Abstract
The effects and consequences of global warming on the productivity of the Patagonian forest are still unknown. The use of Unmanned Aerial Vehicles (UAV) promotes new knowledge of the most pristine and unknown sub-antarctic forests located in Chilean Patagonia. This work presents an initial approach to spatialize biochemicals over the Patagonian forests using ultra-high spatial resolution imagery acquired from UAVs equipped with a multispectral (visible, near-infrared, and thermal) sensor. The images were obtained in multiple flights over the Cerro Castillo National Park (Aysén Region, Chile), and several Vegetation Indices (VIs) were estimated. Leaves of Nothofagus pumilio (Poepp. et Endl.) Krasser (Nothofagaceae) individuals were extracted after the flights and were then used to determine the biochemicals traits of chlorophylls (Chl-a and Chl-b) and carotenoids pigments, as well as the total phenolic content (TPC), total flavonoids content (TFC), and the DPPH radical scavenging assay. Their relationships with multiple VIs was analyzed in order to assess the spatiality of the biochemicals traits in the forest during it most productive phenological stage. Results showed high correlations for the biochemical traits pigments (R2 > 0.75) with the indices DVI, MCARI, and MSAVI1 as the best performing indices, while further spectral availability is needed for significant correlations with biochemicals traits related to the antioxidant capacity. Spatialization of the biochemical traits within UAV imagery was also performed evaluating their representation in the forest. This work allowed us to identify the different spectral behavior of the N. pumilio species, its relation to biochemical traits, and their spatialization, thus presenting the first step to developing a monitoring protocol for the evaluation of the Patagonian forests under the current global warming scenarios in the region.
1. Introduction
The Aysén Region has the most wilderness-dense area of Chile. Including most of the sub-Antarctic forest of Patagonia, covering almost 5 million hectares with a high degree of biodiversity and endemism, this region represents a highly valuable ecosystem and landscape [1,2,3]. Forty-one percent of its surface is protected by national parks and reservoirs, however, it has been threatened by an increase in surface temperatures, tree mortality, massive defoliation, and treeline variation [4,5,6,7,8,9]. Moreover, rising temperatures and decreasing precipitation are still expected in the region due to climate change [10].
The sub-Antarctic forest in the Aysén region extends in a vast uninhabited area with major inaccessibility. Consequently, assessing the forest physiology and its response to climate change implies significant efforts. These are conventionally explained through the determination of biochemicals traits, such as chlorophyll (Chl), carotenoids (Car), or phenols content, that respond relatively quickly to environmental changes (i.e., temperature, solar radiation, precipitation) and site conditions (i.e., soil nutrients, water availability) [11,12,13]. Although useful for initial monitoring, the laboratory analysis of biochemicals is time-consuming and destructive, limiting, even more, the development of monitoring protocols in the region. Therefore, remote sensing techniques play a key role in this context since they could provide precise and non-destructive insights about biochemical traits in largely inaccessible areas like the sub-Antarctic forest, with no similar work released to date in the region. This might be related to the high cloud coverage in the Aysén region, which makes satellite acquisition infrequent. Unmanned Aerial Vehicles (UAVs) have the advantage of getting information under clouds and their reasonable spatial coverage makes them an innovative alternative for under-studied areas thanks to their accessibility [14,15,16,17,18,19,20]. Moreover, they are practical and have innovated research on applications for forests and their traits across the globe, therefore, we present UAVs as the potential main tool for the development of new approaches to monitoring the sub-Antarctic forest and its resilience to climate change.
The ability of remote sensing approaches to estimate biochemical traits has been well established especially in tropical forests [21,22,23]. The quantification of the traits is possible through statistical relationships (regressions) between limited field traits observations and remote sensing imagery; however, they have low transferability as they are site, species, and even time specific. In that sense, relationships between sub-Antarctic forests and their traits still need to be established. Plant pigments such as Chl-a and Chl-b are the most studied traits [24,25,26,27,28], while Car and Anthocyanins pigments have been less studied [29,30,31]. Other traits described through remote sensing include Polyphenols [32], Leaf Water Content [33,34], Leaf Mass per Area, Lignin and Cellulose [35], and multiple plant macronutrients (N, P, K, Mg, Ca) [36,37].
UAVs offer innovative alternatives with great development potential to remote sensing studies [38]. Often, the spatial resolution of the satellite’s sensors is such that a pixel or sampled area contains multiple crowns or a mixture of different trees with the ground and other covers. In the case of UAVs, the enhanced cm-scale spatial detail reduces part of the common issues arising in the remote sensing satellite’s studies, such as the separation of soil with canopy and the direct consideration of structure, background, and shadows [39,40]. Moreover, they could make possible the consideration of within-micro plot variability, as single trees crown, or even different zones of it. Despite this, the quantification of traits at fine spatial resolution still needs the development of methods that take full advantage of it [40].
Thus, the main objective of this work is to perform the first quantification of biochemical traits at ultra-high spatial resolution in the Patagonian sub-Antarctic forest. Combining UAV multispectral imagery enhanced with several vegetation indices and laboratory measurements of biochemical pigments and antioxidants, we assess the spatiality of the biochemicals in the forest and answering the following in the process: (i) How precise and accurate can biochemicals be estimated from UAV imagery at ultra-high resolution in Patagonia? (ii) What are the most suitable spectral combinations for monitoring each biochemical? and (iii) What are some of the advantages that UAVs offer in the Aysén Region context for future monitoring protocols? These questions will be highlighted in our results to contribute to the understanding of the Patagonian sub-Antarctic forest and the development of monitoring methods for its acclimation to new global scenarios.
2. Study Area and Methods
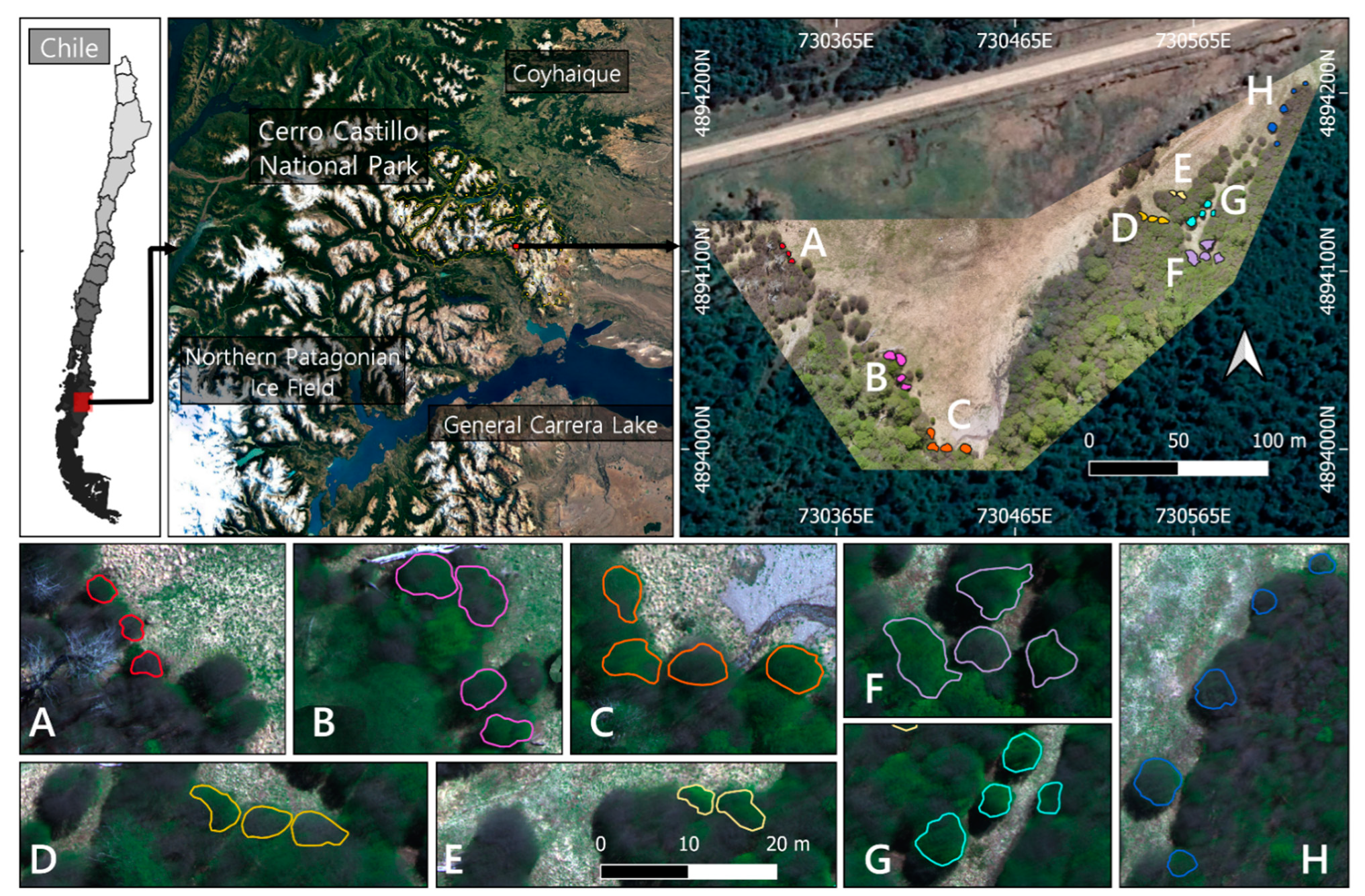
The study was carried out in the Cerro Castillo National Park (46°41′S 72°12′W) at an altitude of 1060 m (see Figure 1). The area belongs to the supra-temperate belt with humid climatic conditions [41,42]. The mean annual precipitation is 1100 mm, with a monthly mean of 250 mm in the growing period between December-March [43]. The mean temperature for the warmest month (January) is 9.1 °C, while the coldest drops to −1.55 °C (July) [44]. The vegetation found here is a Nothofagus pumilio (Poepp. et Endl.) Krasser (Nothofagaceae) monodominance forest, with a mean tree height of 16.9 (±2.8) m and a density of 740 trees (±85) per ha [8]. N. pumilio is a dominant subalpine and treeline monoecious tree, widely distributed from 35° S in central Chile to the southernmost point in South America at 55° S [45]. It covers a diverse variety of climates, showing significant variations in morphological and physiological traits across its distribution due to adaptation and acclimation [6,46,47,48], reaching ages over 400 years old [49,50]. It is deciduous with alternating obtuse coriaceous leaves 2–3 cm long [51]. During the fall, the leaves vary from dark green to red-purple hues until they fall off with the first snowfall [45].
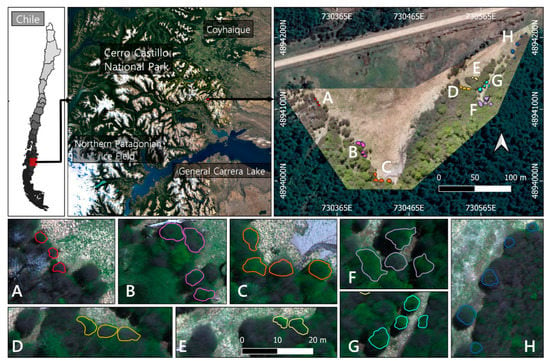
Figure 1.
Study area in Cerro Castillo National Park. The different sampling sites are specified with an approximate area of recollection (A–H). (D,E) are two sample sites representing direct and indirect sunlight exposure, respectively.
2.1. Sample Collection and Laboratory Analysis
Eight sampling sites were defined (see Figure 1) in which only trees above 2 m height with crowns in full exposure to sunlight were selected as samples. The collection of leaves was performed in November (Spring) 2019 with hedge shears fitted with 1.5 m extensors. Only mature branches were taken, then leaves were pulled off and sealed in polyethylene bags to be transported to the laboratory for 1 h and frozen until processing within 2–3 days. Leaves in their whole form and not overly damaged by epiphylls or signs of disease were also included in the collections, performing an analysis of six biochemicals including the pigments Chlorophyll-a (Chl-a), Chlorophyll-b (Chl-b), and Carotenoids (Car), the Phenolic and Flavonoid content, and the total non-enzymatic antioxidant capacity through the free radical scavenging activity estimation.
Chlorophylls and total carotenoids were determined following the method described by Lichtenthaler [52,53] using ethanol as a solvent with some modification. Between 1.2–1.4 g of frozen leaves (at −80 °C) were weighed and macerated in ethanol until all pigments were extracted, the solvent was filtered in the dark and transferred to a 50 mL volumetric flask, completing with ethanol. The solution was diluted four times and the absorbance at 470, 649, and 664 nm was measured. Chlorophyll and Carotenoid content were calculated as micrograms of pigments per milligram of plant material (µg/mg PM).
For the next biochemicals traits, extracts were prepared from dry foliar material, adapted from [54,55,56]. Leaves were dried at 40 °C on a stove and powdered. Foliar extracts were prepared by mixing 10 g of the powdered material with 60 mL of ethanol at 25 °C, shaking gently for 18 h in the dark, then filtered and evaporated in vacuo. Extracts obtained were stored at −20 °C for subsequent analysis. Working solutions were prepared by dissolving the dry extract in ethanol at a final concentration of 1 mg/mL.
The total phenolic content (TPC) determination was based on the method described by [54,55] with some modifications. The mixture of 10 % (v/v) Folin-Ciocalteu reagent with the leaves extract dissolved in ethanol was incubated for 5 min at 25 °C. After that, 350 mM of sodium carbonate was added. The final mixture was incubated for 2 h at 25 °C and the absorbance was determined at 765 nm. A calibration curve of gallic acid was made using different gallic acid solutions (15–75 µg/mL). Results were expressed as milligrams of gallic acid equivalents (GAE) per gram of dry leaf extract (mg GAE/g DLE). Values were reported as means ± standard deviations.
Total flavonoid content (TFC) was determined by using the method described by [56] with some modifications. The leaf ethanolic extract, or (+)-catechin standard solution, or ethanol (blank solution) was added to each of the 96-well microplates, followed by sodium nitrite solution (4.54 mg/mL). The mixture was incubated for 5 min at 25 °C. Then, an aluminum chloride solution (50 g/L) was added and the mixture was incubated for 6 min. After that, sodium hydroxide solution (0.5 M) was added. The plate was stirred vigorously for 2 min and the absorbance was measured immediately against the blank at 510 nm using a spectrophotometer. The standard curve was prepared with (+)-catechin (25–100 µg/mL) and results were expressed as milligrams of catechin equivalents (CE) per gram of dry leaf extract (mg CE/g DLE).
The free radical scavenging activity was determined by using the 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical antioxidant assay, following the method described by [57,58] with some modifications. The ethanolic extract solutions were mixed with a freshly prepared DPPH solution. The mixture was incubated for 30 min at 25 °C and the absorbance was measured at 517 nm. Trolox was used as a positive control. DPPH standard curve (from 20 to 140 µM) was made to quantify its remaining concentration in all experiments. The percentage of DPPH scavenging activity was determined by the equation:
and the IC50 of each extract was determined as the concentration that corresponds to 50% of the DPPH scavenging activity.
2.2. UAV Data Acquisition
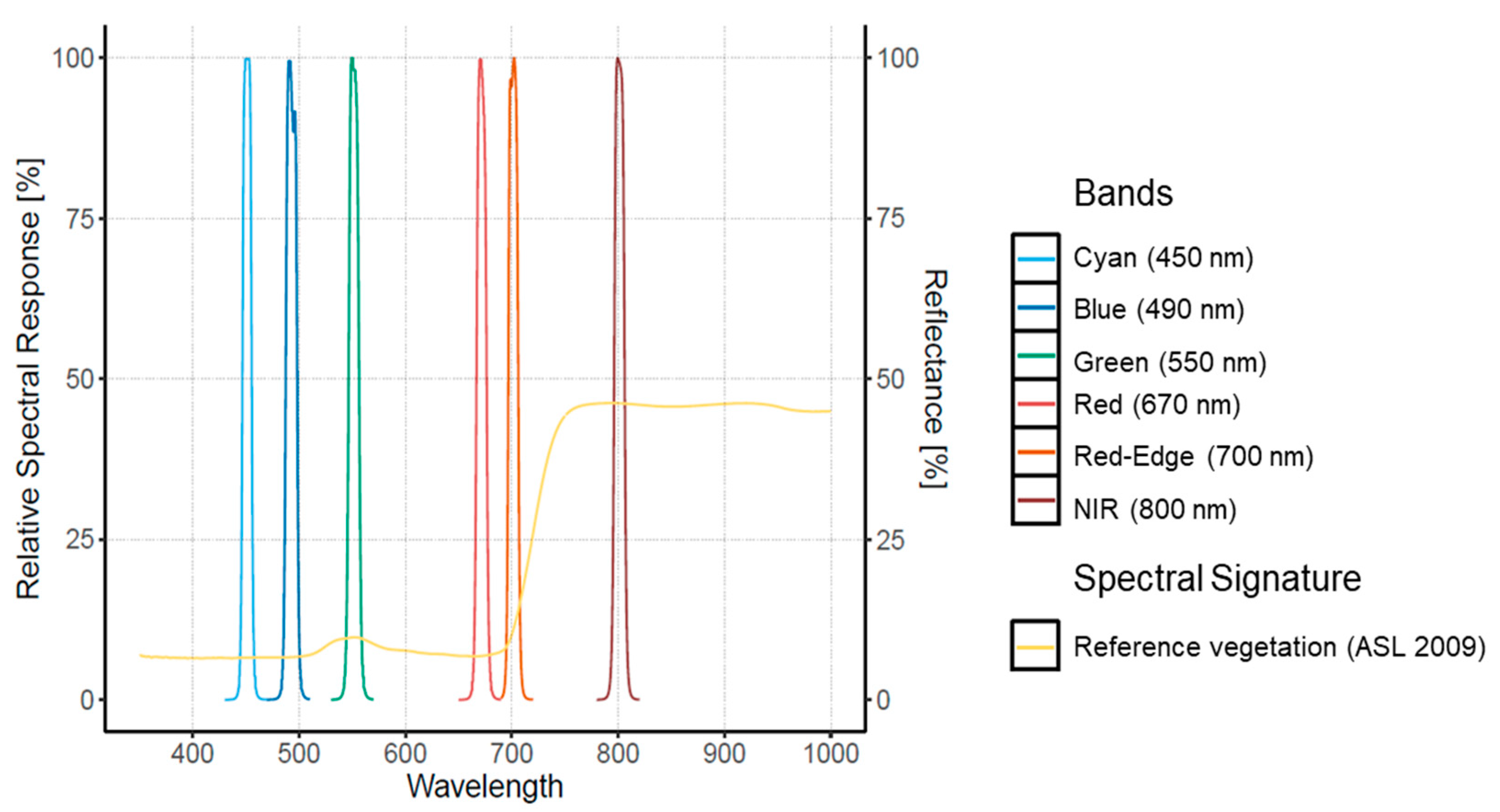
Two cameras were used for this study, embedded separately (different flights) on the quadcopter platform md4-1000 (from microdrones GmbdH, Siegen, Germany). First, the RGB camera Sony Alpha A6300 was set to auto ISO mode, with a speed of 1/1000 s to acquire 6000 × 4000 pixel images in TIFF format. The second is the Macaw (Tetracam, Inc., Chatsworth, CA, USA) multispectral camera with an array of 6 CMOS sensors with a size of 1.3 mega-pixels and an additional thermal sensor FLIR Tau2. The filters for the Macaw were centered at 450, 490, 550, 670, 700, and 800 nm with a spectral resolution of 10 nm and the thermal with the range 7.5–13.5 um (see Figure 2).
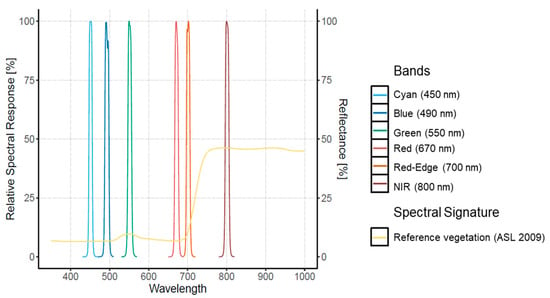
Figure 2.
Relative Spectral Response for the Tetracam Macaw multispectral camera. An example of the spectral signature for pine vegetation trees derived from Aster Spectral Library (ASL 2009) is included from Baldridge et al. (2009).
A total of six flights (three per camera) were conducted during the Spring of 2019, ensuring 80% overlap across and along the route at an altitude of 100 m, corresponding to a spatial resolution of 1.7 cm in the RGB, 3.93 cm in the multispectral and 5.15 cm in the thermal imagery. All flights were performed around solar noon with a clear blue sky and 42.45° and 56.47° azimuth and elevation angles, respectively. Each flight took around 12–15 min, with an extra 10 min for switching out the camera, resulting in a mission of less than 2 h, during which we assume the lighting was stable.
2.3. Imagery Radiometric Calibration and Orthomosaic
Radiometric calibration was carried out using 30 × 30 cm2 Spectralon® reflectance coating plates (Labsphere, Inc. NH, USA, www.labsphere.com, accessed on 25 March 2021) exposed for 30 min before the first flight. The temperature of each plate was measured with a non-contact infrared thermometer (Optris MS Plus, www.optris.es (accessed on 25 March 2021) in the middle of each flight, together with the capture of the absolute irradiance using the Ocean Optics Jaz spectrometer (www.oceaninsight.com, accessed on 25 March 2021) for atmospheric correction. Digital pixels were converted into a measure of reflectance, using a linear interpolation of the reference values from the readings of the panels and spectrometer for each flight.
The mosaicking and orthorectification were achieved with the dense image matching technology of the Pix4Dmapper software (Pix4D SA, www.pix4d.com, accessed on 25 March 2021), in which we integrated the imagery and their GPS references to obtain the Digital Surface Model (DSM) and RGB, thermal and multispectral orthomosaics. Around 70 Ground Control Points (GCP’s) captured in the field were used, considering the position of the road, calibration plates, and specific trees for georectification.
2.4. Vegetation Indices
The reflectance of vegetation canopies at coarser resolutions depends on the radiative properties of leaves, non-photosynthetic canopy elements, and their spatial organization [59,60,61]. The proportional effect of these properties is often generalized with a leaf area index (LAI) value, however, alone it cannot fully describe the effects of canopy structure [62,63]. For this work we assume that, at ultra-high spatial resolution, a direct approach through filtering of the image can allow the exclusive consideration of the leaf’s radiative properties. This is due to the direct differentiation of the non-photosynthetic elements and the spatial organization of the canopy. In fact, at the leaf level, spectra can be linked to biochemical traits directly [64,65], and in some cases, vegetation indices linked with simple linear algebra performed better than complex reflectance derivation methods [25].
Leaf reflectance spectra are determined by chemical and morphological characteristics of the surface of organs or leaves [38], and it changes according to plant type and water content within tissues [66]. The principal applications in remote sensing studies consider the visible region (400–700 nm, VIS), known for the absorption of photosynthetic pigments (i.e., Chlorophyll and Carotenoids) with peaks at blue (400–500 nm) and red (620–680 nm) wavelengths. The Red Edge (700–740 nm), whose position and slope is originated by variation in multiple biochemical traits [67], while the near-infrared (700–1300 nm, NIR) is often related to leaf structure. The shortwave infrared region (1300–2500 nm, SWIR) is considered in several hyperspectral studies and is related to water, protein, and antioxidants (i.e., phenols and flavonoids) [32,68]. Hence, the use of individual bands or the combination of them (as vegetation indices) are often used as a proxy to estimate different plant biochemicals depending on the objective, however, it is considered a complex task since multiple factors might affect the same spectra [38].
The multispectral imagery obtained from the Macaw camera was used to calculate 30 vegetation indices (VIs) selected from literature based on their usefulness for the biochemicals analyzed and the band availability of the sensor (see Figure 2). VIs selected that were originally designed with wavebands different from the Macaw sensor, were reformulated with the closest band available. Most of the VIs are expressed as ratios of two or three wavebands that combine visible light and/or NIR, that indeed, minimize the influence of external factors such as possible variations in the illumination condition during the flights [69]. For example, the vegetation index NDVI is one of the most employed indices for UAVs application [15], it contrasts the reflectivity of the NIR and Red bands, which correspond to a high and low reflectance for chlorophylls, so it is related to estimate LAI, biomass, and greenness in general. Its formulation (see Appendix A, Table A1) allows NDVI to enhance the contrast of NIR and Red reflectivity. Therefore, it is a nonlinear extension of NIR and Red ratios, detailing lower values and reaching saturation more easily, as higher values are suppressed [34]. Other vegetation indices, such as MCARI (modified chlorophyll absorption ratio index), are not necessarily related to NIR bands. They were developed through several measurements at laboratory and further improvement by other author’s modifications (i.e., CARI, MCARI, TCARI). Therefore, every index has been established with a specific purpose derived from theory and practice, but at ultra-high resolution most of the conditions change, though they must be re-evaluated. More detailed information on the different VIs used in this work (Appendix A, Table A1) can be found in [38].
2.5. Extracting Values from Sites
In order to separate the leaves from the non-photosynthetic canopy, shadow, and other background components, pixels with NDVI values greater than 0,4 (green pixels) and DSM higher than 2 m were selected as the reference values in the imagery. All crowns presented heights that exceeded the 2 m minimum criteria, ranging from 3 to 30 m in the inner part of the forest. The selection criteria allowed most of the shadows, branches, and bare soil to be eliminated and was performed using the software QGIS Geographic Information System (version 3.12.2, www.qgis.org, accessed on 25 March 2021). Using the GPS points as reference, a vector cape was generated, encircling separately each tree considered for the sample collection. A total of 29 tree crowns were delineated and separated into eight zones (see Figure 1) that corresponded to each laboratory analysis. Lastly, surface reflectance values from the green pixels of each sampling site were extracted, calculating the mean and standard deviation of the vegetation spectra at tree and sampling site level for further statistical analysis.
2.6. Statistical Analysis
The purpose of this analysis was to assess the performance of each band and vegetation index in the estimation of the biochemicals determined in laboratory. All of the imagery data was filtered according to previous steps, and only the extracted values were considered. Quantitative linkage between biochemicals traits and every spectral band and vegetation index was developed using partial least squares (PLS) regression analysis. This statistical analysis makes it possible to determine the relative contribution of each band to the variation of the different foliar properties, as well as to derive foliar properties from canopy spectral data [70,71,72,73]. Then, the PLS results were evaluated through the determination of the Spearman rank correlation coefficient (R2), establishing significance at p-value < 0.05. A correlation matrix was therefore obtained in order to select the most appropriated vegetation index to describe each biochemical trait. Finally, from the best performing indices, simple linear regressions were carried out for each biochemical trait, assessing the model performance with R2 and p-value specification. Each resulting function was applied in the entire scene spatializing the traits (negative values were replaced for 0). Statistical processes were executed in R (R Core Team 2020, www.r-project.org, accessed on 25 March 2021) [74].
2.7. Transect and Vertical Profiles
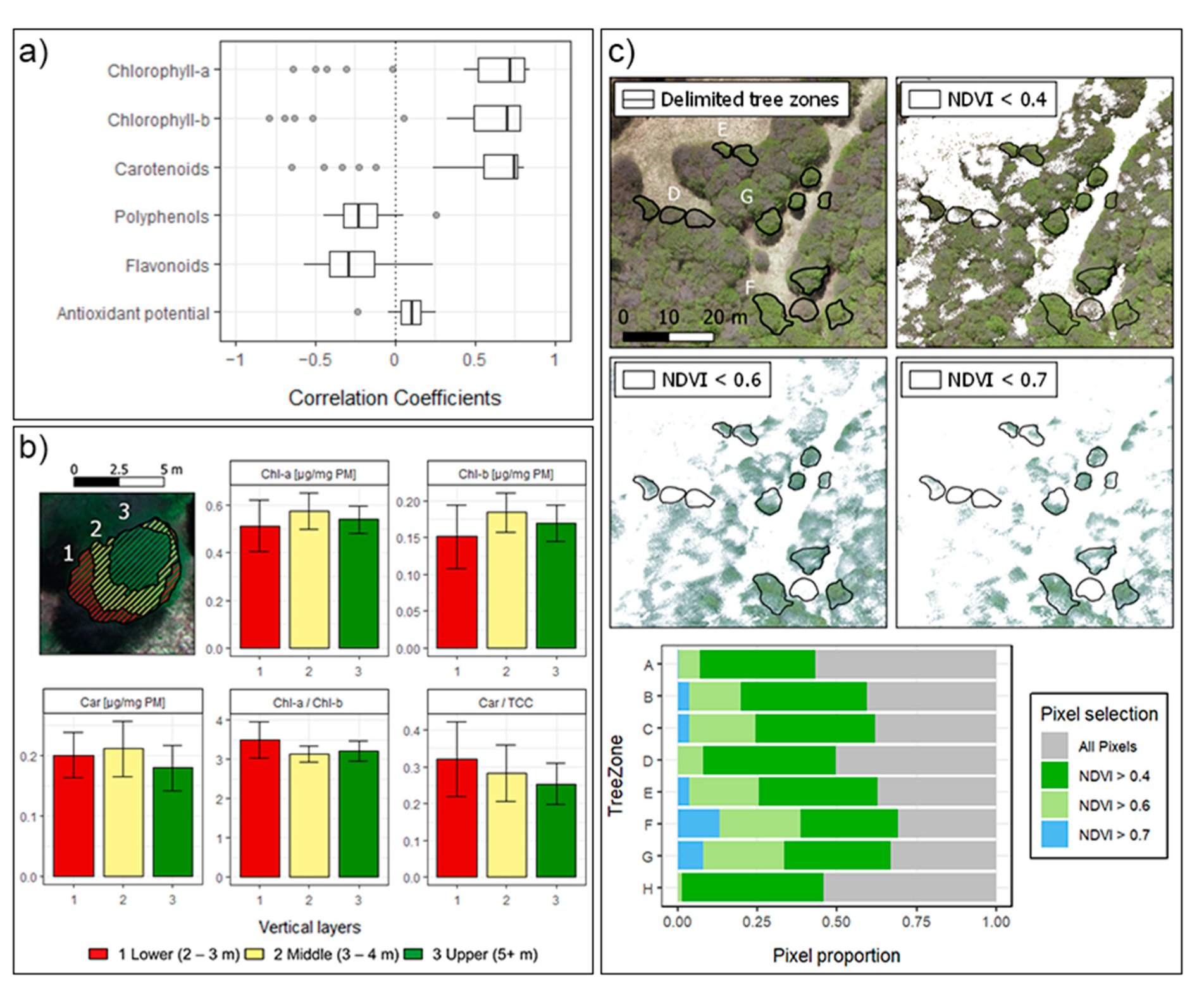
For further understanding and display of the results, a transect and vertical profiles were conducted through the imagery. The transect was defined along 80 m with point values each 0.4 cm. The direction was intended to start at the outside into the inside of the forest, including at least two sampling sites for comparison (west to east). Vertical profiles were defined within a single tree crown, determining three sections according to the DSM and the height presented in the tree. The variables showcased in both examples were chlorophyll content (a and b) and carotenoids, and for vertical profiles pigment ratios were also included.
3. Results and Discussion
3.1. Biochemical Analysis
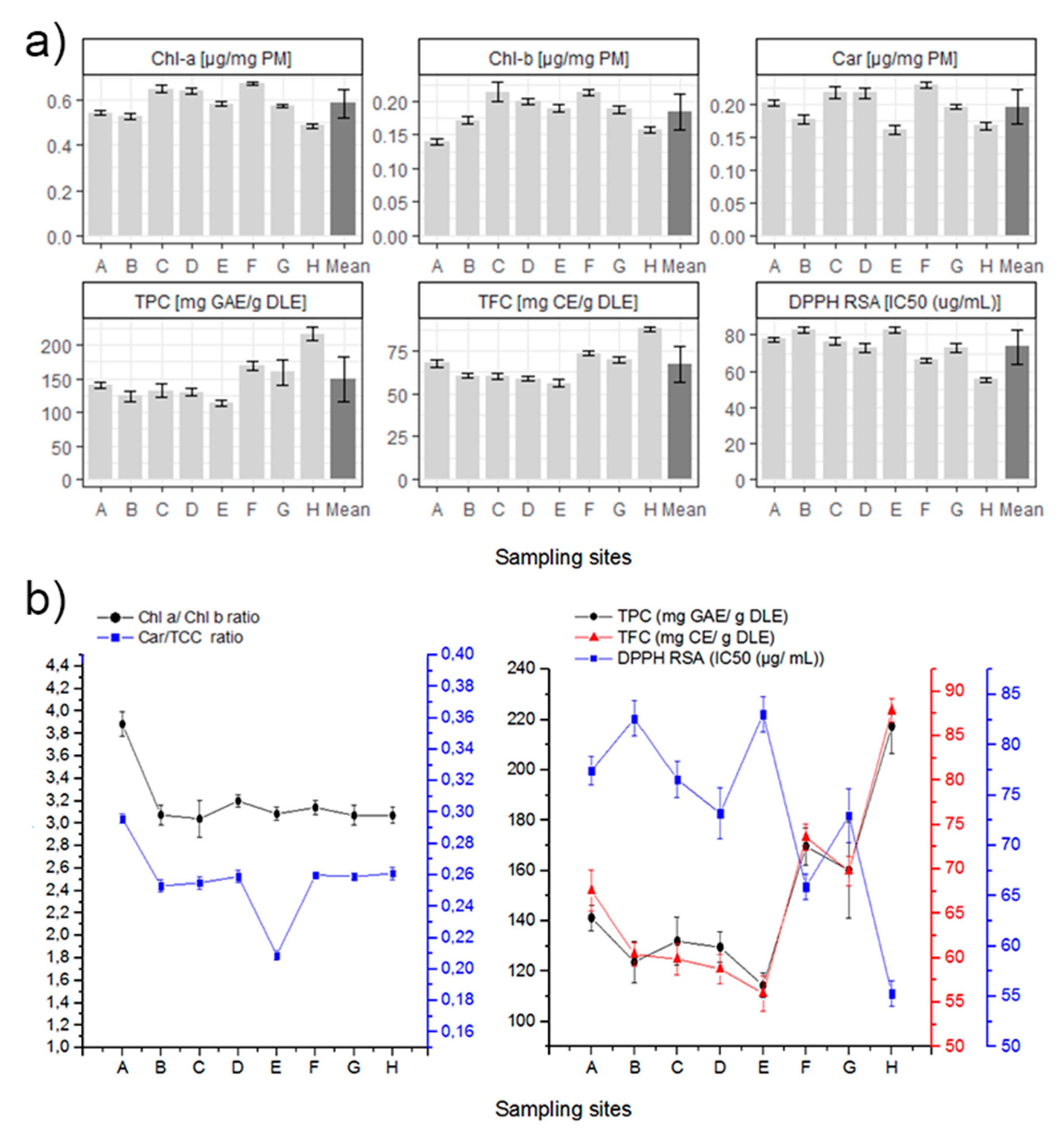
Both Chlorophyll values showed similar results for almost all sampling sites (Figure 3), ranging between 0.4 to almost 0.7 µg/mg PM in the case of Chl-a, and between 0.14 to 0.25 µg/mg PM for Chl-b. The average Chl-a/Chl-b ratio is similar to the reported in literature for beech trees (Fagus sylvatica), another deciduous tree in the Fagaceae family of N. pumilio [75]. Sampling site A is the only one whose average for Chl-a/Chl-b ratio stands apart at 3.216. A high correlation between Chl-a vs. Chl-b was found when excluding the sampling site A (R2: 0.9732, p < 0.0001). We consider that all sampling sites are subjected to the same light intensity, with the exception of zone E. Therefore, the difference in chlorophyll ratio from sample site A might be due to different nitrogen availability. There are reports in the literature that demonstrate a relationship between the ratio of chlorophyll and the availability of nitrogen for the same light intensity [76].
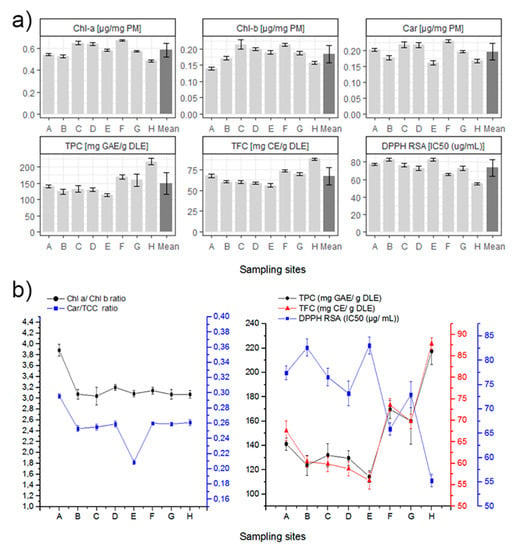
Figure 3.
(a) Summary of laboratory results for each biochemical in all the sampling sites. Mean is included in darker gray, and 1 ± standard deviation is specified for each value. (b) Left. Chlorophyll-a/Chlorophyll-b and Carotenoids/Total Chlorophyll Content ratio for each sampling site. (b) Right. Relationships between the total phenolic content (TPC), total flavonoid content (TFC) and the DPPH free radical scavenging activity at each sampling site.
Carotenoids data are also presented as the Carotenoid/Total Chlorophyll Content (Car/TCC) ratio, similar for almost all sampling sites with an average ratio of 0.2595, with the exception of sites A and E (the highest and lowest respectively). A high correlation of the Car vs. TCC was found when excluding sampling sites A and E (R2: 0.9919, p < 0.0001). The Car/TCC ratio is related to the greenness of the leaves and along with the Chl-a/Chl-b ratio, they are very good markers of the adaptation of chloroplasts to light conditions [77,78,79]. According to these studies, a low Car/TCC ratio in samples in low light conditions would be characteristic of shade chloroplast. As such, shade chloroplasts typically show lower Car/TCC ratios because under low light conditions Carotenoids and Xanthophyll cycles are downregulated and the Total Chlorophyll Content increases [79,80].
The TPC, TFC, and DPPH radical scavenging activity values of each site are shown in Figure 3. A high correlation (R2: 0.9874, p < 0.0001) of the TPC vs. TFC was found. In addition, for the radical scavenging activity and the total content of the previous secondary metabolites (DPPH RSA vs. TPC and TFC), high correlations were also found (R2: 0.9126, p < 0.0002; R2: 0.8325, p = 0.0016). The antioxidant correlation obtained between TPC or TFC and the DPPH RSA is also consistent with laboratory methods. A lower IC50 of DPPH radical scavenging activity value indicates higher non-enzymatic antioxidant capacity in leaves. This is related to higher TPC or TFC [81]. Sample site H exhibits the highest values of TPC, TFC, and non-enzymatic antioxidant capacity, which can be related to a more stressed condition. In fact, site H is the more isolated and exposed sampling site, therefore it might be more susceptible to environmental stressors such as soil moisture, salinity, nutrient availability, soil pH, among others.
3.2. UAV Data Acquisition
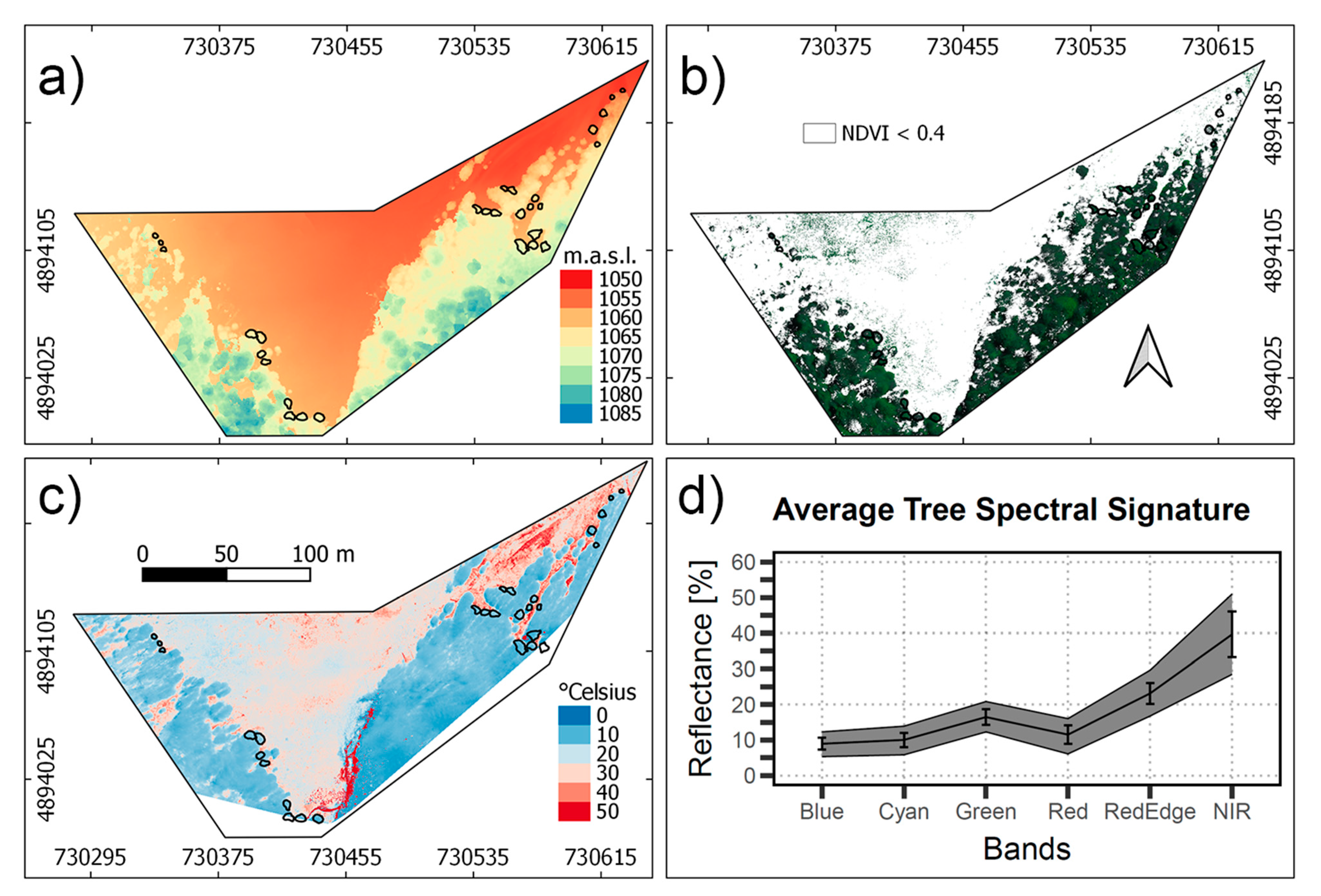
Figure 4a shows the DSM of the study area derived from the RGB orthomosaic of the Sony camera, illustrating the altitude of the sites and trees. Most of the tree zones selected are at 1060 m.a.s.l., avoiding lower altitudes that coincide with zones of water accumulation. Figure 4b presents the green pixels selected with the NDVI > 0.4 criteria, which resulted in the masking of most of the shadows and branches in the canopy. The range of canopy vegetation spectra, atmospherically corrected and converted into surface reflectance, is shown in Figure 4c, according to each band of the Tetracam Macaw. Variation of the reflectance was higher in the NIR spectral range, which is related to the leaf thickness and water content [82]. Visible region presented lower variation, which is related to Chlorophyll, Carotenoids, and Anthocyanin pigments, that are expected to variate less within the forest [83]. The mean spectral reflectance of the trees considered in the Patagonian sub-Antarctic forest resembles the spectral features of healthy green vegetation.
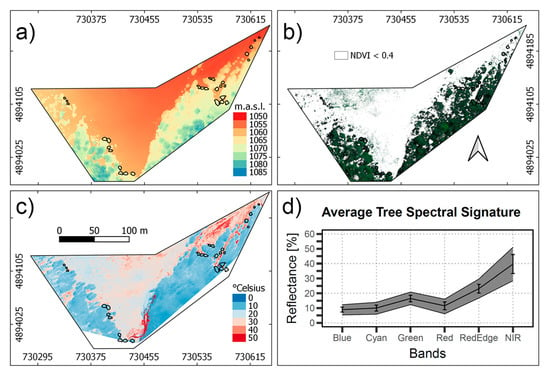
Figure 4.
(a) Digital Surface Model of the study area used for the >2 m height criteria. (b) Multispectral image composite (R, G, B bands) masked with an NDVI < 0.4 criteria. (c) Temperature of the study area. (d) Vegetation spectra for the canopy pixels selected, the black line represents the average spectra, and for each band, the standard deviation is specified.
3.3. Statistical Analysis
Statistical linkage results between biochemicals traits and each spectral band are provided in Table 1. The Red-edge band presented the highest correlation (p < 0.05) with Chl-a and carotenoids (R2: 0.84 and 0.80, respectively). Similar results were obtained from the NIR, including the highest correlation for Chl-b (R2: 0.79). The blue band presented a high correlation (p < 0.05) only for Chl-b (R2: −0.79), while the green and red bands showed high correlations for all the pigments (R2 > 0.7 and R2 > 0.6 respectively). It is possible to notice that in the 450–490 nm range (Blue and Cyan bands), Chl-b content had an inverse correlation with the absorption. On the other hand, polyphenols and flavonoids had the best correlation with Red Edge (R2: −0.38 and −0.47 respectively) while the DPPH assay correlated best with the Cyan band (R2: 0.26), still, none of the antioxidants biochemicals were significantly correlated to any of the bands. According to [32], the spectral range suitable for correlations with phenols belongs to the short-wave infrared (SWIR), specifically around 1.66 μm, as in the VNIR several biochemicals influence the spectral response.

Table 1.
Statistical correlation (R2) between the biochemicals traits and the spectral bands.
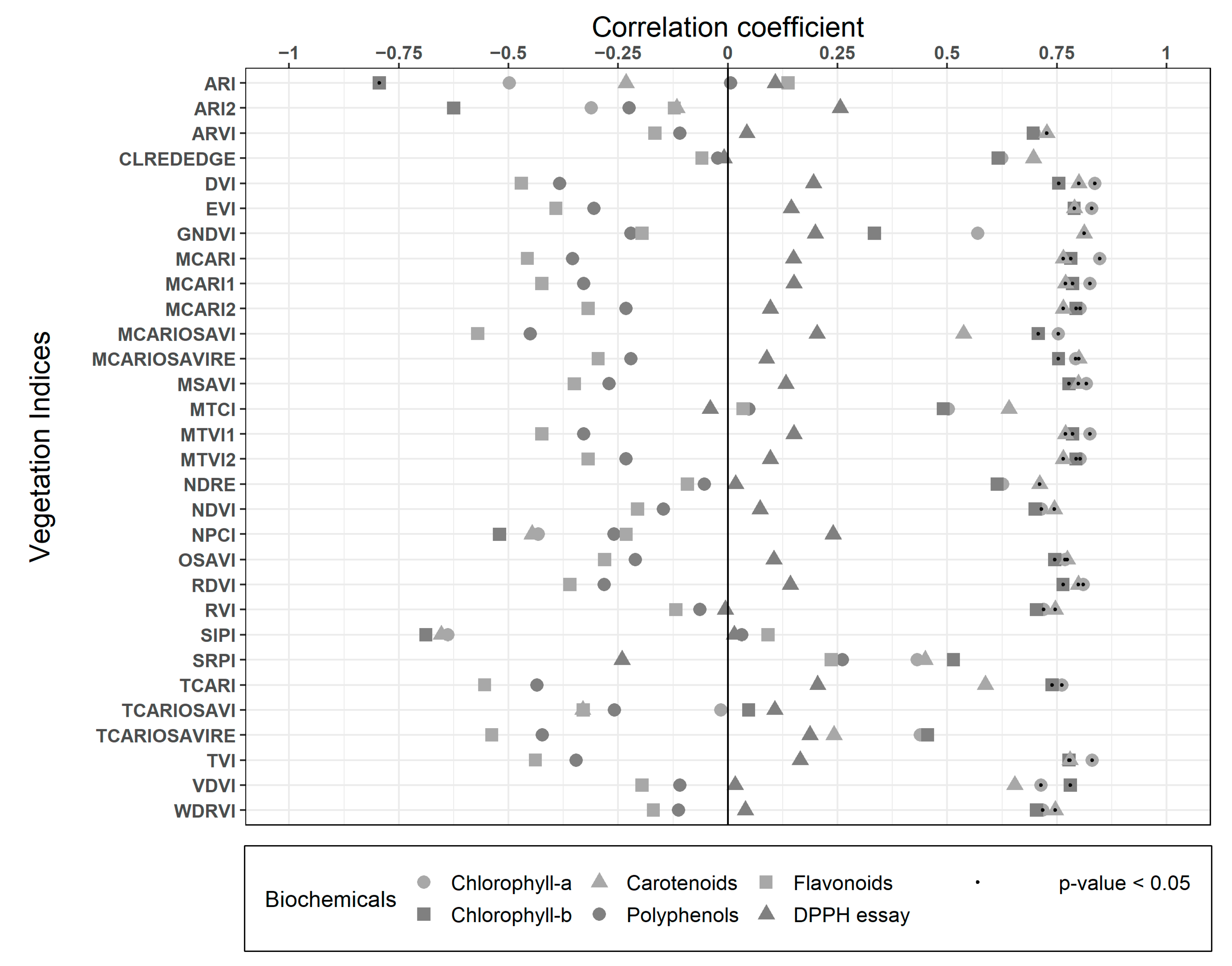
Spearman correlation results between biochemicals traits and VIs are presented in Figure 5. Most of the indices had good correlations (R2 > 0.7) with all the pigments. The simple difference index DVI, which only combines the Red and NIR band in a subtraction (NIR-Red), had significant correlations with Chl-a (R2: 0.84), Chl-b (R2: 0.75), and Carotenoids (R2: 0.8), proving that this spectral difference is very descriptive of the pigments in Nothofagus pumilio forest, as in almost all vegetation. RVI also combines both of these bands, but in a ratio (NIR/Red) that decreases the correlations with the pigments to 0.72, 0.7, and 0.75 R2, respectively, though they are still high and significant. MCARI uses the Green, Red and Red Edge bands, which also have high and significant correlations for the pigments (R2: >0.76). The case is similar for its variations (MCARI1, MCARI2, MCARIOSAVI, MCARIOSAVIRE, TCARI), with correlations higher than 0.7 R2, some of which include the NIR and/or exclude the Green band. MCARIOSAVI considers all of those bands, resulting in the best correlations for Polyphenols and Flavonoids (R2: −0.45 and −0.57 respectively, both not significant), with a similar correlation for Carotenoids (R2: 0.54), but lower than previously specified indices. TVI and its variations (MTVI1 and MTVI2) also use the red, NIR, and green bands, showing similar results (R2 > 0.7 and significant) to the MCARI variations. Most of these indices have different specifications of use according to leaf and canopy structure, leaf area index, the influence of soil and background reflectance, or even atmospheric conditions [28,84,85,86]. However, at the high resolution obtained from UAVs, they perform very similarly, thus most of the differences expected are valid for coarser satellite resolutions. None of the indices presented high (R2 > 0.7) or a significant correlation for the antioxidants assays (TPC, TFC, and DPPH), coinciding with studies suggesting that the absorption features for quantifying Phenolics are in the SWIR spectra [32]. Still, most of the indices showed high and significant performance for the pigments biochemicals (Chl-a, Chl-b, and Car).
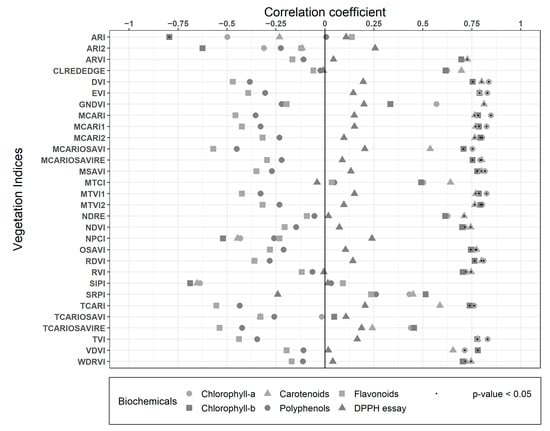
Figure 5.
Spearman correlations between the biochemicals traits and the Vegetation Indices (VIs).
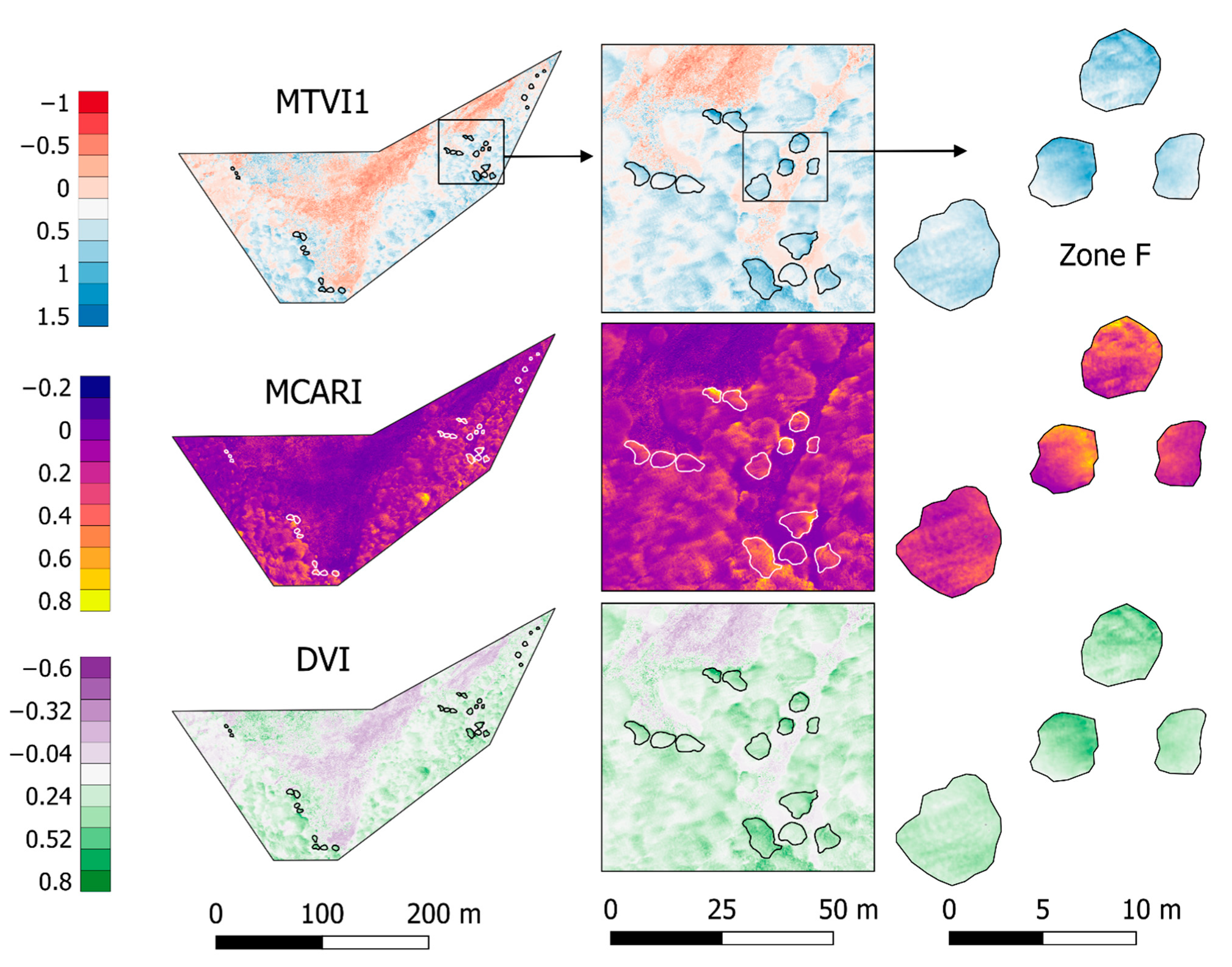
Vegetation indices with the best significant correlations and biochemical trait representativeness were selected (MTVI1, MCARI, and DVI) and spatialized in Figure 6. All three of them highlight the greenness with higher values, representing a heterogeneous canopy. In the case of MCARI, canopies include the full range of values presented in the scenario, representing a very sensitive index to changes among and within trees, specifically for leaf Chlorophyll concentrations [84]. On the other hand, MTVI1 and DVI show less variation inside canopies, though they differentiate with low and negative values the soil and grass cover. The whole zoomed-in area in Figure 6 (on right) represents a single laboratory value in this work, though single tree canopies present significant variations within the same tree crown. The spatial resolution of the imagery allows the forest to be analyzed at nearly leaf level, and therefore more detail and variations can be considered in further studies (i.e., separating each tree crown by exposition or radio segments from the center).
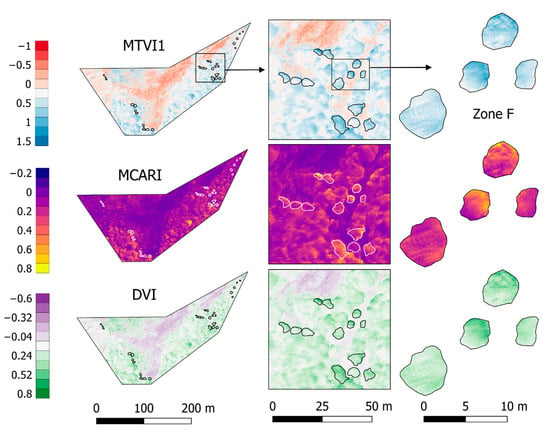
Figure 6.
Vegetation Indices: MTVI1, MCARI, and DVI. These presented the highest and significant correlation coefficients over the study area for most of the biochemicals traits (dimensionless values). Tree zones are specified with white or black lines and Zone F is zoomed in at the right.
3.4. Linear Regressions and Spatialization
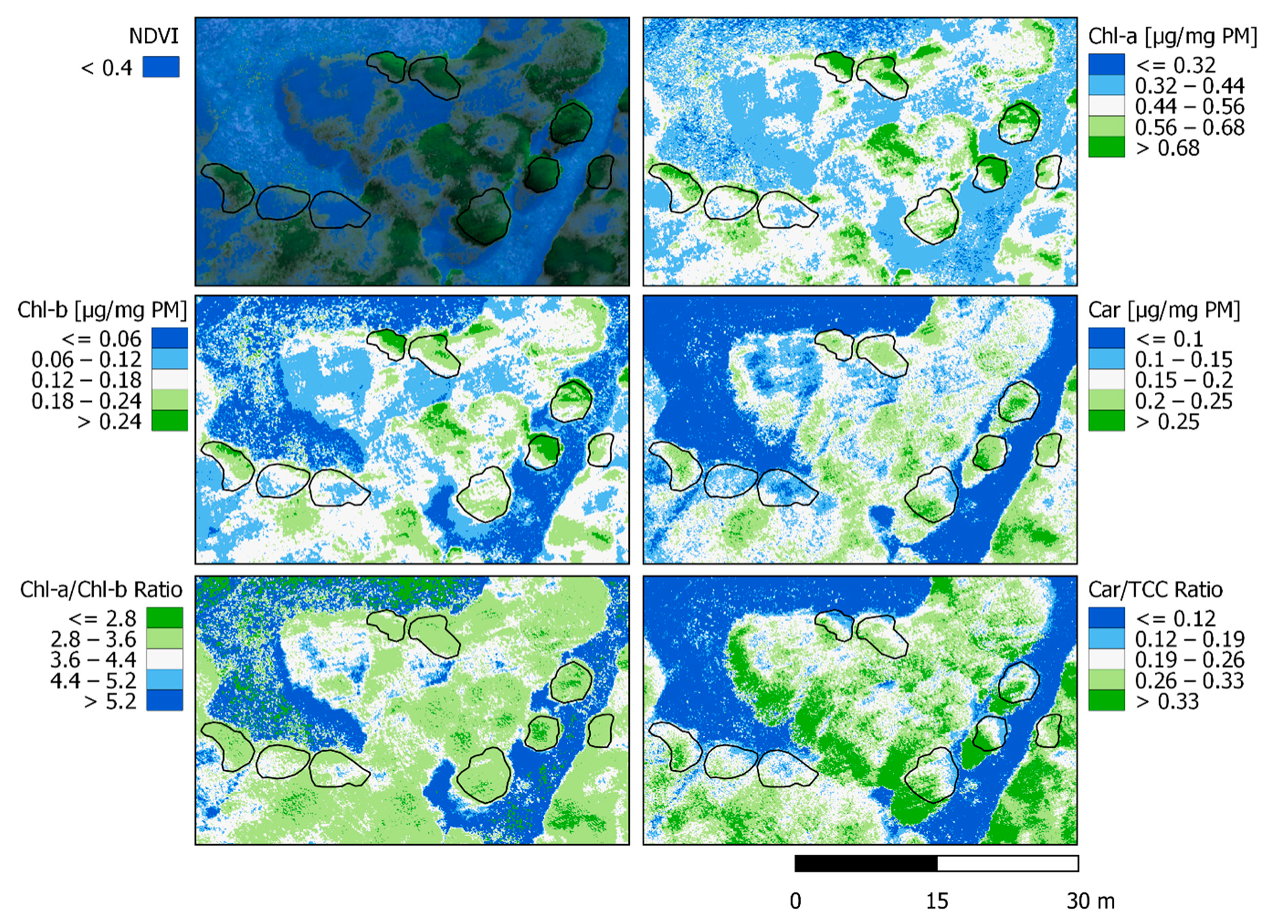
Linear regressions were carried out from the best and significant performing correlation indices (Table 2). A spatialized estimation of Chl-a was performed from MCARI, Chl-b from EVI, and Carotenoids from GNDVI (see Figure 7). From those, UAVs derived Chl-a/Chl-b and Carotenoids/TCC ratios were also calculated for the scene. Both Chlorophyll contents (a and b) are in agreement with the greenest areas, representing them with the highest values at 0.7~ µg/mg PM for Chl-a and 0.25~ µg/mg PM for Chl-b. The spatialization of both Chlorophyll values are very similar, in concordance with their known relationship at different scales (in general, Chl-b content is scarcer). Nothofagus pumilio leaves presented a range between 0.44 to 0.68+ for Chl-a and from 0.12 to 0.24+ in Chl-b. Carotenoids spatialization is also related to greenness, but more homogeneous with less high-value saturation. Only the greener parts of the crowns presented values superior to 0.25+ µg/mg PM while the rest of the leaves showed a range between 0.15 to 0.25 µg/mg PM. It is to notice that Carotenoids show homogeneity in all crowns compared to Chlorophyll. The Chl-a/Chl-b ratio represents the greenest part of the forest with the lower range of values (≤2.8 to 3.6 ratio). It simplifies the differentiation of the crowns with a wider numerical range of values, causing more homogeneity inside the forest and clear limits with the ground and grass. Car/TCC ratio displays differently with more homogeneity outside the forest, but still highlights the differences inside of it. The Car/TCC ratio identifies the border of the open areas in the forest (0.12 to 0.26 ratio), which can be useful for the establishment of solar irradiance incidence zones. The spatialization of both ratios might improve further consideration of forest boundaries in multispectral imagery with enhanced precision than that obtained using vegetation indices alone such as NDVI. The continuous monitoring of these spatialized variables could determine which parts of the forest are more susceptible to changes and whether they are resisting or prevailing as the seasons and years go by.

Table 2.
Biochemicals traits regressions resulted from the best and significant performing vegetation indices (Chl-a from MCARI; Chl-b from EVI; Car from GNDVI). Coefficient of determination (R2) and significance (p-value) for the regressions are specified.
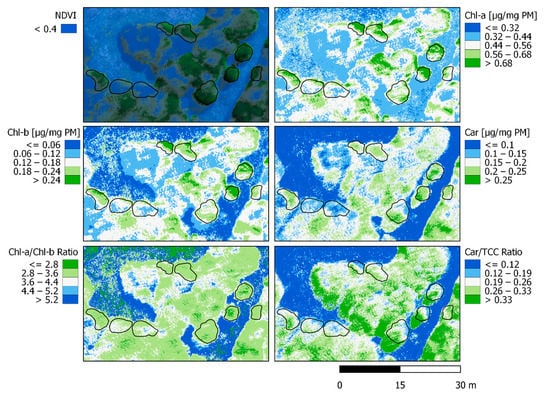
Figure 7.
Spatialization of linear regressions obtained from the best indices for each pigment (Chlorophyll-a with MCARI, Chlorophyll-b with EVI, and Carotenoids with GNDVI). Ratios Chl-a/Chl-b and Car/TCC are also shown, together with the RGB multispectral composite and the green pixel selection for reference.
3.5. Transect
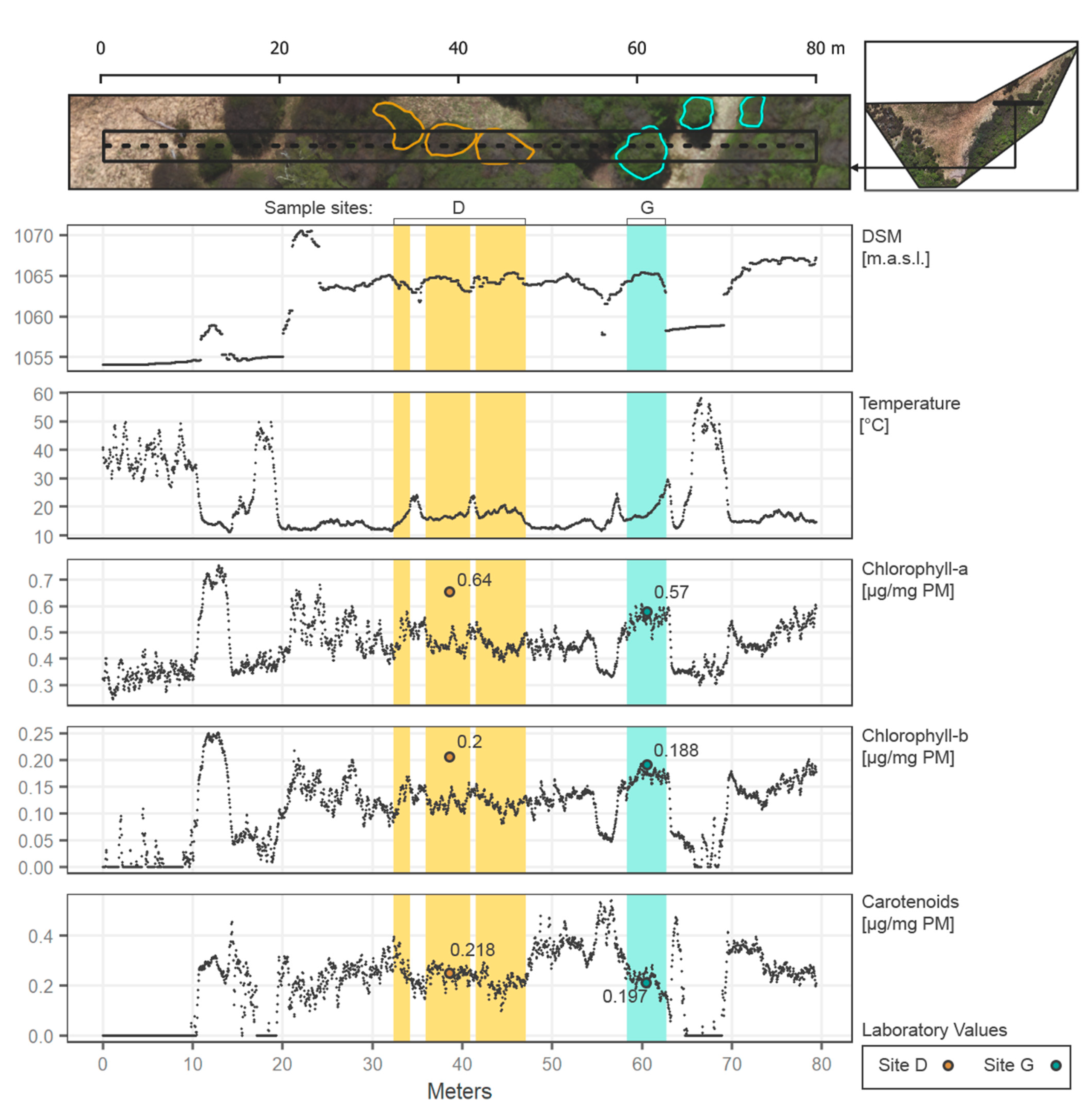
The transect along part of the study area is presented in Figure 8, to highlight key results of the present work in the forest context.
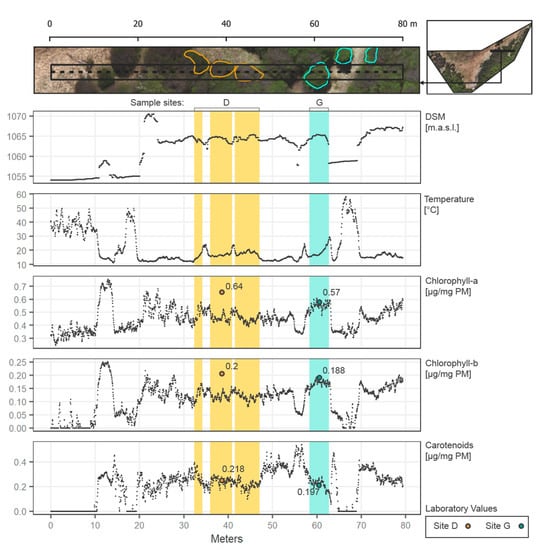
Figure 8.
Transect (west to east) along the study area (80 m length) showing DSM, temperature, and pigments spatialized through the regressions from the vegetation indices. Points values are specified every 4 cm in the transect. Sampling sites D and G are included in the transect and specified.
The transect selected starts (from left to right) with a grass/ground cover at less than 1055 m.a.s.l. over almost 10 m of longitude, introducing the first tree and abruptly increasing the values of all the pigments and a decrease in temperature. Then at 18~ to 20 m, another section of ground is presented, next to the principal section of forest from 20 to 64~ m along the transect. In the forest, temperatures reach stable cooler values then on the exposed ground (around 10 to 20 °C). Moreover, all the pigments increase along the forest, with similar behavior between them. However, a slight increase in pigments is evidenced at higher altitudes (1070 m.a.s.l. in Figure 8). Zone D contrasts with Zone G, as the first has fewer green pixels over the transect, representing a section with considerable branches and gaps between leaves, corresponding to an underestimation of the Chlorophylls according to laboratory values. Zone G in the transect is considered a tree full of mature leaves, coinciding with the laboratory values for all the pigments and representing a good and effective example of the spatialization of the biochemicals. Both zones show clearly the possible variation of the pigments in the forest, with results according to the laboratory expectations. In general, the transect allows the understanding of how each variable relates to the others, and how precise changes can be considered in the UAV scene (every 4 cm in this example).
3.6. Study Limitations and Final Remarks
UAVs ultra-high spatial resolution proved to be useful for the precise separation of the non-photosynthetic canopy, shadows, and other backgrounds from the forest. In fact, for biochemical pigments, most of the VIs showed high correlations (Figure 9a), with several performing at more than 75% of accuracy. As shown in Figure 9c, NDVI filter criteria allows the delimitation of the greenness of the forest at a multispectral resolution of 3.93 cm (while 1.7 cm in the RGB and DSM, and 5.15 cm in the thermal imagery). Similar studies at tropical ecosystems [87] and crops [88], consider NDVI filters from >0.5 to >0.8 for the green pixels selection. However, a slightly broader NDVI criteria (>0.4) was needed for this work to include all the trees sampled. This resulted in the consideration of almost 50% of the crown areas as green pixels for the representation of the spectral variation at all sampling sites.
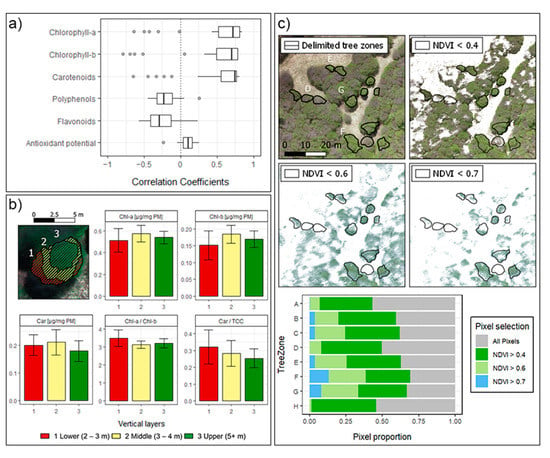
Figure 9.
(a) Distribution of Correlation Coefficients for the biochemicals. (b) Vertical profile of a single Nothofagus pumilio tree from Zone G. Height was measured using RGB DSM, considering surrounding ground as 0 m. (c) Upper. Visualization of different NDVI criteria for the green pixels selection, zones D, E, F, and G are delimited. Lower. Distribution of pixels considering different NDVI criteria within each tree zone.
The N. pumilio forest type represents most of the Patagonian sub-Antarctic forest, and indeed most of the Chilean forestland. The site’s experimental collection of leaves was carried out during the early growing season of N. pumilio (Spring), and as a deciduous tree, the obtained results are strictly related to such season. Further efforts will be made to compare phenology variations in the content of pigments, especially during different leaf growth stages, cover and senescence of the species. Those measurements could lead to further insights into N. pumilio phenology and to the biochemical traits monitoring along years.
Since sampling was performed on a managed National Park, it was spatially restricted to the edge of the forest, with limitations to the trees sampled and to the number of leaves collected. This resulted in the delimitation of eight zones for the study area, including at least two trees per sampling site. The number of samples considered could impact the representativeness of the vegetation indices and possible applications over other similar species and bioclimatic zones. However, as Figure 6 shows, more representativeness could be achieved, by delimiting not only single trees but also parts within them. For example, in Shen et al. [89] vertical profiles are defined within single trees crown, using LiDAR and hyperspectral data. In Figure 9b we present a similar approach using DSM data for the delimitation of three vertical profile zones. The spatialized biochemicals traits show differences in the mean of each zone, consequently within-tree plots consideration might improve further correlations. In that sense, UAVs capacity for monitoring forest traits can be performed in very high detail. Future studies will include trees deep inside the forest, looking to describe the ecosystem at larger areas. This is highly valuable in remotes areas such as the Aysén Region in Chile, where most of the forest is still unmonitored and inaccessible for field techniques, which are often spatially restricted and under-sampled.
4. Conclusions
In this work we studied the combination of multispectral vegetation indices with laboratory measurements of biochemical pigments and antioxidants. This, presented as a first in the development of monitoring methods in the Patagonian sub-Antarctic forest. Vegetation indices from visible spectral bands (such as MTVI1, MCARI and DVI), together with DSM derived from RGB cameras, are sufficient to describe with high and significant accuracy (>0.7 R2) the biochemical trait pigments (Chl-a, Chl-b, and Car). However, for the appropriate consideration of antioxidant traits (polyphenols, flavonoids and antioxidant capacity), SWIR spectra (1.2–2.5 um) should be included, expecting improvement in all the traits correlated. Linear regressions allowed the spatialization of the pigment traits along the study area with high level of detail due to the ultra-high spatial resolution of the UAV imagery, showing that further accuracy can be achieved. In order to scale the results at regional areas, more forest representation is needed, including higher canopies and a greater number of individuals. However, as a first exploratory local-scale study, we highlight the current possibilities to assess the state of the Patagonian forest and its future changes due to new climate scenarios. Further efforts will consider more spatial and seasonal variability, including the scaling up to satellite data and corresponding monitoring protocol for the region.
Author Contributions
Conceptualization, C.M., R.T.-Z. and O.R.-R.; methodology, C.M., R.T.-Z., O.R.-R., M.d.A.-R., F.H.-F. and H.S.; software, R.T.-Z.; validation, R.T.-Z., C.M., O.R.-R., M.d.A.-R. and F.H.-F.; formal analysis, R.T.-Z., C.M., O.R.-R., M.d.A.-R. and F.H.-F.; investigation, R.T.-Z., C.M., O.R.-R., M.d.A.-R., H.S. and F.H.-F.; writing—original draft preparation, R.T.-Z., C.M., O.R.-R., M.d.A.-R., H.S. and F.H.-F.; writing—review and editing, R.T.-Z., C.M., O.R.-R., M.d.A.-R., H.S. and F.H.-F.; visualization, R.T.-Z. and C.M.; supervision, R.T.-Z. and C.M.; project administration, O.R.-R., M.d.A.-R. and C.M.; funding acquisition, O.R.-R., M.d.A.-R. and C.M. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by Fondequip 170060, two Innovation Funds for Competitiveness (Fondo de Innovación para la Competitividad, FIC-Aysén 2017, BIP codes 40000494-0 and 40000496-0) from the Regional Government of Aysén (GORE Aysén), Chile; and the National Student Mobility Program of the Consortium of State Universities of Chile (CUECH).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A

Table A1.
A. Vegetation Indices calculated from Tetracam macaw bands: B = Blue; G = Green; C = Cyan; R = Red; RE = Red Edge; NIR = Near Infrared.
Table A1.
A. Vegetation Indices calculated from Tetracam macaw bands: B = Blue; G = Green; C = Cyan; R = Red; RE = Red Edge; NIR = Near Infrared.
| Name | Acronym | Equation | Source |
|---|---|---|---|
| Normalized Difference Vegetation Index | NDVI | Rouse Jr. et al. [90] | |
| Green NDVI | GNDVI | Gitelson et al. [91] | |
| Normalized Difference Red Edge | NDRE | Gitelson and Merzlyak [92] | |
| Modified Chlorophyll Absorption in Reflectance Index | MCARI | Daughtry et al. [84] | |
| Red Edge Chlorophyll Index | ClRedEdge | Gitelson et al. [93] | |
| Structural Independent Pigment Index | SIPI | J. Peñuelas et al. [94] | |
| Transformed Chlorophyll Absorption in ReflectanceIndex | TCARI | Haboudane et al. [24] | |
| MERIS terrestrial chlorophyll index | MTCI | Dash and Curran [95] | |
| ARVI | where RB = R − (B − R) = | Kaufman and Tanré [96] | |
| Modified Soil-Adjusted Vegetation Index | MSAVI | 0.5[(2 NIR + 1) − Sqrt((2 NIR + 1)2 − 8 (NIR − R))] | Qi et al. [97] |
| Optimized Soil Adjusted Vegetation Index | OSAVI | Rondeaux et al. [98] | |
| Transformed Chlorophyll Absorption in Reflectance Index/Optimized Soil-Adjusted Vegetation Index | TCARI/OSAVI | Haboudane et al. [24] | |
| Modified Chlorophyll Absorption in Reflectance Index/Optimized Soil-Adjusted Vegetation Index | MCARI/OSAVI | Zarco-Tejada et al. [99] | |
| Red Edge-based Transformed Chlorophyll Absorption in Reflectance Index/Optimized Soil-Adjusted Vegetation Index | TCARI/OSAVI_RE | Wu et al. [100] | |
| Red Edge-based Modified Chlorophyll Absorption in Reflectance Index/Optimized Soil-Adjusted Vegetation Index | MCARI/OSAVI_RE | Wu et al. [100] | |
| Ratio Vegetation Index | RVI | Jordan [101] | |
| Difference Vegetation Index | DVI | Tucker [102] | |
| Anthocyanin Reflectance Index | ARI | Gitelson et al. [103] | |
| Anthocyanin Reflectance Index 2 | ARI2 | Gitelson et al. [103] | |
| Simple Ratio Pigment Index | SRPI | Peñuelas et al. [104] | |
| Normalized Pigment Chlorophyll Index | NPCI | Peñuelas et al. [105] | |
| Enhanced Vegetation Index | EVI | Justice et. al. [106] | |
| Visible-Band Difference Vegetation Index | VDVI | Wang et al. [107] | |
| Wide Dynamic Range Vegetation Index | WDRVI | Gitelson [108] | |
| Triangular Vegetation Index | TVI | 0.5 (120 (NIR − G) − 200 (R – G)) | Broge and Leblanc [109] |
| Modified Triangular Vegetation Index 1 | MTVI1 | 1.2 (1.2 (NIR – G) − 2.5 (R − G)) | Haboudane et al. [86] |
| Modified Triangular Vegetation Index 2 | MTVI2 | Haboudane et al. [86] | |
| Modified Chlorophyll Absorption in Reflectance Index 1 | MCARI1 | 1.2 (2.5 (NIR − R) − 1.3 (NIR − G)) | Haboudane et al. [86] |
| Modified Chlorophyll Absorption in Reflectance Index 2 | MCARI2 | Haboudane et al. [86] | |
| Renormalized Difference Vegetation Index | RDVI | Roujean and Breon [110] |
References
- Ramírez, C.; Ortiz, I.; San Matín, C.; Vidal, O.; Álvarez, M.; Pérez, Y.; Solis, J.L.; Alvarez, I. Estudio preliminar de la biodiversidad vegetal terrestre en el Estero Walker (Región de Aysén, Chile): Utilizando líneas base de proyectos de inversión. Gayana Botánica 2014, 71, 227–245. [Google Scholar] [CrossRef][Green Version]
- Bonan, G. Forest and climate change: Forcings, feedbacks, and the climate benefits of forests. Science 2008, 320, 1444–1449. [Google Scholar] [CrossRef]
- Armesto, J.J.; Rozzi, R.; Smith-Ramírez, C.; Arroyo, M.T.K. Conservation targets in South American temperate forests. Science 1998, 282, 1271–1272. [Google Scholar] [CrossRef]
- Olivares-Contreras, V.A.; Mattar, C.; Gutiérrez, A.G.; Jiménez, J.C. Warming trends in Patagonian subantartic forest. Int. J. Appl. Earth Obs. Geoinf. 2019, 76, 51–65. [Google Scholar] [CrossRef]
- Olson, M.; Soriano, D.; Rosell, J.; Anfodillo, T.; Donoghue, M.; Edwards, E.; León-Gómez, C.; Dawson, T.; Camarero Martínez, J.; Castorena, M.; et al. Plant height and hydraulic vulnerability to drought and cold. Proc. Natl. Acad. Sci. USA 2018, 115, 7551–7556. [Google Scholar] [CrossRef]
- Fajardo, A. Are trait-scaling relationships invariant across contrasting elevations in the widely distributed treeline species Nothofagus pumilio? Am. J. Bot. 2016, 103, 821–829. [Google Scholar] [CrossRef]
- Piper, F.; Gundale, M.; Fajardo, A. Extreme defoliation reduces tree growth but not C and N storage in a winter-deciduous species. Ann. Botany 2015, 115, 1093–1103. [Google Scholar] [CrossRef] [PubMed]
- Piper, F.I.; Fajardo, A.; Cavieres, L.A. Simulated warming does not impair seedling survival and growth of Nothofagus pumilio in the southern Andes. Perspect. Plant Ecol. Evol. Syst. 2013, 15, 97–105. [Google Scholar] [CrossRef]
- Fajardo, A.; Piper, F.; Cavieres, L.A. Distinguishing local from global climate influences in the variation of carbon status with altitude in a tree line species. Global. Ecol. Biogeogr. 2011, 20, 307–318. [Google Scholar] [CrossRef]
- Aldunce, P.; Vicuña, S. Adaptación al cambio climático en Chile: Brechas y recomendaciones. In Informe de las Mesas Adaptación y Agua. Comité Científico COP25; Ministerio de Ciencia, Tecnología, Conocimiento e Innovación: Santiago, Chile, 2019. [Google Scholar]
- Belnap, J.; Phillips, S.L.; Miller, M.E. Response of desert biological soil crusts to alterations in precipitation frequency. Oecologia 2004, 141, 306–316. [Google Scholar] [CrossRef] [PubMed]
- Brand, J.D.; Tang, C.; Graham, R.D. The effect of soil moisture on the tolerance of Lupinus pilosus genotypes to a calcareous soil. Plant Soil. 2000, 219, 263–271. [Google Scholar] [CrossRef]
- Chappelle, E.W.; Kim, M.S.; McMurtrey, J.E. Ratio analysis of reflectance spectra (RARS): An algorithm for the remote estimation of the concentrations of chlorophyll a, chlorophyll B, and carotenoids in soybean leaves. Remote Sens. Environ. 1992, 39, 239–247. [Google Scholar] [CrossRef]
- Zhang, D.; Zhou, X.; Zhang, J.; Lan, Y.; Xu, C.; Liang, D. Detection of rice sheath blight using an unmanned aerial system with high resolution color and multispectral imaging. PLoS ONE 2018, 13, e0187470. [Google Scholar] [CrossRef]
- Gago, J.; Douthe, C.; Coopman, R.E.; Gallego, P.P.; Ribas-Carbo, M.; Flexas, J.; Escalona, J.; Medrano, H. UAVs challenge to assess water stress for sustainable agriculture. Agric. Water Manag. 2015, 153, 9–19. [Google Scholar] [CrossRef]
- Sankaran, S.; Khot, L.R.; Espinoza, C.Z.; Jarolmasjed, S.; Sathuvalli, V.R.; Vandemark, G.J.; Miklas, P.N.; Carter, A.H.; Pumphrey, M.O.; Knowles, R.R.N.; et al. Low-altitude, high-resolution aerial imaging systems for row and field crop phenotyping: A review. Eur. J. Agron. 2015, 70, 112–123. [Google Scholar] [CrossRef]
- Van Der Meij, B.; Kooistra, L.; Suomalainen, J.; Barel, J.M.; De Deyn, G.B. Remote sensing of plant trait responses to field-based plant-soil feedback using UAV-based optical sensors. Biogeosciences 2017, 14, 733–749. [Google Scholar] [CrossRef]
- Verger, A.; Vigneau, N.; Chéron, C.; Gilliot, J.M.; Comar, A.; Baret, F. Green area index from an unmanned aerial system over wheat and rapeseed crops. Remote Sens. Environ. 2014, 152, 654–664. [Google Scholar] [CrossRef]
- Zaman-Allah, M.; Vergara, O.; Araus, J.L.; Tarekegne, A.; Magorokosho, C.; Zarco-Tejada, P.J.; Hornero, A.; Albà, A.H.; Das, B.; Craufurd, P.; et al. Unmanned aerial platform-based multi-spectral imaging for field phenotyping of maize. Plant Methods 2015, 11, 35. [Google Scholar] [CrossRef] [PubMed]
- Zarco-Tejada, P.J.; Guillén-Climent, M.L.; Hernández-Clemente, R.; Catalina, A.; González, M.R.; Martín, P. Estimating leaf carotenoid content in vineyards using high resolution hyperspectral imagery acquired from an unmanned aerial vehicle (UAV). Agric. For. Meteorol. 2013, 171, 281–294. [Google Scholar] [CrossRef]
- Durán, S.; Martin, R.E.; Díaz, S.; Maitner, B.; Malhi, Y.; Salinas, N.; Shenkin, A.; Silman, M.; Wieczynski, D.J.; Asner, G.P.; et al. Informing trait based ecology by assessing remotely sensed functional diversity across broad tropical tefajardomperature gradient. Science Adv. 2019, 5, eaaw8114. [Google Scholar] [CrossRef]
- Asner, G.P.; Martin, R.E.; Knapp, D.E.; Tupayachi, R.; Anderson, C.B.; Sinca, F.; Vaughn, N.R.; Llactayo, W. Airborne laser-guided imaging spectroscopy to map forest trait diversity and guide conservation. Science 2017, 355, 385–389. [Google Scholar] [CrossRef]
- Asner, G.P.; Martin, R.E.; Anderson, C.; Kryston, K.; Vaughn, N.; Knapp, D.; Bentley, L.; Shenkin, A.; Salinas, N.; Sinca, F.; et al. Scale dependence of canopy trait distributions along a tropical forest elevation gradient. New Phytol. 2016, 214, 973–988. [Google Scholar] [CrossRef]
- Haboudane, D.; Miller, J.R.; Tremblay, N.; Zarco-Tejada, P.J.; Dextraze, L. Integrated narrow-band vegetation indices for prediction of crop chlorophyll content for application to precision agriculture. Remote Sens. Environ. 2002, 81, 416–426. [Google Scholar] [CrossRef]
- Le Maire, G.; Francois, C.; Dufrene, E. Towards universal broad leaf chlorophyll indices using PROSPECT simulated database and hyperspectral reflectance measurements. Remote Sens. Environ. 2004, 89, 1–28. [Google Scholar] [CrossRef]
- Malenovský, Z.; Homolová, L.; Zurita-Milla, R.; Lukeš, P.; Kaplan, V.; Hanuš, J.; Gastellu-Etchegorry, J.P.; Schaepman, M.E. Retrieval of spruce leaf chlorophyll content from airborne image data using continuum removal and radiative transfer. Remote Sens. Environ. 2013, 131, 85–102. [Google Scholar] [CrossRef]
- Schlerf, M.; Atzberger, C.; Hill, J.; Buddenbaum, H.; Werner, W.; Schuler, G. Retrieval of chlorophyll and nitrogen in Norway spruce (Picea abies L. Karst.) using imaging spectroscopy. Int. J. Appl. Earth Obs. Geoinf. 2010, 12, 17–26. [Google Scholar] [CrossRef]
- Zarco-Tejada, P.J.; Miller, J.R.; Harron, J.; Hu, B.; Noland, T.L.; Goel, N.; Mohammed, G.H.; Sampson, P. Needle chlorophyll content estimation through model inversion using hyperspectral data from boreal conifer forest canopies. Remote Sens. Environ. 2004, 89, 189–199. [Google Scholar] [CrossRef]
- Gitelson, A.A. Assessing carotenoid content in plant leaves with reflectance spectroscopy. Photochem. Photobiol. 2002, 75, 272–281. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Keydan, G.P.; Merzlyak, M.N. Three-band model for noninvasive estimation of chlorophyll, carotenoids, and anthocyanin contents in higher plant leaves. Geophys. Res. Lett. 2006, 33, L11402. [Google Scholar] [CrossRef]
- Hernandez-Clemente, R.; Navarro-Cerrillo, R.M.; Zarco-Tejada, P.J. Carotenoid content estimation in a heterogeneous conifer forest using narrow-band indices and PROSPECT + DART simulations. Remote Sens. Environ. 2012, 127, 298–315. [Google Scholar] [CrossRef]
- Kokaly, R.F.; Skidmore, A.K. Plant phenolics and absorption features in vegetation reflectance spectra near 1.66 microns. Int. J. Appl. Earth Obs. Geoinf. (JAG) 2015, 43, 55–83. [Google Scholar] [CrossRef]
- Clevers, J.G.P.W.; Kooistra, L.; Schaepman, M.E. Estimating canopy water content using hyperspectral remote sensing data. Int. J. Appl. Earth Obs. Geoinf. 2010, 12, 119–125. [Google Scholar] [CrossRef]
- Colombo, R.; Meroni, M.; Marchesi, A.; Busetto, L.; Rossini, M.; Giardino, C.; Panigada, C. Estimation of leaf and canopy water content in poplar plantations by means of hyperspectral indices and inverse modeling. Remote Sens. Environ. 2008, 112, 1820–1834. [Google Scholar] [CrossRef]
- Kokaly, R.F.; Asner, G.P.; Ollinger, S.V.; Martin, M.E.; Wessman, C.A. Characterizing canopy biochemistry from imaging spectroscopy and its application to ecosystem studies. Remote Sens. Environ. 2009, 113, S78–S91. [Google Scholar] [CrossRef]
- Mutanga, O.; Skidmore, A.K.; Prins, H.H.T. Predicting in situ pasture quality in the Kruger National Park, South Africa, using continuum-removed absorption features. Remote Sens. Environ. 2004, 89, 393–408. [Google Scholar] [CrossRef]
- Pimstein, A.; Karnieli, A.; Bansal, S.K.; Bonfil, D.J. Exploring remotely sensed technologies for monitoring wheat potassium and phosphorus using field spectroscopy. Field Crop. Res. 2011, 121, 125–135. [Google Scholar] [CrossRef]
- Xue, J.; Su, B. Significant remote sensing vegetation indices: A review of developments and applications. J. Sens. 2017, 2017, 17. [Google Scholar] [CrossRef]
- Sylvester, G.E. Agriculture in Action: Drones for Agriculture; FAO (Food and Agriculture Organization of the United Nations) and ITU (International Telecommunication Union): Bangkok, Thailand, 2018; p. 126. [Google Scholar]
- Houborg, R.; Fisher, J.; Skidmore, A. Advances in remote sensing of vegetation function and traits. Int. J. Appl. Earth Obs. Geoinf. 2015, 43, 1–6. [Google Scholar] [CrossRef]
- Almeyda, E.; Sáez, F. Recopilación de Datos Climáticos de Chile y Mapas Sinópticos Respectivos; Ministerio de Agricultura: Santiago, Chile, 1958. [Google Scholar]
- Amigo, J.; Ramírez, C. A bioclimatic classification of Chile: Woodland communities in the temperate zone. Plant Ecol. 1998, 136, 9–26. [Google Scholar] [CrossRef]
- DGA (Dirección General de Aguas). Informe Meteorológico de Chile; Dirección General de Agua: Santiago, Chile, 2008. [Google Scholar]
- Hijmans, R.J.; Cameron, S.E.; Parra, J.L.; Jones, P.G.; Jarvis, A.; Richardson, K.; WorldClim. Global Climate Data. 2005. Available online: http://www.worldclim.org (accessed on 25 March 2021).
- Veblen, T.T.; Donoso, C.; Kitzberger, T.; Rebertus, A. Ecology of Southern Chilean and Argentinean Nothofagus forests. In The Ecology and Biogeography of Nothofagus Forests; Veblen, T.T., Hill, R.S., Read, J., Eds.; Yale University Press: New Haven, CT, USA, 1996; pp. 293–353. [Google Scholar]
- Premoli, A.C.; Raffaele, E.; Mathiassen, P. Morphological and phenological differences in Nothofagus pumilio from contrasting elevations: Evidence from a common garden. Aust. Ecol. 2007, 32, 515–523. [Google Scholar] [CrossRef]
- Premoli, A.C.; Brewer, C.A. Environmental versus genetically driven variation in ecophysiological traits of Nothofagus pumilio from contrasting elevations. Aust. J. Bot. 2007, 55, 585–591. [Google Scholar] [CrossRef]
- Fajardo, A.; Piper, F. Intraspecific trait variation and covariation in a widespread trees species (Nothofagus pumilio) in southern Chile. New Phytol. 2011, 189, 259–271. [Google Scholar] [CrossRef] [PubMed]
- Rebertus, A.; Veblen, T.T. Structure and tree-fall gap dynamics of old-growth Nothofagus forests in Tierra del Fuego, Argentina. J. Veg. Sci. 1993, 4, 461–654. [Google Scholar] [CrossRef]
- González, M.E. Fire History of Araucaria-Nothofagus Forests in the Andean Cordillera of South-Central Chile; University of Colorado: Boulder, CO, USA, 2002; p. 158. [Google Scholar]
- Parodi, L.R. Enciclopedia Argentina de Agricultura y Jardinería; Editorial ACME, S.A.C.I.: Buenos Aires, Argentina, 1987. [Google Scholar]
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. Meth. Enzymol. 1987, 148, 350–382. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Buschmann, C. Chlorophylls and carotenoids: Measurement and characterization by UV-VIS spectroscopy. Curr. Protoc. Food Anal. Chem. 2001, 1, F4.3.1–F4.3.8. [Google Scholar] [CrossRef]
- Ainsworth, E.A.; Gillespie, K.M. Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin–Ciocalteu reagent. Nat. Protoc. 2007, 2, 875–877. [Google Scholar] [CrossRef]
- Bridi, R.; Atala, E.; Pizarro, P.N.; Montenegro, G. Honey bee pollen Load: Phenolic composition and antimicrobial activity and antioxidant capacity. J. Nat. Prod. 2019, 82, 559–565. [Google Scholar] [CrossRef]
- Dewanto, V.; Wu, X.; Adom, K.K.; Liu, R.H. Thermal processing enhances the nutritional value of tomatoes by increasing total antioxidant activity. J. Agric. Food Chem. 2002, 50, 3010–3014. [Google Scholar] [CrossRef]
- Ben Mrid, R.; Bouchmaa, N.; Bouargalne, Y.; Ramdan, B.; Karrouchi, K.; Kabach, I.; El Karbane, M.; Idir, A.; Zyad, A.; Nhiri, M. Phytochemical characterization, antioxidant and in vitro cytotoxic activity evaluation of Juniperus oxycedrus Subsp. oxycedrus needles and berries. Molecules 2019, 24, 502. [Google Scholar] [CrossRef]
- Hartwig, V.G.; Brumovsky, L.A.; Fretes, R.M.; Boado, L.S. A novel procedure to measure the antioxidant capacity of Yerba maté extracts. Food Sci. Technol. 2012, 32, 126–133. [Google Scholar] [CrossRef]
- Kumar, L.; Schmidt, K.; Dury, S.J.; Skidmore, A. Imaging spectrometry and vegetation sciences. In Imaging Spectrometry. Basic Principles and Prospective Applications; Van der Meer, F.D., de Jong, S.M., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2001; pp. 111–155. [Google Scholar]
- Disney, M.; Lewis, P.; Saich, P. 3D modelling of forest canopy structure for remote sensing simulations in the optical and microwave domains. Remote Sens. Environ. 2006, 100, 114–132. [Google Scholar] [CrossRef]
- Homolová, L.; Malenovský, Z.; Clevers, J.; García-Santos, G.; Schaepman, M. Review of optical-based remote sensing for plant trait mapping. Ecol. Complex. 2013, 15, 1–16. [Google Scholar] [CrossRef]
- Fernandes, R.A.; Miller, J.R.; Chen, J.M.; Rubinstein, I.G. Evaluating image-based estimates of leaf area index in boreal conifer stands over a range of scales using high-resolution CASI imagery. Remote Sens. Environ. 2004, 89, 200–216. [Google Scholar] [CrossRef]
- Turner, D.P.; Cohen, W.B.; Kennedy, R.E.; Fassnacht, K.S.; Briggs, J.M. Relationships between leaf area index and Landsat TM spectral vegetation indices across three temperate zone sites. Remote Sens. Environ. 1999, 70, 52–68. [Google Scholar] [CrossRef]
- Xiao, X.; Hollinger, D.; Aber, J.; Goltz, M.; Davidson, E.A.; Zhang, Q.; Moore, B., III. Satellite-based modeling of gross primary production in an evergreen needleleaf forest. Remote Sens. Environ. 2004, 89, 519–534. [Google Scholar] [CrossRef]
- Yi, Q.X.; Huang, J.F.; Wang, F.M.; Wang, X.Z. Quantifying biochemical variables of corn by hyperspectral reflectance at leaf scale. J. Zhejiang Univ. Sci. B 2008, 9, 378–384. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.; Peng-Sen, S.; Liu, S.-R. A review of plant spectral reflectance response to water physiological changes. Chin. J. Plant Ecol. 2016, 40, 80–91. [Google Scholar] [CrossRef]
- Sun, J.; Shi, S.; Gong, W.; Yang, J.; Du, L.; Song, S.; Chen, B.; Zhang, Z. Evaluation of hyperspectral LiDAR for monitoring rice leaf nitrogen by comparison with multispectral LiDAR and passive spectrometer. Sci. Rep. 2017, 7, 40362. [Google Scholar] [CrossRef] [PubMed]
- Asner, G.; Martin, R.E.; Knapp, D.; Tupayachi, R.; Anderson, C.; Carranza, L.; Martinez, P.; Houcheime, M.; Sinca, F.; Weiss, P. Spectroscopy of canopy chemicals in humid tropical forests. Remote Sens. Environ. 2011, 115, 3587–3598. [Google Scholar] [CrossRef]
- Jay, S.; Baret, F.; Dutartre, D.; Malatesta, G.; Héno, S.; Comar, A.; Weiss, M.; Maupas, F. Exploiting the centimeter resolution of UAV multispectral imagery to improve remote-sensing estimates of canopy structure and biochemistry in sugar beet crops. Rem. Sens. Environ. 2019, 231, 110898. [Google Scholar] [CrossRef]
- Ollinger, S.V.; Smith, M.L.; Martin, M.E.; Hallett, R.A.; Goodale, C.L.; Aber, J.D. Regional variation in foliar chemistry and N cycling among forests of diverse history and composition. Ecology 2002, 83, 339–355. [Google Scholar]
- Smith, M.L.; Ollinger, S.V.; Martin, M.E.; Aber, J.D.; Hallett, R.A.; Goodale, C.L. Direct estimation of aboveground forest productivity through hyperspectral remote sensing of canopy nitrogen. Ecol. Appl. 2002, 12, 1286–1302. [Google Scholar] [CrossRef]
- Smith, M.L.; Martin, M.E.; Plourde, L.; Ollinger, S.V. Analysis of hyperspectral data for estimation of temperate forest canopy nitrogen concentration: Comparison between airborne (AVIRIS) and spaceborne (Hyperion) sensor. IEEE Trans. Geosci. Remote Sens. 2003, 41, 1332–1337. [Google Scholar] [CrossRef]
- Asner, G.; Martin, R.E. Spectral and chemical analysis of tropical forests: Scaling from leaf to canopy levels. Remote Sens. Environ. 2008, 112, 3958–3970. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2020. [Google Scholar]
- Lichtenthaler, H.K.; Buschmann, C.; Döll, M.; Fietz, H.J.; Bach, T.; Kozel, U.; Meir, D.; Rahmsdorf, U. Photosynthetic activity, chloroplasts ultrastructure, and leaf characteristics of high-light and low-light plants and of sun and shade leaves. Photosynth. Res. 1981, 2, 115–141. [Google Scholar] [CrossRef]
- Kitajima, K.; Hogan, K.P. Increases of chlorophyll a/b ratios during acclimation of tropical woody seedlings to nitrogen limitation and high light. Plant Cell Environ. 2003, 26, 857–865. [Google Scholar] [CrossRef] [PubMed]
- Papageorgiou, G.C.; Stamatakis, K. Water and solute transport in cyanobacteria as probed by chlorophyll fluorescence. In Chlorophyll a Fluorescence: A Signature of Photosynthesis, Advances in Photosynthesis and Respiration; Sharkey, T.D., Ed.; Springer: Dordrecht, Switzerland, 2004; Volume 19, pp. 663–678. [Google Scholar]
- Yamazaki, J.; Suzuki, T.; Maruta, E.; Kamimura, Y. The stoichiometry and antenna size of the two photosystems in marine green algae, Bryopsis maxima and Ulva pertusa, in relation to the light environment of their natural habitat. J. Exp. Bot. 2005, 56, 1517–1523. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.T. The Effect of Light Exposure on the Total Chlorophyll Content, Chl a/b ratio, and Car/chl Ratio in the Barks of Fraxinus latifolia Seedlings. University Honors, Bachelor of Science Thesis, Portland State University, Porland, OR, USA, 2018. [Google Scholar]
- Levizou, E.; Petropoulou, Y.; Manetas, Y. Carotenoid composition of peridermal twigs does not fully conform to a shade acclimation hypothesis. Photosynthetica 2004, 42, 591–596. [Google Scholar] [CrossRef]
- Molyneux, P. The use of stable free radical Diphenylpicrylhydrazyl (DPPH) for estimating antioxidant activity. Songklanakarin J. Sci. Technol. 2004, 26, 211–219. [Google Scholar]
- Jacquemoud, S.; Verhoef, W.; Baret, F.; Bacour, C.; Zarco-Tejada, P.J.; Asner, G.P.; Francois, C.; Ustin, S.L. PROSPECT + SAIL models: A review of use for vegetation characterization. Remote Sens. Environ. 2009, 113, S56–S66. [Google Scholar] [CrossRef]
- Sims, D.A.; Gamon, J.A. Relationships between leaf pigment content and spectral reflectance across a wide range of species, leaf structures and developmental stages. Remote Sens. Environ. 2002, 81, 337–354. [Google Scholar] [CrossRef]
- Daughtry, C.S.T.; Walthall, C.L.; Kim, M.S.; Brown de Colstoun, E.; McMurtrey, J.E. Estimating corn leaf chlorophyll concentration from leaf and canopy reflectance. Remote Sens. Environ. 2000, 74, 229–239. [Google Scholar] [CrossRef]
- Huete, A.; Didan, K.; Miura, T.; Rodriguez, E.P.; Gao, X.; Ferreira, L.G. Overview of the radiometric and biophysical performance of the MODIS vegetation indices. Remote Sens. Environ. 2002, 83, 195–213. [Google Scholar] [CrossRef]
- Haboudane, D.; Miller, J.R.; Pattey, E.; Zarco-Tejada, P.J.; Strachan, I.B. Hyperspectral vegetation indices and novel algorithms for predicting green LAI of crop canopies: Modeling and validation in the context of precision agriculture. Remote Sens. Environ. 2004, 90, 337–352. [Google Scholar] [CrossRef]
- Asner, G.; Anderson, C.; Martin, R.; Tupayachi, R.; Knapp, D.E.; Sinca, F. Landscape biogeochemistry reflected in shifting distributions of chemical traits in the Amazon Forest canopy. Nat. Geosci. 2015, 8, 567–573. [Google Scholar] [CrossRef]
- Potgieter, A.; George-Jaeggli, B.; Chapman, S.; Laws, K.; Suárez, L.; Wixted, J.; Watson, J.; Eldridge, M.; Jordan, D.; Hammer, G. Multi-spectral imaging from an unmanned aerial vehicle enables the assessment of seasonal leaf area dynamics of sorghum breeding lines. Front. Plant Sci. 2017, 8, 1532. [Google Scholar] [CrossRef]
- Shen, X.; Cao, L.; Coops, N.; Fan, H.; Wu, X.; Liu, H.; Wang, G.; Cao, F. Quantifying vertical profiles of biochemical traits for forest plantation species using advanced remote sensing approaches. Remote Sens. Environ. 2020, 250, 112041. [Google Scholar] [CrossRef]
- Rouse, J.W.; Haas, R.H.; Schell, J.A.; Deering, D.W. Monitoring vegetation systems in the Great Plains with ERTS. In Proceedings of the Third ERTS Symposium, Washington, DC, USA, 10–14 December 1973; NASA Special Publication-351. US. Government Printing Office: Washington, DC, USA, 1973; Volume 1, pp. 309–317. [Google Scholar]
- Gitelson, A.A.; Kaufman, Y.J.; Merzlyak, M.N. Use of a green channel in remote sensing of global vegetation from EOS-MODIS. Remote Sens. Environ. 1996, 58, 289–298. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Merzlyak, M.N. Remote estimation of chlorophyll content in higher plant leaves. Int. J. Remote Sens. 1997, 18, 2691–2697. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Viña, A.; Ciganda, V.; Rundquist, D.C.; Arkebauer, T.J. Remote estimation of canopy chlorophyll content in crops. Geophys. Res. Lett. 2005, 32, L08403. [Google Scholar] [CrossRef]
- Peñuelas, J.; Baret, F.; Filella, I. Semi-empirical indices to assess carotenoids/chlorophyll-a ratio from leaf spectral reflectance. Photosynthetica 1995, 31, 221–230. [Google Scholar]
- Dash, J.; Curran, P.J. The MERIS terrestrial chlorophyll index. Int. J. Remote Sens. 2004, 25, 5403–5413. [Google Scholar] [CrossRef]
- Kaufman, Y.J.; Tanré, D. Atmospherically Resistant Vegetation Index (ARVI) for EOS-MODIS. IEEE Trans. Geosci. Remote Sens. 1992, 30, 261–270. [Google Scholar] [CrossRef]
- Qi, J.; Chehbouni, A.; Huete, A.R.; Kerr, Y.H.; Sorooshian, S. A modified soil adjusted vegetation index. Remote Sens. Environ. 1994, 48, 119–126. [Google Scholar] [CrossRef]
- Rondeaux, G.; Steven, M.; Baret, F. Optimization of soil-adjusted vegetation indices. Remote Sens. Environ. 1996, 55, 95–107. [Google Scholar] [CrossRef]
- Zarco-Tejada, P.J.; Berjón, A.; López-Lozano, R.; Miller, J.R.; Martín, P.; Cachorro, P.; González, M.R.; de Frutos, A. Assessing vineyard condition with hyperspectral indices: Leaf and canopy reflectance simulation in a row-structured discontinuous canopy. Remote Sens. Environ. 2005, 99, 271–287. [Google Scholar] [CrossRef]
- Wu, C.; Niu, Z.; Tang, Q.; Huang, W. Estimating chlorophyll content from hyperspectral vegetation indices: Modeling and validation. Agric. For. Meteorol. 2008, 148, 1230–1241. [Google Scholar] [CrossRef]
- Jordan, C.F. Derivation of leaf-area index from quality of light on the forest floor. Ecology 1969, 50, 663–666. [Google Scholar] [CrossRef]
- Tucker, C.J. Red and photographic infrared linear combinations for monitoring vegetation. Remote Sens. Environ. 1979, 8, 127–150. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Merzlyak, M.N.; Chivkunova, O.B. Optical properties and nondestructive estimation of anthocyanin content in plant leaves. Photochem. Photobiol. 2001, 74, 38–45. [Google Scholar] [CrossRef]
- Peñuelas, J.; Filella, I.; Lloret, P.; Muñoz, F.; Vilajeliu, M. Reflectance assessment of mite effects on apple trees. Int. J. Remote Sens. 1995, 16, 2727–2733. [Google Scholar] [CrossRef]
- Peñuelas, J.; Gamon, J.A.; Fredeen, A.L.; Merino, J.; Field, C.B. Reflectance indices associated with physiological changes in nitrogen- and water-limited sunflower leaves. Remote Sens. Environ. 1994, 48, 135–146. [Google Scholar] [CrossRef]
- Justice, C.O.; Vermote, E.; Townshend, J.R.G.; Defries, R.; Roy, D.P.; Hall, D.K.; Salomonson, V.V.; Privette, J.L.; Riggs, G.; Strahler, A.; et al. The Moderate Resolution Imaging Spectroradiometer (MODIS): Land remote sensing for global change research. IEEE Trans. Geosci. Remote Sens. 1998, 36, 1228–1249. [Google Scholar] [CrossRef]
- Wang, X.; Wang, M.; Wang, S.; Wu, Y. Extraction of vegetation information from visible unmanned aerial vehicle images. Nongye Gongcheng Xuebao/Trans. Chin. Soc. Agric. Eng. 2015, 31, 152–159. [Google Scholar] [CrossRef]
- Gitelson, A.A. Wide dynamic range vegetation index for remote quantification of biophysical characteristics of vegetation. J. Plant Physiol. 2004, 161, 165–173. [Google Scholar] [CrossRef]
- Broge, N.H.; Leblanc, E. Comparing prediction power and stability of broadband and hyperspectral vegetation indices for estimation of green leaf area index and canopy chlorophyll density. Remote Sens. Environ. 2001, 76, 156–172. [Google Scholar] [CrossRef]
- Roujean, J.L.; Breon, F.M. Estimating PAR absorbed by vegetation from bidirectional reflectance measurements. Remote Sens. Environ. 1995, 51, 375–384. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).