Remote Sensing Phenology of the Brazilian Caatinga and Its Environmental Drivers
Abstract
1. Introduction
2. Material and Methods
2.1. Study Areas
2.2. Data Processing
2.2.1. MODIS Data
2.2.2. TerraClimate e CHIRPS Data
2.2.3. Phenological Metrics
2.2.4. Seasonal Variability Analysis
3. Results
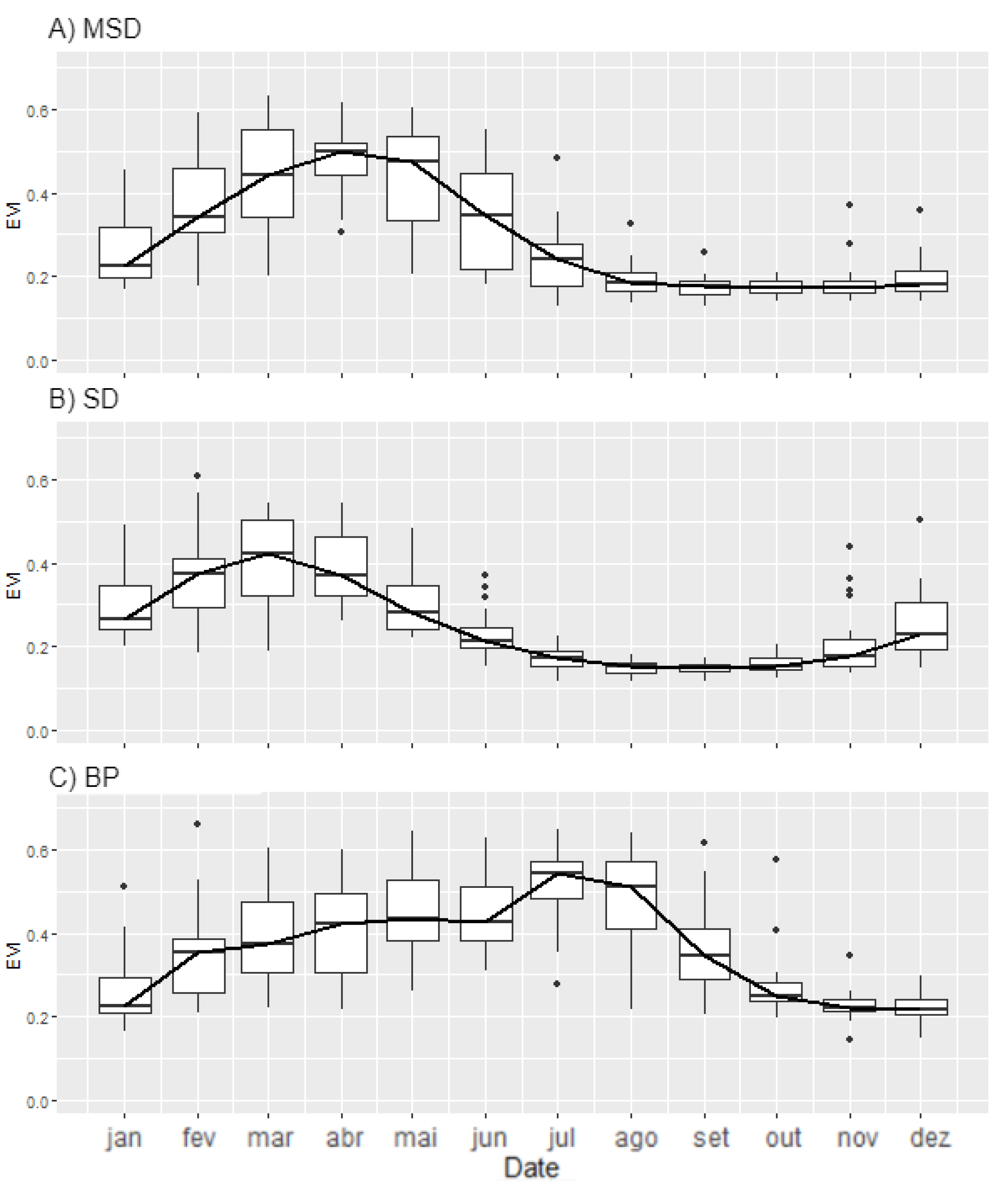
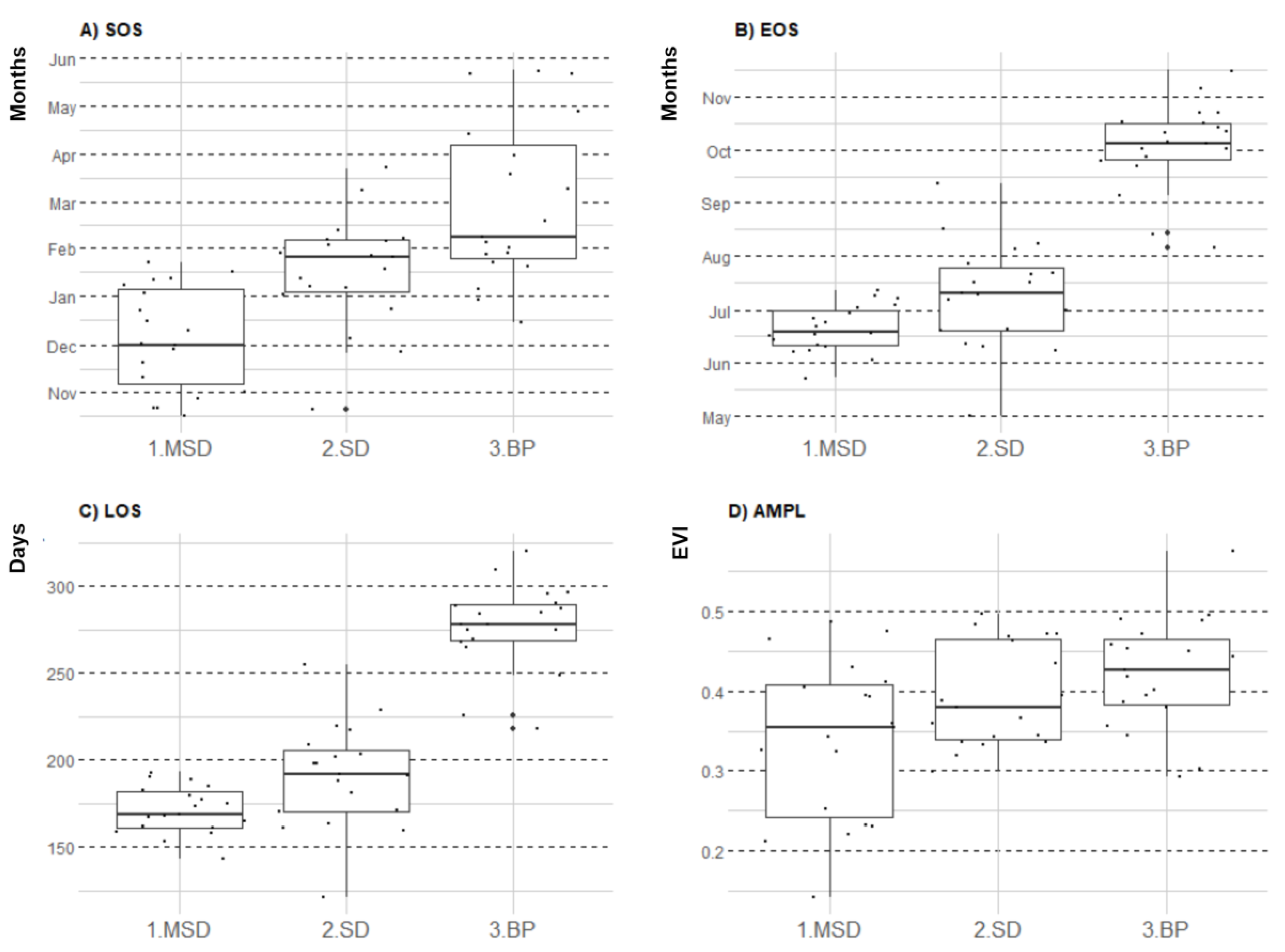
3.1. Seasonal Profiles and Phenology of SDTF Studied Sites
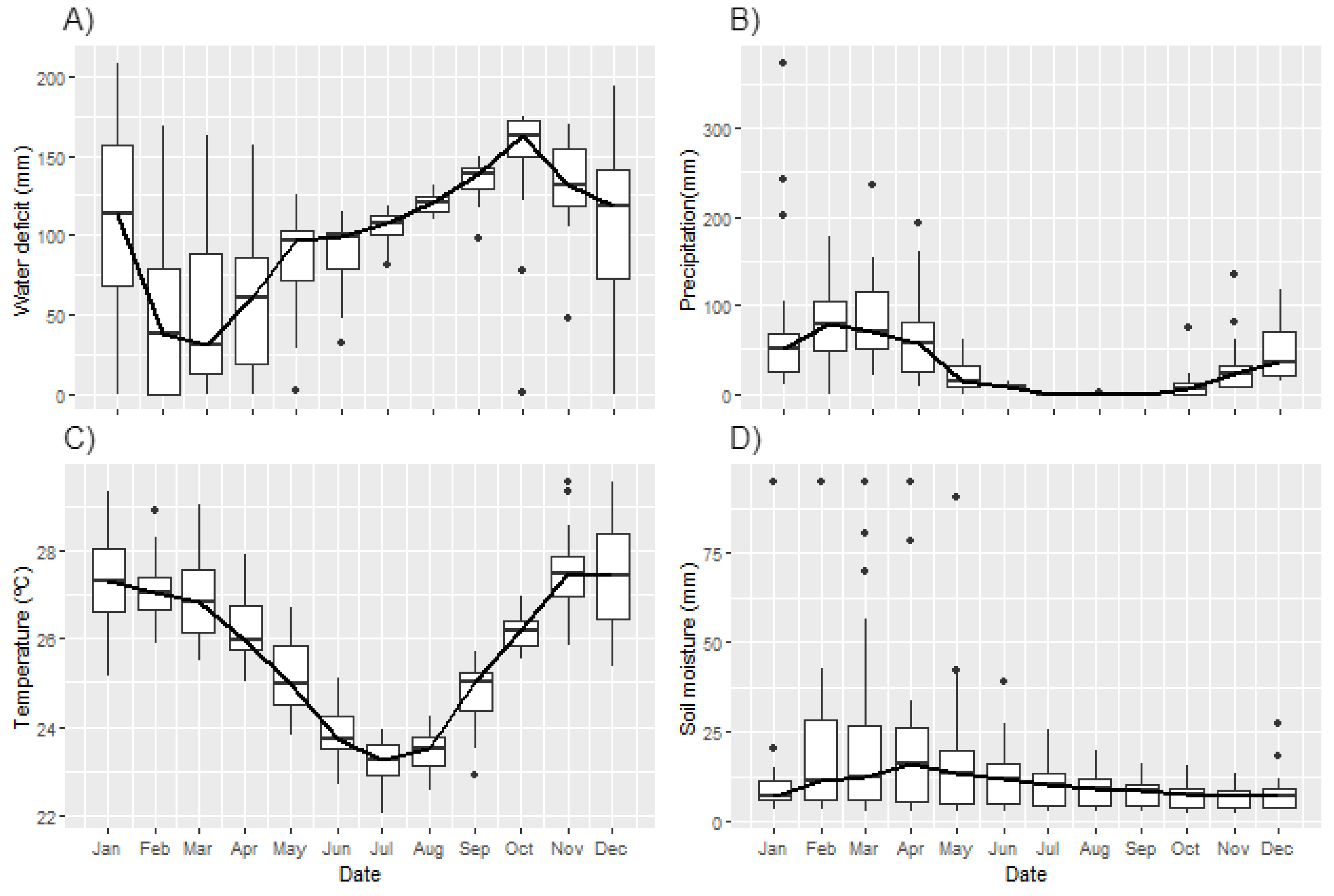
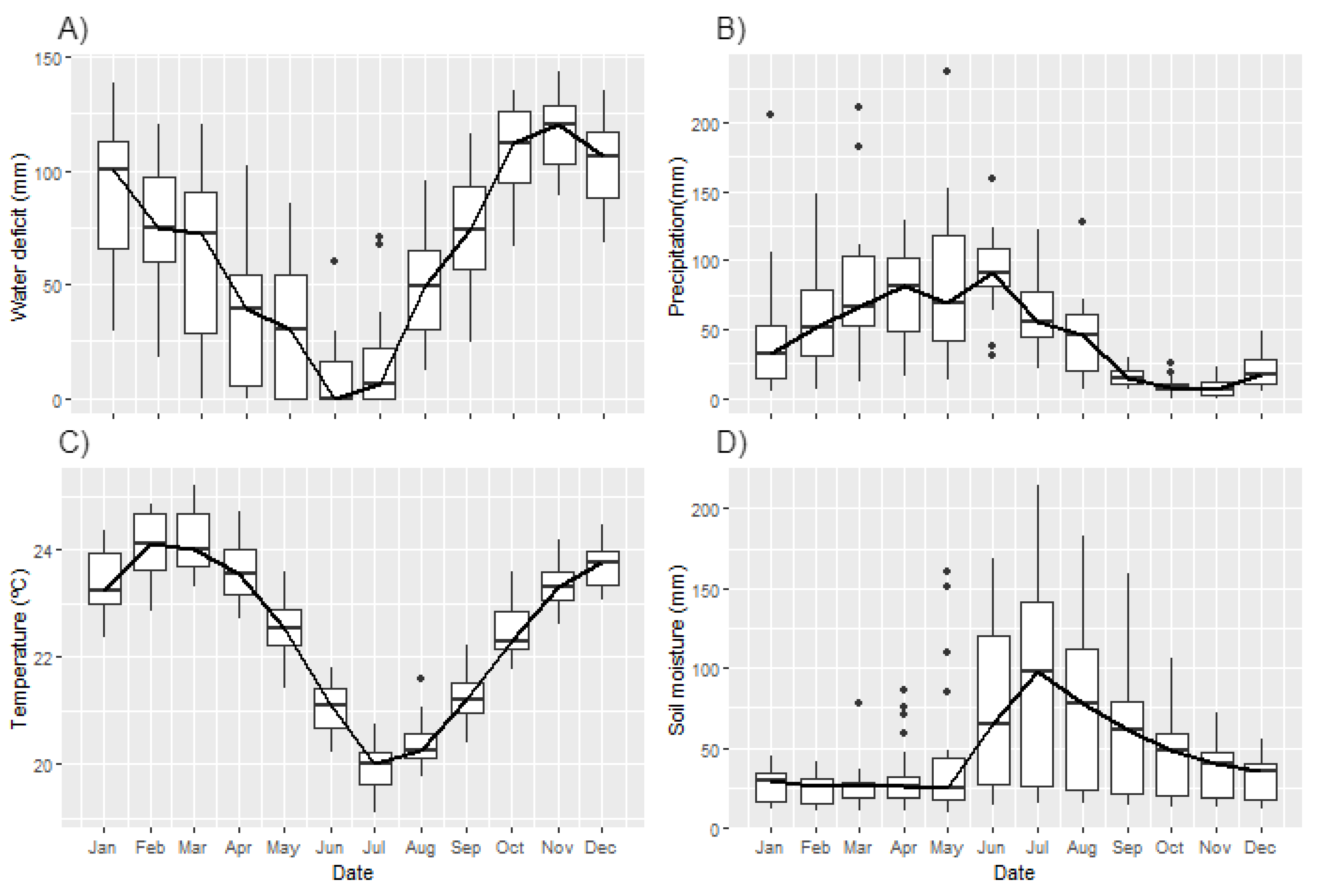
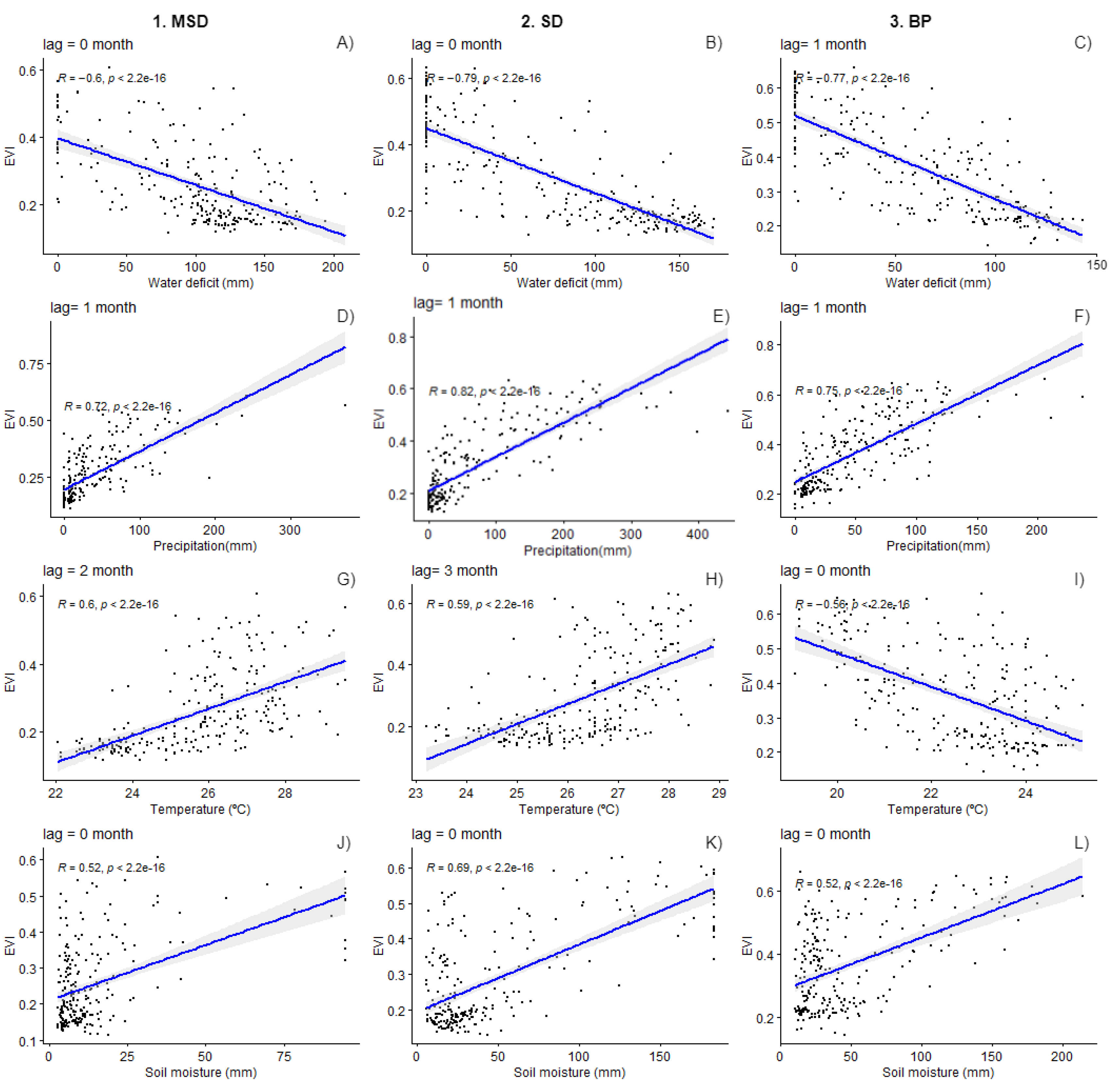
3.2. Environmental Drivers
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pennington, R.T.; Lehmann, C.; Rowland, L.M. Tropical savannas and dry forests. Curr. Biol. 2018, 28, R541–R545. [Google Scholar] [CrossRef] [PubMed]
- Moro, M.F.; Nic Lughadha, E.; de Araújo, F.S.; Martins, F.R. A Phytogeographical Metaanalysis of the Semiarid Caatinga Domain in Brazil. Bot. Rev. 2016, 82, 91–148. [Google Scholar] [CrossRef]
- De Queiroz, L.P.; Cardoso, D.; Fernandes, M.F.; Moro, M.F. Diversity and Evolution of Flowering Plants of the Caatinga Domain. Caatinga 2017, 23–63. [Google Scholar] [CrossRef]
- Fernandes, M.F.; Cardoso, D.; de Queiroz, L.P. An updated plant checklist of the Brazilian Caatinga seasonally dry forests and woodlands reveals high species richness and endemism. J. Arid Environ. 2020, 174, 104079. [Google Scholar] [CrossRef]
- Leith, H. Phenology and Seasonality Modeling. Soil Sci. 1974, 120, 461. [Google Scholar] [CrossRef]
- Morisette, J.T.; Richardson, A.D.; Knapp, A.K.; Fisher, J.I.; Graham, E.A.; Abatzoglou, J.; Wilson, B.E.; Breshears, D.D.; Henebry, G.M.; Hanes, J.M.; et al. Tracking the rhythm of the seasons in the face of global change: Phenological research in the 21st century. Front. Ecol. Environ. 2009, 7, 253–260. [Google Scholar] [CrossRef]
- Machado, I.C.S.; Barros, L.M.; Sampaio, E.V.S.B. Phenology of Caatinga Species at Serra Talhada, PE, Northeastern Brazil. Biotropica 1997, 29, 57–68. [Google Scholar] [CrossRef]
- Alberton, B.; Torres, R.D.S.; Cancian, L.F.; Borges, B.D.; Almeida, J.; Mariano, G.C.; dos Santos, J.; Morellato, P. Introducing digital cameras to monitor plant phenology in the tropics: Applications for conservation. Perspect. Ecol. Conserv. 2017, 15, 82–90. [Google Scholar] [CrossRef]
- Paloschi, R.; Ramos, D.; Ventura, D.; Souza, R.; Souza, E.; Morellato, L.; Nóbrega, R.; Coutinho, I.A.C.; Verhoef, A.; Körting, T.; et al. Environmental Drivers of Water Use for Caatinga Woody Plant Species: Combining Remote Sensing Phenology and Sap Flow Measurements. Remote Sens. 2020, 13, 75. [Google Scholar] [CrossRef]
- Vico, G.; Thompson, S.E.; Manzoni, S.; Molini, A.; Albertson, J.D.; Almeida-Cortez, J.S.; Fay, P.A.; Feng, X.; Guswa, A.J.; Liu, H.; et al. Climatic, ecophysiological, and phenological controls on plant ecohydrological strategies in seasonally dry ecosystems. Ecohydrology 2015, 8, 660–681. [Google Scholar] [CrossRef]
- Riahi, K.; Schaeffer, R.; Arango, J.; Calvin, K.; Guivarch, C.; Hasegawa, T.; Jiang, K.; Kriegler, E.; Matthews, R.; Peters, G.P.; et al. Mitigation pathways compatible with long-term goals. In IPCC, 2022: Climate Change 2022: Mitigation of Climate Change. Contribution of Working Group III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Shukla, P.R., Skea, J., Slade, R., Al Khourdajie, A., van Diemen, R., McCollum, D., Pathak, M., Some, S., Vyas, P., Fradera, R., et al., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2022. [Google Scholar] [CrossRef]
- Torres, R.R.; Lapola, D.M.; Gamarra, N.L.R. Future Climate Change in the Caatinga. Caatinga 2017, 383–410. [Google Scholar] [CrossRef]
- Marengo, J.A.; Cunha, A.P.M.A.; Nobre, C.A.; Neto, G.G.R.; Magalhaes, A.R.; Torres, R.R.; Sampaio, G.; Alexandre, F.; Alves, L.M.; Cuartas, L.A.; et al. Assessing drought in the drylands of northeast Brazil under regional warming exceeding 4 °C. Nat. Hazards 2020, 103, 2589–2611. [Google Scholar] [CrossRef]
- Xu, L.; Myneni, R.; Iii, F.S.C.; Callaghan, T.V.; Pinzon, J.E.; Tucker, C.J.; Zhu, Z.; Bi, J.; Ciais, P.; Tømmervik, H.; et al. Temperature and vegetation seasonality diminishment over northern lands. Nat. Clim. Chang. 2013, 3, 581–586. [Google Scholar] [CrossRef]
- Tong, X.; Tian, F.; Brandt, M.; Liu, Y.; Zhang, W.; Fensholt, R. Trends of land surface phenology derived from passive microwave and optical remote sensing systems and associated drivers across the dry tropics 1992–2012. Remote Sens. Environ. 2019, 232. [Google Scholar] [CrossRef]
- Brando, P.M.; Goetz, S.J.; Baccini, A.; Nepstad, D.C.; Beck, P.S.A.; Christman, M.C. Seasonal and interannual variability of climate and vegetation indices across the Amazon. Proc. Natl. Acad. Sci. USA 2010, 107, 14685–14690. [Google Scholar] [CrossRef]
- Olmos-Trujillo, E.; González-Trinidad, J.; Júnez-Ferreira, H.; Pacheco-Guerrero, A.; Bautista-Capetillo, C.; Avila-Sandoval, C.; Galván-Tejada, E. Spatio-Temporal Response of Vegetation Indices to Rainfall and Temperature in A Semiarid Region. Sustainability 2020, 12, 1939. [Google Scholar] [CrossRef]
- Andrade, J.; Cunha, J.; Silva, J.; Rufino, I.; Galvão, C. Evaluating single and multi-date Landsat classifications of land-cover in a seasonally dry tropical forest. Remote Sens. Appl. Soc. Environ. 2021, 22, 100515. [Google Scholar] [CrossRef]
- Huete, A.; Didan, K.; Miura, T.; Rodriguez, E.P.; Gao, X.; Ferreira, L.G. Overview of the radiometric and biophysical performance of the MODIS vegetation indices. Remote Sens. Environ. 2002, 83, 195–213. [Google Scholar] [CrossRef]
- Adole, T.; Dash, J.; Atkinson, P. A systematic review of vegetation phenology in Africa. Ecol. Inform. 2016, 34, 117–128. [Google Scholar] [CrossRef]
- Suepa, T.; Qi, J.; Lawawirojwong, S.; Messina, J. Understanding spatio-temporal variation of vegetation phenology and rainfall seasonality in the monsoon Southeast Asia. Environ. Res. 2016, 147, 621–629. [Google Scholar] [CrossRef]
- Htitiou, A.; Boudhar, A.; Lebrini, Y.; Hadria, R.; Lionboui, H.; Elmansouri, L.; Tychon, B.; Benabdelouahab, T. The Performance of Random Forest Classification Based on Phenological Metrics Derived from Sentinel-2 and Landsat 8 to Map Crop Cover in an Irrigated Semi-arid Region. Remote Sens. Earth Syst. Sci. 2019, 2, 208–224. [Google Scholar] [CrossRef]
- Pastor-Guzman, J.; Dash, J.; Atkinson, P. Remote sensing of mangrove forest phenology and its environmental drivers. Remote Sens. Environ. 2018, 205, 71–84. [Google Scholar] [CrossRef]
- De Jesus, J.B.; Kuplich, T.M.; Barreto, D.D.C.; da Rosa, C.N.; Hillebrand, F.L. Temporal and phenological profiles of open and dense Caatinga using remote sensing: Response to precipitation and its irregularities. J. For. Res. 2021, 32, 1067–1076. [Google Scholar] [CrossRef]
- Songsom, V.; Koedsin, W.; Ritchie, R.J.; Huete, A. Mangrove Phenology and Environmental Drivers Derived from Remote Sensing in Southern Thailand. Remote Sens. 2019, 11, 955. [Google Scholar] [CrossRef]
- Wang, G.; Huang, Y.; Wei, Y.; Zhang, W.; Li, T.; Zhang, Q. Inner Mongolian grassland plant phenological changes and their climatic drivers. Sci. Total Environ. 2019, 683, 1–8. [Google Scholar] [CrossRef]
- Huang, J.-G.; Ma, Q.; Rossi, S.; Biondi, F.; Deslauriers, A.; Fonti, P.; Liang, E.; Mäkinen, H.; Oberhuber, W.; Rathgeber, C.B.K.; et al. Photoperiod and temperature as dominant environmental drivers triggering secondary growth resumption in Northern Hemisphere conifers. Proc. Natl. Acad. Sci. USA 2020, 117, 20645–20652. [Google Scholar] [CrossRef]
- Godoy-Veiga, M.; Cintra, B.B.L.; Stríkis, N.M.; Cruz, F.W.; Grohmann, C.H.; Santos, M.S.; Regev, L.; Boaretto, E.; Ceccantini, G.; Locosselli, G.M. The value of climate responses of individual trees to detect areas of climate-change refugia, a tree-ring study in the Brazilian seasonally dry tropical forests. For. Ecol. Manag. 2021, 488, 118971. [Google Scholar] [CrossRef]
- Whitecross, M.; Witkowski, E.; Archibald, S. No two are the same: Assessing variability in broad-leaved savanna tree phenology, with watering, from 2012 to 2014 at Nylsvley, South Africa. S. Afr. J. Bot. 2016, 105, 123–132. [Google Scholar] [CrossRef]
- Ryan, C.M.; Williams, M.; Grace, J.; Woollen, E.; Lehmann, C.E.R. Pre-rain green-up is ubiquitous across southern tropical Africa: Implications for temporal niche separation and model representation. New Phytol. 2016, 213, 625–633. [Google Scholar] [CrossRef]
- Sampaio, E.V. Overview of the Brazilian caatinga. Seas. Dry Trop. For. 1995, 1, 35–63. [Google Scholar] [CrossRef]
- Gutiérrez, A.P.A.; Engle, N.L.; De Nys, E.; Molejón, C.; Martins, E.S. Drought preparedness in Brazil. Weather Clim. Extremes 2014, 3, 95–106. [Google Scholar] [CrossRef]
- Alberton, B.; Torres, R.D.S.; Silva, T.S.F.; da Rocha, H.R.; Moura, M.S.B.; Morellato, L.P.C. Leafing Patterns and Drivers across Seasonally Dry Tropical Communities. Remote Sens. 2019, 11, 2267. [Google Scholar] [CrossRef]
- Alvares, C.A.; Stape, J.L.; Sentelhas, P.C.; Moraes, G.J.L.; Sparovek, G. Köppen’s climate classification map for Brazil. Meteorol. Z. 2013, 22, 711–728. [Google Scholar] [CrossRef]
- Sampaio, E.V.S.B. Características e potencialidades. In Uso Sustentável e Conservação dos Recursos Florestais da Caatinga; Gariglio, M.A., Sampaio, E.V.S.B., Cestaro, L.A., Kageyama, P.Y., Eds.; Serviço Florestal Brasileiro: Brasília, Brazil, 2010; pp. 29–48. [Google Scholar]
- Prado, D. As caatingas da América do Sul. In Ecologia e Conservação da Caatinga; Leal, I.R., Tabarelli, M., Silva, J.M.C., Eds.; Universitária da UFPE: Recife, Brazil, 2003; pp. 3–73. [Google Scholar]
- Nimer, E. Climatologia da região Nordeste do Brasil. Introdução à climatologia dinâmica. Rev. Bras. Geogr. 1972, 34, 3–51. [Google Scholar]
- Velloso, A.L.; Sampaio, E.V.S.B.; Giulietti, A.M.; Barbosa, M.R.V.; Castri, A.A.J.F.; Queiroz, L.P.; Fernandes, A.; Oren, D.C.; Cestaro, L.A.; Carvalho, A.J.E.; et al. Ecorregiões: Propostas para o Bioma Caatinga. In Associação Plantas do Nordeste; The Nature Conservancy do Brasil: Recife, Brazil, 2002; p. 75. [Google Scholar]
- Souza, R.; Feng, X.; Antonino, A.; Montenegro, S.; Souza, E.; Porporato, A. Vegetation response to rainfall seasonality and interannual variability in tropical dry forests. Hydrol. Process. 2016, 30, 3583–3595. [Google Scholar] [CrossRef]
- Kill, L.H.P. Caracterização da vegetação da Reserva Legal da Embrapa Semiárido. Embrapa Semiárido Pet. 2017, 1, 1–27. [Google Scholar]
- Tavares-Damasceno, J.P.; Silveira, J.L.G.D.S.; Câmara, T.; Stedile, P.D.C.; Macario, P.; Toledo-Lima, G.S.; Pichorim, M. Effect of drought on demography of Pileated Finch (Coryphospingus pileatus: Thraupidae) in northeastern Brazil. J. Arid Environ. 2017, 147, 63–70. [Google Scholar] [CrossRef]
- Althoff, T.D.; Menezes, R.; de Carvalho, A.L.; Pinto, A.D.S.; Santiago, G.A.C.F.; Ometto, J.; Von Randow, C.; Sampaio, E.V.D.S.B. Climate change impacts on the sustainability of the firewood harvest and vegetation and soil carbon stocks in a tropical dry forest in Santa Teresinha Municipality, Northeast Brazil. For. Ecol. Manag. 2016, 360, 367–375. [Google Scholar] [CrossRef]
- Cunha, J.E.B.L.; Rufino, I.A.A.; Ideião, S.M.A. Determinação da temperatura da superfície na cidade de Campina Grande-PB a partir de imagens do satélite Landsat 5-TM. In Anais XIV Simpósio Brasileiro de Sensoriamento Remoto; INPE: Natal, Brazil, 2009. [Google Scholar]
- INMET, National Institute of Meteorology of Brazil, 2021. Available online: https://bdmep.inmet.gov.br/# (accessed on 1 October 2021).
- Embrapa, Empresa Brasileira de Pesquisa Aagropecuária. Centro Nacional de Pesquisa de Solos. Sistema Brasileiro de Classificação de Solo, 2nd ed.; Embrapa Solos: Rio de Janeiro, Brazil, 2006. [Google Scholar]
- Gorelick, N.; Hancher, M.; Dixon, M.; Ilyushchenko, S.; Thau, D.; Moore, R. Google Earth Engine: Planetary-scale geospatial analysis for everyone. Remote Sens. Environ. 2017, 202, 18–27. [Google Scholar] [CrossRef]
- Guerschman, J.P.; Scarth, P.F.; McVicar, T.R.; Renzullo, L.J.; Malthus, T.J.; Stewart, J.B.; Rickards, J.E.; Trevithick, R. Assessing the effects of site heterogeneity and soil properties when unmixing photosynthetic vegetation, non-photosynthetic vegetation and bare soil fractions from Landsat and MODIS data. Remote Sens. Environ. 2015, 161, 12–26. [Google Scholar] [CrossRef]
- Abatzoglou, J.T.; Dobrowski, S.; Parks, S.A.; Hegewisch, K.C. TerraClimate, a high-resolution global dataset of monthly climate and climatic water balance from 1958–2015. Sci. Data 2018, 5, 170191. [Google Scholar] [CrossRef] [PubMed]
- Funk, C.; Peterson, P.; Landsfeld, M.; Pedreros, D.; Verdin, J.; Shukla, S.; Husak, G.; Rowland, J.; Harrison, L.; Hoell, A.; et al. The climate hazards infrared precipitation with stations—A new environmental record for monitoring extremes. Sci. Data 2015, 2, 150066. [Google Scholar] [CrossRef] [PubMed]
- Jönsson, P.; Eklundh, L. TIMESAT—A program for analyzing time-series of satellite sensor data. Comput. Geosci. 2004, 30, 833–845. [Google Scholar] [CrossRef]
- Jönsson, P.; Eklundh, L. TIMESAT 3.1—Software Manual; Lund University: Lund, Sweden, 2012; p. 82. Available online: http://web.nateko.lu.se/timesat/timesat.asp (accessed on 1 May 2021).
- Savitzky, A.; Golay, M.J.E. Smoothing and Differentiation of Data by Simplified Least Squares Procedures. Anal. Chem. 1964, 36, 1627–1639. [Google Scholar] [CrossRef]
- Kong, F.; Li, X.; Wang, H.; Xie, D.; Li, X.; Bai, Y. Land Cover Classification Based on Fused Data from GF-1 and MODIS NDVI Time Series. Remote Sens. 2016, 8, 741. [Google Scholar] [CrossRef]
- Streher, A.S.; Sobreiro, J.F.F.; Morellato, P.; Silva, T. Land Surface Phenology in the Tropics: The Role of Climate and Topography in a Snow-Free Mountain. Ecosystems 2017, 20, 1436–1453. [Google Scholar] [CrossRef]
- Diem, P.K.; Pimple, U.; Sitthi, A.; Varnakovida, P.; Tanaka, K.; Pungkul, S.; Leadprathom, K.; LeClerc, M.Y.; Chidthaisong, A. Shifts in Growing Season of Tropical Deciduous Forests as Driven by El Niño and La Niña during 2001–2016. Forests 2018, 9, 448. [Google Scholar] [CrossRef]
- Wang, Y.; Zang, S.; Tian, Y. Mapping paddy rice with the random forest algorithm using MODIS and SMAP time series. Chaos Solitons Fractals 2020, 140. [Google Scholar] [CrossRef]
- Ramírez-Cuesta, J.; Minacapilli, M.; Motisi, A.; Consoli, S.; Intrigliolo, D.; Vanella, D. Characterization of the main land processes occurring in Europe (2000–2018) through a MODIS NDVI seasonal parameter-based procedure. Sci. Total Environ. 2021, 799, 149346. [Google Scholar] [CrossRef]
- Doussoulin-Guzmán, M.-A.; Pérez-Porras, F.-J.; Triviño-Tarradas, P.; Ríos-Mesa, A.-F.; Porras, A.G.-F.; Mesas-Carrascosa, F.-J. Grassland Phenology Response to Climate Conditions in Biobio, Chile from 2001 to 2020. Remote Sens. 2022, 14, 475. [Google Scholar] [CrossRef]
- Conover, W.J.; Johnson, M.E.; Johnson, M.M. A Comparative Study of Tests for Homogeneity of Variances, with Applications to the Outer Continental Shelf Bidding Data. Technometrics 1981, 23, 351–361. [Google Scholar] [CrossRef]
- Zhu, W.; Zheng, Z.; Jiang, N.; Zhang, D. A comparative analysis of the spatio-temporal variation in the phenologies of two herbaceous species and associated climatic driving factors on the Tibetan Plateau. Agric. For. Meteorol. 2018, 248, 177–184. [Google Scholar] [CrossRef]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019; Available online: https://www.r-project.org (accessed on 20 February 2019).
- De Souza, L.S.B.; Silva, M.T.L.; Alba, E.; de Moura, M.S.B.; Neto, J.F.D.C.; de Souza, C.A.A.; da Silva, T.G.F. New method for estimating reference evapotranspiration and comparison with alternative methods in a fruit-producing hub in the semi-arid region of Brazil. Arch. Meteorol. Geophys. Bioclimatol. Ser. B 2022, 1–10. [Google Scholar] [CrossRef]
- Dos Santos, C.A.C.; Mariano, D.A.; Nascimento, F.D.C.A.D.; Dantas, F.R.D.C.; de Oliveira, G.; Silva, M.T.; da Silva, L.L.; da Silva, B.B.; Bezerra, B.G.; Safa, B.; et al. Spatio-temporal patterns of energy exchange and evapotranspiration during an intense drought for drylands in Brazil. Int. J. Appl. earth Obs. Geoinf. ITC J. 2019, 85, 101982. [Google Scholar] [CrossRef]
- Mendes, K.R.; Campos, S.; Da Silva, L.L.; Mutti, P.R.; Ferreira, R.R.; Medeiros, S.S.; Perez-Marin, A.M.; Marques, T.V.; Ramos, T.M.; Vieira, M.M.D.L.; et al. Seasonal variation in net ecosystem CO2 exchange of a Brazilian seasonally dry tropical forest. Sci. Rep. 2020, 10, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Bezerra, M.V.C.; Da Silva, B.B.; Bezerra, B.G. Avaliação dos efeitos atmosféricos no albedo e NDVI obtidos com imagens de satélite. Rev. Bras. Eng. Agrícola Ambient. 2011, 15, 709–717. [Google Scholar] [CrossRef]
- Allen, K.; Dupuy, J.M.; Gei, M.G.; Hulshof, C.; Medvigy, D.; Pizano, C.; Salgado-Negret, B.; Smith, C.M.; Trierweiler, A.; Van Bloem, S.J.; et al. Will seasonally dry tropical forests be sensitive or resistant to future changes in rainfall regimes? Environ. Res. Lett. 2017, 12, 023001. [Google Scholar] [CrossRef]
- Trovão, D.M.D.B.M.; Fernandes, P.D.; De Andrade, L.A.; Neto, J.D. Variações sazonais de aspectos fisiológicos de espécies da Caatinga. Rev. Bras. Eng. Agrícola Ambient. 2007, 11, 307–311. [Google Scholar] [CrossRef][Green Version]
- Miranda, R.D.Q.; Nóbrega, R.L.B.; de Moura, M.S.B.; Raghavan, S.; Galvíncio, J.D. Realistic and simplified models of plant and leaf area indices for a seasonally dry tropical forest. Int. J. Appl. Earth Obs. Geoinf. ITC J. 2020, 85, 101992. [Google Scholar] [CrossRef]
- Marengo, J.A.; Torres, R.R.; Alves, L.M. Drought in Northeast Brazil—past, present, and future. Theor. Appl. Climatol. 2016, 129, 1189–1200. [Google Scholar] [CrossRef]
- Flerchinger, G.N.; Hanson, C.L.; Wight, J.R. Modeling Evapotranspiration and Surface Energy Budgets across a Watershed. Water Resour. Res. 1996, 32, 2539–2548. [Google Scholar] [CrossRef]
- Langvall, O.; Löfvenius, M.O. Long-term standardized forest phenology in Sweden: A climate change indicator. Int. J. Biometeorol. 2021, 65, 381–391. [Google Scholar] [CrossRef] [PubMed]
- Marin, F.R.; Assad, E.D.; Barbarisi, B.F.; Pilau, F.G.; Pacheco, L.R.F.; Zullo, J.J.; Pinto, H.S. Efeito das mudanças climáticas sobre a aptidão climática para cana-de-açúcar no Estado de São Paulo. In Congresso Brasileiro de Agrometeorologia; Embrapa/SBAgro: Aracaju, Brazil, 2007; Volume 15. [Google Scholar]
- Nepomuceno, A.L.; Farias, J.R.B.; Salinet, L.H.; Polizel, A.M.; Neumaier, N.; Beneventi, M.A.; Stolf, R.; Rolla, A.A.P. Engenharia genética como ferramenta no desenvolvimento de plantas de soja adaptadas a cenários futuros de mudanças climáticas. In Congresso Brasileiro de Agrometeorologia; Embrapa/SBAgro: Aracaju, Brazil, 2007. [Google Scholar]
- Bergamaschi, H. O clima como fator determinante da fenologia das plantas. In Fenologia: Ferramenta para Conservação, Melhoramento e Manejo de Recursos Vegetais Arbóreos; Embrapa Florestas: Colombo, Brazil, 2007; Volume 1, pp. 291–310. [Google Scholar]


| Site | Scenarios | Air Temperature (°C) | Water Deficit (mm) | Soil Moisture (mm) | Precipitation (mm) |
|---|---|---|---|---|---|
| 1. MSD | 1 | 0.28 | −0.25 | NS | 0.48 |
| 2 | 0.46 | −0.27 | 0.26 | - | |
| 3 | 0.26 | −0.34 | - | 0.52 | |
| 4 | 0.34 | - | 0.26 | 0.49 | |
| 5 | - | −0.32 | NS | 0.58 | |
| 2. SD | 1 | 0.09 | −0.41 | 0.11 | 0.51 |
| 2 | 0.26 | −0.40 | 0.40 | - | |
| 3 | 0.06 | −0.50 | - | 0.61 | |
| 4 | 0.36 | - | 0.33 | 0.51 | |
| 5 | - | −0.52 | 0.08 | 0.55 | |
| 3. BP | 1 | NS | −0.29 | 0.18 | 0.48 |
| 2 | NS | −0.62 | 0.16 | - | |
| 3 | −0.14 | −0.32 | - | 0.47 | |
| 4 | −0.28 | - | 0.23 | 0.69 | |
| 5 | - | −0.39 | 0.22 | 0.48 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Medeiros, R.; Andrade, J.; Ramos, D.; Moura, M.; Pérez-Marin, A.M.; dos Santos, C.A.C.; da Silva, B.B.; Cunha, J. Remote Sensing Phenology of the Brazilian Caatinga and Its Environmental Drivers. Remote Sens. 2022, 14, 2637. https://doi.org/10.3390/rs14112637
Medeiros R, Andrade J, Ramos D, Moura M, Pérez-Marin AM, dos Santos CAC, da Silva BB, Cunha J. Remote Sensing Phenology of the Brazilian Caatinga and Its Environmental Drivers. Remote Sensing. 2022; 14(11):2637. https://doi.org/10.3390/rs14112637
Chicago/Turabian StyleMedeiros, Rodolpho, João Andrade, Desirée Ramos, Magna Moura, Aldrin Martin Pérez-Marin, Carlos A. C. dos Santos, Bernardo Barbosa da Silva, and John Cunha. 2022. "Remote Sensing Phenology of the Brazilian Caatinga and Its Environmental Drivers" Remote Sensing 14, no. 11: 2637. https://doi.org/10.3390/rs14112637
APA StyleMedeiros, R., Andrade, J., Ramos, D., Moura, M., Pérez-Marin, A. M., dos Santos, C. A. C., da Silva, B. B., & Cunha, J. (2022). Remote Sensing Phenology of the Brazilian Caatinga and Its Environmental Drivers. Remote Sensing, 14(11), 2637. https://doi.org/10.3390/rs14112637







