Drone-Sensed and Sap Flux-Derived Leaf Phenology in a Cool Temperate Deciduous Forest: A Tree-Level Comparison of 17 Species
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site and Samples
2.2. Observation of Leaf Phenology
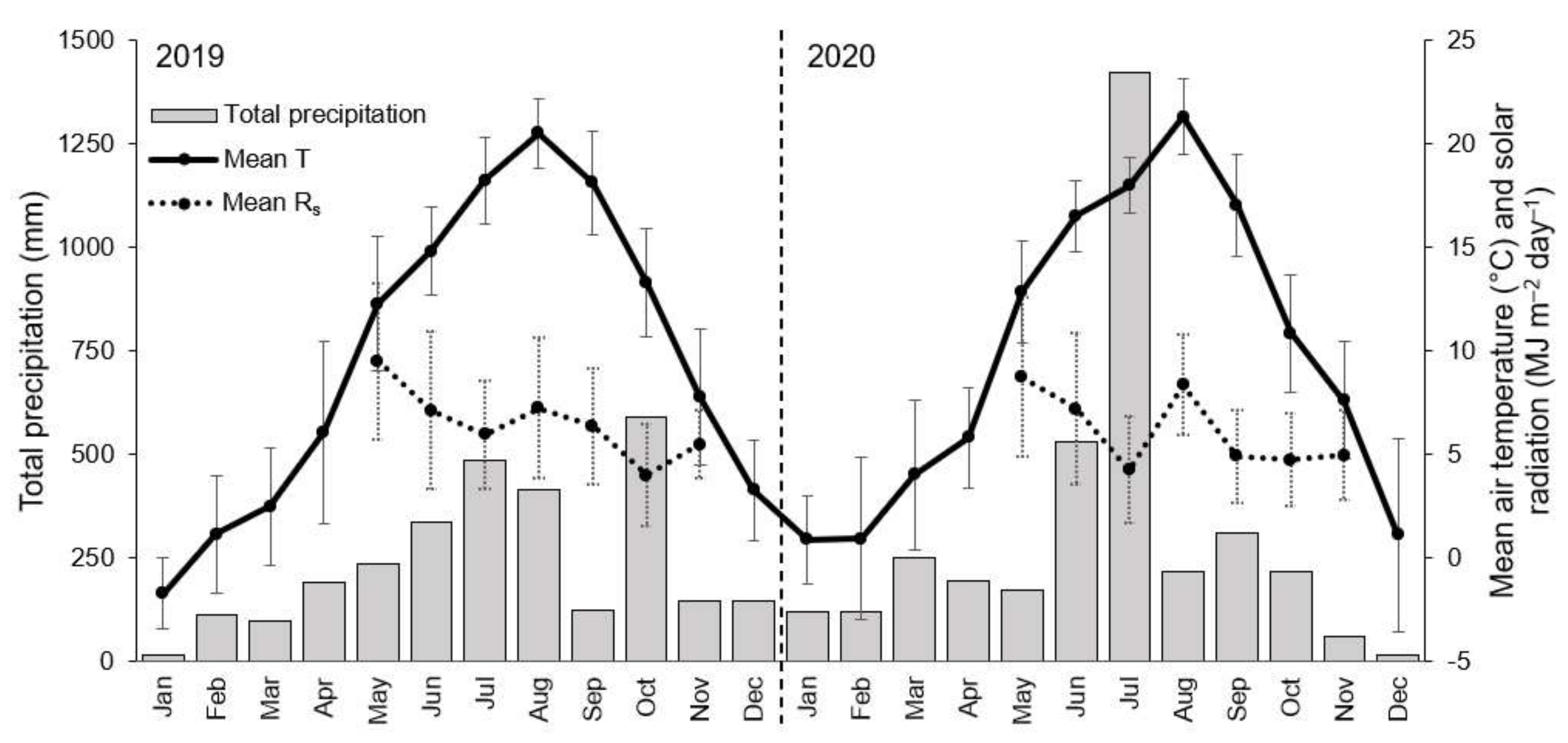
2.3. Measurements of Sap Flow and Environmental Factors
2.4. Data Analysis
2.4.1. UAV-Derived Leaf Phenological Metrics
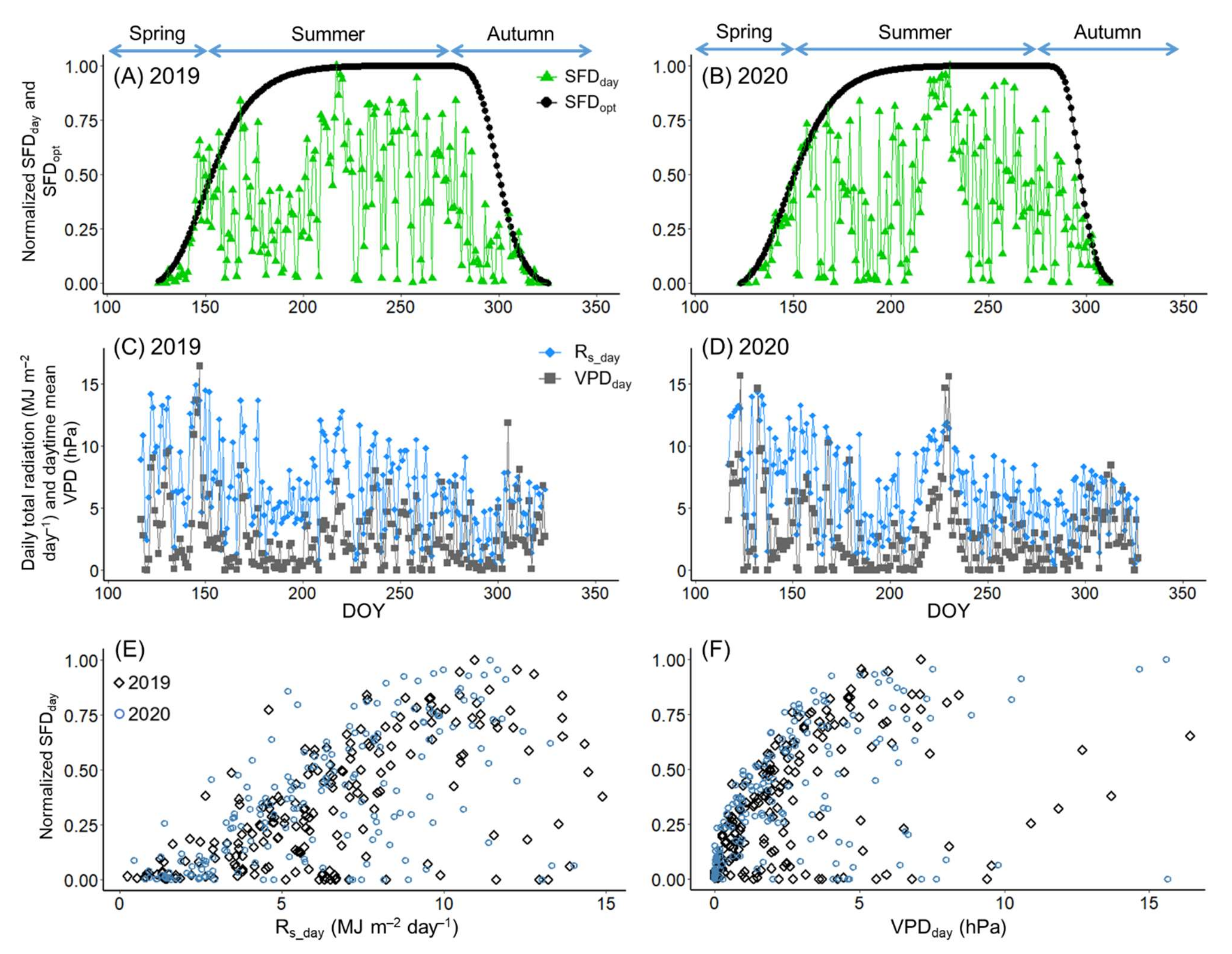
2.4.2. Calculation and Normalization of Daytime Total Sap Flow
2.4.3. Modelling the Phenology of Sap Flow
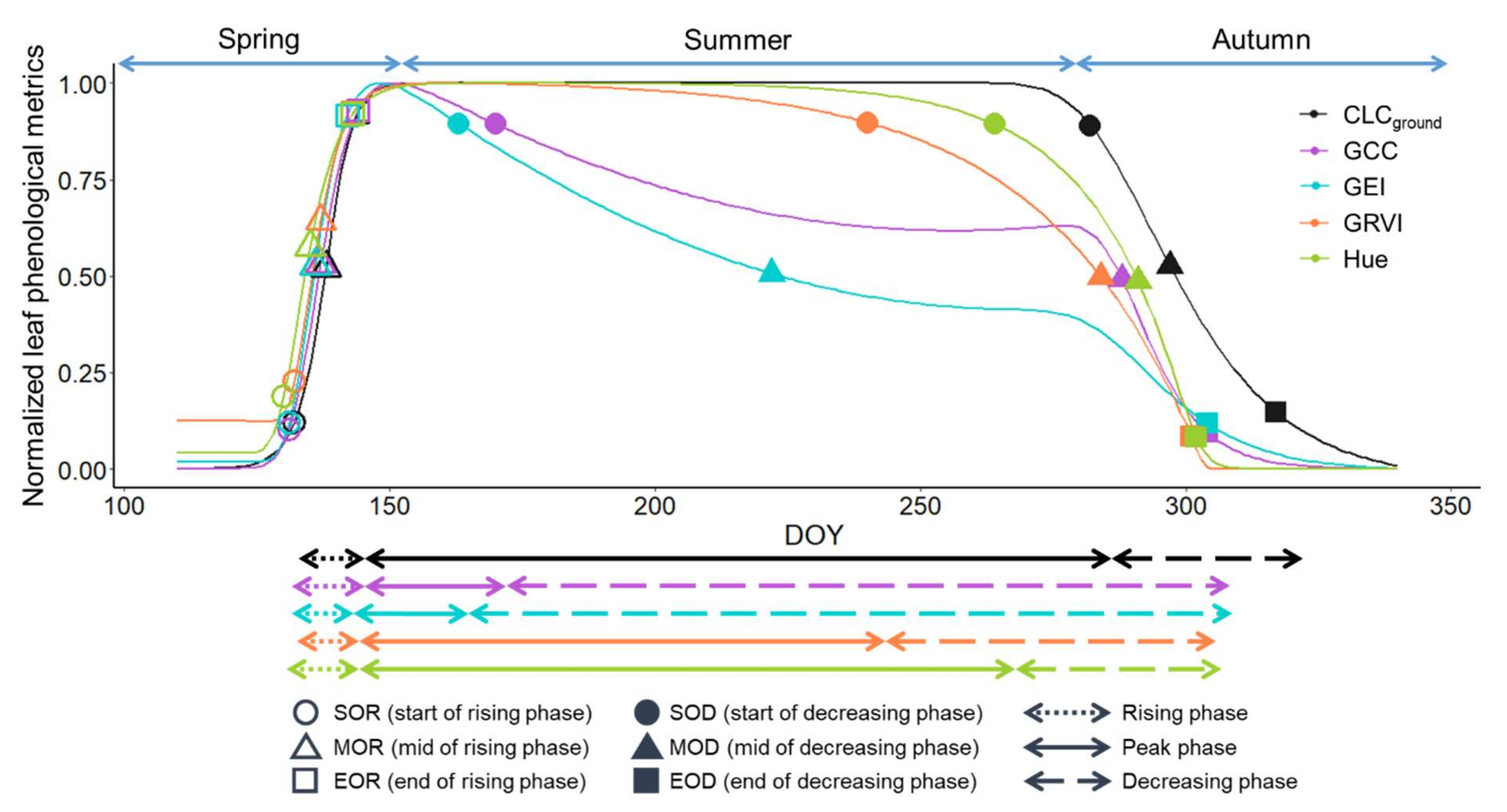
2.4.4. Calculation of Phenological Transition Dates
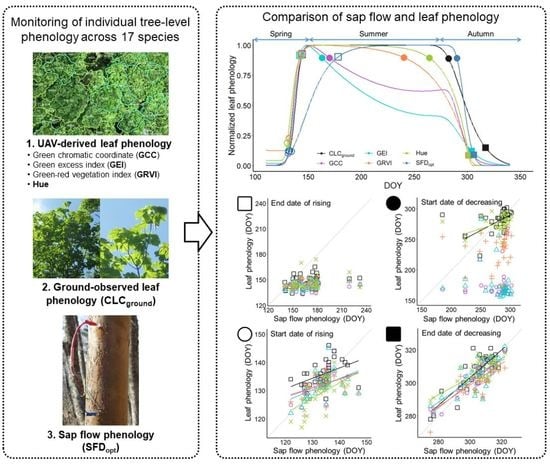
3. Results
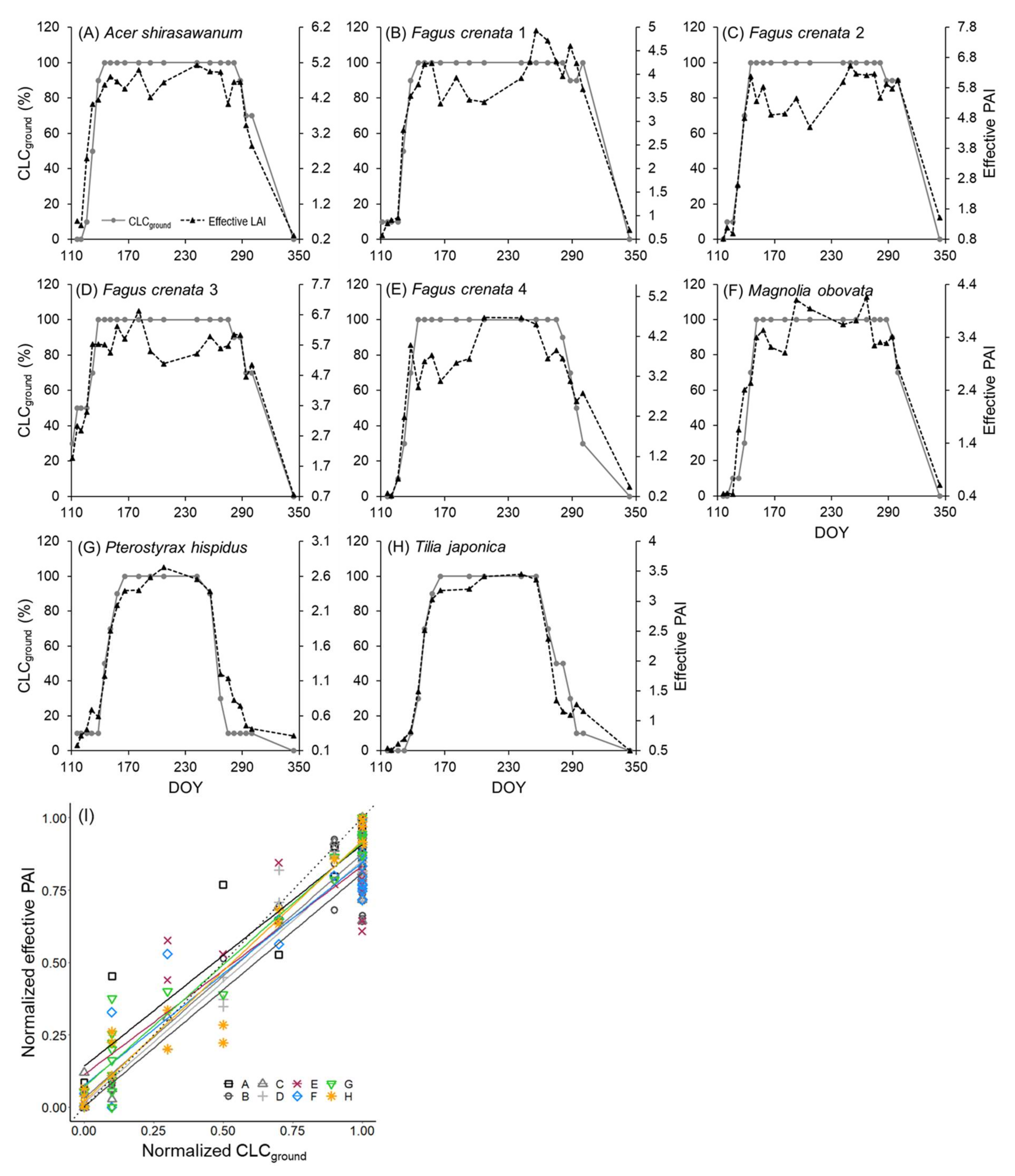
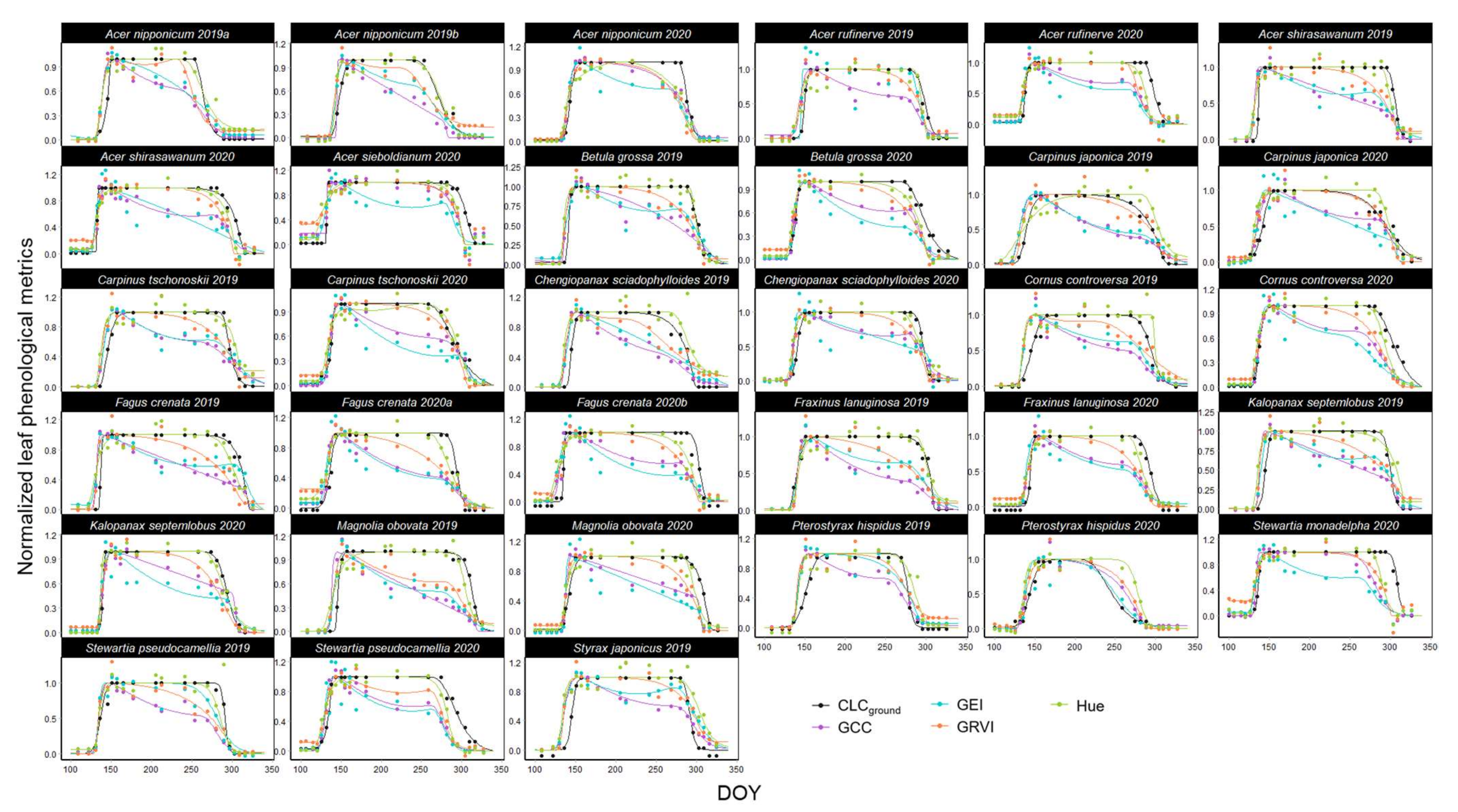
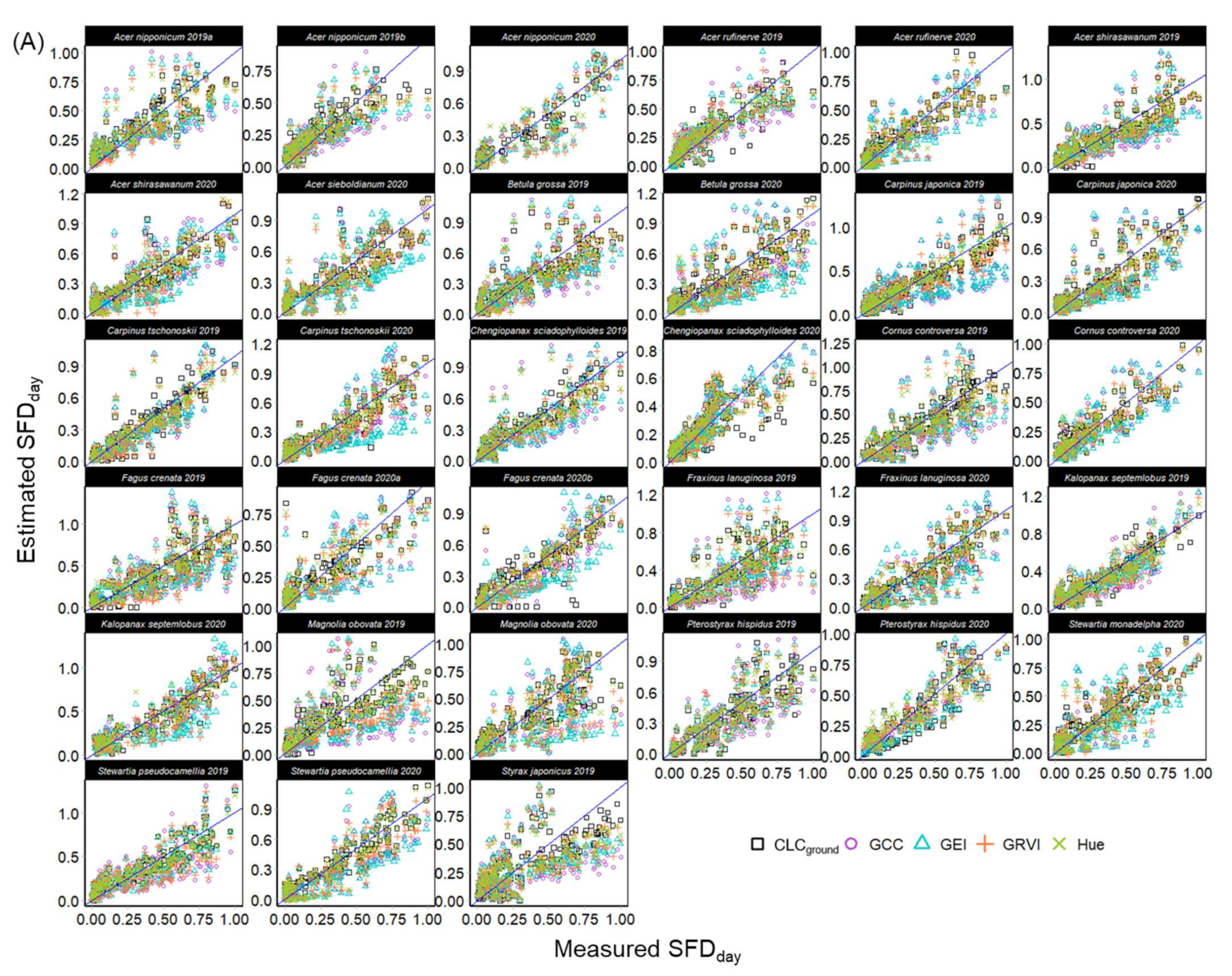
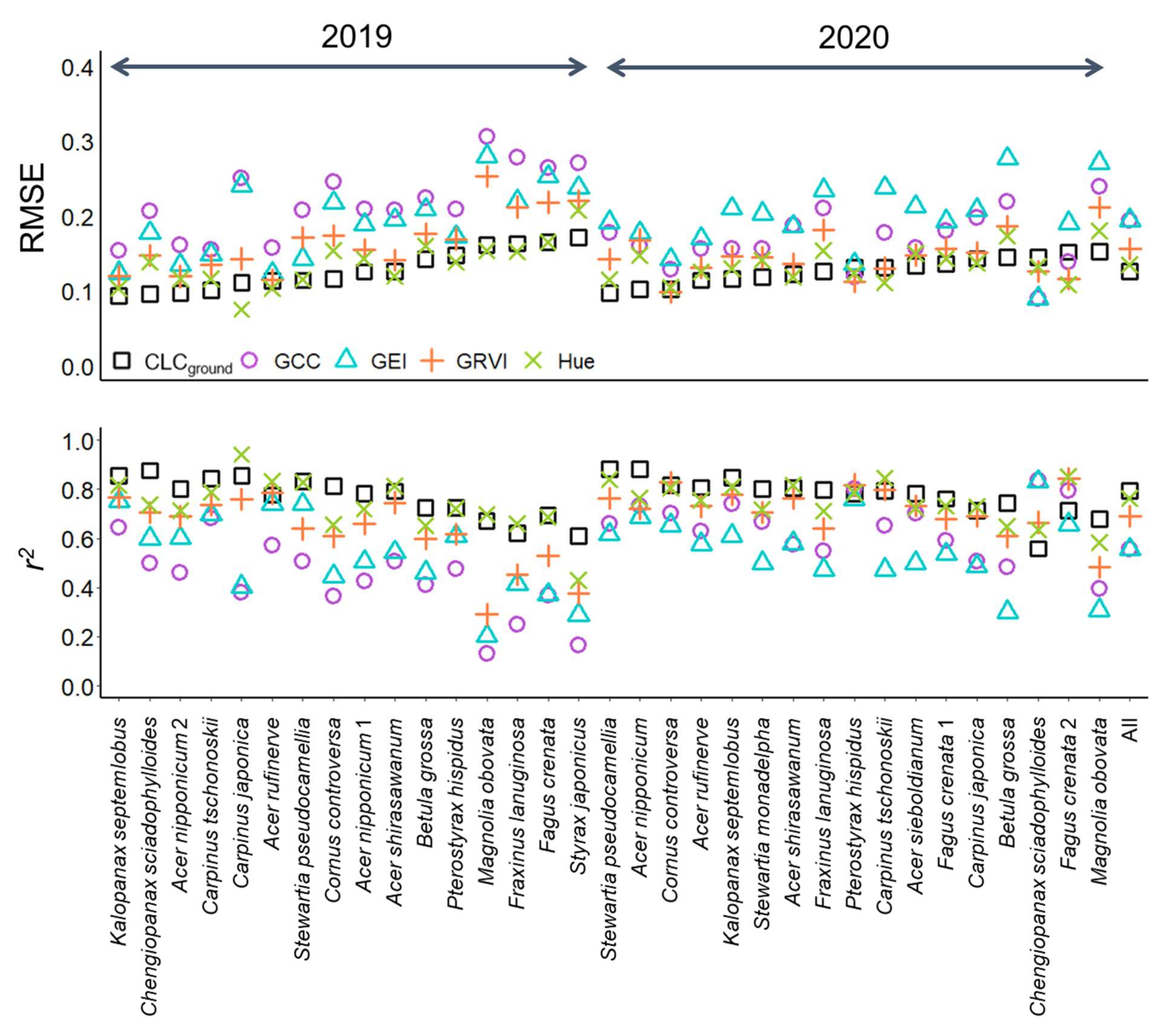
3.1. Leaf Phenology Derived from Ground Observation and UAV Imagery
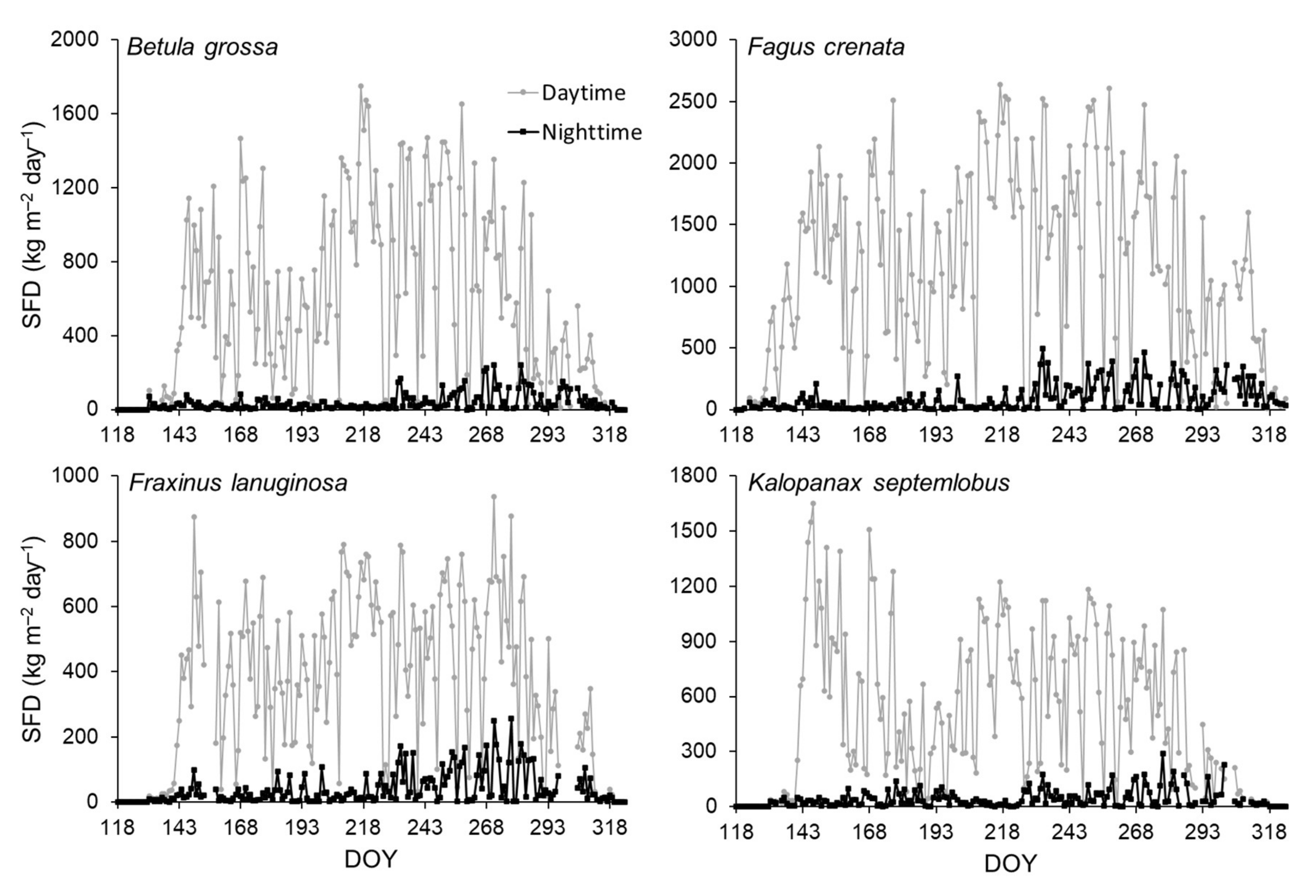
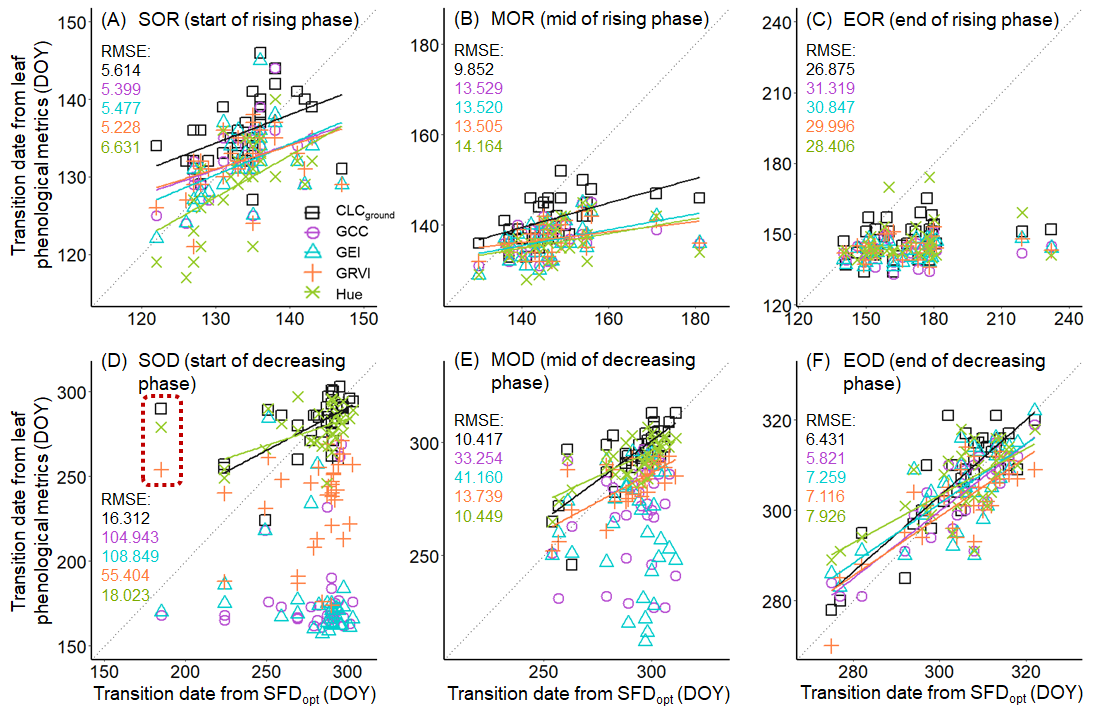
3.2. Sap Flow Phenology and Comparison with Leaf Phenology
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CLCground | Crown leaf cover derived from ground observation |
| EOD | End of the decreasing phase |
| EOR | End of the rising phase |
| GEI | Green excess index |
| GRVI | Green-red vegetation index |
| gs | Stomatal conductance |
| LAI | Leaf area index |
| MOD | Mid of the decreasing phase |
| MOR | Mid of the rising phase |
| RMSE | Root-mean-square error |
| Rs | Solar radiation |
| SFD | Sap flux density |
| SFDday | Daytime (05:00–20:00) total sap flux density |
| SFDopt | Optimized daytime sap flux density |
| SOD | Start of the decreasing phase |
| SOR | Start of the rising phase |
| UAV | Unmanned aerial vehicle |
| VPD | Vapor pressure deficit |
Appendix A



| Year | Date | DOY | Flight Time | Mean ± SD Radiation during the Flight (J m−2 s−1) | Number of Photos Used for Orthophoto Production | Average Ground Resolution (cm Pixel−1) | RMSE of Reprojection (Pixel) | 3D RSME of GCPs (m) |
|---|---|---|---|---|---|---|---|---|
| 2019 | 18 April | 108 | 10:30–12:19 | N/A | 3012 | 1.62 | 0.62 | 0.003 |
| 02 May | 122 | 09:44–10:51 | 466.8 ± 38.7 | 1264 | 1.47 | 0.58 | 0.021 | |
| 10 May | 130 | 10:09–12:28 | 453.1 ± 14.2 | 3099 | 1.55 | 0.69 | 0.000 | |
| 16 May | 136 | 11:05–12:28 | 262.7 ± 26.4 | 3092 | 1.15 | 0.84 | 0.001 | |
| 25 May | 145 | 11:05–13:24 | 505.0 ± 18.5 | 1208 | 1.17 | 0.72 | 0.001 | |
| 31 May | 151 | 10:02–12:22 | 222.2 ± 80.6 | 1178 | 0.91 | 0.71 | 0.001 | |
| 06 May | 157 | 10:24–11:29 | 532.5 ± 103.7 | 1703 | 1.30 | 0.80 | 0.001 | |
| 14 June | 165 | 09:25–11:19 | 191.9 ± 67.6 | 3008 | 1.40 | 0.81 | 0.001 | |
| 26 June | 177 | 10:00–11:44 | 440.9 ± 37.6 | 1693 | 1.00 | 0.85 | 0.048 | |
| 24 July | 205 | 12:15–12:35 | 398.3 ± 85.6 | 769 | 1.27 | 0.74 | 0.001 | |
| 31 July | 212 | 11:08–11:47 | 379.6 ± 151.3 | 536 | 1.26 | 0.72 | 0.000 | |
| 29 August | 241 | 15:03–16:23 | 108.2 ± 8.4 | 2068 | 1.11 | 0.80 | 0.052 | |
| 10 September | 253 | 11:26–13:17 | 388.8 ± 47.4 | 2182 | 1.20 | 0.67 | 0.055 | |
| 26 September | 269 | 12:10–13:44 | 249.2 ± 78.0 | 1850 | 1.29 | 0.74 | 0.048 | |
| 07 October | 280 | 10:59–12:31 | 275.3 ± 42.3 | 2410 | 1.10 | 0.72 | 0.074 | |
| 16 October | 289 | 14:39–15:31 | 96.4 ± 45.4 | 1266 | 1.01 | 0.70 | 0.044 | |
| 23 October | 296 | 10:36–11:50 | 220.3 ± 29.0 | 1412 | 1.02 | 0.67 | 0.060 | |
| 30 October | 303 | 10:30–11:48 | 336.5 ± 13.4 | 1042 | 1.04 | 0.60 | 0.023 | |
| 05 November | 309 | 10:37–12:08 | 305.7 ± 36.8 | nd | nd | nd | nd | |
| 12 November | 316 | 10:15–11:33 | 298.44 ± 18.0 | nd | nd | nd | nd | |
| 21 November | 325 | 10:39–12:27 | N/A | nd | nd | nd | nd | |
| 2020 | 09 April | 100 | 12:06–13:57 | 438.9 ± 28.3 | 2475 | 1.37 | 0.62 | 0.054 |
| 15 April | 106 | 10:07–12:00 | 434.6 ± 41.0 | 2506 | 1.46 | 0.60 | 0.040 | |
| 24 April | 115 | 09:25–11:45 | 439.4 ± 49.1 | 2271 | 1.24 | 0.62 | 1.172 * | |
| 29 April | 120 | 10:32–12:18 | 469.3 ± 13.7 | 2318 | 1.19 | 0.61 | 0.966 * | |
| 05 May | 126 | 10:17–12:17 | 315.8 ± 119.7 | 2280 | 1.23 | 0.60 | 0.027 | |
| 11 May | 132 | 10:04–11:40 | 477.9 ± 43.7 | 2158 | 1.27 | 0.71 | 0.096 | |
| 14 May | 135 | 10:10–12:35 | 480.8 ± 19.0 | 2614 | 1.14 | 0.77 | 0.089 | |
| 17 May | 138 | 10:19–11:46 | 460.9 ± 68.8 | 2571 | 1.05 | 0.71 | 0.096 | |
| 22 May | 143 | 10:25–12:17 | 292.7 ± 73.7 | 2493 | 1.12 | 0.77 | 0.050 | |
| 29 May | 150 | 09:59–11:58 | 394.3 ± 111.9 | 2305 | 1.05 | 0.70 | 0.106 | |
| 03 June | 155 | 09:49–11:25 | 372.4 ± 41.7 | 3367 | 0.98 | 0.67 | 0.049 | |
| 09 June | 161 | 10:04–11:41 | 348.7 ± 76.7 | 2310 | 0.88 | 0.69 | 0.090 | |
| 17 June | 169 | 13:24–14:33 | 183.7 ± 61.7 | 1534 | 1.03 | 0.79 | 0.054 | |
| 29 June | 181 | 09:55–11:16 | 299.1 ± 94.9 | 1873 | 0.89 | 0.74 | 0.062 | |
| 07 August | 220 | 10:24–12:06 | 392.9 ± 33.1 | 1886 | 1.06 | 0.80 | 0.060 | |
| 14 September | 258 | 10:20–12:31 | 341.7 ± 52.8 | 2218 | 0.94 | 0.66 | 0.102 | |
| 30 September | 274 | 08:18–10:24 | 280.9 ± 66.7 | 2322 | 1.36 | 0.60 | 0.047 | |
| 07 October | 281 | 10:15–11:35 | 262.9 ± 47.1 | 2036 | 0.99 | 0.69 | 0.062 | |
| 14 October | 288 | 09:16–10:58 | 183.3 ± 32.7 | 1920 | 0.91 | 0.60 | 0.053 | |
| 20 October | 294 | 11:10–12:58 | 353.9 ± 10.4 | 2585 | 0.98 | 0.63 | 0.063 | |
| 30 October | 304 | 09:16–10:58 | 199.7 ± 119.6 | 2407 | 0.89 | 0.60 | 0.116 | |
| 04 November | 309 | 14:17–14:58 | 213.8 ± 40.2 | 1074 | 1.15 | 0.61 | 0.055 | |
| 11 November | 316 | 10:05–11:05 | 305.3 ± 29.9 | 1445 | 1.36 | 0.57 | 0.246 | |
| 22 November | 327 | 10:43–11:55 | 82.0 ± 16.6 | 1430 | 1.25 | 0.58 | 0.042 |
References
- Vitasse, Y.; Porté, A.J.; Kremer, A.; Michalet, R.; Delzon, S. Responses of canopy duration to temperature changes in four temperate tree species: Relative contributions of spring and autumn leaf phenology. Oecologia 2009, 161, 187–198. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Yuan, W.; Liu, S.; Dong, W. Divergent responses of leaf phenology to changing temperature among plant species and geographical regions. Ecosphere 2015, 6, art250. [Google Scholar] [CrossRef]
- Shen, M.; Tang, Y.; Chen, J.; Yang, X.; Wang, C.; Cui, X.; Yang, Y.; Han, L.; Li, L.; Du, J.; et al. Earlier-Season Vegetation Has Greater Temperature Sensitivity of Spring Phenology in Northern Hemisphere. PLoS ONE 2014, 9, e88178. [Google Scholar] [CrossRef] [PubMed]
- Gill, A.L.; Gallinat, A.S.; Sanders-DeMott, R.; Rigden, A.J.; Short Gianotti, D.J.; Mantooth, J.A.; Templer, P.H. Changes in autumn senescence in northern hemisphere deciduous trees: A meta-analysis of autumn phenology studies. Ann. Bot. 2015, 116, 875–888. [Google Scholar] [CrossRef] [PubMed]
- Richardson, A.D.; Keenan, T.F.; Migliavacca, M.; Ryu, Y.; Sonnentag, O.; Toomey, M. Climate change, phenology, and phenological control of vegetation feedbacks to the climate system. Agric. For. Meteorol. 2013, 169, 156–173. [Google Scholar] [CrossRef]
- Vitasse, Y.; Basler, D. What role for photoperiod in the bud burst phenology of European beech. Eur. J. For. Res. 2013, 132, 1–8. [Google Scholar] [CrossRef]
- Fu, Y.S.H.; Campioli, M.; Vitasse, Y.; De Boeck, H.J.; Van Den Berge, J.; AbdElgawad, H.; Asard, H.; Piao, S.; Deckmyn, G.; Janssens, I.A. Variation in leaf flushing date influences autumnal senescence and next year’s flushing date in two temperate tree species. Proc. Natl. Acad. Sci. USA 2014, 111, 7355–7360. [Google Scholar] [CrossRef]
- Xie, Y.; Wang, X.; Wilson, A.M.; Silander, J.A. Predicting autumn phenology: How deciduous tree species respond to weather stressors. Agric. For. Meteorol. 2018, 250–251, 127–137. [Google Scholar] [CrossRef]
- Toomey, M.; Friedl, M.A.; Frolking, S.; Hufkens, K.; Klosterman, S.; Sonnentag, O.; Baldocchi, D.D.; Bernacchi, C.J.; Biraud, S.C.; Bohrer, G.; et al. Greenness indices from digital cameras predict the timing and seasonal dynamics of canopy-scale photosynthesis. Ecol. Appl. 2015, 25, 99–115. [Google Scholar] [CrossRef]
- Ahrends, H.E.; Etzold, S.; Kutsch, W.L.; Stoeckli, R.; Bruegger, R.; Jeanneret, F.; Wanner, H.; Buchmann, N.; Eugster, W. Tree phenology and carbon dioxide fluxes: Use of digital photography for process-based interpretation at the ecosystem scale. Clim. Res. 2009, 39, 261–274. [Google Scholar] [CrossRef]
- Richardson, A.D.; Braswell, B.H.; Hollinger, D.Y.; Jenkins, J.P.; Ollinger, S.V. Near-surface remote sensing of spatial and temporal variation in canopy phenology. Ecol. Appl. 2009, 19, 1417–1428. [Google Scholar] [CrossRef]
- Nagai, S.; Maeda, T.; Gamo, M.; Muraoka, H.; Suzuki, R.; Nasahara, K.N. Using digital camera images to detect canopy condition of deciduous broad-leaved trees. Plant Ecol. Divers. 2011, 4, 79–89. [Google Scholar] [CrossRef]
- Keenan, T.F.; Darby, B.; Felts, E.; Sonnentag, O.; Friedl, M.A.; Hufkens, K.; O’Keefe, J.; Klosterman, S.; Munger, J.W.; Toomey, M.; et al. Tracking forest phenology and seasonal physiology using digital repeat photography: A critical assessment. Ecol. Appl. 2014, 24, 1478–1489. [Google Scholar] [CrossRef] [PubMed]
- Wingate, L.; Ogeé, J.; Cremonese, E.; Filippa, G.; Mizunuma, T.; Migliavacca, M.; Moisy, C.; Wilkinson, M.; Moureaux, C.; Wohlfahrt, G.; et al. Interpreting canopy development and physiology using a European phenology camera network at flux sites. Biogeosciences 2015, 12, 5995–6015. [Google Scholar] [CrossRef]
- Liu, Z.; Hu, H.; Yu, H.; Yang, X.; Yang, H.; Ruan, C.; Wang, Y.; Tang, J. Relationship between leaf physiologic traits and canopy color indices during the leaf expansion period in an oak forest. Ecosphere 2015, 6, 1–9. [Google Scholar] [CrossRef]
- Liu, Z.; An, S.; Lu, X.; Hu, H.; Tang, J. Using canopy greenness index to identify leaf ecophysiological traits during the foliar senescence in an oak forest. Ecosphere 2018, 9, 1–14. [Google Scholar] [CrossRef]
- Toda, M.; Richardson, A.D. Estimation of plant area index and phenological transition dates from digital repeat photography and radiometric approaches in a hardwood forest in the Northeastern United States. Agric. For. Meteorol. 2018, 249, 457–466. [Google Scholar] [CrossRef]
- Richardson, A.D.; Jenkins, J.P.; Braswell, B.H.; Hollinger, D.Y.; Ollinger, S.V.; Smith, M.L. Use of digital webcam images to track spring green-up in a deciduous broadleaf forest. Oecologia 2007, 152, 323–334. [Google Scholar] [CrossRef]
- Richardson, A.D.; Hollinger, D.Y.; Dail, D.B.; Lee, J.T.; Munger, J.W.; O’Keefe, J. Influence of spring phenology on seasonal and annual carbon balance in two contrasting New England forests. Tree Physiol. 2009, 29, 321–331. [Google Scholar] [CrossRef]
- Moore, C.E.; Brown, T.; Keenan, T.F.; Duursma, R.A.; van Dijk, A.I.J.M.; Beringer, J.; Culvenor, D.; Evans, B.; Huete, A.; Hutley, L.B.; et al. Reviews and syntheses: Australian vegetation phenology: New insights from satellite remote sensing and digital repeat photography. Biogeosciences 2016, 13, 5085–5102. [Google Scholar] [CrossRef]
- Zhou, L.; He, H.L.; Sun, X.M.; Zhang, L.; Yu, G.R.; Ren, X.L.; Wang, J.Y.; Zhao, F.H. Modeling winter wheat phenology and carbon dioxide fluxes at the ecosystem scale based on digital photography and eddy covariance data. Ecol. Inform. 2013, 18, 69–78. [Google Scholar] [CrossRef]
- Ide, R.; Nakaji, T.; Motohka, T.; Oguma, H. Advantages of visible-band spectral remote sensing at both satellite and near-surface scales for monitoring the seasonal dynamics of GPP in a Japanese larch forest. J. Agric. Meteorol. 2011, 67, 75–84. [Google Scholar] [CrossRef]
- Saitoh, T.M.; Nagai, S.; Saigusa, N.; Kobayashi, H.; Suzuki, R.; Nasahara, K.N.; Muraoka, H. Assessing the use of camera-based indices for characterizing canopy phenology in relation to gross primary production in a deciduous broad-leaved and an evergreen coniferous forest in Japan. Ecol. Inform. 2012, 11, 45–54. [Google Scholar] [CrossRef]
- Mizunuma, T.; Wilkinson, M.; Eaton, E.L.; Mencuccini, M.; Morison, J.I.L.; Grace, J. The relationship between carbon dioxide uptake and canopy colour from two camera systems in a deciduous forest in southern England. Funct. Ecol. 2013, 27, 196–207. [Google Scholar] [CrossRef]
- Muraoka, H.; Koizumi, H. Photosynthetic and structural characteristics of canopy and shrub trees in a cool-temperate deciduous broadleaved forest: Implication to the ecosystem carbon gain. Agric. For. Meteorol. 2005, 134, 39–59. [Google Scholar] [CrossRef]
- Yang, J.C.; Magney, T.S.; Yan, D.; Knowles, J.F.; Smith, W.K.; Scott, R.L.; Barron-Gafford, G.A. The photochemical reflectance index (PRI) captures the ecohydrologic sensitivity of a semiarid mixed conifer forest. J. Geophys. Res. Biogeosci. 2020, 125, e2019JG005624. [Google Scholar] [CrossRef]
- Klosterman, S.; Richardson, A.D. Observing spring and fall phenology in a deciduous forest with aerial drone imagery. Sensors 2017, 17, 2852. [Google Scholar] [CrossRef]
- Budianti, N.; Mizunaga, H.; Iio, A. Crown structure explains the discrepancy in leaf phenology metrics derived from ground- and UAV-based observations in a Japanese cool temperate deciduous forest. Forests 2021, 12, 425. [Google Scholar] [CrossRef]
- Medhurst, J.; Parsby, J.; Linder, S.; Wallin, G.; Ceschia, E.; Slaney, M. A whole-tree chamber system for examining tree-level physiological responses of field-grown trees to environmental variation and climate change. Plant Cell Environ. 2006, 29, 1853–1869. [Google Scholar] [CrossRef]
- Michelot, A.; Eglin, T.; Dufrene, E.; Lelarge-Trouverie, C.; Damesin, C. Comparison of seasonal variations in water-use efficiency calculated from the carbon isotope composition of tree rings and flux data in a temperate forest. Plant. Cell Environ. 2011, 34, 230–244. [Google Scholar] [CrossRef]
- Du, B.; Zheng, J.; Ji, H.; Zhu, Y.; Yuan, J.; Wen, J.; Kang, H.; Liu, C. Stable carbon isotope used to estimate water use efficiency can effectively indicate seasonal variation in leaf stoichiometry. Ecol. Indic. 2021, 121, 107250. [Google Scholar] [CrossRef]
- Niinemets, Ü.; Valladares, F. Tolerance to shade, drought, and waterlogging of temperate northern hemisphere trees and shrubs. Ecol. Monogr. 2006, 76, 521–547. [Google Scholar] [CrossRef]
- Bentley ContextCapture User Guide. Available online: https://docs.bentley.com/LiveContent/web/ContextCapture_User_Guide_EN_PDF-v18/en/ContextCapture User Guide EN.pdf (accessed on 13 April 2022).
- Granier, A. Evaluation of transpiration in a Douglas-fir stand by means of sap flow measurements. Tree Physiol. 1987, 3, 309–320. [Google Scholar] [CrossRef] [PubMed]
- Tetens, O. Uber einige meteorologische Begriffe. Z. Geophys. 1930, 6, 297–309. [Google Scholar]
- Steppe, K.; De Pauw, D.J.W.; Doody, T.M.; Teskey, R.O. A comparison of sap flux density using thermal dissipation, heat pulse velocity and heat field deformation methods. Agric. For. Meteorol. 2010, 150, 1046–1056. [Google Scholar] [CrossRef]
- Ford, C.R.; McGuire, M.A.; Mitchell, R.J.; Teskey, R.O. Assessing variation in the radial profile of sap flux density in Pinus species and its effect on daily water use. Tree Physiol. 2004, 24, 241–249. [Google Scholar] [CrossRef]
- Shinohara, Y.; Tsuruta, K.; Ogura, A.; Noto, F.; Komatsu, H.; Otsuki, K.; Maruyama, T. Azimuthal and radial variations in sap flux density and effects on stand-scale transpiration estimates in a Japanese cedar forest. Tree Physiol. 2013, 33, 550–558. [Google Scholar] [CrossRef]
- Wullschleger, S.D.; Hanson, P.J.; Todd, D.E. Transpiration from a multi-species deciduous forest as estimated by xylem sap flow techniques. For. Ecol. Manag. 2001, 143, 205–213. [Google Scholar] [CrossRef]
- Gu, L.; Post, W.M.; Baldocchi, D.D.; Black, T.A.; Suyker, A.E.; Verma, S.B.; Vesala, T.; Wofsy, S.C. Characterizing the seasonal dynamics of plant community photosynthesis across a range of vegetation types. In Phenology of Ecosystem Processes: Applications in Global Change Research; Noormets, A., Ed.; Springer: New York, NY, USA, 2009; pp. 35–58. ISBN 978-1-4419-0026-5. [Google Scholar]
- Quo, J.; Gabry, J.; Goodrich, B.; Weber, S. RStan: The R interface to Stan, Version 2.21.2. 2020. Available online: https://mc-stan.org/rstan (accessed on 9 November 2020).
- Filippa, G.; Cremonese, E.; Migliavacca, M.; Galvagno, M.; Forkel, M.; Wingate, L.; Tomelleri, E.; Morra di Cella, U.; Richardson, A.D. Phenopix: A R package for image-based vegetation phenology. Agric. For. Meteorol. 2016, 220, 141–150. [Google Scholar] [CrossRef]
- Barnard, D.M.; Bauerle, W.L. The implications of minimum stomatal conductance on modeling water flux in forest canopies. J. Geophys. Res. Biogeosci. 2013, 118, 1322–1333. [Google Scholar] [CrossRef]
- Matsumoto, K.; Ohta, T.; Tanaka, T. Dependence of stomatal conductance on leaf chlorophyll concentration and meteorological variables. Agric. For. Meteorol. 2005, 132, 44–57. [Google Scholar] [CrossRef]
- Croft, H.; Chen, J.M.; Luo, X.; Bartlett, P.; Chen, B.; Staebler, R.M. Leaf chlorophyll content as a proxy for leaf photosynthetic capacity. Glob. Chang. Biol. 2017, 23, 3513–3524. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Civco, D.L.; Silander, J.A. Species-specific spring and autumn leaf phenology captured by time-lapse digital cameras. Ecosphere 2018, 9, 1–21. [Google Scholar] [CrossRef]
- Tie, Q.; Hu, H.; Tian, F.; Guan, H.; Lin, H. Environmental and physiological controls on sap flow in a subhumid mountainous catchment in North China. Agric. For. Meteorol. 2017, 240–241, 46–57. [Google Scholar] [CrossRef]
- Hiyama, T.; Kochi, K.; Kobayashi, N.; Sirisampan, S. Seasonal variation in stomatal conductance and physiological factors observed in a secondary warm-temperate forest. Ecol. Res. 2005, 20, 333–346. [Google Scholar] [CrossRef]
- Čatský, J.; Solárová, J.; Pospišilová, J.; Tichá, I. Conductances for Carbon Dioxide Transfer in the Leaf. In Photosynthesis during leaf Development; Šestăk, Z., Ed.; Springer: Dordrecht, The Netherlands, 1985; pp. 217–249. ISBN 978-94-009-5530-1. [Google Scholar]
- Hoshika, Y.; Watanabe, M.; Inada, N.; Koike, T. Ozone-induced stomatal sluggishness develops progressively in Siebold’s beech (Fagus crenata). Environ. Pollut. 2012, 166, 152–156. [Google Scholar] [CrossRef]
- Schaberg, P.G.; Van Den Berg, A.K.; Murakami, P.F.; Shane, J.B.; Donnelly, J.R. Factors influencing red expression in autumn foliage of sugar maple trees. Tree Physiol. 2003, 23, 325–333. [Google Scholar] [CrossRef]
- Renner, S.S.; Zohner, C.M. The occurrence of red and yellow autumn leaves explained by regional differences in insolation and temperature. New Phytol. 2019, 224, 1464–1471. [Google Scholar] [CrossRef]
- Yang, H.; Yang, X.; Heskel, M.; Sun, S.; Tang, J. Seasonal variations of leaf and canopy properties tracked by ground-based NDVI imagery in a temperate forest. Sci. Rep. 2017, 7, 1–10. [Google Scholar] [CrossRef]
- Filippa, G.; Cremonese, E.; Migliavacca, M.; Galvagno, M.; Sonnentag, O.; Humphreys, E.; Hufkens, K.; Ryu, Y.; Verfaillie, J.; Morra di Cella, U.; et al. NDVI derived from near-infrared-enabled digital cameras: Applicability across different plant functional types. Agric. For. Meteorol. 2018, 249, 275–285. [Google Scholar] [CrossRef]
- Junker, L.V.; Ensminger, I. Relationship between leaf optical properties, chlorophyll fluorescence and pigment changes in senescing Acer saccharum leaves. Tree Physiol. 2016, 36, 694–711. [Google Scholar] [CrossRef] [PubMed]
- Viña, A.; Gitelson, A.A.; Nguy-Robertson, A.L.; Peng, Y. Comparison of different vegetation indices for the remote assessment of green leaf area index of crops. Remote Sens. Environ. 2011, 115, 3468–3478. [Google Scholar] [CrossRef]
- Motohka, T.; Nasahara, K.N.; Oguma, H.; Tsuchida, S. Applicability of Green-Red Vegetation Index for remote sensing of vegetation phenology. Remote Sens. 2010, 2, 2369–2387. [Google Scholar] [CrossRef]
- Urban, J.; Bednářová, E.; Plichta, R.; Kučera, J. Linking phenological data to ecophysiology of European beech. Acta Hortic. 2013, 991, 293–300. [Google Scholar] [CrossRef]
- Graham, E.A.; Yuen, E.M.; Robertson, G.F.; Kaiser, W.J.; Hamilton, M.P.; Rundel, P.W. Budburst and leaf area expansion measured with a novel mobile camera system and simple color thresholding. Environ. Exp. Bot. 2009, 65, 238–244. [Google Scholar] [CrossRef]
- Klosterman, S.; Hufkens, K.; Richardson, A.D. Later springs green-up faster: The relation between onset and completion of green-up in deciduous forests of North America. Int. J. Biometeorol. 2018, 62, 1645–1655. [Google Scholar] [CrossRef]
- Nadezhdina, N.; Čermák, J.; Ceulemans, R. Radial patterns of sap flow in woody stems of dominant and understory species: Scaling errors associated with positioning of sensors. Tree Physiol. 2002, 22, 907–918. [Google Scholar] [CrossRef]
- Phillips, N.; Oren, R.; Zimmermann, R. Radial patterns of xylem sap flow in non-, diffuse- and ring-porous tree species. Plant, Cell Environ. 1996, 19, 983–990. [Google Scholar] [CrossRef]
- Gartner, K.; Nadezhdina, N.; Englisch, M.; Čermak, J.; Leitgeb, E. Sap flow of birch and Norway spruce during the European heat and drought in summer 2003. For. Ecol. Manag. 2009, 258, 590–599. [Google Scholar] [CrossRef]
- Fiora, A.; Cescatti, A. Diurnal and seasonal variability in radial distribution of sap flux density: Implications for estimating stand transpiration. Tree Physiol. 2006, 26, 1217–1225. [Google Scholar] [CrossRef]
- Fawcett, D.; Bennie, J.; Anderson, K. Monitoring spring phenology of individual tree crowns using drone-acquired NDVI data. Remote Sens. Ecol. Conserv. 2021, 7, 227–244. [Google Scholar] [CrossRef]
- Gonsamo, A.; Walter, J.M.N.; Pellikka, P. CIMES: A package of programs for determining canopy geometry and solar radiation regimes through hemispherical photographs. Comput. Electron. Agric. 2011, 79, 207–215. [Google Scholar] [CrossRef]


| No. | Species | Successional Type | Number of Samples | Tree Size | |||
|---|---|---|---|---|---|---|---|
| 2019 | 2020 | DBH (cm) | H (m) | CA (m2) | |||
| 1. | Acer nipponicum H.Hara | Unknown | 2 | 1 | 19.62–41.11 | 13.70–16.90 | 14.72–28.87 |
| 2. | Acer rufinerve Siebold & Zucc. | Mid | 1 | 1 | 28.63–41.80 | 14.00–15.40 | 15.61–19.81 |
| 3. | Acer shirasawanum Koidz. | Late | 1 | 1 | 28.69–42.44 | 13.50–14.70 | 21.50–45.52 |
| 4. | Acer sieboldianum Miq. | Unknown | 0 | 1 | 23.03 | N/A | 15.14 |
| 5. | Betula grossa Siebold & Zucc. * | Early | 1 | 1 | 32.58 | 17.00 | 43.41 |
| 6. | Carpinus japonica Blume | Mid | 1 | 1 | 26.75–61.77 | 12.50–15.00 | 26.46–68.75 |
| 7. | Carpinus tschonoskii Maxim. | Mid | 1 | 1 | 31.07–36.10 | 15.30–22.00 | 25.49–45.47 |
| 8. | Chengiopanax sciadophylloides (Franch. & Sav.) C.B.Shang & J.Y.Huang | Mid | 1 | 1 | 34.87–41.50 | 14.30 | 17.80–29.49 |
| 9. | Cornus controversa Hemsl. * | Mid | 1 | 1 | 55.48 | 20.95 | 117.35 |
| 10. | Fagus crenata Blume | Late | 1 | 2 | 22.96–59.46 | 13.60–23.40 | 12.18–132.99 |
| 11. | Fraxinus lanuginosa Koidz. | Mid | 1 | 1 | 29.01–32.47 | 15.20–15.30 | 31.94–50.00 |
| 12. | Kalopanax septemlobus (Thunb.) Koidz. * | Mid | 1 | 1 | 34.04 | 16.50 | 29.96 |
| 13. | Magnolia obovata Thunb. * | Unknown | 1 | 1 | 50.44 | 16.60 | 63.16 |
| 14. | Pterostyrax hispidus Siebold & Zucc. | Early | 1 | 1 | 21.83–31.34 | 9.70–12.20 | 13.57–30.21 |
| 15. | Stewartia monadelpha Siebold & Zucc. | Unknown | 0 | 1 | 38.51 | N/A | 43.27 |
| 16. | Stewartia pseudocamellia Maxim. | Unknown | 1 | 1 | 26.55–28.70 | 13.40–13.80 | 9.21–12.23 |
| 17. | Styrax japonicus Siebold & Zucc. | Mid | 1 | 0 | 21.26 | 10.60 | 5.90 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Budianti, N.; Naramoto, M.; Iio, A. Drone-Sensed and Sap Flux-Derived Leaf Phenology in a Cool Temperate Deciduous Forest: A Tree-Level Comparison of 17 Species. Remote Sens. 2022, 14, 2505. https://doi.org/10.3390/rs14102505
Budianti N, Naramoto M, Iio A. Drone-Sensed and Sap Flux-Derived Leaf Phenology in a Cool Temperate Deciduous Forest: A Tree-Level Comparison of 17 Species. Remote Sensing. 2022; 14(10):2505. https://doi.org/10.3390/rs14102505
Chicago/Turabian StyleBudianti, Noviana, Masaaki Naramoto, and Atsuhiro Iio. 2022. "Drone-Sensed and Sap Flux-Derived Leaf Phenology in a Cool Temperate Deciduous Forest: A Tree-Level Comparison of 17 Species" Remote Sensing 14, no. 10: 2505. https://doi.org/10.3390/rs14102505
APA StyleBudianti, N., Naramoto, M., & Iio, A. (2022). Drone-Sensed and Sap Flux-Derived Leaf Phenology in a Cool Temperate Deciduous Forest: A Tree-Level Comparison of 17 Species. Remote Sensing, 14(10), 2505. https://doi.org/10.3390/rs14102505