HYDROPT: An Open-Source Framework for Fast Inverse Modelling of Multi- and Hyperspectral Observations from Oceans, Coastal and Inland Waters
Abstract
:1. Introduction
2. Materials and Methods
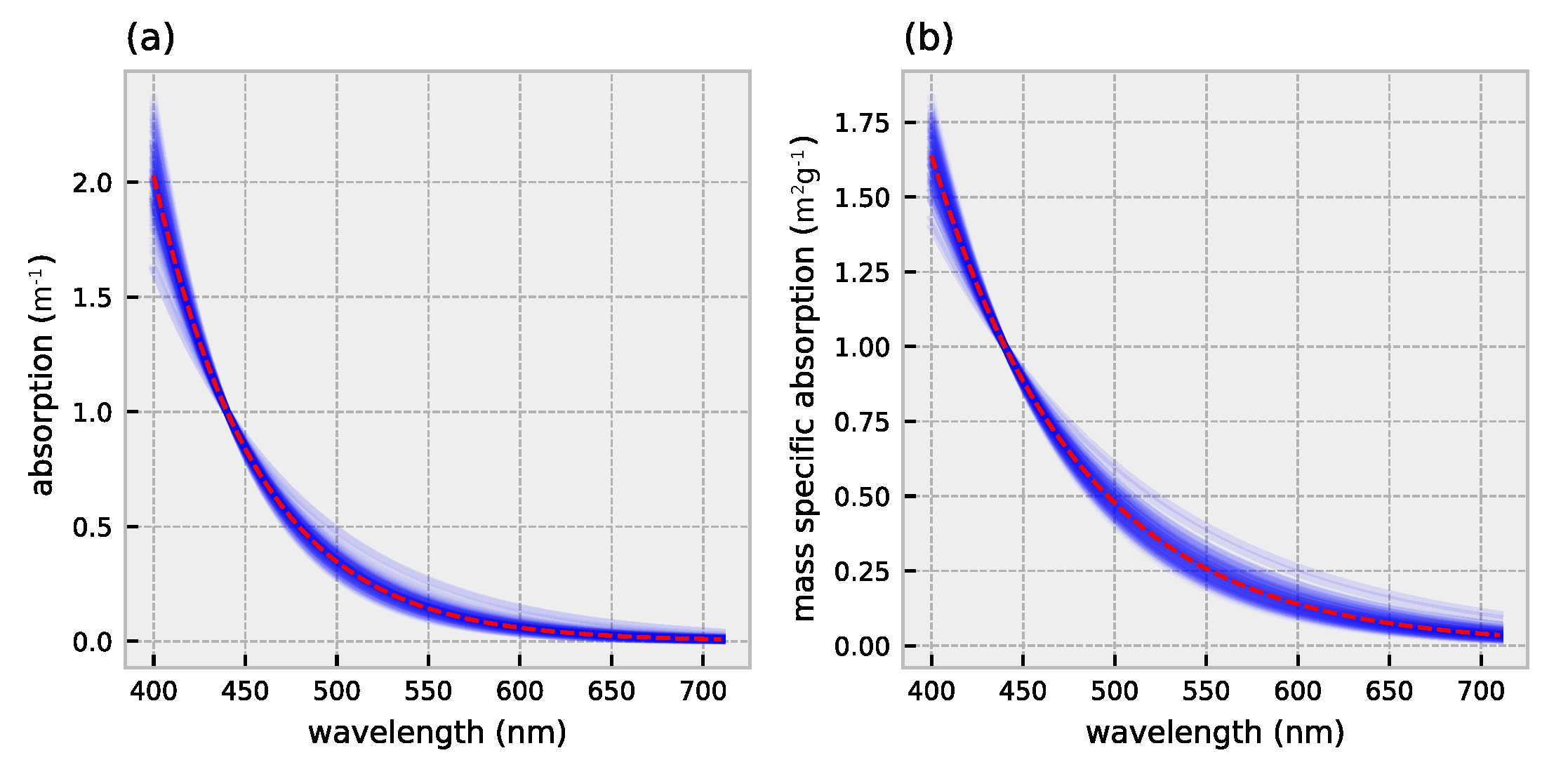
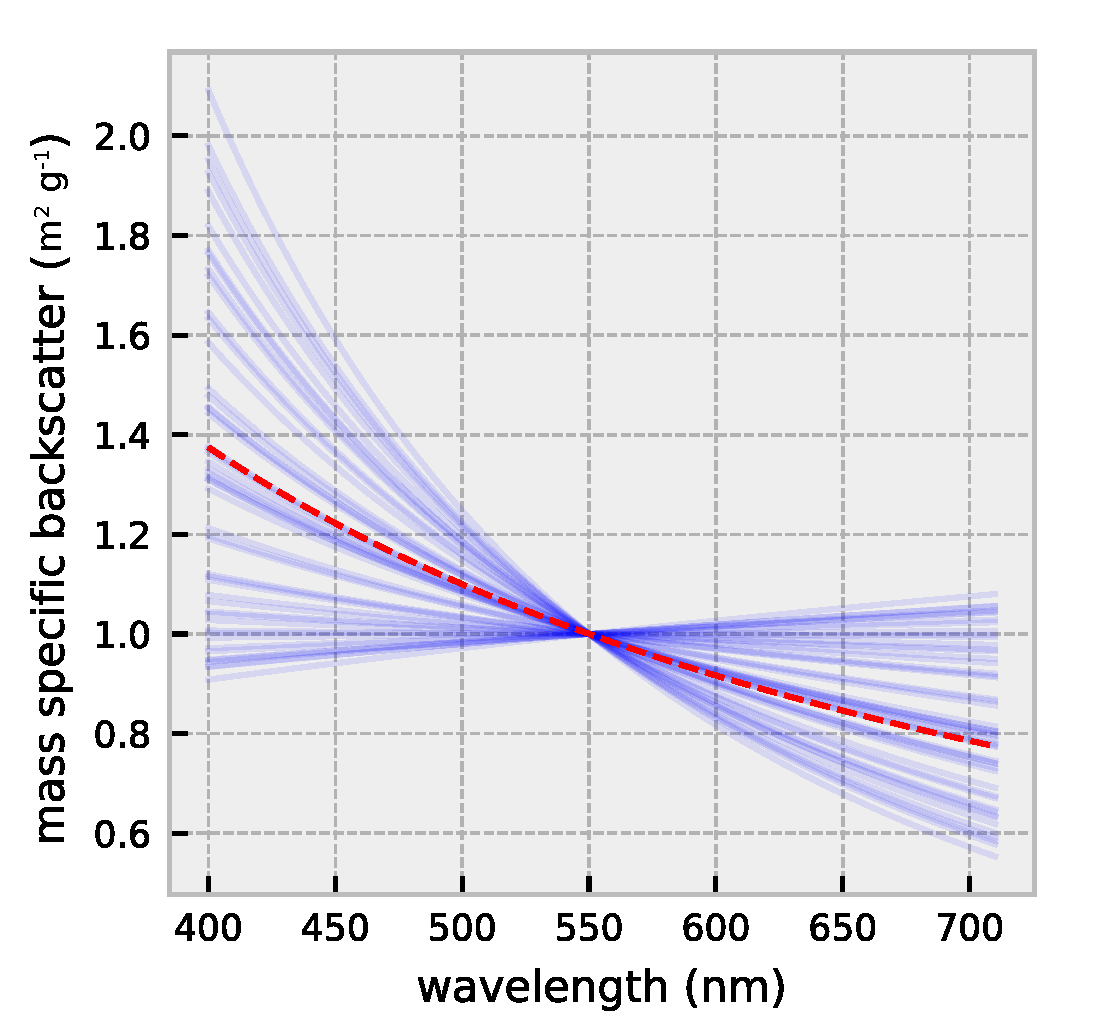
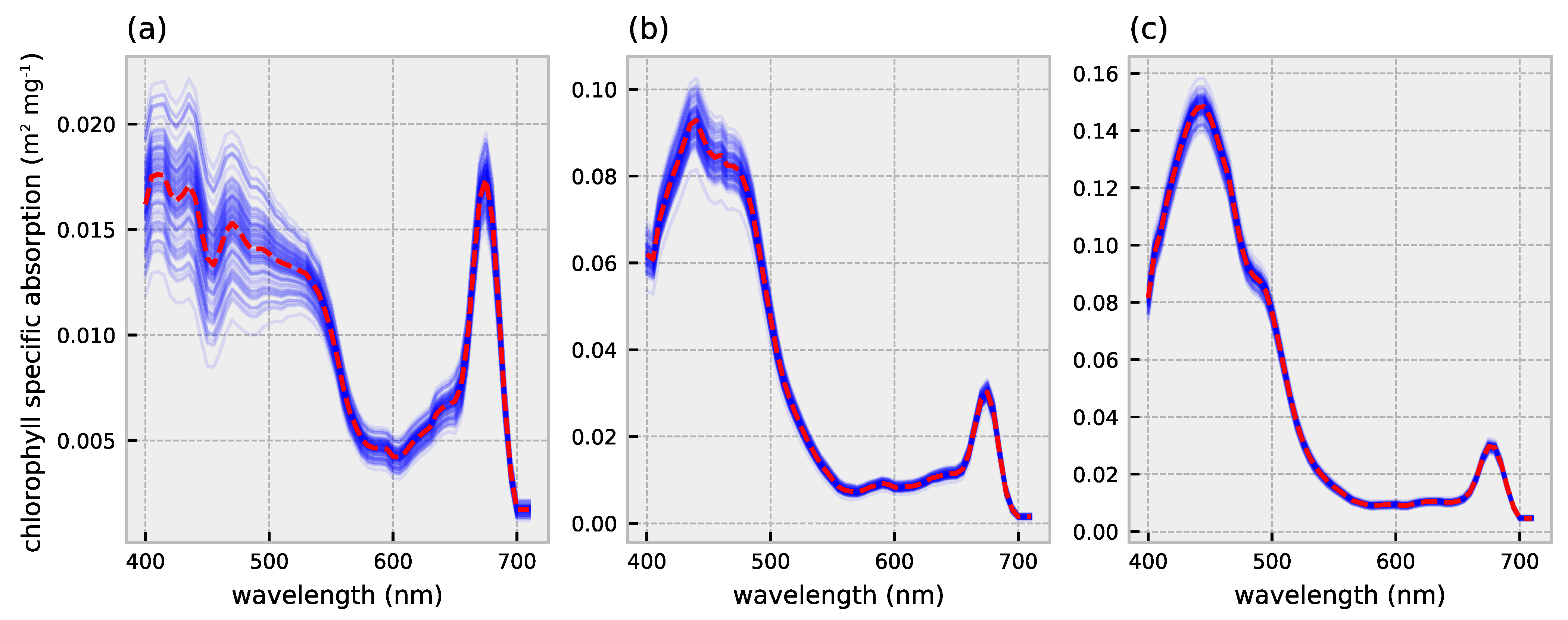
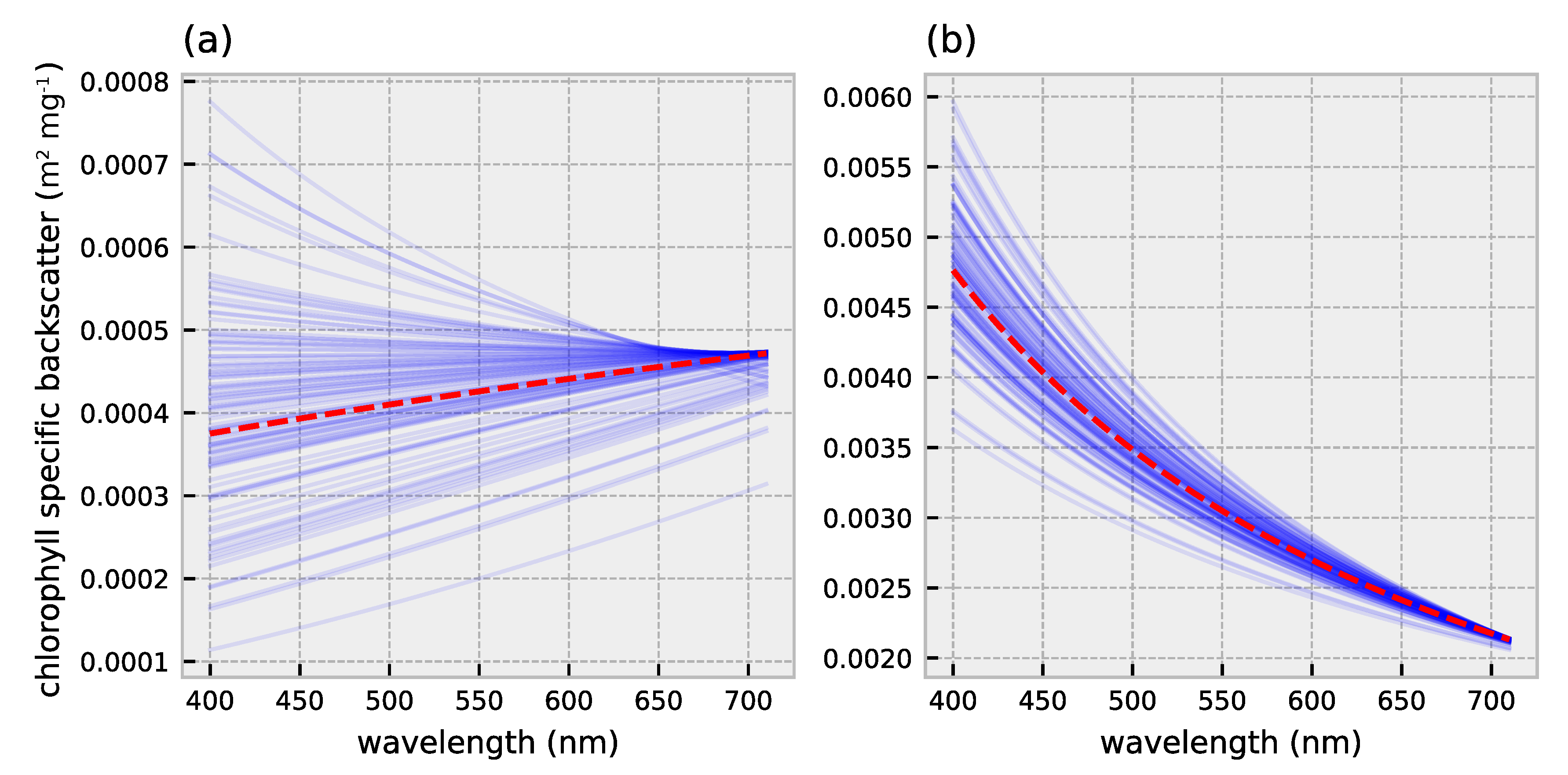
2.1. HydroLight Simulations
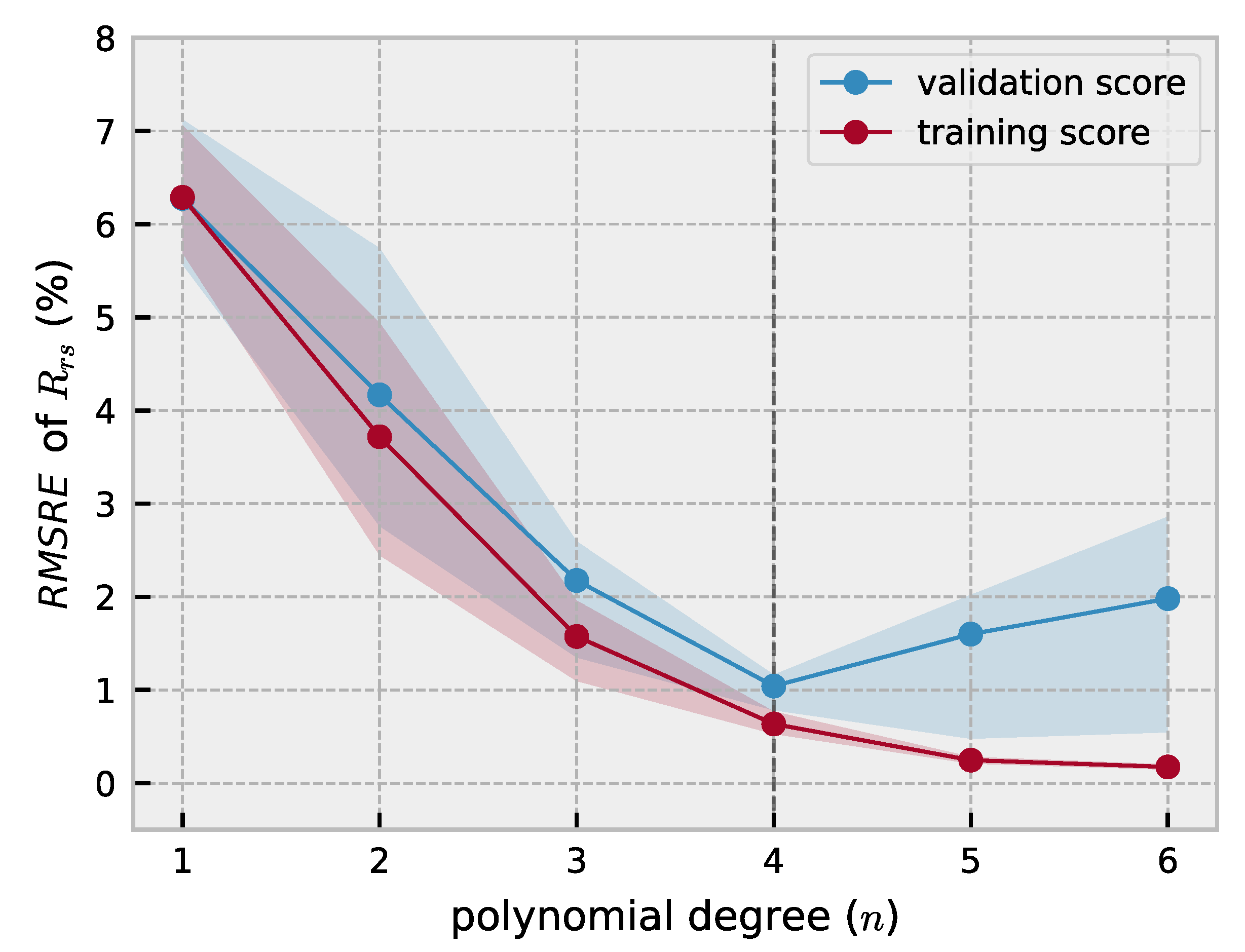
2.2. Polynomial Forward Model
2.3. Optimization
2.4. IOCCG Dataset
2.5. Hyperspectral Phytoplankton Size Class Dataset
2.6. Ocean-Color Instruments
3. Results
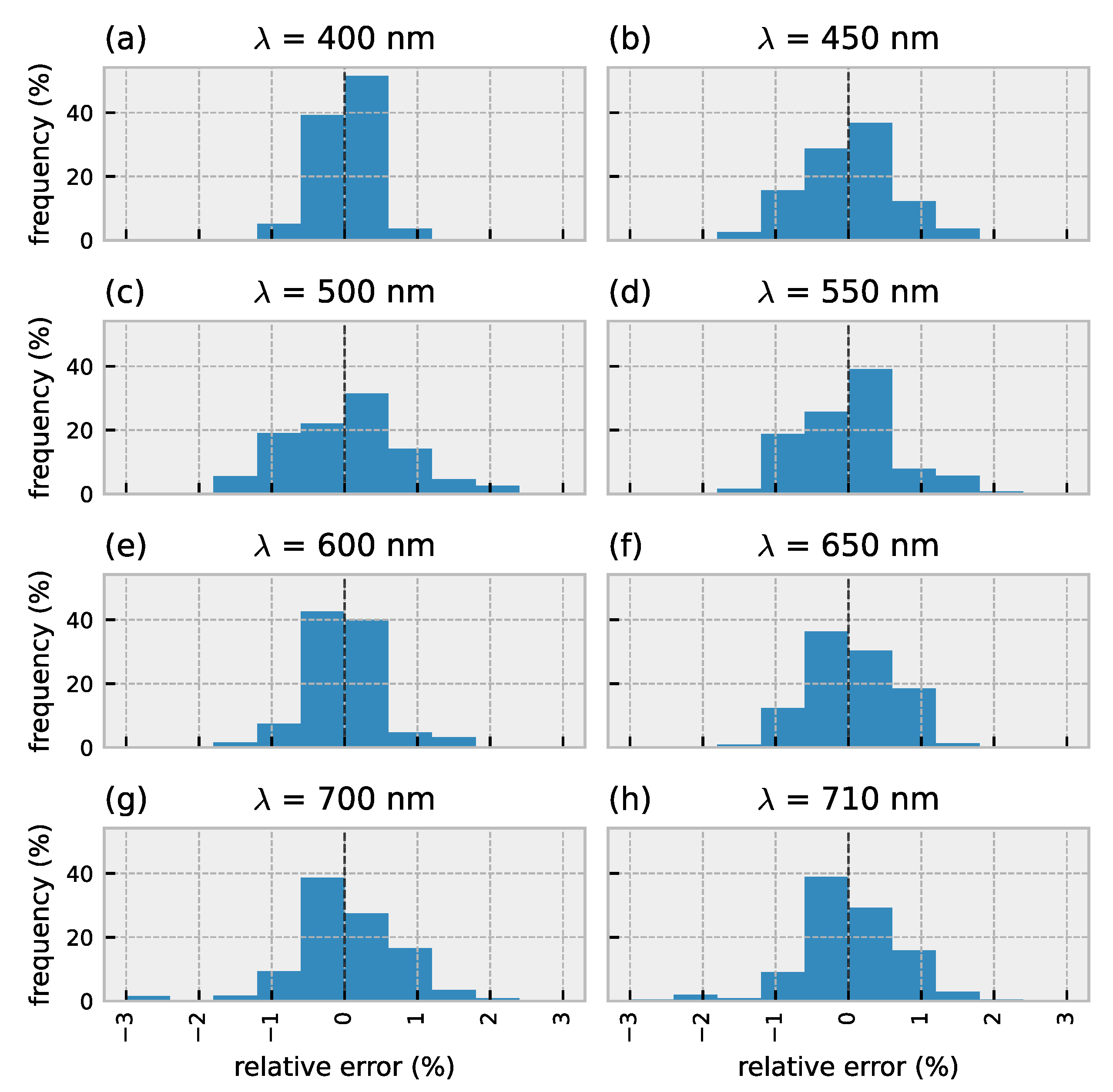
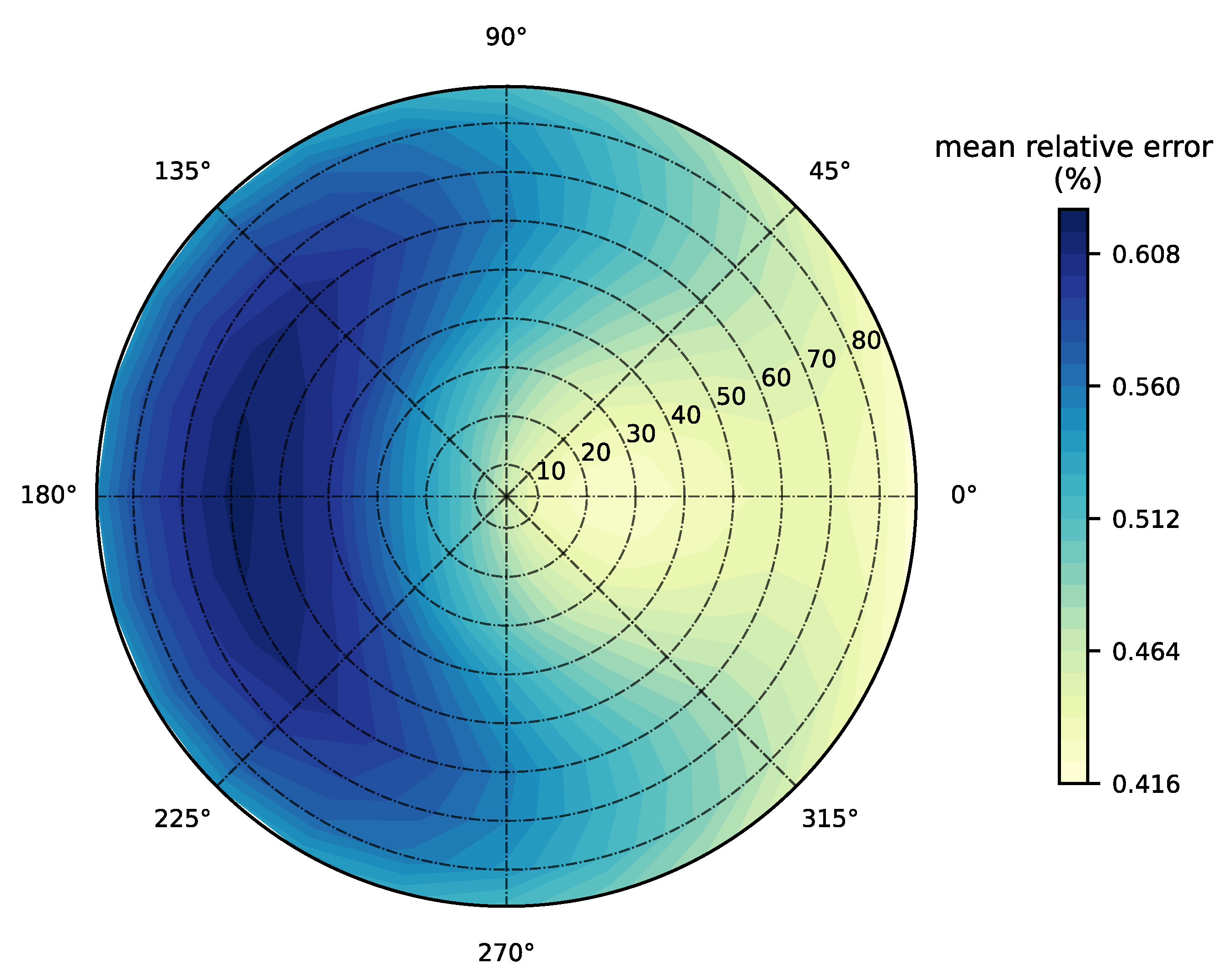
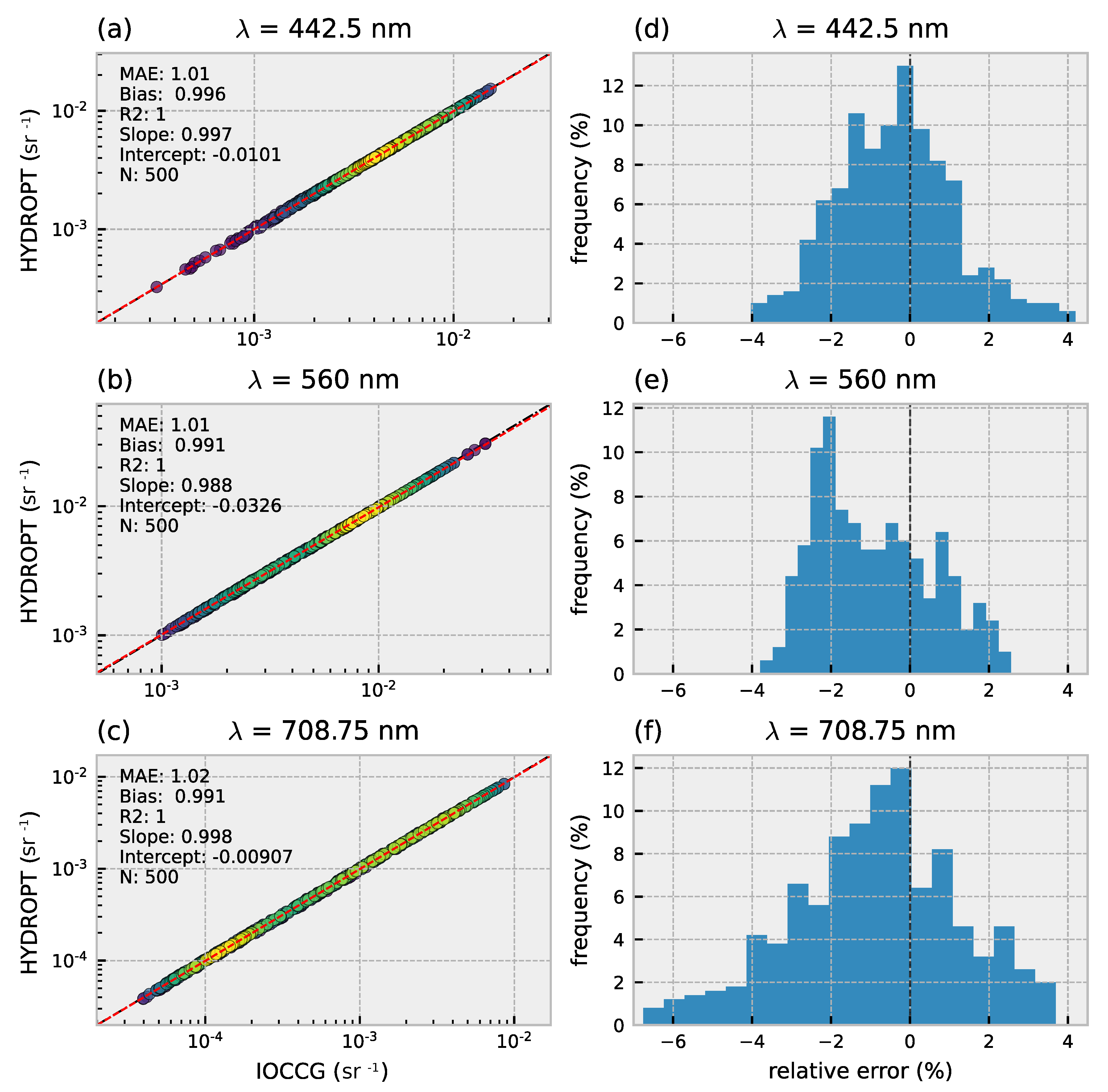
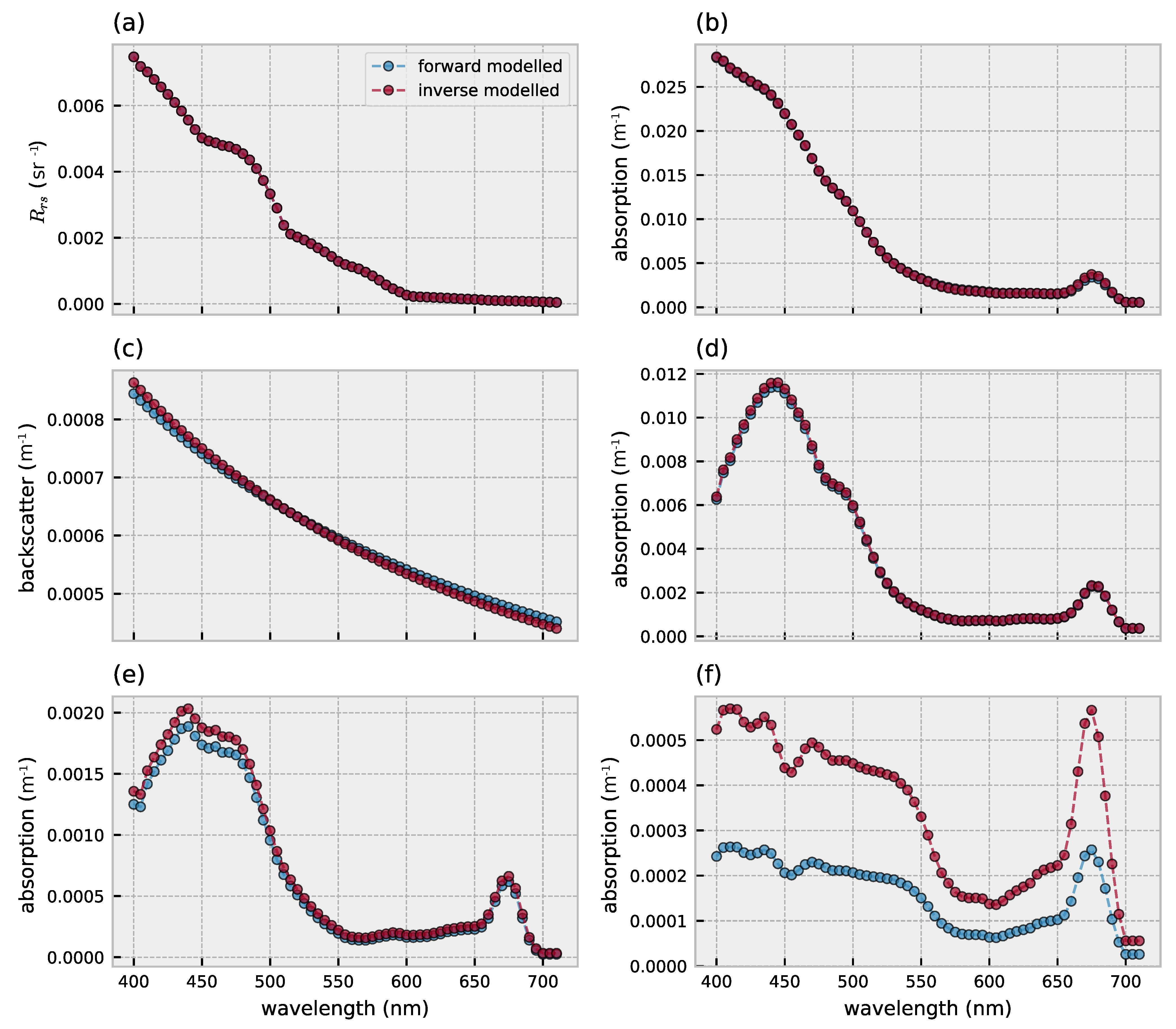
3.1. Forward Model Validation
3.2. Hyperspectral Inversion
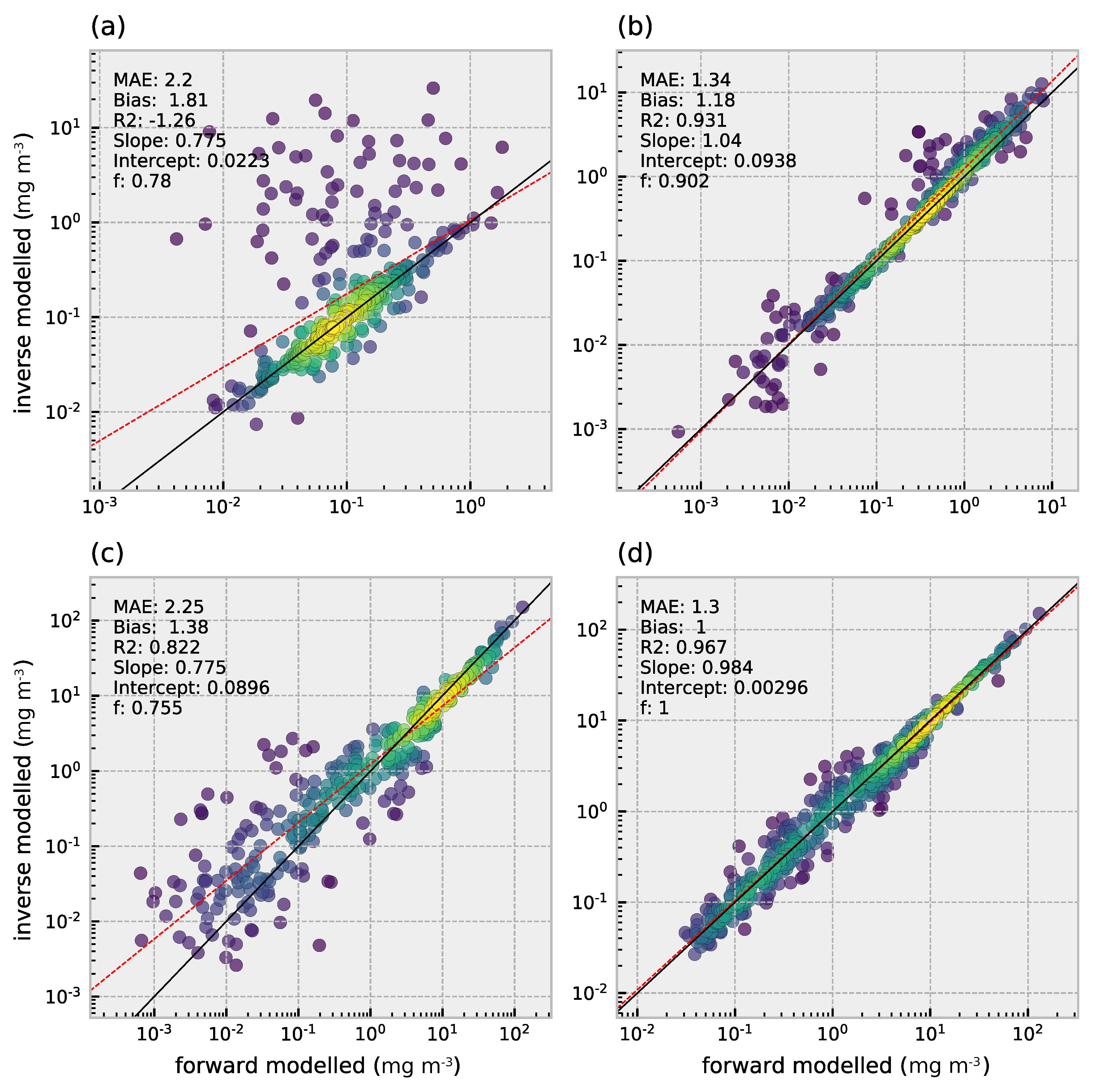
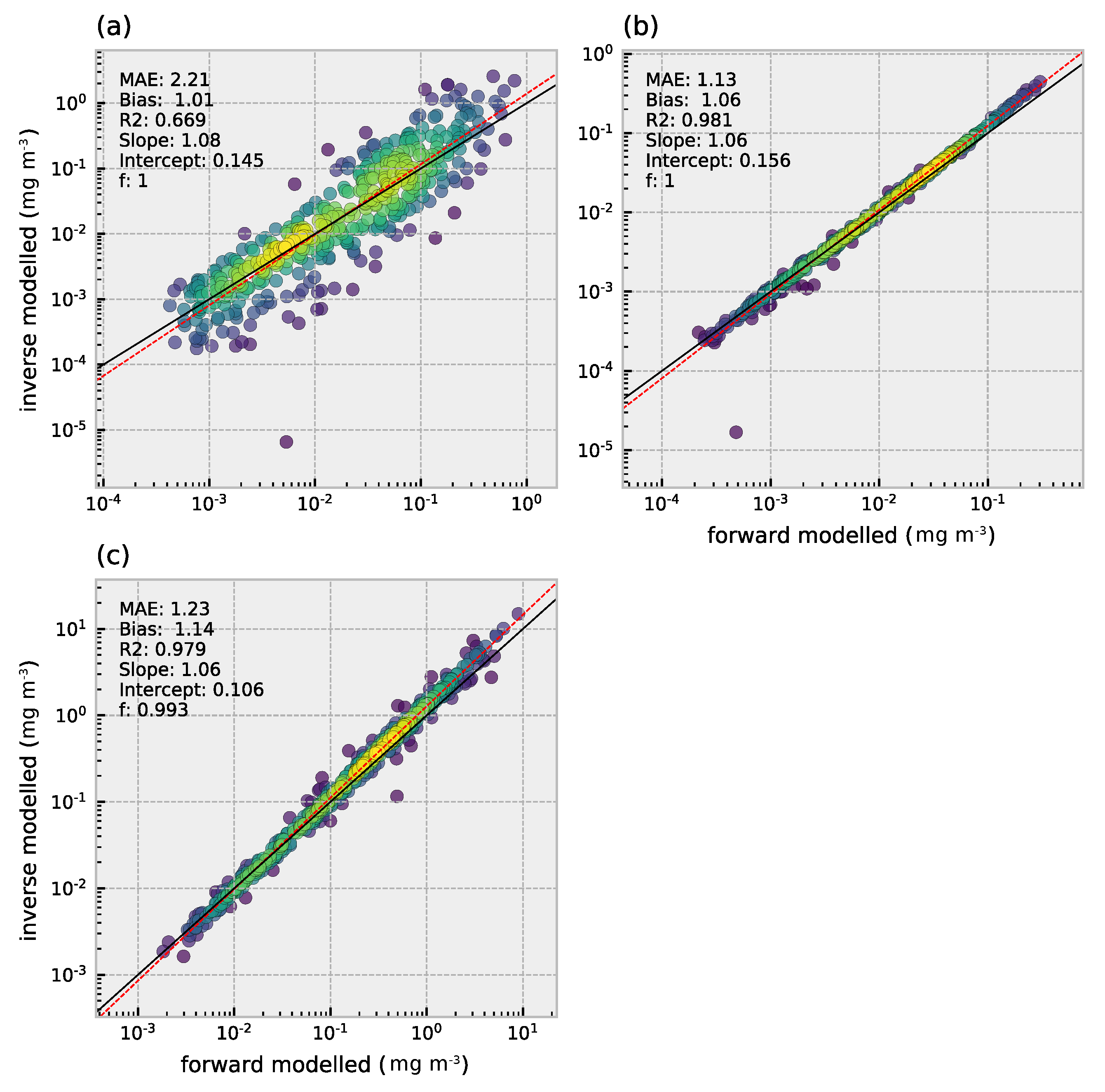
3.3. Retrieval of Phytoplankton Size Classes
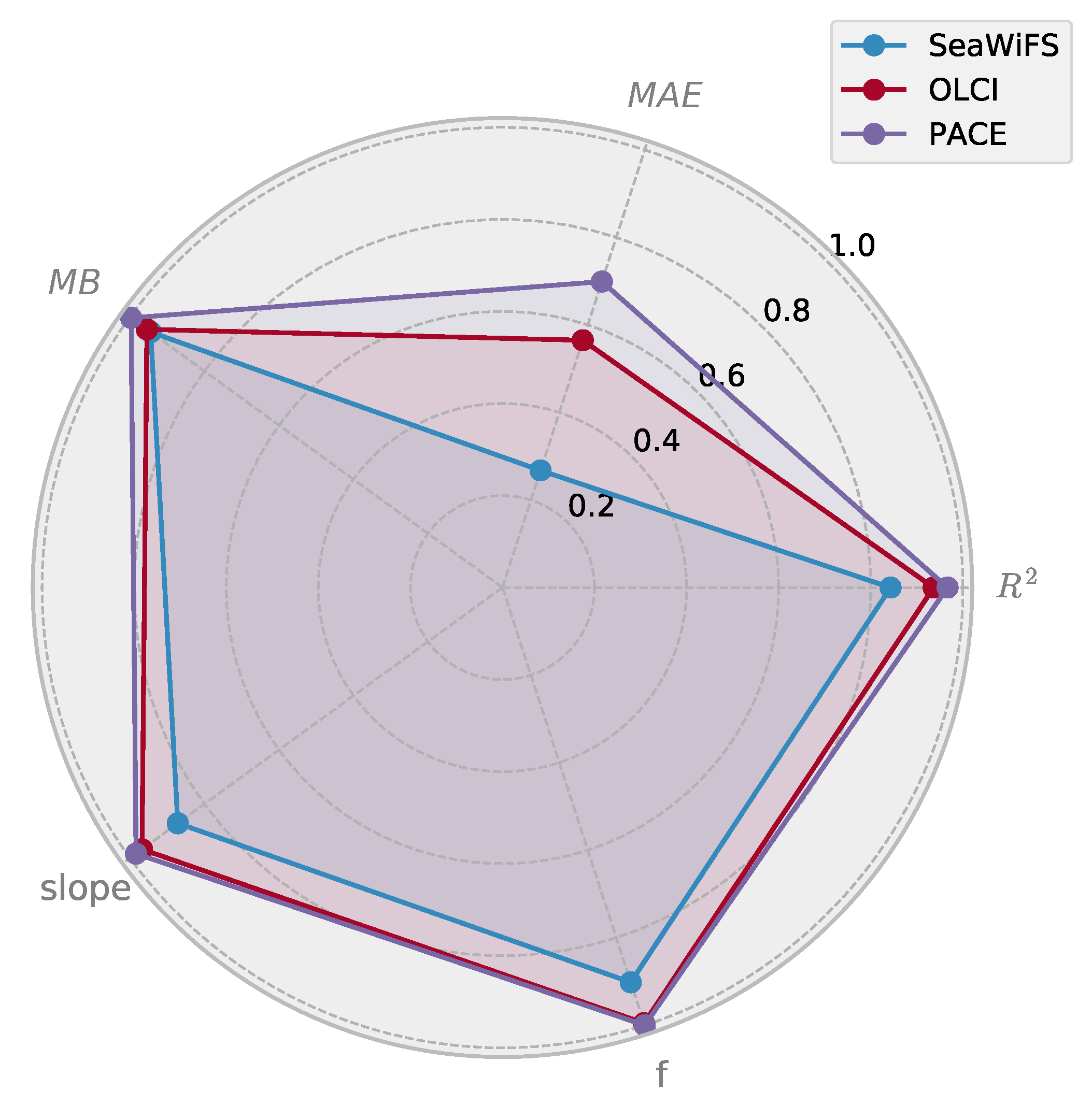
3.4. Comparison of Multi- and Hyperspectral Retrievals
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| RT | Radiative transfer |
| HYDROPT | HydroLight Optimization |
| IOP | Inherent optical properties |
| CDOM | Colored dissolved organic matter |
| VSF | Volume scattering function |
| FF | Fournier–Forand phase function |
| PACE | Plankton, Aerosol, Cloud, ocean Ecosystem |
| MERIS | MEdium Resolution Imaging Spectrometer |
| CZCS | Coastal Zone Color Scanner |
| OLCI | Ocean Land Color Instrument |
| SeaWiFS | Sea-viewing Wide Field-of-view Sensor |
| Observed remote sensing reflectance-either from HydroLight simulations or synthetic dataset | |
| Predicted remote sensing reflectance by the HYDROPT forward model | |
| Natural log-transformed remote sensing reflectance | |
| a | Absorption coefficient |
| Backscatter coefficient | |
| Backscatter ratio | |
| , | chlorophyll-a- or mass specific absorption and backscatter coefficient |
| Concentration of constituent i or absorption by CDOM at reference wavelength | |
| Estimated concentration or absorption of optical constituent i | |
| RMSRE | Root mean squared relative error |
| MAE | Mean absolute error |
Appendix A
| Parameter | Value | Units | Notes | References |
|---|---|---|---|---|
| Case-II bio-optical model | ||||
| Sea-water | ||||
| Absorption | - | m | See references | Pope and Fry [67] for >550 nm Mason et al. [20] <550 nm |
| Phase function | - | sr | See reference | Equation 3.30 in Mobley [68] |
| Elastic scattering | - | m | See reference | Equation. 3.31 in Mobley [68] |
| Inelastic (Raman) scattering | - | m | No inelastic scattering | |
| Phytoplankton | ||||
| Absorption | - | m | See reference for spectral absorption | Prieur and Sathyendranath [69] |
| Phase function | - | sr | Fournier-Forand (1.4% backscatter ratio) | |
| Scattering | - | m | Spectral backscatter according to: | |
| Fluorescence | - | - | No chlorophyll fluorescence | |
| Concentration | 0.01–31.62 | mg m | ||
| Colored dissolved organic matter | ||||
| 0.005–1 | m | |||
| Absorption | - | m | See reference for spectral absorption | Babin et al. [26] |
| Slope | 0.017 | nm | Exponential decay function with reference at 440 nm | Babin et al. [26] |
| Non-algal particles | ||||
| concentration | 0.01–100 | g m | ||
| Absorption | - | m | See reference for spectral absorption | Babin et al. [26] |
| Slope (spectral absorption) | 0.0123 | nm | Exponential decay function with reference at 443 nm | Babin et al. [26] |
| Phase function | - | sr | Fournier-Forand (1.4% backscatter ratio) | |
| Backscatter | - | m | See reference for spectral backscatter | Babin et al. [70] |
| Slope (spectral backscatter) | −1 | nm | power-law with reference wavelength at 550 nm | Babin et al. [70] |
| Sea-surface boundary model | ||||
| Wind speed | 5 | m s | ||
| Real index of refraction of water | 1.34 | - | Wavelength indepedent | |
| Atmospheric model (RADTRAN-X) | ||||
| Solar zenith angle | 0–80 | degrees | 10 degree intervals | |
| Cloud cover | 0 | percent | Clear sky | |
| Earth-sun distance | - | - | Yearly average | |
| 24-h averaged wind speed | 5 | m s | ||
| Horizontal visibility | 15 | km | ||
| Relative humidity | 80 | percent | ||
| Precipitable water content | 2.5 | cm | ||
| Total ozone | 300 | Dobson units | Yearly average | |
| Airmass type | 1 | - | Marine | |
| Bottom reflection model | ||||
| Depth | - | m | Infinitely deep (no bottom reflection) | |
| Output | ||||
| Wavebands | 400–710 | nm | 5-nm resolution | |
| Radiance (upwelling) | - | W sr m nm | Radiance distribution for all HydroLight quads | |
| Irradiance (downwelling) | - | W m nm | ||
| Abbreviation | Definition | Formula |
|---|---|---|
| MAE | Mean absolute error | |
| MB | Mean bias | |
| Coefficient of determination | ||
| slope | slope of linear regression model | |
| f | fraction of successful retrievals |
References
- IOCCG. Phytoplankton Functional Types from Space; Reports of the International Ocean Colour Coordinating Group; IOCCG: Dartmouth, NS, Canada, 2014; Volume 15. [Google Scholar] [CrossRef]
- Alvain, S.; Moulin, C.; Dandonneau, Y.; Bréon, F.M. Remote sensing of phytoplankton groups in case 1 waters from global SeaWiFS imagery. Deep. Sea Res. Part I Oceanogr. Res. Pap. 2005, 52, 1989–2004. [Google Scholar] [CrossRef] [Green Version]
- Bracher, A.; Bouman, H.A.; Brewin, R.J.W.; Bricaud, A.; Brotas, V.; Ciotti, A.M.; Clementson, L.; Devred, E.; Di Cicco, A.; Dutkiewicz, S.; et al. Obtaining Phytoplankton Diversity from Ocean Color: A Scientific Roadmap for Future Development. Front. Mar. Sci. 2017, 4, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Stomp, M.; Huisman, J.; Stal, L.J.; Matthijs, H.C.P. Colorful niches of phototrophic microorganisms shaped by vibrations of the water molecule. ISME J. 2007, 1, 271–282. [Google Scholar] [CrossRef]
- Brewin, R.J.W.; Sathyendranath, S.; Hirata, T.; Lavender, S.J.; Barciela, R.M.; Hardman-Mountford, N.J. A three-component model of phytoplankton size class for the Atlantic Ocean. Ecol. Model. 2010, 221, 1472–1483. [Google Scholar] [CrossRef]
- Mouw, C.B.; Hardman-Mountford, N.J.; Alvain, S.; Bracher, A.; Brewin, R.J.W.; Bricaud, A.; Ciotti, A.M.; Devred, E.; Fujiwara, A.; Hirata, T.; et al. A Consumer’s Guide to Satellite Remote Sensing of Multiple Phytoplankton Groups in the Global Ocean. Front. Mar. Sci. 2017, 4. [Google Scholar] [CrossRef] [Green Version]
- Holtrop, T.; Huisman, J.; Stomp, M.; Biersteker, L.; Aerts, J.; Grébert, T.; Partensky, F.; Garczarek, L.; Van Der Woerd, H.J. Vibrational modes of water predict spectral niches for photosynthesis in lakes and oceans. Nat. Ecol. Evol. 2020, 5, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Kramer, S.J.; Siegel, D.A. How Can Phytoplankton Pigments Be Best Used to Characterize Surface Ocean Phytoplankton Groups for Ocean Color Remote Sensing Algorithms? J. Geophys. Res. Ocean. 2019, 124, 7557–7574. [Google Scholar] [CrossRef]
- Kostadinov, T.S.; Siegel, D.A.; Maritorena, S. Global variability of phytoplankton functional types from space: Assessment via the particle size distribution. Biogeosciences 2010, 7, 3239–3257. [Google Scholar] [CrossRef] [Green Version]
- Bricaud, A.; Claustre, H.; Ras, J.; Oubelkheir, K. Natural variability of phytoplanktonic absorption in oceanic waters: Influence of the size structure of algal populations. J. Geophys. Res. 2004, 109, 1–12. [Google Scholar] [CrossRef]
- Uitz, J.; Huot, Y.; Bruyant, F.; Babin, M.; Claustre, H. Relating phytoplankton photophysiological properties to community structure on large scales. Limnol. Oceanogr. 2008, 53, 614–630. [Google Scholar] [CrossRef] [Green Version]
- Brewin, R.J.; Dall’Olmo, G.; Sathyendranath, S.; Hardman-Mountford, N.J. Particle backscattering as a function of chlorophyll and phytoplankton size structure in the open-ocean. Opt. Express 2012, 20, 17632. [Google Scholar] [CrossRef]
- Ciotti, A.M.; Lewis, M.R.; Cullen, J.J. Assessment of the relationships between dominant cell size in natural phytoplankton communities and the spectral shape of the absorption coefficient. Limnol. Oceanogr. 2002, 47, 404–417. [Google Scholar] [CrossRef] [Green Version]
- Brewin, R.J.W.; Hardman-Mountford, N.J.; Lavender, S.J.; Raitsos, D.E.; Hirata, T.; Uitz, J.; Devred, E.; Bricaud, A.; Ciotti, A.; Gentili, B. An intercomparison of bio-optical techniques for detecting dominant phytoplankton size class from satellite remote sensing. Remote Sens. Environ. 2011, 115, 325–339. [Google Scholar] [CrossRef]
- Werdell, P.J.; Roesler, C.S.; Goes, J.I. Discrimination of phytoplankton functional groups using an ocean reflectance inversion model. Appl. Opt. 2014, 53, 4833. [Google Scholar] [CrossRef]
- Xi, H.; Hieronymi, M.; Krasemann, H.; Röttgers, R. Phytoplankton Group Identification Using Simulated and In situ Hyperspectral Remote Sensing Reflectance. Front. Mar. Sci. 2017, 4, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Evers-King, H.; Bernard, S.; Robertson Lain, L.; Probyn, T.A. Sensitivity in reflectance attributed to phytoplankton cell size: Forward and inverse modelling approaches. Opt. Express 2014, 22, 11536. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mobley, C.; Sundman, L. HydroLight 5.3-EcoLight 5.3 Users’ Guide; Technical Report; Sequoia Scientific: Bellevue, WA, USA, 2016. [Google Scholar]
- Van Der Woerd, H.J.; Pasterkamp, R. HYDROPT: A fast and flexible method to retrieve chlorophyll-a from multispectral satellite observations of optically complex coastal waters. Remote Sens. Environ. 2008, 112, 1795–1807. [Google Scholar] [CrossRef]
- Mason, J.D.; Cone, M.T.; Fry, E.S. Ultraviolet (250–550 nm) absorption spectrum of pure water. Appl. Opt. 2016, 55, 7163. [Google Scholar] [CrossRef]
- Holtrop, T.; Van Der Woerd, H. HYDROPT: A Python Framework for Fast Inverse Modelling of Multi- and Hyperspectral Ocean Color Data. 2021. [Google Scholar] [CrossRef]
- Werdell, P.J.; Behrenfeld, M.J.; Bontempi, P.S.; Boss, E.; Cairns, B.; Davis, G.T.; Franz, B.A.; Gliese, U.B.; Gorman, E.T.; Hasekamp, O.; et al. The plankton, aerosol, cloud, ocean ecosystem mission status, science, advances. Bull. Am. Meteorol. Soc. 2019, 100, 1775–1794. [Google Scholar] [CrossRef]
- Gordon, H.R.; Brown, O.B.; Evans, R.H.; Brown, J.W.; Smith, R.C.; Baker, K.S.; Clark, D.K. A Semianalytical Radiance Model of Ocean Color. J. Geophys. Res. 1988, 93, 10909–10924. [Google Scholar]
- Morel, A.; Gentili, B. Diffuse reflectance of oceanic waters: Its dependence on sun angle as influenced by the molecular scattering contribution. Appl. Opt. 1991, 30, 4427–4438. [Google Scholar] [CrossRef]
- Morel, A.; Antoine, D.; Gentili, B. Bidirectional reflectance of oceanic waters: Accounting for Raman emission and varying particle scattering phase function. Appl. Opt. 2002, 41, 6289. [Google Scholar] [CrossRef] [PubMed]
- Babin, M.; Stramski, D.; Ferrari, G.M.; Claustre, H.; Bricaud, A.; Obolensky, G.; Hoepffner, N. Variations in the light absorption coefficients of phytoplankton, nonalgal particles, and dissolved organic matter in coastal waters around Europe. J. Geophys. Res. 2003, 108, 1–20. [Google Scholar] [CrossRef]
- IOCCG. Remote Sensing of Inherent Optical Properties: Fundamentals, Tests of Algorithms, and Applications; Reports of the International Ocean Colour Coordinating Group; IOCCG: Dartmouth, NS, Canada, 2006; Volume 5. [Google Scholar] [CrossRef]
- Göçken, M.; Özçalici, M.; Boru, A.; Dosdogru, A.T. Integrating metaheuristics and Artificial Neural Networks for improved stock price prediction. Expert Syst. Appl. 2016, 44, 320–331. [Google Scholar] [CrossRef]
- Despotovic, M.; Nedic, V.; Despotovic, D.; Cvetanovic, S. Evaluation of empirical models for predicting monthly mean horizontal diffuse solar radiation. Renew. Sustain. Energy Rev. 2016, 56, 246–260. [Google Scholar] [CrossRef]
- James, G.; Witten, D.; Hastie, T.; Tibshirani, R. An Introduction to Statistical Learning: With Applications in R; Springer Publishing Company, Incorporated: New York, NY, USA, 2014. [Google Scholar] [CrossRef]
- Virtanen, P.; Gommers, R.; Oliphant, T.E.; Haberland, M.; Reddy, T.; Cournapeau, D.; Burovski, E.; Peterson, P.; Weckesser, W.; Bright, J.; et al. {SciPy} 1.0: Fundamental Algorithms for Scientific Computing in Python. Nat. Methods 2020, 17, 261–272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Newville, M.; Otten, R.; Nelson, A.; Ingargiola, A.; Stensitzki, T.; Maier, D.A.; Fox, A.; Carter, F.; Pustakhod, D.; Weigand, S.; et al. Lmfit: Non-Linear Least-Square Minimization and Curve-Fitting for Python. 2021. [Google Scholar] [CrossRef]
- Tuchow, N.; Broughton, J.; Kudela, R. Sensitivity analysis of volume scattering phase functions. Opt. Express 2016, 24, 18559. [Google Scholar] [CrossRef]
- Lain, L.R.; Bernard, S.; Matthews, M.W. Understanding the contribution of phytoplankton phase functions to uncertainties in the water colour signal. Opt. Express 2017, 25, A151–A165. [Google Scholar] [CrossRef]
- Holtrop, T.; Van Der Woerd, H. A Synthetic Hyperspectral Dataset for Development and Validation of Phytoplankton Size Class Retrieval Models. 2021. [Google Scholar] [CrossRef]
- Sathyendranath, S.; Cota, G.; Stuart, V.; Maass, H.; Platt, T. Remote sensing of phytoplankton pigments: A comparison of empirical and theoretical approaches. Int. J. Remote Sens. 2001, 22, 249–273. [Google Scholar] [CrossRef]
- Devred, E.; Sathyendranath, S.; Stuart, V.; Maass, H.; Ulloa, O.; Platt, T. A two-component model of phytoplankton absorption in the open ocean: Theory and applications. J. Geophys. Res. Ocean. 2006, 111, 1–11. [Google Scholar] [CrossRef]
- Irigoien, X.; Huisman, J.; Harris, R.P. Global biodiversity patterns of marine phytoplankton and zooplankton. Nature 2004, 429, 863–867. [Google Scholar] [CrossRef]
- Zhang, X.; Huot, Y.; Bricaud, A.; Sosik, H.M. Inversion of spectral absorption coefficients to infer phytoplankton size classes, chlorophyll concentration, and detrital matter. Appl. Opt. 2015, 54, 5805. [Google Scholar] [CrossRef]
- Loisel, H.; Nicolas, J.M.; Sciandra, A.; Stramski, D.; Poteau, A. Spectral dependency of optical backscattering by marine particles from satellite remote sensing of the global ocean. J. Geophys. Res. Ocean. 2006, 111, 1–14. [Google Scholar] [CrossRef]
- Seegers, B.N.; Stumpf, R.P.; Schaeffer, B.A.; Loftin, K.A.; Werdell, P.J. Performance metrics for the assessment of satellite data products: An ocean color case study. Opt. Express 2018, 26, 7404. [Google Scholar] [CrossRef] [Green Version]
- Van Der Woerd, H.J.; Wernand, M.; Peters, M.; Bala, M. True color analysis of natural waters with SeaWiFS, MODIS, MERIS and OLCI by SNAP. In Proceedings of the Ocean Optics XXIII, Victoria, BC, Canada, 23–28 October 2016. [Google Scholar]
- Hirata, T.; Hardman-Mountford, N.J.; Brewin, R.J.W.; Aiken, J.; Barlow, R.; Suzuki, K.; Isada, T.; Howell, E.; Hashioka, T.; Noguchi-Aita, M.; et al. Synoptic relationships between surface Chlorophyll-a and diagnostic pigments specific to phytoplankton functional types. Biogeosciences 2011, 8, 311–327. [Google Scholar] [CrossRef] [Green Version]
- Uitz, J.; Stramski, D.; Reynolds, R.A.; Dubranna, J. Assessing phytoplankton community composition from hyperspectral measurements of phytoplankton absorption coefficient and remote-sensing reflectance in open-ocean environments. Remote Sens. Environ. 2015, 171, 58–74. [Google Scholar] [CrossRef]
- Bracher, A.; Taylor, M.H.; Taylor, B.; Dinter, T.; Röttgers, R.; Steinmetz, F. Using empirical orthogonal functions derived from remote-sensing reflectance for the prediction of phytoplankton pigment concentrations. Ocean. Sci. 2015, 11, 139–158. [Google Scholar] [CrossRef] [Green Version]
- Gittings, J.A.; Brewin, R.J.; Raitsos, D.E.; Kheireddine, M.; Ouhssain, M.; Jones, B.H.; Hoteit, I. Remotely sensing phytoplankton size structure in the Red Sea. Remote Sens. Environ. 2019, 234, 111387. [Google Scholar] [CrossRef]
- Devred, E.; Sathyendranath, S.; Stuart, V.; Platt, T. A three component classification of phytoplankton absorption spectra: Application to ocean-color data. Remote Sens. Environ. 2011, 115, 2255–2266. [Google Scholar] [CrossRef]
- Kostadinov, T.S.; Milutinovi, S.; Marinov, I.; Cabré, A. Carbon-based phytoplankton size classes retrieved via ocean color estimates of the particle size distribution. Ocean. Sci. 2016, 12, 561–575. [Google Scholar] [CrossRef] [Green Version]
- Werdell, P.J.; McKinna, L.I.; Boss, E.; Ackleson, S.G.; Craig, S.E.; Gregg, W.W.; Lee, Z.; Maritorena, S.; Roesler, C.S.; Rousseaux, C.S.; et al. An overview of approaches and challenges for retrieving marine inherent optical properties from ocean color remote sensing. Prog. Oceanogr. 2018, 160, 186–212. [Google Scholar] [CrossRef] [PubMed]
- Brito, A.C.; Sá, C.; Brotas, V.; Brewin, R.J.; Silva, T.; Vitorino, J.; Platt, T.; Sathyendranath, S. Effect of phytoplankton size classes on bio-optical properties of phytoplankton in the Western Iberian coast: Application of models. Remote Sens. Environ. 2015, 156, 537–550. [Google Scholar] [CrossRef] [Green Version]
- Brewin, R.J.; Sathyendranath, S.; Jackson, T.; Barlow, R.; Brotas, V.; Airs, R.; Lamont, T. Influence of light in the mixed-layer on the parameters of a three-component model of phytoplankton size class. Remote Sens. Environ. 2015, 168, 437–450. [Google Scholar] [CrossRef]
- Sun, X.; Shen, F.; Liu, D.; Bellerby, R.G.J.; Liu, Y.; Tang, R. In Situ and Satellite Observations of Phytoplankton Size Classes in the Entire Continental Shelf Sea, China. J. Geophys. Res. Ocean. 2018, 123, 3523–3544. [Google Scholar] [CrossRef] [Green Version]
- Morel, A.; Huot, Y.; Gentili, B.; Werdell, P.J.; Hooker, S.B.; Franz, B.A. Examining the consistency of products derived from various ocean color sensors in open ocean (Case 1) waters in the perspective of a multi-sensor approach. Remote Sens. Environ. 2007, 111, 69–88. [Google Scholar] [CrossRef]
- Sathyendranath, S.; Brewin, R.J.W.; Brockmann, C.; Brotas, V.; Calton, B.; Chuprin, A.; Cipollini, P.; Couto, A.B.; Dingle, J.; Doerffer, R.; et al. An Ocean-Colour Time Series for Use in Climate Studies: The Experience of the Ocean-Colour Climate Change Initiative (OC-CCI). Sensors 2019, 19, 4285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morel, A.; Prieur, L. Analysis of variations in ocean color. Limnol. Oceanogr. 1977, 22, 709–722. [Google Scholar] [CrossRef]
- Chami, M.; McKee, D.; Leymarie, E.; Khomenko, G. Influence of the angular shape of the volume-scattering function and multiple scattering on remote sensing reflectance. Appl. Opt. 2006, 45, 9210–9220. [Google Scholar] [CrossRef] [PubMed]
- Zhai, P.W.; Hu, Y.; Trepte, C.R.; Winker, D.M.; Lucker, P.L.; Lee, Z.; Josset, D.B. Uncertainty in the bidirectional reflectance model for oceanic waters. Appl. Opt. 2015, 54, 4061. [Google Scholar] [CrossRef]
- Park, Y.J.; Ruddick, K. Model of remote-sensing reflectance including bidirectional effects for case 1 and case 2 waters. Appl. Opt. 2005, 44, 1236–1249. [Google Scholar] [CrossRef]
- He, S.; Fischer, J.; Schaale, M.; He, M.X. Optical closure of parameterized bio-optical relationships. Chin. J. Oceanol. Limnol. 2014, 32, 480–489. [Google Scholar] [CrossRef]
- Salama, M.S.; Stein, A. Error decomposition and estimation of inherent optical properties. Appl. Opt. 2009, 48, 4947–4962. [Google Scholar] [CrossRef]
- Maritorena, S.; Siegel, D.A.; Peterson, A.R. Optimization of a semianalytical ocean color model for global-scale applications. Appl. Opt. 2002, 41, 2705. [Google Scholar] [CrossRef]
- Frouin, R.; Pelletier, B. Bayesian methodology for inverting satellite ocean-color data. Remote Sens. Environ. 2015, 159, 332–360. [Google Scholar] [CrossRef]
- He, S.; Zhang, X.; Xiong, Y.; Gray, D. A Bidirectional Subsurface Remote Sensing Reflectance Model Explicitly Accounting for Particle Backscattering Shapes. J. Geophys. Res. Ocean. 2017, 122, 8614–8626. [Google Scholar] [CrossRef]
- Twardowski, M.; Tonizzo, A. Ocean color analytical model explicitly dependent on the volume scattering function. Appl. Sci. 2018, 8, 2684. [Google Scholar] [CrossRef] [Green Version]
- Werdell, P.J.; Bailey, S.W. An improved in-situ bio-optical data set for ocean color algorithm development and satellite data product validation. Remote Sens. Environ. 2005, 98, 122–140. [Google Scholar] [CrossRef]
- Soja-Woźniak, M.; Laiolo, L.; Baird, M.E.; Matear, R.; Clementson, L.; Schroeder, T.; Doblin, M.A.; Suthers, I.M. Effect of phytoplankton community size structure on remote-sensing reflectance and chlorophyll a products. J. Mar. Syst. 2020, 211. [Google Scholar] [CrossRef]
- Pope, R.M.; Fry, E.S. Absorption spectrum (380–700 nm) of pure water. II. Integrating cavity measurements. Appl. Opt. 1997, 36, 8710–8723. [Google Scholar] [CrossRef] [PubMed]
- Mobley, C.D. Ligth and Water: Radiative Transfer in Natural Waters; Academic Press: San Diego, CA, USA, 1994; p. 592. [Google Scholar]
- Prieur, L.; Sathyendranath, S. An optical classification of coastal and oceanic waters based on the specific spectral absorption curves of phytoplankton pigments, dissolved organic matter, and other particulate materials 1. Limnol. Oceanogr. 1981, 26, 671–689. [Google Scholar] [CrossRef]
- Babin, M.; Morel, A.; Fournier-Sicre, V.; Fell, F.; Stramski, D. Light scattering properties of marine particles in coastal and open ocean waters as related to the particle mass concentration. Limnol. Oceanogr. 2003, 48, 843–859. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Holtrop, T.; Van Der Woerd, H.J. HYDROPT: An Open-Source Framework for Fast Inverse Modelling of Multi- and Hyperspectral Observations from Oceans, Coastal and Inland Waters. Remote Sens. 2021, 13, 3006. https://doi.org/10.3390/rs13153006
Holtrop T, Van Der Woerd HJ. HYDROPT: An Open-Source Framework for Fast Inverse Modelling of Multi- and Hyperspectral Observations from Oceans, Coastal and Inland Waters. Remote Sensing. 2021; 13(15):3006. https://doi.org/10.3390/rs13153006
Chicago/Turabian StyleHoltrop, Tadzio, and Hendrik Jan Van Der Woerd. 2021. "HYDROPT: An Open-Source Framework for Fast Inverse Modelling of Multi- and Hyperspectral Observations from Oceans, Coastal and Inland Waters" Remote Sensing 13, no. 15: 3006. https://doi.org/10.3390/rs13153006
APA StyleHoltrop, T., & Van Der Woerd, H. J. (2021). HYDROPT: An Open-Source Framework for Fast Inverse Modelling of Multi- and Hyperspectral Observations from Oceans, Coastal and Inland Waters. Remote Sensing, 13(15), 3006. https://doi.org/10.3390/rs13153006







