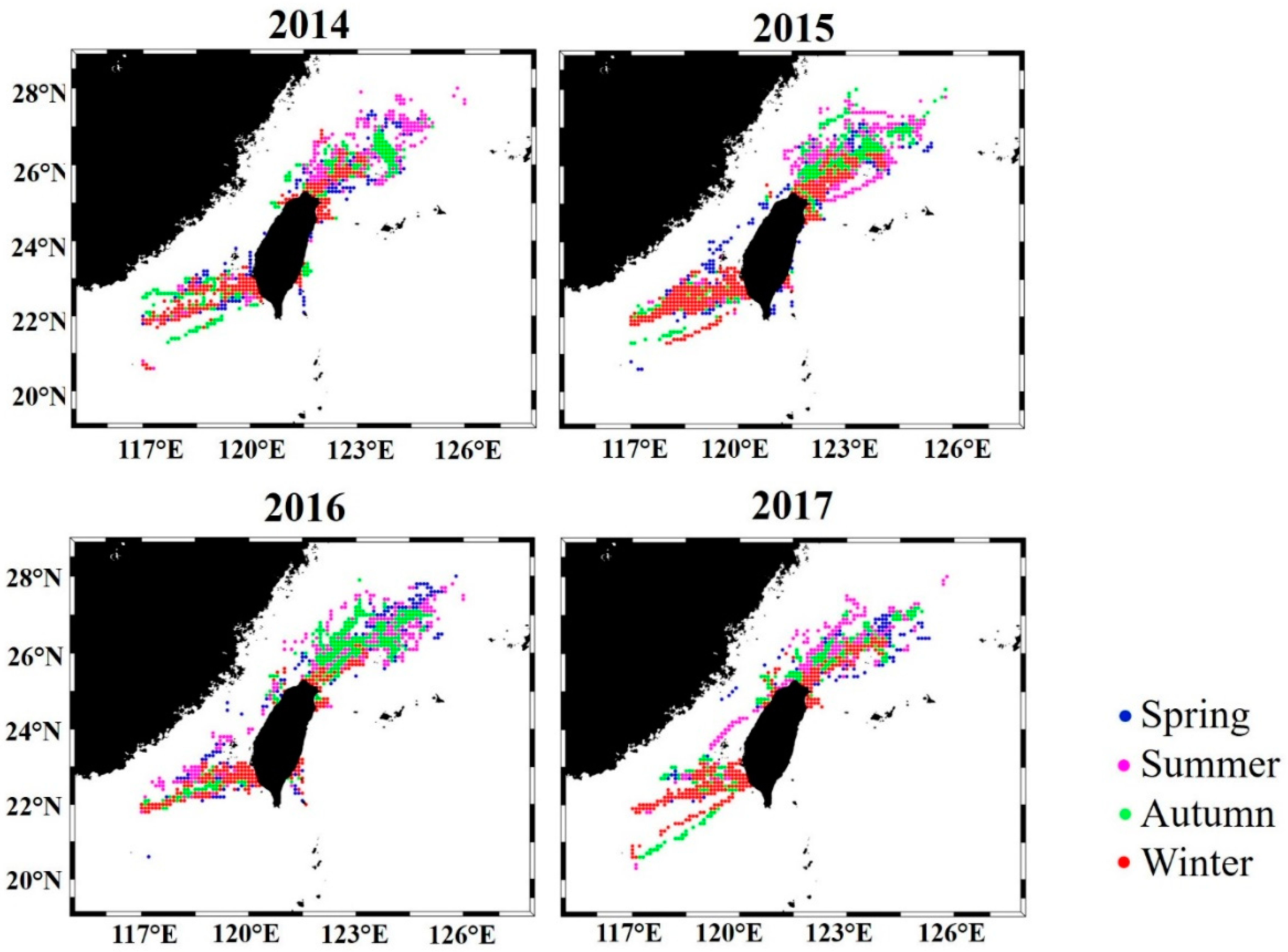
3.1. Spatial and Temporal Distribution Pattern of S. dumerili in the Taiwan Strait
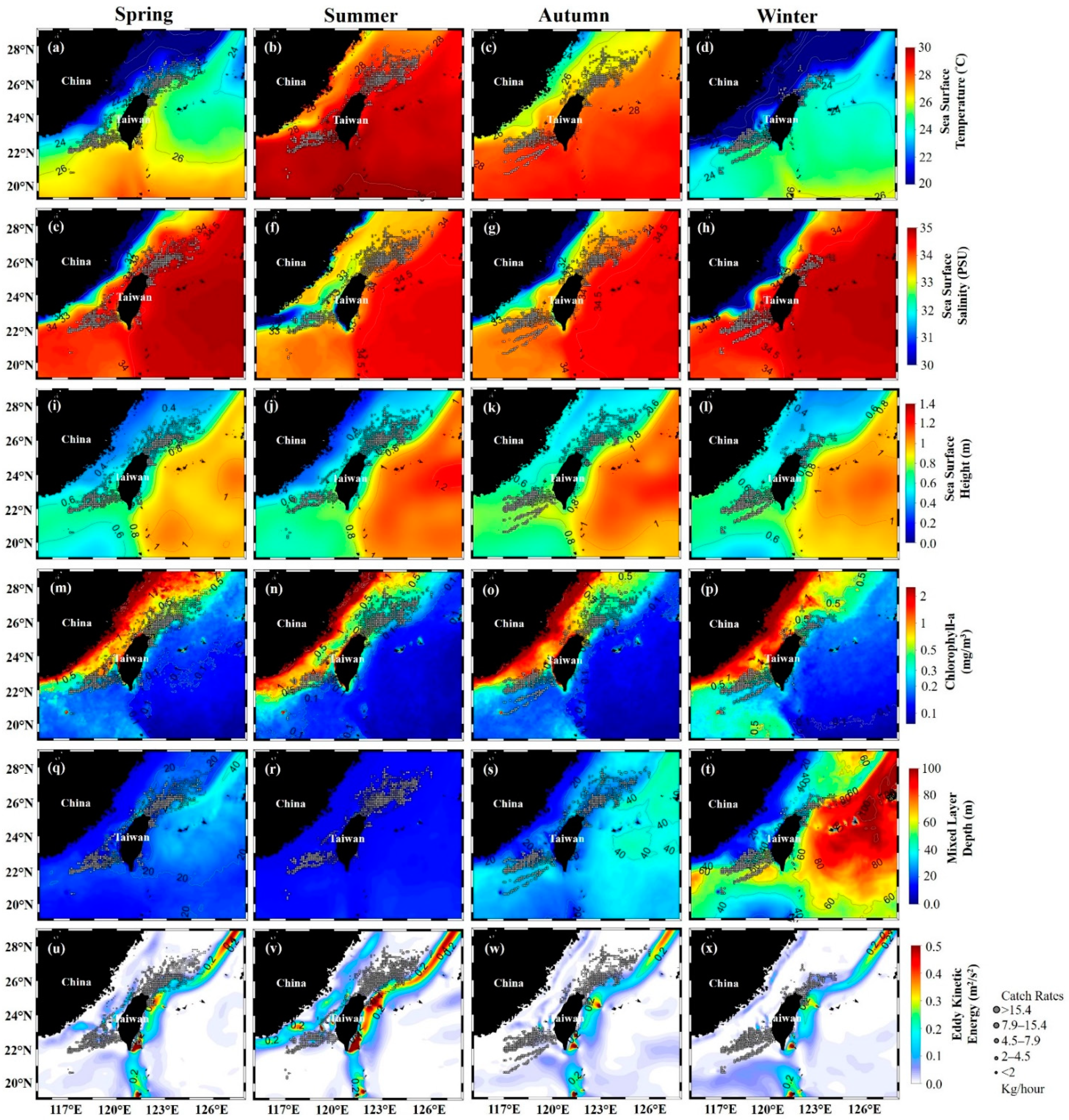
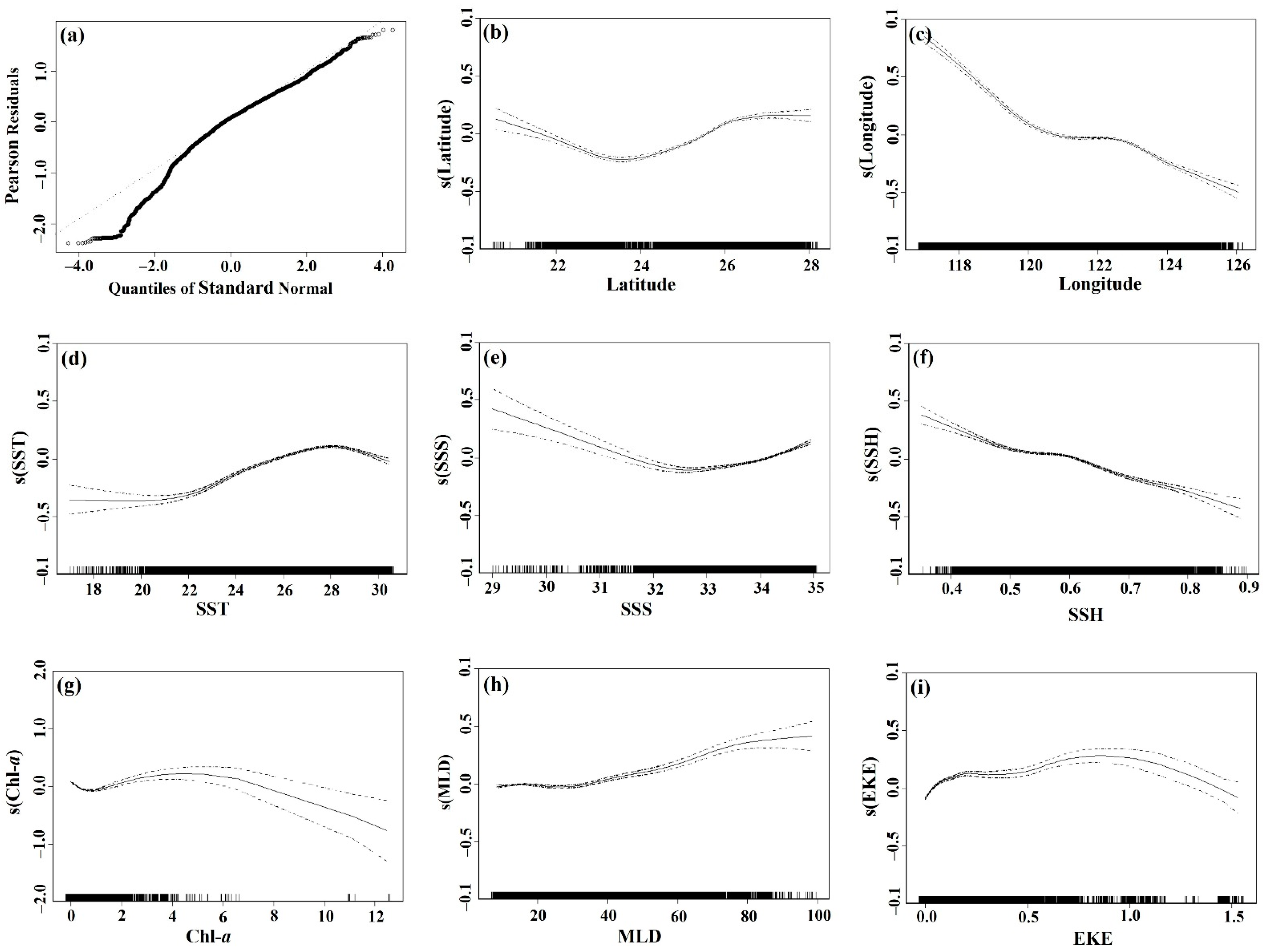
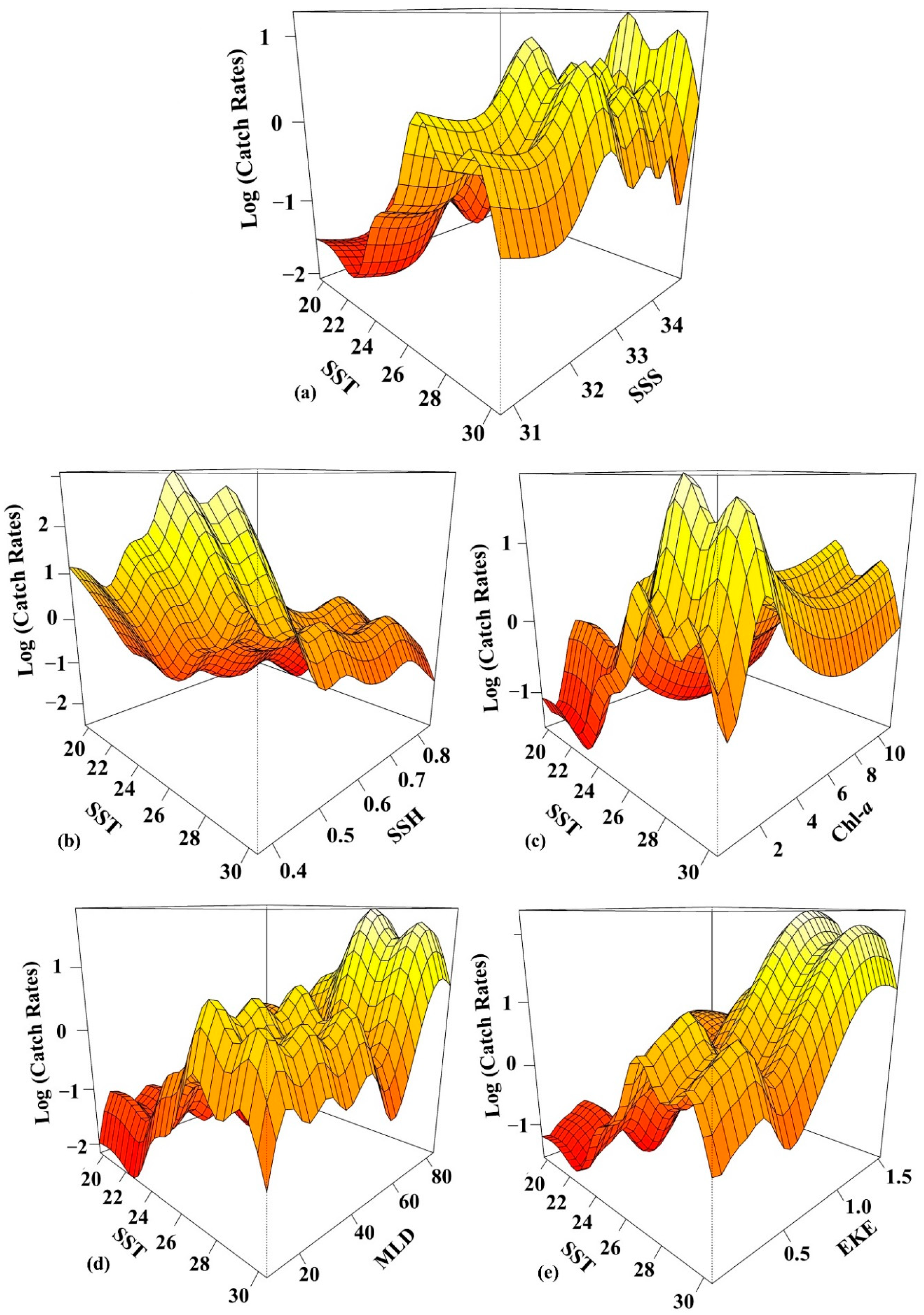
3.2. Environmental Effect on S. dumerili Catch Rates by Statistical Model
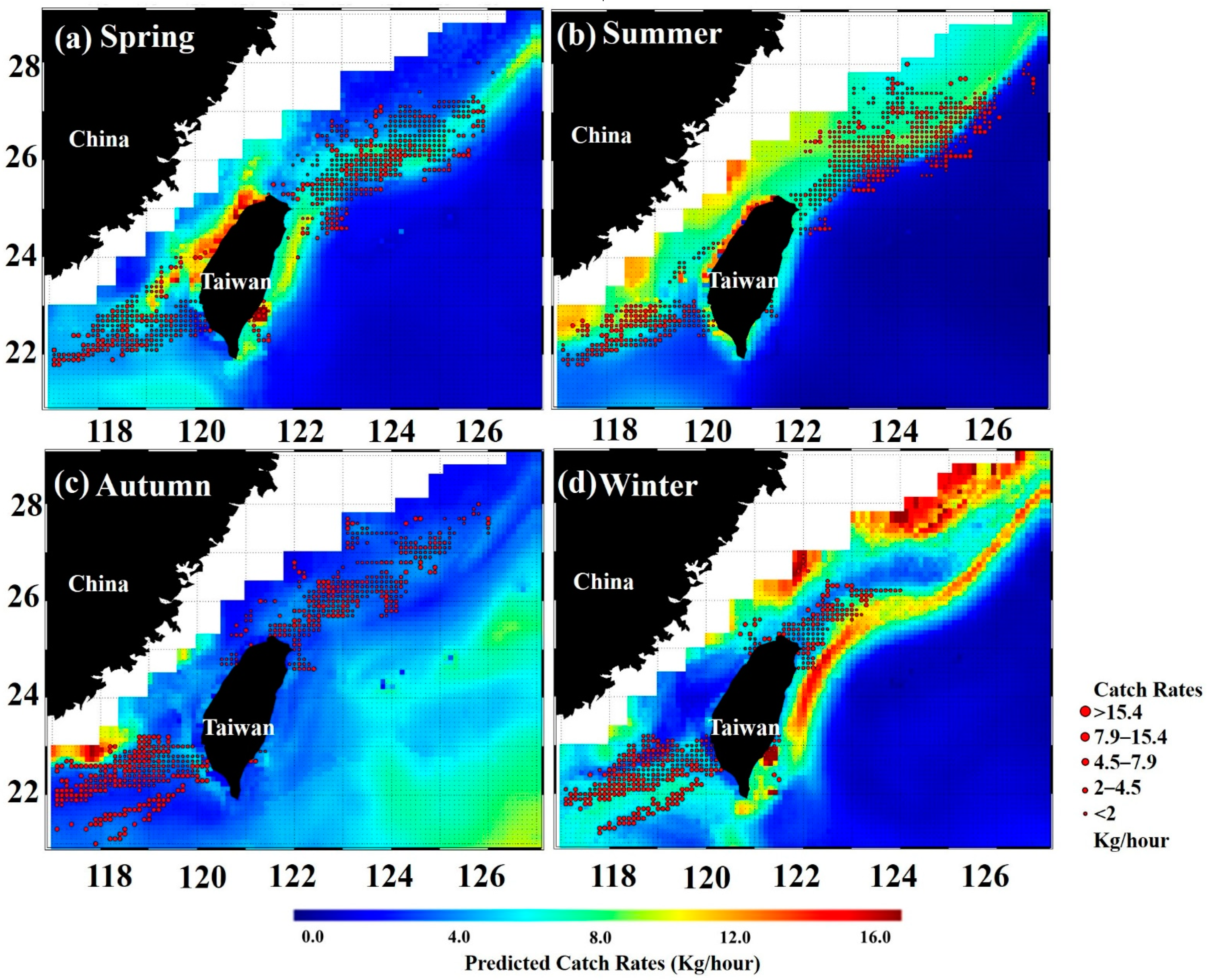
3.3. Predicted Spatial Distribution Pattern of the Greater Amberjack
4. Discussion
4.1. Distribution of Greater Amberjack in the Taiwan Strait
4.2. Environmental Factors Affecting the Greater Amberjack
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Saraux, C.; Fromentin, J.M.; Bigot, J.L.; Bourdeix, J.H.; Morfin, M.; Roos, D.; Van Beveren, E.; Bez, N. Spatial structure and distribution of small pelagic fish in the northwestern Mediterranean Sea. PLoS ONE 2014, 9, 111211. [Google Scholar]
- Garrison, L.P.; Michaels, W.; Link, J.S.; Fogarty, M.J. Spatial distribution and overlap between ichthyoplankton and pelagic fish and squids on the southern flank of Georges Bank. Fish. Oceanogr. 2002, 11, 267–285. [Google Scholar] [CrossRef]
- Azevedo, M.; Silva, C. A framework to investigate fishery dynamics and species size and age spatio-temporal distribution patterns based on daily resolution data: A case study using Northeast Atlantic horse mackerel. ICES J. Mar. Sci. 2020, 77, 2933–2944. [Google Scholar] [CrossRef]
- Ward, E.J.; Jannot, J.E.; Lee, Y.W.; Ono, K.; Shelton, A.O.; Thorson, J.T. Using spatiotemporal species distribution models to identify temporally evolving hotspots of species co-occurrence. Ecol. Appl. 2015, 25, 2198–2209. [Google Scholar] [CrossRef]
- Kai, M.; Thorson, J.T.; Piner, K.R.; Maunder, M.N. Predicting the spatio-temporal distributions of pelagic sharks in the western and central North Pacific. Fish. Oceanogr. 2017, 26, 569–582. [Google Scholar] [CrossRef]
- Lee, M.A.; Vayghan, A.H.; Liu, D.C.; Yang, W.C. Potential and prospective seasonal distribution of hotspot habitat of albacore tuna (Thunnus alalunga) in the South Indian Ocean using the satellite data. In Proceedings of the 2017 IEEE International Geoscience and Remote Sensing Symposium (IGARSS), Fort Worth, TX, USA, 23–28 July 2017; pp. 5747–5750. [Google Scholar]
- Vayghan, A.H.; Lee, M.A.; Weng, J.S.; Mondal, S.; Lin, C.T.; Wang, Y.C. Multisatellite-based feeding habitat suitability modeling of albacore tuna in the Southern Atlantic Ocean. Remote Sens. 2020, 12, 2515. [Google Scholar] [CrossRef]
- Mansor, S.; Tan, C.K.; Ibrahim, H.M.; Shariff, A.R.M. Satellite fish forecasting in south China sea. In Proceedings of the 22nd Asian Conference on Remote Sensing, Singapore, 5–9 November 2001; Volume 5. No. 9. [Google Scholar]
- Zhang, X.; Saitoh, S.I.; Hirawake, T. Predicting potential fishing zones of japanese common squid (Todarodes Pacificus) using remotely sensed images in coastal waters of south-western Hokkaido, Japan. Int. J. Remote Sens. 2017, 38, 6129–6146. [Google Scholar] [CrossRef]
- Lee, D.; Son, S.; Kim, W.; Park, J.M.; Joo, H.; Lee, S.H. Spatio-temporal variability of the habitat suitability index for Chub Mackerel (Scomber japonicus) in the East/Japan sea and the South sea of South Korea. Remote Sens. 2018, 10, 938. [Google Scholar] [CrossRef] [Green Version]
- Abdul Azeez, P.; Raman, M.; Rohit, P.; Shenoy, L.; Jaiswar, A.K.; Mohammed Koya, K.; Damodaran, D. Predicting potential fishing grounds of ribbonfish (Trichiurus lepturus) in the north-eastern Arabian Sea, using remote sensing data. Int. J. Remote Sens. 2021, 42, 322–342. [Google Scholar] [CrossRef]
- Taki, Y.; Kohno, H.; Sakamaot, K.; Hosoya, K. Illustrated Fishes in Colour Revised Edition; Hokuryukan Co., Ltd.: Tokyo, Japan, 2005. (In Japanese) [Google Scholar]
- Hasegawa, T.; Lu, C.P.; Hsiao, S.T.; Uchino, T.; Yeh, H.M.; Chiang, W.C.; Chen, J.R.; Sassa, C.; Komeyama, K.; Kawabe, R.; et al. Distribution and genetic variability of young-of-the-year greater amberjack (Seriola dumerili) in the East China Sea. Environ. Biol. Fishes 2020, 103, 833–846. [Google Scholar] [CrossRef]
- Thompson, B.A.; Beasley, M.; Wilson, C.A. Age distribution and growth of greater amberjack, Seriola dumerili, from the north-central Gulf of Mexico. Fish. Bull. 1999, 97, 362–371. [Google Scholar]
- Wells, R.J.; Rooker, J.R. Distribution, age, and growth of young-of-the year greater amberjack (Seriola dumerili) associated with pelagic Sargassum. Fish. Bull. 2004, 102, 545–554. [Google Scholar]
- Hasegawa, T.; Yeh, H.M.; Chen, J.R.; Kuo, C.L.; Kawabe, R.; Sakakura, Y. Collection and aging of greater amberjack Seriola dumerili larvae and juveniles around the Penghu Islands, Taiwan. Ichthyol. Res. 2017, 64, 145–150. [Google Scholar] [CrossRef] [Green Version]
- Sassa, C.; Tsukamoto, Y.; Nishiuchi, K.; Konishi, Y. Spawning ground and larval transport processes of jack mackerel Trachurus japonicus in the shelf-break region of the southern East China Sea. Cont. Shelf Res. 2008, 28, 2574–2583. [Google Scholar] [CrossRef]
- Sassa, C.; Tsukamoto, Y. Distribution and growth of Scomber japonicus and S. australasicus larvae in the southern East China Sea in response to oceanographic conditions. Mar. Ecol. Prog. Ser. 2010, 419, 185–199. [Google Scholar] [CrossRef] [Green Version]
- Fisher, W.; Bianchi, G.; Scott, W.B. FAO Species Identification Guides for Fishery Purposes; Volume 3, Eastern Central Atlantic, Fishing Area 34 and Part of 47; Food and Agriculture Organization: Ottawa, ON, Canada, 1981. [Google Scholar]
- Huh, C.A.; Chen, W.; Hsu, F.H.; Su, C.C.; Chiu, J.K.; Lin, S.; Liu, C.S.; Huang, B.J. Modern (<100 years) sedimentation in the Taiwan Strait: Rates and source-to-sink pathways elucidated from radionuclides and particle size distribution. Cont. Shelf Res. 2011, 31, 47–63. [Google Scholar] [CrossRef]
- Naimullah, M.; Lan, K.W.; Liao, C.H.; Hsiao, P.Y.; Liang, Y.R.; Chiu, T.C. Association of environmental factors in the Taiwan strait with distributions and habitat characteristics of three swimming crabs. Remote Sens. 2020, 12, 2231. [Google Scholar] [CrossRef]
- Zainuddin, M.; Saitoh, S.I.; Saitoh, K. Detection of potential fishing ground for albacore tuna using synoptic measurements of ocean color and thermal remote sensing in the northwestern North Pacific. Geophys. Res. Lett. 2004, 31. [Google Scholar] [CrossRef]
- Chen, I.C.; Lee, P.F.; Tzeng, W.N. Distribution of albacore (Thunnus alalunga) in the Indian Ocean and its relation to environmental factors. Fish. Oceanogr. 2005, 14, 71–80. [Google Scholar] [CrossRef]
- Tian, Y.; Kidokoro, H.; Watanabe, T.; Igeta, Y.; Sakaji, H.; Ino, S. Response of yellowtail, Seriola quinqueradiata, a key large predatory fish in the Japan Sea, to sea water temperature over the last century and potential effects of global warming. J. Mar. Syst. 2012, 91, 1–10. [Google Scholar] [CrossRef]
- Brodie, S.; Hobday, A.J.; Smith, J.A.; Everett, J.D.; Taylor, M.D.; Gray, C.A.; Suthers, I.M. Modelling the oceanic habitats of two pelagic species using recreational fisheries data. Fish. Oceanogr. 2015, 24, 463–477. [Google Scholar] [CrossRef]
- Champion, C.; Hobday, A.J.; Zhang, X.; Pecl, G.T.; Tracey, S.R. Changing windows of opportunity: Past and future climate-driven shifts in temporal persistence of kingfish (Seriola lalandi) oceanographic habitat within south-eastern Australian bioregions. Mar. Freshw. Res. 2018, 70, 33–42. [Google Scholar] [CrossRef]
- Furukawa, S.; Kozuka, A.; Tsuji, T.; Kubota, H. Horizontal and vertical movement of yellowtails Seriola quinqueradiata during summer to early winter recorded by archival tags in the northeastern Japan Sea. Mar. Ecol. Prog. Ser. 2020, 636, 139–156. [Google Scholar] [CrossRef]
- Sassa, C.; Takahashi, M.; Konishi, Y.; Yoshimasa, A.; Tsukamoto, Y. The rapid expansion of yellowtail (Seriola quinqueradiata) spawning ground in the East China Sea is linked to increasing recruitment and spawning stock biomass. ICES J. Mar. Sci. 2020, 77, 581–592. [Google Scholar] [CrossRef]
- Mondal, S.; Vayghan, A.H.; Lee, M.A.; Wang, Y.C.; Semedi, B. Habitat suitability modeling for the feeding ground of immature albacore in the Southern Indian Ocean using satellite-derived sea surface temperature and chlorophyll data. Remote Sens. 2021, 13, 2669. [Google Scholar] [CrossRef]
- Teng, S.Y.; Su, N.J.; Lee, M.A.; Lan, K.W.; Chang, Y.; Weng, J.S.; Wang, Y.C.; Sihombing, R.I.; Vayghan, A.H. Modeling the habitat distribution of Acanthopagrus schlegelii in the coastal waters of the Eastern Taiwan strait using MAXENT with fishery and remote sensing data. J. Mar. Sci. Eng. 2021, 9, 1442. [Google Scholar] [CrossRef]
- Lee, M.A.; Weng, J.S.; Lan, K.W.; Vayghan, A.H.; Wang, Y.C.; Chan, J.W. Empirical habitat suitability model for immature albacore tuna in the North Pacific Ocean obtained using multisatellite remote sensing data. Int. J. Remote Sens. 2020, 41, 5819–5837. [Google Scholar] [CrossRef]
- Guisan, A.; Zimmermann, N.E. Predictive habitat distribution models in ecology. Ecol. Modell. 2000, 135, 147–186. [Google Scholar] [CrossRef]
- Wang, L.; Kerr, L.A.; Record, N.R.; Bridger, E.; Tupper, B.; Mills, K.E.; Armstrong, E.M.; Pershing, A.J. Modeling marine pelagic fish species spatiotemporal distributions utilizing a maximum entropy approach. Fish. Oceanogr. 2018, 27, 571–586. [Google Scholar] [CrossRef]
- Elith, J.; Leathwick, J.R. Species distribution models: Ecological explanation and prediction across space and time. Annu. Rev. Ecol. Evol. Syst. 2009, 40, 677–697. [Google Scholar] [CrossRef]
- Solanki, H.U.; Bhatpuria, D.; Chauhan, P. Applications of generalized additive model (GAM) to satellite-derived variables and fishery data for prediction of fishery resources distributions in the Arabian Sea. Geocarto Int. 2017, 32, 30–43. [Google Scholar] [CrossRef]
- Liao, C.H.; Lan, K.W.; Ho, H.Y.; Wang, K.Y.; Wu, Y.L. Variation in the catch rate and distribution of swordtip squid Uroteuthis edulis associated with factors of the oceanic environment in the Southern East China Sea. Mar. Coast. Fish. 2018, 10, 452–464. [Google Scholar] [CrossRef] [Green Version]
- Hastie, T.; Tibshirani, R. Generalized additive models. Stat. Sci. 1986, 1, 297–310. [Google Scholar] [CrossRef]
- Zainuddin, M.; Saitoh, K.; Saitoh, S.I. Albacore (Thunnus alalunga) fishing ground in relation to oceanographic conditions in the western North Pacific Ocean using remotely sensed satellite data. Fish. Oceanogr. 2008, 17, 61–73. [Google Scholar] [CrossRef] [Green Version]
- Lan, K.W.; Shimada, T.; Lee, M.A.; Su, N.J.; Chang, Y. Using remote-sensing environmental and fishery data to map potential yellowfin tuna habitats in the tropical Pacific Ocean. Remote Sens. 2017, 9, 444. [Google Scholar] [CrossRef] [Green Version]
- QGIS Development Team. QGIS 3.6 Noosa. Available online: https://qgis.org/en/site (accessed on 30 September 2019).
- Lan, K.W.; Evans, K.; Lee, M.A. Effects of climate variability on the distribution and fishing conditions of yellowfin tuna (Thunnus albacares) in the western Indian Ocean. Clim. Chang. 2013, 119, 63–77. [Google Scholar] [CrossRef] [Green Version]
- Chai, T.; Draxler, R.R. Root mean square error (RMSE) or mean absolute error (MAE)?—Arguments against avoiding RMSE in the literature. Geosci. Model Dev. 2014, 7, 1247–1250. [Google Scholar] [CrossRef] [Green Version]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Hsiao, S.T.; Li, C.H.; Yeh, H.M. Seasonal changes in the gonadosomatic index of the greater amberjack in Taiwanese waters. 2016; Unpublished raw data. [Google Scholar]
- Yue, J.; Noman, M.A.; Sun, J. Kuroshio intrusion drives the Trichodesmium assemblage and shapes the phytoplankton community during spring in the East China Sea. J. Oceanol. Limnol. 2021, 39, 536–549. [Google Scholar] [CrossRef]
- Tone, K.; Nakamura, Y.; Chiang, W.C.; Yeh, H.M.; Hsiao, S.T.; Li, C.H.; Komeyama, K.; Tomisaki, M.; Hasegawa, T.; Sakamoto, T.; et al. Migration and spawning behavior of the greater amberjack Seriola dumerili in eastern Taiwan. Fish. Oceanogr. 2022, 31, 1–18. [Google Scholar] [CrossRef]
- Yin, W.; Huang, D. Short-term variations in the surface upwelling off northeastern Taiwan observed via satellite data. J. Geophys. Res. Oceans 2019, 124, 939–954. [Google Scholar] [CrossRef]
- Zhuang, Z.; Zheng, Q.; Zhang, X.; Yang, G.; Zhao, X.; Cao, L.; Zhang, T.; Yuan, Y. Variability of Kuroshio surface axis northeast of Taiwan island derived from satellite altimeter data. Remote Sens. 2020, 12, 1059. [Google Scholar] [CrossRef] [Green Version]
- Kang, J.; Na, H. Long-term variability of the Kuroshio shelf intrusion and its relationship to upper-ocean current and temperature variability in the East China Sea. Front. Mar. Sci. 2022, 9, 812911. [Google Scholar] [CrossRef]
- Chen, L.; Dong, C.; Wang, G. GOCI-observed chlorophyll belts associated with sea-surface fronts in the East China Sea. IEEE Geosci. Remote. Sens. Lett. 2019, 17, 1299–1302. [Google Scholar] [CrossRef]
- Park, M.S.; Lee, S.; Ahn, J.H.; Lee, S.J.; Choi, J.K.; Ryu, J.H. Decadal measurements of the first Geostationary Ocean Color Satellite (GOCI) compared with MODIS and VIIRS data. Remote Sens. 2021, 14, 72. [Google Scholar] [CrossRef]
- Lan, K.W.; Kawamura, H.; Lee, M.A.; Chang, Y.; Chan, J.W.; Liao, C.H. Summertime sea surface temperature fronts associated with upwelling around the Taiwan Bank. Cont. Shelf Res. 2009, 29, 903–910. [Google Scholar] [CrossRef]
- Chang, Y.; Shimada, T.; Lee, M.A.; Lu, H.J.; Sakaida, F.; Kawamura, H. Wintertime sea surface temperature fronts in the Taiwan Strait. Geophys. Res. Lett. 2006, 33. [Google Scholar] [CrossRef]
- Chang, Y.; Lee, M.A.; Shimada, T.; Sakaida, F.; Kawamura, H.; Chan, J.W.; Lu, H.J. Wintertime high-resolution features of sea surface temperature and chlorophyll-a fields associated with oceanic fronts in the southern East China Sea. Int. J. Remote Sens. 2008, 29, 6249–6261. [Google Scholar] [CrossRef]
- Lee, M.A.; Chang, Y.; Shimada, T. Seasonal evolution of fine-scale sea surface temperature fronts in the East China Sea. Deep Sea Res. Part II Top. Stud. Oceanogr. 2015, 119, 20–29. [Google Scholar] [CrossRef]
- Snyder, S.; Franks, P.J.; Talley, L.D.; Xu, Y.; Kohin, S. Crossing the line: Tunas actively exploit submesoscale fronts to enhance foraging success. Limnol. Oceanogr. Lett. 2017, 2, 187–194. [Google Scholar] [CrossRef]
- Vayghan, A.H.; Lee, M.A. Hotspot habitat modeling of skipjack tuna (Katsuwonus pelamis) in the Indian Ocean by using multisatellite remote sensing. Turk. J. Fish. Aquat. Sci. 2022, 22, TRJFAS19107. [Google Scholar] [CrossRef]
- Arrizabalaga, H.; Dufour, F.; Kell, L.; Merino, G.; Ibaibarriaga, L.; Chust, G.; Irigoien, X.; Santiago, J.; Murua, H.; Fraile, I.; et al. Global habitat preferences of commercially valuable tuna. Deep-Sea Res. II Top. Stud. Oceanogr. 2015, 113, 102–112. [Google Scholar] [CrossRef] [Green Version]
- Lien, Y.H.; Su, N.J.; Sun, C.L.; Punt, A.; Yeh, S.Z.; DiNardo, G. Spatial and environmental determinants of the distribution of Striped Marlin (Kajikia audax) in the western and central North Pacific Ocean. Environ. Biol. Fishes 2014, 97, 267–276. [Google Scholar] [CrossRef]
- Hirata, Y.; Hamasaki, K.; Imai, A.; Teruya, K.; Iwasaki, T.; Hamada, K.; Mushiake, K. Effects of different photoperiods and water temperatures on survival, growth, feeding and initial swim bladder inflation of greater amberjack Seriola dumerili larvae. Nippon Suisan Gakkaishi 2009, 75, 995–1003, (In Japanese with English abstract). [Google Scholar] [CrossRef] [Green Version]
- Zainuddin, M.; Kiyofuji, H.; Saitoh, K.; Saitoh, S.I. Using multi-sensor satellite remote sensing and catch data to detect ocean hot spots for albacore (Thunnus alalunga) in the northwestern North Pacific. Deesp-Sea Res. II Top. Stud. Oceanogr. 2006, 53, 419–431. [Google Scholar] [CrossRef]
- Tzeng, M.T.; Lan, K.W.; Chan, J.W. Interannual variability of wintertime sea surface temperatures in the eastern Taiwan Strait. J. Mar. Sci. Tech-Taiw. 2012, 20, 14. [Google Scholar]
- Turner, S.C.; Cummings, N.J.; Porch, C.P. Stock assessments of Gulf of Mexico greater amberjack using data through 1998. In Southeast Data, Assessment and Review; S9RD06; SEDAR: North Charleston, SC, USA, 2000. [Google Scholar]
- Castillo, J.; Barbieri, M.A.; Gonzalez, A. Relationships between sea surface temperature, salinity, and pelagic fish distribution off northern Chile. ICES J. Mar. Sci. 1996, 53, 139–146. [Google Scholar] [CrossRef]
- Jan, S.; Tseng, Y.H.; Dietrich, D.E. Sources of water in the Taiwan Strait. J. Oceanogr. 2010, 66, 211–221. [Google Scholar] [CrossRef]
- Chen, X.; Li, G.; Feng, B.; Tian, S. Habitat suitability index of chub mackerel (Scomber japonicus) from July to September in the East China Sea. J. Oceanogr. 2009, 65, 93–102. [Google Scholar] [CrossRef]
- Lan, K.W.; Kawamura, H.; Lee, M.A.; Lu, H.J.; Shimada, T.; Hosoda, K.; Sakaida, F. Relationship between Albacore (Thunnus alalunga) fishing grounds in the Indian Ocean and the thermal environment revealed by cloud-free microwave sea surface temperature. Fish. Res. 2012, 113, 1–7. [Google Scholar] [CrossRef]
- Smith, R.C.; Dustan, P.; Au, D.; Baker, K.S.; Dunlap, E.A. Distribution of cetaceans and sea-surface chlorophyll concentrations in the California Current. Mar. Biol. 1986, 91, 385–402. [Google Scholar] [CrossRef]
- Chen, L.C.; Weng, J.S.; Naimullah, M.; Hsiao, P.Y.; Tseng, C.T.; Lan, K.W.; Chuang, C.C. Distribution and catch rate characteristics of narrow-barred Spanish mackerel (Scomberomorus commerson) in relation to oceanographic factors in the waters around Taiwan. Front. Mar. Sci. 2021, 8, 770722. [Google Scholar] [CrossRef]
- Murie, D.; Parkyn, D.; Austin, J. Seasonal movement and mixing rates of greater amberjack in the Gulf of Mexico and assessment of exchange with the South Atlantic spawning stock. In Southeast Data, Assessment and Review; 33-DW12; SEDAR: North Charleston, SC, USA, 2011. [Google Scholar]
- Poore, R.Z.; Tedesco, K.A.; Spear, J.W. Seasonal flux and assemblage composition of planktonic foraminifers from a sediment-trap study in the northern Gulf of Mexico. J. Coast. Res. 2013, 63, 6–19. [Google Scholar] [CrossRef]
- Jan, S.; Wang, J.; Chern, C.S.; Chao, S.Y. Seasonal variation of the circulation in the Taiwan Strait. J. Mar. Syst. 2002, 35, 249–268. [Google Scholar] [CrossRef]
- Naimullah, M.; Wu, Y.L.; Lee, M.A.; Lan, K.W. Effect of the El Nino-Southern Oscillation (ENSO) cycle on the catches and habitat patterns of three swimming crabs in the Taiwan Strait. Front. Mar. Sci. 2021, 8, 763543. [Google Scholar] [CrossRef]
- Lan, K.W.; Lian, L.J.; Li, C.H.; Hsiao, P.Y.; Cheng, S.Y. Validation of a primary production algorithm of vertically generalized production model derived from multi-satellite data around the waters of Taiwan. Remote Sens. 2020, 12, 1627. [Google Scholar] [CrossRef]
- Tseng, H.C.; You, W.L.; Huang, W.; Chung, C.C.; Tsai, A.Y.; Chen, T.Y.; Lan, K.W.; Gong, G.C. Seasonal variations of marine environment and primary production in the Taiwan Strait. Front. Mar. Sci. 2020, 7, 38. [Google Scholar] [CrossRef]
- Alabia, I.D.; Saitoh, S.I.; Mugo, R.; Igarashi, H.; Ishikawa, Y.; Usui, N.; Kamachi, M.; Awaji, T.; Seito, M. Seasonal potential fishing ground prediction of neon flying squid (Ommastrephes bartramii) in the western and central North Pacific. Fish. Oceanogr. 2015, 24, 190–203. [Google Scholar] [CrossRef]


| Environmental Variables | Units | Data Source | Resolution |
|---|---|---|---|
| Sea Surface Temperature (SST) | °C | https://marine.copernicus.eu | 1/12° |
| Sea Surface Salinity (SSS) | PSU | https://marine.copernicus.eu | 1/12° |
| Sea Surface Height (SSH) | m | https://marine.copernicus.eu | 1/12° |
| Sea Surface Chlorophyll-a Concentration (Chl-a) | mg/m3 | oceancolor.gsfc.nasa.gov | 4.6 km × 4.6 km |
| Mixed Layer Depth (MLD) | m | https://marine.copernicus.eu | 1/12° |
| Eddy Kinetic Energy (EKE) | m2/s2 | https://marine.copernicus.eu | 1/12° |
| Variables | Deviance Explained (%) | AIC | Residual Factor | p-Value |
|---|---|---|---|---|
| Latitude | 1.72 | 6178.84 | 992.59 | <0.001 |
| Longitude | 1.47 | 6192.58 | 995.14 | <0.001 |
| SST | 13.78 | 5476.31 | 870.79 | <0.001 |
| SSS | 2.94 | 6112.02 | 980.31 | <0.001 |
| SSH | 2.54 | 6133.77 | 984.29 | <0.001 |
| Chl-a | 0.11 | 6266.09 | 1008.9 | <0.05 |
| MLD | 0.11 | 6266.36 | 1008.9 | <0.05 |
| EKE | 2.03 | 6161.71 | 989.43 | <0.001 |
| Total Deviance Explained (%) | 19 |
| Variables | Deviance Explained (%) | AIC | Residual Factor | p-Value |
|---|---|---|---|---|
| +s (Latitude) | 15.1 | 5408.49 | 876.34 | <0.001 |
| +s (Longitude) | 16.8 | 5302.51 | 877.45 | <0.001 |
| +s (SST) | 22.9 | 4891.28 | 809.42 | <0.001 |
| +s (SSS) | 11.5 | 5633.03 | 947.86 | <0.001 |
| +s (SSH) | 9.75 | 5736.73 | 945.99 | <0.001 |
| +s (Chl-a) | 5.91 | 5959.21 | 957.43 | <0.001 |
| +s (MLD) | 2.46 | 6154.00 | 995.34 | <0.001 |
| +s (EKE) | 2.64 | 6134.40 | 982.02 | <0.001 |
| Total Deviance Explained (%) | 47.3 | |||
| r2 | 0.47 |
| Season | Observed Catch Rates | Predicted Catch Rates | RMSD |
|---|---|---|---|
| Spring | 372 | 189 | 0.46 |
| Summer | 378 | 592 | 0.39 |
| Autumn | 201 | 239 | 0.35 |
| Winter | 110 | 89 | 0.48 |
| Year | 1061 | 1109 | 0.42 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mammel, M.; Naimullah, M.; Vayghan, A.H.; Hsu, J.; Lee, M.-A.; Wu, J.-H.; Wang, Y.-C.; Lan, K.-W. Variability in the Spatiotemporal Distribution Patterns of Greater Amberjack in Response to Environmental Factors in the Taiwan Strait Using Remote Sensing Data. Remote Sens. 2022, 14, 2932. https://doi.org/10.3390/rs14122932
Mammel M, Naimullah M, Vayghan AH, Hsu J, Lee M-A, Wu J-H, Wang Y-C, Lan K-W. Variability in the Spatiotemporal Distribution Patterns of Greater Amberjack in Response to Environmental Factors in the Taiwan Strait Using Remote Sensing Data. Remote Sensing. 2022; 14(12):2932. https://doi.org/10.3390/rs14122932
Chicago/Turabian StyleMammel, Mubarak, Muhamad Naimullah, Ali Haghi Vayghan, Jhen Hsu, Ming-An Lee, Jun-Hong Wu, Yi-Chen Wang, and Kuo-Wei Lan. 2022. "Variability in the Spatiotemporal Distribution Patterns of Greater Amberjack in Response to Environmental Factors in the Taiwan Strait Using Remote Sensing Data" Remote Sensing 14, no. 12: 2932. https://doi.org/10.3390/rs14122932
APA StyleMammel, M., Naimullah, M., Vayghan, A. H., Hsu, J., Lee, M.-A., Wu, J.-H., Wang, Y.-C., & Lan, K.-W. (2022). Variability in the Spatiotemporal Distribution Patterns of Greater Amberjack in Response to Environmental Factors in the Taiwan Strait Using Remote Sensing Data. Remote Sensing, 14(12), 2932. https://doi.org/10.3390/rs14122932








