Fishing for Feral Cats in a Naturally Fragmented Rocky Landscape Using Movement Data
Abstract
:1. Introduction
2. Materials and Methods
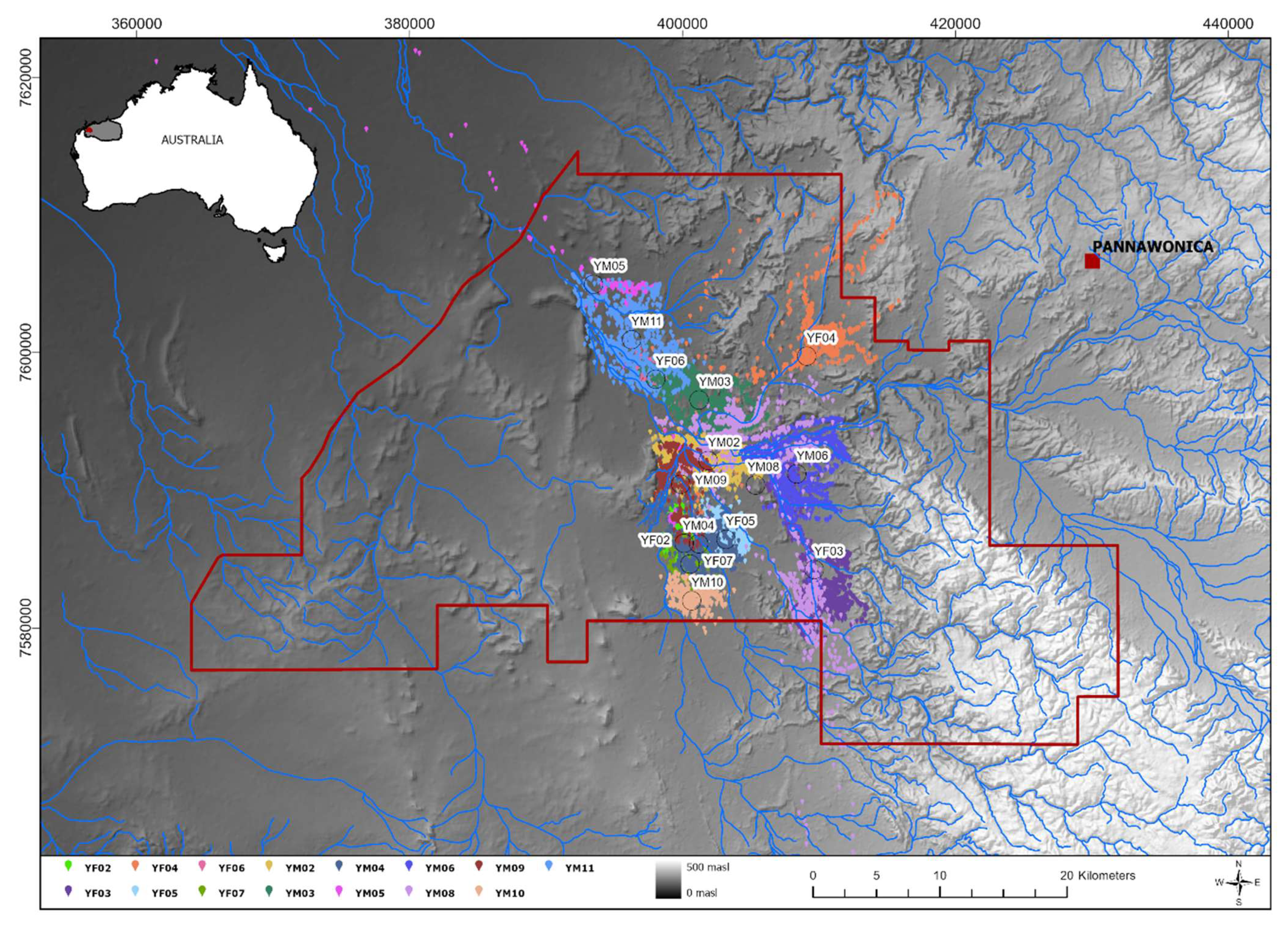
2.1. Study Site and Sample Collection
2.2. Spatial Layers
2.3. Home Range Analysis
2.4. Species Distribution Models
3. Results
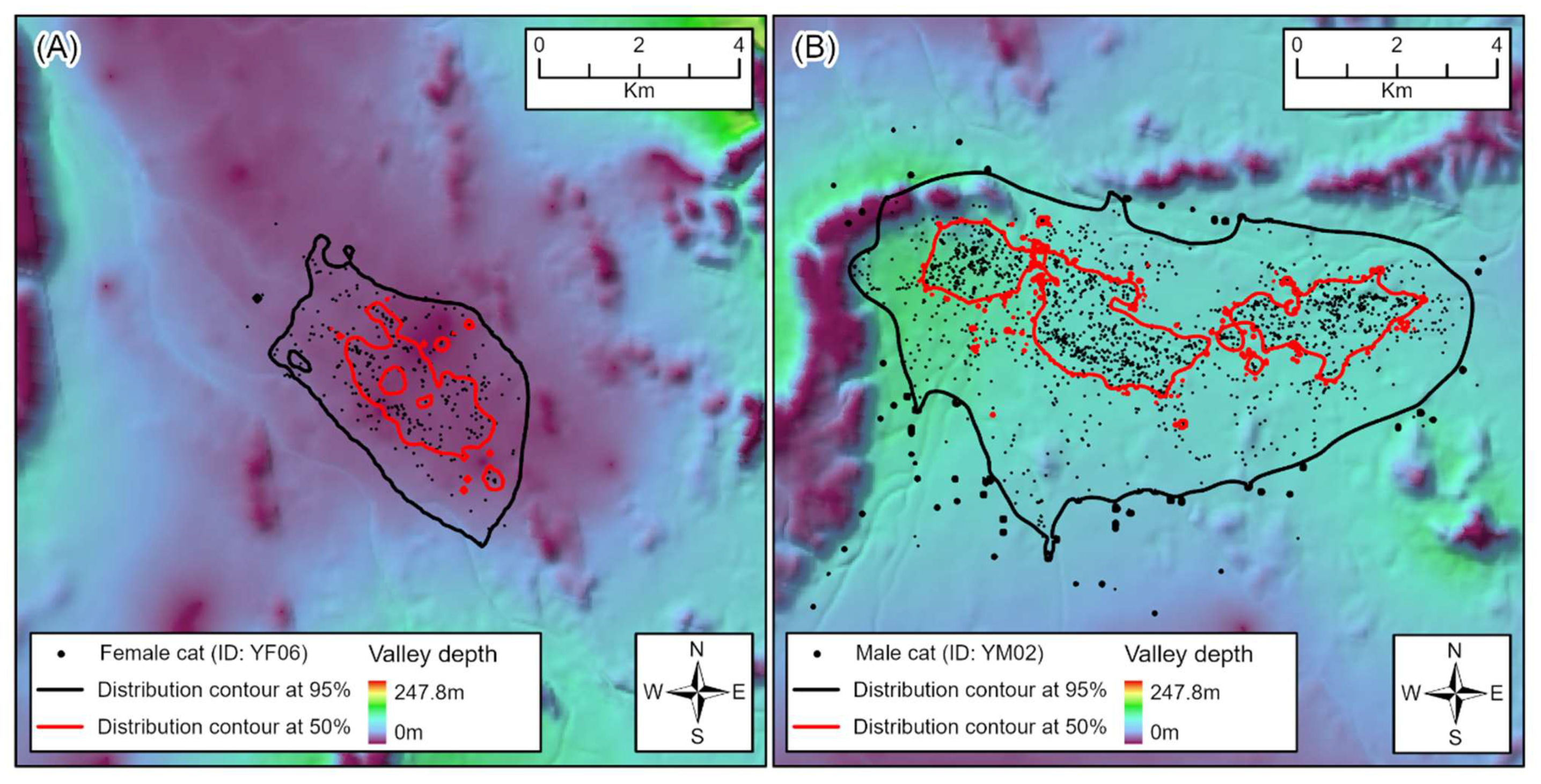
3.1. Home Ranges
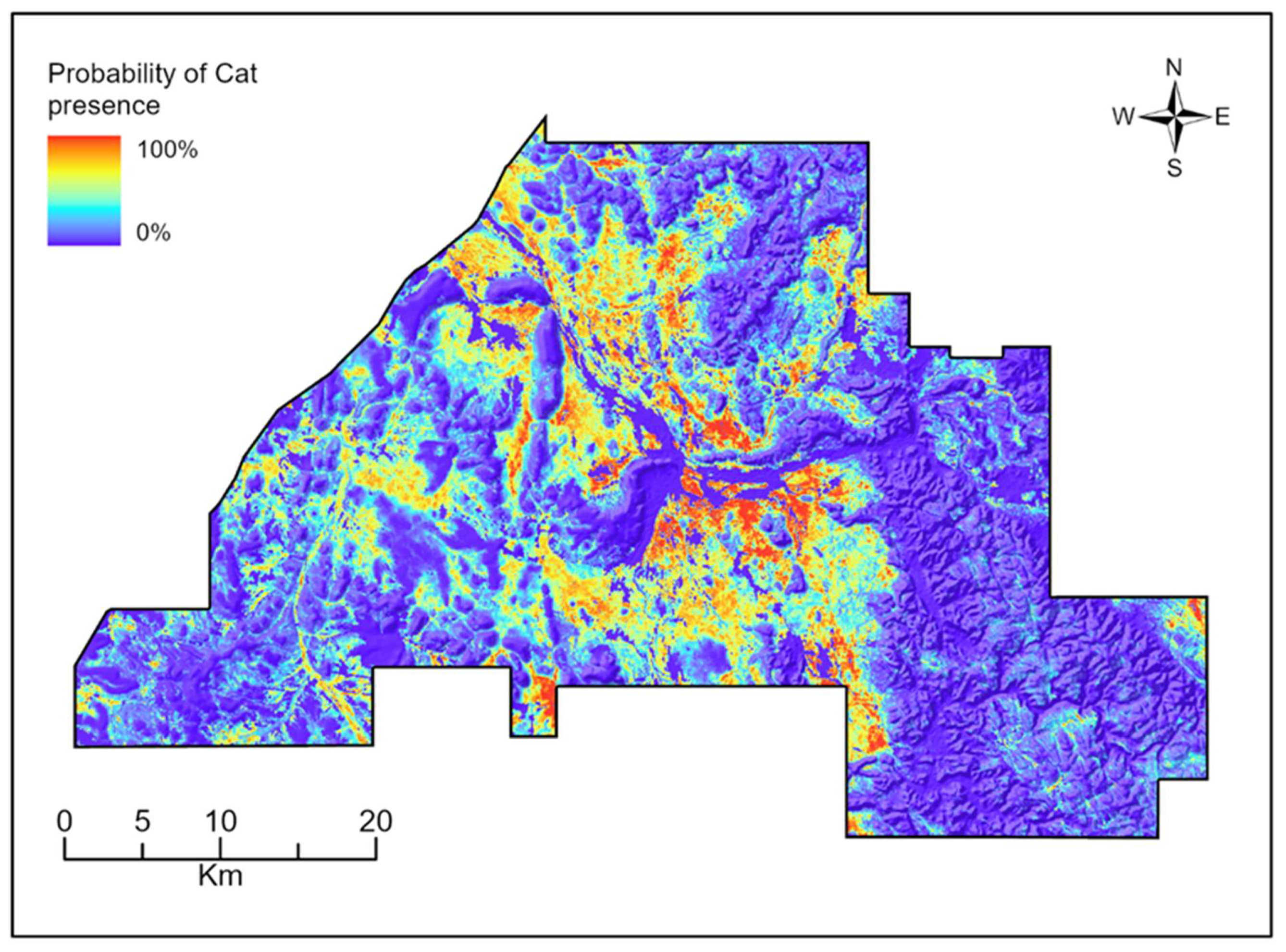
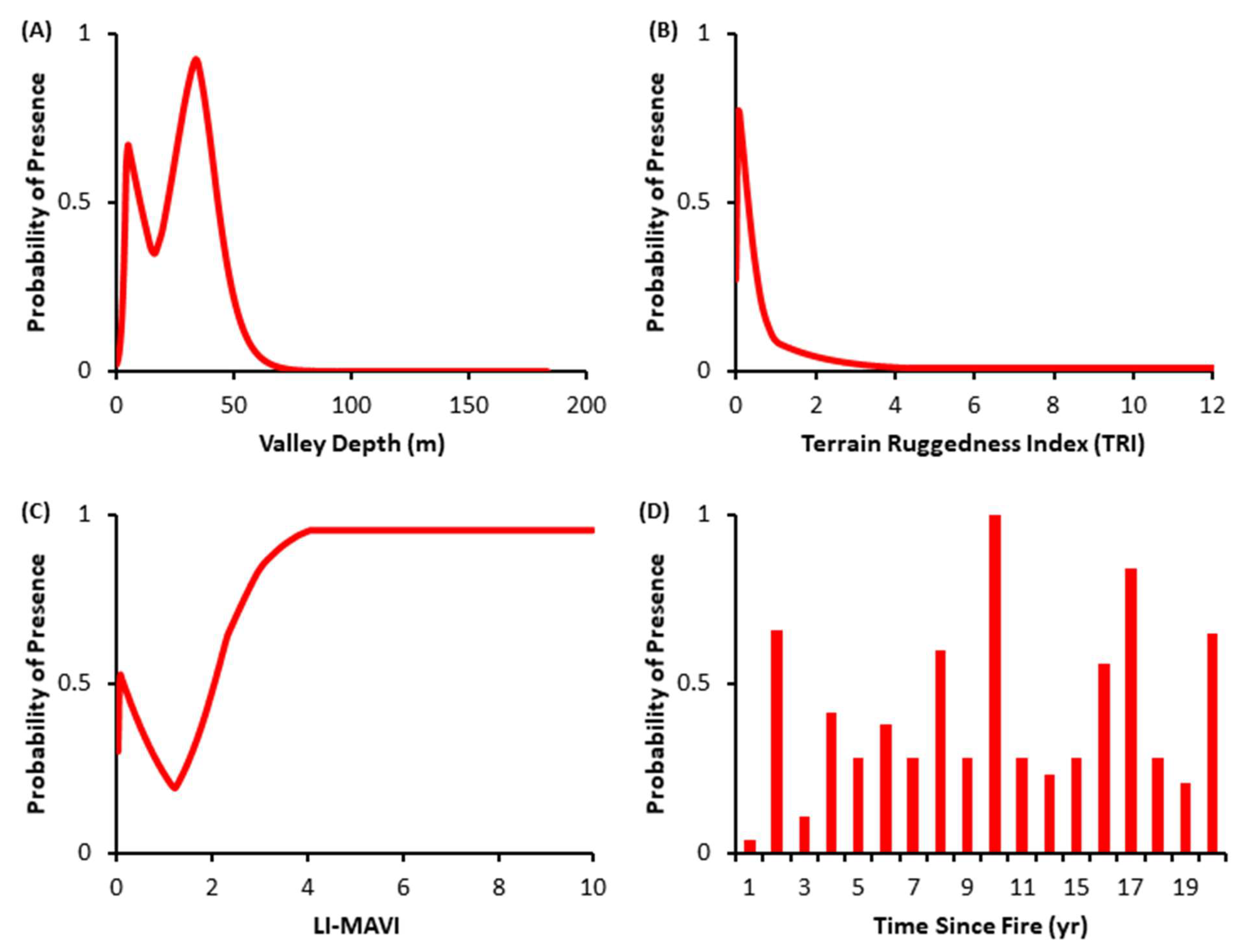
3.2. Species Distribution Models
4. Discussion
4.1. Home Ranges
4.2. Species Distribution Models
4.3. Conservation Implications
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pimentel, D. (Ed.) Non-Native Invasive Species of Arthopods and Plant Pathogens in the British Isles. In Biological Invasions; CRC: Boca Raton, FL, USA, 2002; pp. 151–158. [Google Scholar]
- Hoffmann, B.; Broadhurst, L. The economic cost of managing invasive species in Australia. NeoBiota 2016, 31, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Schartel, T.; Bayles, B.; Cooper, M.; Simmons, G.; Thomas, S.; Varela, L.; Daugherty, M. Reconstructing the European Grapevine Moth (Lepidoptera: Tortricidae), Invasion in California: Insights from a successful eradication. Ann. Entomol. Soc. Am. 2019, 112, 107–117. [Google Scholar] [CrossRef]
- Smart, A.; Tingley, R.; Phillips, B. Estimating the benefit of quarantine: Eradicating invasive cane toads from islands. NeoBiota 2020, 60, 117–136. [Google Scholar] [CrossRef]
- Fleming, P.; Ballard, G.; Reid, N.; Tracey, J. Invasive species and their impacts on agri-ecosystems: Issues and solutions for restoring ecosystem processes. Rangel. 2017, 39, 523–535. [Google Scholar] [CrossRef] [Green Version]
- Gause, G. The Struggle for Existence; Hafner: New York, NY, USA, 1934. [Google Scholar]
- Loss, S.R.; Marra, P.P. Population impacts of free-ranging domestic cats on mainland vertebrates. Front. Ecol. Environm. 2017, 15, 502–509. [Google Scholar] [CrossRef]
- Bengsen, A.; Algar, D.; Ballard, G.; Buckmaster, T.; Comer, S.; Fleming, P.; Friend, J.A.; Johnston, M.; McGregor, H.; Moseby, K.; et al. Feral cat home-range size varies predictably with landscape productivity and population density. J. Zool. 2016, 298, 112–120. [Google Scholar] [CrossRef]
- McGranahan, D.; Geaumont, B.; Spiess, W. Assessment of a livestock GPS collar based on an open-source datalogger informs best practices for logging intensity. Ecol. Evol. 2018, 8, 5649–5660. [Google Scholar] [CrossRef] [PubMed]
- Burt, W.H. Territoriality and Home Range Concepts as Applied to Mammals. J. Mammal. 1943, 24, 346–352. [Google Scholar] [CrossRef]
- Kranstauber, B. Modelling animal movement as Brownian bridges with covariates. Mov. Ecol. 2019, 7, 22. [Google Scholar] [CrossRef] [Green Version]
- Fleming, C.; Calabrese, J. A new kernel density estimator for accurate home-range and species-range area estimation. Methods Ecol. Evol. 2017, 8, 571–579. [Google Scholar] [CrossRef]
- Robley, A.; Reddiex, B.; Arthur, A.; Pech, R.; Forsyth, D. Interactions Between Feral Cats, Foxes, Native Carnivores, and Rabbits in Australia; Department of the Environment and Heritage: Canberra, Australia, 2004. [Google Scholar]
- Guisan, A.; Zimmerman, N. Predictive habitat distribution models in ecology. Ecol. Modell. 2000, 135, 147–186. [Google Scholar] [CrossRef]
- Guisan, A.; Tingley, R.; Baumgartner, J.; Naujokaitis-Lewis, I.; Sutcliffe, P.; Tulloch, A.; Regan, T.; Brotons, L.; McDonald-Madden, E.; Mantyka-Pringle, C. Predicting species distributions for conservation decisions. Ecol. Lett. 2013, 16, 1424–1435. [Google Scholar] [CrossRef]
- Rocchini, D.; Andreo, V.; Förster, M.; Garzon-Lopez, C.; Gutierrez, A.; Gillespie, T.; Hauffe, H.; He, K.; Kleinschmidt, B.; Mairota, P. Potential of remote sensing to predict species invasions: A modelling perspective. Prog. Phys. Geogr. 2015, 39, 283–309. [Google Scholar] [CrossRef] [Green Version]
- Legge, S.; Murphy, B.; McGregor, H.; Woinarski, J.; Augusteyn, J.; Ballard, G.; Baseler, M.; Buckmaster, T.; Dickman, C.; Doherty, T.; et al. Enumerating a continental-scale threat: How many feral cats are in Australia? Biol. Conserv. 2017, 206, 293–303. [Google Scholar] [CrossRef]
- Woinarski, J.C.; Legge, S.M.; Dickman, C.R. Cats in Australia: Companion and Killer; CSIRO Publishing: Clayton, Australia, 2019. [Google Scholar]
- Woinarski, J.; Burbidge, A.; Harrison, P. The Action Plan for Australian Mammals 2012; Commonwealth Scientific and Industrial Research Organisation Publising (CSIRO Publishing): Clayton, Australia, 2014. [Google Scholar]
- Lohr, C.A.; Algar, D. Managing feral cats through an adaptive framework in an arid landscape. Sci. Total. Environ. 2020, 720, 137631. [Google Scholar] [CrossRef]
- Palmer, R.; Anderson, H.; Richards, B.; Craig, M.D.; Gibson, L. Does aerial baiting for controlling feral cats in a heterogeneous landscape confer benefits to a threatened native meso-predator? PLoS ONE 2021, 16, e0251304. [Google Scholar] [CrossRef] [PubMed]
- McKenzie, N.; Burbidge, A.; Baynes, A.; Brereton, R.; Dickman, C.; Gordon, G.; Gibson, L.A.; Menkhorst, P.W.; Robinson, A.C.; Williams, M.R.; et al. Analysis of factors implicated in the recent decline of Australia’s mammal fauna. J. Biogeogr. 2007, 34, 597–611. [Google Scholar] [CrossRef]
- Carwardine, J.; Nicol, S.; van Leeuwen, S.; Walters, B.; Firn, J.; Reeson, A.; Martin, T.G.; Chadés, I. Priority Threat Management for Pilbara Species of Conservation Significance; CSIRO Ecosystems Sciences: Brisbane, Australia, 2014. [Google Scholar]
- Martin, G.R.; Twigg, L.E.; Robinson, D.J. Comparison of the diet of feral cats from rural and pastoral Western Australia. Wildl. Res. 1996, 23, 475–484. [Google Scholar] [CrossRef]
- Thackway, R.; Cresswell, I. An Interim Biogeographical Regionalisation for Australia; Australian Nature Conservation Agency: Canberra, Australia, 1995.
- Van Vreeswyk, A.; Leighton, K.; Payne, A.; Hennig, P. An Inventory and Condition Survey of the Pilbara Region, Western Australia; Technical Bulletin 92; Department of Agriculture and Food: Perth, Australia, 2004. [Google Scholar]
- Gallant, J.; Wilson, N.; Dowling, T.; Read, A.; Inskeep, C. SRTM-Derived 1 Second Digital Elevation Models Version 1.0.; Record 1; Geoscience Australia: Canberra, Australia, 2011. [Google Scholar]
- Conrad, O.; Bechtel, B.; Bock, M.; Dietrich, H.; Fischer, E.; Gerlitz, L.; Wehberg, J.; Wichmann, V.; Böhner, J. System for automated geoscientific analyses (SAGA) v. 2.1.4. Geosci. Model Dev. Discuss. 2015, 8, 1991–2007. [Google Scholar] [CrossRef] [Green Version]
- Zhu, G.; Ju, W.; Chen, J.; Liu, Y. A Novel Moisture Adjusted Vegetation Index (MAVI) to reduce background reflectance and topographical effects on LAI retrieval. PLoS ONE 2014, 9, e102560. [Google Scholar] [CrossRef] [Green Version]
- Van Leeuwen, W.J.; Davison, J.E.; Casady, G.M.; Marsh, S.E. Phenological characterization of desert sky island vegetation communities with remotely sensed and climate time series data. Remote Sens. 2010, 2, 388–415. [Google Scholar] [CrossRef] [Green Version]
- Department Biodiversity, Conservation and Attractions. Yarraloola Annual Desktop Fire Regime Monitoring: 2019; Department of Biodiversity, Conservation and Attractions: Perth, Australia, 2020. [Google Scholar]
- Byrne, M.; McCoy, J.; Hinton, J.; Chamberlain, M.; Collier, B.A. Using dynamic Brownian bridge movement modelling to measure temporal patterns of habitat selection. J. Anim. Ecol. 2014, 83, 1234–1243. [Google Scholar] [CrossRef]
- Kranstauber, B.; Kays, R.; LaPoint, S.D.; Wikelski, M.; Safi, K. A dynamic Brownian bridge movement model to estimate utilization distributions for heterogeneous animal movement. J. Anim. Ecol. 2012, 81, 738–746. [Google Scholar] [CrossRef]
- Kranstauber, B.; Smolla, M.; Scharf, A.K. Move: Visualizing and Analyzing Animal Track Data. R Package Version 4.0.4. 2020. Available online: https://cran.r-project.org/web/packages/move/move.pdf (accessed on 17 July 2020).
- Clark, J.; Wang, Y.; August, P. Assessing current and projected suitable habitats for tree-of-heaven along the Appalachian Trail. Philos. Trans. R. Soc. B Biol. Sci. 2014, 369, 20130192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baldwin, R. Use of Maximum Entropy Modeling in Wildlife Research. Entropy 2009, 11, 854–866. [Google Scholar] [CrossRef]
- Robinson, T.; van Klinken, R.; Metternicht, G. Comparison of alternative strategies for invasive species distribution modeling. Ecol. Modell. 2010, 221, 2261–2269. [Google Scholar] [CrossRef] [Green Version]
- West, A.; Kumar, S.; Brown, C.; Stohlgren, T.; Bromberg, J. Field validation of an invasive species Maxent model. Ecol. Inform. 2016, 36, 126–134. [Google Scholar] [CrossRef] [Green Version]
- Phillips, S. A Brief Tutorial on Maxent. 2017. Available online: https://biodiversityinformatics.amnh.org/open_source/maxent/Maxent_tutorial2017.pdf (accessed on 16 June 2020).
- Fielding, A.; Bell, J. A review of methods for the assessment of prediction errors in conservation presence/absence models. Environ. Conserv. 1997, 24, 38–49. [Google Scholar] [CrossRef]
- Warren, D.; Seifert, S. Ecological niche modeling in Maxent: The importance of model complexity and the performance of model selection criteria. Ecol. Appl. 2011, 21, 335–342. [Google Scholar] [CrossRef] [Green Version]
- Kays, R.; Dunn, R.R.; Parsons, A.W.; Mcdonald, B.; Perkins, T.; Powers, S.A.; Shell, L.; McDonald, J.L.; Cole, H.; Kikillus, H.; et al. The small home ranges and large local ecological impacts of pet cats. Anim. Conserv. 2020, 23, 516–523. [Google Scholar] [CrossRef]
- Spotte, S. Free-Ranging Cats: Behavior, Ecology, Management; John Wiley & Sons: Chicester, UK, 2014. [Google Scholar]
- McGregor, H.; Cliff, H.; Kanowski, J. Habitat preference for fire scars by feral cats in Cape York Peninsula, Australia. Wildl. Res. 2016, 43, 623–633. [Google Scholar] [CrossRef]
- Stobo-Wilson, A.; Stokeld, D.; Einoder, L.; Davies, H.; Fisher, A.; Hill, B.M.; Mahney, T.; Murphy, B.P.; Stevens, A.; Woinarksi, C.Z.; et al. Habitat structural complexity explains patterns of feral cat and dingo occurrence in monsoonal Australia. Divers. Distrib. 2020, 26, 832–842. [Google Scholar] [CrossRef]
- Moseby, K.; Stott, J.; Crisp, H. Movement patterns of feral predators in an arid environment–implications for control through poison baiting. Wildl. Res. 2009, 36, 422–435. [Google Scholar] [CrossRef]
- Fancourt, B.A.; Cremasco, P.; Wilson, C.; Gentle, M.N. Do introduced apex predators suppress introduced mesopredators? A multiscale spatiotemporal study of dingoes and feral cats in Australia suggests not. J. Appl. Ecol. 2019, 56, 2584–2595. [Google Scholar] [CrossRef]
- Hohnen, R.; Tuft, K.; McGregor, H.; Legge, S.; Radford, I.; Johnson, C. Occupancy of the invasive feral cat varies with habitat complexity. PLoS ONE 2016, 11, e0152520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hernandez-Santin, L.; Goldizen, A.W.; Fisher, D.O. Introduced predators and habitat structure influence range contraction of an endangered native predator, the northern quoll. Biol. Cons. 2016, 203, 160–167. [Google Scholar] [CrossRef]
- Moore, H.A.; Michael, D.R.; Ritchie, E.G.; Dunlop, J.A.; Valentine, L.E.; Hobbs, R.J.; Nimmo, D.G. A rocky heart in a spinifex sea: Occurrence of an endangered marsupial predator is multiscale dependent in naturally fragmented landscapes. Landsc. Ecol. 2021, 36, 1359–1376. [Google Scholar] [CrossRef]
- Recio, M.; Seddon, P.; Moore, A. Niche and movement models identify corridors of introduced feral cats infringing ecologically sensitive areas in New Zealand. Biol. Conserv. 2015, 192, 48–56. [Google Scholar] [CrossRef]
- McGregor, H.; Legge, S.; Jones, M.; Johnson, C. Feral cats are better killers in open habitats, revealed by animal-borne video. PLoS ONE 2015, 10, e0133915. [Google Scholar] [CrossRef] [Green Version]
- Comer, S.; Speldewinde, P.; Tiller, C.; Clausen, L.; Pinder, J.; Cowen, S.; Algar, D. Evaluating the efficacy of a landscape scale feral cat control program using camera traps and occupancy models. Sci. Rep. 2018, 8, 5335. [Google Scholar] [CrossRef] [Green Version]
- Allsop, S.E.; Dundas, S.J.; Adams, P.J.; Kreplins, T.L.; Bateman, P.W.; Fleming, P.A. Reduced efficacy of baiting programs for invasive species: Some mechanisms and management implications. Pac. Conserv. Biol. 2017, 23, 240–257. [Google Scholar] [CrossRef] [Green Version]

| Cat ID | Sex | Body Mass (g) | Area of Estimated Home Range (km2) | |
|---|---|---|---|---|
| 50% | 95% | |||
| YF02 |  | 1700 | 0.59 | 3.91 |
| YF03 |  | 3390 | 6.41 | 46.55 |
| YF04 |  | 3120 | 1.61 | 25.81 |
| YF05 |  | 2630 | 1.53 | 7.83 |
| YF06 |  | 2930 | 2.04 | 7.85 |
| YF07 |  | 4040 | 1.08 | 7.98 |
| YM02 |  | 4170 | 5.63 | 25.70 |
| YM03 |  | 4200 | 13.07 | 69.79 |
| YM04 |  | 5000 | 3.69 | 27.91 |
| YM05 |  | 2050 | 0.75 | 8.63 |
| YM06 |  | 4630 | 10.62 | 47.21 |
| YM08 |  | 3080 | 5.99 | 66.01 |
| YM09 |  | 4400 | 2.47 | 15.99 |
| YM10 |  | 4075 | 2.10 | 11.04 |
| YM11 |  | 4535 | 4.27 | 37.64 |
| Mean | 4.12 | 27.32 | ||
| Median | 2.47 | 25.70 | ||
| Std Dev | 3.69 | 21.72 | ||
| Spatial Layer | Variable Importance (%) | |||
|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | Model 4 | |
| Valley depth | 47.0 | 50.1 | 53.0 | 100 |
| Ruggedness | 36.1 | 38.7 | 47.0 | |
| Time since fire | 10.3 | 11.2 | ||
| LI-MAVI | 6.6 | |||
| AUC | 0.90 | 0.88 | 0.85 | 0.74 |
| AICc | 23,260 | 23,557 | 23,599 | 24,778 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Williamson, S.D.; van Dongen, R.; Trotter, L.; Palmer, R.; Robinson, T.P. Fishing for Feral Cats in a Naturally Fragmented Rocky Landscape Using Movement Data. Remote Sens. 2021, 13, 4925. https://doi.org/10.3390/rs13234925
Williamson SD, van Dongen R, Trotter L, Palmer R, Robinson TP. Fishing for Feral Cats in a Naturally Fragmented Rocky Landscape Using Movement Data. Remote Sensing. 2021; 13(23):4925. https://doi.org/10.3390/rs13234925
Chicago/Turabian StyleWilliamson, Sandra D., Richard van Dongen, Lewis Trotter, Russell Palmer, and Todd P. Robinson. 2021. "Fishing for Feral Cats in a Naturally Fragmented Rocky Landscape Using Movement Data" Remote Sensing 13, no. 23: 4925. https://doi.org/10.3390/rs13234925
APA StyleWilliamson, S. D., van Dongen, R., Trotter, L., Palmer, R., & Robinson, T. P. (2021). Fishing for Feral Cats in a Naturally Fragmented Rocky Landscape Using Movement Data. Remote Sensing, 13(23), 4925. https://doi.org/10.3390/rs13234925







