Abstract
In this study, we examine leaf reflectance as the main optical property used in remote sensing of vegetation. The total leaf reflectance consists of two main components: a diffuse component, originating from the leaf interior, and a component reflected directly from the leaf surface. The latter contains specular (mirror-like) reflectance (SR) and surface particle scattering, driven by the surface roughness. Our study aimed to (1) reveal the effects of key leaf structural traits on SR in 400–2500 nm, and (2) compare the performance of PLSR models of leaf biophysical properties based on the total reflectance and SR removal reflectance. Four Arabidopsis thaliana structural surface mutants and six Hieracium species differing in trichome properties were studied. PCA did not reveal any systematic effect of trichome density, length, and morphology on SR. Therefore, the results do not support the hypothesis that leaves with denser and longer trichomes have lower SR and higher total reflectance than the smooth leaves. SR removal did not remarkably improve PLSR models of biophysical traits (up to 2% of RMSE). Thus, in herbaceous dorsiventral leaves with relatively sparse trichomes of various morphology and without apparent waxy surface, we cannot confirm that SR removal significantly improves biophysical trait prediction.
1. Introduction
Leaf optical properties, i.e., reflectance, absorbance, and transmittance, are defined as the fractions of incoming light that are reflected, absorbed, and transmitted by a leaf, respectively. Leaf reflectance as the main optical property used in the evaluation of vegetation status on a large scale using remote sensing approaches [1] and plant phenotyping [2,3].
Glossy green leaves may appear white in some view directions, meaning they have a strong reflectance in one specific direction compared to others [4,5]. This simple observation demonstrates that the leaf reflectance exhibits an angular dependency on incident and viewing angle [6]. A previous assumption that a leaf acts as a Lambertian reflector (meaning that reflected light is perfectly diffused and constant in all directions) has been rejected by many earlier studies, including [7,8].
The light reflected from a leaf consists of two components with different origins. First, the radiation reflected from a leaf interior, which is diffuse and determined primarily by leaf biochemical components, water content [1,9], intercellular air spaces [10,11], and the orientation of cell walls [12] in the leaf mesophyll. Second, the radiation reflected from a leaf surface, which is independent of leaf internal composition and structure, and is determined by the surface properties of the epidermis. Light reflected at an angle equal to the angle of incidence produces specular (mirror-like) reflection [13]. This specular fraction of leaf reflectance, in particular, contributes to the bias from a Lambertian reflection model [14]. The light scattered on very small particles (comparable in size to the wavelength of incident light) at the leaf surface is reflected away from the specular direction and is likely neglected in most plant studies [15].
Separation of the specular component from the diffuse reflectance can be achieved by various optical methods. In the case of bidirectional reflectance factor measurement [16], it is typical to use optical devices measuring the degree of light polarisation [15,17,18,19], as the light reflected from the leaf surface is polarised in comparison to the diffuse light reflected from inside of the leaf [5]. Another common measurement set-up for bidirectional spectra measurements is placing a sensor at an angle equal to that of the incident light source [20]. If the light reflected from a leaf is integrated over the whole hemisphere using an integrating sphere, the specular component can be removed by placing a specular exclusion light trap at the designated angle of incidence [21].
Because specular reflectance does not contain information from the leaf interior, it was considered a possible source of error in the retrieval of leaf biochemical compounds [18]. The idea of removing the specular component of reflectance from vegetation indices has been investigated for decades [22,23,24]. Recently, improved chlorophyll content estimation after removing the specular reflectance from total reflectance was achieved [18]. Efforts have also been made to model the specular component in the PROSPECT leaf-level radiative transfer model [25], or to include leaf surface parameters and their effects in the PROSPECT model [26].
Due to the lack of interactions with light-absorbing pigments, specular reflectance is assumed to be constant in visible (VIS) wavelengths [18] with a tendency to increase through the near-infrared spectral region [27,28]. Specular reflectance strongly depends on the angle of incident light [8,29]. McClendon [20] investigated the specular reflectance of several trees, shrubs, and herbs with different epidermal anatomy in the wavelengths around 632 nm. Regardless of the character of leaf surface (waxy, glabrous, or hairy), the specular reflection was several folds higher than the diffuse reflection. The authors of [15] also studied the effect of leaf surface on specular reflectance and showed that the less hairy and glabrous leaves had noticeably higher specular reflectance in comparison to densely pubescent leaves with lower specular reflectance. The undulation of leaf surface and venation has been shown to increase specular reflectance [15]. The effect of epidermal surface properties on reflectance in VIS and near-infrared (NIR) spectral regions was recently explored using measurements from multiple angular geometries [18,27,30] on leaves with very distinct surface properties: leathery, smooth, and shiny; intermediate epidermal roughness with venation or sparse trichomes; and finally, leaves with rough undulating surface and denser trichomes. The three studies confirmed that the apparently smooth and shiny leaves show their sharp maximal reflection peak in the specular viewing direction, i.e., having equal incident and viewing angles. This peak of reflection in the specular direction was almost absent in rough leaves with trichomes. However, epidermal traits were not quantified in any of the above cited studies, and only qualitative measures of leaf surface were used.
From an ecophysiological point of view, leaf optical properties may reflect an adaptation to a particular environment and contribute to efficient water relations and light capture. Understory species have relatively high photosynthetic rates under diffuse light, and exhibit decrease in the photosynthetic rate when wet [31]. Vanderbilt [32] proposed that the specular reflectance originates from cuticle and waxes, which also have water repulsion functions at the leaf surface [33]. The golden surface shine of Tradescantia leaves was proven to exhibit specular reflectivity from epidermal nanostructures having a photoprotection purpose [34]. Greater light scattering caused by trichomes was hypothesised by Gates and Tantraporn [35] and confirmed by Qiu et al. [26] as a protection mechanism against excessive photosynthetically active radiation [36].
To assess the influence of trichomes on leaf optical properties, some authors have measured optical properties on hairy leaves before and after removing trichomes [36,37,38,39]; however, this may have resulted in artifacts caused by the removal of trichomes. The results were, nevertheless, ambiguous regarding the trichome effect on the reflectance along the VIS–NIR spectrum range. Gausman and Cardenas [37] observed a reflectance drop in the range of 750–1000 nm, which they explained by both trichome removal and loss of phenolic compounds due to trichome shaving.
The aims of our study were: (1) to investigate the effects of selected quantitative epidermal traits (trichome density and length, and adaxial epidermis thickness) on specular reflectance in visible and near-infrared spectral regions, and (2) to compare the performance of partial least squares regression (PLSR) prediction models of leaf biophysical properties based on the total reflectance and the reflectance after specular component removal. To avoid artefacts caused by trichome removal, the study was conducted on intact leaves of two groups of plants with different surface variability: (1) four selected Arabidopsis thaliana surface structural mutants, and (2) six selected Hieracium species with various trichome density and morphology. We hypothesised that the epidermal traits affect the specular reflectance and that leaves with denser and longer trichomes have lower specular and higher total reflectance than the smooth leaves with fewer or no trichomes. We expected that the exclusion of leaf specular reflectance would improve the performance of PLSR models for leaf biochemical traits estimation.
2. Materials and Methods
2.1. Plant Material and Anatomical Structure Assessment
Two narrow taxonomical groups of the herbaceous plant genus (A. thaliana and Hieracium species) with dorsoventral leaf anatomy were selected to minimise the variation in other leaf traits but epidermal surface structure. We used closely related taxonomic units to ensure comparable leaf internal structure and minimise the influence of internal structural and biochemical traits on leaf optical properties.
2.1.1. Plant Material and Cultivation
We studied the anatomical and optical properties of wild type (WT, Columbia-0) and four structural mutants (exo70h4-1, tbr1, arpc5, and gl1) of Arabidopsis thaliana (L.) Heynh. (Table 1).

Table 1.
Leaf epidermal structural traits of studied plants: wild type Columbia-0 and four A. thaliana structural mutants, with descriptions of surface structural mutation from a referenced study.
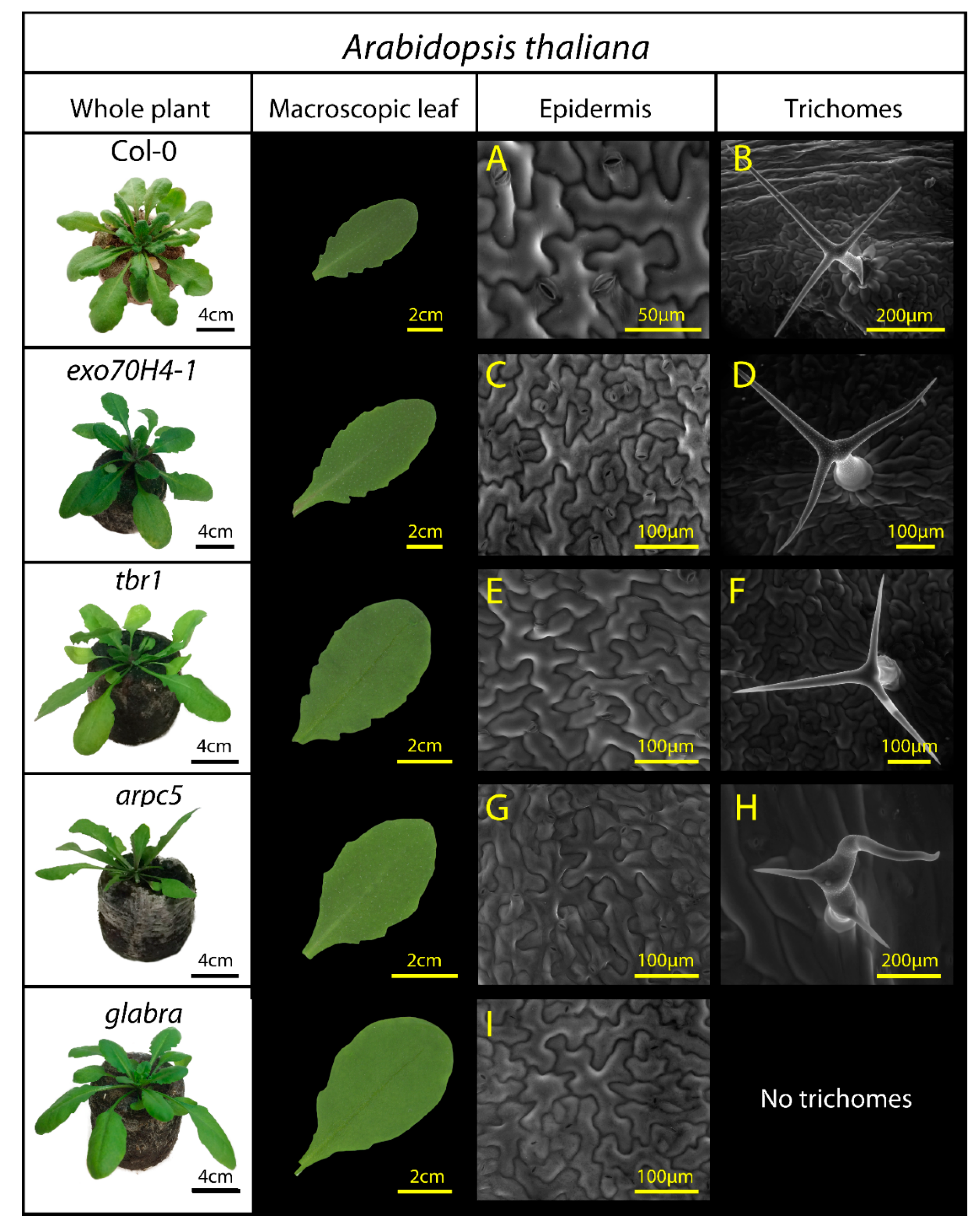
The studied lineages exhibit modified epidermal structure, particularly in trichome presence or trichome morphology (Table 1, Figure 1).
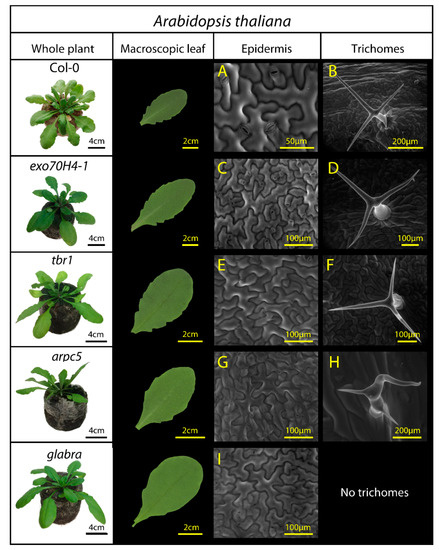
Figure 1.
A. thaliana lineages used in the study: Col-0 (wild type), exo70H4-1, tbr1, arpc5, and glabra. Third and fourth columns present epidermal surface observations by environmental scanning electron microscopy, including structure of pavement epidermal cells (A,C,E,G,I) and structure of trichomes (B,D,F,H), if present. Epidermal mutations are detailed in Table 1.
In Arabidopsis, the differences in epidermal properties among mutants were given by their specific genetic mutations. Apart from the glabra mutant, where trichomes are not observed, we expected no significant differences in trichome density, but rather in trichome structure. Distorted trichomes with short branches are characteristic of arpc5 mutant, as is the absence of secondary cell wall in exo70H4-1 trichomes and reduced crystalline cellulose deposition in tbr1 trichomes.
A. thaliana plants were cultivated in a growing room with a long day (16 h light and 8 h dark (16/8)) in temperature 24 °C, with 100 µmol m−2 s−1 without any artificial illumination, for 14 days in August 2018 to achieve suitable germination. To prevent early maturation and flowering of A. thaliana plants in long-day conditions, they were moved to the greenhouse without controlled temperature conditions and ambient, natural illumination and grown for the next 2 months from August to September 2018 until leaf area of their leaves was sufficient for the measurements in the integrating sphere and all biochemical and structural analyses.
In addition to the Arabidopsis experimental system, we selected six species of the Hieracium genus with differences in indumentum: H. prenanthoides Vill., H. intybaceum All., H. sabaudum L., H. nigrescens Willd., H. setigerum Tausch, and H. coldei Szeląg (Table 2).

Table 2.
Leaf description including epidermal surface structure, ecology of six Hieracium species from a referenced study.
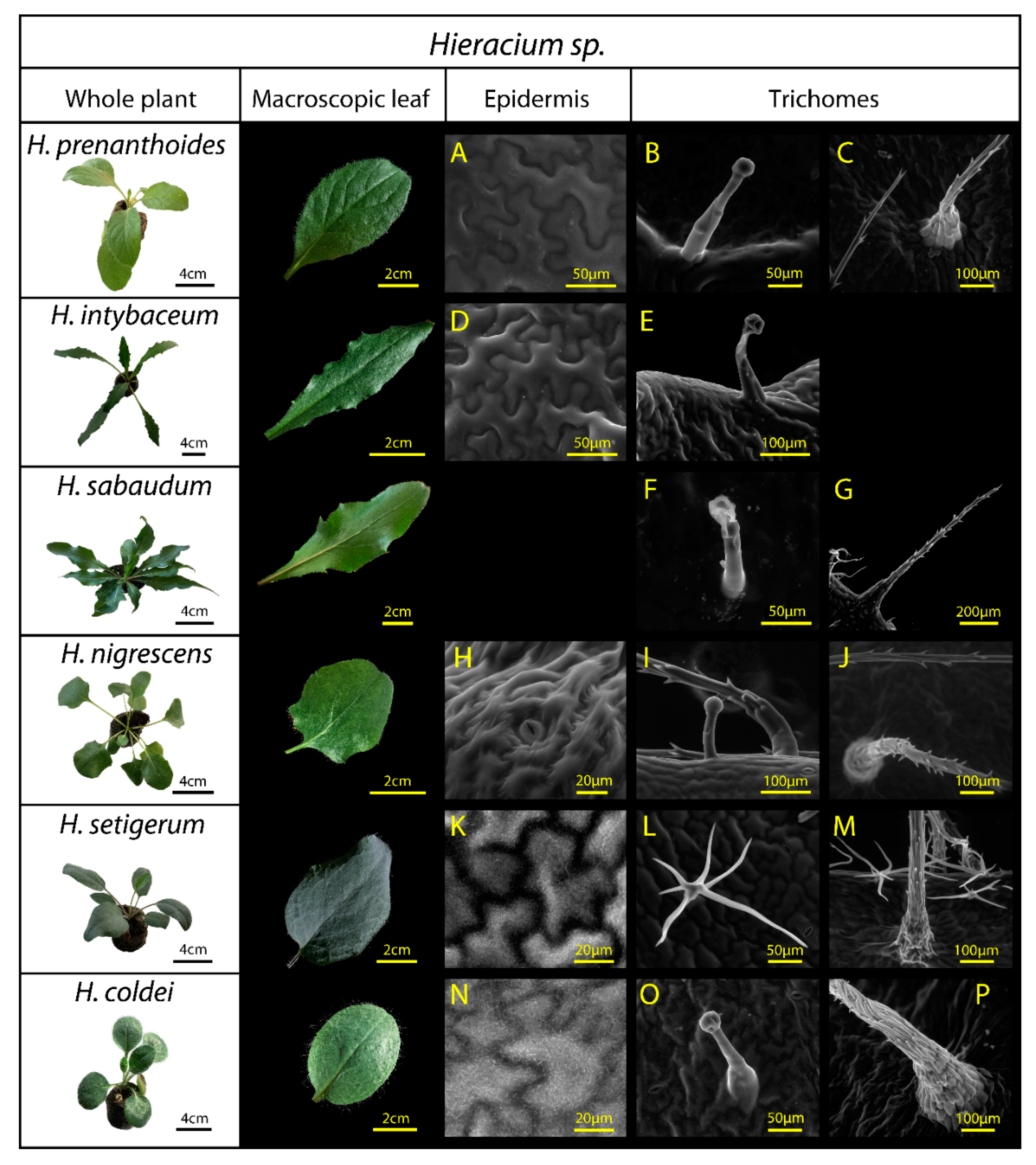
These Hieracium species exhibit natural variation in the epidermal structure, including differences in trichome length and density, and in the epicuticular wax abundance and structure (Table 2, Figure 2). We observed three trichome morphology types in the studied Hieracium species: mostly glandular trichomes (H. prenanthoides, H. intybaceum, H. sabaudum, H. nigrescens, H. coldei), (Figure 2B,E,F,I,O), followed by stiff trichomes (H. prenanthoides, H. sabaudum, H. nigrescens, H. setigerum, H. coldei), (Figure 2C,G,J,M,P) and branched (stellate) trichomes (H. setigerum) (Figure 2L). All these differences may affect specular reflectance.
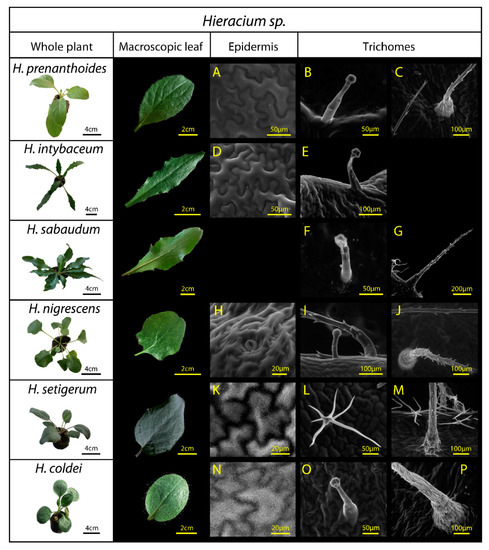
Figure 2.
Hieracium species used in the study: H. prenanthoides, H. intybaceum, H. sabaudum, H. nigrescens, H. setigerum, and H. coldei. The three columns on the right (A–P) present epidermal surface observations by environmental scanning electron microscopy. Third column: leaf epidermal pavement cells without waxes (A,D,H), and with a wax layer on the epidermal pavement cells (K,N). Fourth column: glandular trichomes (B,E,F,I (a smaller one),O) and branched (stellate) trichome in H. setigerum (L). Fifth column: multicellular stiff trichomes (C,G,I (base of the trichome at right)) J,M (base of the trichome in front), P (base of the trichome). For expanded description of surface structures, see Table 2. Due to the epidermal cell collapsing during ESEM acquisition, we did not include H. sabaudum epidermal cells in the figure; however, we did not observe there any surface waxes, or roughness on the surface, which could influence leaf reflectance.
Hieracium plants were cultivated in a growing room under short-day conditions (8/16), to prevent plants from early flowering at a temperature of 24 °C, with 120 µmol m−2 s−1, without any additional artificial illumination, for approximately 2 months from May to July 2019.
For optical measurements, we selected leaves with sufficiently large leaf area to fully cover a field of view in the integrating sphere (diameter = 1.5 cm), regardless of leaf insertion. The same leaves used for optical measurements were used also for analysing leaf structural parameters.
2.1.2. Anatomical Structure Assessment
Specific leaf area (SLA) was determined for A. thaliana plants and Hieracium species on leaves sampled during the day of harvesting. Leaves were weighed, scanned, then placed into an oven for 3 days at 60 °C. After drying, the samples were weighed again and their leaf areas were expressed as leaf area per unit of dry mass as determined using ImageJ software (https://imagej.net/ (accessed on 15 October 2021)) while following the protocol of [52,53].
Microscopic visualisation of leaf epidermal surface structures of A. thaliana and Hieracium species (including size and type of trichomes and presence of epicuticular wax deposition) was performed using a Quanta 200 Environmental Scanning Electron Microscope (ESEM), (FEI Technologies Inc. Hillsboro, Oregon 97124, USA;Thermo Fischer Scientific) up to 1 week from the day of optical properties measurements. Fresh leaves were scanned in the native state in the environmental chamber of the microscope. The specimen was observed under pressure of 260 Pa and temperature −12 °C with water equilibrium and 100% relative humidity. Quantification of trichome density for A. thaliana leaves was performed from scanned fresh true leaves with 600 dpi resolution while using a systematically uniform sampling approach [41] and point-counting method with superimposed point grid in ImageJ.
Quantitative anatomical analysis of trichome density was performed for Hieracium species on fixed leaf samples. Leaf segments (squares of 0.5 cm2) of all samples were fixed in formalin–acetic acid–alcohol (FAA) fixative (70% ethanol, 40% formaldehyde, acetic acid in volume ratio 90:5:5). Images of fixed leaf segments stained with toluidine blue to visualise cell walls [54] were acquired by a Cannon EOS100D camera mounted on an Olympus BX40 microscope with 30× magnification. To assess trichome length and density on equal leaf area, a square mask of 12.8 mm2 was applied on each image in AdobePhotoshop Lightroom 3.0 software (https://www.adobe.com/products/photoshop-lightroom.html (accessed on 15 October 2021)) and trichomes were counted manually in ImageJ using an unbiased counting frame.
Leaf cross sections (about 80 µm thick) of Hieracium leaves were prepared from previously FAA-fixed leaf samples using a hand microtome and then stained by toluidine blue [54]. Using a light microscope, bright field images (five per each leaf segment) were acquired using the same microscope and camera and processed in ImageJ to measure leaf thickness parameters [52]. These were whole leaf thickness; thicknesses of mesophyll, palisade, and spongy parenchyma; and thicknesses of adaxial (upper) and abaxial (lower) epidermis. Each parameter was measured three times on each image in leaf cross section at regular intervals. The ratio of palisade to spongy parenchyma (PP/SP) was calculated.
Based on our observation, there was no significant difference in SLA among the genotypes of A. thaliana with the exception that there was a significant difference between Col-0 and arpc5. We decided, however, not to perform leaf sectioning.
2.2. Leaf Optical Properties Assessment
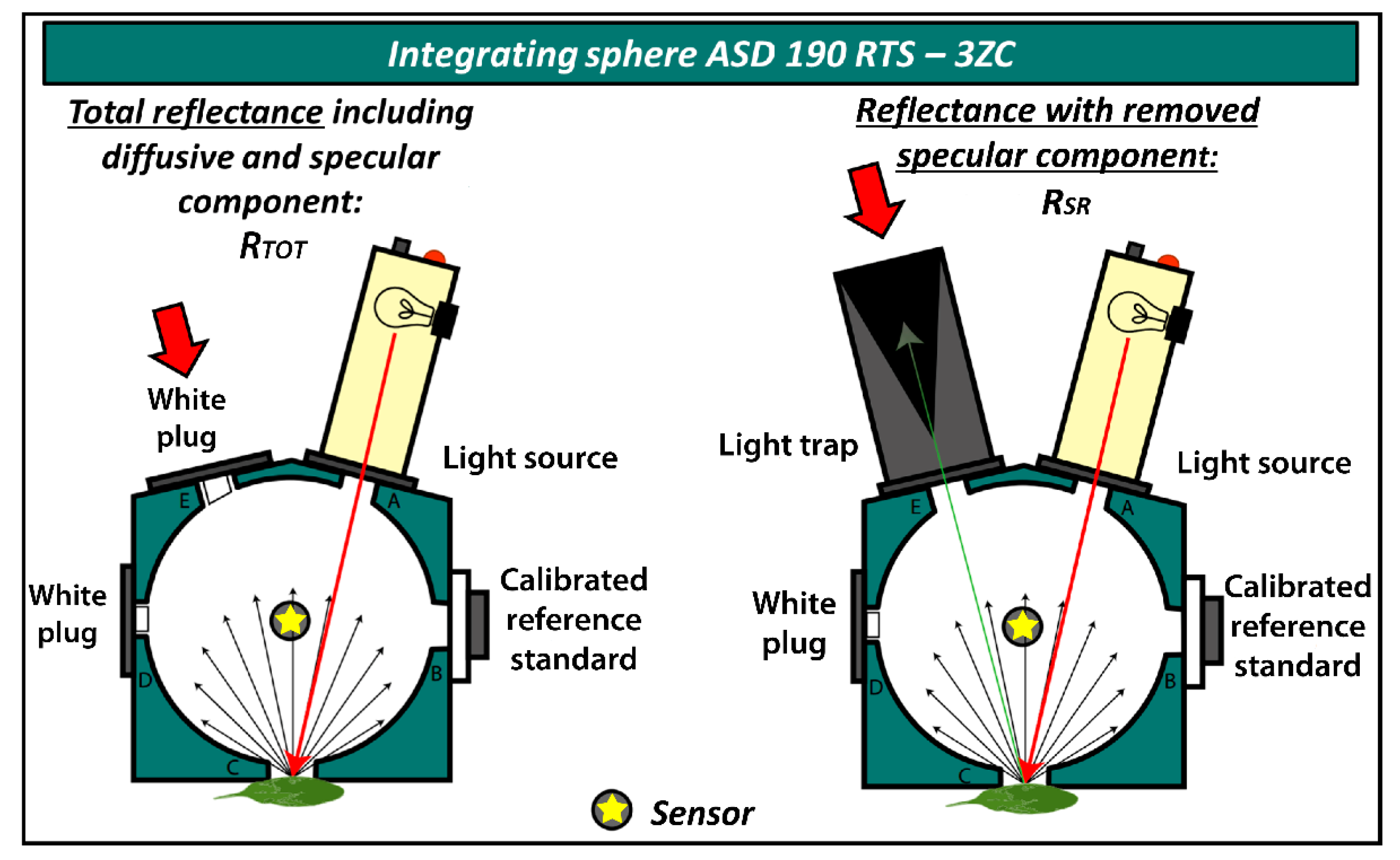
Leaf optical properties in the 350–2500 nm spectral range were measured by spectroradiometer ASD FieldSpec 4 with an integrating sphere ASD 190 RTS—3ZC (both Malvern Panalytical, Cambridge, UK [55]. This allowed us to measure hemispherical integrated reflectance as the directional–hemispherical reflectance factor (DHRF) [16] of the adaxial side of the leaf. For Arabidopsis, whole leaves were used for completely covering the field of view of the integrating sphere; for Hieracium, leaves were measured excluding the midrib region if possible. Measurements were taken in digital number (DN) values and the integration time was 136 ms, averaging 25 readings for sample measurements and 50 readings for instrument optimisation. White calibration measurements were taken after each sample, no longer than 15-minute intervals between. The sphere was specifically designed to allow specular reflectance measurements by placing a light trap in the corresponding specular reflection measurement geometry. Two reflectance quantities, in the same sample positions, can be measured: (1) total directional–hemispherical reflectance including specular component (RTOT), and (2) leaf directional–hemispherical reflectance with the specular component removed from the total reflectance (RSR). The latter corresponds to diffuse reflectance measurements when the light trap is placed in the angle equal to that of the light source relative to the sample surface (Figure 3).
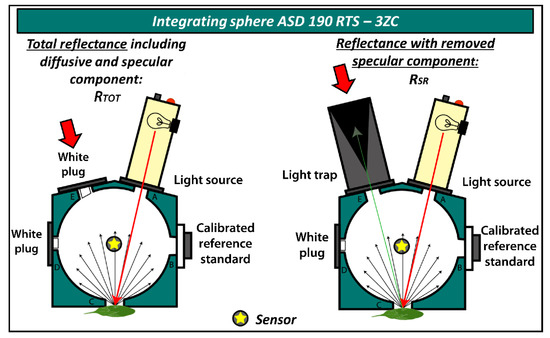
Figure 3.
Measuring leaf reflectance by two integrating sphere set-ups: total reflectance (RTOT) and reflectance with specular component removed (RSR). Yellow star in grey circle represents the optical cable sensor, which was placed on the surface of the integrating sphere.
The specular component of reflectance (RSPEC) can then be calculated as the difference RTOT—RSR. Due to technical problems, measurements of RSR for wild type of A. thaliana, (Col-0) were not possible to accomplish. However, missing wild type (Col-0) used as a control in most studies on mutants is not crucial in this study. The main emphasis was put on variation in trichome density, which has been preserved even if Col-0 was left out. For example, tbr1 shows the same trichome density as Col-0 (Figure 4D). In the case of A. thaliana lineages, spectral data were analysed only for the four mutants (arpc5, exo70H4-1, tbr1, and glabra). In total, 36 leaf samples were measured for A. thaliana and 45 for Hieracium.
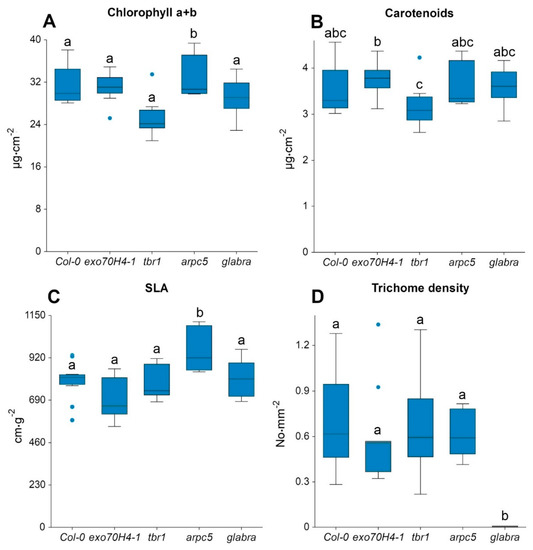
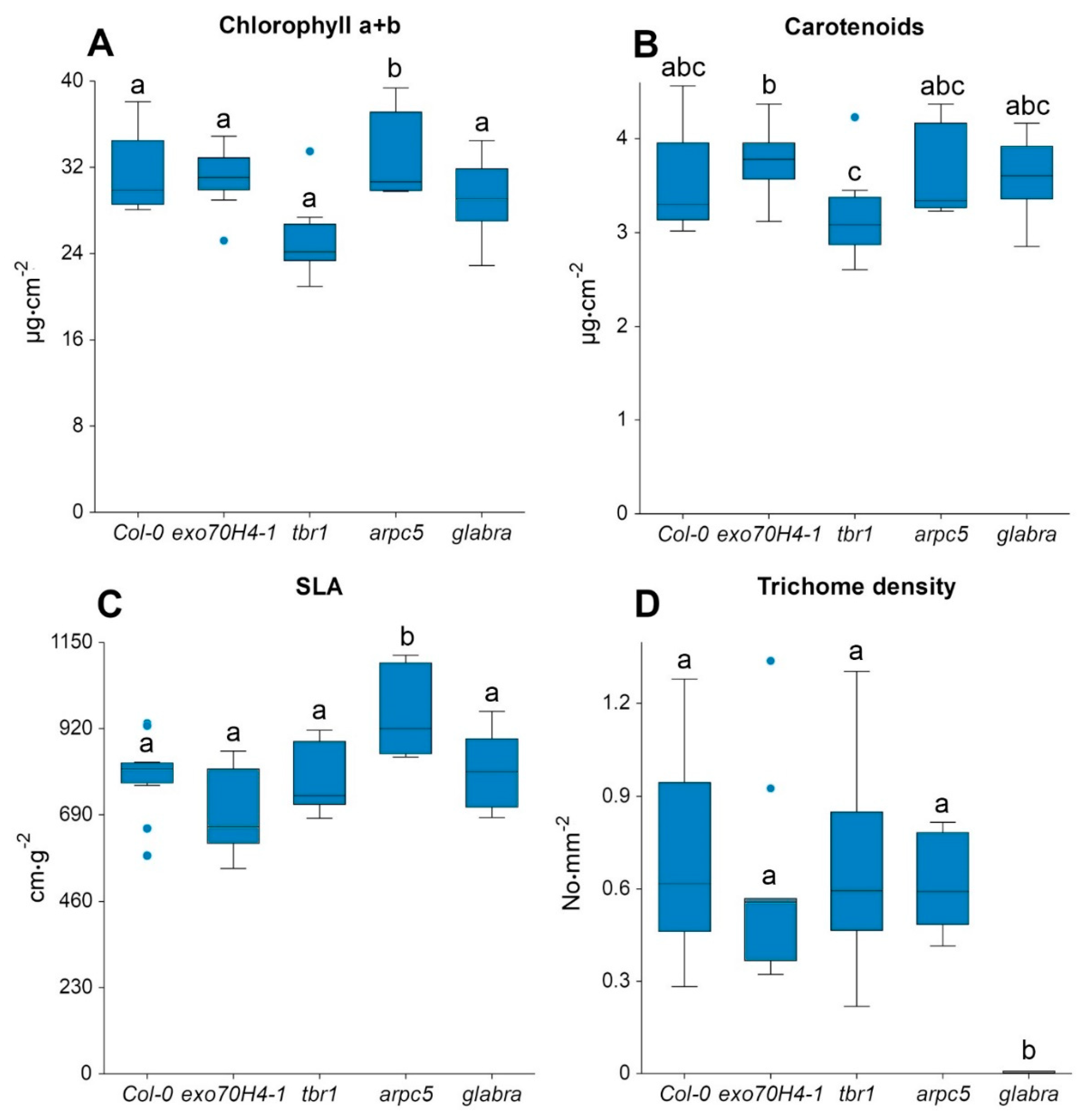
Figure 4.
Leaf biochemical and structural parameters for A. thaliana lineages (Col-0, exo70H4-1, tbr1, arpc5, and glabra): (A) chlorophyll a + b content, (B) carotenoids content, (C) specific leaf area (SLA), and (D) trichome density. Differences among lineages were tested by one-way ANOVA. In the case of normal distribution, the Tukey–Kramer multiple comparison test was used; otherwise, the Kruskal–Wallis test was applied. Significance was set at a p-value < 0.05. Lines in boxes show median, bars show the inter-quartile range, and dots correspond to outliers.
2.3. Biochemical Assessment of Leaf Chlorophyll and Carotenoids Contents
After measurement of leaf optical properties by spectroradiometer, the same leaf was sampled to determine leaf pigment composition biochemically by spectrophotometer (i.e., chlorophyll a + b and total carotenoids). A leaf disc of 50 mm2 was cut and pigments were extracted in dimethylformamide [56] until the leaf disc was bleached. Absorbances at 480 nm, 647 nm, 664 nm, and 750 nm were measured using an Evolution 201 spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA) and the contents of chlorophyll a + b (Chl a + b) and carotenoids (Car) were calculated using equations from Wellburn [57] and related to leaf area.
2.4. Data Processing and Statistical Analysis
2.4.1. Biophysical Properties
Statistical comparison of selected leaf biochemical and structural traits (Chl a + b, Car, SLA, trichome length and density, and leaf thickness) were performed using NCSS 9 software by means of one-way ANOVA separately for Arabidopsis and Hieracium plant groups. When residuals were normally distributed, the Tukey–Kramer multiple comparison test was used. Otherwise, the Kruskal–Wallis test was applied. Significance was set at p-value < 0.05.
2.4.2. Visualisation of Spectral Curves
For each species of Hieracium and mutants of Arabidopsis, an average and standard deviation of measured total reflectance (RTOT) and reflectance with removed specular component (RSR) was calculated from 5 to 12 individual samples. For visualisation purposes, a Savitzky–Golay filter with 3rd degree polynomial and 21 nm frame length was applied to remove spectral noise in the data. The dataset was limited to the 400–2400 nm spectra region to exclude unreliable observations with excessive noise. The RTOT and RSR of species of Hieracium and mutant of Arabidopsis were visualised as a shaded error bar with average reflectance as line and standard deviation as vertical area, and RSPEC on the secondary y-axis as line. For further analysis, no filtered data were used, and to exclude noisy character of spectra below 400–450 and above 1800 nm, we used spectral ranges described below.
2.4.3. Exploring of Leaf Specular Reflectance by Using PCA
Principal component analysis (PCA) was applied on specular reflectance spectra in 450–1800 nm. PCA was based on the NIPALS algorithm [58]. To reveal patterns in specular reflectance related to the selected surface traits (trichome density for both model systems; trichome length and adaxial epidermis thickness for Hieracium), categorical variables (mutant or species) and gradual ranges of structural traits were used for sample grouping in PCA score plots. Trichome densities in both species and epidermal thickness in Hieracium were log-transformed before the analysis. For Arabidopsis, the trichome density was not transformed as there were zero values in glabra mutants. The five gradual ranges of trichome density, trichome length, and adaxial epidermis thickness were automatically created by the software Unscrambler 11 (CAMO Analytics, Oslo, Norway), and were applied as categorical variables for scores grouping of surface properties.
2.4.4. PLSR Modelling
Prediction models for selected leaf biophysical and structural traits were established, using PLSR analysis with the same NIPALS algorithm as the PCA. For each leaf trait, two models were created: the first based on RTOT and the second based on RSR. The model input variables were the measured leaf spectra (RTOT or RSR between 450 and 1800 nm) representing independent variables and the leaf biophysical and surface traits were then dependent variables. The PLSR modelling included the standard calibration and the validation stages [59,60]. To assess the model performance, a random cross-validation was conducted. The model was cross validated with random segments: five segments for Arabidopsis and eight segments for Hieracium. The models were evaluated based on the standard measures [61] such as the lowest number of factors (a measure of model robustness), the lowest root mean square error of prediction (RMSEP), %RMSR (% of root mean square error (RMSE) related to average values of parameters), and the coefficient of determination (R2). PLSR modelling was performed in Unscrambler 11 (CAMO Analytics, Oslo, Norway). Finally, the performance of models based on RTOT and RSR was compared.
3. Results
3.1. Leaf Biochemical and Structural Traits
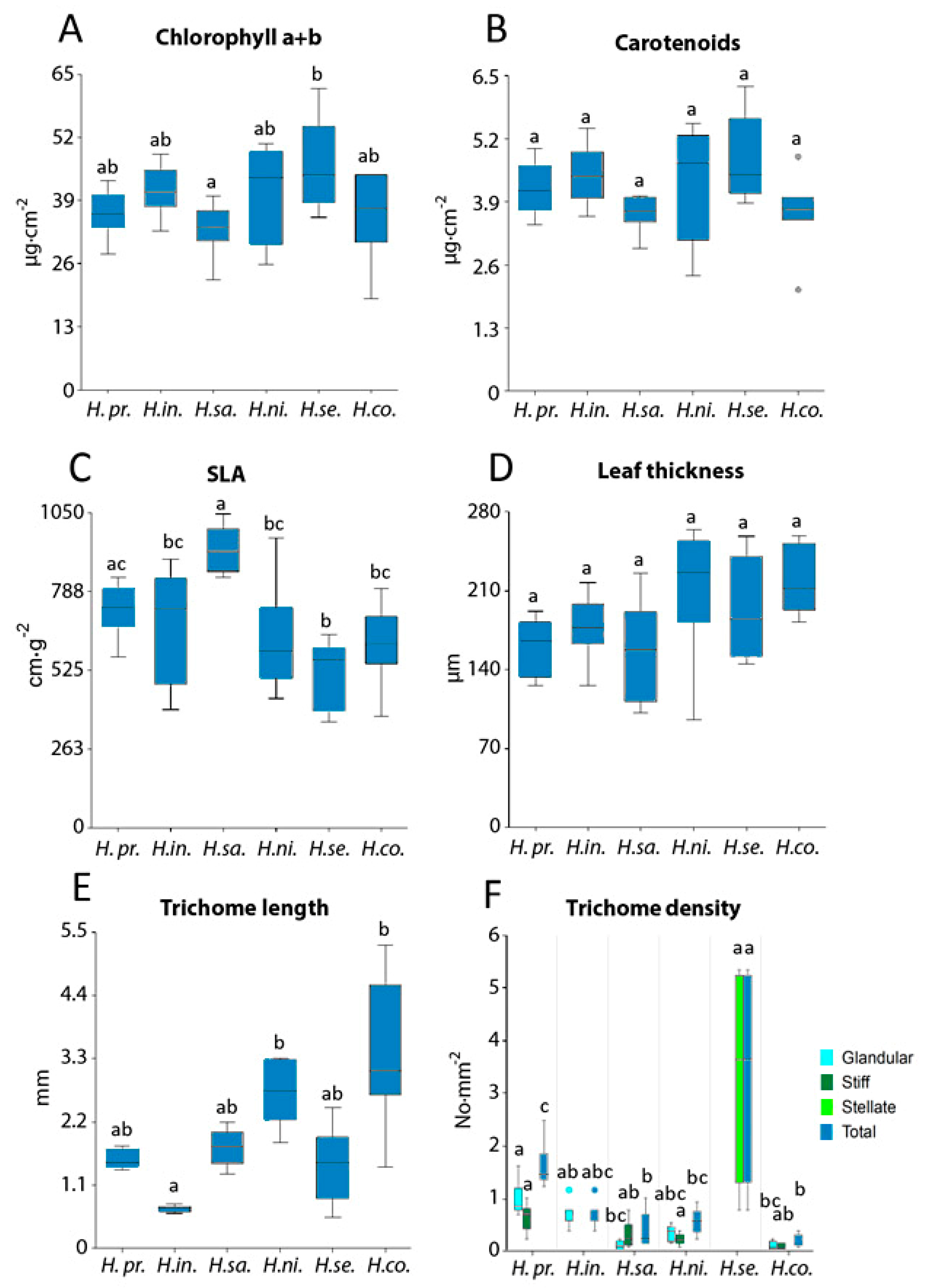
Figure 5F shows the presence and abundance of trichome types (glandular, stiff, and stellate) for each species and differences in trichome type density among the species. H. setigerum, with branched trichomes, showed the highest total trichome density, which was significantly higher than in other Hieracium species, and simultaneously the highest variance in trichome density among all studied species.
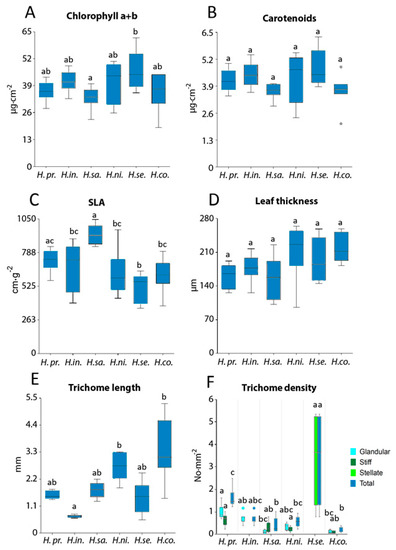
Figure 5.
Leaf biochemical and structural parameters for six Hieracium species (H. prenanthoides, H. intybaceum, H. sabaudum, H. nigrescens, H. setigerum, and H. coldei). (A) Chlorophyll a + b content, (B) carotenoids content, (C) specific leaf area (SLA), (D) leaf thickness, (E) trichome length, and (F) trichome density. Different letters above columns correspond to statistical differences among species as tested by one-way ANOVA. In (F), one-way ANOVA was performed separately for each trichome type, and the only statistically significant difference in trichome density was found for stellate trichomes in comparison to glandular and stiff ones. In the case of normal distribution, the Tukey–Kramer multiple comparison test was used; otherwise, the Kruskal–Wallis test was applied. Significance was set at p-value < 0.05. Lines in boxes show median, bars show inter-quartile range; dots correspond to outliers.
We observed a similar pattern of Chl a + b and SLA among the Arabidopsis lineages, with only exception for arpc5 with significantly higher values compared to other lineages (Figure 4A,C). For Car, the only significant differences were observed between exo70h4-1 and tbr1 mutants (Figure 4B). Significant differences in trichome density occurred between glabra and the other lineages (Figure 4D), as had been expected based on the descriptions of the mutants (Table 1).
Hieracium species showed no significant differences for Car (Figure 5B) and leaf thickness (Figure 5D) whereas significant differences for Chl a + b (Figure 5A) and SLA (Figure 5C) were observed among the Hieracium species studied. H. sabaudum had the lowest Chl a + b and highest SLA values among the species, and H. setigerum had the highest Chl a + b and lowest SLA values.
In Figure 5E, we present total trichome length for Hieracium species regardless of the type of trichomes. In Figure 5F, we present total trichome density for Hieracium species with respect to the type of trichomes—glandular, stiff, and stellate—and as a total density regardless of trichome type. In case of trichome length and density, H. coldei had the longest stiff trichomes (up to 5 mm, Figure 2P and Figure 5E). H. intybaceum (Figure 5E) had the shortest trichomes with low variance, which is given by the presence of only glandular trichomes (Figure 2E). H. setigerum showed the highest density of stellate (Figure 2L) trichomes compared to the rest of the Hieracium species with glandular or stiff trichomes (Figure 5F).
3.2. Leaf Optical Properties: Total, Diffuse, and Specular Reflectance
Leaf directional–hemispherical reflectance factor for RTOT and RSR (DHRF) and for specular component RSPEC (RTOT-RSR), i.e., DHRF specular, were visualised in the spectral range 400–2400 nm (Figure 6 and Figure 7). For individual plant mutants or species, three spectral curves, RTOT, RSR, and RSPEC, are shown in separate graphs: the average of the DHRF is marked by a solid black line for RTOT and a red dashed line for RSR. Grey and light red shadow areas show standard deviation belonging to the same coloured DHRF line. The blue line exhibits the average of the specular component of DHRF, RSPEC.
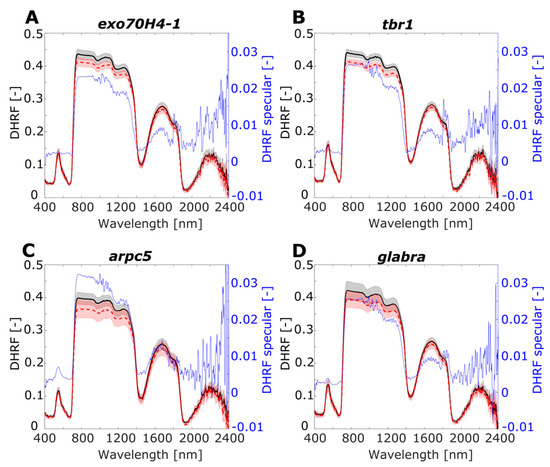
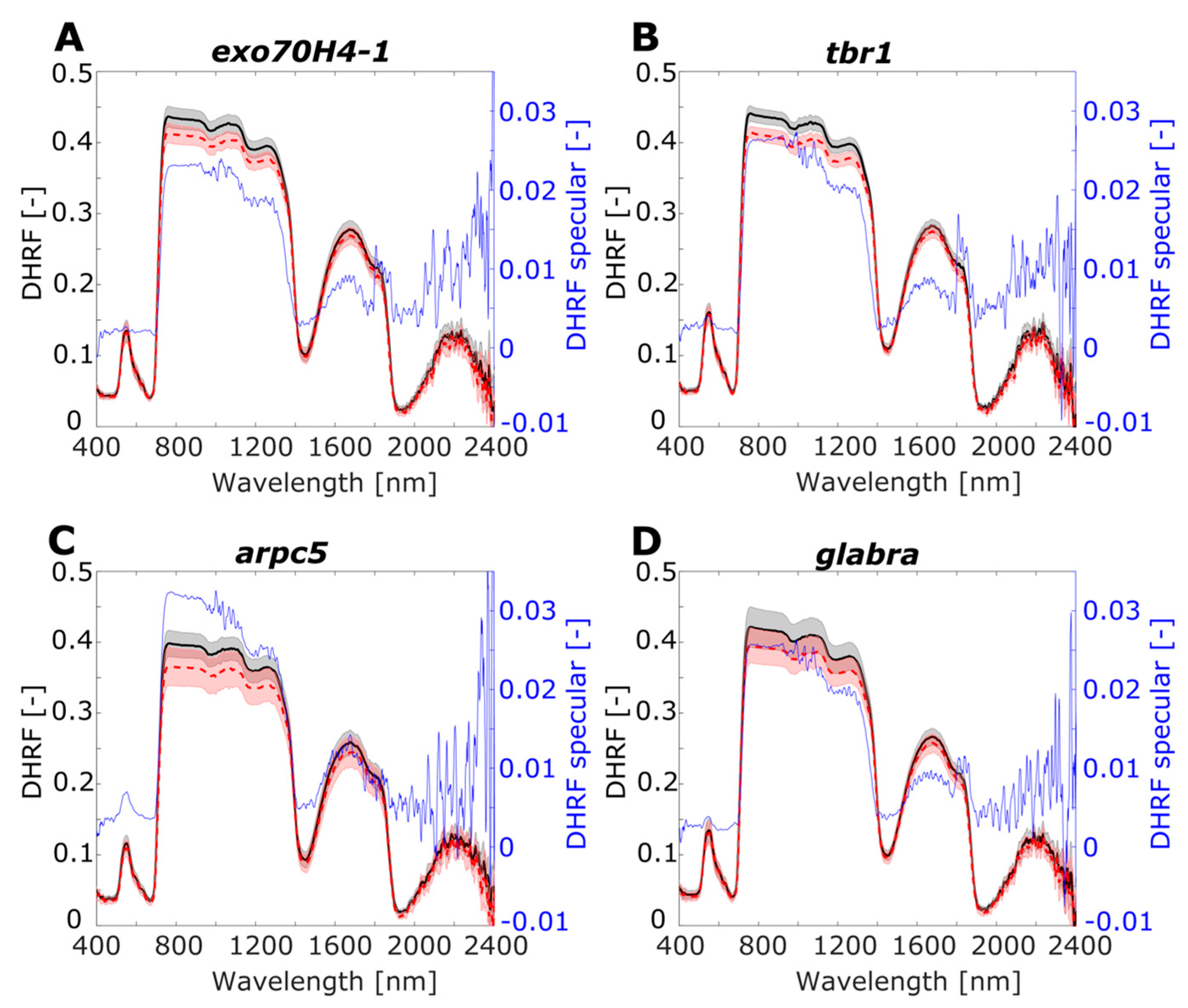
Figure 6.
Leaf optical properties averaged for RTOT, RSR, and RSPEC for A. thaliana lineages: (A) exo70H4-1, (B) tbr1, (C) arpc5, (D) glabra. Left y-axis shows directional–hemispherical reflectance factor (DHRF) of RTOT and RSR, and right y-axis corresponds to DHRF of specular component RSPEC. Grey and light red shadows of RTOT and for RSR curves display variance among the samples by standard deviation value. Black axes (left) correspond to DHRF of RTOT and RSR and blue axes (right) correspond to DHRF of RSPEC.
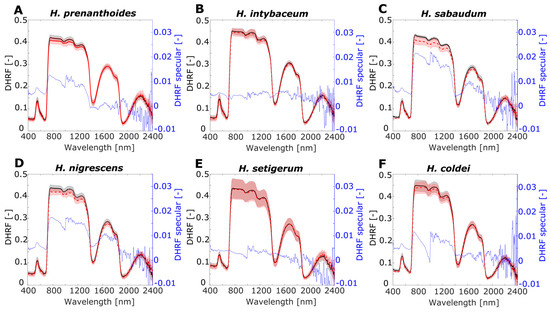
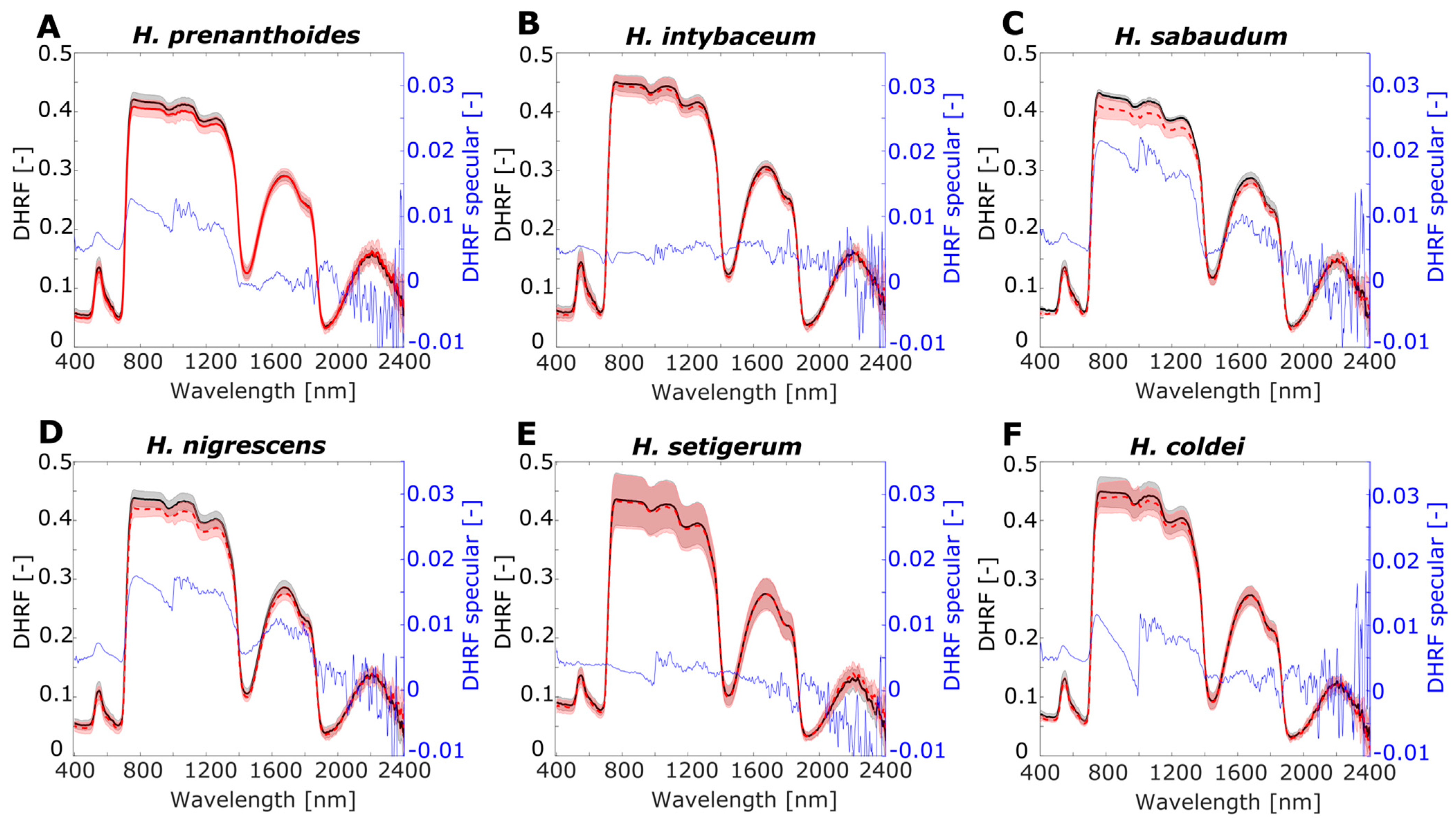
Figure 7.
Leaf optical properties averaged for RTOT, RSR, and RSPEC for Hieracium species (A) H. prenanthoides, (B) H. intybaceum, (C) H. sabaudum, (D) H. nigrescens, (E) H. setigerum, and (F) H. coldei. Left y-axis shows directional–hemispherical reflectance factor (DHRF) of RTOT and RSR, and right y-axis corresponds to DHRF of specular component RSPEC. Grey and light red shadows of RTOT and for RSR curves display variance among the samples by standard deviation value. Black axes (left) correspond to DHRF of RTOT, RSR and blue axes (right) correspond to DHRF of RSPEC.
For both experimental groups of plants A. thaliana and Hieracium, there was higher variance of DHFR in NIR spectral region, compared to the VIS spectral region. For the SWIR spectral region, the most pronounced variance was in the spectral interval 1500–1900 nm. The RTOT and RSR spectra were noisy in longer wavelengths for both groups of plants, which was also apparent for RSPEC: the noise was more pronounced for A. thaliana than for Hieracium. Note that the range of RSPEC is from −0.01 to 0.035 of DHRF, showing negative values due to noise only in longer SWIR wavelengths. For A. thaliana, RSPEC is almost zero in VIS and SWIR regions, except the low green peak in arpc5 and with higher plateau in NIR (0.025–0.03). For Hieracium, RSPEC has no uniform pattern through the VIS, NIR, and SWIR spectral regions, and the shape of the curves differs among the species.
For all Arabidopsis mutants, the difference between the RTOT and RSR was almost negligible in VIS and RSR was always lower than RTOT in NIR. In NIR, arpc5 (Figure 6C) and glabra (Figure 6D) showed remarkably lower RSR than RTOT although having quite high variance, in contrast to exo70H4-1 and tbr1 with low variances (Figure 6A,B). In SWIR, the difference between RTOT and RSR was also negligible, and this spectral region was noisy for all the mutants. The shape of the RSPEC curve in NIR and SWIR up to 1700 nm had the similar shape as RTOT and RSR, with the highest values in NIR reaching 0.03 of DHRF. In the VIS region, only arpc5 mutant displayed a small peak in the green spectral region (0.007 DHRF), in contrast to other three mutants, where the RSPEC was almost flat.
For Hieracium, the difference between RSR and RTOT was negligible in VIS for all studied species (Figure 7). In NIR, H. prenanthoides, H. sabaudum, and H. nigrescens (Figure 7A,C,D) showed lower RSR than RTOT in contrast to H. intybaceum, H. setigerum, and H. coldei (Figure 7B,E,F) with almost no difference in RSR and RTOT. In SWIR, minutely lower RSR than RTOT were detected in H. intybaceum, H. sabaudum, and H. nigrescens (Figure 7B–D). In the rest of the species, RSR and RTOT were almost equal in the SWIR region. RTOT and RSR also have the highest variance in NIR, especially H. setigerum and H. coldei (Figure 7E,F); medium variance was observed for H. intybaceum and H. nigrescens (Figure 7B,D). For H. sabaudum, the variance in NIR was remarkably higher for RSR than for RTOT (Figure 7C). RSPEC is very small for Hieracium species and does not exhibit any pattern, except for the higher values in NIR for H. sabaudum and H. nigrescens with values of DHRF 0.02 (Figure 7C,D).
3.3. PCA of Specular Reflectance—Effect of Leaf Surface Properties
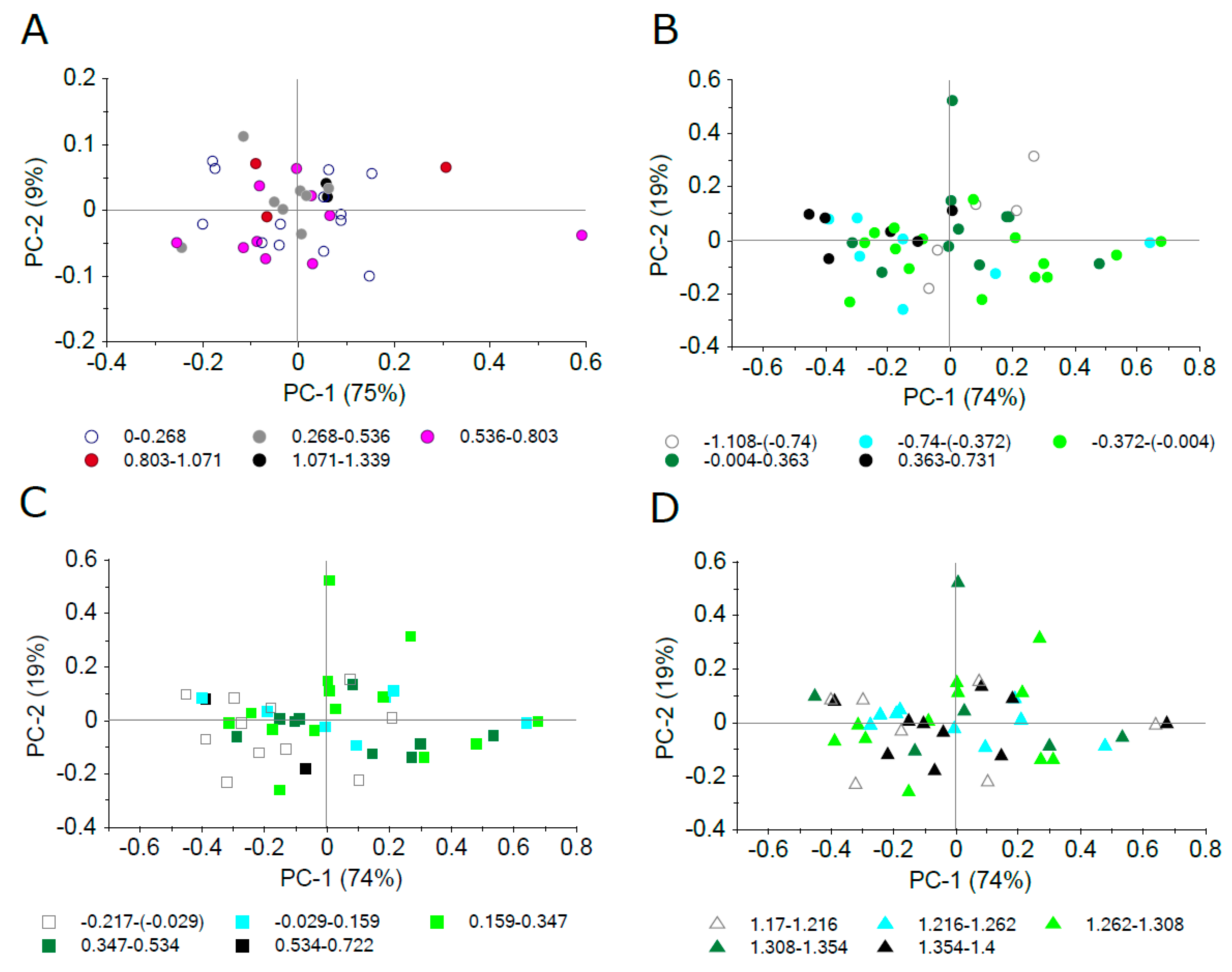
PCA was applied on specular reflectance in the 450–1800 nm spectral range in both plant model systems, A. thaliana and Hieracium, to reveal RSPEC relation to trichome density and length. To find a common pattern of structure in spectral data, we first grouped the scores according to mutants (in A. thaliana) or species (in Hieracium). Despite the significant differences in trichome density in both model plant lineages and trichome length in Hieracium confirmed by the ANOVA analysis (Figure 3 and Figure 4), there was not any apparent clustering linked to mutant or species categories (data not shown). As there was a considerable variation in epidermal traits, we designed five gradual ranges of trichome density, trichome length, and adaxial epidermis thickness which were applied as categorical variables for scores grouping. Figure 8 shows score plots of PCA applied on specular reflectance between 450 and 1800 nm. Different colours and shapes correspond to five intervals of studied epidermal surface traits. As the representatives for all five intervals are distributed evenly along the PC1 and PC2 coordinate space, we conclude that there is no systematic relationship of RSPEC to trichome density in studied A. thaliana lineages and Hieracium species, similarly as for trichome length and adaxial epidermal thickness in Hieracium.
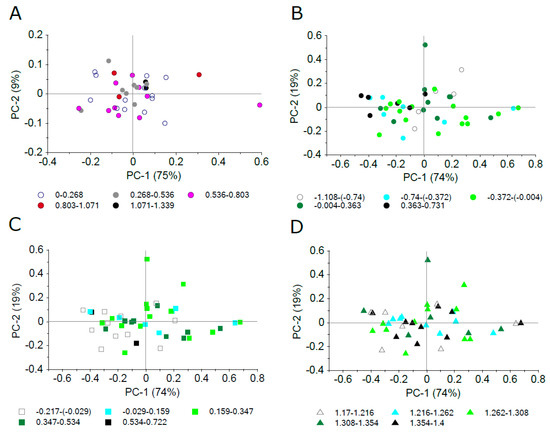
Figure 8.
Principal component analysis of specular reflectance in 450–1800 nm. Sample scores are grouped into five gradual intervals of surface properties, which were automatically created by the software Unscrambler 11 (CAMO Analytics, Oslo, Norway). (A) A. thaliana trichome density, (B) Hieracium sp. trichome density (log-transformed), (C) Hieracium sp. trichome length (log-transformed), (D) Hieracium sp. adaxial epidermis thickness (log-transformed).
3.4. Predicting Leaf Biochemical and Structural Traits by PLSR Models
We hypothesised that the exclusion of leaf specular reflectance would improve the performance of PLSR models for leaf biochemical traits estimation, as the specular reflectance does not origin from the leaf interior. The results of PLSR models based on RTOT and RSR for prediction of biophysical and structural traits of A. thaliana mutants and Hieracium species are given in Table 3.

Table 3.
Parameters of calibration and validation of PLSR models based on RTOT and RSR spectra between 450–1800 nm, for A. thaliana mutants and Hieracium sp. for biophysical traits. Left side of the table represents calibration and validation of PLSR models based on RTOT spectra and right side of the table represents models based on RSR. Columns represent: Factors (number of components used in model creation), RMSE (root mean square error), RMSE% (% of RMSE related to average values of parameters), R2 (Coefficient of determination) of calibration and validation of models.
The evaluation of model performances was based on the following parameters: R2, RMSE, %RMSE, and the number of factors explaining the variability of a modelled trait. In the case of A. thaliana, only biophysical traits (chlorophyll and carotenoids contents SLA, DW) and trichome density were modelled (Table 3); for Hieracium, we present prediction models for both biophysical and anatomical traits (Table 3). These include trichome density and length, leaf thickness, and thicknesses of palisade and spongy parenchyma.
When comparing the A. thaliana and Hieracium PLSR models, the prediction of leaf biochemical pigments (Chl a + b, Car) and SLA performed better for A. thaliana than for Hieracium sp. In contrast, the model calibration for leaf anatomical properties prediction was better for Hieracium sp. than for the Arabidopsis group. The model validations show slightly better results for A. thaliana models based on RTOT than for RSR. In Hieracium, the validations are better for models based on RSR than for RTOT.
The effect of specular reflectance removal on PLSR model performance in A. thaliana was neutral or slightly negative in comparison to models based on RTOT. In A. thaliana, the most remarkable difference between PLSR models based on RTOT and RSR was for SLA, where the model was better for RTOT than for RSR. In Hieracium, the effect of removing specular reflectance had both positive and negative effects on models; however, neither the improving nor the worsening of the model performance was considerable.
4. Discussion
Several authors [36,39] have studied species with and without trichomes in relation to their leaf reflectance; however, investigations of the relationship between reflectance and quantitative epidermal parameters—trichome length and density quantification—are missing in the literature. We discuss the results of our investigation as follows: in Section 4.1, we focus on variation in biochemical and surface structural leaf traits to confirm our assumption about the variability of these traits in plant model systems. In Section 4.2, we address our first hypothesis (i.e., that epidermal traits affect the specular reflectance and that leaves with denser and longer trichomes have lower specular and higher total reflectance) and we discuss the relation of leaf specular reflectance to its surface properties. Finally, in Section 4.3, we evaluate how the specular reflectance removal affects PLSR modelling of biophysical traits.
4.1. Structural and Biochemical Parameters Variation
4.1.1. Trichome Density
Variation in trichome morphology in A. thaliana mutants and Hieracium species were confirmed qualitatively via ESEM images (Figure 1 and Figure 2, respectively). Moreover, quantitative differences were detected in total trichome density for A. thaliana and in density of trichome among the species of Hieracium (Figure 5F). In Arabidopsis, it is known that the trichome distribution on the leaf surface corresponds to its vegetative phase (juvenile, adult; [62]) and also depends on leaf insertion. In the present study, measurements of leaf optical properties in Arabidopsis were conducted on mature vegetative leaves so that the size of the leaf would fit the field of a view of the integrating sphere. Our A. thaliana leaves exhibited relatively low trichome density of 0–1.2 mm−2 in comparison to the range of 0.3–3.84 trichomes mm−2 observed in 26 A. thaliana ecotypes [63]. We emphasise that the trichome quantitative and qualitative descriptions presented in Figure 2 correspond to mature vegetative leaves of ontogenetically relatively young Hieracium individuals sampled at the time of measuring the leaf optical properties and sampling for ESEM analysis. The trichome densities in Hieracium leaves (0.2–5.5 mm−2 on average) are also rather low in comparison with literature—e.g., 15.4 trichomes mm−2 in H. albiflorum [64]—which, however, was not included in our study.
4.1.2. Ecophysiological Function of Leaf Surface
The selected Hieracium species are adapted to mountain environments (see references in Table 1), where the trichomes play an important role in reflecting excessive UV radiation, increasing the leaf boundary layer, and preventing leaf overheating. Similar protection is provided by leaf surface waxes, but, in contrast to leaf trichomes, the waxes have already been shown to increase the specular component of reflectance [34]. Due to the common genus and habitat of all the studied Hieracium species, there is a similarity in trichome composition in terms of morphology and density (except that the H. setigerum is adapted to a drier environment) and leaf thicknesses [65]. The Hieracium genus is known for its ability to hybridise among the species [66], and differences in trichome parameters are often observed [67]. We selected this genus to take advantage of such a variation in trichome parameters.
4.1.3. Leaf Biochemical Traits
The biochemical traits studied (chlorophyll a + b and carotenoids) exhibited small variability in both A. thaliana (Figure 4A,B) and Hieracium (Figure 5A,B). This was in accordance with the macroscopic plant homogeneity, stable cultivation conditions, and absence of stress factors. Differences in leaf chlorophyll content and SLA (Figure 5A,C) showed inverse trends among the Hieracium species, which corresponds to their adaptation to a specific environment or species area occurrence. Based on our study, mountain or foothill species (H. prenanthoides, H. intybaceum, H. nigrescens, and H. coldei) with similar ecology (see Table 1) also did not differ in either chlorophyll a + b content or SLA. Leaves of H. sabaudum showed greater SLA, indicating a lower density of mesophyll tissue and low chlorophyll a + b content, corresponding to their adaptation for growth in forest understory. Waxy leaves (Figure 2K,N) of H. setigerum with frequently branched, stellate trichomes (Figure 2L) contained high chlorophyll a + b content and low SLA, thus indicating a trend towards more xeromorphic adaptation to fit the conditions of south and south-eastern Europe.
4.2. Optical Properties Related to Leaf Surface Traits
Previous studies [36,68,69] have described that hairy leaves reflect more light in VIS compared to leaves without trichomes. We did not confirm this observation on Arabidopsis, as the green peak of VIS reflectance of the glabra mutant (hairless lineage) was very similar to exo70H4-1 and slightly higher than arpc5, both lineages bearing trichomes (Figure 6). We explain this contradiction by the fact that the studies cited above focused on leaves with high density of trichomes such as Artemisia tridentata and Salix commutata (very hairy leaves), comparing them with, e.g., Populus tremuloides and Salix lemmonii (smooth leaves) [68].
A study of Mershon et al. [70] suggests that trichome morphology rather than density affects leaf reflectance. Those authors showed that a presence of dendritic trichomes resulted in greater reflectance in the VIS spectral region compared to the effect of simple trichomes. Our results point to a similar trend, as H. setigerum, the only species having branched (stellate) trichomes among the Hieracium genus (Figure 2L), showed the highest reflectance values (both total and diffuse) in blue and red parts of the VIS spectral region in comparison to the other Hieracium species having only stiff and glandular trichomes (Figure 7E). However, the PCA did not reveal species clustering based on trichome morphology.
Spectral measurements of both studied groups of plants, A. thaliana mutants and Hieracium species, confirmed the assumption [8] that exclusion of RSPEC from RTOT decreases DHRF in the 400–2400 nm range (Figure 6 and Figure 7). Numerous studies [8,13,26,34] indicate that smooth leaves have higher ratios of RSPEC to RSR than leaves with trichomes. A. thaliana, which had lower trichome density than Hieracium, showed noticeably higher RSPEC in NIR (Figure 6 and Figure 7). The effect of trichomes on leaf optical properties is the most obvious in H. setigerum with the highest trichome density and the biggest variance in RTOT and RSR in NIR accompanied by almost zero RSPEC. We tested the potential of RSPEC to optically distinguish plants according to their trichome density and length using PCA (Figure 8). We reject the hypothesis that the leaves with denser and longer trichomes we studied have lower specular reflectance than the smooth leaves with fewer or no trichomes. We agree with [15] that specular reflectance does not serve for prediction about trichome density. The authors of [27] demonstrated that glossy leaves have the sharpest peak of bidirectional reflectance distribution function in the specular direction (i.e., the highest RSPEC in our measurement approach) compared to rarely or densely haired leaves. None of the model plants used in the present study had apparently glossy leaves and it seems plausible that the gradient in leaf surface properties was insufficient to reveal the above-cited effects of epidermal structure on specular reflection.
Qiu et al. [26] estimated that surface reflectance, and particularly its specular component, is predominantly driven by thick cuticle and heavy wax cover, but none of our studied plants exhibited these highly xeromorphic leaf traits. The only two Hieracium species showing indistinctive, rather smooth waxy structures on ESEM were H. setigerum and H. coldei (Figure 2K,N); however, the effect of waxes was nevertheless weak, as we did not detect lower RSR in their cases in comparison to the other studied species.
4.3. PLSR Modelling of Structural and Biochemical Traits
Spectral-based estimation of chlorophyll a + b and carotenoids contents depends on optical information from the leaf interior, and the specular component could, as previously hypothesised [18], bring additional noise to the spectral signal. Grant [8] showed that exclusion of the specular component from total reflectance could successfully be used to improve chlorophyll retrieval from leaf optical properties. In Hieracium, we achieved very slight improvement in PLSR model performance after the specular reflectance was removed in most biophysical parameters (except for leaf thickness, spongy parenchyma thickness, and trichome length). However, these improvements were less than 2% of RMSE. In A. thaliana, the effect of IS setup on the PLSR models’ performance was almost negligible and rather random. Bell and Curran [71] observed that the exclusion of specular reflectance improved correlation at the canopy level between leaf area, fresh and dry weights, and optical properties in VIS spectral region more so than in NIR. We tested PLSR models based on RSR and RTOT at the leaf level, which involved continual spectrum from 450 to 1800 nm, and we achieved subtle improvement of models for SLA and FW/DW ratio only in Hieracium, not in A. thaliana. This finding is rather surprising because A. thaliana had higher RSPEC than Hieracium, thus more significant model improvement could have been expected. Due to the very small differences in model performance based on RSR compared to RTOT, we cannot confirm that specular reflectance removal can significantly improve biophysical traits prediction in herbaceous dorsiventral leaves with relatively sparse trichomes of various morphology and without apparent pronounced waxy surface causing leaf surface roughness.
Although specular reflectance may not affect biophysical parameters’ retrieval in sparsely hairy leaves such as the leaves analysed in the present study, it is worth considering it in radiative transfer modelling (RTM). Difficulties in estimating chlorophyll content in plants with extremely high surface reflectance in RTM PROSPECT were previously confirmed, and chlorophyll retrieval was improved after incorporating “leaf surface reflectance parameter” (which is the difference between the refractive indices of leaf surface and interior layers) in RTM PROSPECT-Rsurf [26]. Systematic quantification of leaf surface parameters such as trichome branching, length, and density, but also wax structure, wax patterns, and cuticle thickness, could help to characterise the refractive indices of the additional surface layer incorporated by Qiu et al. [26] into radiative transfer models such as PROSPECT-Rsurf.
5. Conclusions
The measurement of specular reflectance using an integrating sphere with an additional light trap appeared to be well-suited for research on specular reflectance and its effect on biophysical and structural traits retrieval at the leaf level. We confirmed our assumptions on differences in trichome morphology (ESEM imaging) and on trichome length, density, and biophysical leaf traits (quantitative microscopic assay, biochemistry) on our two model plant systems—A. thaliana trichome structural mutants and various Hieracium species. However, PCA applied to the spectra did not reveal any systematic effect of trichome density, length, or morphology on specular reflectance between 450 and 1800 nm. Our results do not support the hypothesis that leaves with denser and longer trichomes such as those analysed in our study have lower specular and higher total reflectance than the smooth leaves.
PLSR models for the prediction of selected biophysical and structural traits were successfully constructed. Their performance was compared for models based on the total reflectance and reflectance after specular component removal. Contrastingly to our expectations, the removal of specular reflectance did not remarkably improve PLSR models—the best improvements were only up to 2% of RMSE. Therefore, we cannot draw a clear conclusion regarding the effect of specular component removal on performance of PLSR models for estimation of herbaceous dorsiventral leaves with relatively sparse trichomes of various morphology and without apparent waxy surface based on a set of plant material selected for this study.
To minimise the variation in leaf traits other than epidermal surface structure, the study was conducted on closely related taxonomic units (one species A. thaliana and one genus Hieracium), which probably resulted in a too narrow range of quantitative epidermal traits than expected. However, we are convinced that specular reflectance should be further explored in plant model systems including glossy leaves and a broader gradient in trichome parameters but similar internal leaf structure. Measurements of specular reflectance using an integrating sphere with a light trap in the specular direction is possible only at the leaf level. To reveal effects of specular reflectance on biophysical traits’ retrieval at the canopy level, leaf surface parameters such as trichome branching, length, and density, as well as wax structure, wax patterns, and cuticle thickness could be incorporated into radiative transfer models.
Author Contributions
Conceptualisation, P.L., E.N., Z.L. and J.A.; methodology, E.N., P.L. and Z.L.; software, E.N., P.L. and Z.L.; data curation, E.N., P.L. and Z.L.; writing—original draft preparation, E.N., Z.L., P.L. and J.A.; writing—review and editing, E.N., Z.L., P.L. and J.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Charles University Grant Agency (GAUK), 1752218, and partly in initial experimental phases by the NPUI LO1417 project of the Ministry of Education, Youth and Sports of the Czech Republic.
Institutional Review Board Statement
Not Applicable.
Informed Consent Statement
Not Applicable.
Data Availability Statement
The data presented in this study are available on reasonable request from the corresponding author.
Acknowledgments
We thank Ing. Jiří Machač, from the Institute of Botany of the Czech Academy of Sciences, for the environmental scanning electron microscopy image acquisition; Kateřina Schwarzerová and Petra Cifrová from the Department of Experimental Plant Biology at Charles University for precious advice regarding Arabidopsis cultivation; Jan Pinc, from the Department of Botany at Charles University, for the Hieracium seeds; for proof-reading of the paper by a native speaker, Lena Hunt from the USA, who is a student of J. Albrechtová; and Miroslav Barták for graphical technical help.
Conflicts of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Ustin, S.L.; Gitelson, A.A.; Jacquemoud, S.; Schaepman, M.; Asner, G.P.; Gamon, J.A.; Zarco-Tejada, P. Retrieval of Foliar Information about Plant Pigment Systems from High Resolution Spectroscopy. Remote Sens. Environ. 2009, 113, S67–S77. [Google Scholar] [CrossRef]
- Flood, P.J.; Kruijer, W.; Schnabel, S.K.; van der Schoor, R.; Jalink, H.; Snel, J.F.H.; Harbinson, J.; Aarts, M.G.M. Phenomics for Photosynthesis, Growth and Reflectance in Arabidopsis Thaliana Reveals Circadian and Long-Term Fluctuations in Heritability. Plant Methods 2016, 12, 1–14. [Google Scholar] [CrossRef]
- Cotrozzi, L.; Peron, R.; Tuinstra, M.R.; Mickelbart, M.V.; Couture, J.J. Spectral Phenotyping of Physiological and Anatomical Leaf Traits Related with Maize Water Status. Plant Physiol. 2020, 184, 1363–1377. [Google Scholar] [CrossRef] [PubMed]
- Nicodemus, F.E.; Richmond, J.C.; Hsia, J.J. Geometrical Considerations and Nomenclature for Reflectance; Institute for Basic Standards, National Bureau of Standards: Washington, DC, USA, 1977.
- Jacquemoud, S.; Ustin, S. Leaf Optical Properties; Cambridge University Press: Cambridge, UK, 2019; ISBN 978-1-108-48126-7. [Google Scholar]
- Breece, H.T.; Holmes, R.A. Bidirectional Scattering Characteristics of Healthy Green Soybean and Corn Leaves in Vivo. Appl. Opt. 1971, 10, 119. [Google Scholar] [CrossRef]
- Gates, D.M.; Keegan, H.J.; Schleter, J.C.; Weidner, V.R. Spectral Properties of Plants. Appl. Opt. 1965, 4, 11–20. [Google Scholar] [CrossRef]
- Grant, L. Diffuse and Specular Characteristics of Leaf Reflectance. Remote Sens. Environ. 1987, 22, 309–322. [Google Scholar] [CrossRef]
- Kokaly, R.F.; Asner, G.P.; Ollinger, S.V.; Martin, M.E.; Wessman, C.A. Characterizing Canopy Biochemistry from Imaging Spectroscopy and Its Application to Ecosystem Studies. Remote Sens. Environ. 2009, 113, S78–S91. [Google Scholar] [CrossRef]
- Allen, W.A.; Gausman, H.W.; Richardson, A.J. Willstätter-Stoll Theory of Leaf Reflectance Evaluated by Ray Tracing. Appl. Opt. AO 1973, 12, 2448–2453. [Google Scholar] [CrossRef]
- Xiao, Y.; Tholen, D.; Zhu, X.-G. The Influence of Leaf Anatomy on the Internal Light Environment and Photosynthetic Electron Transport Rate: Exploration with a New Leaf Ray Tracing Model. J. Exp. Bot. 2016, 67, 6021–6035. [Google Scholar] [CrossRef]
- Sinclair, T.R.; Schreiber, M.M.; Hoffer, R.M. Diffuse Reflectance Hypothesis for the Pathway of Solar Radiation Through Leaves1. Agron. J. 1973, 65, 276–283. [Google Scholar] [CrossRef]
- Vanderbilt, V.C.; Grant, L.; Daughtry, C.S.T. Polarization of Light Scattered by Vegetation. Proc. IEEE 1985, 73, 1012–1024. [Google Scholar] [CrossRef]
- Greiner, M.A.; Duncan, B.D.; Dierking, M.P. Bidirectional Scattering Distribution Functions of Maple and Cottonwood Leaves. Appl. Opt. 2007, 46, 6485. [Google Scholar] [CrossRef] [PubMed]
- Grant, L.; Daughtry, C.S.T.; Vanderbilt, V.C. Polarized and Specular Reflectance Variation with Leaf Surface Features. Physiol. Plant. 1993, 88, 1–9. [Google Scholar] [CrossRef]
- Schaepman-Strub, G.; Schaepman, M.E.; Painter, T.H.; Dangel, S.; Martonchik, J.V. Reflectance Quantities in Optical Remote Sensing—Definitions and Case Studies. Remote Sens. Environ. 2006, 103, 27–42. [Google Scholar] [CrossRef]
- Brakke, T.W.; Smith, J.A.; Harnden, J.M. Bidirectional Scattering of Light from Tree Leaves. Remote Sens. Environ. 1989, 29, 175–183. [Google Scholar] [CrossRef]
- Li, Y.; Chen, Y.; Huang, J. An Approach to Improve Leaf Pigment Content Retrieval by Removing Specular Reflectance Through Polarization Measurements. IEEE Trans. Geosci. Remote Sens. 2019, 57, 2173–2186. [Google Scholar] [CrossRef]
- Vanderbilt, V.C.; Grant, L. Polarization Photometer to Measure Bidirectional Reflectance Factor R(55°, 0°; 55°,180°) of Leaves. Opt. Eng. 1986, 24, 566–571. [Google Scholar] [CrossRef]
- McClendon, J.H. The Micro-Optics of Leaves. I. Patterns of Reflection from the Epidermis. Am. J. Bot. 1984, 71, 1391–1397. [Google Scholar] [CrossRef]
- ASD Inc. Integrating Sphere User Manual. Available online: https://www.mapping-solutions.co.uk/downloads/data/ASD/Accessories_Brochure/A1A15.pdf (accessed on 15 October 2021).
- Datt, B. Remote Sensing of Chlorophyll a, Chlorophyll b, Chlorophyll A+ b, and Total Carotenoid Content in Eucalyptus Leaves. Remote Sens. Environ. 1998, 66, 111–121. [Google Scholar] [CrossRef]
- Datt, B. A New Reflectance Index for Remote Sensing of Chlorophyll Content in Higher Plants: Tests Using Eucalyptus Leaves. J. Plant Physiol. 1999, 154, 30–36. [Google Scholar] [CrossRef]
- Sims, D.A.; Gamon, J.A. Relationships between Leaf Pigment Content and Spectral Reflectance across a Wide Range of Species, Leaf Structures and Developmental Stages. Remote Sens. Environ. 2002, 81, 337–354. [Google Scholar] [CrossRef]
- Jay, S.; Bendoula, R.; Hadoux, X.; Féret, J.-B.; Gorretta, N. A Physically-Based Model for Retrieving Foliar Biochemistry and Leaf Orientation Using Close-Range Imaging Spectroscopy. Remote Sens. Environ. 2016, 177, 220–236. [Google Scholar] [CrossRef]
- Qiu, F.; Chen, J.; Croft, H.; Li, J.; Zhang, Q. Retrieving Leaf Chlorophyll Content by Incorporating Variable Leaf Surface Reflectance in the PROSPECT Model. Remote Sens. 2019, 11, 1572. [Google Scholar] [CrossRef]
- Bousquet, L.; Lachérade, S.; Jacquemoud, S.; Moya, I. Leaf BRDF Measurements and Model for Specular and Diffuse Components Differentiation. Remote Sens. Environ. 2005, 98, 201–211. [Google Scholar] [CrossRef]
- Vogelmann, T.C.; Gorton, H.L. Leaf: Light Capture in the Photosynthetic Organ. In The Structural Basis of Biological Energy Generation; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar]
- Woolley, J.T. Reflectance and Transmittance of Light by Leaves. Plant Physiol. 1971, 47, 656–662. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Sun, Z.; Lu, S.; Omasa, K. Estimation of the Leaf Chlorophyll Content Using Multiangular Spectral Reflectance Factor. Plant Cell Environ. 2019, 42, 3152–3165. [Google Scholar] [CrossRef]
- Berry, Z.C.; Goldsmith, G.R. Diffuse Light and Wetting Differentially Affect Tropical Tree Leaf Photosynthesis. New Phytol. 2020, 225, 143–153. [Google Scholar] [CrossRef]
- Vanderbilt, V.C.; Grant, L.; Biehl, L.L.; Robinson, B.F. Specular, Diffuse, and Polarized Light Scattered by Two Wheat Canopies. Appl. Opt. 1985, 24, 2408. [Google Scholar] [CrossRef]
- Smith, W.K.; McClean, T.M. Adaptive Relationship Between Leaf Water Repellency, Stomatal Distribution, and Gas Exchange. Am. J. Bot. 1989, 76, 465–469. [Google Scholar] [CrossRef]
- van de Kerkhof, G.T.; Schertel, L.; Poon, R.; Jacucci, G.; Glover, B.J.; Vignolini, S. Disordered Wax Platelets on Tradescantia Pallida Leaves Create Golden Shine. Faraday Discuss. 2020, 223, 207–215. [Google Scholar] [CrossRef]
- Gates, D.M.; Tantraporn, W. The Reflectivity of Deciduous Trees and Herbaceous Plants in the Infrared to 25 Microns. Science 1952, 115, 613–616. [Google Scholar] [CrossRef] [PubMed]
- Holmes, M.G.; Keiller, D.R. Effects of Pubescence and Waxes on the Reflectance of Leaves in the Ultraviolet and Photosynthetic Wavebands: A Comparison of a Range of Species: Ultraviolet Leaf Reflectance. Plant Cell Environ. 2002, 25, 85–93. [Google Scholar] [CrossRef]
- Gausman, H.W.; Cardenas, R. Effect of Leaf Pubescence of Gynura Aurantiaca on Light Reflectance. Bot. Gaz. 1969, 130, 158–162. [Google Scholar] [CrossRef]
- Levizou, E.; Drilias, P.; Psaras, G.K.; Manetas, Y. Nondestructive Assessment of Leaf Chemistry and Physiology through Spectral Reflectance Measurements May Be Misleading When Changes in Trichome Density Co-Occur. New Phytol. 2005, 165, 463–472. [Google Scholar] [CrossRef]
- Buschmann, C.; Lenk, S.; Lichtenthaler, H.K. Reflectance Spectra and Images of Green Leaves with Different Tissue Structure and Chlorophyll Content. Isr. J. Plant Sci. 2012, 60, 49–64. [Google Scholar] [CrossRef]
- Meyerowitz, E.M.; Pruitt, R.E. Arabidopsis Thaliana and Plant Molecular Genetics. Sci. New Ser. 1985, 229, 1214–1218. [Google Scholar] [CrossRef]
- Kulich, I.; Vojtíková, Z.; Glanc, M.; Ortmannová, J.; Rasmann, S.; Žárský, V. Cell Wall Maturation of Arabidopsis Trichomes Is Dependent on Exocyst Subunit EXO70H4 and Involves Callose Deposition. Plant Physiol. 2015, 168, 120–131. [Google Scholar] [CrossRef] [PubMed]
- Potikha, T.; Delmer, D.P. A Mutant of Arabidopsis Thaliana Displaying Altered Patterns of Cellulose Deposition. Plant J. 1995, 7, 453–460. [Google Scholar] [CrossRef]
- Li, S.; Blanchoin, L.; Yang, Z.; Lord, E.M. The Putative Arabidopsis Arp2/3 Complex Controls Leaf Cell Morphogenesis. Plant Physiol. 2003, 132, 2034–2044. [Google Scholar] [CrossRef]
- McKelvie, A.D. A list of mutant genes in arabldopsis thaliana (l.) heynh. Radiat. Bot. 1961, 1, 233–241. [Google Scholar] [CrossRef]
- Marks, M.D.; Feldmann, K.A. Trichome Development in Arabidopsis Thaliana. 1. T-DNA Tagging GLABROUS1 Gene. Plant Cell 1989, 1, 1043–1050. [Google Scholar] [CrossRef]
- Monnier, A. Essai Monographique Sur Les Hieracium Et Quelques Genres Voisins; Hissette: Nancy, France, 1829. [Google Scholar]
- Zahradníček, J.; Chrtek, J. Cytotype Distribution and Phylogeography of Hieracium Intybaceum (Asteraceae): Cyto and Phylogeography of Hieracium Intybaceum. Bot. J. Linn. Soc. 2015, 179, 487–498. [Google Scholar] [CrossRef][Green Version]
- Grass, S.; Zidorn, C.; Ellmerer, E.P.; Stuppner, H. Eudesmane Derivatives from Hieracium Intybaceum. Chem. Biodivers. 2004, 1, 353–360. [Google Scholar] [CrossRef]
- Haveman, R. Enkele opmerkelijke vondsten van Hieracium sabaudum L. s. str. op de Veluwe. Gorteria 2010, 34, 137–143. [Google Scholar]
- Chrtek, J. Hieracium Decipientiforme (the H. Nigrescens Group)—An Interesting Species of the Ukrainian Carpathians. Folia Geobot. 1997, 69, 121–128. [Google Scholar]
- Szeląg, Z. Taxonomic and Nomenclatural Notes on Hieracium Tubulare (Asteraceae) with Description of a New Species from the Eastern Carpathians. Ann. Bot. Fennici. 2006, 43, 310–314. [Google Scholar]
- Albrechtová, J.; Kubínová, Z.; Soukup, A.; Janáček, J. Image Analysis: Basic Procedures for Description of Plant Structures. In Plant Cell Morphogenesis: Methods and Protocols; Cvrčková, F., Žárský, V., Eds.; Springer: New York, NY, USA, 2019; pp. 109–119. ISBN 978-1-4939-9469-4. [Google Scholar]
- Gundersen, H.J.G.; Jensen, E.B. The Efficiency of Systematic Sampling in Stereology and Its Prediction. J. Microsc. 1987, 147, 229–263. [Google Scholar] [CrossRef]
- O’Brien, T.P.; Feder, N.; McCully, M.E. Polychromatic Staining of Plant Cell Walls by Toluidine Blue O. Protoplasma 1964, 59, 368–373. [Google Scholar] [CrossRef]
- Potůčková, M.; Červená, L.; Kupková, L.; Lhotáková, Z.; Lukeš, P.; Hanuš, J.; Novotný, J.; Albrechtová, J. Comparison of Reflectance Measurements Acquired with a Contact Probe and an Integration Sphere: Implications for the Spectral Properties of Vegetation at a Leaf Level. Sensors 2016, 16, 1801. [Google Scholar] [CrossRef]
- Porra, R.; Thompson, W.; Kriedemann, P. Determination of Accurate Extinction Coefficients and Simultaneous-Equations for Assaying Chlorophyll-a and Chlorophyll-B Extracted with 4 Different Solvents—Verification of the Concentration. Biochim. Biophys. Acta 1989, 975, 384–394. [Google Scholar] [CrossRef]
- Wellburn, A.R. The Spectral Determination of Chlorophylls a and b, as Well as Total Carotenoids, Using Various Solvents with Spectrophotometers of Different Resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- Esbensen, K.; Swarbrick, B.; Westad, F.; Whitcomb, P.J.; Anderson, M.J. Multivariate Data Analysis: An Introduction to Multivariate Analysis, Process Analytical Technology and Quality by Design; CAMO Software AS: Oslo, Norway, 2018; ISBN 978-82-691104-0-1. [Google Scholar]
- Zhao, N.; Wu, Z.; Zhang, Q.; Shi, X.; Ma, Q.; Qiao, Y. Optimization of Parameter Selection for Partial Least Squares Model Development. Sci. Rep. 2015, 5, 11647. [Google Scholar] [CrossRef]
- Kopačková, V.; Ben-Dor, E.; Carmon, N.; Notesco, G. Modelling Diverse Soil Attributes with Visible to Longwave Infrared Spectroscopy Using PLSR Employed by an Automatic Modelling Engine. Remote Sens. 2017, 9, 134. [Google Scholar] [CrossRef]
- Heise, H.M.; Damm, U.; Lampen, P.; Davies, A.N.; McIntyre, P.S. Spectral Variable Selection for Partial Least Squares Calibration Applied to Authentication and Quantification of Extra Virgin Olive Oils Using Fourier Transform Raman Spectroscopy. Appl. Spectrosc. AS 2005, 59, 1286–1294. [Google Scholar] [CrossRef]
- Telfer, A.; Bollman, K.M.; Poethig, R.S. Phase Change and the Regulation of Trichome Distribution in Arabidopsis Thaliana. Development 1997, 124, 645–654. [Google Scholar] [CrossRef]
- Hauser, M.-T.; Harr, B.; Schlötterer, C. Trichome Distribution in Arabidopsis Thaliana and Its Close Relative Arabidopsis Lyrata: Molecular Analysis of the Candidate Gene GLABROUS1. Mol. Biol. Evol. 2001, 18, 1754–1763. [Google Scholar] [CrossRef]
- Schreuder, M.D.J.; Brewer, C.A.; Heine, C. Modelled Influences of Non-Exchanging Trichomes on Leaf Boundary Layers and Gas Exchange. J. Theor. Biol. 2001, 210, 23–32. [Google Scholar] [CrossRef]
- Gottschlich, G.; Drenckhahn, D. Iconography of the Genus Hieracium in Central Europe—Part 1 General Description and Morphotypes. Forum Geobot. 2005, 2, 1–7. [Google Scholar]
- Chrtek, J.; Mráz, P.; Belyayev, A.; Paštová, L.; Mrázová, V.; Caklová, P.; Josefiová, J.; Zagorski, D.; Hartmann, M.; Jandová, M.; et al. Evolutionary History and Genetic Diversity of Apomictic Allopolyploids in Hieracium s.Str.: Morphological versus Genomic Features. Am. J. Bot. 2020, 107, 66–90. [Google Scholar] [CrossRef]
- Krak, K.; Mráz, P. Trichomes in the Tribe Lactuceae (Asteraceae)—Taxonomic Implications. Biologia 2008, 63, 616–630. [Google Scholar] [CrossRef]
- Billings, W.D.; Morris, R.J. Reflection of Visible and Infrared Radiation from Leaves of Different Ecological Groups. Am. J. Bot. 1951, 38, 327–331. [Google Scholar] [CrossRef]
- Shull, C.A. A Spectrophotometric Study of Reflection of Light from Leaf Surfaces. Bot. Gaz. 1929, 87, 583–607. [Google Scholar] [CrossRef]
- Mershon, J.P.; Becker, M.; Bickford, C.P. Linkage between Trichome Morphology and Leaf Optical Properties in New Zealand Alpine Pachycladon (Brassicaceae). N. Z. J. Bot. 2015, 53, 175–182. [Google Scholar] [CrossRef]
- Bell, C.C.; Curran, P.J. The Effect of Specular Reflectance on the Relationship between Reflectance and Vegetation Amount. Int. J. Remote Sens. 1992, 13, 2751–2757. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).