Assessment of UAV-Onboard Multispectral Sensor for Non-Destructive Site-Specific Rapeseed Crop Phenotype Variable at Different Phenological Stages and Resolutions
Abstract
1. Introduction
2. Materials and Methods
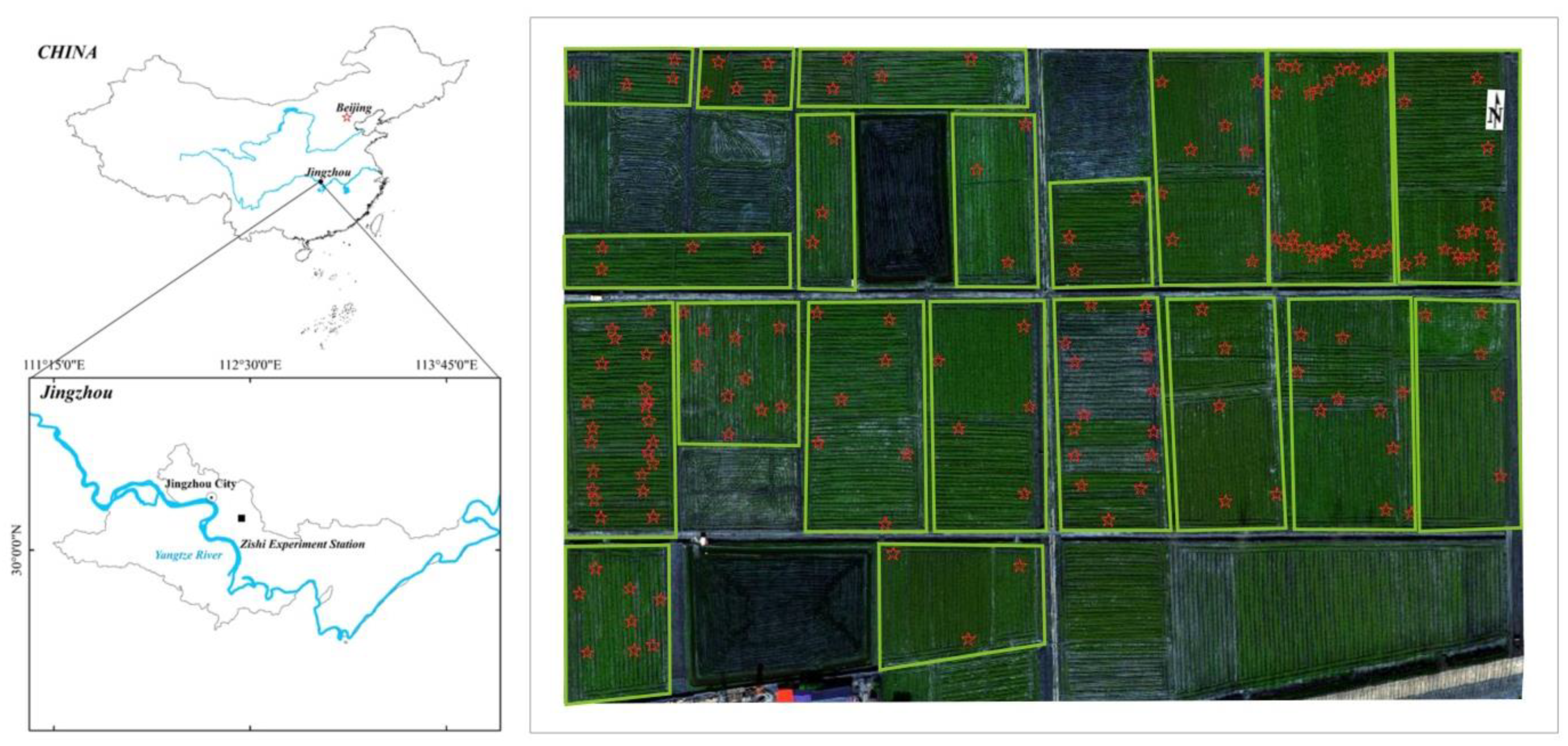
2.1. Study Area
2.2. Biophysical Parameter Measurements
2.3. UAV Image Acquisition
2.4. Image Processing
2.5. Multispectral VIs
2.6. Statistical Analysis
3. Results
3.1. Association of VIs to the Phenolgical Stages
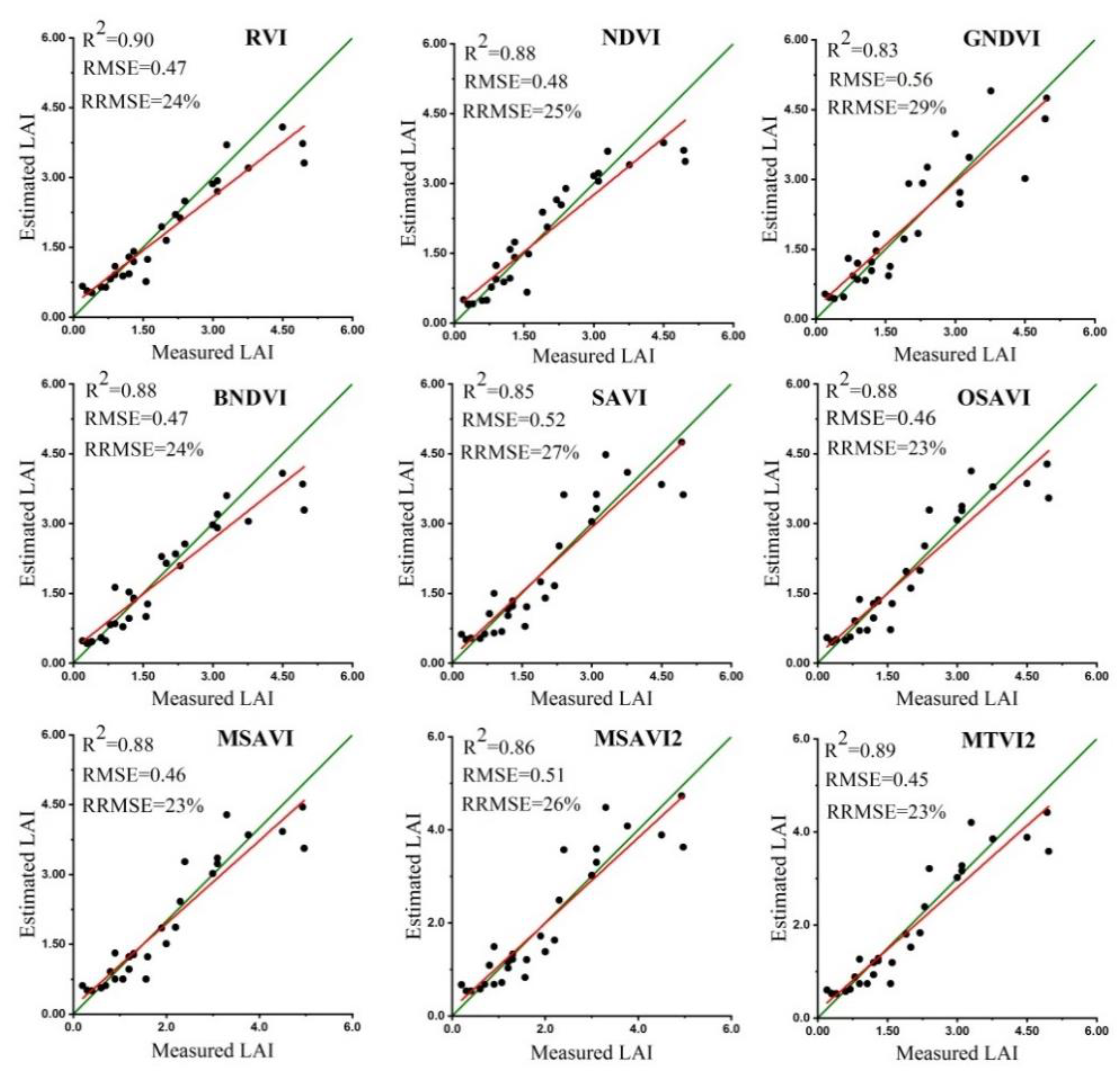
3.2. Evaluation of Spectral VIs for Estimation of Rapeseed LAI
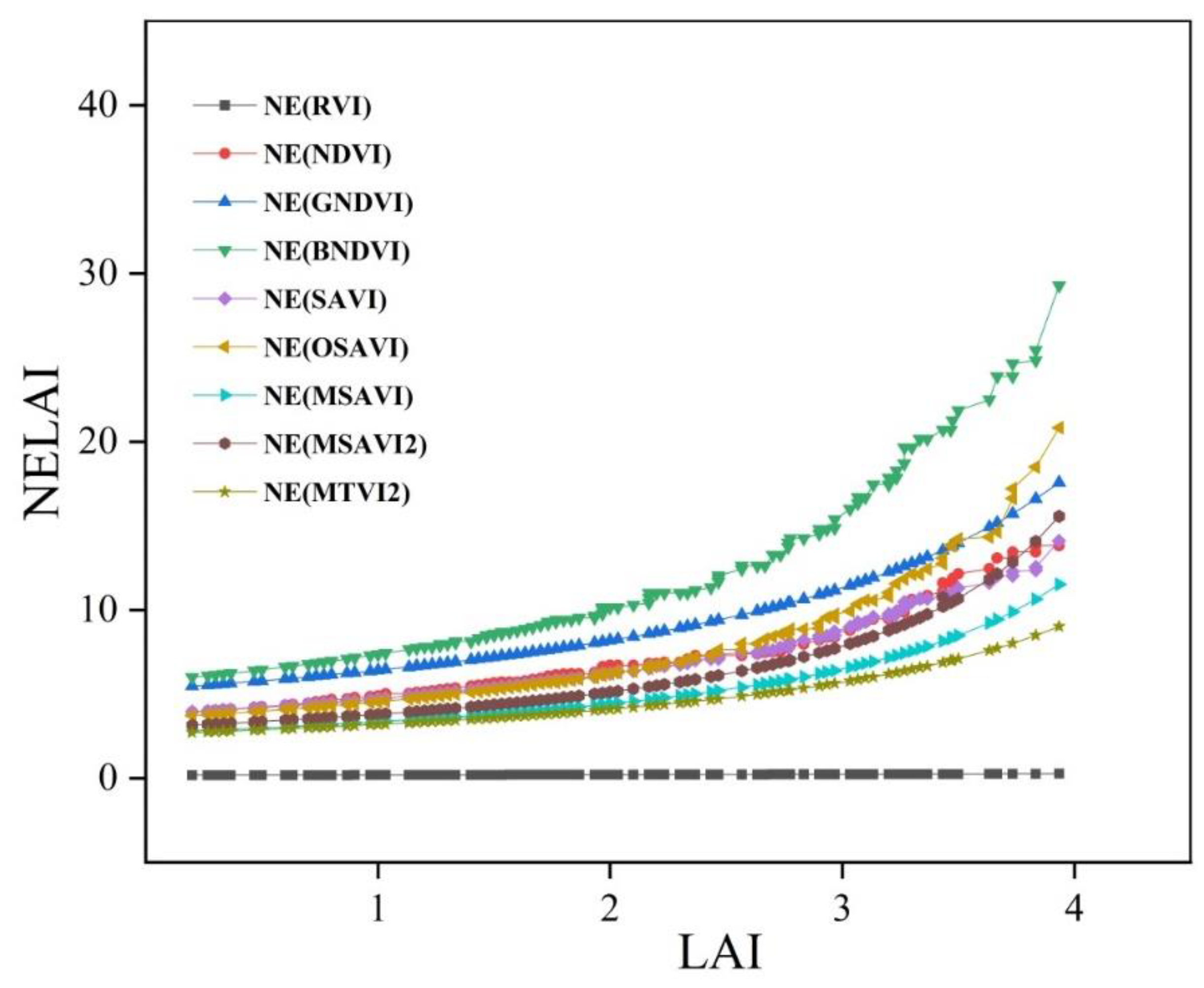
3.3. Sensitivity Analysis
3.4. Relationship of VIs and Leaf DW
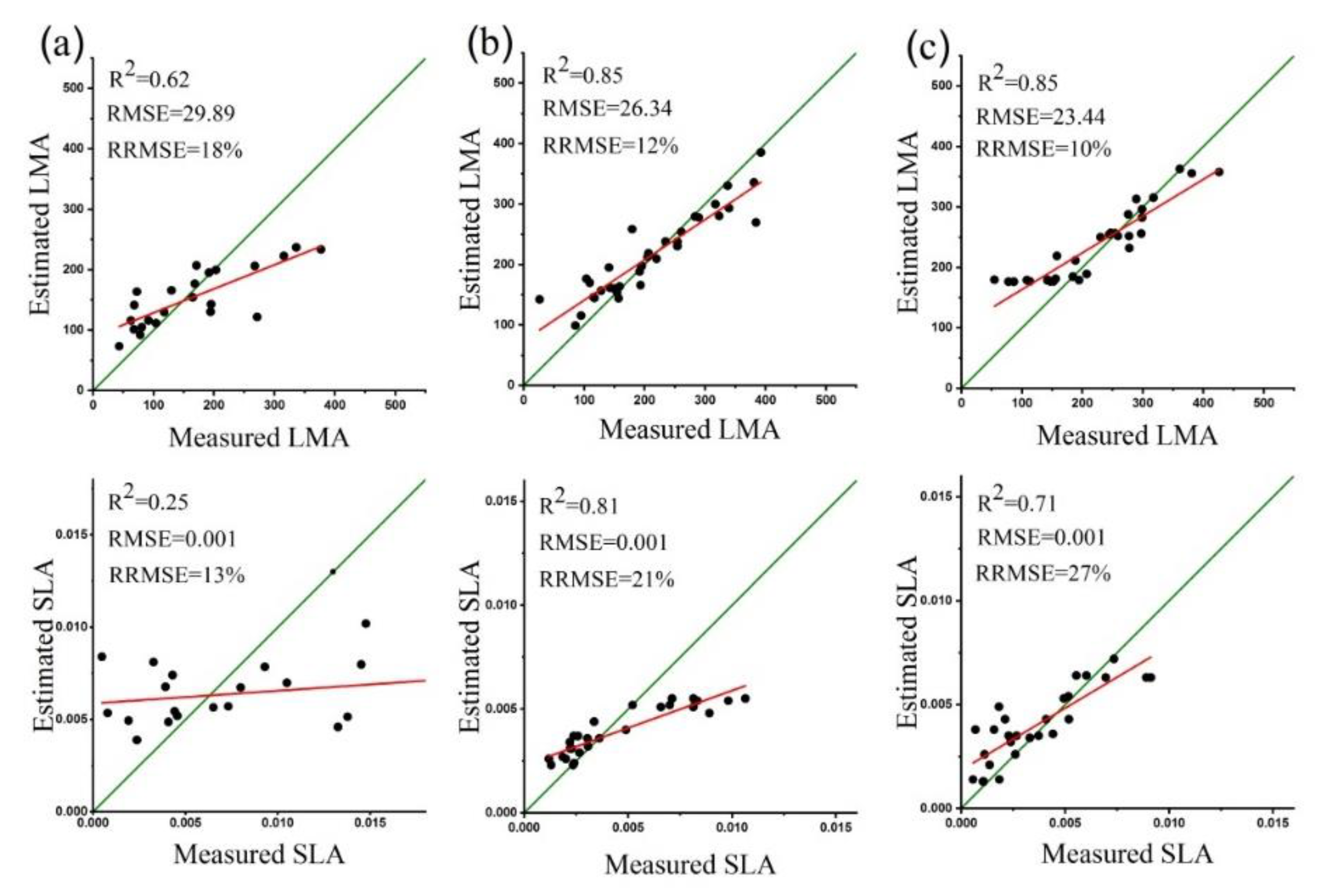
3.5. Estimation LMA and SLA using Spectral VIs
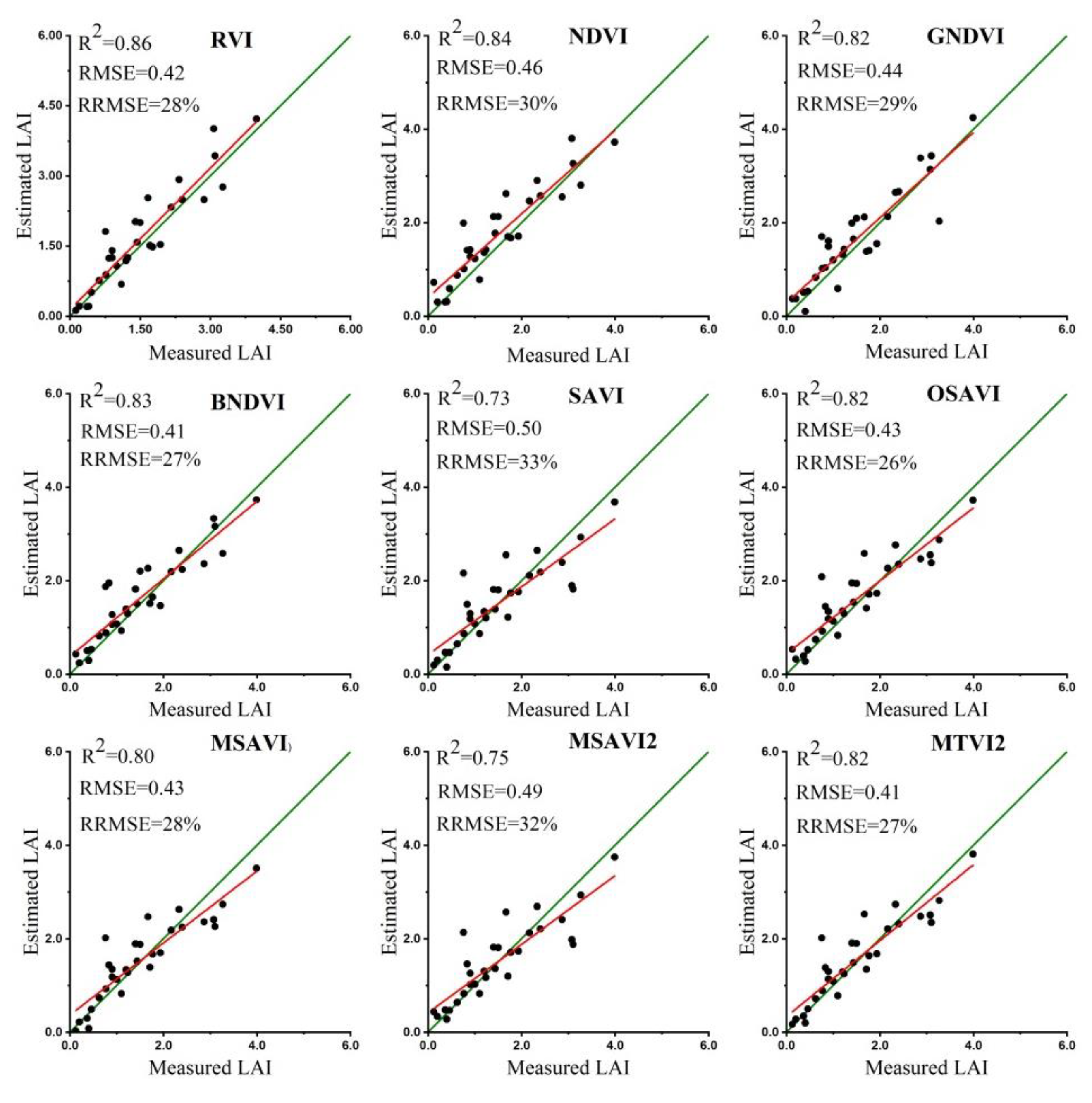
3.6. Validation of LAI, LMA and SLA Estimates
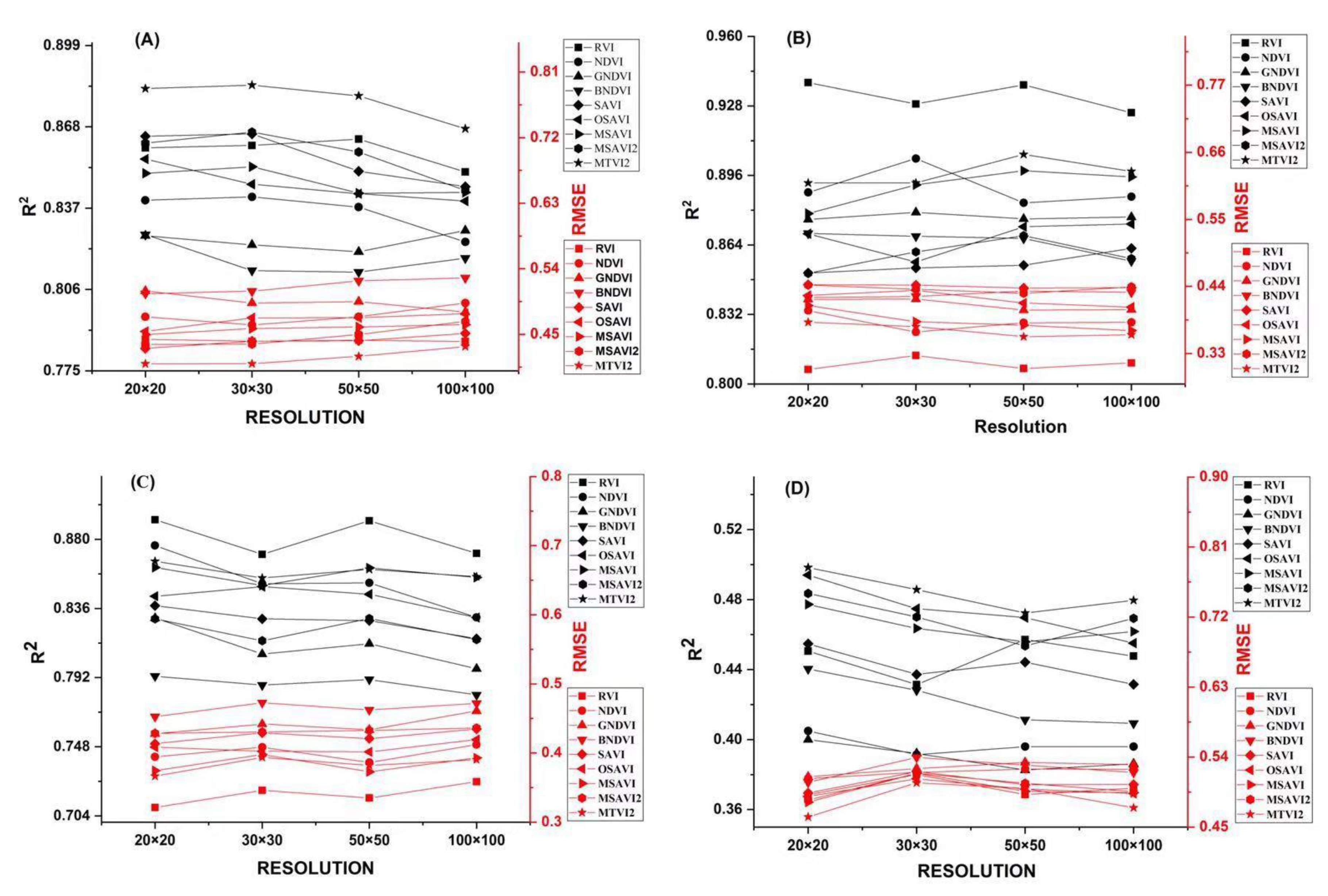
3.7. Evaluation of Image Resolution Effect
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Turner, D.; Lucieer, A.; Watson, C. An Automated Technique for Generating Georectified Mosaics from Ultra-High Resolution Unmanned Aerial Vehicle (UAV) Imagery, Based on Structure from Motion (SfM) Point Clouds. Remote Sens. 2012, 4, 1392–1410. [Google Scholar] [CrossRef]
- Vega, F.A.; Ramirez, F.C.; Saiz, M.P.; Rosua, F.O. ScienceDirect Multi-temporal imaging using an unmanned aerial vehicle for monitoring a sunflower crop. Biosyst. Eng. 2015, 132, 19–27. [Google Scholar] [CrossRef]
- Zhang, C.; Kovacs, J.M. The application of small unmanned aerial systems for precision agriculture: A review. Precis. Agric. 2015, 13, 693–712. [Google Scholar] [CrossRef]
- Tremblay, N.; Vigneault, P.; Bélec, C.; Fallon, E.; Bouroubi, M.Y. A comparison of performance between UAV and satellite imagery for N status assessment in corn. In Proceedings of the 12th International Conference on Precision Agriculture, Sacramento, CA, USA, 20–23 July 2014. [Google Scholar]
- Hunt, E.R.; Daughtry, C.S.T.; Mirsky, S.B.; Hively, W.D. Remote sensing with unmanned aircraft systems for precision agriculture applications. In Proceedings of the 2013 Second International Conference on Agro-Geoinformatics (Agro-Geoinformatics), Fairfax, VA, USA, 12–16 August 2013. [Google Scholar]
- Zhao, G.; Miao, Y.; Wang, H.; Su, M.; Fan, M.; Zhang, F.; Jiang, R.; Zhang, Z.; Liu, C.; Liu, P.; et al. A preliminary precision rice management system for increasing both grain yield and nitrogen use efficiency. Field Crops Res. 2013, 154, 23–30. [Google Scholar] [CrossRef]
- Hatfield, J.L.; Prueger, J.H. Value of Using Different Vegetative Indices to Quantify Agricultural Crop Characteristics at Different Growth Stages under Varying Management Practices. Remote Sens. 2010, 2, 562–578. [Google Scholar] [CrossRef]
- Warren, G.; Metternicht, G. Agricultural Applications of High-Resolution Digital Multispectral Imagery: Evaluating Within-Field Spatial Variability of Canola (Brassica napus) in Western Australia. Photogramm. Eng. Remote Sens. 2005, 71, 595–602. [Google Scholar] [CrossRef]
- Primicerio, J.; Di Gennaro, S.F.; Fiorillo, E.; Genesio, L.; Lugato, E.; Matese, A.; Vaccari, F.P. A flexible unmanned aerial vehicle for precision agriculture. Precis. Agric. 2012, 13, 517–523. [Google Scholar] [CrossRef]
- Din, M.; Zheng, W.; Rashid, M.; Wang, S.; Shi, Z. Evaluating Hyperspectral Vegetation Indices for Leaf Area Index Estimation of Oryza sativa L. at Diverse Phenological Stages. Front. Plant Sci. 2017, 8, 1–17. [Google Scholar] [CrossRef]
- Lan, Y.; Thomson, S.J.; Huang, Y.; Hoffmann, W.C.; Zhang, H. Current status and future directions of precision aerial application for site-specific crop management in the USA. Comput. Electron. Agric. 2010, 74, 34–38. [Google Scholar] [CrossRef]
- Hunt, E.-R.; Horneck, D.; Gadler, D.; Bruce, A.; Turner, R.; Spinelli, C.; Brungardt, J.; Hamm, P. Detection of nitrogen deficiency in potatoes using small unmanned aircraft systems. In Proceedings of the 12th International Conference on Precision Agriculture, Sacramento, CA, USA, 20–23 July 2014. [Google Scholar]
- Swain, K.C.; Zaman, Q.U. Rice Crop Monitoring with Unmanned Helicopter Remote Sensing Images. In Remote Sensing of Biomass—Principles and Applications; Fatoyinbo, T., Ed.; InTech: Rijeca, Croatia, 2012; pp. 253–272. [Google Scholar]
- Laliberte, A. Unmanned aerial vehicle-based remote sensing for rangeland assessment, monitoring, and management. J. Appl. Remote Sens. 2009, 3, 033542. [Google Scholar] [CrossRef]
- Lelong, C.; Burger, P.; Jubelin, G.; Roux, B.; Labbé, S.; Baret, F. Assessment of Unmanned Aerial Vehicles Imagery for Quantitative Monitoring of Wheat Crop in Small Plots. Sensors 2008, 8, 3557–3585. [Google Scholar] [CrossRef] [PubMed]
- Tilman, D. Functional Diversity. In Encyclopedia of Biodiversity; Elsevier: Amsterdam, The Netherlands, 2001; Volume 3, pp. 109–120. [Google Scholar] [CrossRef]
- Ali, A.M.; Darvishzadeh, R.; Skidmore, A.K.; van Duren, I.; Heiden, U.; Heurich, M. Prospect inversion for indirect estimation of leaf dry matter content and specific leaf area. Int. Arch. Photogramm. Remote Sens. Spat. Inf. Sci. 2015, 40, 277–284. [Google Scholar] [CrossRef]
- Jin, X.; Diao, W.; Xiao, C.; Wang, F.; Chen, B.; Wang, K.; Li, S. Estimation of Wheat Agronomic Parameters using New Spectral Indices. PLoS ONE 2013, 8, e72736. [Google Scholar] [CrossRef] [PubMed]
- Cheng, T.; Rivard, B.; Sánchez-Azofeifa, A.G.; Féret, J.-B.; Jacquemoud, S.; Ustin, S.L. Deriving leaf mass per area (LMA) from foliar reflectance across a variety of plant species using continuous wavelet analysis. ISPRS J. Photogramm. Remote Sens. 2014, 87, 28–38. [Google Scholar] [CrossRef]
- De Riva, E.G.; Olmo, M.; Poorter, H.; Ubera, J.L.; Villar, R. Leaf Mass per Area (LMA) and Its Relationship with Leaf Structure and Anatomy in 34 Mediterranean Woody Species along a Water Availability Gradient. PloS ONE 2016, 11, 1–18. [Google Scholar] [CrossRef]
- Homolova, L.; Malenovsky, Z.; Clevers, J.G.P.W.; Garcı´a-Santos, G. Review of optical-based remote sensing for plant trait mapping. Ecol. Complex. 2013, 15, 1–16. [Google Scholar] [CrossRef]
- Wei, Z.; Zhang, B.; Liu, Y.; Xu, D. The Application of a Modified Version of the SWAT Model at the Daily Temporal Scale and the Hydrological Response unit Spatial Scale: A Case Study Covering an Irrigation District in the Hei River Basin. Water 2018, 10, 1064. [Google Scholar] [CrossRef]
- Singh, G.; Saraswat, D. Development and evaluation of targeted marginal land mapping approach in SWAT model for simulating water quality impacts of selected second generation biofeedstock. Environ. Model. Softw. 2016, 81, 26–39. [Google Scholar] [CrossRef]
- Xie, Q.; Huang, W.; Liang, D.; Chen, P.; Wu, C.; Yang, G.; Zhang, J.; Huang, L.; Zhang, D. Leaf area index estimation using vegetation indices derived from airborne hyperspectral images in winter wheat. IEEE J. Sel. Top. Appl. Earth Obs. Remote Sens. 2014, 7, 3586–3594. [Google Scholar] [CrossRef]
- Viña, A.; Gitelson, A.A.; Nguy-Robertson, A.L.; Peng, Y. Comparison of different vegetation indices for the remote assessment of green leaf area index of crops. Remote Sens. Environ. 2011, 115, 3468–3478. [Google Scholar]
- Marques, P.; Martins, M.; Baptista, A.; Torres, J.P.N. Communication Antenas for UAVs. J. Eng. Sci. Technol. Rev. 2018, 11, 90–102. [Google Scholar] [CrossRef]
- Hardin, P.J.; Hardin, T.J. Small-Scale Remotely Piloted Vehicles in Environmental Research. Geogr. Compass 2010, 4, 1297–1311. [Google Scholar] [CrossRef]
- Taylor, J.A.; Jacob, F.; Galleguillos, M.; Prévot, L.; Guix, N.; Lagacherie, P. The utility of remotely-sensed vegetative and terrain covariates at different spatial resolutions in modelling soil and watertable depth (for digital soil mapping). Geoderma 2013, 193–194, 83–93. [Google Scholar] [CrossRef]
- Xu, Y.; Smith, S.E.; Grunwald, S.; Abd-Elrahman, A.; Wani, S.P. Evaluating the effect of remote sensing image spatial resolution on soil exchangeable potassium prediction models in smallholder farm settings. J. Environ. Manag. 2017, 200, 423–433. [Google Scholar] [CrossRef]
- Kim, J.; Grunwald, S.; Rivero, R.G. Soil Phosphorus and Nitrogen Predictions Across Spatial Escalating Scales in an Aquatic Ecosystem Using Remote Sensing Images. IEEE Trans. Geosci. Remote Sens. 2014, 52, 6724–6737. [Google Scholar]
- Vergara-Díaz, O.; Zaman-Allah, M.A.; Masuka, B.; Hornero, A.; Zarco-Tejada, P.; Prasanna, B.M.; Cairns, J.E.; Araus, J.L. A Novel Remote Sensing Approach for Prediction of Maize Yield Under Different Conditions of Nitrogen Fertilization. Front. Plant Sci. 2016, 7, 1–13. [Google Scholar]
- Liu, S.; Li, L.; Gao, W.; Zhang, Y.; Liu, Y.; Wang, S.; Lu, J. Diagnosis of Nitrogen Status In Winter Oilseed Rape (Brassica napus L.) Using In-situ Hyperspectral Data and Unmanned Aerial Vehicle (UAV) Multispectral Images. Comput. Electron. Agric. 2018, 151, 185–195. [Google Scholar] [CrossRef]
- Pearson, R.L.; Miller, L.D. Remote Mapping of Standing Crop Biomass for Estimation of the Productivity of the Shortgrass Prairie; Colorado State University: Fort Collins, CO, USA, 1972. [Google Scholar]
- J Rouse, J.W.; Haas, R.W.; Schell, J.A.; Deering, D.H.; Harlan, J.C. Monitoring the Vernal Advancement and Retrogradation (Greenwave effect) of Natural Vegetation; NASA/GSFC: Greenbelt, MD, USA, 1974.
- Gitelson, A.A.; Kaufman, Y.J.; Merzlyak, M.N. Use of a green channel in remote sensing of global vegetation from EOS-MODIS. Remote Sens. Environ. 1996, 58, 289–298. [Google Scholar] [CrossRef]
- Wang, F.M.; Huang, J.F.; Tang, Y.L.; Wang, X.Z. New Vegetation Index and Its Application in Estimating Leaf Area Index of Rice. Rice Sci. 2007, 14, 195–203. [Google Scholar] [CrossRef]
- Huete, A. A soil-adjusted vegetation index (SAVI). Remote Sens. Environ. 1988, 25, 295–309. [Google Scholar] [CrossRef]
- Haboudane, D. Hyperspectral vegetation indices and novel algorithms for predicting green LAI of crop canopies: Modeling and validation in the context of precision agriculture. Remote Sens. Environ. 2004, 90, 337–352. [Google Scholar] [CrossRef]
- Rondeaux, G.; Steven, M.; Baret, F. Optimization of soil-adjusted vegetation indices. Remote Sens. Environ. 1996, 55, 95–107. [Google Scholar] [CrossRef]
- Whiting, M.L.; Ustin, S.L.; Zarco-Tejada, P.; Palacios-Orueta, A.; Vanderbilt, V.C. Hyperspectral mapping of crop and soils for precision agriculture. In Remote Sensing and Modeling of Ecosystems for Sustainability III; Gao, W., Ustin, S.L., Eds.; SPIE: Bellingham, WA, USA, 2006; Volume 6298, p. 62980B. [Google Scholar]
- Qi, J.; Chehbouni, A.; Huete, A.R.; Keer, Y.H.; Sorooshian, S. A modified soil adusted vegetation index. Remote Sens. Environ. 1994, 48, 119–126. [Google Scholar] [CrossRef]
- Li, F.; Mao, L.; Hennig, S.D.; Gnyp, M.L.; Chen, X.P.; Jia, L.L.; Bareth, G. Evaluating hyperspectral vegetation indices for estimating nitrogen concentration of winter wheat at different growth stages. Precis. Agric. 2010, 11, 335–357. [Google Scholar] [CrossRef]
- Din, M.; Ming, J.; Hussain, S.; Ata-Ul-Karim, S.T.; Rashid, M.; Tahir, M.N.; Hua, S.; Wang, S. Estimation of dynamic canopy variables using hyperspectral derived vegetation indices under varying N rates at diverse phenological stages of rice. Front. Plant Sci. 2019, 9, 1–16. [Google Scholar] [CrossRef]
- Taylor, P.; Gitelson, A.A. Remote estimation of crop fractional vegetation cover : the use of noise equivalent as an indicator of performance of vegetation indices. Int. J. Remote Sens. 2013, 34, 37–41. [Google Scholar]
- Nguy-Robertson, A.L.; Peng, Y.; Gitelson, A.A.; Arkebauer, T.J.; Pimstein, A.; Herrmann, I.; Karnieli, A.; Rundquist, D.C.; Bonfil, D.J. Estimating green LAI in four crops: Potential of determining optimal spectral bands for a universal algorithm. Agric. For. Meteorol. 2014, 192–193, 140–148. [Google Scholar] [CrossRef]
- Roelofsen, H.D.; Van Bodegom, P.M.; Kooistra, L.; Witte, J.P.M. Predicting leaf traits of herbaceous species from their spectral characteristics. Ecol. Evol. 2014, 4, 706–719. [Google Scholar] [CrossRef]
- Wang, S.Q.; Gao, W.H.; Ming, J.; Li, L.T.; Xu, D.H.; Liu, S.S.; Lu, J.W. A TPE based inversion of PROSAIL for estimating canopy biophysical and biochemical variables of oilseed rape. Comput. Electron. Agric. 2018, 152, 350–362. [Google Scholar] [CrossRef]
- Houborg, R.; Anderson, M.; Daughtry, C. Utility of an image-based canopy reflectance modeling tool for remote estimation of LAI and leaf chlorophyll content at the field scale. Remote Sens. Environ. 2009, 113, 259–274. [Google Scholar] [CrossRef]
- Li, F.; Mistele, B.; Hu, Y.; Chen, X.; Schmidhalter, U. Comparing hyperspectral index optimization algorithms to estimate aerial N uptake using multi-temporal winter wheat datasets from contrasting climatic and geographic zones in China and Germany. Agric. For. Meteorol. 2013, 180, 44–57. [Google Scholar] [CrossRef]
- Xiong, D.; Wang, D.; Liu, X.; Peng, S.; Huang, J.; Li, Y. Leaf density explains variation in leaf mass per area in rice between cultivars and nitrogen treatments. Ann. Bot. 2016, 117, 963–971. [Google Scholar] [CrossRef] [PubMed]
- Jégo, G.; Pattey, E.; Liu, J. Using Leaf Area Index, retrieved from optical imagery, in the STICS crop model for predicting yield and biomass of field crops. Field Crops Res. 2012, 131, 63–74. [Google Scholar]
- Carvalho, S.; Van der Putten, W.H.; Hol, W.H.G. The Potential of Hyperspectral Patterns of Winter Wheat to Detect Changes in Soil Microbial Community Composition. Front. Plant Sci. 2016, 7, 1–11. [Google Scholar] [CrossRef]
- Huang, M.; Yang, C.; Ji, Q.; Jiang, L.; Tan, J.; Li, Y. Tillering responses of rice to plant density and nitrogen rate in a subtropical environment of southern China. Field Crops Res. 2013, 149, 187–192. [Google Scholar] [CrossRef]
- Tian, Y.-C.; Yang, J.; Yao, X.; Zhu, Y.; Cao, W.-X. Quantitative relationships between hyper-spectral vegetation indices and leaf area index of rice. J. Appl. Ecol. 2009, 20, 1685–1690. [Google Scholar]
- Zhang, F.; Zhou, G.; Nilsson, C. Remote estimation of the fraction of absorbed photosynthetically active radiation for a maize canopy in Northeast China. J. Plant Ecol. 2015, 8, 429–435. [Google Scholar] [CrossRef]
- Darvishzadeh, R.; Atzberger, C.; Skidmore, A.K.; Abkar, A.A. Leaf Area Index derivation from hyperspectral vegetation indicesand the red edge position. Int. J. Remote Sens. 2009, 30, 6199–6218. [Google Scholar] [CrossRef]
- Xiao, Y.; Zhao, W.; Zhou, D.; Gong, H. Sensitivity Analysis of Vegetation Reflectance to Biochemical and Biophysical Variables at Leaf, Canopy, and Regional Scales. IEEE Trans. Geosci. Remote Sens. 2014, 52, 4014–4024. [Google Scholar] [CrossRef]
- Marshall, M.; Thenkabail, P.; Biggs, T.; Post, K. Hyperspectral narrowband and multispectral broadband indices for remote sensing of crop evapotranspiration and its components (transpiration and soil evaporation). Agric. For. Meteorol. 2016, 218–219, 122–134. [Google Scholar] [CrossRef]
- Reich, P.B.; Ellsworth, D.S.; Walters, M.B. Leaf structure (specific leaf area) modulates photosynthesis–nitrogen relations : Evidence from within and across species and functional groups. Funct. Ecol. 1998, 12, 948–958. [Google Scholar] [CrossRef]
- Nautiyal, P.C.; Rao, N.; Joshi, Y.C. Moisture-deficit-induced changes in leaf-water content, leaf carbon exchange rate and biomass production in groundnut cultivars differing in specific leaf area. Field Crops Res. 2002, 74, 67–79. [Google Scholar] [CrossRef]
- Vergara-díaz, O.; Zaman-allah, M.A.; Masuka, B.; Hornero, A. A Novel Remote Sensing Approach for Prediction of Maize Yield Under Different Conditions of Nitrogen Fertilization. Front. Plant Sci. 2016, 7, 1–13. [Google Scholar]
- Li, C.; Wulf, H.; Schmid, B.; He, J.; Schaepman, M.E.; Member, S. Estimating Plant Traits of Alpine Grasslands on the Qinghai-Tibetan Plateau Using Remote Sensing. IEEE J. Sel. Top. Appl. Earth Obs. Remote Sens. 2018, 11, 1–13. [Google Scholar] [CrossRef]
- Anser, G.P.; Martin, R.E. Airborne spectranomics : mapping canopy chemical and taxonomic diversity in tropical forests. Environ. Front. Ecol. 2009, 7, 269–276. [Google Scholar]
- Myneni, R.B.; Williams, D.L. On the relationship between FAPAR and NDVI. Remote Sens. Environ. 1994, 49, 200–211. [Google Scholar] [CrossRef]
- Steinberg, A.; Chabrillat, S.; Stevens, A.; Segl, K.; Foerster, S. Prediction of common surface soil properties based on Vis-NIR airborne and simulated EnMAP imaging spectroscopy data: Prediction accuracy and influence of spatial resolution. Remote Sens. 2016, 8, 613. [Google Scholar] [CrossRef]
- Maynard, J.J.; Johnson, M.G. Scale-dependency of LiDAR derived terrain attributes in quantitative soil-landscape modeling: Effects of grid resolution vs. neighborhood extent. Geoderma 2014, 230–231, 29–40. [Google Scholar] [CrossRef]

| Indices | Formulas | Description | References |
|---|---|---|---|
| RVI | Sensitive to nitrogen | [33,10] | |
| NDVI | Structure (LAI, fraction) Chlorophyll content | [34] | |
| GNDVI | Biomass, LAI, photosynthesis and plant stress | [7,35] | |
| BNDVI | Chlorophyll content | [36] | |
| SAVI | Structure (LAI, fraction) | [37,38] | |
| OSAVI | Structure (LAI, fraction) | [39,40] | |
| MSAVI | Structure (LAI, sensitive to canopy effects) | [41] | |
| MSAVI2 | Structure (LAI, fraction) | [41] | |
| MTVI2 | Structure (Sensitive to LAI, resistant to chlorophyll influence) | [38] |
| Phonological stage | Statistics | LAI (m2m−2) | DW (g·cm−2) | LMA (g·cm−2) | SLA (cm2g−1) |
|---|---|---|---|---|---|
| Seedling stage | Minimum value | 0.2 | 0.005 | 0.004 | 0.001 |
| Maximum value | 4.9 | 0.051 | 0.037 | 0.055 | |
| Mean value | 1.72 | 0.025 | 0.016 | 0.012 | |
| Standard deviation | 1.13 | 0.014 | 0.009 | 0.012 | |
| Elongation stage | Minimum value | 0.2 | 0.008 | 0.003 | 0.001 |
| Maximum value | 5.03 | 0.083 | 0.087 | 0.024 | |
| Mean value | 2.23 | 0.038 | 0.023 | 0.005 | |
| Standard deviation | 1.15 | 0.019 | 0.014 | 0.004 | |
| Flowering stage | Minimum value | 0.1 | 0.010 | 0.002 | 0.001 |
| Maximum value | 4.83 | 0.044 | 0.082 | 0.032 | |
| Mean value | 1.60 | 0.027 | 0.023 | 0.006 | |
| Standard deviation | 0.99 | 0.010 | 0.014 | 0.006 | |
| Maturity stage | Minimum value | 0.1 | 0.001 | 0.002 | 0.001 |
| Maximum value | 3.72 | 0.052 | 0.046 | 0.045 | |
| Mean value | 1.74 | 0.022 | 0.013 | 0.007 | |
| Standard deviation | 0.70 | 0.010 | 0.009 | 0.008 |
| Phenological stages | ||||||||
|---|---|---|---|---|---|---|---|---|
| R2 | RMSE | |||||||
| VIs | Seedling | Elongation | Flowering | Maturity | Seedling | Elongation | Flowering | Maturity |
| RVI | 0.86 | 0.93 | 0.89 | 0.45 | 0.44 | 0.30 | 0.32 | 0.49 |
| NDVI | 0.86 | 0.88 | 0.87 | 0.41 | 0.47 | 0.40 | 0.35 | 0.54 |
| GNDVI | 0.82 | 0.87 | 0.82 | 0.41 | 0.50 | 0.41 | 0.42 | 0.51 |
| BNDVI | 0.82 | 0.86 | 0.79 | 0.44 | 0.50 | 0.42 | 0.44 | 0.50 |
| SAVI | 0.86 | 0.85 | 0.83 | 0.45 | 0.43 | 0.44 | 0.41 | 0.49 |
| OSAVI | 0.85 | 0.86 | 0.84 | 0.49 | 0.45 | 0.42 | 0.40 | 0.48 |
| MSAVI | 0.85 | 0.87 | 0.86 | 0.47 | 0.44 | 0.40 | 0.37 | 0.49 |
| MSAVI2 | 0.86 | 0.85 | 0.82 | 0.48 | 0.43 | 0.44 | 0.42 | 0.48 |
| MTVI2 | 0.88 | 0.89 | 0.86 | 0.52 | 0.40 | 0.38 | 0.36 | 0.46 |
| Phenological Stage | VIs | Model | R2 | RRMSE |
|---|---|---|---|---|
| Seedling Stage | RVI | y = 0.0462x2 − 0.1042x + 0.3819 | 0.86 | 24% |
| NDVI | y = 43.705x2 − 47.037x + 13.056 | 0.84 | 26% | |
| GNDVI | y = 65.417x2 −54.631x + 11.848 | 0.82 | 28% | |
| BNDVI | y = 104.91x2 − 130.44x + 40.965 | 0.82 | 28% | |
| SAVI | y = 46.485x2 − 30.462x + 5.4871 | 0.86 | 24% | |
| OSAVI | y = 44.048x2 − 38.005x + 8.6542 | 0.85 | 25% | |
| MSAVI | y = 24.965x2 − 6.9701x + 0.9874 | 0.86 | 25% | |
| MSAVI2 | y = 28.964x2 − 16.621x + 2.9121 | 0.86 | 24% | |
| MTVI2 | y = 22.307x2 − 4.5415x + 0.7493 | 0.88 | 22% | |
| Elongation Stage | RVI | y = 0.011x2 + 0.5195x − 1.6884 | 0.93 | 13% |
| NDVI | y = 68.09x2 − 79.03x + 23.531 | 0.88 | 17% | |
| GNDVI | y = 60.213x2 − 48.337x + 10.064 | 0.87 | 18% | |
| BNDVI | y = 119.49x2 − 142.14x + 42.779 | 0.86 | 18% | |
| SAVI | y = 31.046x2 −17.785x + 2.8609 | 0.85 | 19% | |
| OSAVI | y = 43.247x2 − 37.807x + 8.7777 | 0.86 | 18% | |
| MSAVI | y = 18.643x2 − 3.1952x + 0.433 | 0.88 | 17% | |
| MSAVI2 | y = 19.239x2 − 8.6972x + 1.2314 | 0.85 | 19% | |
| MTVI2 | y = 17.139x2 −1.3713x + 0.3066 | 0.89 | 16% | |
| Flowering Stage | RVI | y = 0.1402x2 − 0.273x − 0.2612 | 0.89 | 19% |
| NDVI | y = 49.059x2 − 46.541x + 11.289 | 0.87 | 21% | |
| GNDVI | y = 42.303x2 − 27.235x + 4.1857 | 0.82 | 25% | |
| BNDVI | y = 94.642x2 − 102.03x + 27.677 | 0.79 | 27% | |
| SAVI | y = 27.79x2 − 17.512x + 2.8845 | 0.83 | 25% | |
| OSAVI | y = 38.042x2 − 31.109x + 6.6067 | 0.84 | 24% | |
| MSAVI | y = 12.155x2 + 1.2127x − 0.3412 | 0.86 | 22% | |
| MSAVI2 | y = 26.44x2 − 17.359x + 3.1088 | 0.82 | 25% | |
| MTVI2 | y = 15.947x2 − 0.3135x + 0.0678 | 0.86 | 22% |
| Phenological Stage | VIs | Model | R2 | RRMSE |
|---|---|---|---|---|
| Seedling Stage | RVI | y = 23.32562x1.31245 | 0.59 | 38% |
| NDVI | y = 1047.82027x3.91124 | 0.60 | 38% | |
| GNDVI | y = 1803.29932x3.25556 | 0.53 | 41% | |
| BNDVI | y = 1626.95299x5.62912 | 0.59 | 38% | |
| SAVI | y = 1403.98804x2.28148 | 0.68 | 34% | |
| OSAVI | y = 1135.78629x2.72497 | 0.62 | 37% | |
| MSAVI | y = 947.33922x1.16294 | 0.59 | 38% | |
| MSAVI2 | y = 1014.56073x1.83346 | 0.63 | 36% | |
| MTVI2 | y = 725.25602x0.84641 | 0.45 | 44% | |
| Elongation Stage | RVI | y= 16.96029x1.69583 | 0.75 | 27% |
| NDVI | y = 2130.44779x5.10758 | 0.70 | 29% | |
| GNDVI | y = 4586.364x4.33196 | 0.75 | 26% | |
| BNDVI | y = 3529.73179x6.18505 | 0.66 | 31% | |
| SAVI | y = 2522.0947x2.78668 | 0.67 | 31% | |
| OSAVI | y = 2119.82125x3.48711 | 0.67 | 30% | |
| MSAVI | y = 1879.90964x1.59398 | 0.68 | 30% | |
| MSAVI2 | y = 1548.72146x2.08531 | 0.65 | 31% | |
| MTVI2 | y = 1885.3379x1.47159 | 0.68 | 30% | |
| Flowering Stage | RVI | y = 18.43701x1.82708 | 0.70 | 25% |
| NDVI | y = 1884.4817x3.95822 | 0.71 | 24% | |
| GNDVI | y = 2335.00995x3.18035 | 0.66 | 26% | |
| BNDVI | y = 3603.56195x5.83675 | 0.61 | 28% | |
| SAVI | y = 1742.20092x2.77482 | 0.71 | 24% | |
| OSAVI | y = 1710.05981x3.23342 | 0.66 | 26% | |
| MSAVI | y = 1496.06954x1.47261 | 0.72 | 24% | |
| MSAVI2 | y = 1466.35551x2.51978 | 0.71 | 24% | |
| MTVI2 | y = 1295.23183x1.1978 | 0.70 | 24% |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hussain, S.; Gao, K.; Din, M.; Gao, Y.; Shi, Z.; Wang, S. Assessment of UAV-Onboard Multispectral Sensor for Non-Destructive Site-Specific Rapeseed Crop Phenotype Variable at Different Phenological Stages and Resolutions. Remote Sens. 2020, 12, 397. https://doi.org/10.3390/rs12030397
Hussain S, Gao K, Din M, Gao Y, Shi Z, Wang S. Assessment of UAV-Onboard Multispectral Sensor for Non-Destructive Site-Specific Rapeseed Crop Phenotype Variable at Different Phenological Stages and Resolutions. Remote Sensing. 2020; 12(3):397. https://doi.org/10.3390/rs12030397
Chicago/Turabian StyleHussain, Sadeed, Kaixiu Gao, Mairaj Din, Yongkang Gao, Zhihua Shi, and Shanqin Wang. 2020. "Assessment of UAV-Onboard Multispectral Sensor for Non-Destructive Site-Specific Rapeseed Crop Phenotype Variable at Different Phenological Stages and Resolutions" Remote Sensing 12, no. 3: 397. https://doi.org/10.3390/rs12030397
APA StyleHussain, S., Gao, K., Din, M., Gao, Y., Shi, Z., & Wang, S. (2020). Assessment of UAV-Onboard Multispectral Sensor for Non-Destructive Site-Specific Rapeseed Crop Phenotype Variable at Different Phenological Stages and Resolutions. Remote Sensing, 12(3), 397. https://doi.org/10.3390/rs12030397





