Red-Green-Blue and Multispectral Imaging as Potential Tools for Estimating Growth and Nutritional Performance of Cassava under Deficit Irrigation and Potassium Fertigation
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design and Plant Material
2.2. Measurements of Ground Data
2.3. Image Data Collection
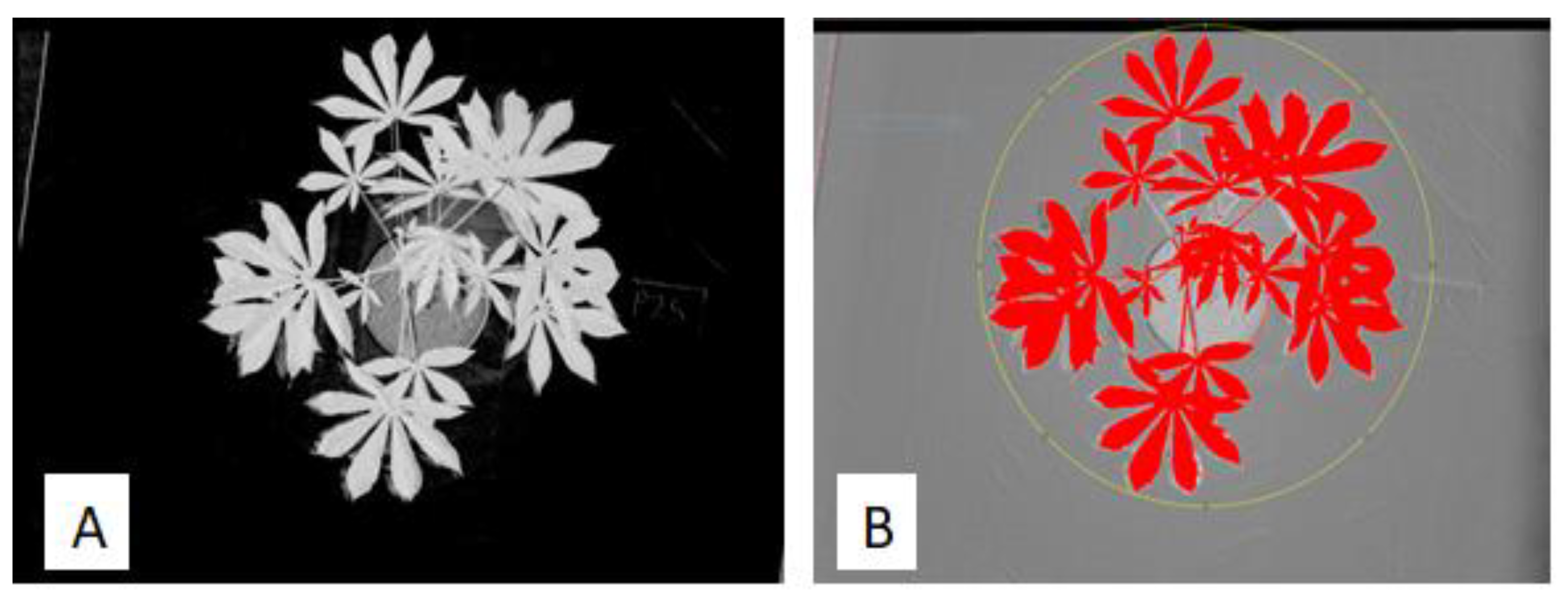
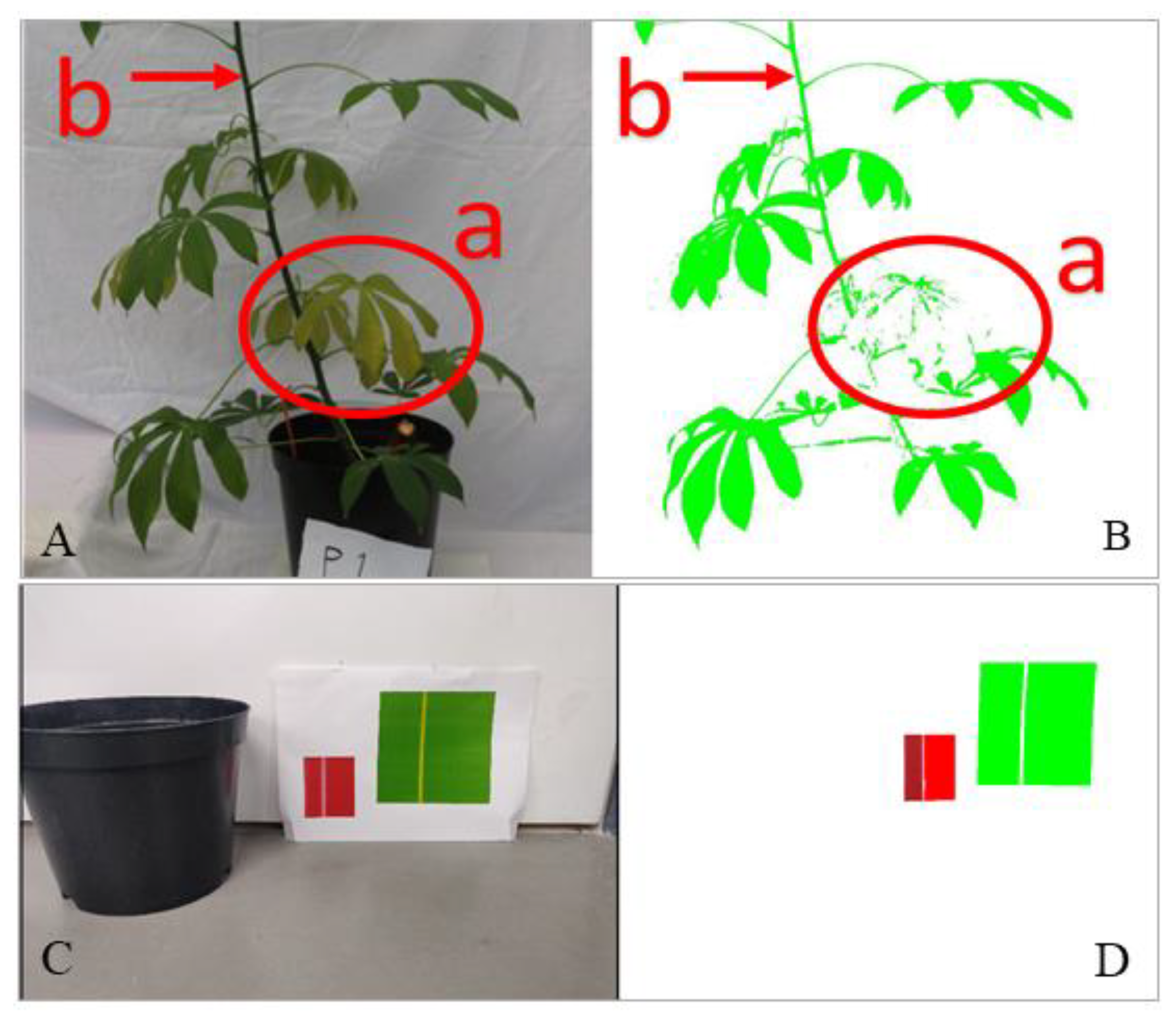
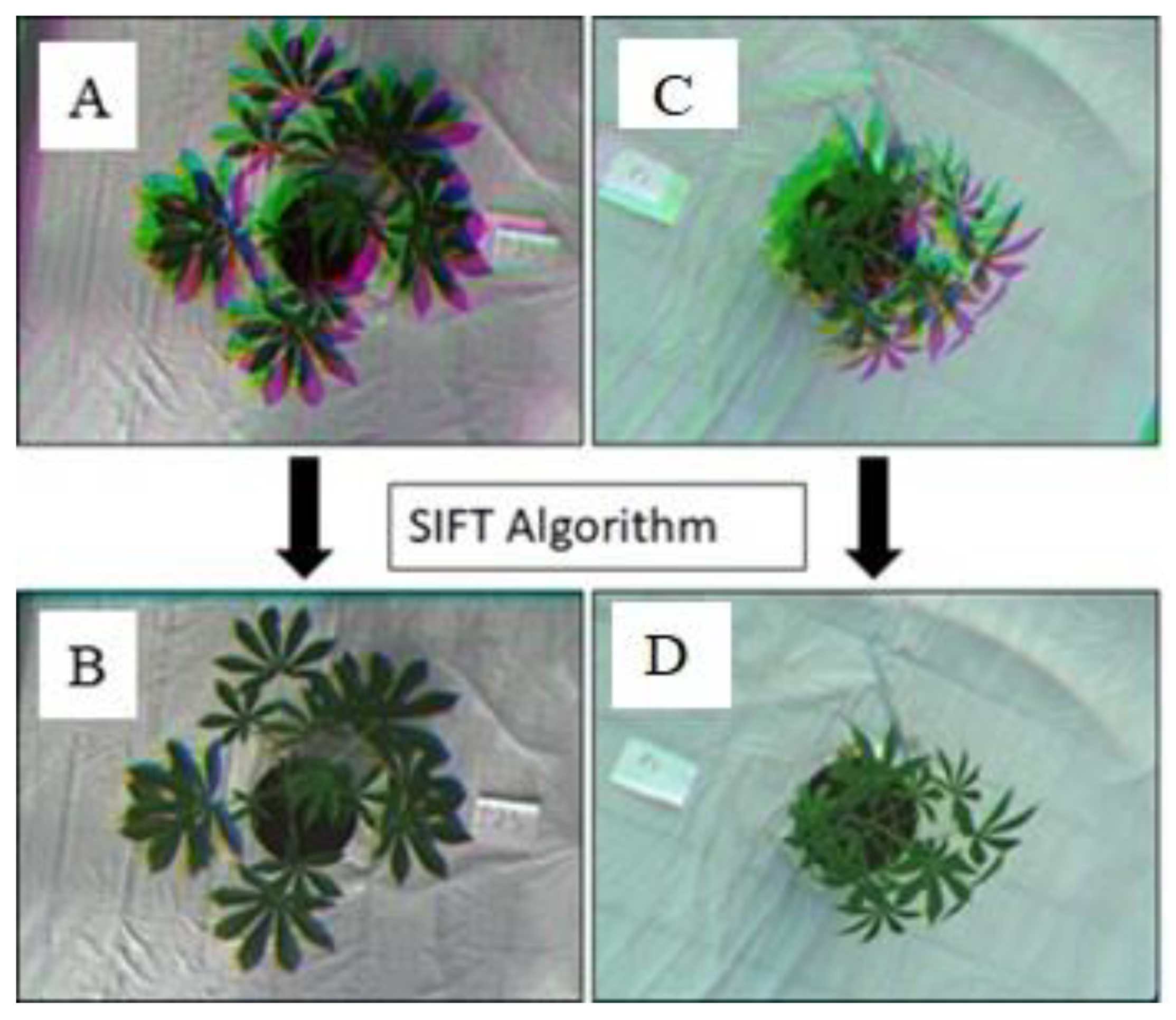
2.4. Red–Green–Blue Image Processing
2.5. Multispectral Image Processing
2.6. Data Analysis
3. Results
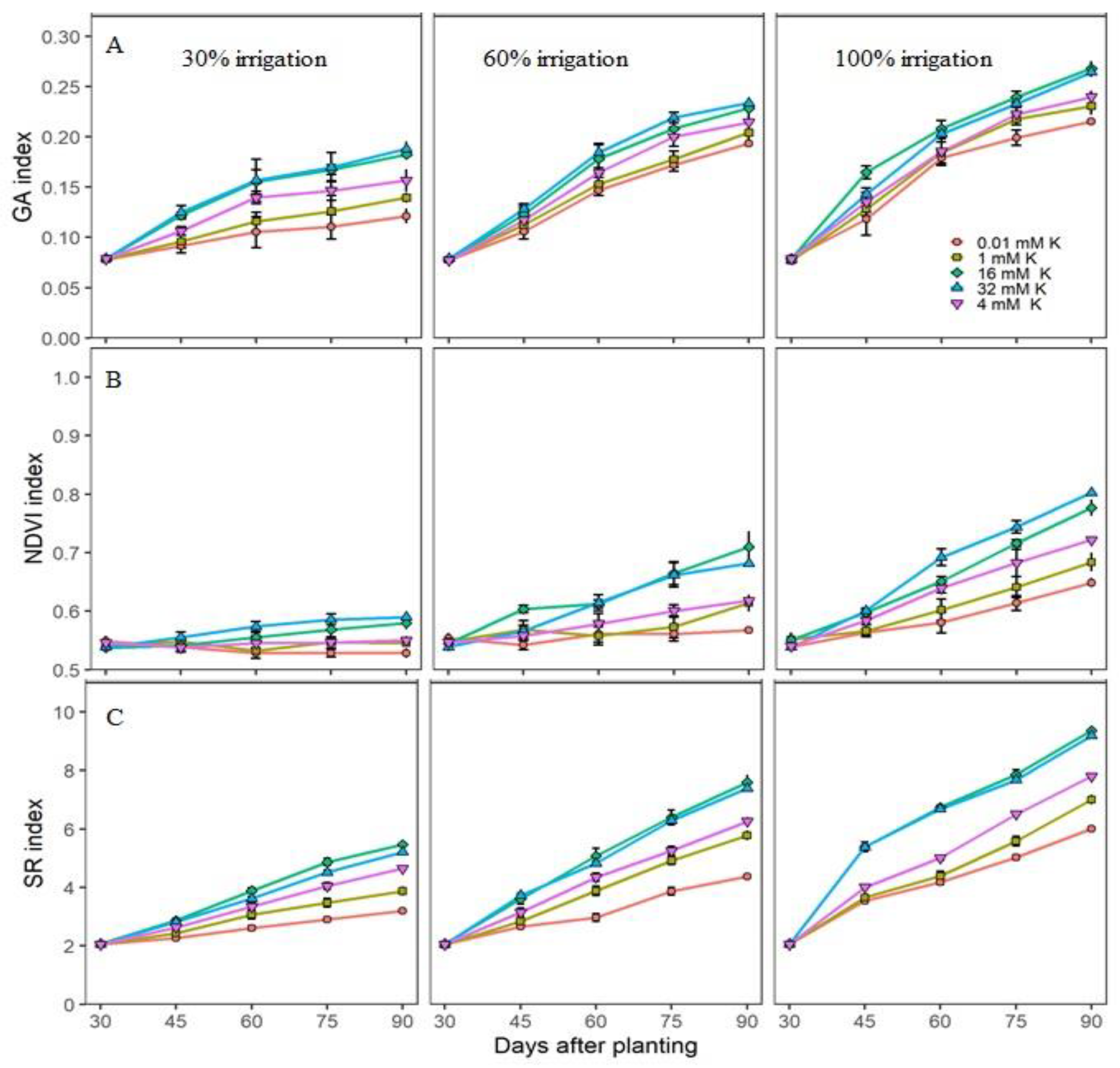
3.1. Treatment Effects on the Spectral Reflectance Indices
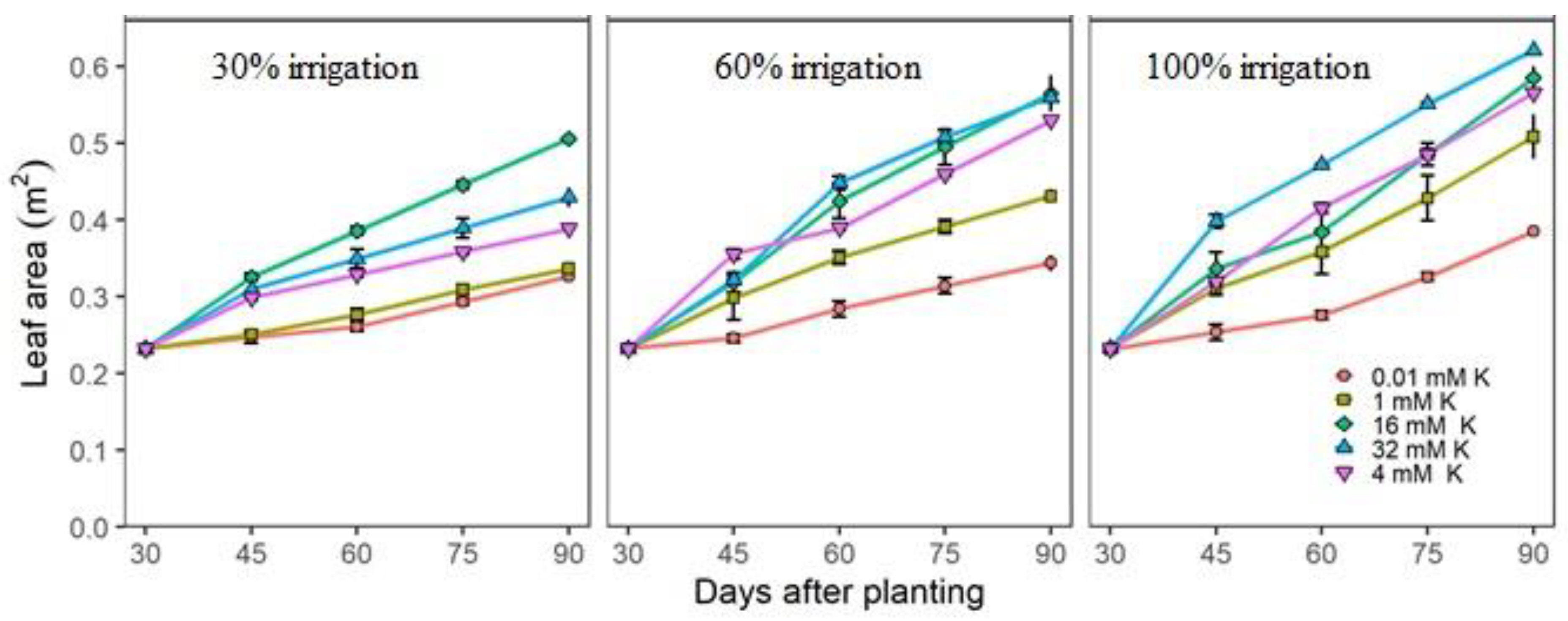
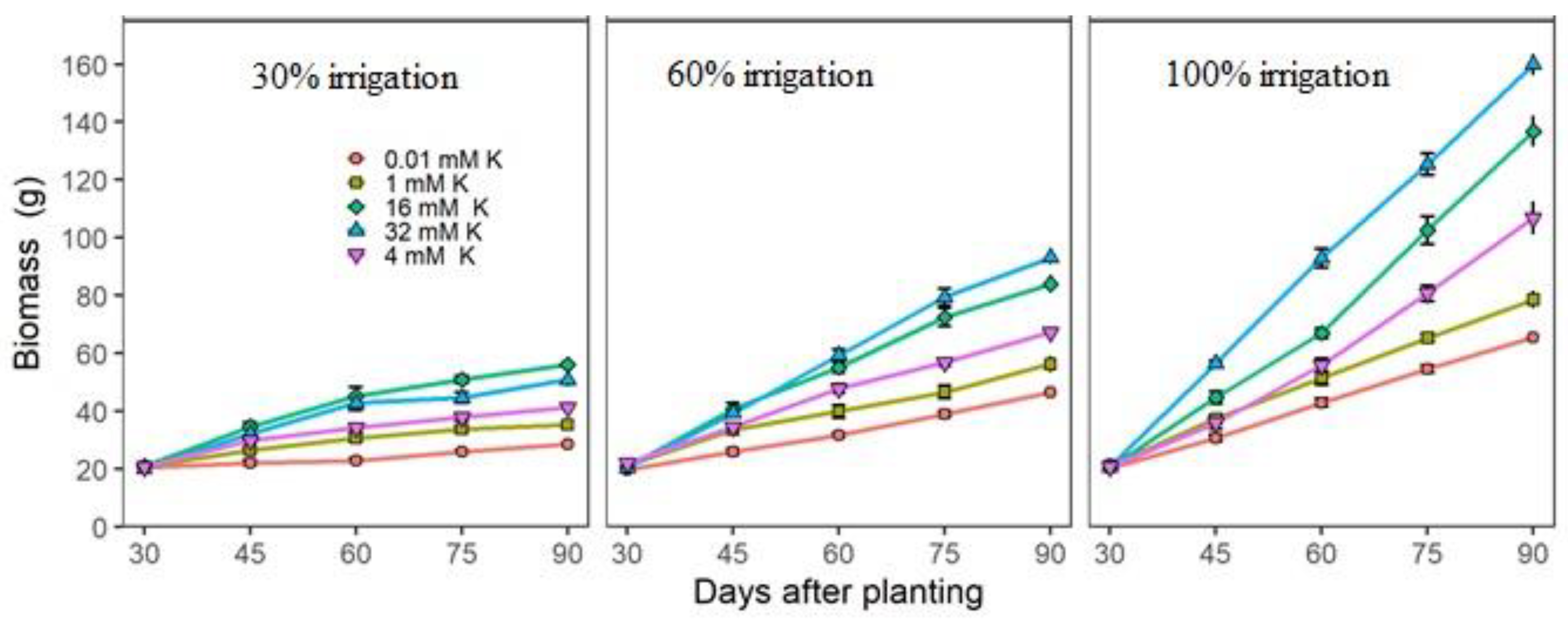
3.2. Actual Growth and Nutritional Measurements
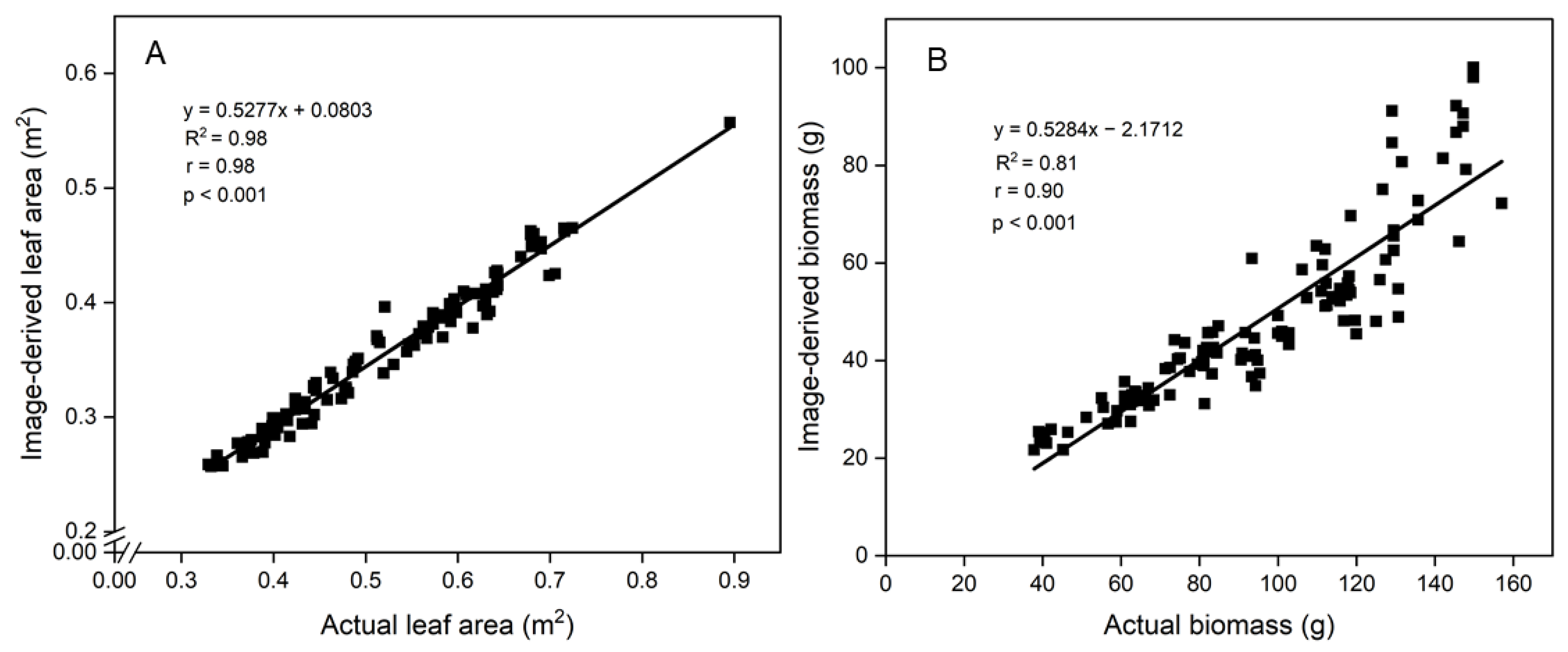
3.3. Correlations between Image-Derived and Actual Measurements
3.4. Estimation of Cassava Growth Traits
3.5. Estimation of Cassava Nutritional Traits
4. Discussion
4.1. Interaction between Growth Traits and Spectral Reflectance Indices
4.2. Interaction between Nutritional Traits and Spectral Reflectance Indices
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Burns, A.; Gleadow, R.; Cliff, J.; Zacarias, A.; Cavagnaro, T. Cassava the drought, war and famine crop in a changing world. Sustainability 2010, 2, 3572–3607. [Google Scholar] [CrossRef]
- Iglesias, C.; Mayer, J.; Chávez, A.L.; Calle, F. Genetic potential and stability of carotene content in cassava roots. Euphytica 1997, 94, 367–373. [Google Scholar] [CrossRef]
- Bakayoko, S.; Tschannen, A.; Nindjin, C.; Dao, D.; Girardin, O.; Assa, A. Impact of water stress on fresh tuber yield and dry matter content of cassava (Manihot esculenta Crantz) in Côte d’Ivoire. Afr. J. Agric. Res. 2009, 4, 21–27. [Google Scholar]
- Okogbenin, E.; Setter, T.L.; Ferguson, M.; Mutegi, R.; Ceballos, H.; Olasanmi, B.; Fregene, M. Phenotypic approaches to drought in cassava: Review. Front. Physiol. 2013, 4, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Cakmak, I. Potassium for better crop production and quality. Plant Soil. 2010, 335, 1–2. [Google Scholar] [CrossRef]
- Pardales, J.R.; Esquibel, C.B. Effect of drought during the establishment period on the root system development of cassava. Jpn. J. Crop Sci. 1996, 65, 93–97. [Google Scholar] [CrossRef]
- Cakmak, I. The role of potassium in alleviating determinal effects of abiotic stresses in plants. J. Plant Nutr. Soil Sci. 2005, 168, 521–530. [Google Scholar] [CrossRef]
- Oosterhuis, D.M.; Loka, D.A.; Kawakami, E.M.; Pettigrew, W.T. The physiology of potassium in crop production. Adv. Agron. 2014, 126, 203–233. [Google Scholar]
- Yol, E.; Toker, C.; Uzun, B. Traits for Phenotyping. In Phenomics in Crop Plants: Trends, Options and Limitations; Springer: Berlin/Heidelberg, Germany, 2015; pp. 11–26. [Google Scholar]
- Fiorani, F.; Schurr, U. Future scenarios for plant phenotyping. Annu. Rev. Plant Biol. 2013, 64, 267–291. [Google Scholar] [CrossRef]
- Araus, J.L.; Cairns, J.E. Field high-throughput phenotyping: The new crop breeding frontier. Trends Plant Sci. 2014, 19, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Walter, A.; Liebisch, F.; Hund, A. Plant phenotyping: From bean weighing to image analysis. Plant Methods 2015, 11, 14. [Google Scholar] [PubMed]
- Peñuelas, J.; Filella, I. Visible and near-infrared reflectance techniques for diagnosing plant physiological status. Trends Plant Sci. 1998, 3, 151–156. [Google Scholar] [CrossRef]
- Silva-Perez, V.; Molero, G.; Serbin, S.P.; Condon, A.G.; Reynolds, M.P.; Furbank, R.T.; Evans, J.R. Hyperspectral reflectance as a tool to measure biochemical and physiological traits in wheat. J. Exp. Bot. 2018, 69, 483–496. [Google Scholar] [PubMed]
- Lobos, G.A.; Escobar-Opazo, A.; Estrada, F.; Romero-Bravo, S.; Garriga, M.; Pozo, A.; Poblete-Echeverría, C.; Gonzalez-Talice, J.; González-Martinez, L.; Caligari, P. Spectral reflectance modeling by wavelength selection: Studying the scope for blueberry physiological breeding under contrasting water supply and heat conditions. Remote Sens. 2019, 11, 329. [Google Scholar] [CrossRef]
- Lopes, M.S.; Araus, J.L.; Van Heerden, P.D.R.; Foyer, C.H. Enhancing drought tolerance in C4 crops. J. Exp. Bot. 2011, 62, 3135–3153. [Google Scholar] [CrossRef]
- Knipling, E.B. Physical and physiological basis for the reflectance of visible and near-infrared radiation from vegetation. Remote Sens. Environ. 1970, 1, 155–159. [Google Scholar]
- Milton, E.J.; Schaepman, M.; Anderson, K.; Kneubhler, M.; Fox, N. Progress in field spectroscopy. Remote Sens. Environ. 2009, 113, S92–S109. [Google Scholar] [CrossRef]
- Reynolds, D.; Baret, F.; Welcker, C.; Bostrom, A.; Ball, J.; Cellini, F.; Lorence, A.; Chawade, A.; Khafif, M.; Noshita, K.; et al. What is cost-efficient phenotyping? Optimizing costs for different scenarios. Plant Sci. 2019, 282, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhang, Q.; Huang, D. A review of imaging techniques for plant phenotyping. Sensors 2014, 14, 20078–20111. [Google Scholar] [CrossRef]
- Ghanem, M.E.; Marrou, H.; Sinclair, T.R. Physiological phenotyping of plants for crop improvement. Trends Plant Sci. 2015, 20, 139–144. [Google Scholar] [CrossRef]
- Deery, D.; Jimenez-Berni, J.; Jones, H.; Sirault, X.; Furbank, R. Proximal remote sensing buggies and potential applications for field-based phenotyping. Agronomy 2014, 4, 349–379. [Google Scholar] [CrossRef]
- Hamza, M.; Anderson, W. Soil compaction in cropping systems: A review of the nature, causes and possible solutions. Soil Tillage Res. 2005, 82, 121–145. [Google Scholar] [CrossRef]
- Shafiekhani, A.; Kadam, S.; Fritschi, F.B.; DeSouza, G.N. Vinobot and Vinoculer: Two Robotic Platforms for High-Throughput Field Phenotyping. Sensors 2017, 17, 214. [Google Scholar] [CrossRef] [PubMed]
- Van der Merwe, D.; Burchfield, D.R.; Witt, T.D.; Price, K.P.; Sharda, A. Drones in agriculture. Adv. Agron. 2020, 162, 1–30. [Google Scholar]
- Jansen, M.; Pinto, F.; Nagel, K.A.; van Dusschoten, D.; Fiorani, F.; Rascher, U.; Schneider, H.U.; Walter, A.; Schurr, U. Non-invasive Phenotyping Methodologies Enable the Accurate Characterization of Growth and Performance of Shoots and Roots. In Genomics of Plant Genetic Resources; Tuberosa, R., Graner, A., Frison, E., Eds.; Springer: Dordrecht, The Netherlands, 2014; pp. 173–203. [Google Scholar]
- Casadesús, J.; Kaya, Y.; Bort, J.; Nachit, M.M.; Araus, J.L.; Amor, S.; Ferrazzano, G.; Maalouf, F.; Maccaferri, M.; Martos, V.; et al. Using vegetation indices derived from conventional digital cameras as selection criteria for wheat breeding in water-limited environments. Ann. Appl. Biol. 2007, 150, 227–236. [Google Scholar] [CrossRef]
- Cabrera-Bosquet, L.; Molero, G.; Stellacci, A.M.; Bort, J.; Nogués, S.; Araus, J.L. NDVI as a Potential Tool for Predicting Biomass, Plant Nitrogen Content and Growth in Wheat Genotypes Subjected to Different Water and Nitrogen Conditions. Cereal Res. Commun. 2011, 39, 147–159. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Merzlyak, M.N. Remote sensing of chlorophyll concentration in higher plant leaves. Adv. Space Res. 1998, 22, 689–692. [Google Scholar] [CrossRef]
- Bannari, A.; Morin, D.; Bonn, F.; Huete, A.R. A review of vegetation indices. Remote Sens. Rev. 1995, 13, 95–120. [Google Scholar] [CrossRef]
- Armoniené, R.; Odilbekov, F.; Vivekanand, V.; Chawade, A. Affordable Imaging Lab for Noninvasive Analysis of Biomass and Early Vigour in Cereal Crops. BioMed Res. Int. 2018, 2018, 1–9. [Google Scholar] [CrossRef]
- Casadesús, J.; Villegas, D. Conventional digital cameras as a tool for assessing leaf area index and biomass for cereal breeding. J. Integr. Plant Biol. 2014, 56, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Tackenberg, O. A new method for non-destructive measurement of biomass, growth rates, vertical biomass distribution and dry matter content based on digital image analysis. Ann. Bot. 2007, 99, 777–783. [Google Scholar] [CrossRef]
- Fernandez-Gallego, J.A.; Kefauver, S.C.; Vatter, T.; Gutiérrez, N.A.; Nieto-Taladriz, M.T.; Araus, J.L. Low-cost assessment of grain yield in durum wheat using RGB images. Eur. J. Agron. 2019, 105, 146–156. [Google Scholar] [CrossRef]
- Fu, Z.; Jiang, J.; Gao, Y.; Krienke, B.; Wang, M.; Zhong, K.; Cao, Q.; Tian, Y.; Zhu, Y.; Cao, W.; et al. Wheat Growth Monitoring and Yield Estimation based on Multi-Rotor Unmanned Aerial Vehicle. Remote Sens. 2020, 12, 508. [Google Scholar] [CrossRef]
- Pandey, P.; Ge, Y.; Stoerger, V.; Schnable, J.C. High throughput in vivo analysis of plant leaf chemical properties using hyperspectral imaging. Front. Plant Sci. 2017, 8, 1348. [Google Scholar] [CrossRef]
- Nguyen, H.T.; Lee, B.W. Assessment of rice leaf growth and nitrogen status by hyperspectral canopy reflectance and partial least square regression. Eur. J. Agron. 2006, 24, 349–356. [Google Scholar] [CrossRef]
- Pimstein, A.; Karnieli, A.; Bonfil, D.J. Wheat and Maize Monitoring Based on Ground Spectral Measurements and Multivariate Data Analysis. J. Appl. Remote Sens. 2007, 1, 013530. [Google Scholar]
- Leardi, R.; Seasholtz, M.B.; Pell, R.J. Variable selection for multivariate calibration using a genetic algorithm: Prediction of additive concentrations in polymer films from Fourier transform-infrared spectral data. Anal. Chim. Acta-Comp. 2002, 461, 189–200. [Google Scholar] [CrossRef]
- Abdi, H. Partial least squares regression and projection on latent structure regression (PLS regression). Wiley Interdiscip. Rev. Comput. Stat. 2010, 2, 97–106. [Google Scholar] [CrossRef]
- Wold, S.; Ruhe, H.; Wold, H.; Dunn, W.J. The collinearity problem in linear regression. The partial least squares (PLS) approach to generalized inverses. SIAM J. Sci. Stat. Comput. 1984, 5, 735–743. [Google Scholar] [CrossRef]
- Curran, P.J.; Dungan, J.L.; Peterson, D.L. Estimating the foliar biochemical concentration of leaves with reflectance spectrometry: Testing the Kokaly and Clark methodologies. Remote Sens. Environ. 2001, 76, 349–359. [Google Scholar] [CrossRef]
- Zhai, Y.; Cui, L.; Zhou, X.; Gao, Y.; Fei, T.; Gao, W. Estimation of nitrogen, phosphorus, and potassium contents in the leaves of different plants using laboratory-based visible and near-infrared reflectance spectroscopy: Comparison of partial least-square regression and support vector machine regression methods. Int. J. Remote Sens. 2013, 34, 2502–2518. [Google Scholar]
- Silva, S.P.P.; e Sousa, M.B.; de Oliveira, E.J. Prediction models and selection of agronomic and physiological traits for tolerance to water deficit in cassava. Euphytica 2019, 215, 73. [Google Scholar] [CrossRef]
- Vitor, A.B.; Diniz, R.P.; Morgante, C.V.; Antônio, R.P.; Oliveira, E.J. Early prediction models for cassava root yield in different water regimes. Field Crop. Res. 2019, 239, 149–158. [Google Scholar] [CrossRef]
- Selvaraj, M.G.; Valderrama1, M.; Guzman, D.; Valencia, M.; Ruiz, H.; Acharjee, A. Machine learning for high-throughput field phenotyping and image processing provides insight into the association of above and below-ground traits in cassava (Manihot esculenta Crantz). Plant Methods 2020, 16, 87. [Google Scholar] [CrossRef] [PubMed]
- Latif, S.; Müller, J. Potential of cassava leaves in human nutrition: A review. Trends Food Sci. Technol. 2015, 44, 147–158. [Google Scholar] [CrossRef]
- Wasonga, D.; Kleemola, J.; Alakukku, L.; Mäkelä, P. Growth response of cassava to deficit irrigation and potassium fertigation during the early growth phase. Agronomy 2020, 10, 321. [Google Scholar] [CrossRef]
- Hoagland, D.R.; Arnon, D.I. The water-culture method for growing plants without soil. Calif. Agric. Exp. Stat. 1950, 347, 32. [Google Scholar]
- Oliveira, R.G.A.; de Carvalho, M.J.L.; Nutti, R.M.; de Carvalho, L.V.J.; Fukuda, W.G. Assessment and degradation study of total carotenoid and ß-carotene in bitter yellow cassava (Manihot esculenta Crantz) varieties. Afr. J. Food Sci. 2010, 4, 148–155. [Google Scholar]
- Williams, S. (Ed.) Hydrocyanic acid in beans, alkaline titration method. In Official Method of Analysis of the Association of Official Analytical Chemists; AOAC Inc.: Arlington, VA, USA, 1990; p. 1213. [Google Scholar]
- Wasonga, D.O.; Kleemola, J.; Alakukku, L.; Mäkelä, P.S.A. Potassium fertigation with deficit irrigation improves the nutritive quality of cassava. Front. Sustain. Food Syst. 2020, 4, 575353. [Google Scholar] [CrossRef]
- Rueden, C.T.; Schindelin, J.; Hiner, M.C.; DeZonia, B.E.; Walter, A.E.; Arena, E.T.; Eliceiri, K.W. ImageJ2: ImageJ for the next generation of scientific image data. BMC Bioinform. 2017, 18, 529. [Google Scholar] [CrossRef]
- Kefauver, S.C.; Vicente, R.; Vergara-Díaz, O.; Fernandez-Gallego, J.A.; Kerfal, S.; Lopez, A.; Melichar, J.P.E.; Molins, M.D.S.; Araus, J.L. Comparative UAV and field phenotyping to assess yield and nitrogen use efficiency in hybrid and conventional barley. Front. Plant Sci. 2017, 8, 1733. [Google Scholar] [CrossRef]
- Easlon, H.M.; Bloom, A.J. Easy leaf area: Automated digital image analysis for rapid and accurate measurement of leaf area. Appl. Plant Sci. 2014, 2, 1400033. [Google Scholar] [CrossRef]
- Lindeberg, T. Scale invariant feature transform. Scholarpedia 2012, 7, 10491. [Google Scholar] [CrossRef]
- Boegh, E.; Soegaard, H.; Broge, N.; Hasager, C.; Jensen, N.; Schelde, K.; Thomsen, A. Airborne multispectral data for quantifying leaf area index, nitrogen concentration, and photosynthetic efficiency in agriculture. Remote Sens. Environ. 2002, 81, 179–193. [Google Scholar] [CrossRef]
- Freeman, K.W.; Girma, K.; Arnall, D.B.; Mullen, R.W.; Martin, K.L. By-plant prediction of corn forage biomass and nitrogen uptake at various growth stages using remote sensing and plant height. Agon. J. 2007, 99, 530–536. [Google Scholar] [CrossRef]
- Cawley, G.C.; Talbot, N.L.C. Efficient leave-one-out cross-validation of Kernel Fischer discriminant classifiers. Pattern Recognit. 2003, 36, 2585–2592. [Google Scholar] [CrossRef]
- Boshkovski, B.; Tzerakis, C.; Doupis, G.; Zapolska, A.; Kalaitzidis, C.; Koubouris, G. Relationships of spectral reflectance with plant tissue mineral elements of common bean (Phaseolus vulgaris L.) under drought and salinity stresses. Commun. Soil Sci. Plant Anal. 2020, 51, 675–686. [Google Scholar] [CrossRef]
- Merzlyak, M.N.; Gitelson, A.A.; Chivkunova, O.B.; Rakitin, V.Y. Non-destructive optical detection of pigment changes during leaf senescence and fruit ripening. Physiol. Plant. 1999, 106, 135–141. [Google Scholar] [CrossRef]
- Ahmad, I.; Maathuis, F.J.M. Cellular and tissue distribution of potassium; physiological relevance, mechanisms and regulation. J. Plant Physiol. 2014, 171, 708–714. [Google Scholar] [CrossRef]
- Chen, D.; Shi, R.; Pape, J.M.; Neumann, K.; Arend, D.; Graner, A.; Chen, M.; Klukas, C. Predicting plant biomass accumulation from image-derived parameters. GigaScience 2018, 7, 1–13. [Google Scholar] [CrossRef]
- Niu, Y.; Zhang, L.; Zhang, H.; Han, W.; Peng, X. Estimating above-ground biomass of maize using features derived from UAV-Based RGB Imagery. Remote Sens. 2019, 11, 1261. [Google Scholar] [CrossRef]
- Hosoi, F.; Omasa, K. Factors contributing to accuracy in the estimation of the woody canopy leaf area density profile using 3D portable lidar imaging. J. Exp. Bot. 2007, 58, 3463–3473. [Google Scholar] [CrossRef] [PubMed]
- Smith, W.K.; Schoettle, A.W.; Cui, M. Importance of the method of leaf area measurement to the interpretation of gas exchange of complex shoots. Tree Physiol. 1991, 8, 121–127. [Google Scholar] [CrossRef]
- Neuwirthová, E.; Lhotáková, Z.; Albrechtová, J. The Effect of Leaf Stacking on Leaf Reflectance and Vegetation Indices Measured by Contact Probe during the Season. Sensors 2017, 17, 1202. [Google Scholar] [CrossRef]
- Glenn, D.M.; Tabb, A. Evaluation of five methods to measure normalized difference vegetation index (NDVI) in apple and citrus. Int. J. Fruit Sci. 2019, 19, 191–210. [Google Scholar] [CrossRef]
- Blackburn, G.A. Hyperspectral remote sensing of plant pigments. J. Exp. Bot. 2007, 58, 855–867. [Google Scholar] [CrossRef]
- Christenson, B.S.; Schapaugh, W.T.; An, N.; Price, K.P.; Prasad, V.; Fritz, A.K. Predicting soybean relative maturity and seed yield using canopy reflectance. Crop Sci. 2016, 56, 625–643. [Google Scholar] [CrossRef]
- Mutanga, O.; Skidmore, A.K. Narrow band vegetation indices overcome the saturation problem in biomass estimation. Int. J. Remote Sens. 2004, 25, 3999–4014. [Google Scholar] [CrossRef]
- Ruban, A.V.; Horton, P.; Young, A.J. Aggregation of higher plant xanthophylls: Differences in absorption spectra and in the dependency on solvent polarity. J. Photochem. Photobiol. B Biol. 1993, 21, 229–234. [Google Scholar] [CrossRef]
- McCleary, B.V.; Solah, V.; Gibson, T.S. Quantitative measurement of total starch in cereal flours and products. J. Cereal Sci. 1994, 20, 51–58. [Google Scholar] [CrossRef]
- Stoscheck, C.M. Quantification of proteins. Meth. Enzymol. 1990, 182, 50–68. [Google Scholar]
- Delaquis, E.; Haan, S.; Wyckhuys, K.A.G. On-farm diversity offsets environmental pressures in tropical agro-ecosystems: A synthetic review for cassava-based systems. Agric. Ecosyst. Environ. 2018, 251, 226–235. [Google Scholar] [CrossRef]
- Moran, M.S.; Inoue, Y.; Barnes, E.M. Opportunities and limitations for image-based remote sensing in precision crop management. Remote Sens. Environ. 1997, 61, 319–346. [Google Scholar] [CrossRef]

| Actual Measurements | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Growth Traits | Nutritive Traits | |||||||||||||||||||
| Indices | Pn | Chl | Leaf Area | Plant Biomass | Ca | P | K | S | Na | Mg | Fe | Zn | Cu | Mn | CP | TC | Starch | Energy | TDF | Cyanide |
| NDVI | 0.85 ** | 0.86 ** | 0.74 ** | 0.85 ** | 0.51 ** | 0.41 ** | 0.41 ** | 0.51 ** | −0.28 ** | 0.32 ** | 0.38 ** | 0.36 ** | 0.22 * | −0.32 ** | 0.45 ** | 0.77 ** | 0.78 ** | 0.84 ** | 0.64 ** | −0.75 ** |
| GA | 0.89 ** | 0.90 ** | 0.78 ** | 0.88 ** | 0.59 ** | 0.40 ** | 0.47 ** | 0.50 ** | −0.28 ** | 0.33 ** | 0.41 ** | 0.31 ** | 0.19 * | −0.25 ** | 0.46 ** | 0.81 ** | 0.79 ** | 0.85 ** | 0.76 ** | −0.87 ** |
| GGA | 0.84 ** | 0.85 ** | 0.71 ** | 0.84 ** | 0.55 ** | 0.33 ** | 0.44 ** | 0.46 ** | −0.31 ** | 0.30 ** | 0.33 ** | 0.29 ** | 0.25 ** | −0.23 * | 0.43 ** | 0.72 ** | 0.73 ** | 0.82 ** | 0.69 ** | −0.87 ** |
| CSI | −0.32 ** | −0.36 ** | −0.25 * | −0.36 ** | −0.29 * | 0.03 | −0.29 * | −0.22 | 0.14 | −0.23 | −0.05 | −0.17 | −0.12 | 0.1 | −0.26 * | −0.21 | −0.26 * | −0.38 ** | −0.21 | 0.45 ** |
| SR | 0.94 ** | 0.94 ** | 0.84 ** | 0.94 ** | 0.65 ** | 0.48 ** | 0.48 ** | 0.57 ** | −0.28 ** | 0.31 ** | 0.46 ** | 0.43 ** | 0.22 * | −0.35 ** | 0.51 ** | 0.89 ** | 0.86 ** | 0.92 ** | 0.73 ** | −0.83 ** |
| GRVI | 0.84 ** | 0.83 ** | 0.78 ** | 0.84 ** | 0.64 ** | 0.36 ** | 0.44 ** | 0.59 ** | −0.19 * | 0.23 * | 0.44 ** | 0.41 ** | 0.16 | −0.34 ** | 0.42 ** | 0.79 ** | 0.78 ** | 0.82 ** | 0.69 ** | −0.82 ** |
| GNDVI | 0.78 ** | 0.74 ** | 0.66 ** | 0.75 ** | 0.51 ** | 0.40 ** | 0.34 ** | 0.43 ** | −0.29 ** | 0.28 ** | 0.34 ** | 0.30 ** | 0.12 | −0.15 | 0.38 ** | 0.72 ** | 0.68 ** | 0.83 ** | 0.59 ** | −0.61 ** |
| RENDVI | 0.90 ** | 0.91 ** | 0.73 ** | 0.89 ** | 0.56 ** | 0.37 ** | 0.37 ** | 0.51 ** | −0.24 ** | 0.29 ** | 0.29 ** | 0.29 ** | 0.27 ** | −0.25 ** | 0.47 ** | 0.81 ** | 0.76 ** | 0.92 ** | 0.73 ** | −0.82 ** |
| Growth Traits | Spectral Index | Regression Coefficient | R2 | p-Value | Model R2 |
|---|---|---|---|---|---|
| Leaf chlorophyll | NDVI | 7.26 | 0.74 | 0.006 | 0.92 |
| GA | 10.01 | 0.81 | 0.004 | ||
| GGA | 6.34 | 0.73 | 0.03 | ||
| SR | 25.37 | 0.88 | <0.001 | ||
| Net photosynthesis | NDVI | 0.17 | 0.72 | 0.101 | 0.91 |
| GA | 0.28 | 0.78 | 0.041 | ||
| GGA | 0.21 | 0.71 | 0.06 | ||
| SR | 1.16 | 0.89 | <0.001 | ||
| Total plant biomass | NDVI | 3.36 | 0.72 | 0.056 | 0.91 |
| GA | 5.61 | 0.77 | 0.003 | ||
| SR | 17.65 | 0.89 | <0.001 | ||
| GRVI | 3.08 | 0.71 | 0.074 | ||
| CSI | −1.81 | 0.12 | 0.052 | ||
| Leaf area | NDVI | 0.02 | 0.55 | 0.151 | 0.76 |
| GA | 0.03 | 0.6 | 0.009 | ||
| SR | 0.09 | 0.71 | <0.001 | ||
| GRVI | 0.02 | 0.6 | 0.052 |
| Nutritional Traits | Spectral Index | Regression Coefficient | R2 | p-Value | Model R2 |
|---|---|---|---|---|---|
| Energy content | NDVI | 0.34 | 0.71 | 0.093 | 0.89 |
| SR | 0.74 | 0.84 | 0.016 | ||
| GNDVI | 0.59 | 0.69 | 0.001 | ||
| RENDVI | 0.95 | 0.84 | 0.002 | ||
| Starch content | NDVI | 12.65 | 0.6 | 0.035 | 0.77 |
| GA | 15.08 | 0.62 | 0.026 | ||
| SR | 44.71 | 0.73 | <0.001 | ||
| GRVI | 8.91 | 0.6 | 0.109 | ||
| RENDVI | −24.68 | 0.58 | 0.004 | ||
| Total carotenoid | SR | 11.17 | 0.8 | <0.001 | 0.82 |
| CSI | 1.14 | 0.04 | 0.028 | ||
| Total dietary fiber | GA | 4.94 | 0.58 | <0.001 | 0.61 |
| GRVI | 1.89 | 0.48 | 0.026 | ||
| Crude Protein | SR | 1.16 | 0.26 | <0.001 | 0.27 |
| Cyanide concentration | GA | −1.11 | 0.76 | 0.019 | 0.84 |
| GGA | −1.84 | 0.76 | <0.001 | ||
| SR | −1.01 | 0.69 | 0.051 | ||
| GRVI | −1.38 | 0.68 | <0.001 | ||
| Calcium | SR | 1.53 | 0.43 | 0.001 | 0.48 |
| GRVI | 0.79 | 0.41 | 0.012 | ||
| RENDVI | −0.70 | 0.31 | 0.090 | ||
| Potassium | GGA | 3.10 | 0.20 | <0.001 | 0.34 |
| CSI | 1.22 | 0.02 | 0.016 | ||
| SR | 3.14 | 0.23 | <0.001 | ||
| Phosphorus | SR | 0.71 | 0.23 | <0.001 | 0.28 |
| RENDVI | −0.36 | 0.14 | 0.030 | ||
| Sulphur | SR | 0.14 | 0.33 | 0.078 | 0.38 |
| GRVI | 0.21 | 0.35 | 0.007 | ||
| Magnesium | GA | 0.59 | 0.11 | 0.031 | 0.17 |
| GGA | −0.48 | 0.09 | 0.162 | ||
| Sodium | GRVI | 0.22 | 0.03 | 0.041 | 0.18 |
| RENDVI | 1.67 | 0.05 | 0.026 | ||
| Iron | GA | 63.00 | 0.16 | <0.001 | 0.39 |
| SR | 56.30 | 0.21 | <0.001 | ||
| Zinc | GRVI | 2.74 | 0.18 | <0.001 | 0.28 |
| SR | 4.05 | 0.16 | <0.001 | ||
| Copper | RENDVI | 8.53 | 0.07 | 0.003 | 0.14 |
| CSI | −0.04 | 0.04 | 0.015 | ||
| Manganese | GRVI | −13.71 | 0.11 | <0.001 | 0.23 |
| SR | −5.66 | 0.11 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wasonga, D.O.; Yaw, A.; Kleemola, J.; Alakukku, L.; Mäkelä, P.S.A. Red-Green-Blue and Multispectral Imaging as Potential Tools for Estimating Growth and Nutritional Performance of Cassava under Deficit Irrigation and Potassium Fertigation. Remote Sens. 2021, 13, 598. https://doi.org/10.3390/rs13040598
Wasonga DO, Yaw A, Kleemola J, Alakukku L, Mäkelä PSA. Red-Green-Blue and Multispectral Imaging as Potential Tools for Estimating Growth and Nutritional Performance of Cassava under Deficit Irrigation and Potassium Fertigation. Remote Sensing. 2021; 13(4):598. https://doi.org/10.3390/rs13040598
Chicago/Turabian StyleWasonga, Daniel O., Afrane Yaw, Jouko Kleemola, Laura Alakukku, and Pirjo S.A. Mäkelä. 2021. "Red-Green-Blue and Multispectral Imaging as Potential Tools for Estimating Growth and Nutritional Performance of Cassava under Deficit Irrigation and Potassium Fertigation" Remote Sensing 13, no. 4: 598. https://doi.org/10.3390/rs13040598
APA StyleWasonga, D. O., Yaw, A., Kleemola, J., Alakukku, L., & Mäkelä, P. S. A. (2021). Red-Green-Blue and Multispectral Imaging as Potential Tools for Estimating Growth and Nutritional Performance of Cassava under Deficit Irrigation and Potassium Fertigation. Remote Sensing, 13(4), 598. https://doi.org/10.3390/rs13040598










