Using Unmanned Aerial Vehicle and Ground-Based RGB Indices to Assess Agronomic Performance of Wheat Landraces and Cultivars in a Mediterranean-Type Environment
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Field Setup and Agronomic Data Recording
2.2. Remote Sensing Images Acquisition
2.2.1. Ground-Based RGB Vegetation Indices
2.2.2. Multispectral Images Acquired with the UAV
2.3. Statistical Analysis
3. Results
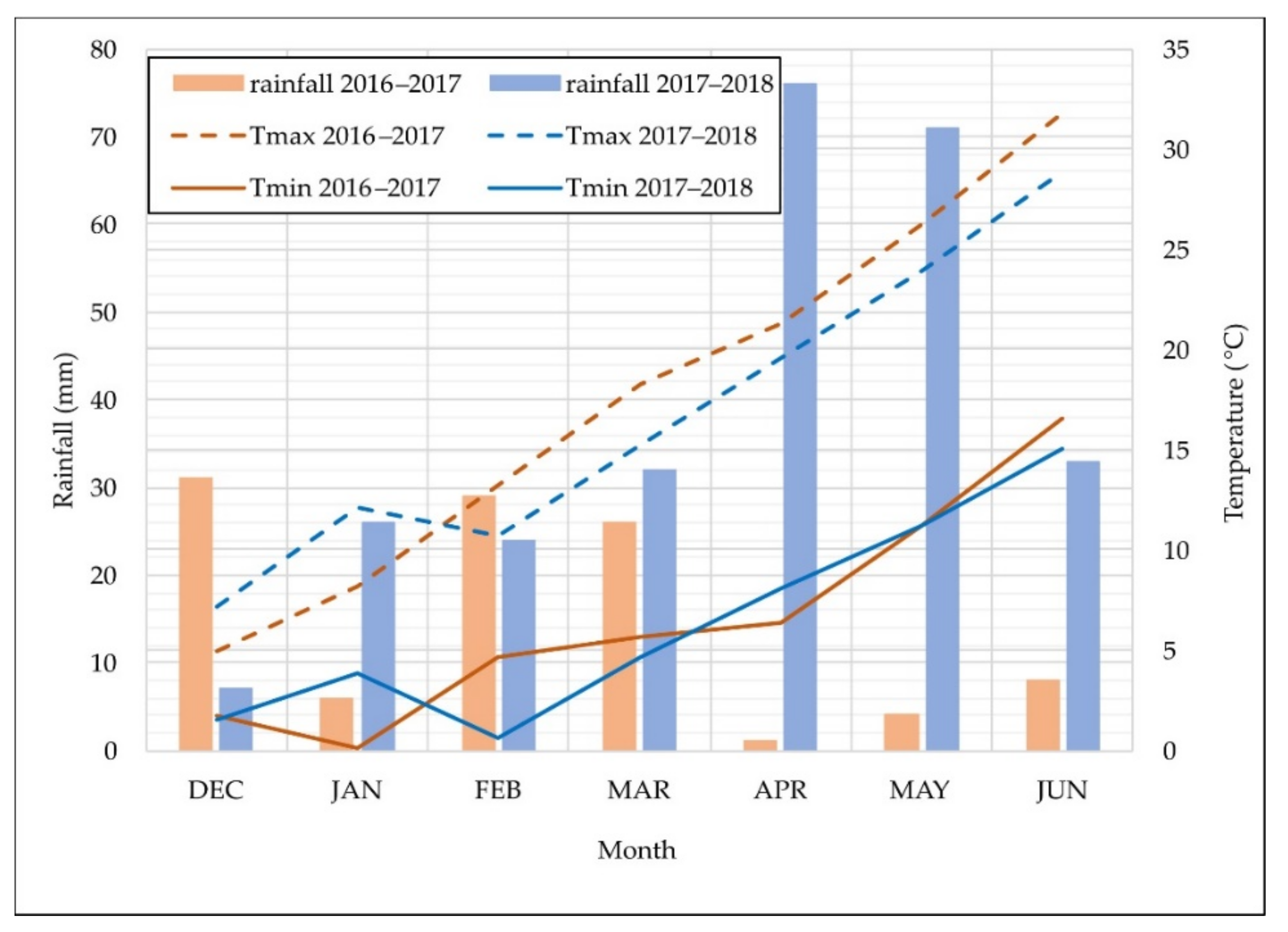
3.1. Environmental Conditions
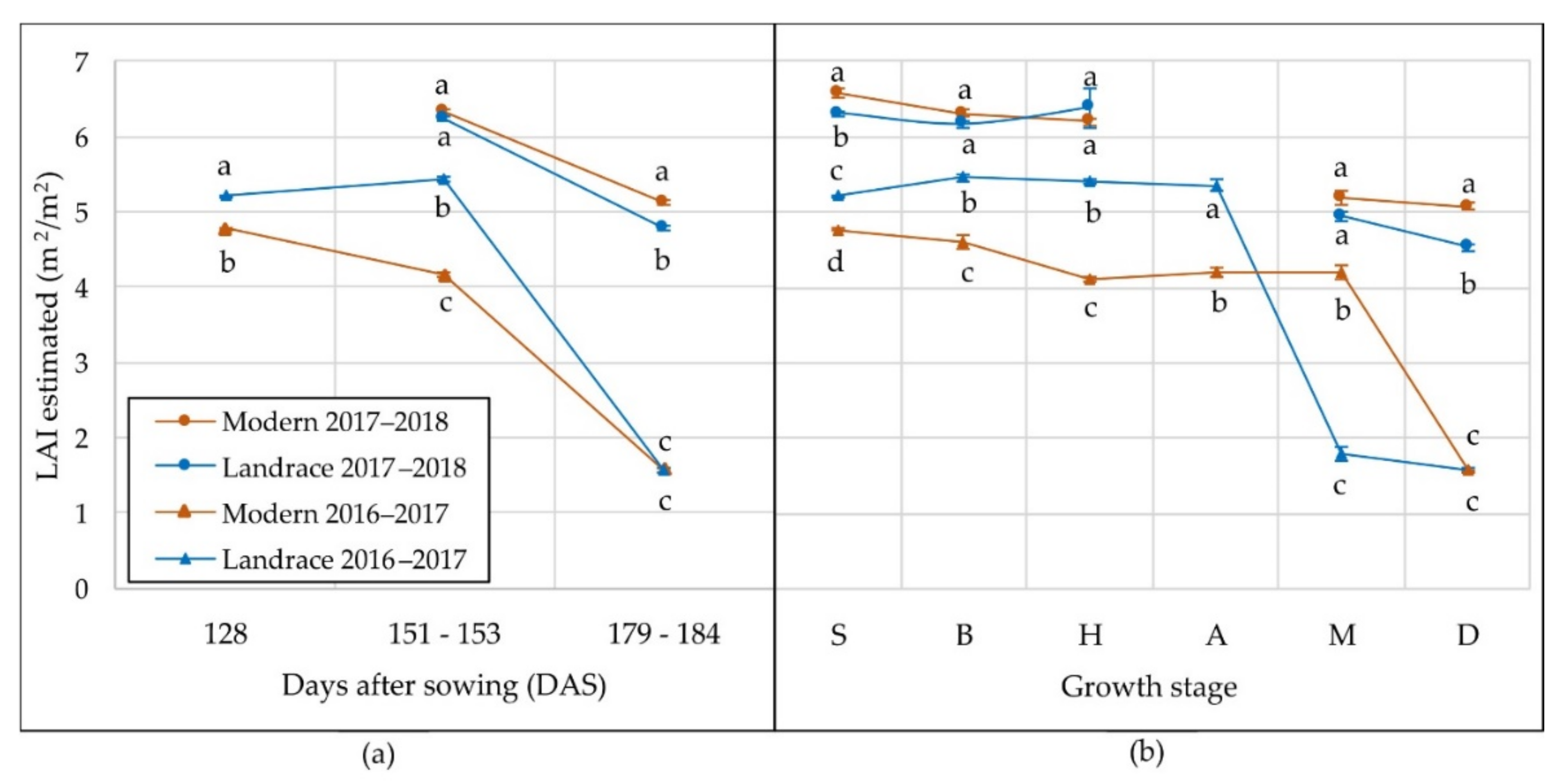
3.2. Agronomic Performance
3.3. LAI Prediction through Vegetation Indices
3.4. Performance of Stepwise Regression Models
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Leegood, R.C.; Evans, J.R.; Furbank, R.T. Food security requires genetic advances to increase farm yields. Nature 2010, 464, 831. [Google Scholar] [CrossRef]
- Fischer, R.A.T.; Edmeades, G.O. Breeding and Cereal Yield Progress. Crop Sci. 2010, 50, S-85–S-98. [Google Scholar] [CrossRef]
- Araus, J.L.; Slafer, G.A.; Reynolds, M.P.; Royo, C. Plant breeding and drought in C3 cereals: What should we breed for? Ann. Bot. 2002, 89, 925–940. [Google Scholar] [CrossRef] [PubMed]
- Bates, B.C.; Kundzewicz, Z.W.; Wu, S.; Palutikof, J.P. Climate Change and Water. In The American Midland Naturalist; Technical Paper of the Intergovernmental Panel on Climate Change; Intergovernmental Panel on Climate Change Secretariat: Geneva, Switzerland, 2008; ISBN 9789291691234. [Google Scholar]
- Bhatta, M.; Morgounov, A.; Belamkar, V.; Baenziger, P. Genome-Wide Association Study Reveals Novel Genomic Regions for Grain Yield and Yield-Related Traits in Drought-Stressed Synthetic Hexaploid Wheat. Int. J. Mol. Sci. 2018, 19, 3011. [Google Scholar] [CrossRef] [PubMed]
- Araus, J.L.; Cairns, J.E. Field high-throughput phenotyping: The new crop breeding frontier. Trends Plant Sci. 2014, 19, 52–61. [Google Scholar] [CrossRef]
- Reynolds, M.; Tuberosa, R. Translational research impacting on crop productivity in drought-prone environments. Curr. Opin. Plant Biol. 2008, 11, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.; Yang, C. A review on plant high-throughput phenotyping traits using UAV-based sensors. Comput. Electron. Agric. 2020, 178, 105731. [Google Scholar] [CrossRef]
- Gracia-Romero, A.; Kefauver, S.C.; Vergara-Díaz, O.; Zaman-Allah, M.A.; Prasanna, B.M.; Cairns, J.E.; Araus, J.L. Comparative Performance of Ground vs. Aerially Assessed RGB and Multispectral Indices for Early-Growth Evaluation of Maize Performance under Phosphorus Fertilization. Front. Plant Sci. 2017, 8, 2004. [Google Scholar] [CrossRef]
- Herrmann, I.; Bdolach, E.; Montekyo, Y.; Rachmilevitch, S.; Townsend, P.A.; Karnieli, A. Assessment of maize yield and phenology by drone-mounted superspectral camera. Precis. Agric. 2020, 21, 51–76. [Google Scholar] [CrossRef]
- White, J.; Andrade-Sanchez, P.; Gore, M. Field-based phenomics for plant genetics research. Field Crop. Res. 2012, 133, 101–113. [Google Scholar] [CrossRef]
- Kyratzis, A.C.; Skarlatos, D.P.; Menexes, G.C.; Vamvakousis, V.F.; Katsiotis, A. Assessment of vegetation indices derived by UAV imagery for durum wheat phenotyping under a water limited and heat stressed Mediterranean environment. Front. Plant Sci. 2017, 8, 1114. [Google Scholar] [CrossRef]
- Araus, J.L.; Kefauver, S.C.; Zaman-Allah, M.; Olsen, M.S.; Cairns, J.E. Translating High-Throughput Phenotyping into Genetic Gain. Trends Plant Sci. 2018, 23, 451–466. [Google Scholar] [CrossRef]
- Gracia-Romero, A.; Kefauver, S.C.; Fernandez-Gallego, J.A.; Vergara-Díaz, O.; Nieto-Taladriz, M.T.; Araus, J.L. UAV and Ground Image-Based Phenotyping: A Proof of Concept with Durum Wheat. Remote Sens. 2019, 11, 1244. [Google Scholar] [CrossRef]
- Aparicio, N.; Villegas, D.; Casadesus, J.; Araus, J.L.; Royo, C. Spectral vegetation indices as nondestructive tools for determining durum wheat yield. Agron. J. 2000, 92, 83–91. [Google Scholar] [CrossRef]
- Aparicio, N.; Villegas, D.; Araus, J.L.; Casadesús, J.; Royo, C. Relationship between growth traits and spectral vegetation indices in durum wheat. Crop Sci. 2002, 42, 1547–1555. [Google Scholar] [CrossRef]
- Royo, C.; Aparicio, N.; Villegas, D.; Casadesus, J.; Monneveux, P.; Araus, J.L. Usefulness of spectral reflectance indices as durum wheat yield predictors under contrasting Mediterranean conditions. Int. J. Remote Sens. 2003, 24, 4403–4419. [Google Scholar] [CrossRef]
- Zhao, Y.; Potgieter, A.B.; Zhang, M.; Wu, B.; Hammer, G.L. Predicting Wheat Yield at the Field Scale by Combining High-Resolution Sentinel-2 Satellite Imagery and Crop Modelling. Remote Sens. 2020, 12, 1024. [Google Scholar] [CrossRef]
- Zhou, X.; Kono, Y.; Win, A.; Matsui, T.; Tanaka, T.S.T. Predicting within-field variability in grain yield and protein content of winter wheat using UAV-based multispectral imagery and machine learning approaches. Plant Prod. Sci. 2020, 1–15. [Google Scholar] [CrossRef]
- Berger, K.; Rivera Caicedo, J.P.; Martino, L.; Wocher, M.; Hank, T.; Verrelst, J. A survey of active learning for quantifying vegetation traits from terrestrial earth observation data. Remote Sens. 2021, 13, 287. [Google Scholar] [CrossRef]
- Bellvert, J.; Nieto, H.; Pelechá, A.; Jofre-Čekalović, C.; Zazurca, L.; Miarnau, X. Remote Sensing Energy Balance Model for the Assessment of Crop Evapotranspiration and Water Status in an Almond Rootstock Collection. Front. Plant Sci. 2021, 12, 288. [Google Scholar] [CrossRef]
- Mahajan, G.R.; Das, B.; Murgaokar, D.; Herrmann, I.; Berger, K.; Sahoo, R.N.; Patel, K.; Desai, A.; Morajkar, S.; Kulkarni, R.M. Monitoring the Foliar Nutrients Status of Mango Using Spectroscopy-Based Spectral Indices and PLSR-Combined Machine Learning Models. Remote Sens. 2021, 13, 641. [Google Scholar] [CrossRef]
- Elsayed, S.; Elhoweity, M.; Ibrahim, H.H.; Dewir, Y.H.; Migdadi, H.M.; Schmidhalter, U. Thermal imaging and passive reflectance sensing to estimate the water status and grain yield of wheat under different irrigation regimes. Agric. Water Manag. 2017, 189, 98–110. [Google Scholar] [CrossRef]
- Deery, D.; Jimenez-Berni, J.; Jones, H.; Sirault, X.; Furbank, R. Proximal Remote Sensing Buggies and Potential Applications for Field-Based Phenotyping. Agronomy 2014, 4, 349–379. [Google Scholar] [CrossRef]
- Casadesús, J.; Kaya, Y.; Bort, J.; Nachit, M.M.; Araus, J.L.; Amor, S.; Ferrazzano, G.; Maalouf, F.; Maccaferri, M.; Martos, V.; et al. Using vegetation indices derived from conventional digital cameras as selection criteria for wheat breeding in water-limited environments. Ann. Appl. Biol. 2007, 150, 227–236. [Google Scholar] [CrossRef]
- Casadesús, J.; Villegas, D. Conventional digital cameras as a tool for assessing leaf area index and biomass for cereal breeding. J. Integr. Plant Biol. 2014, 56, 7–14. [Google Scholar] [CrossRef]
- Kefauver, S.C.; Vicente, R.; Vergara-Díaz, O.; Fernandez-Gallego, J.A.; Kerfal, S.; Lopez, A.; Melichar, J.P.E.; Serret Molins, M.D.; Araus, J.L. Comparative UAV and field phenotyping to assess yield and nitrogen use efficiency in hybrid and conventional barley. Front. Plant Sci. 2017, 8, 1733. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Candon, D.; Bellvert Rios, J.; Royo, C. Performance of the two-sourceenergy balance (TSEB) model as a tool for monitoring the response of durum wheat to drought by high-throughput field phenotyping. Front. Plant Sci. 2021. (under revision). [Google Scholar]
- Rufo, R.; Alvaro, F.; Royo, C.; Soriano, J.M. From landraces to improved cultivars: Assessment of genetic diversity and population structure of Mediterranean wheat using SNP markers. PLoS ONE 2019, 14, e0219867. [Google Scholar] [CrossRef] [PubMed]
- Zadoks, J.C.; Chang, T.T.; Konzak, C.F. A decimal code for the growth stages of cereals. Weed Res. 1974, 14, 415–421. [Google Scholar] [CrossRef]
- Norman, J.M.; Jarvis, P.G. Photosynthesis in Sitka Spruce (Picea sitchensis (Bong.) Carr.). III. Measurements of Canopy Structure and Interception of Radiation. J. Appl. Ecol. 1974, 11, 375. [Google Scholar] [CrossRef]
- Trussell, H.J.; Saber, E.; Vrhel, M.; Joel, H. Color Image Processing: Basics and Special Issue Overview. IEEE Signal Process. Mag. 2005, 22, 14–22. [Google Scholar] [CrossRef]
- Gonzalez-Dugo, V.; Hernandez, P.; Solis, I.; Zarco-Tejada, P.J. Using high-resolution hyperspectral and thermal airborne imagery to assess physiological condition in the context of wheat phenotyping. Remote Sens. 2015, 7, 13586–13605. [Google Scholar] [CrossRef]
- Roujean, J.L.; Breon, F.M. Estimating PAR absorbed by vegetation from bidirectional reflectance measurements. Remote Sens. Environ. 1995, 51, 375–384. [Google Scholar] [CrossRef]
- Qi, J.; Chehbouni, A.; Huete, A.R.; Kerr, Y.H.; Sorooshian, S. A modified soil adjusted vegetation index. Remote Sens. Environ. 1994, 48, 119–126. [Google Scholar] [CrossRef]
- Haboudane, D.; Miller, J.R.; Pattey, E.; Zarco-tejada, P.J.; Strachan, I.B. Hyperspectral vegetation indices and novel algorithms for predicting green LAI of crop canopies: Modeling and validation in the context of precision agriculture. Remote Sens. 2004, 90, 337–352. [Google Scholar] [CrossRef]
- Haboudane, D.; Miller, J.R.; Tremblay, N.; Zarco-Tejada, P.J.; Dextraze, L. Integrated narrow-band vegetation indices for prediction of crop chlorophyll content for application to precision agriculture. Remote Sens. Environ. 2002, 81, 416–426. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Kaufman, Y.J.; Merzlyak, M.N. Use of a green channel in remote sensing of global vegetation from EOS-MODIS. Remote Sens. Environ. 1996, 58, 289–298. [Google Scholar] [CrossRef]
- Lobos, G.A.; Matus, I.; Rodriguez, A.; Romero-Bravo, S.; Araus, J.L.; del Pozo, A. Wheat genotypic variability in grain yield and carbon isotope discrimination under Mediterranean conditions assessed by spectral reflectance. J. Integr. Plant Biol. 2014, 56, 470–479. [Google Scholar] [CrossRef]
- Costa, J.M.; Grant, O.M.; Chaves, M.M. Thermography to explore plant-environment interactions. J. Exp. Bot. 2013, 64, 3937–3949. [Google Scholar] [CrossRef]
- Royo, C.; Nazco, R.; Villegas, D. The climate of the zone of origin of Mediterranean durum wheat (Triticum durum Desf.) landraces affects their agronomic performance. Genet. Resour. Crop Evol. 2014, 61, 1345–1358. [Google Scholar] [CrossRef]
- García Del Moral, L.F.; Rharrabti, Y.; Elhani, S.; Martos, V.; Royo, C. Yield formation in Mediterranean durum wheats under two contrasting water regimes based on path-coefficient analysis. Euphytica 2005, 146, 203–212. [Google Scholar] [CrossRef]
- Brocklehurst, P.A. Factors controlling grain weight in wheat. Nature 1977, 266, 348–349. [Google Scholar] [CrossRef]
- Roselló, M.; Villegas, D.; Álvaro, F.; Soriano, J.M.; Lopes, M.S.; Nazco, R.; Royo, C. Unravelling the relationship between adaptation pattern and yield formation strategies in Mediterranean durum wheat landraces. Eur. J. Agron. 2019, 107, 43–52. [Google Scholar] [CrossRef]
- Moragues, M.; Del Moral, L.F.G.; Moralejo, M.; Royo, C. Yield formation strategies of durum wheat landraces with distinct pattern of dispersal within the Mediterranean basin I: Yield components. Field Crop. Res. 2006, 95, 194–205. [Google Scholar] [CrossRef]
- Royo, C.; Dreisigacker, S.; Ammar, K.; Villegas, D. Agronomic performance of durum wheat landraces and modern cultivars and its association with genotypic variation in vernalization response (Vrn-1) and photoperiod sensitivity (Ppd-1) genes. Eur. J. Agron. 2020, 120, 126129. [Google Scholar] [CrossRef]
- Villegas, D.; Aparicio, N.; Blanco, R.; Royo, C. Biomass accumulation and main stem elongation of durum wheat grown under Mediterranean conditions. Ann. Bot. 2001, 88, 617–627. [Google Scholar] [CrossRef]
- Moragues, M.; García Del Moral, L.F.; Moralejo, M.; Royo, C. Yield formation strategies of durum wheat landraces with distinct pattern of dispersal within the Mediterranean basin: II. Biomass production and allocation. Field Crop. Res. 2006, 95, 182–193. [Google Scholar] [CrossRef]
- Royo, C.; Aparicio, N.; Blanco, R.; Villegas, D. Leaf and green area development of durum wheat genotypes grown under Mediterranean conditions. Eur. J. Agron. 2004, 20, 419–430. [Google Scholar] [CrossRef]
- Soriano, J.M.; Villegas, D.; Sorrells, M.E.; Royo, C. Durum wheat landraces from east and west regions of the mediterranean basin are genetically distinct for yield components and phenology. Front. Plant Sci. 2018, 9, 1–9. [Google Scholar] [CrossRef]
- Subira, J.; Álvaro, F.; García del Moral, L.F.; Royo, C. Breeding effects on the cultivar×environment interaction of durum wheat yield. Eur. J. Agron. 2015, 68, 78–88. [Google Scholar] [CrossRef]
- Subira, J.; Ammar, K.; Álvaro, F.; García del Moral, L.F.; Dreisigacker, S.; Royo, C. Changes in durum wheat root and aerial biomass caused by the introduction of the Rht-B1b dwarfing allele and their effects on yield formation. Plant Soil 2016, 403, 291–304. [Google Scholar] [CrossRef]
- Adamsen, F.J.; Pinter, P.J.; Barnes, E.M.; LaMorte, R.L.; Wall, G.W.; Leavitt, S.W.; Kimball, B.A. Measuring Wheat Senescence with a Digital Camera. Crop Sci. 1999, 39, 719–724. [Google Scholar] [CrossRef]
- Din, M.; Zheng, W.; Rashid, M.; Wang, S.; Shi, Z. Evaluating hyperspectral vegetation indices for leaf area index estimation of Oryza sativa L. at diverse phenological stages. Front. Plant Sci. 2017, 8, 820. [Google Scholar] [CrossRef]
- Condorelli, G.E.; Maccaferri, M.; Newcomb, M.; Andrade-Sanchez, P.; White, J.W.; French, A.N.; Sciara, G.; Ward, R.; Tuberosa, R. Comparative Aerial and Ground Based High Throughput Phenotyping for the Genetic Dissection of NDVI as a Proxy for Drought Adaptive Traits in Durum Wheat. Front. Plant Sci. 2018, 9, 893. [Google Scholar] [CrossRef]
- Yao, X.; Wang, N.; Liu, Y.; Cheng, T.; Tian, Y.; Chen, Q.; Zhu, Y. Estimation of wheat LAI at middle to high levels using unmanned aerial vehicle narrowband multispectral imagery. Remote Sens. 2017, 9, 1304. [Google Scholar] [CrossRef]
- Vergara-Díaz, O.; Zaman-Allah, M.A.; Masuka, B.; Hornero, A.; Zarco-Tejada, P.; Prasanna, B.M.; Cairns, J.E.; Araus, J.L. A Novel Remote Sensing Approach for Prediction of Maize Yield under Different Conditions of Nitrogen Fertilization. Front. Plant Sci. 2016, 7, 666. [Google Scholar] [CrossRef]
- Zarco-Tejada, P.J.; Ustin, S.L.; Whiting, M.L. Temporal and spatial relationships between within-field yield variability in cotton and high-spatial hyperspectral remote sensing imagery. Agron. J. 2005, 97, 641–653. [Google Scholar] [CrossRef]
- Xing, N.; Huang, W.; Xie, Q.; Shi, Y.; Ye, H.; Dong, Y.; Wu, M.; Sun, G.; Jiao, Q. A transformed triangular vegetation index for estimating winter wheat leaf area index. Remote Sens. 2020, 12, 16. [Google Scholar] [CrossRef]
- Hassan, M.; Yang, M.; Rasheed, A.; Jin, X.; Xia, X.; Xiao, Y.; He, Z. Time-Series Multispectral Indices from Unmanned Aerial Vehicle Imagery Reveal Senescence Rate in Bread Wheat. Remote Sens. 2018, 10, 809. [Google Scholar] [CrossRef]
- Gizaw, S.A.; Garland-Campbell, K.; Carter, A.H. Evaluation of agronomic traits and spectral reflectance in Pacific Northwest winter wheat under rain-fed and irrigated conditions. Field Crop. Res. 2016, 196, 168–179. [Google Scholar] [CrossRef]
- Gracia-Romero, A.; Vergara-Díaz, O.; Thierfelder, C.; Cairns, J.; Kefauver, S.; Araus, J. Phenotyping Conservation Agriculture Management Effects on Ground and Aerial Remote Sensing Assessments of Maize Hybrids Performance in Zimbabwe. Remote Sens. 2018, 10, 349. [Google Scholar] [CrossRef]
- Lukina, E.V.; Stone, M.L.; Raun, W.R. Estimating vegetation coverage in wheat using digital images. J. Plant Nutr. 1999, 22, 341–350. [Google Scholar] [CrossRef]
- Rezzouk, F.Z.; Gracia-Romero, A.; Kefauver, S.C.; Gutiérrez, N.A.; Aranjuelo, I.; Serret, M.D.; Araus, J.L. Remote sensing techniques and stable isotopes as phenotyping tools to assess wheat yield performance: Effects of growing temperature and vernalization. Plant Sci. 2020, 295, 110281. [Google Scholar] [CrossRef] [PubMed]
- Bort, J.; Casadesus, J.; Nachit, M.M.; Araus, J.L. Factors affecting the grain yield predicting attributes of spectral reflectance indices in durum wheat: Growing conditions, genotype variability and date of measurement. Int. J. Remote Sens. 2005, 26, 2337–2358. [Google Scholar] [CrossRef]
- Gutierrez, M.; Reynolds, M.P.; Raun, W.R.; Stone, M.L.; Klatt, A.R. Spectral Water Indices for Assessing Yield in Elite Bread Wheat Genotypes under Well-Irrigated, Water-Stressed, and High-Temperature Conditions. Crop Sci. 2010, 50, 197–214. [Google Scholar] [CrossRef]
- Lopes, M.S.; Saglam, D.; Ozdogan, M.; Reynolds, M. Traits associated with winter wheat grain yield in Central and West Asia. J. Integr. Plant Biol. 2014, 56, 673–683. [Google Scholar] [CrossRef] [PubMed]
- Rutkoski, J.; Poland, J.; Mondal, S.; Autrique, E.; Pérez, L.G.; Crossa, J.; Reynolds, M.; Singh, R. Canopy temperature and vegetation indices from high-throughput phenotyping improve accuracy of pedigree and genomic selection for grain yield in wheat. G3 Genes Genomes Genet. 2016, 6, 2799–2808. [Google Scholar] [CrossRef]
- Shibayama, M.; Wiegand, C.L.; Richardson, A.J. Diurnal patterns of bidirectional vegetation indices for wheat canopies. Int. J. Remote Sens. 1986, 7, 233–246. [Google Scholar] [CrossRef]
- Perry, M.W.; D’Antuono, M.F. Yield improvement and associated characteristics of some Australian spring wheat cultivars introduced between 1860 and 1982. Aust. J. Agric. Res. 1989, 40, 457–472. [Google Scholar]
- Donmez, E.; Sears, R.G.; Shroyer, J.P.; Paulsen, G.M. Genetic Gain in Yield Attributes of Winter Wheat in the Great Plains. Crop Sci. 2001, 41, 1412–1419. [Google Scholar] [CrossRef]
- Ferrio, J.P.; Bertran, E.; Nachit, M.; Royo, C.; Araus, J.L. Near infrared reflectance spectroscopy as a potential surrogate method for the analysis of δ13C in mature kernels of durum wheat. Aust. J. Agric. Res. 2001, 52, 809–816. [Google Scholar] [CrossRef]
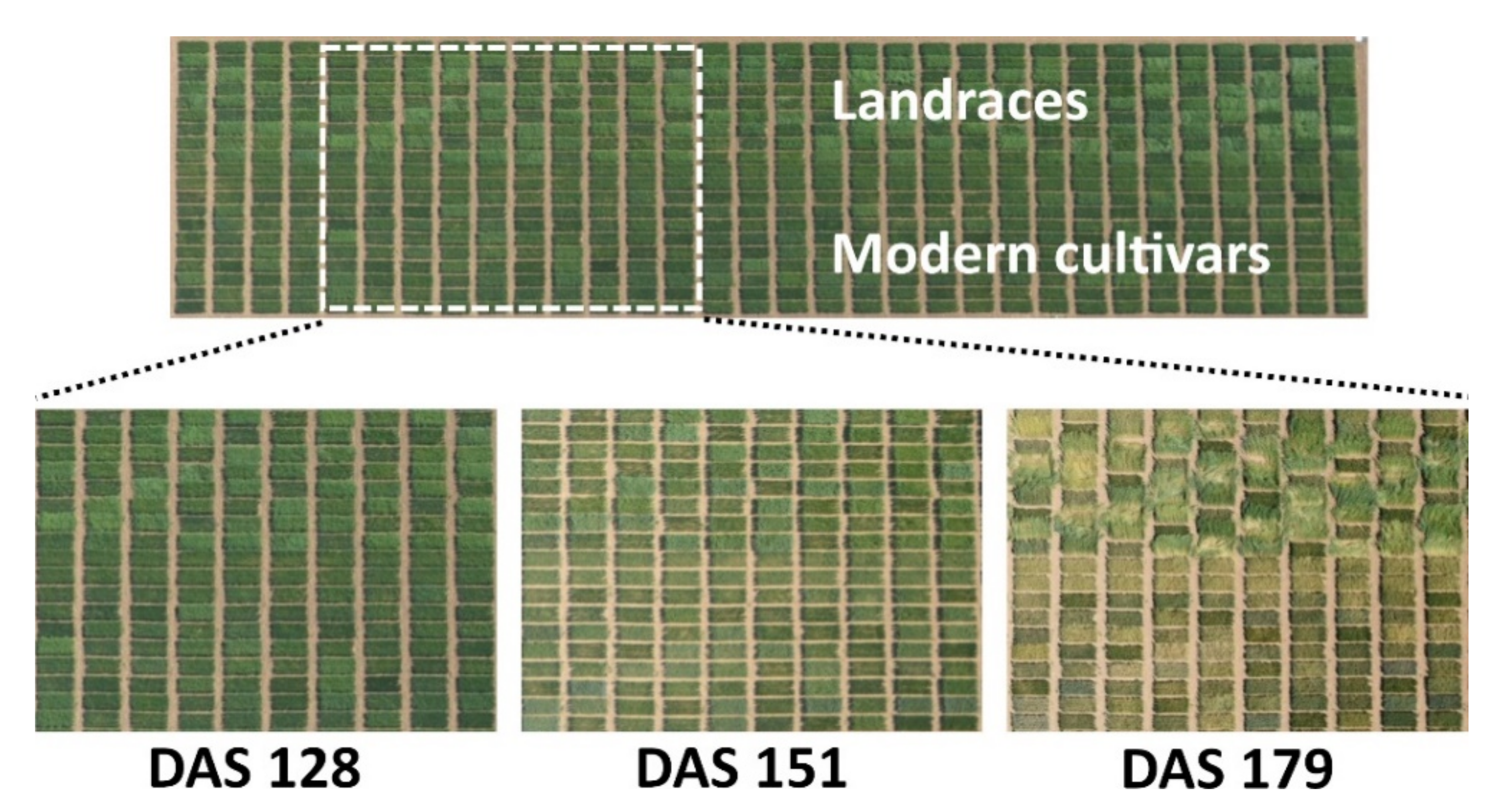

| Landraces 2016–2017 | ||||
|---|---|---|---|---|
| Date | Days after Sowing | Growth Stage | Number of Genotypes | (%) |
| 28 March 2017 | 128 | Stem elongation | 181 | 100 |
| 21 April 2017 | 151 | Booting | 95 | 53 |
| Heading | 53 | 29 | ||
| Anthesis | 29 | 16 | ||
| Milk development | 4 | 2 | ||
| 19 May 2017 | 179 | Milk development | 52 | 29 |
| Dough development | 129 | 71 | ||
| Modern 2016–2017 | ||||
| 28 March 2017 | 128 | Stem elongation | 169 | 92 |
| Booting | 15 | 8 | ||
| 21 April 2017 | 151 | Booting | 8 | 4 |
| Heading | 72 | 39 | ||
| Anthesis | 45 | 25 | ||
| Milk development | 59 | 32 | ||
| 19 May 2017 | 179 | Dough development | 184 | 100 |
| Landraces 2017–2018 | ||||
| 17 April 2018 | 153 | Stem elongation | 97 | 53 |
| Booting | 83 | 46 | ||
| Heading | 1 | 1 | ||
| 18 May 2018 | 184 | Milk development | 109 | 60 |
| Dough development | 72 | 40 | ||
| Modern 2017–2018 | ||||
| 17 April 2018 | 153 | Stem elongation | 26 | 14 |
| Booting | 126 | 69 | ||
| Heading | 32 | 17 | ||
| 18 May 2018 | 184 | Milk development | 66 | 36 |
| Dough development | 118 | 64 | ||
| Parameter | Definition | Reference |
|---|---|---|
| Intensity | Brightness of the image from black to white | [32] |
| Hue | Color tint | |
| Saturation | Amount of tint | |
| Lightness | Overall albedo from the HIS color space | |
| a* | Red‒green spectrum of chromaticity | |
| u* | ||
| b* | Yellow‒blue color spectrum | |
| v* | ||
| GA | Green area | [25] |
| GGA | Greener area |
| Vegetation Index | Equation | Reference |
|---|---|---|
| NDVI | (R790 – R660)/(R790 + R660) | [33] |
| RDVI | (R790 – R660)/ | [34] |
| MSAVI | ½ [2 R790 + 1 ‒ ] | [35] |
| MTVI2 | [36] | |
| TCARI/OSAVI | [37] | |
| GNDVI | (R790 – R550)/(R790 + R550) | [38] |
| Set | Agronomic Traits | Training | Test | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Min | Max | Mean | SD | N | Min | Max | Mean | SD | ||
| Landrace 2016–2017 | Yield (t/ha) | 84 | 3.0 | 8.3 | 5.0 | 0.9 | 85 | 3.2 | 8.5 | 5.2 | 0.9 |
| Biomass (t/ha) | 8.6 | 24.5 | 15.5 | 3.5 | 6.5 | 24.5 | 15.9 | 3.4 | |||
| NSm2 | 386 | 761 | 544 | 73 | 381 | 686 | 542 | 63 | |||
| NGm2 | 7438 | 20,154 | 12,764 | 2481 | 8180 | 23,003 | 13,082 | 2742 | |||
| TKW (g) | 27.0 | 51.6 | 38.9 | 5.2 | 23.3 | 52.7 | 39.8 | 5.1 | |||
| Landrace 2017–2018 | Yield (t/ha) | 84 | 3.6 | 7.2 | 5.5 | 0.7 | 85 | 4.1 | 9.0 | 5.8 | 0.9 |
| Biomass (t/ha) | 7.1 | 29.9 | 16.1 | 4.7 | 6.7 | 33.9 | 16.9 | 5.2 | |||
| NSm2 | 372 | 824 | 580 | 94 | 345 | 889 | 583 | 95 | |||
| NGm2 | 13,035 | 22,227 | 16,357 | 1645 | 12,917 | 24,836 | 17,130 | 2650 | |||
| TKW (g) | 19.9 | 49.3 | 33.9 | 6.3 | 17.9 | 49.0 | 34.6 | 7.5 | |||
| Modern 2016– 2017 | Yield (t/ha) | 92 | 7.1 | 11.8 | 9.5 | 0.9 | 92 | 6.5 | 11.7 | 9.4 | 1.1 |
| Biomass (t/ha) | 8.5 | 22.9 | 16.4 | 2.9 | 10.2 | 22.9 | 16.3 | 3.0 | |||
| NSm2 | 253 | 820 | 486 | 117 | 280 | 813 | 471 | 108 | |||
| NGm2 | 14,276 | 31,452 | 22,630 | 3628 | 12,520 | 33,852 | 22,170 | 4251 | |||
| TKW (g) | 31.3 | 58.8 | 42.8 | 5.2 | 32.6 | 58.1 | 43.3 | 5.1 | |||
| Modern 2017–2018 | Yield (t/ha) | 92 | 6.9 | 12.0 | 10.0 | 1.0 | 92 | 7.3 | 12.4 | 10.0 | 1.0 |
| Biomass (t(ha) | 10.4 | 39.0 | 19.2 | 4.6 | 6.2 | 29.4 | 19.6 | 3.9 | |||
| NSm2 | 200 | 973 | 583 | 149 | 220 | 920 | 585 | 142 | |||
| NGm2 | 17,002 | 34,191 | 26,848 | 3706 | 17,752 | 41,629 | 26,718 | 4082 | |||
| TKW (g) | 29.7 | 51.1 | 37.7 | 4.2 | 24.4 | 51.3 | 38.1 | 4.6 | |||
| Landraces | Modern | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Yield (t/ha) | Biomass (t/ha) | NSm2 | NGm2 | TKW (g) | Yield (t/ha) | Biomass (t/ha) | NSm2 | NGm2 | TKW (g) | ||
| SS Year (%) | 8.4 | 0.8 | 5.3 | 38.3 | 15.0 | 6.2 | 14.2 | 14.2 | 23.9 | 22.5 | |
| SS Genotype (%) | 63.7 | 52.6 | 55.9 | 40.1 | 64.8 | 64.7 | 42.9 | 50.6 | 62.5 | 64.2 | |
| SS Year × Genotype (%) | 27.9 | 46.6 | 38.8 | 21.5 | 20.1 | 29.1 | 42.9 | 35.2 | 13.6 | 13.3 | |
| F year | 50.3 *** | 2.8 | 23.1 *** | 296.7 *** | 125.6 *** | 38.8 *** | 61.0 *** | 74.0 *** | 322.1 *** | 309.2 *** | |
| F genotype | 2.3 *** | 1.1 | 1.4 ** | 1.8 *** | 3.2 *** | 2.2 *** | 1.0 | 1.4 ** | 4.6 *** | 4.8 *** | |
| CV (%) | 2016–2017 | 17.9 | 22.2 | 12.5 | 20.2 | 13.1 | 10.7 | 18.0 | 23.5 | 17.6 | 11.9 |
| 2017–2018 | 14.7 | 30.1 | 16.2 | 13.4 | 20.1 | 9.9 | 22.1 | 24.9 | 14.5 | 11.6 | |
| Mean | 2016–2017 | 5.1 | 15.7 | 543 | 12,923 | 39.4 | 9.5 | 16.4 | 479 | 22,400 | 43.0 |
| 2017–2018 | 5.6 | 16.5 | 582 | 16,746 | 34.3 | 10.0 | 19.4 | 584 | 26,783 | 37.9 | |
| Minimum | 2016–2017 | 3.0 | 6.5 | 381 | 7438 | 23.3 | 6.5 | 8.5 | 253 | 12,520 | 31.3 |
| 2017–2018 | 3.6 | 6.7 | 345 | 12,917 | 17.9 | 6.9 | 6.2 | 200 | 17,002 | 24.4 | |
| Maximum | 2016–2017 | 8.5 | 24.5 | 761 | 23,003 | 52.7 | 11.8 | 22.9 | 820 | 33,852 | 58.8 |
| 2017–2018 | 9.0 | 33.9 | 889 | 24,835 | 49.3 | 12.4 | 39.0 | 973 | 41,629 | 51.3 | |
| Method | VI | Equation | R2 | RMSE | Equation | R2 | RMSE | Equation | R2 | RMSE |
|---|---|---|---|---|---|---|---|---|---|---|
| Landraces + Modern (N = 640) | Landraces (N = 320) | Modern (N = 320) | ||||||||
| UAV Multispectral | NDVI | y = 11.63x − 5.55 | 0.38 | 1.48 | y = 10.74x − 4.49 | 0.16 | 1.45 | y = 11.06x − 5.22 | 0.43 | 1.47 |
| GNDVI | y = 8.89x − 2.54 | 0.18 | 1.70 | ns | y = 9.42x − 3.35 | 0.26 | 1.67 | |||
| MTVI2 | y = 7.45x − 1.01 | 0.61 | 1.17 | y = 7.11x − 0.72 | 0.39 | 1.24 | y = 7.58x − 1.10 | 0.66 | 1.12 | |
| Ground-based RGB | GA | y = 7.18x − 1.36 | 0.41 | 1.43 | y = 8.04x − 2.00 | 0.20 | 1.41 | y = 6.71x − 1.09 | 0.45 | 1.43 |
| GGA | y = 4.52x − 1.86 | 0.39 | 1.45 | y = 4.47x + 2.07 | 0.29 | 1.33 | y = 4.27x + 1.89 | 0.38 | 1.52 | |
| Hue | y = 0.09x − 2.91 | 0.45 | 1.40 | y = 0.10x − 3.59 | 0.33 | 1.29 | y = 0.08x − 2.44 | 0.45 | 1.44 | |
| a* | y = 0.18x − 2.04 | 0.22 | 1.66 | ns | y = −0.18x + 1.96 | 0.21 | 1.73 | |||
| u* | y = 0.19x − 3.05 | 0.3 | 1.57 | y = -0.16x + 3.54 | 0.15 | 1.46 | y = −0.18x + 2.99 | 0.3 | 1.63 | |
| UAV Multispectral | Ground-Based RGB | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Set | Traits | Training | Test | Training | Test | ||||||||
| N | Equation | R2 | N | R2 | RMSE | N | Equation | R2 | N | R2 | RMSE | ||
| Landraces 2016–2017 | Yield | 84 | −26.69 + 31.89GNDVI_1 + 5.98NDVI_3 | 0.18 ** | 85 | 0.18 ** | 0.45 | 84 | 40.45 + 2.26GA_3 + 12.77S_3 − 0.91L_1 − 0.15b*_2 | 0.45 ** | 85 | 0.28 ** | 0.66 |
| Biomass | −130.27 + 29.05MSAVI_2 + 135.54GNDVI_2 | 0.18 ** | ns | 1.41 | −17.65 + 40.58GGA_1 | 0.11 ** | ns | 1.04 | |||||
| NSm2 | −2167.28 + 2958.35GNDVI_2 | 0.15 * | ns | 27.22 | −731.73 + 8062.69I_3 − 58.49L_3 − 64.60a*_2 | 0.24 ** | 0.10 ** | 37.05 | |||||
| NGm2 | −73,937 + 9059.90NDVI_3 + 92,920GNDVI_1 | 0.27 ** | 0.08 ** | 1204 | 34,836 − 544.90L_2 | 0.12 ** | ns | 867 | |||||
| TKW | 172.87 − 173.71GNDVI_2 + 40.10GNDVI_3 | 0.15** | ns | 1.86 | −115.24 + 614.25I_2 − 106.53I_3 | 0.17** | ns | 2.06 | |||||
| Landraces 2017–2018 | Yield | 84 | 0.006 + 7.01GNDVI_3 | 0.18 ** | 85 | 0.36 ** | 0.28 | 84 | −4.53 + 0.08Hue_2 − 0.18a*_3 | 0.33 ** | 85 | 0.25 ** | 0.34 |
| Biomass | −26.24 + 46.70MSAVI_2 | 0.10 ** | ns | 1.34 | ns | ns | ns | ns | |||||
| NSm2 | ns | ns | ns | ns | ns | ns | ns | ns | |||||
| NGm2 | −2,167,724 + 2,631,346GNDVI_2 + 12,018GNDVI_3 | 0.24 ** | 0.19 ** | 768 | 19,077 − 25,561I_2 − 453.53a*_3 | 0.10 ** | 0.16 ** | 590 | |||||
| TKW | −11.97 + 57.72RDVI_2 | 0.15 ** | ns | 2.22 | 52.23 − 209.47S_2 − 1.72a*_2 | 0.29 ** | 0.11 ** | 2.92 | |||||
| Modern 2016–2017 | Yield | 92 | −9.23 + 8.09NDVI_3 + 19.68MTVI_2 | 0.28 ** | 92 | 0.43 ** | 0.39 | 92 | 4.93 − 0.15a*_3 − 0.27u*_1 | 0.34 ** | 92 | 0.37 ** | 0.49 |
| Biomass | −63.23 + 21.86MSAVI_3 + 102.20MTVI_2 | 0.28 ** | 0.11 ** | 1.48 | 0.28 + 0.20Hue_2 | 0.24 ** | 0.22 ** | 1.14 | |||||
| NSm2 | −13,857 + 11,036TCARI/OSAVI_2 + 15,315GNDVI_2 | 0.22 ** | 0.16 ** | 56.49 | 916.14 − 33.34L_1 − 44.65a*_1 | 0.21 ** | 0.18 ** | 55.83 | |||||
| NGm2 | −221,638 + 272,852GNDVI_2 | 0.33 ** | 0.35 ** | 1863 | 34,087 + 5840.71GGA_3 − 99,931I_1 − 1085.73a*_2 | 0.45 ** | 0.45 ** | 1780 | |||||
| TKW | 292.96 − 279.44GNDVI_2 | 0.17 ** | 0.11 ** | 2.23 | −11.24 + 26.68GA_2 + 163.80I_1 + 2.27a*_2 + 0.59v*_3 | 0.36 ** | 0.11 ** | 5.17 | |||||
| Modern 2017–2018 | Yield | 92 | −13 + 25.25NDVI_3 | 0.29 ** | 92 | 0.24 ** | 0.38 | 92 | −1.04 − 16.13GA_2 + 9.05GA_3 + 0.19Hue_2 | 0.45 ** | 92 | 0.22 ** | 0.54 |
| biomass | −143.78 + 177.75MSAVI_2 | 0.07 ** | ns | 1.28 | −21.58 − 55.66I_3 + 0.59Hue_2 | 0.12 ** | ns | 1.84 | |||||
| NSm2 | 10,710 − 14,953MTVI2_3 + 2604GNDVI_2 | 0.22 ** | 0.08 ** | 72.94 | −594.57 − 84.23u*_2 | 0.06 * | 0.06 * | 37.29 | |||||
| NGm2 | −52,520 + 34,921MSAVI_3 + 59,546GNDVI_2 | 0.49 ** | 0.38 ** | 1835 | 34,497 − 447.30L_3 − 2198.99u*_3 | 0.32 ** | 0.21 ** | 1825 | |||||
| TKW | 82.24 − 52.04GNDVI_2 | 0.15 ** | 0.10 ** | 1.55 | 57.85 − 0.87b*_2 | 0.14 ** | 0.04 * | 1.34 | |||||
| UAV Multispectral | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Set | Traits | Training | Test 2016–2017 | Test 2017–2018 | Test 2016–2017+2017–2018 | ||||||||
| N | Equation | R2 | N | R2 | RMSE | N | R2 | RMSE | N | R2 | RMSE | ||
| Landraces 2016–2017+2017–2018 | Yield | 168 | 0.30 + 11.26NDVI_3 − 5.11MTVI2_3 | 0.25 ** | 85 | 0.17 ** | 0.27 | 85 | 0.27 ** | 0.26 | 170 | 0.28 ** | 0.32 |
| Biomass | −50.03 + 35.23MTVI2_2 + 38.30GNDVI_2 | 0.11 ** | ns | - | ns | - | ns | - | |||||
| NSm2 | ns | ns | ns | - | ns | - | ns | - | |||||
| NGm2 | −713.21 + 21637GNDVI_3 | 0.44 ** | ns | 545 | 0.17 ** | 980.73 | 0.42 ** | 1376 | |||||
| TKW | −80.01 + 29.67RDVI_2 + 109.65GNDVI_2 | 0.16 ** | ns | 0.99 | ns | - | 0.14 ** | 2.20 | |||||
| Modern 2016–2017+2017–2018 | Yield | 184 | −5.19 + 9.47GNDVI_2 + 13.63NDVI_3 − 6.46MTVI2_3 | 0.30 ** | 92 | 0.51 ** | 0.34 | 92 | 0.33 ** | 0.38 | 184 | 0.46 ** | 0.38 |
| Biomass | −63.23 + 102.20MTVI2_2 + 21.86MSAVI_3 | 0.28 ** | 0.11 ** | 1.48 | 0.01 | 2.38 | 0.20 ** | 17.73 | |||||
| NSm2 | −1906.39 + 1047.64NDVI_2 + 1265.34GNDVI_2 + 477.01GNDVI_3 | 0.27 ** | 0.24 ** | 46.45 | 0.10 ** | 55.94 | 0.29 ** | 60.88 | |||||
| NGm2 | −69,278 + 20,340NDVI_2 + 22,033NDVI_3 + 66,300GNDVI_2 | 0.54 ** | 0.49 ** | 1419 | 0.32 ** | 2058.47 | 0.53 ** | 2091 | |||||
| TKW | 92.05 − 37.35GNDVI_2 − 26.21GNDVI_3 | 0.31** | 0.19 ** | 1.13 | 0.10 ** | 1.67 | 0.33 ** | 2.42 | |||||
| Ground-based RGB | |||||||||||||
| Landraces 2016–2017+2017–2018 | Yield | 168 | 3.91 − 0.07L_2 − 0.10a*_2 − 0.14b*_2 + 0.05Hue_3 + 9.19S_3 | 0.36 ** | 85 | 0.21 ** | 0.50 | 85 | 0.30 ** | 0.28 | 170 | 0.28 ** | 0.42 |
| biomass | ns | ns | ns | - | ns | - | ns | - | |||||
| NSm2 | 412.15 − 13.54u*_2 − 84.37GGA_3 | 0.09 ** | ns | - | ns | - | 0.09 ** | 24.31 | |||||
| NGm2 | 25,069 + 6675.08GGA_2 − 34739I_2 − 292.79L_2 + 11,005GA_3 + 426.02u*_3 | 0.50 ** | ns | - | 0.14 ** | 804.53 | 0.39 ** | 1604 | |||||
| TKW | 32.37 − 27.23GGA_2 − 3.93b*_2 + 4.06v*_2 + 0.13Hue_3 | 0.28 ** | ns | 1.94 | 0.04 | 2.50 | 0.15 ** | 2.98 | |||||
| Modern 2016–2017+2017–2018 | Yield | 184 | 10.64 − 4.95GA_3 − 7.68I_3 + 0.07Hue_3 − 0.22u*_3 | 0.28 ** | 92 | 0.27 ** | 0.51 | 92 | 0.24 ** | 0.39 | 184 | 0.30 ** | 0.54 |
| Biomass | −0.28 + 0.20Hue_2 | 0.24 ** | 0.09 ** | 1.90 | ns | - | 0.20** | 2.09 | |||||
| NSm2 | 238.83 − 18.41a*_2 | 0.19 ** | 0.20 ** | 30.24 | 0.05* | 30.87 | 0.26 ** | 50.66 | |||||
| NGm2 | 21,688 − 37,911I_3 + 143.07Hue_3 − 373.30a*_2 | 0.45 ** | 0.45 ** | 1314 | 0.19 ** | 1257.45 | 0.45 ** | 2221 | |||||
| TKW | 24.78 + 39.88GA_2 + 0.63a*_2 + 1.04u*_2 | 0.36** | 0.28 ** | 1.45 | 0.11 ** | 1.31 | 0.36 ** | 2.37 | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rufo, R.; Soriano, J.M.; Villegas, D.; Royo, C.; Bellvert, J. Using Unmanned Aerial Vehicle and Ground-Based RGB Indices to Assess Agronomic Performance of Wheat Landraces and Cultivars in a Mediterranean-Type Environment. Remote Sens. 2021, 13, 1187. https://doi.org/10.3390/rs13061187
Rufo R, Soriano JM, Villegas D, Royo C, Bellvert J. Using Unmanned Aerial Vehicle and Ground-Based RGB Indices to Assess Agronomic Performance of Wheat Landraces and Cultivars in a Mediterranean-Type Environment. Remote Sensing. 2021; 13(6):1187. https://doi.org/10.3390/rs13061187
Chicago/Turabian StyleRufo, Rubén, Jose Miguel Soriano, Dolors Villegas, Conxita Royo, and Joaquim Bellvert. 2021. "Using Unmanned Aerial Vehicle and Ground-Based RGB Indices to Assess Agronomic Performance of Wheat Landraces and Cultivars in a Mediterranean-Type Environment" Remote Sensing 13, no. 6: 1187. https://doi.org/10.3390/rs13061187
APA StyleRufo, R., Soriano, J. M., Villegas, D., Royo, C., & Bellvert, J. (2021). Using Unmanned Aerial Vehicle and Ground-Based RGB Indices to Assess Agronomic Performance of Wheat Landraces and Cultivars in a Mediterranean-Type Environment. Remote Sensing, 13(6), 1187. https://doi.org/10.3390/rs13061187








