Assessment and Attribution of Mangrove Forest Changes in the Indian Sundarbans from 2000 to 2020
Abstract
:1. Introduction
- (1)
- To detect changes in mangrove forest area, genus composition, and indicators of health across the Indian Sundarbans at various temporal and spatial scales, using Landsat and MODIS satellite imagery.
- (2)
- To assess the possible drivers of the observed mangrove dynamics, including legacy drivers (e.g., decline in sediment supply and river flow), contemporary progressive drivers (e.g., relative sea-level rise), and shocks (e.g., cyclone landfall).
2. Materials and Methods
2.1. The Sundarban Biosphere Reserve, India
2.2. Mangrove Extent
2.3. Mangrove Community Classification
2.4. Mangrove Health Indicators
2.5. Meteorological Data
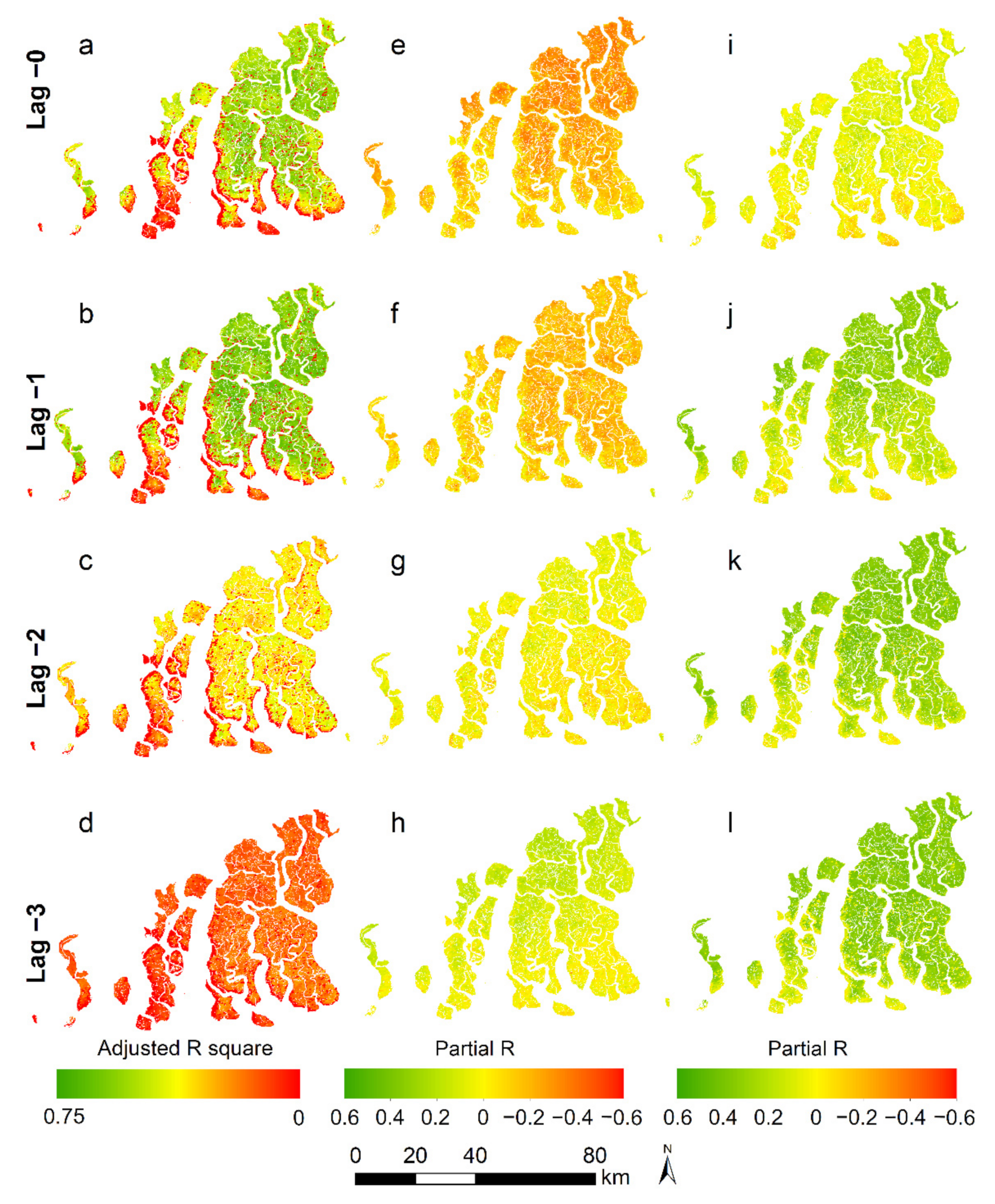
2.6. Relation of Vegetation Health Indicators to Climate Variability
2.7. Relation of Vegetation Health Indicators to Cyclone Impact
2.7.1. Canopy Density
2.7.2. EVI
3. Results and Analysis
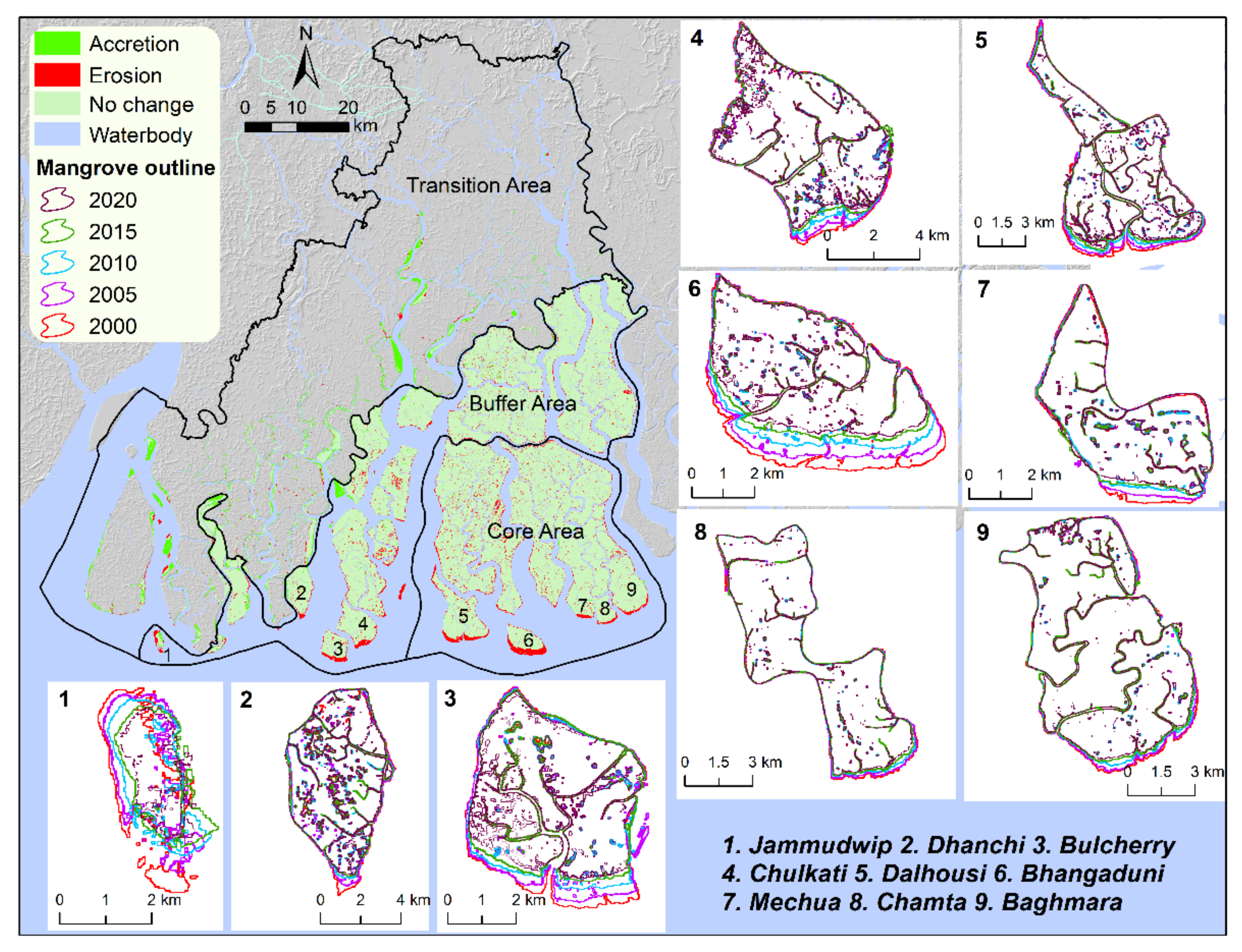
3.1. Change in Mangrove Forest Area
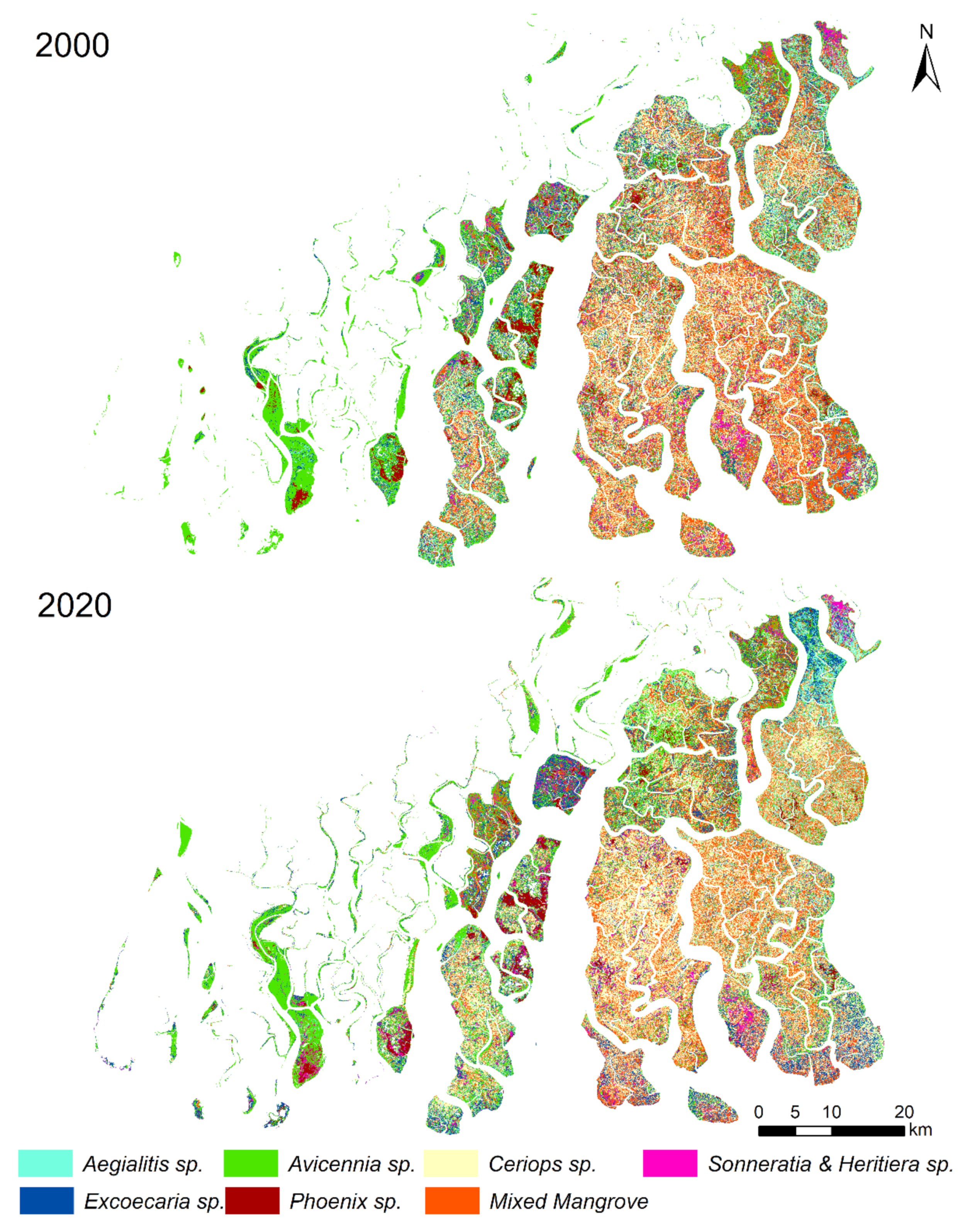
3.2. Changes in Mangrove Community Composition
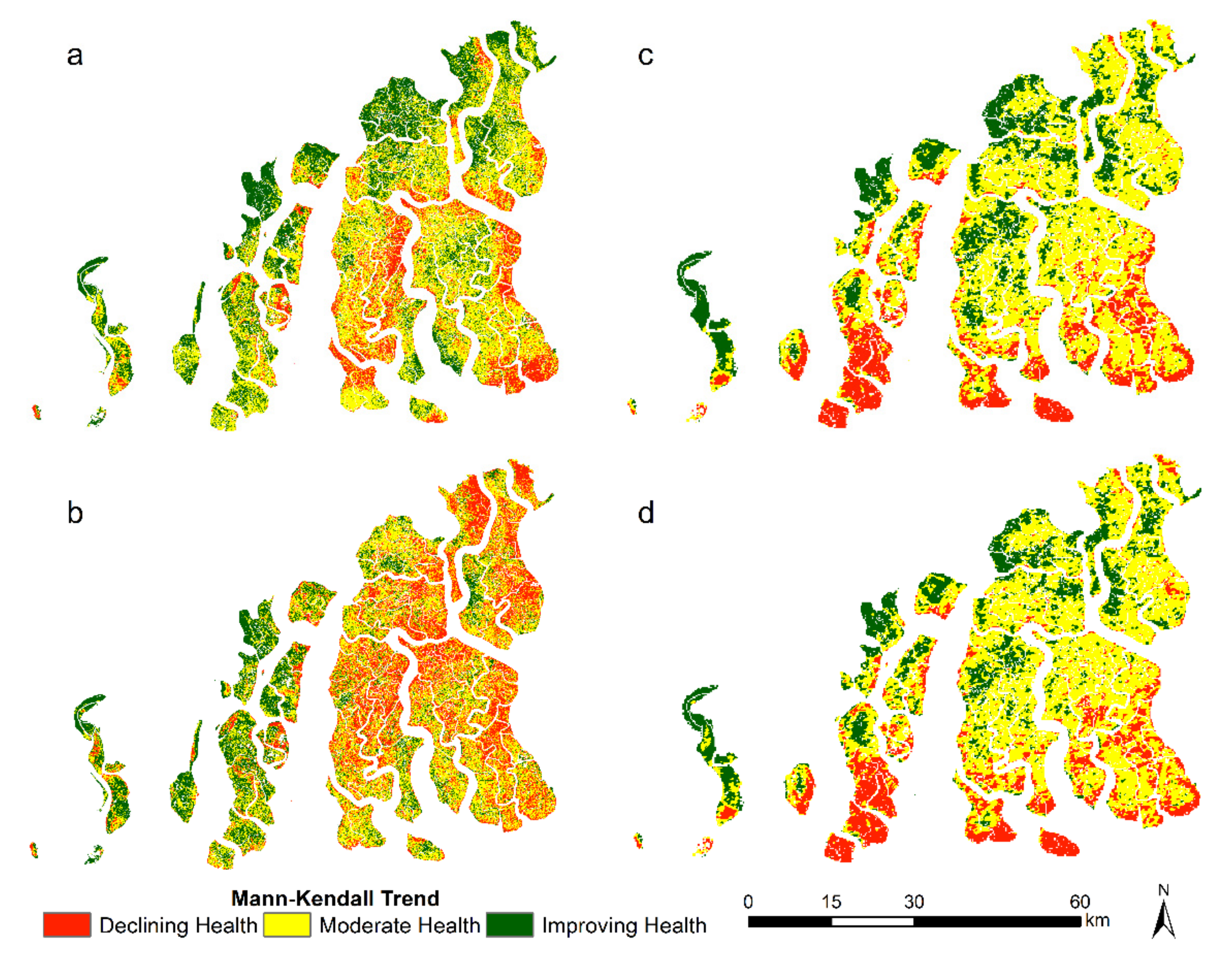
3.3. Change in Mangrove Health Indicators
3.4. Drivers of Change
3.4.1. Sediment Supply
3.4.2. Salinization
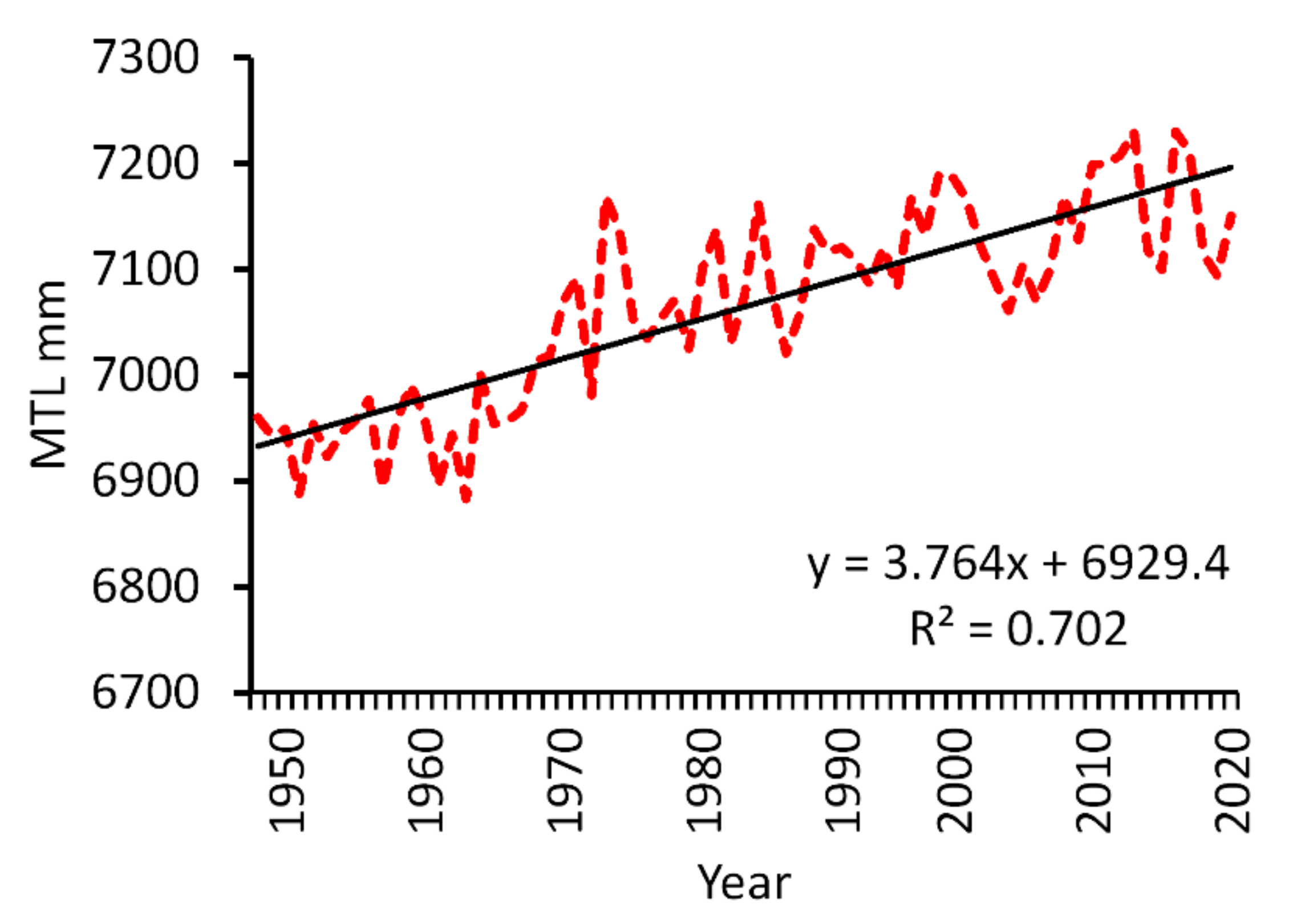
3.4.3. Relative Sea-Level Rise
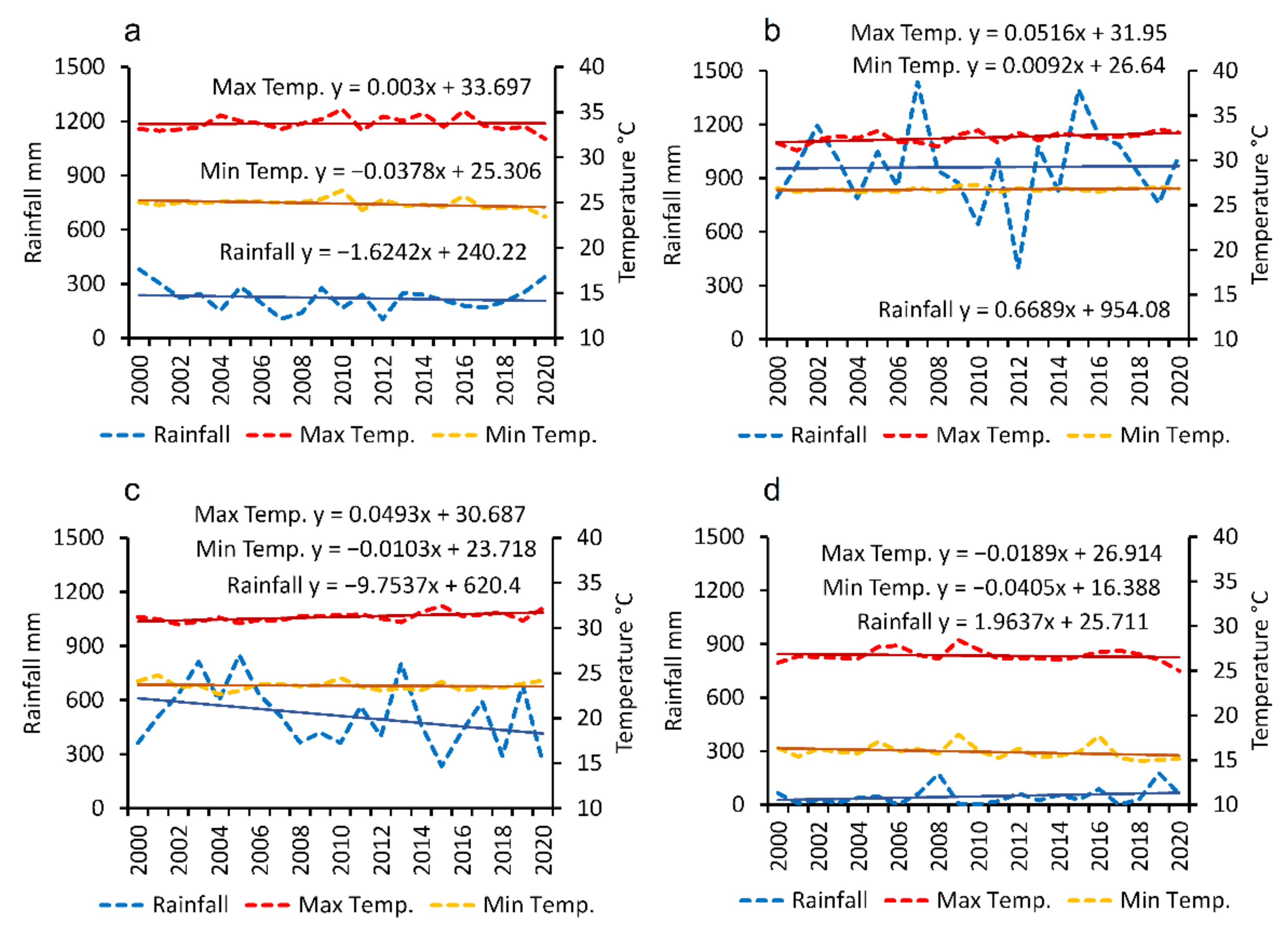
3.4.4. Changes in Air Temperature
3.4.5. Changes in Rainfall
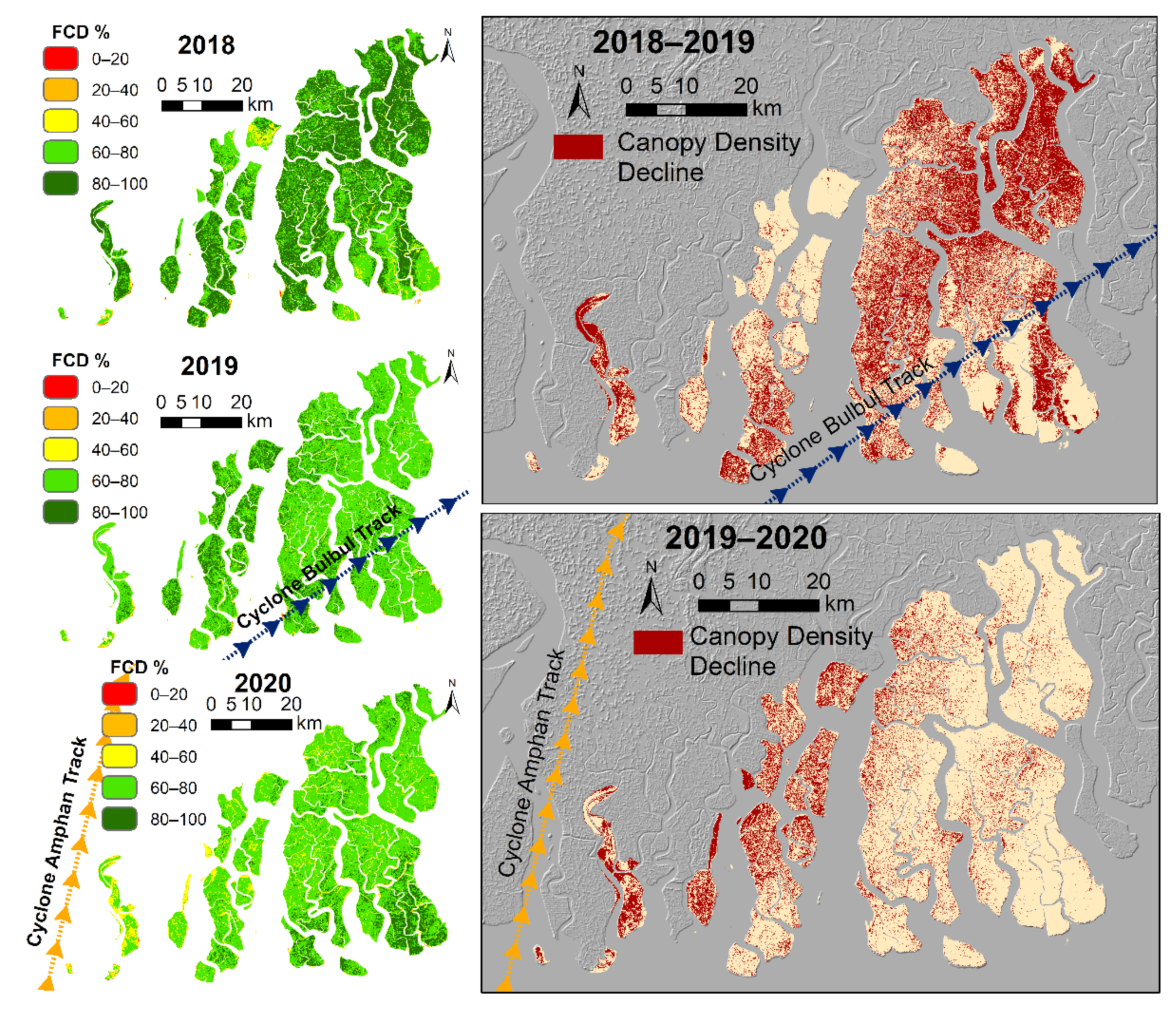
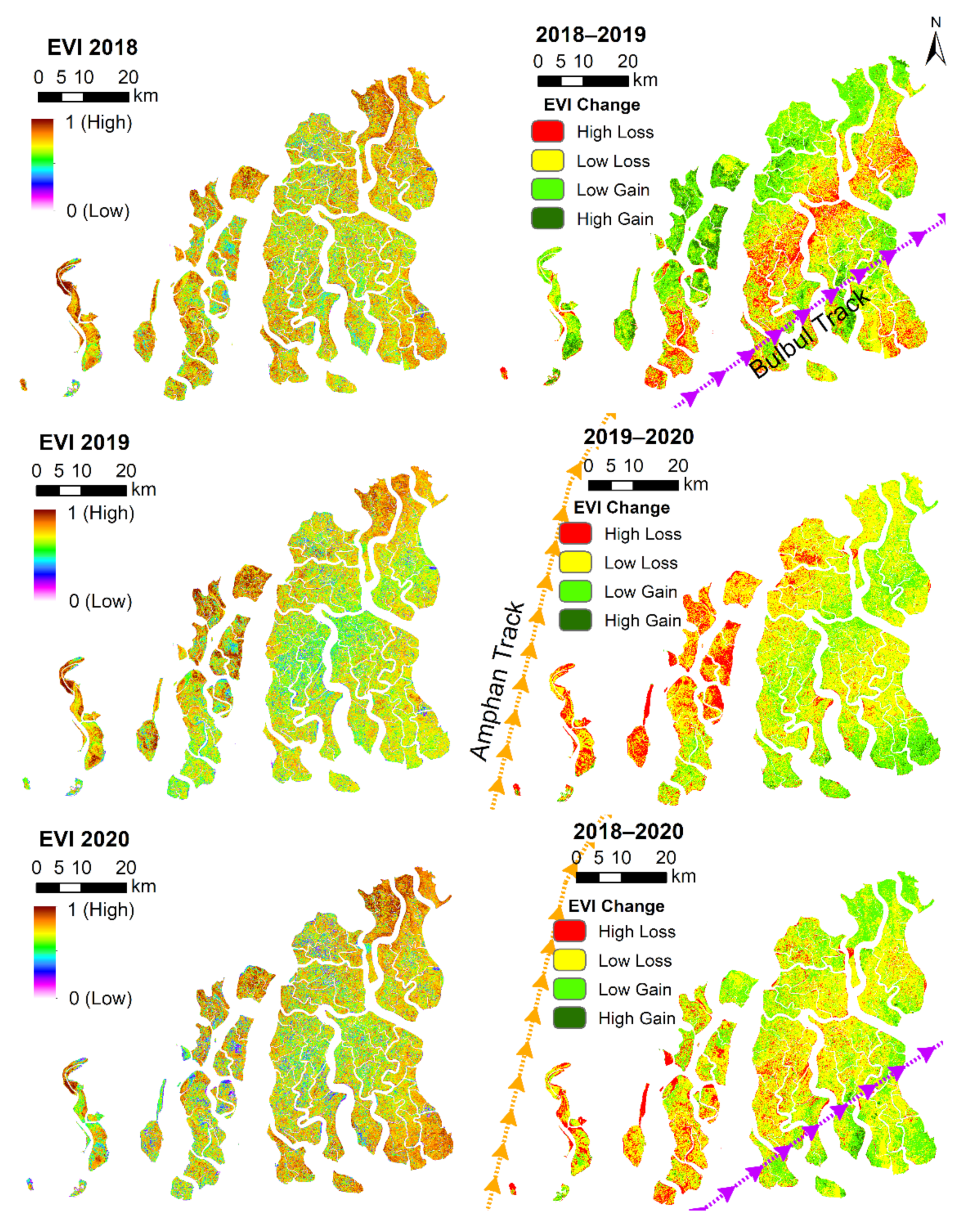
3.4.6. Short-Term Degradation Due to Cyclones
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Giri, C.; Ochieng, E.; Tiszen, L.L.; Zhu, Z.; Singh, A.; Loveland, T.; Masek, J.; Duke, N. Status and distribution of mangrove forests of the world using earth observation satellite data. Glob. Ecol. Biogeogr. 2010, 20, 154–159. [Google Scholar] [CrossRef]
- Alongi, D.M. The Energetics of Mangrove Forests; Springer: Dordrecht, The Netherlands, 2009. [Google Scholar]
- Field, C.B.; Osborn, J.G.; Hoffman, L.L.; Polsenberg, J.F.; Ackerly, D.D.; Berry, J.A.; Björkman, O.; Held, A.; Matson, P.A.; Mooney, H.A. Mangrove biodiversity and ecosystem function. Glob. Ecol. Biogeogr. Lett. 1998, 7, 3–14. [Google Scholar] [CrossRef]
- Dodd, R.S.; Ong, J.E. Future of mangrove ecosystems to 2025. In Aquatic Ecosystems: Trends and Global Prospects; Polunin, N.V.C., Ed.; Cambridge University Press: Cambridge, UK, 2008; pp. 172–187. [Google Scholar]
- Nagelkerken, I.; Blaber, S.J.M.; Bouillon, S.; Green, P.; Haywood, M.; Kirton, L.G.; Meynecke, J.-O.; Pawlik, J.; Penrose, H.M.; Sasekumar, A.; et al. The habitat function of mangroves for terrestrial and marine fauna: A review. Aquat. Bot. 2008, 89, 155–185. [Google Scholar] [CrossRef] [Green Version]
- Wolanski, E. Transport of sediment in mangrove swamps. In Asia-Pacific Symposium on Mangrove Ecosystems. Developments in Hydrobiology; Wong, Y.S., Tam, N.F.Y., Eds.; Springer: Dordrecht, The Netherlands, 1995; Volume 106. [Google Scholar] [CrossRef]
- Dittmar, T.; Hertkorn, N.; Kattner, G.; Lara, R.J. Mangroves, a major source of dissolved organic carbon to the oceans. Glob. Biogeochem. Cycles 2006, 20, GB1012. [Google Scholar] [CrossRef]
- Zhang, K.; Liu, H.; Li, Y.; Xu, H.; Shen, J.; Rhome, J.; Smith, T.J. The role of mangroves in attenuating storm surges. Estuar. Coast. Shelf Sci. 2012, 102–103, 11–23. [Google Scholar] [CrossRef]
- Arkema, K.K.; Guannel, G.; Verutes, G.; Wood, S.A.; Guerry, A.; Ruckelshaus, M.; Kareiva, P.; Lacayo, M.; Silver, J.M. Coastal habitats shield people and property from sea-level rise and storms. Nat. Clim. Chang. 2013, 3, 913–918. [Google Scholar] [CrossRef]
- Menéndez, P.; Losada, I.J.; Torres-Ortega, S.; Narayan, S.; Beck, M.W. The Global Flood Protection Benefits of Mangroves. Sci. Rep. 2020, 10, 4404. [Google Scholar] [CrossRef]
- Donato, D.C.; Kauffman, J.B.; Murdiyarso, D.; Kurnianto, S.; Stidham, M.; Kanninen, M. Mangroves among the most carbon-rich forests in the tropics. Nat. Geosci. 2011, 4, 293–297. [Google Scholar] [CrossRef]
- Alongi, D.M. Carbon cycling and storage in mangrove forests. Annu. Rev. Mar. Sci. 2014, 6, 195–219. [Google Scholar] [CrossRef]
- Duarte, C.M.; Losada, I.J.; Hendriks, I.E.; Mazarrasa, I.; Marbà, N. The role of coastal plant communities for climate change mitigation and adaptation. Nat. Clim. Chang. 2013, 3, 961–968. [Google Scholar] [CrossRef] [Green Version]
- Valiela, I.; Bowen, J.L.; York, J.K. Mangrove forests: One of the world’s threatened major tropical environments. Bioscience 2001, 51, 807–815. [Google Scholar] [CrossRef] [Green Version]
- Thomas, N.; Lucas, R.; Bunting, P.; Hardy, A.; Rosenqvist, A.; Simard, M. Distribution and drivers of global mangrove forest change, 1996–2010. PLoS ONE 2017, 12, e0179302. [Google Scholar] [CrossRef] [Green Version]
- Polidoro, B.A.; Carpenter, K.E.; Collins, L.; Duke, N.C.; Ellison, A.M.; Ellison, J.C.; Farnsworth, E.J.; Fernando, E.S.; Kathiresan, K.; Koedam, N.E.; et al. The loss of species: Mangrove extinction risk and geographic areas of global concern. PLoS ONE 2010, 5, e10095. [Google Scholar] [CrossRef]
- Hamilton, S.E.; Casey, D. Creation of a high spatio-temporal resolution global database of continuous mangrove forest cover for the 21st century (CGMFC-21). Glob. Ecol. Biogeogr. 2016, 25, 729–738. [Google Scholar] [CrossRef]
- Goldberg, L.; Lagomasino, D.; Thomas, N.; Fatoyinbo, T. Global declines in human-driven mangrove loss. Glob. Chang. Biol. 2020, 26, 5844–5855. [Google Scholar] [CrossRef]
- Doughty, C.L.; Langley, J.A.; Walker, W.S.; Feller, I.C.; Schaub, R.; Chapman, S.K. Mangrove range expansion rapidly increases coastal wetland carbon storage. Estuar. Coast. 2016, 39, 385–396. [Google Scholar] [CrossRef]
- Alongi, D.M. Mangrove forests: Resilience, protection from tsunamis, and responses to global climate change. Estuar. Coast. Shelf Sci. 2008, 76, 1–13. [Google Scholar] [CrossRef]
- Kuenzer, C.L.; Bluemel, A.; Gebhardt, S.; Quoc, T.V.; Dech, S. Remote sensing of mangrove ecosystems: A review. Remote Sens. 2011, 3, 878–928. [Google Scholar] [CrossRef] [Green Version]
- Simard, M.; Fatoyinbo, L.; Smetanka, C.; Rivera-Monroy, V.H.; Castañeda-Moya, E.; Thomas, N.; Van Der Stocken, T. Mangrove canopy height globally related to precipitation, temperature and cyclone frequency. Nat. Geosci. 2019, 12, 40–45. [Google Scholar] [CrossRef]
- Lee, S.-K. Mangrove height estimates from TanDEM-X data. Korea J. Rem. Sens. 2020, 36, 325–335. [Google Scholar] [CrossRef]
- Atwood, T.B.; Connolly, R.M.; Almahasheer, H.; Carnell, P.E.; Duarte, C.M.; Lewis, C.J.E.; Irigoien, X.; Kelleway, J.J.; Lavery, P.S.; Macreadie, P.I.; et al. Global patterns in mangrove soil carbon stocks and losses. Nat. Clim. Chang. 2017, 7, 523–528. [Google Scholar] [CrossRef]
- Tang, W.; Zheng, M.; Zhao, X.; Shi, J.; Yang, J.; Trettin, C.C. Big geospatial data analytics for global mangrove biomass and carbon estimation. Sustainability 2018, 10, 472. [Google Scholar] [CrossRef] [Green Version]
- Bryan-Brown, D.N.; Connolly, R.M.; Richards, D.R.; Adame, F.; Friess, D.A.; Brown, C.J. Global trends in mangrove forest fragmentation. Sci. Rep. 2020, 10, 7117. [Google Scholar] [CrossRef] [PubMed]
- Manna, S.; Raychaudhuri, B. Mapping distribution of Sundarban mangroves using Sentinel-2 data and new spectral metric for detecting their health condition. Geocarto Int. 2020, 35, 434–452. [Google Scholar] [CrossRef]
- Giri, C.; Pengra, B.; Zhu, Z.; Singh, A.; Tieszen, L.L. Monitoring mangrove forest dynamics of the Sundarbans in Bangladesh and India using multi-temporal satellite data from 1973 to 2000. Estuar. Coast. Shelf Sci. 2007, 73, 91–100. [Google Scholar] [CrossRef]
- Chellamani, P.; Prakash Singh, C.; Panigrahy, S. Assessment of the health status of Indian mangrove ecosystems using multi temporal remote sensing data. Tropic. Ecol. 2014, 55, 245–253. [Google Scholar]
- Pastor-Guzman, J.; Dash, J.; Atkinson, P.M. Remote sensing of mangrove forest phenology and its environmental drivers. Rem. Sens. Environ. 2018, 205, 71–84. [Google Scholar] [CrossRef] [Green Version]
- Huete, A.; Didan, K.; Miura, T.; Rodriguez, E.P.; Gao, X.; Ferreira, L.G. Overview of the radiometric and biophysical performance of the MODIS vegetation indices. Remote Sens. Environ. 2002, 83, 195–213. [Google Scholar] [CrossRef]
- Maryantika, N.; Lin, C. Exploring changes of land use and mangrove distribution in the economic area of Sidoarjo District, East Java using multi-temporal Landsat images. Info. Process. Agric. 2017, 4, 321–332. [Google Scholar] [CrossRef]
- Abd-El Monsef, H.; Smith, S.E. A new approach for estimating mangrove canopy cover using Landsat 8 imagery. Comp. Electron. Agric. 2017, 135, 183–194. [Google Scholar] [CrossRef]
- Ishtiaque, A.; Myint, S.W.; Wang, C. Examining the ecosystem health and sustainability of the world’s largest mangrove forest using multi-temporal MODIS products. Sci. Total Environ. 2016, 569–570, 1241–1254. [Google Scholar] [CrossRef]
- Rahman, M.M.; Rahman, M.M.; Islam, K.S. The causes of deterioration of Sundarban mangrove forest ecosystem of Bangladesh: Conservation and sustainable management issues. Aquac. Aquar. Conserv. Legis. 2010, 3, 77–90. [Google Scholar]
- Paul, A.K.; Ray, R.; Kamila, A.; Jana, S. Mangrove degradation in the Sundarbans. In Coastal Wetlands: Alteration and Remediation; Finkl, C., Makowski, C., Eds.; Springer: Cham, Switzerland, 2017; pp. 357–392. [Google Scholar] [CrossRef]
- Islam, M.J.; Alam, M.S.; Elahi, K.M. Remote sensing for change detection in the Sundarbans, Bangladesh. Geocarto Int. 1997, 12, 91–100. [Google Scholar] [CrossRef]
- Kundu, K.; Halder, P.; Mandal, J.K. Detection and prediction of sundarban reserve forest using the CA-Markov chain model and remote sensing data. Earth Sci. Inf. 2021, 14, 1503–1520. [Google Scholar] [CrossRef]
- Kundu, K.; Halder, P.; Mandal, J.K. Change Detection and Patch Analysis of Sundarban Forest During 1975–2018 Using Remote Sensing and GIS Data. SN Comput. Sci. 2021, 2, 1–14. [Google Scholar] [CrossRef]
- Chatterjee, N.; Mukhopadhyay, R.; Mitra, D. Decadal changes in shoreline patterns in Sundarbans, India. J. Coast Sci. 2015, 2, 54–64. [Google Scholar]
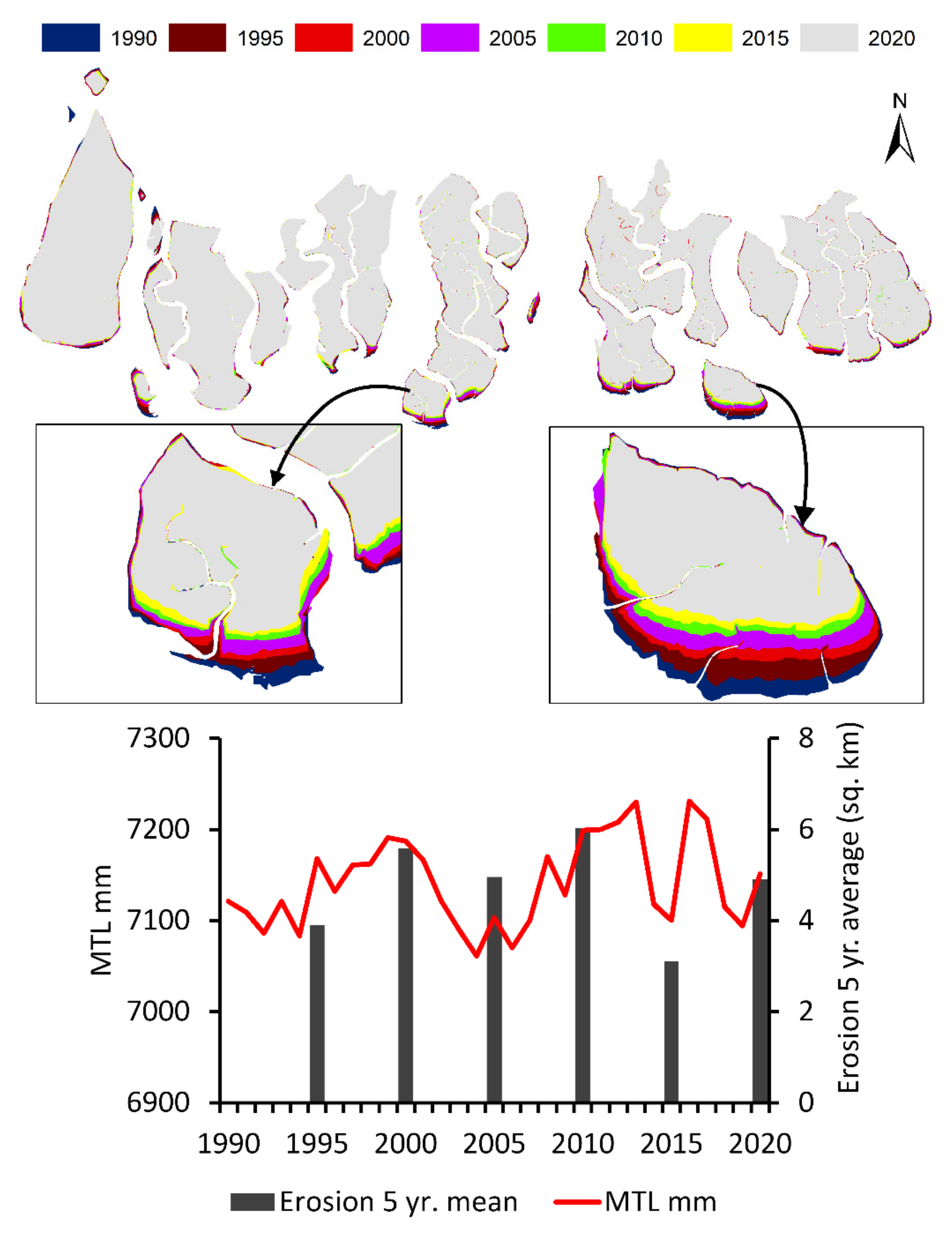
- Bera, R.; Maiti, R. Quantitative analysis of erosion and accretion (1975–2017) using DSAS—A study on Indian Sundarbans. Reg. Stud. Mar. Sci. 2019, 28, 100583. [Google Scholar] [CrossRef]
- Rahman, A.F.; Dragoni, D.; El-Masri, B. Response of the Sundarbans coastline to sea level rise and decreased sediment flow: A remote sensing assessment. Rem. Sens. Environ. 2011, 115, 3121–3128. [Google Scholar] [CrossRef]
- Sánchez-Arias, L.E.; Rodriguez, J.P.; Caballer, M.; Asmussen, M.V.; Medina, G. Diagnostic of health status in Mangrove ecosystems. Adv. Environ. Res. 2011, 3, 235–262. [Google Scholar]
- Sahana, M.; Sajjad, H.; Ahmed, R. Assessing spatio-temporal health of forest cover using forest canopy density model and forest fragmentation approach in Sundarban reserve forest, India. Model. Earth Syst. Environ. 2015, 1, 49. [Google Scholar] [CrossRef]
- Rahman, M.M. Impact of increased salinity on the plant community of the Sundarbans Mangrove of Bangladesh. Commun. Ecol. 2020, 21, 273–284. [Google Scholar] [CrossRef]
- Pitchaikani, J.S. Vertical current structure in a macro-tidal, well mixed Sundarban ecosystem, India. J. Coast Conserv. 2020, 24, 63. [Google Scholar] [CrossRef]
- Ali, S.A.; Khatun, R.; Ahmad, A.; Ahmad, S.N. Assessment of cyclone vulnerability, hazard evaluation and mitigation capacity for analyzing cyclone risk using GIS technique: A study on Sundarban biosphere reserve, India. Earth Syst. Environ. 2020, 4, 71–92. [Google Scholar] [CrossRef]
- Padhy, S.R.; Bhattacharyya, P.; Dash, P.K.; Reddy, C.S.; Chakraborty, A.; Pathak, H. Seasonal fluctuation in three mode of greenhouse gases emission in relation to soil labile carbon pools in degraded mangrove, Sundarban, India. Sci. Total Environ. 2020, 705, 135909. [Google Scholar] [CrossRef]
- Ghosh, A.; Schmidt, S.; Fickert, T.; Nüsser, M. The Indian Sundarban mangrove forests: History, utilization, conservation strategies and local perception. Diversity 2015, 7, 149–169. [Google Scholar] [CrossRef]
- Barik, J.; Chowdhury, S. True mangrove species of Sundarbans delta, West Bengal, eastern India. Check List 2014, 10, 329–334. [Google Scholar] [CrossRef]
- Barik, J.; Mukhopadhyay, A.; Ghosh, T.; Mukhopadhyay, S.K.; Chowdhury, S.M.; Hazra, S. Mangrove species distribution and water salinity: An indicator species approach to Sundarban. J. Coast. Conserv. 2018, 22, 361–368. [Google Scholar] [CrossRef]
- Banerjee, K.; Gatti, R.C.; Mitra, A. Climate change-induced salinity variation impacts on a stenoecious mangrove species in the Indian Sundarbans. Ambio 2017, 46, 492–499. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.; Wan, B.; Qiu, P.; Su, Y.; Guo, Q.; Wang, R.; Sun, F.; Wu, X. Evaluating the performance of Sentinel-2, Landsat 8 and Pléiades-1 in mapping mangrove extent and species. Remote Sens. 2018, 10, 1468. [Google Scholar] [CrossRef] [Green Version]
- Giri, S.; Mukhopadhyay, A.; Hazra, S.; Mukherjee, S.; Roy, D.; Ghosh, S.; Ghosh, T.; Mitra, D. A study on abundance and distribution of mangrove species in Indian Sundarban using remote sensing technique. J. Coast. Conserv. 2014, 18, 359–367. [Google Scholar] [CrossRef]
- Ghosh, M.K.; Kumar, L.; Roy, C. Mapping long-term changes in mangrove species composition and distribution in the Sundarbans. Forests 2016, 7, 305. [Google Scholar] [CrossRef] [Green Version]
- Mitra, D.; Karmaker, S. Mangrove classification in Sundarban using high resolution multi-spectral remote sensing data and GIS. Asian J. Environ. Disaster Manag. 2010, 2, 197–207. [Google Scholar]
- Kumar, T.; Mandal, A.; Dutta, D.; Nagaraja, R.; Dadhwal, V.K. Discrimination and classification of mangrove forests using EO-1 Hyperion data: A case study of Indian Sundarbans. Geocarto Int. 2019, 34, 415–442. [Google Scholar] [CrossRef]
- Mukhopadhyay, A.; Wheeler, D.; Dasgupta, S.; Dey, A.; Sobhan, I. Aquatic salinization and mangrove species in a changing climate: Impact in the Indian Sundarbans. World Bank Policy Res. Work. Pap. 2018, 8532, 1–21. [Google Scholar]
- Hati, J.P.; Samanta, S.; Chaube, N.R.; Misra, A.; Giri, S.; Pramanick, N.; Gupta, K.; Majumdar, S.D.; Chanda, A.; Mukhopadhyay, A.; et al. Mangrove classification using airborne hyperspectral AVIRIS-NG and comparing with other spaceborne hyperspectral and multispectral data. Egypt. J. Remote Sens. Space Sci. 2021, 24, 273–281. [Google Scholar] [CrossRef]
- Didan, K. MOD13Q1 MODIS/Terra Vegetation Indices 16-Day L3 Global 250m SIN Grid V006. NASA EOSDIS Land Processes DAAC. 2015. Available online: https://lpdaac.usgs.gov/data/ (accessed on 6 June 2020).
- AppEEARS Team. Application for Extracting and Exploring Analysis Ready Samples (AppEEARS); Ver. 2.42.1; NASA EOSDIS Land Processes Distributed Active Archive Center (LP DAAC), USGS/Earth Resources Observation and Science (EROS) Center: Sioux Falls, SD, USA, 2020. Available online: https://lpdaacsvc.cr.usgs.gov/appeears (accessed on 6 June 2020).
- Kovács, F.; Gulácsi, A. Spectral index-based monitoring (2000–2017) in lowland forests to evaluate the effects of climate change. Geosciences 2019, 9, 411. [Google Scholar] [CrossRef] [Green Version]
- Neeti, N.; Eastman, R. A contextual Mann-Kendall approach for the assessment of trend significance in image time series. Trans. GIS 2011, 15, 599–611. [Google Scholar] [CrossRef]
- Rikimaru, A.; Roy, P.S.; Miyatake, S. Tropical Forest cover density mapping. Trop. Ecol. 2002, 43, 39–47. [Google Scholar]
- Sarwar, M.G.M.; Woodroffe, C.D. Rates of shoreline change along the coast of Bangladesh. J. Coast. Conserv. 2013, 17, 515–526. [Google Scholar] [CrossRef] [Green Version]
- Rahman, M.M.; Ghosh, T.; Salehin, M.; Ghosh, A.; Haque, A.; Hossain, M.A.; Das, S.; Hazra, S.; Islam, N.; Sarker, M.H.; et al. Ganges-Brahmaputra-Meghna Delta, Bangladesh, and India: A Transnational Mega-Delta. In Deltas in the Anthropocene; Nicholls, R., Adger, W., Hutton, C., Hanson, S., Eds.; Palgrave Macmillan: Cham, Switzerland, 2020; pp. 23–51. [Google Scholar] [CrossRef] [Green Version]
- Rahman, M.S.; Sass-Klaassen, U.; Zuidema, P.A.; Chowdhury, M.Q.; Beeckman, H. Salinity drives growth dynamics of the mangrove tree Sonneratia apetala Buch. -Ham. in the Sundarbans, Bangladesh. Dendrochronologia 2020, 62, 125711. [Google Scholar] [CrossRef]
- Akhand, A.; Chanda, A.; Manna, S.; Das, S.; Hazra, S.; Roy, R. A comparison of CO2 dynamics and air-water fluxes in a river-dominated estuary and a mangrove-dominated marine estuary. Geophys. Res. Lett. 2016, 43, 11726–11735. [Google Scholar] [CrossRef]
- Chowdhury, R.; Sutradhar, T.; Begam, M.M.; Mukherjee, C.; Chatterjee, K.; Basak, S.K.; Ray, K. Effects of nutrient limitation, salinity increase, and associated stressors on mangrove forest cover, structure, and zonation across Indian Sundarbans. Hydrobiologia 2019, 842, 191–217. [Google Scholar] [CrossRef]
- Feher, L.C.; Osland, M.J.; Griffith, K.T.; Grace, J.B.; Howard, R.J.; Stagg, C.L.; Enwright, N.M.; Krauss, K.W.; Gabler, C.A.; Day, R.H.; et al. Linear and nonlinear effects of temperature and precipitation on ecosystem properties in tidal saline wetlands. Ecosphere 2017, 8, e01956. [Google Scholar] [CrossRef]
- Ward, R.D.; Friess, D.A.; Day, R.H.; MacKenzie, R.A. Impacts of climate change on mangrove ecosystems: A region-by-region overview. Ecosyst. Health Sustain. 2016, 2, e01211. [Google Scholar] [CrossRef] [Green Version]
- Krauss, K.W.; Osland, M.J. Tropical cyclones and the organization of mangrove forests: A review. Ann. Bot. 2020, 125, 213–234. [Google Scholar] [CrossRef]
- Mandal, M.S.H.; Hosaka, T. Assessing cyclone disturbances (1988–2016) in the Sundarbans mangrove forests using Landsat and Google Earth Engine. Nat. Hazards 2020, 102, 133–150. [Google Scholar] [CrossRef]
- Morgan, J.P.; McIntire, W.G. Quaternary geology of the Bengal basin, East Pakistan and India. Geol. Soc. Amer. Bull. 1959, 70, 319–342. [Google Scholar] [CrossRef]
- Basu, S.K. A geotechnical assessment of the Farakka Barrage Project, Murshidabad and Maldah Districts, West Bengal. Bull. Geol. Surv. India 1982, 47, 2–3. [Google Scholar]
- Gupta, H.; Kao, S.; Dai, M. The role of mega dams in reducing sediment fluxes: A case study of large Asian rivers. J. Hydrol. 2012, 464–465, 447–458. [Google Scholar] [CrossRef]
- Rudra, K. Changing river courses in the western part of the Ganga—Brahmaputra delta. Geomorphology 2014, 227, 87–100. [Google Scholar] [CrossRef]
- Bhadra, T.; Mukhopadhyay, A.; Hazra, S. Identification of river discontinuity using geo-informatics to improve freshwater flow and ecosystem services in Indian Sundarban Delta. In Environment and Earth Observation; Hazra, S., Mukhopadhyay, A., Ghosh, A., Mitra, D., Dadhwal, V., Eds.; Springer: Cham, Switzerland, 2017; pp. 137–152. [Google Scholar] [CrossRef]
- Mitra, S.; Chanda, A.; Das, S.; Ghosh, T.; Hazra, S. Salinity Dynamics in the Hooghly-Matla Estuarine System and Its Impact on the Mangrove Plants of Indian Sundarbans. In Sundarbans Mangrove Systems; Mukhopadhyay, A., Mitra, D., Hazra, S., Eds.; CRC Press: Boca Raton, FL, USA, 2021; pp. 305–328. [Google Scholar] [CrossRef]
- Brown, S.; Nicholls, R.J. Subsidence and human influences in mega deltas: The case of the Ganges–Brahmaputra–Meghna. Sci. Total Environ. 2015, 527, 362–374. [Google Scholar] [CrossRef] [Green Version]
- Becker, M.; Papa, F.; Karpytchev, M.; Delebecque, C.; Krien, Y.; Khan, J.U.; Ballu, V.; Durand, F.; Cozannet, G.L.; Saiful Islam, A.K.M.; et al. Water level changes, subsidence, and sea-level rise in the Ganges–Brahmaputra–Meghna delta. Proc. Natl. Acad. Sci. USA 2020, 117, 1867–1876. [Google Scholar] [CrossRef]
- Ghosh, M.K.; Hazra, S.; Nandy, S.; Mondal, P.P.; Watham, T.; Kushwaha, S.P.S. Trends of sea level in the Bay of Bengal using altimetry and other complementary techniques. J. Spat. Sci. 2018, 63, 49–62. [Google Scholar] [CrossRef]
- Hazra, S.; Mukhopadhyay, A.; Chanda, A.; Mondal, P.; Ghosh, T.; Mukherjee, S.; Salehin, M. Characterizing the 2D shape complexity dynamics of the islands of Sundarbans, Bangladesh: A fractal dimension approach. Environ. Earth Sci. 2016, 75, 1367. [Google Scholar] [CrossRef]
- Brown, S.; Nicholls, R.J.; Lázár, A.N.; Hornby, D.D.; Hill, C.; Hazra, S.; Addo, K.A.; Haque, A.; Caesar, J.; Tompkins, E.L. What are the implications of sea-level rise for a 1.5, 2 and 3° C rise in global mean temperatures in the Ganges-Brahmaputra-Meghna and other vulnerable deltas? Reg. Environ. Chang. 2018, 18, 1829–1842. [Google Scholar] [CrossRef] [Green Version]
- Wong, P.P.; Losada, I.J.; Gattuso, J.-P.; Hinkel, J.; Khattabi, A.; McInnes, K.L.; Saito, Y.; Sallenger, A. Coastal systems and low-lying areas. In Climate Change 2014: Impacts, Adaptation, and Vulnerability. Part A: Global and Sectoral Aspects. Contribution of Working Group II to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Field, C.B., Barros, V.R., Dokken, D.J., Mach, K.J., Mastrandrea, M.D., Bilir, T.E., Chatterjee, M., Ebi, K.L., Estrada, Y.O., Genova, R.C., et al., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2014; pp. 361–409. [Google Scholar]
- Tomlinson, P.B. The Botany of Mangroves; Cambridge University Press: Cambridge, UK, 1986. [Google Scholar]
- Krauss, K.W.; Lovelock, C.E.; McKee, K.L.; Lopez-Hoffman, L.; Ewe, S.M.L.; Sousa, W.P. Environmental drivers in mangrove establishment and early development: A review. Aquat. Bot. 2008, 89, 105–127. [Google Scholar] [CrossRef]
- McKee, K.L. Growth and physiological responses of neotropical mangrove seedlings to root zone hypoxia. Tree Physiol. 1996, 16, 883–889. [Google Scholar] [CrossRef] [Green Version]
- Mukhopadhyay, S.K.; Biswas, H.; De, T.K.; Jana, T.K. Fluxes of nutrients from the tropical River Hooghly at the land–ocean boundary of Sundarbans, NE Coast of Bay of Bengal, India. J. Mar. Syst. 2006, 62, 9–21. [Google Scholar] [CrossRef]
- Dube, S.K.; Jain, I.; Rao, A.D.; Murty, T.S. Storm surge modelling for the Bay of Bengal and Arabian Sea. Nat. Hazards 2009, 51, 3–27. [Google Scholar] [CrossRef]
- Singh, D.; Singh, V. Impact of tropical cyclone on total ozone measured by TOMS–EP over the Indian region. Curr. Sci. 2007, 93, 471–476. [Google Scholar]
- Sattar, A.M.; Cheung, K.K. Comparison between the active tropical cyclone seasons over the Arabian Sea and Bay of Bengal. Int. J. Clim. 2019, 39, 5486–5502. [Google Scholar] [CrossRef]
- Bhardwaj, P.; Singh, O.; Pattanaik, D.R.; Klotzbach, P.J. Modulation of Bay of Bengal tropical cyclone activity by the Madden-Julian oscillation. Atmos. Res. 2019, 229, 23–38. [Google Scholar] [CrossRef]
- Bhardwaj, P.; Singh, O. Climatological characteristics of Bay of Bengal tropical cyclones: 1972–2017. Theor. Appl. Clim. 2020, 139, 615–629. [Google Scholar] [CrossRef]
- Sannigrahi, S.; Zhang, Q.; Joshi, P.K.; Sutton, P.C.; Keesstra, S.; Roy, P.S. Examining effects of climate change and land use dynamic on biophysical and economic values of ecosystem services of a natural reserve region. J. Clean. Produc. 2020, 257, 120424. [Google Scholar] [CrossRef]
- Sannigrahi, S.; Chakraborti, S.; Banerjee, A.; Rahmat, S.; Bhatt, S.; Jha, S.; Singh, L.; Paul, S.K.; Sen, S. Ecosystem service valuation of a natural reserve region for sustainable management of natural resources. Environ. Sustain. Indic. 2020, 5, 100014. [Google Scholar] [CrossRef]
- Ray, R.; Ganguly, D.; Chowdhury, C.; Dey, M.; Das, S.; Dutta, M.K.; Mandal, S.; Majumder, N.; De, T.K.; Mukhopadhyay, S.; et al. Carbon sequestration and annual increase of carbon stock in a mangrove forest. Atmos. Environ. 2011, 45, 5016–5024. [Google Scholar] [CrossRef]
- Hazra, S.; Bhadra, T.; Ghosh, S.; Barman, B.C. Assessing Environmental Flows for Indian Sundarban: A suggested approach. River Behav. Control 2015, 35, 65–74. [Google Scholar]
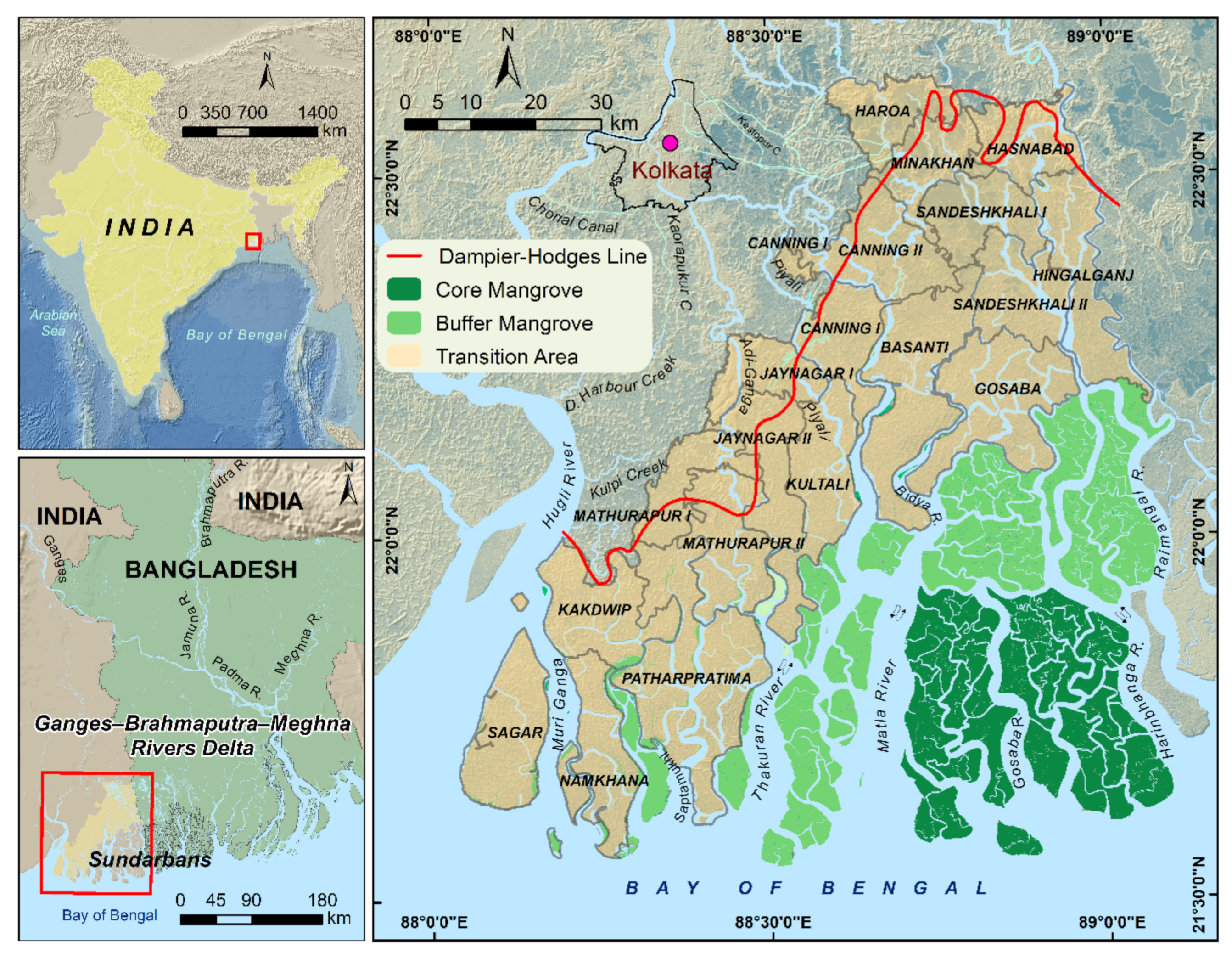

| Year | Core Area (km2) | Buffer Area (km2) | Transition Area (km2) | Total Area (km2) |
|---|---|---|---|---|
| 2000 | 903.2 | 1084.7 | 86.2 | 2074.1 |
| 2005 | 880.9 (−4.5) | 1067.6 (−3.4) | 100.3 (+2.8) | 2048.8 |
| 2010 | 869.9(−2.2) | 1053.2(−2.9) | 109.5 (+1.8) | 2032.6 |
| 2015 | 855.9 (−2.8) | 1046.6 (−1.3) | 128.7 (+3.8) | 2031.2 |
| 2020 | 845.2 (−2.2) | 1032.9 (−2.7) | 167.4 (+7.7) | 2045.4 |
| 2000–2020 (% change) | −6.42% | −4.78% | +94.20% | −1.38% |
| Island | 2000 (km2) | 2005 (km2) | 2010 (km2) | 2015 (km2) | 2020 (km2) | 2000 to 2020 | ||
|---|---|---|---|---|---|---|---|---|
| (km2) | (km2/yr) | |||||||
| 1 | Jammudwip | 3.9 | 3.8 | 3.7 | 3.4 | 2.6 | −1.3 | −0.1 |
| 2 | Dhanchi | 32.1 | 31.0 | 30.2 | 29.4 | 28.0 | −4.1 | −0.2 |
| 3 | Bulcherry | 22.1 | 20.4 | 19.0 | 18.2 | 17.2 | −4.9 | −0.2 |
| 4 | Chulkati | 38.5 | 37.5 | 35.2 | 34.9 | 32.9 | −5.6 | −0.3 |
| 5 | Dalhousi | 63.0 | 60.9 | 56.7 | 55.4 | 51.4 | −11.6 | −0.6 |
| 6 | Bhangaduni | 30.0 | 27.2 | 23.7 | 21.5 | 19.1 | −10.9 | −0.5 |
| 7 | Mechua | 18.3 | 17.6 | 16.6 | 16.2 | 15.7 | −2.6 | −0.1 |
| 8 | Chamta | 37.0 | 36.3 | 35.4 | 35.1 | 34.0 | −3.0 | −0.1 |
| 9 | Baghmara | 58.2 | 57.4 | 55.3 | 55.0 | 53.6 | −4.7 | −0.2 |
| Mangrove Genus | Area (km2) | % Area | ||
|---|---|---|---|---|
| 2000 | 2020 | 2000 | 2020 | |
| Aegialitis sp. | 159.4 | 144.3 | 7.7 | 7.1 |
| Avicennia sp. | 425.9 | 456.5 | 20.5 | 22.3 |
| Ceriops sp. | 459.3 | 537.4 | 22.1 | 26.3 |
| Excoecaria sp. | 253.0 | 278.2 | 12.2 | 13.6 |
| Phoenix sp. | 60.1 | 67.8 | 2.9 | 3.3 |
| Excoecaria & Ceriops sp. | 536.6 | 427.7 | 25.9 | 20.9 |
| Sonneratia & Heritiera sp. | 109.2 | 78.9 | 5.3 | 3.9 |
| Mixed Mangrove | 70.9 | 54.5 | 3.4 | 2.7 |
| Driver (and Type) | Cause | Scale and Duration | Potential Impact on the Mangroves | Strength of Evidence | Sources |
|---|---|---|---|---|---|
| Declining sediment supply (L) | Avulsion of river courses eastward with a consequent reduction in freshwater flow and sediment supply to the SBR | Regional, Centuries | Reduced resilience of mangrove to relative sea-level rise and increased coastal erosion | High based on greater losses in the Indian Sundarbans versus the Bangladesh Sundarbans | [65,66] |
| Salinization (L, C) | Avulsion of river courses eastward with a consequent reduction in freshwater inflow to the SBR, and increasing marine influence due to sea-level rise | Regional, Centuries | Salinity stress, loss/decline of low salinity mangroves, stunted growth and mangrove health deterioration, | High, based on an observed shift in mangrove community composition towards more salt-tolerant varieties | [65,67] |
| Relative sea-level rise (L, C) | Climate-induced sea-level rise and deltaic subsidence | Global and regional, decadal and longer | Loss of mangroves due to erosion and inundation | High based on tide gauge measurements across the region | [68,69] |
| Temperature rise (C) | Continuing warming of land and Sea | Global and regional, decadal and longer | Stress on mangrove germination and propagation, with potential adverse impacts on ecosystem functions | Hypothesized globally; insufficient observations in south Asia to confirm a causal relationship with mangrove health/loss | [70] |
| Change in rainfall (C) | Seasonal rainfall change and variability including in the monsoon | Regional, decadal and longer | Stress on germination and propagation, and potential for stress on established mangrove forest | Hypothesized globally; insufficient observations in south Asia to confirm a causal relationship with mangrove health/loss | [71] |
| Cyclones and Storm surges (S) | High wind speeds and extreme water levels | Local, Days to years | Abrupt loss of mangrove canopy cover, reduced leaf area, death along river margins due to storm/surge thrust | High, based on studies of past cyclones in the Sundarbans and also the remote sensing analysis presented in this paper | [72,73] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Samanta, S.; Hazra, S.; Mondal, P.P.; Chanda, A.; Giri, S.; French, J.R.; Nicholls, R.J. Assessment and Attribution of Mangrove Forest Changes in the Indian Sundarbans from 2000 to 2020. Remote Sens. 2021, 13, 4957. https://doi.org/10.3390/rs13244957
Samanta S, Hazra S, Mondal PP, Chanda A, Giri S, French JR, Nicholls RJ. Assessment and Attribution of Mangrove Forest Changes in the Indian Sundarbans from 2000 to 2020. Remote Sensing. 2021; 13(24):4957. https://doi.org/10.3390/rs13244957
Chicago/Turabian StyleSamanta, Sourav, Sugata Hazra, Partho P. Mondal, Abhra Chanda, Sandip Giri, Jon R. French, and Robert J. Nicholls. 2021. "Assessment and Attribution of Mangrove Forest Changes in the Indian Sundarbans from 2000 to 2020" Remote Sensing 13, no. 24: 4957. https://doi.org/10.3390/rs13244957
APA StyleSamanta, S., Hazra, S., Mondal, P. P., Chanda, A., Giri, S., French, J. R., & Nicholls, R. J. (2021). Assessment and Attribution of Mangrove Forest Changes in the Indian Sundarbans from 2000 to 2020. Remote Sensing, 13(24), 4957. https://doi.org/10.3390/rs13244957






