Application of UAV Imagery to Detect and Quantify Submerged Filamentous Algae and Rooted Macrophytes in a Non-Wadeable River
Abstract
1. Introduction
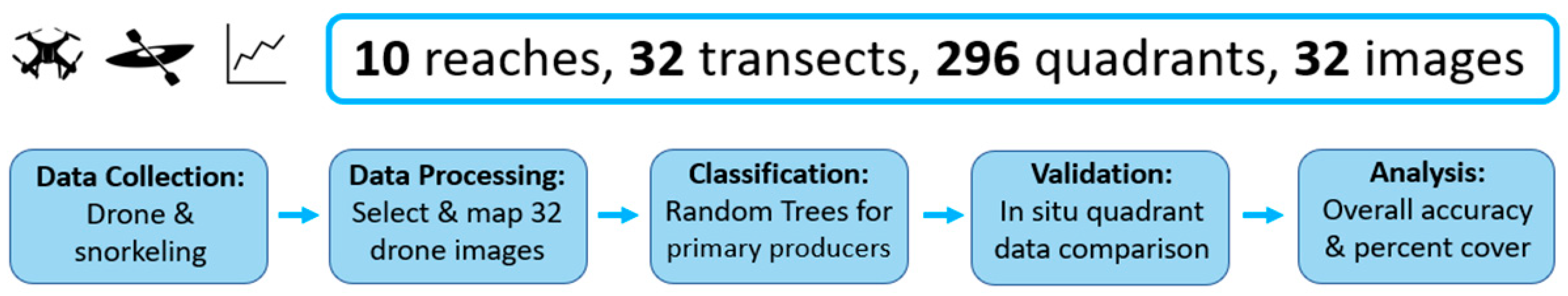
2. Materials and Methods
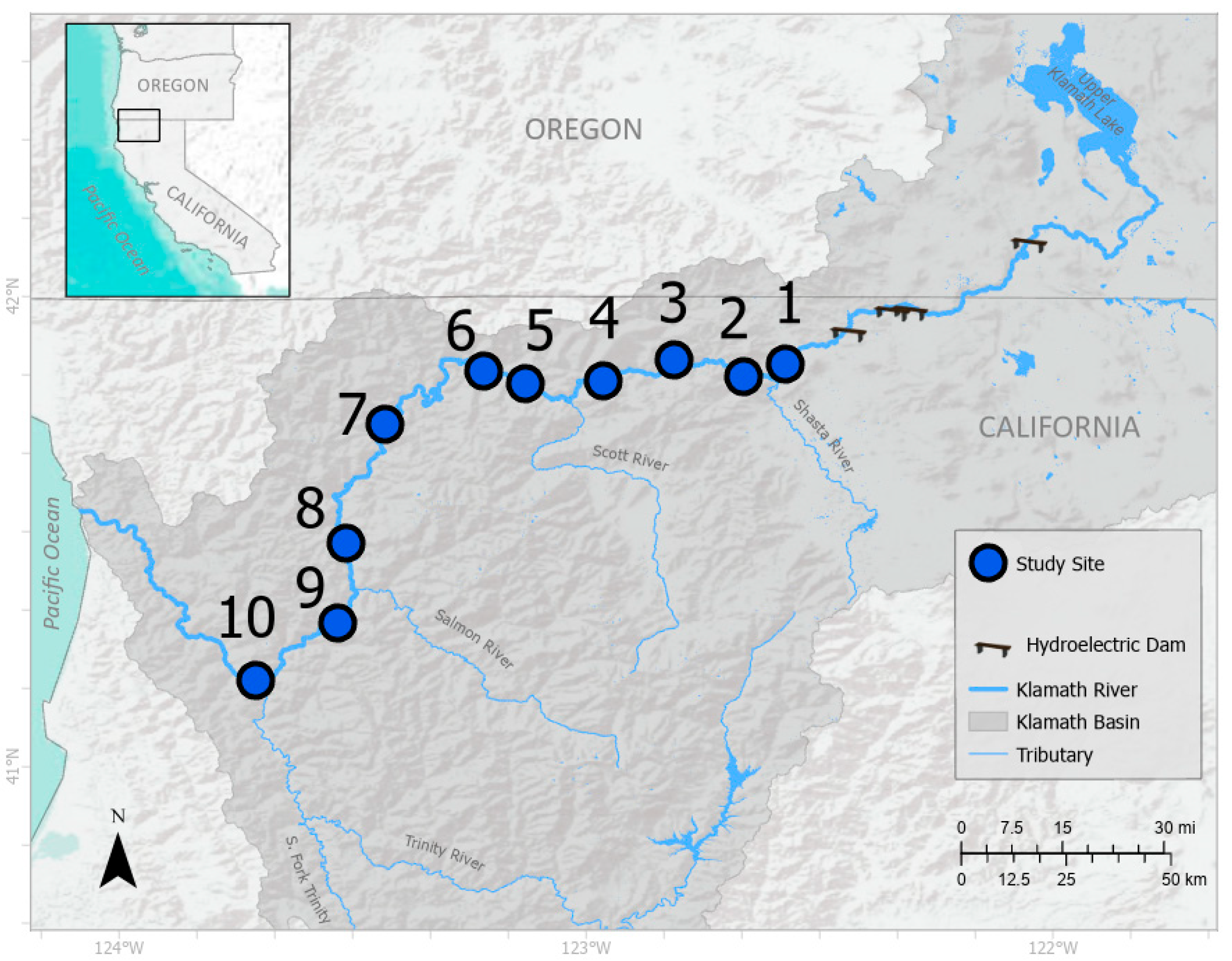
2.1. Study Sites
2.2. Data Acquisition
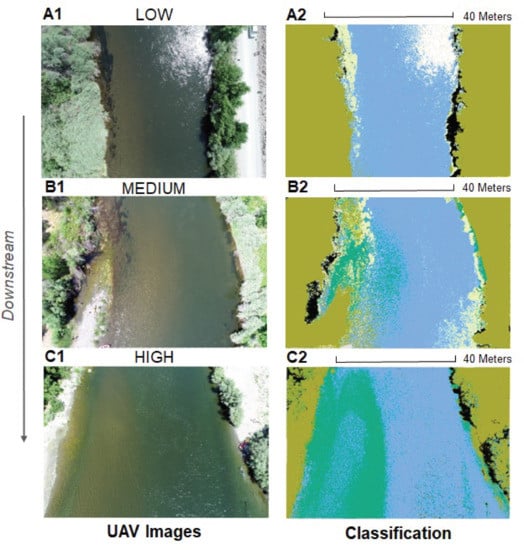
2.2.1. UAV Data
2.2.2. In-Situ Surveys
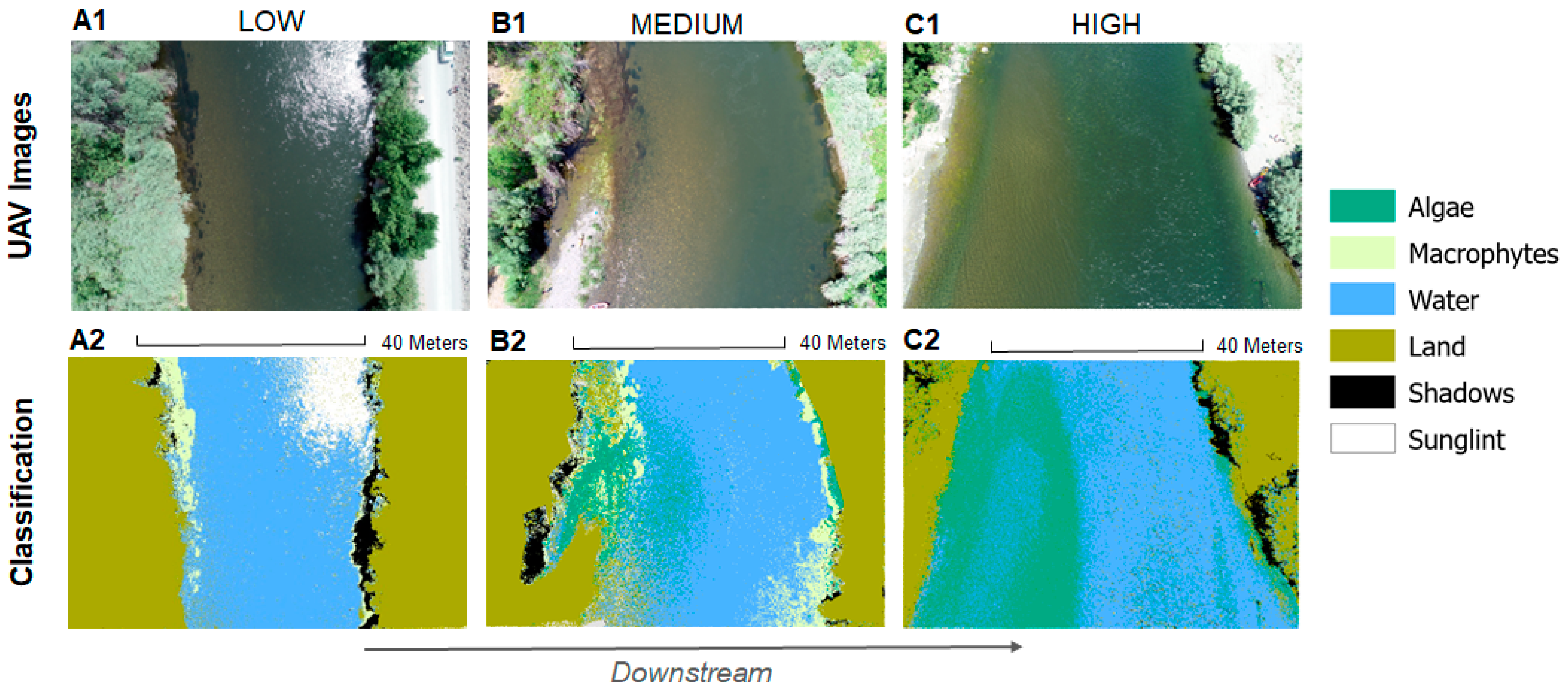
2.3. Image Classification
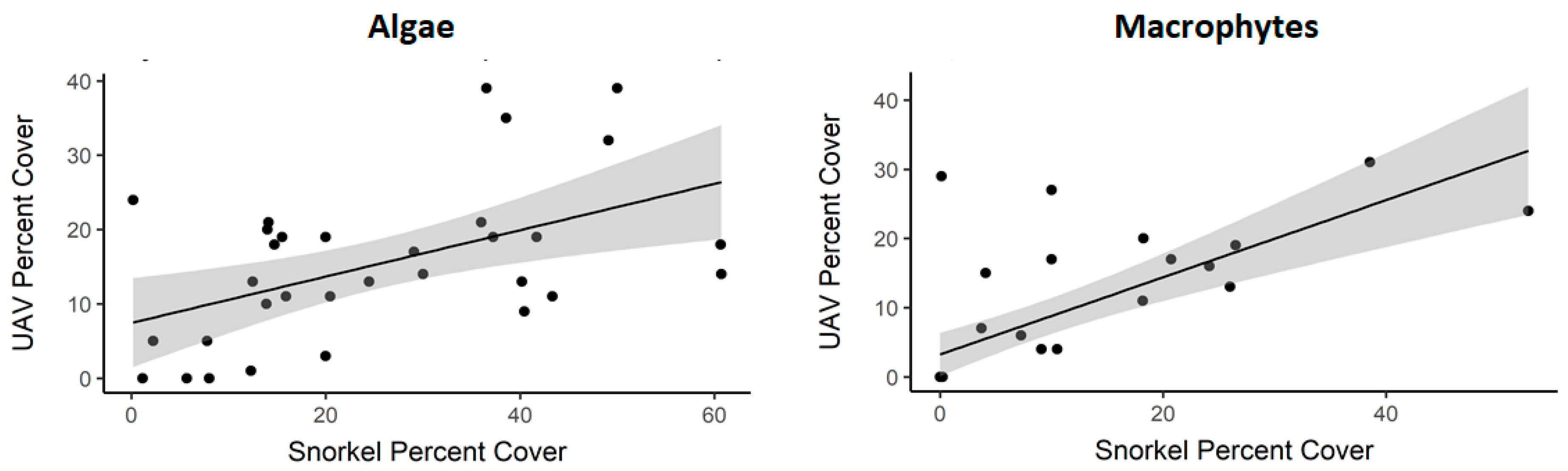
2.4. Validation
2.5. Percent Cover Estimates
3. Results
3.1. Random Trees Classification Results
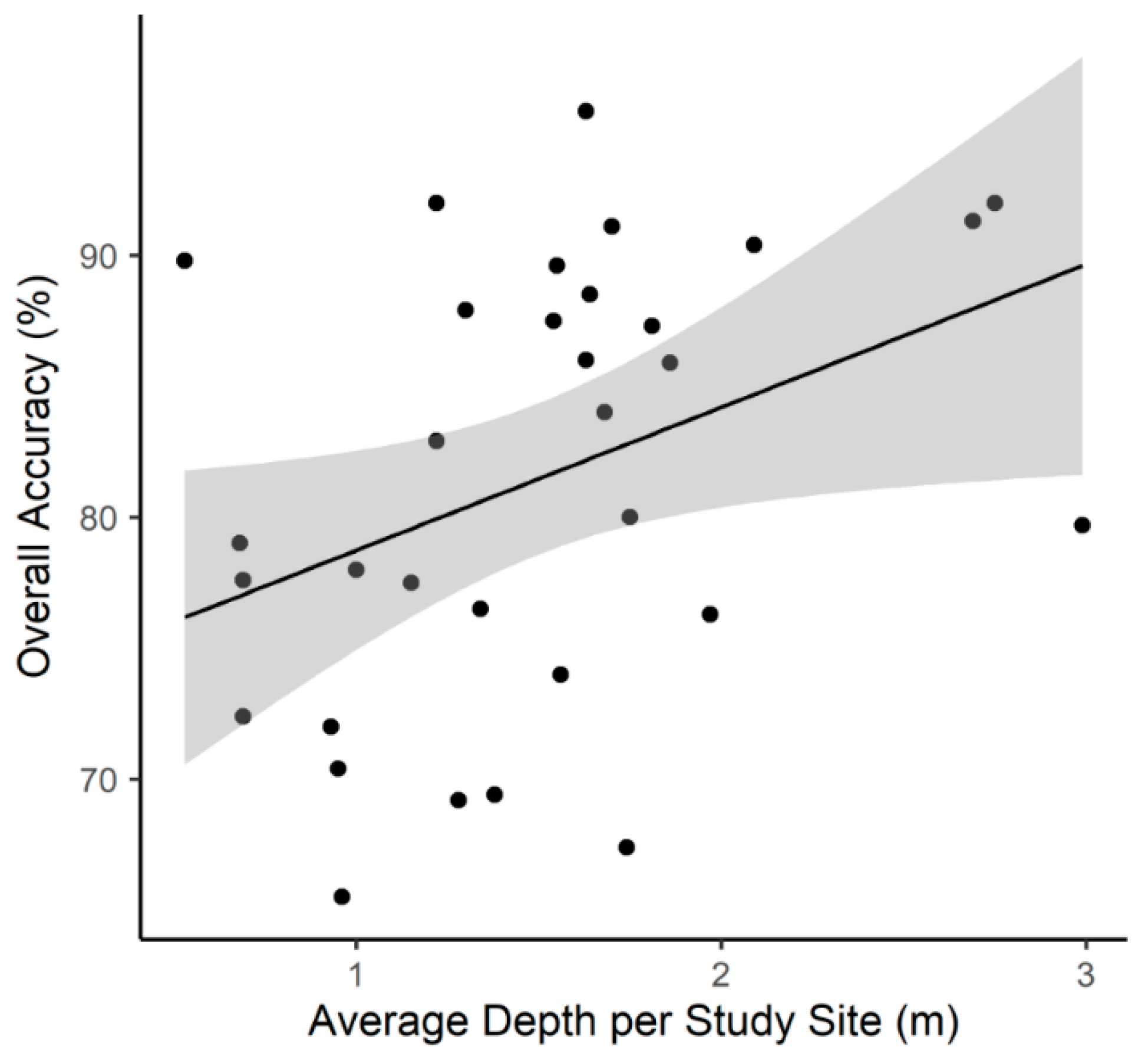
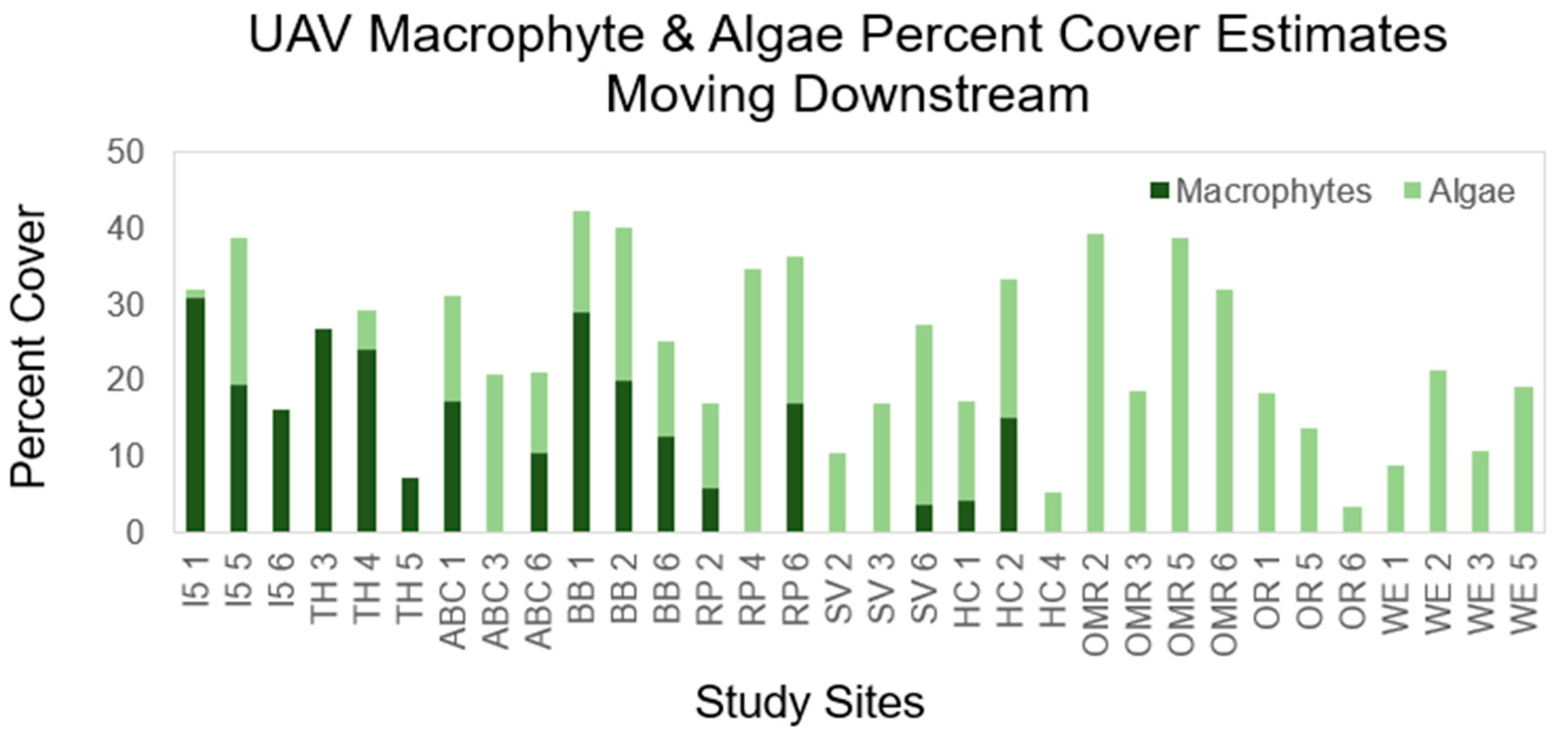
3.2. Study Site Characteristics and Percent Cover Estimates
4. Discussion
4.1. UAV Monitoring of Benthic Primary Producers
4.2. Recommendations
4.3. Management Applications of UAV Monitoring in Non-Wadeable Rivers
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A

| Reach | Name | Site | Overall Accuracy (%) | Algae (%) | Macro-phyte (%) | Estimated GSD (cm) | Secchi Depth (m) | Habitat | Avg. Depth (m) | Solar Elevation (Degrees) | Flight Latitude | Flight Longitude |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | I5 | 1 | 76 | 1 | 31 | 1.72 | 1.98 | run | 1.34 | 59.26 | 41.87464 | −122.55732 |
| 1 | I5 | 5 | 88 | 19 | 19 | 2.48 | 1.98 | run | 1.54 | 49.54 | 41.86690 | −122.56314 |
| 1 | I5 | 6 | 78 | 0 | 16 | 1.39 | 1.98 | run | 1.00 | 37.75 | 41.86408 | −122.56460 |
| 2 | TH | 3 | 80 | 0 | 27 | 1.18 | 2.53 | run | 1.75 | 71.06 | 41.82669 | −122.65785 |
| 2 | TH | 4 | 72 | 5 | 24 | 1.64 | 2.53 | run | 0.69 | 70.77 | 41.82578 | −122.65799 |
| 2 | TH | 5 | 90 | 0 | 7 | 1.46 | 2.53 | riffle | 0.53 | 66.38 | 41.82821 | −122.66158 |
| 3 | ABC | 1 | 72 | 14 | 17 | 1.53 | 3.05 | run | 0.93 | 63.76 | 41.86546 | −122.79469 |
| 3 | ABC | 3 | 83 | 21 | 0 | 2.85 | 3.05 | run | 1.22 | 70.72 | 41.86691 | −122.80595 |
| 3 | ABC | 6 | 76 | 11 | 11 | 1.04 | 3.05 | run | 1.97 | 69.87 | 41.86733 | −122.80884 |
| 4 | BB | 1 | 69 | 13 | 29 | 1.85 | 2.29 | run | 1.38 | 56.39 | 41.83120 | −122.95339 |
| 4 | BB | 2 | 69 | 20 | 20 | 1.33 | 2.29 | run | 1.28 | 62.72 | 41.83123 | −122.95296 |
| 4 | BB | 6 | 79 | 13 | 13 | 1.58 | 2.29 | run | 0.68 | 59.41 | 41.82330 | −122.96155 |
| 5 | RP | 2 | 86 | 11 | 6 | 0.52 | 2.74 | run | 1.86 | 58.33 | 41.80778 | −123.11062 |
| 5 | RP | 4 | 66 | 35 | 0 | 0.55 | 2.74 | riffle | 0.96 | 71.20 | 41.81437 | −123.11721 |
| 5 | RP | 6 | 67 | 19 | 17 | 1.69 | 2.74 | run | 1.74 | 60.93 | 41.81620 | −123.12726 |
| 6 | SV | 2 | 88 | 10 | 0 | 0.92 | 2.29 | run | 1.30 | 45.70 | 41.84317 | −123.22069 |
| 6 | SV | 3 | 74 | 17 | 0 | 1.15 | 2.29 | run | 1.56 | 54.31 | 41.84914 | −123.22803 |
| 6 | SV | 6 | 92 | 24 | 4 | 0.96 | 2.29 | riffle | 1.22 | 70.72 | 41.85475 | −123.23216 |
| 7 | HC | 1 | 70 | 13 | 4 | 1.61 | 2.59 | run | 0.95 | 70.86 | 41.79296 | −123.36820 |
| 7 | HC | 2 | 78 | 18 | 15 | 1.48 | 2.59 | run | 0.69 | 69.26 | 41.79229 | −123.37003 |
| 7 | HC | 4 | 86 | 5 | 0 | 1.21 | 2.59 | run | 1.63 | 48.51 | 41.78779 | −123.38213 |
| 8 | OMR | 2 | 90 | 39 | 0 | 1.65 | 2.13 | run | 1.55 | 56.89 | 41.48296 | −123.51500 |
| 8 | OMR | 3 | 90 | 19 | 0 | 1.39 | 2.13 | run | 2.09 | 67.62 | 41.48145 | −123.51385 |
| 8 | OMR | 5 | 84 | 39 | 0 | 1.46 | 2.13 | run | 1.68 | 68.94 | 41.47694 | −123.51273 |
| 8 | OMR | 6 | 88 | 32 | 0 | 1.85 | 2.13 | run | 1.64 | 62.69 | 41.47543 | −123.51309 |
| 9 | OR | 1 | 92 | 18 | 0 | 2.20 | 4.42 | run | 2.75 | 54.08 | 41.31895 | −123.52479 |
| 9 | OR | 5 | 78 | 14 | 0 | 1.68 | 4.42 | riffle | 1.15 | 62.59 | 41.31102 | −123.52683 |
| 9 | OR | 6 | 87 | 3 | 0 | 0.63 | 4.42 | run | 1.81 | 52.02 | 41.30547 | −123.53338 |
| 10 | WE | 1 | 91 | 9 | 0 | 1.67 | 2.59 | run | 2.69 | 58.72 | 41.22923 | −123.65160 |
| 10 | WE | 2 | 95 | 21 | 0 | 0.57 | 2.59 | riffle | 1.63 | 68.89 | 41.20333 | −123.66201 |
| 10 | WE | 3 | 80 | 11 | 0 | 1.60 | 2.59 | run | 2.99 | 70.70 | 41.19162 | −123.67278 |
| 10 | WE | 5 | 91 | 19 | 0 | 1.16 | 2.59 | run | 1.70 | 57.54 | 41.18659 | −123.69190 |
| Red | Green | Blue | |
|---|---|---|---|
| Algae | 0 | 168 | 132 |
| Macrophytes | 233 | 255 | 190 |
| Water | 115 | 178 | 255 |
| Land | 168 | 168 | 0 |
| Shadows | 0 | 0 | 0 |
| Sun glint | 255 | 255 | 255 |
References
- Dennison, W.C.; Orth, R.J.; Moore, K.A.; Stevenson, J.C.; Carter, V.; Kollar, S.; Bergstrom, P.W.; Batiuk, R.A. Assessing Water Quality with Submersed Aquatic Vegetation Habitat requirements as barometers of Chesapeake Bay health. Bioscience 1993, 43, 86–94. [Google Scholar] [CrossRef]
- Carpenter, S.R.; Lodge, D.M. Effects of submersed macrophytes on ecosystem processes. Aquat. Bot. 1986, 26, 341–370. [Google Scholar] [CrossRef]
- Wetzel, R.G. A Comparative Study of the Primary Production of Higher Aquatic Plants, Periphyton, and Phytoplankton in a Large, Shallow Lake. Int. Rev. Ges. Hydrobiol. Hydrogr. 1964, 49, 1–61. [Google Scholar] [CrossRef]
- Jones, J.; Collins, A.; Naden, P.; Sear, D. The Relationship between Fine Sediment and Macrophytes in Rivers. River Res. Appl. 2011, 28, 1006–1018. [Google Scholar] [CrossRef]
- Jan Stevenson, R.; Bothwell, M.L.; Lowe, R.L. (Eds.) Algal Ecology: Freshwater Benthic Ecosystem; Academic Press: Cambridge, MA, USA, 1996. [Google Scholar]
- Vadeboncoeur, Y.; Power, M.E. Attached Algae: The Cryptic Base of Inverted Trophic Pyramids in Freshwaters. Annu. Rev. Ecol. Evol. Syst. 2017, 48, 255–279. [Google Scholar] [CrossRef]
- Lusardi, R.A.; Jeffres, C.A.; Moyle, P.B. Stream macrophytes increase invertebrate production and fish habitat utilization in a California stream. River Res. Appl. 2018, 34, 1003–1012. [Google Scholar] [CrossRef]
- Duarte, C.M. Submerged aquatic vegetation in relation to different nutrient regimes. Ophelia 1995, 41, 87–112. [Google Scholar] [CrossRef]
- Torn, K.; Martin, G. Response of submerged aquatic vegetation to eutrophication-related environment descriptors in coastal waters of the NE Baltic Sea. Estonian J. Ecol. 2012, 61, 106. [Google Scholar] [CrossRef]
- Welch, E.B.; Jacoby, J.M.; Horner, R.R.; Seeley, M.R. Nuisance biomass levels of periphytic algae in streams. Hydrobiologia 1988, 157, 161–168. [Google Scholar] [CrossRef]
- Flinders, C.A.; Hart, D.D. Effects of pulsed flows on nuisance periphyton growths in rivers: A mesocosm study. River Res. Appl. 2009, 25, 1320–1330. [Google Scholar] [CrossRef]
- Dodds, W.K.; Gudder, D.A. The Ecology of Cladophora. J. Phycol. 1992, 28, 415–427. [Google Scholar] [CrossRef]
- Rørslett, B.; Johansen, S.W. Remedial measures connected with aquatic macrophytes in Norwegian regulated rivers and reservoirs. Regul. Rivers Res. Manag. 1996, 12, 509–522. [Google Scholar] [CrossRef]
- Biggs, B.J.F. Patterns in benthic algae of streams. In Algal Ecology: Freshwater Benthic Ecosystems; Academic Press: Cambridge, MA, USA, 1996; pp. 31–56. [Google Scholar]
- Biggs, B.J.F. Eutrophication of streams and rivers: Dissolved nutrient-chlorophyll relationships for benthic algae. J. N. Am. Benthol. Soc. 2000, 19, 17–31. [Google Scholar] [CrossRef]
- Hilton, J.; O’Hare, M.; Bowes, M.J.; Jones, J.I. How green is my river? A new paradigm of eutrophication in rivers. Sci. Total. Environ. 2006, 365, 66–83. [Google Scholar] [CrossRef]
- Smith, V.; Tilman, G.; Nekola, J. Eutrophication: Impacts of excess nutrient inputs on freshwater, marine, and terrestrial ecosystems. Environ. Pollut. 1999, 100, 179–196. [Google Scholar] [CrossRef]
- Aguirre-Gómez, R.; Salmerón-García, O.; Gómez-Rodríguez, G.; Peralta-Higuera, A. Use of unmanned aerial vehicles and remote sensors in urban lakes studies in Mexico. Int. J. Remote. Sens. 2016, 38, 2771–2779. [Google Scholar] [CrossRef]
- Husson, E.; Hagner, O.; Ecke, F. Unmanned aircraft systems help to map aquatic vegetation. Appl. Veg. Sci. 2014, 17, 567–577. [Google Scholar] [CrossRef]
- Brooks, C.N.; Grimm, A.G.; Marcarelli, A.M.; Dobson, R.J. Multiscale collection and analysis of submerged aquatic vegetation spectral profiles for Eurasian watermilfoil detection. J. Appl. Remote. Sens. 2019, 13, 037501. [Google Scholar] [CrossRef]
- Van Der Merwe, D.; Price, K.P. Harmful Algal Bloom Characterization at Ultra-High Spatial and Temporal Resolution Using Small Unmanned Aircraft Systems. Toxins 2015, 7, 1065–1078. [Google Scholar] [CrossRef]
- Kislik, C.; Dronova, I.; Kelly, M. UAVs in Support of Algal Bloom Research: A Review of Current Applications and Future Opportunities. Drones 2018, 2, 35. [Google Scholar] [CrossRef]
- Flynn, K.F.; Chapra, S.C. Remote Sensing of Submerged Aquatic Vegetation in a Shallow Non-Turbid River Using an Unmanned Aerial Vehicle. Remote. Sens. 2014, 6, 12815–12836. [Google Scholar] [CrossRef]
- Nowak, M.M.; Dziób, K.; Bogawski, P. Unmanned Aerial Vehicles (UAVs) in environmental biology: A review. Eur. J. Ecol. 2019, 4, 56–74. [Google Scholar] [CrossRef]
- Yang, B.; Hawthorne, T.L.; Torres, H.R.; Feinman, M. Using Object-Oriented Classification for Coastal Management in the East Central Coast of Florida: A Quantitative Comparison between UAV, Satellite, and Aerial Data. Drones 2019, 3, 60. [Google Scholar] [CrossRef]
- Díaz-Delgado, R.; Ónodi, G.; Kröel-Dulay, G.; Kertész, M. Enhancement of Ecological Field Experimental Research by Means of UAV Multispectral Sensing. Drones 2019, 3, 7. [Google Scholar] [CrossRef]
- Manfreda, S.; McCabe, M.; Miller, P.E.; Lucas, R.M.; Madrigal, V.P.; Mallinis, G.; Ben Dor, E.; Helman, D.; Estes, L.; Ciraolo, G.; et al. On the Use of Unmanned Aerial Systems for Environmental Monitoring. Remote. Sens. 2018, 10, 641. [Google Scholar] [CrossRef]
- Preskitt, L.B.; Vroom, P.S.; Smith, C.M. A Rapid Ecological Assessment (REA) Quantitative Survey Method for Benthic Algae Using Photoquadrats with Scuba. Pac. Sci. 2004, 58, 201–209. [Google Scholar] [CrossRef][Green Version]
- Priddle, J. The Production Ecology of Benthic Plants in Some Antarctic Lakes: I. In Situ Production Studies. J. Ecol. 1980, 68, 141. [Google Scholar] [CrossRef]
- Pennuto, C.M.; Howell, E.; Makarewicz, J. Relationships among round gobies, Dreissena mussels, and benthic algae in the south nearshore of Lake Ontario. J. Great Lakes Res. 2012, 38, 154–160. [Google Scholar] [CrossRef]
- Suplee, M.W.; Watson, V.; Teply, M.; McKee, H. How Green is Too Green? Public Opinion of What Constitutes Undesirable Algae Levels in Streams. JAWRA J. Am. Water Resour. Assoc. 2009, 45, 123–140. [Google Scholar] [CrossRef]
- Beijbom, O.; Edmunds, P.J.; Roelfsema, C.; Smith, J.; Kline, D.I.; Neal, B.P.; Dunlap, M.J.; Moriarty, V.; Fan, T.-Y.; Tan, C.-J.; et al. Towards Automated Annotation of Benthic Survey Images: Variability of Human Experts and Operational Modes of Automation. PLoS ONE 2015, 10, e0130312. [Google Scholar] [CrossRef]
- Visser, F.; Wallis, C.; Sinnott, A.M. Optical remote sensing of submerged aquatic vegetation: Opportunities for shallow clearwater streams. Limnologica 2013, 43, 388–398. [Google Scholar] [CrossRef]
- Stanfield, K. Developing Methods to Differentiate Species and Estimate Coverage of Benthic Autotrophs in the Potomac Using Digital Imaging. Master’s Thesis, Hood College, Frederick, MD, USA, 2018. Available online: https://mdsoar.org/handle/11603/8764 (accessed on 1 March 2020).
- Silva, T.S.F.; Costa, M.P.F.; Melack, J.M.; Novo, E.M.L.M. Remote sensing of aquatic vegetation: Theory and applications. Environ. Monit. Assess. 2008, 140, 131–145. [Google Scholar] [CrossRef] [PubMed]
- Legleiter, C.J.; Roberts, D.A.; Marcus, A.; Fonstad, M.A. Passive optical remote sensing of river channel morphology and in-stream habitat: Physical basis and feasibility. Remote. Sens. Environ. 2004, 93, 493–510. [Google Scholar] [CrossRef]
- Kwon, Y.S.; Pyo, J.; Kwon, Y.-H.; Duan, H.; Cho, K.H.; Park, Y. Drone-based hyperspectral remote sensing of cyanobacteria using vertical cumulative pigment concentration in a deep reservoir. Remote. Sens. Environ. 2020, 236, 111517. [Google Scholar] [CrossRef]
- Mount, R. Acquisition of Through-water Aerial Survey Images. Photogramm. Eng. Remote. Sens. 2005, 71, 1407–1415. [Google Scholar] [CrossRef]
- Flynn, K.F.; Chapra, S.C. Evaluating Hydraulic Habitat Suitability of Filamentous Algae Using an Unmanned Aerial Vehicle and Acoustic Doppler Current Profiler. J. Environ. Eng. 2020, 146, 04019126. [Google Scholar] [CrossRef]
- Taddia, Y.; Russo, P.; Lovo, S.; Pellegrinelli, A. Multispectral UAV monitoring of submerged seaweed in shallow water. Appl. Geomatics 2019, 12, 19–34. [Google Scholar] [CrossRef]
- Brinkhoff, J.; Hornbuckle, J.; Barton, J.L. Assessment of Aquatic Weed in Irrigation Channels Using UAV and Satellite Imagery. Water 2018, 10, 1497. [Google Scholar] [CrossRef]
- Tait, L.; Bind, J.; Charan-Dixon, H.; Hawes, I.; Pirker, J.; Schiel, D.R. Unmanned Aerial Vehicles (UAVs) for Monitoring Macroalgal Biodiversity: Comparison of RGB and Multispectral Imaging Sensors for Biodiversity Assessments. Remote. Sens. 2019, 11, 2332. [Google Scholar] [CrossRef]
- Slocum, R.K.; Wright, W.; Parrish, C.; Costa, B.; Sharr, M.; Battista, T.A. Guidelines for Bathymetric Mapping and Orthoimage Generation using sUAS and SfM, An Approach for Conducting Nearshore Coastal Mapping. Available online: https://repository.library.noaa.gov/view/noaa/22923 (accessed on 26 February 2020).
- Genzoli, L.; Hall, R.O. Shifts in Klamath River metabolism following a reservoir cyanobacterial bloom. Freshw. Sci. 2016, 35, 795–809. [Google Scholar] [CrossRef]
- Gillett, N.D.; Pan, Y.; Asarian, J.E.; Kann, J. Spatial and temporal variability of river periphyton below a hypereutrophic lake and a series of dams. Sci. Total. Environ. 2016, 541, 1382–1392. [Google Scholar] [CrossRef] [PubMed]
- Biggs, B.J.; Kilroy, C. Stream Periphyton Monitoring Manual; Niwa: Christchurch, New Zealand, 2000. [Google Scholar]
- Ode, P.R.; Fetscher, A.E.; Busse, L.B. Standard Operating Procedures (SOP) for the Collection of Field Data for Bioassessments of California Wadeable Streams: Benthic Macroinvertebrates, Algae, and Physical Habitat; California State Water Resources Control Board Surface Water Ambient Monitoring Program: Sacramento, CA, USA; Available online: https://meadows.ucdavis.edu/files/SWAMP_combined_sop_031116_reduced.pdf (accessed on 15 August 2020).
- Bellmore, J.R.; Pess, G.R.; Duda, J.J.; O’Connor, J.E.; East, A.E.; Foley, M.M.; Wilcox, A.C.; Major, J.J.; Shafroth, P.B.; Morley, S.A.; et al. Conceptualizing Ecological Responses to Dam Removal: If You Remove It, What’s to Come? Bioscience 2019, 69, 26–39. [Google Scholar] [CrossRef] [PubMed]
- Van Kirk, R.W.; Naman, S.W. Relative Effects of Climate and Water Use on Base-Flow Trends in the Lower Klamath Basin. JAWRA J. Am. Water Resour. Assoc. 2008, 44, 1035–1052. [Google Scholar] [CrossRef]
- Marcus, A.; Fonstad, M.A. Optical remote mapping of rivers at sub-meter resolutions and watershed extents. Earth Surf. Process. Landforms J. Br. Geomorphol. Res. Group 2007, 33, 4–24. [Google Scholar] [CrossRef]
- Snyder, D.T.; Morace, J.L. Nitrogen and Phosphorus Loading from Drained Wetlands Adjacent to Upper Klamath and Agency Lakes, Oregon; US Department of the Interior, U.S. Geological Survey, Branch of Information Services: Denver, CO, USA, 1997. [Google Scholar]
- Bartholow, J.M.; Campbell, S.G.; Flug, M. Predicting the thermal effects of dam removal on the Klamath River. Environ. Manag. 2004, 34, 856–874. [Google Scholar] [CrossRef]
- Holmquist-Johnson, C.L.; Milhous, R.T. Channel Maintenance and Flushing Flows for the Klamath River Below Iron Gate Dam, California. 2010. Available online: https://pubs.usgs.gov/of/2010/1086/ (accessed on 8 January 2020).
- Peppa, M.V.; Hall, J.; Goodyear, J.; Mills, J.P. Photogrammetric assessment and comparison of DJI Phantom 4 pro and phantom 4 RTK small unmanned aircraft systems. In Proceedings of the 4th ISPRS Geospatial Week, Enschede, The Netherlands, 10–14 June 2019; Available online: https://eprints.ncl.ac.uk/file_store/production/258436/916EAB96-2294-430D-A832-CA872DB0A2CD.pdf (accessed on 3 March 2020).
- Su, T.-C.; Chou, H.-T. Application of Multispectral Sensors Carried on Unmanned Aerial Vehicle (UAV) to Trophic State Mapping of Small Reservoirs: A Case Study of Tain-Pu Reservoir in Kinmen, Taiwan. Remote. Sens. 2015, 7, 10078–10097. [Google Scholar] [CrossRef]
- Lyons, A.; R Development Core Team. Uasimg: Drone Images Utilities. R Package Version 1.3.4. Available online: https://github.com/ucanr-igis/uasimg (accessed on 17 August 2020).
- Yang, X. An Assessment of Algorithmic Parameters Affecting Image Classification Accuracy by Random Forests. Photogramm. Eng. Remote. Sens. 2016, 82, 407–417. [Google Scholar] [CrossRef]
- Gerke, M. Supervised Classification of Multiple View Images in Object Space for Seismic Damage Assessment. In Proceedings of the ISPRS Conference, Photogrammetric Image Analysis 2011, Munich, Germany, 5–7 October 2011; Springer: Berlin/Heidelberg, Germany, 2011; pp. 221–232. [Google Scholar]
- Benediktsson, J.A.; Chanussot, J.; Fauvel, M. Multiple Classifier Systems in Remote Sensing: From Basics to Recent Developments. In Proceedings of the 7th International Workshop, Multiple Classifier Systems 2007, Prague, Czech Republic, 23–25 May 2007; Springer: Berlin/Heidelberg, Germany, 2007; pp. 501–512. [Google Scholar]
- Maxwell, A.E.; Warner, T.A.; Fang, F. Implementation of machine-learning classification in remote sensing: An applied review. Int. J. Remote. Sens. 2018, 39, 2784–2817. [Google Scholar] [CrossRef]
- Gomez-Chova, L.; Muñoz-Marí, J.; Laparra, V.; Malo, J.; Camps-Valls, G.; Camps-Valls, G. A Review of Kernel Methods in Remote Sensing Data Analysis. In Optical Remote Sensing: Advances in Signal Processing and Exploitation Techniques; Prasad, S., Bruce, L.M., Chanussot, J., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 171–206. [Google Scholar]
- Crisci, C.; Ghattas, B.; Perera, G. A review of supervised machine learning algorithms and their applications to ecological data. Ecol. Model. 2012, 240, 113–122. [Google Scholar] [CrossRef]
- Rodriguez, F.; Ponce, R.A.; Pérez-Rodríguez, F.; Agueda, B.; Martín-García, S.; Martínez-Rodrigo, R.; Lizarralde, I. Comparison of Machine Learning Algorithms for Wildland-Urban Interface Fuelbreak Planning Integrating ALS and UAV-borne LiDAR Data and Multispectral Images. Drones 2020, 4, 21. [Google Scholar] [CrossRef]
- Ghimire, B.; Rogan, J.; Miller, J. Contextual land-cover classification: Incorporating spatial dependence in land-cover classification models using random forests and the Getis statistic. Remote. Sens. Lett. 2010, 1, 45–54. [Google Scholar] [CrossRef]
- Asarian, J.E.; Pan, Y.; Gillett, N.D.; Kann, J. Spatial and Temporal Variation of Periphyton Assemblages in the Klamath River 2004—Prepared by Kier Associates, Portland State University, and Aquatic Ecosystem Sciences LLC for the Klamath Basin Tribal Water Quality Work Group. Available online: https://www.researchgate.net/profile/J_Asarian/publication/272788109_Spatial_and_temporal_variation_of_periphyton_assemblages_in_the_Klamath_River_2004-2012/links/5eb032c845851592d6b880e8/Spatial-and-temporal-variation-of-periphyton-assemblages-in-the-Klamath-River-2004-2012.pdf (accessed on 29 August 2020).
- Foody, G.M. Explaining the unsuitability of the kappa coefficient in the assessment and comparison of the accuracy of thematic maps obtained by image classification. Remote. Sens. Environ. 2020, 239, 111630. [Google Scholar] [CrossRef]
- Pontius, R.G.; Millones, M. Death to Kappa: Birth of quantity disagreement and allocation disagreement for accuracy assessment. Int. J. Remote. Sens. 2011, 32, 4407–4429. [Google Scholar] [CrossRef]
- Hughes, B.B.; Haskins, J.C.; Wasson, K.; Watson, E. Identifying factors that influence expression of eutrophication in a central California estuary. Mar. Ecol. Prog. Ser. 2011, 439, 31–43. [Google Scholar] [CrossRef]
- lm Function|R Documentation. 2017. Available online: https://www.rdocumentation.org/packages/stats/versions/3.6.2/topics/lm (accessed on 17 March 2020).
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2013. [Google Scholar]
- Jay Kerns, G. Introduction to Probability and Statistics Using R. 2010. Available online: http://www.atmos.albany.edu/facstaff/timm/ATM315spring14/R/IPSUR.pdf (accessed on 18 August 2020).
- Shintani, C.; Fonstad, M.A. Comparing remote-sensing techniques collecting bathymetric data from a gravel-bed river. Int. J. Remote. Sens. 2017, 38, 2883–2902. [Google Scholar] [CrossRef]
- Zinke, P.; Flener, C. Experiences from the use of unmanned aerial vehicles (UAV) for river bathymetry modelling in Norway. Vann 2013, 48, 351–360. [Google Scholar]
- PacifiCorps. 2019 KHSA Final Datasets. Available online: https://www.pacificorp.com/energy/hydro/klamath-river/water-quality.html (accessed on 14 August 2020).
- Oliver, A.A.; Dahlgren, R.A.; Deas, M.L. The upside-down river: Reservoirs, algal blooms, and tributaries affect temporal and spatial patterns in nitrogen and phosphorus in the Klamath River, USA. J. Hydrol. 2014, 519, 164–176. [Google Scholar] [CrossRef]
- Ahmad, A.; Quegan, S. Comparative analysis of supervised and unsupervised classification on multispectral data. Appl. Math. Sci. 2013, 7, 3681–3694. [Google Scholar] [CrossRef]
- Hasmadi, M.; Pakhriazad, H.Z.; Shahrin, M.F. Evaluating supervised and unsupervised techniques for land cover mapping using remote sensing data. Geogr. Malays. J. Soc. Space 2009, 5, 1–10. [Google Scholar]
- Nelson, S.A.; Cheruvelil, K.S.; Soranno, P.A. Satellite remote sensing of freshwater macrophytes and the influence of water clarity. Aquat. Bot. 2006, 85, 289–298. [Google Scholar] [CrossRef]
- Yadav, S.; Yoneda, M.; Susaki, J.; Tamura, M.; Ishikawa, K.; Yamashiki, Y.A. A Satellite-Based Assessment of the Distribution and Biomass of Submerged Aquatic Vegetation in the Optically Shallow Basin of Lake Biwa. Remote. Sens. 2017, 9, 966. [Google Scholar] [CrossRef]
- Keshava, N. A survey of spectral unmixing algorithms. Lincoln Lab. J. 2003, 14, 55–78. [Google Scholar]
- Yan, G.; Li, L.; Coy, A.; Mu, X.; Chen, S.; Xie, D.; Zhang, W.; Shen, Q.; Zhou, H. Improving the estimation of fractional vegetation cover from UAV RGB imagery by colour unmixing. ISPRS J. Photogramm. Remote. Sens. 2019, 158, 23–34. [Google Scholar] [CrossRef]
- Govender, M.; Chetty, K.; Bulcock, H. A review of hyperspectral remote sensing and its application in vegetation and water resource studies. Water SA 2007, 33. [Google Scholar] [CrossRef]
- Dronova, I. Object-Based Image Analysis in Wetland Research: A Review. Remote. Sens. 2015, 7, 6380–6413. [Google Scholar] [CrossRef]
- Husson, E.; Ecke, F.; Reese, H. Comparison of Manual Mapping and Automated Object-Based Image Analysis of Non-Submerged Aquatic Vegetation from Very-High-Resolution UAS Images. Remote. Sens. 2016, 8, 724. [Google Scholar] [CrossRef]
- Chabot, D.; Dillon, C.; Ahmed, O.; Shemrock, A. Object-based analysis of UAS imagery to map emergent and submerged invasive aquatic vegetation: A case study. J. Unmanned Veh. Syst. 2017, 5, 27–33. [Google Scholar] [CrossRef]
- Becker, R.; Sayers, M.; Dehm, D.; Shuchman, R.; Quintero, K.; Bosse, K.; Sawtell, R. Unmanned aerial system based spectroradiometer for monitoring harmful algal blooms: A new paradigm in water quality monitoring. J. Great Lakes Res. 2019, 45, 444–453. [Google Scholar] [CrossRef]
- Joyce, K.E.; Duce, S.; Leahy, S.M.; Leon, J.; Maier, S.W. Principles and practice of acquiring drone-based image data in marine environments. Mar. Freshw. Res. 2019, 70, 952. [Google Scholar] [CrossRef]
- Ventura, D.; Bonifazi, A.; Gravina, M.F.; Belluscio, A.; Ardizzone, G. Mapping and Classification of Ecologically Sensitive Marine Habitats Using Unmanned Aerial Vehicle (UAV) Imagery and Object-Based Image Analysis (OBIA). Remote. Sens. 2018, 10, 1331. [Google Scholar] [CrossRef]
- Hardin, P.J.; Jensen, R.R. Small-Scale Unmanned Aerial Vehicles in Environmental Remote Sensing: Challenges and Opportunities. GISci. Remote. Sens. 2011, 48, 99–111. [Google Scholar] [CrossRef]
- Kutser, T.; Vahtmäe, E.; Praks, J. A sun glint correction method for hyperspectral imagery containing areas with non-negligible water leaving NIR signal. Remote. Sens. Environ. 2009, 113, 2267–2274. [Google Scholar] [CrossRef]
- Bandini, F.; Jakobsen, J.; Olesen, D.; Reyna-Gutierrez, J.A.; Bauer-Gottwein, P. Measuring water level in rivers and lakes from lightweight Unmanned Aerial Vehicles. J. Hydrol. 2017, 548, 237–250. [Google Scholar] [CrossRef]
- Kay, S.; Hedley, J.; Lavender, S. Sun Glint Correction of High and Low Spatial Resolution Images of Aquatic Scenes: A Review of Methods for Visible and Near-Infrared Wavelengths. Remote. Sens. 2009, 1, 697–730. [Google Scholar] [CrossRef]
- Benavides, M.T.; Fodrie, F.J.; Johnston, D.W. Shark detection probability from aerial drone surveys within a temperate estuary. J. Unmanned Veh. Syst. 2020, 8, 44–56. [Google Scholar] [CrossRef]
- Tamondong, A.; Nakamura, T.; Kobayashi, Y.; Garcia, M.; Nadaoka, K. Investigating the Effects of River Discharges on Submerged Aquatic Vegetation Using Uav Images and GIS Techniques. ISPRS Ann. Photogramm. Remote. Sens. Spat. Inf. Sci. 2020, 5, 93–99. [Google Scholar] [CrossRef]
- Thomsen, M.S.; Mondardini, L.; Alestra, T.; Gerrity, S.; Tait, L.; South, P.M.; Lilley, S.A.; Schiel, D.R. Local Extinction of Bull Kelp (Durvillaea spp.) Due to a Marine Heatwave. Front. Mar. Sci. 2019, 6, 84. [Google Scholar] [CrossRef]
- Duffy, J.P.; Pratt, L.; Anderson, K.; Land, P.E.; Shutler, J.D. Spatial assessment of intertidal seagrass meadows using optical imaging systems and a lightweight drone. Estuarine Coast. Shelf Sci. 2018, 200, 169–180. [Google Scholar] [CrossRef]
- Mora-Soto, A.; Palacios, M.; Macaya, E.C.; Gómez, I.; Huovinen, P.; Pérez-Matus, A.; Young, M.A.; Golding, N.; Toro, M.; Yaqub, M.; et al. A High-Resolution Global Map of Giant Kelp (Macrocystis pyrifera) Forests and Intertidal Green Algae (Ulvophyceae) with Sentinel-2 Imagery. Remote. Sens. 2020, 12, 694. [Google Scholar] [CrossRef]
- Green, D.R.; Gregory, B.J.; Karachok, A.R. Unmanned Aerial Remote Sensing: UAS for Environmental Applications; CRC Press: Boca Raton, FL, USA, 2020. [Google Scholar]
- Rossiter, T.; Furey, T.; McCarthy, T.; Stengel, D.B. UAV-mounted hyperspectral mapping of intertidal macroalgae. Estuarine Coast. Shelf Sci. 2020, 242, 106789. [Google Scholar] [CrossRef]
- Gallant, A.L. The Challenges of Remote Monitoring of Wetlands. Remote. Sens. 2015, 7, 10938–10950. [Google Scholar] [CrossRef]
- Abati, S.; Minciardi, M.R.; Ciadamidaro, S.; Fattorini, S.; Ceschin, S. Response of macrophyte communities to flow regulation in mountain streams. Environ. Monit. Assess. 2016, 188. [Google Scholar] [CrossRef] [PubMed]
- Power, M.E.; Parker, M.S.; Dietrich, W.E. Seasonal Reassembly of a River Food Web: Floods, Droughts, and Impacts of Fish. Ecol. Monogr. 2008, 78, 263–282. [Google Scholar] [CrossRef]
- Wehr, J.D. Analysis of seasonal succession of attached algae in a mountain stream, the North Alouette River, British Columbia. Can. J. Bot. 1981, 59, 1465–1474. [Google Scholar] [CrossRef]
- Banish, N.J. Factors Influencing Cladophora Biomass Abundance in the Upper Clark Fork River, Montana. Available online: https://scholarworks.umt.edu/cgi/viewcontent.cgi?article=12162&context=etd (accessed on 1 October 2020).
- Rusnák, M.; Sládek, J.; Kidová, A.; Lehotský, M. Template for high-resolution river landscape mapping using UAV technology. Measurement 2018, 115, 139–151. [Google Scholar] [CrossRef]
- Nahirnick, N.K.; Hunter, P.; Costa, M.; Schroeder, S.; Sharma, T. Benefits and Challenges of UAS Imagery for Eelgrass (Zostera marina) Mapping in Small Estuaries of the Canadian West Coast. J. Coast. Res. 2019, 35, 673–683. [Google Scholar] [CrossRef]
- Hashemi-Beni, L.; Jones, J.; Thompson, G.; Johnson, C.; Gebrehiwot, A. Challenges and Opportunities for UAV-Based Digital Elevation Model Generation for Flood-Risk Management: A Case of Princeville, North Carolina. Sensors 2018, 18, 3843. [Google Scholar] [CrossRef]
- Kutser, T.; Vahtmäe, E.; Martin, G. Assessing suitability of multispectral satellites for mapping benthic macroalgal cover in turbid coastal waters by means of model simulations. Estuarine Coast. Shelf Sci. 2006, 67, 521–529. [Google Scholar] [CrossRef]
- Boon, M.A.; Drijfhout, A.P.; Tesfamichael, S.G. Comparison of a fixed-wing and multi-rotor uav for environmental mapping applications: A case study. ISPRS Int. Arch. Photogramm. Remote. Sens. Spat. Inf. Sci. 2017, 42, 47–54. [Google Scholar] [CrossRef]
- Bunn, S.E.; Abal, E.G.; Smith, M.J.; Choy, S.C.; Fellows, C.S.; Harch, B.D.; Kennard, M.J.; Sheldon, F. Integration of science and monitoring of river ecosystem health to guide investments in catchment protection and rehabilitation. Freshw. Biol. 2010, 55, 223–240. [Google Scholar] [CrossRef]
- Schneider, S.C.; Hilt, S.; Vermaat, J.E.; Kelly, M. The “Forgotten” Ecology Behind Ecological Status Evaluation: Re-Assessing the Roles of Aquatic Plants and Benthic Algae in Ecosystem Functioning. In Progress in Botany; Cánovas, F.M., Lüttge, U., Matyssek, R., Eds.; Springer: Cham, Switzerland, 2016; Volume 78, pp. 285–304. [Google Scholar]
- Kornijów, R.; Gulati, R.D.; Ozimek, T. Food preference of freshwater invertebrates: Comparing fresh and decomposed angiosperm and a filamentous alga. Freshw. Biol. 1995, 33, 205–212. [Google Scholar] [CrossRef]
- Stevenson, R.J.; Bennett, B.J.; Jordan, D.N.; French, R.D. Phosphorus regulates stream injury by filamentous green algae, DO, and pH with thresholds in responses. Hydrobiologia 2012, 695, 25–42. [Google Scholar] [CrossRef]
- Deas, M.; Vaughn, J. Characterization of Organic Matter Fate and Transport in the Klamath River Below Link Dam to Assess Treatment/Reduction Potential; Watercourse Engineering Inc.: Davis, CA, USA, 2006; Available online: https://www.researchgate.net/profile/Michael_Deas/publication/228985030_Characterization_of_organic_matter_fate_and_transport_in_the_Klamath_River_below_Link_Dam_to_assess_treatmentreduction_potential/links/00b7d534ffacabfad8000000/Characterization-of-organic-matter-fate-and-transport-in-the-Klamath-River-below-Link-Dam-to-assess-treatment-reduction-potential.pdf (accessed on 1 October 2020).
- Poikane, S.; Kelly, M.; Cantonati, M. Benthic algal assessment of ecological status in European lakes and rivers: Challenges and opportunities. Sci. Total. Environ. 2016, 568, 603–613. [Google Scholar] [CrossRef] [PubMed]
- Ebert, D.; Austin, D.; Deas, M.; Carlson, K. Interim Measure 11, Activity 6—Study of Algal Conditions Management within a Reservoir Cove Using Physical Measures. Available online: https://www.pacificorp.com/content/dam/pcorp/documents/en/pacificorp/energy/hydro/klamath-river/khsa-implementation/technical-documents/2016-IM11-Act6TRptF(7-12-16_v3).pdf (accessed on 1 October 2020).
- Blinn, D.W.; Shannon, J.P.; Benenati, P.L.; Wilson, K.P. Algal ecology in tailwater stream communities: The Colorado River below Glen Canyon Dam, Arizona. J. Phycol. 1998, 34, 734–740. [Google Scholar] [CrossRef]
- Sabater, S.; Bregoli, F.; Acuña, V.; Barceló, D.; Elosegi, A.; Ginebreda, A.; Arce, M.I.; Muñoz, I.; Sabater-Liesa, L.; Ferreira, V. Effects of human-driven water stress on river ecosystems: A meta-analysis. Sci. Rep. 2018, 8, 11462. [Google Scholar] [CrossRef] [PubMed]
- Klamath River Renewal Corporation. Definite Plan for the Lower Klamath Project. Available online: https://play.google.com/store/books/details?id=9HXEvQEACAAJ (accessed on 1 October 2020).
- Visser, F.; Buis, K.; Verschoren, V.; Meire, P. Depth Estimation of Submerged Aquatic Vegetation in Clear Water Streams Using Low-Altitude Optical Remote Sensing. Sensors 2015, 15, 25287–25312. [Google Scholar] [CrossRef]

| Error Matrix | Algae | Macro-phytes | Water | Land | Shadows | Sun Glint | Total | Commission Error |
|---|---|---|---|---|---|---|---|---|
| Algae | 209 | 4 | 58 | 11 | 1 | 2 | 285 | 27% |
| Macro-phytes | 7 | 91 | 47 | 5 | 3 | 0 | 153 | 41% |
| Water | 22 | 4 | 628 | 3 | 0 | 7 | 664 | 5% |
| Land | 9 | 1 | 23 | 89 | 4 | 9 | 135 | 34% |
| Shadows | 5 | 3 | 1 | 5 | 171 | 2 | 187 | 9% |
| Sun glint | 0 | 0 | 32 | 18 | 1 | 129 | 180 | 28% |
| Total | 252 | 103 | 789 | 131 | 180 | 149 | 1604 | |
| Omission Error | 17% | 12% | 20% | 32% | 5% | 13% | 82% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kislik, C.; Genzoli, L.; Lyons, A.; Kelly, M. Application of UAV Imagery to Detect and Quantify Submerged Filamentous Algae and Rooted Macrophytes in a Non-Wadeable River. Remote Sens. 2020, 12, 3332. https://doi.org/10.3390/rs12203332
Kislik C, Genzoli L, Lyons A, Kelly M. Application of UAV Imagery to Detect and Quantify Submerged Filamentous Algae and Rooted Macrophytes in a Non-Wadeable River. Remote Sensing. 2020; 12(20):3332. https://doi.org/10.3390/rs12203332
Chicago/Turabian StyleKislik, Chippie, Laurel Genzoli, Andy Lyons, and Maggi Kelly. 2020. "Application of UAV Imagery to Detect and Quantify Submerged Filamentous Algae and Rooted Macrophytes in a Non-Wadeable River" Remote Sensing 12, no. 20: 3332. https://doi.org/10.3390/rs12203332
APA StyleKislik, C., Genzoli, L., Lyons, A., & Kelly, M. (2020). Application of UAV Imagery to Detect and Quantify Submerged Filamentous Algae and Rooted Macrophytes in a Non-Wadeable River. Remote Sensing, 12(20), 3332. https://doi.org/10.3390/rs12203332