Adjustments to SIF Aid the Interpretation of Drought Responses at the Caatinga of Northeast Brazil
Abstract
1. Introduction
- Describe seasonal and spatial patterns of chlorophyll fluorescence dynamics as estimated by the GOME-2 orbital instrument in a eleven-year period from February 2007 until December 2017;
- Model Sun-induced fluorescence (SIF) and spectrally adjusted SIF, as functions of environmental parameters, testing their responses to climate in the period;
- Compare the responses of vegetation from the different ecoregions of the Caatinga, as defined by Velloso et al. [35], to the observed environmental variation in the period.
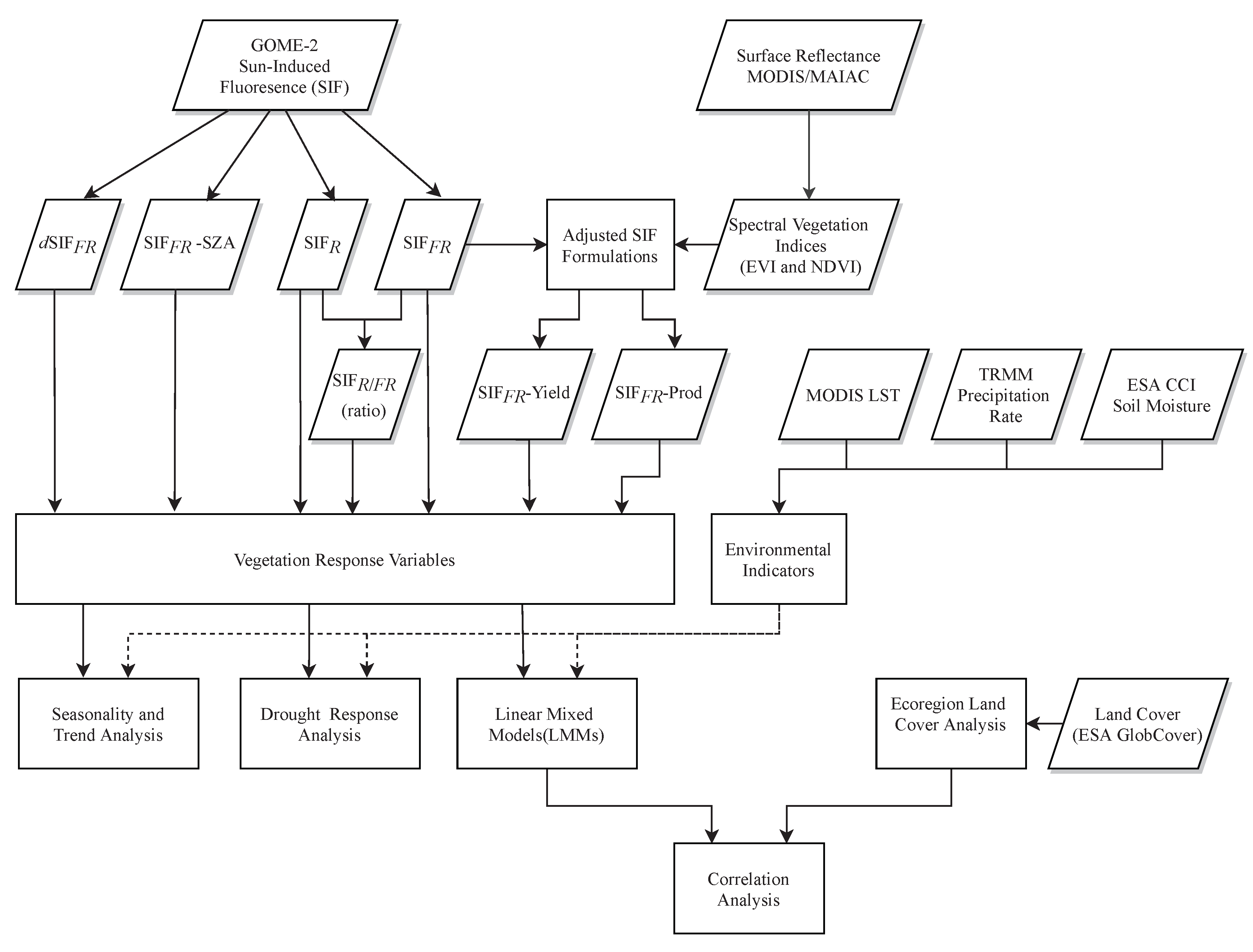
2. Materials and Methods
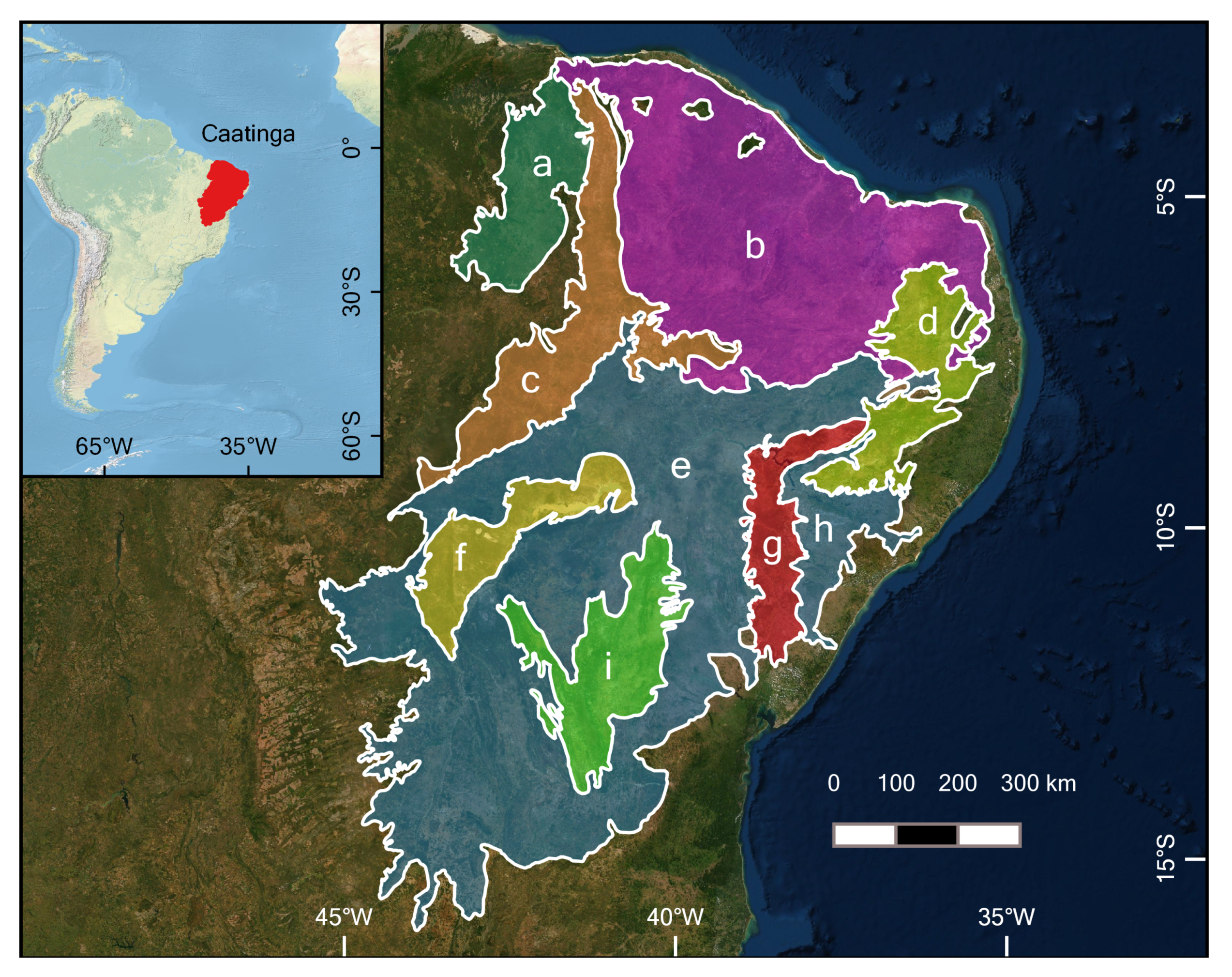
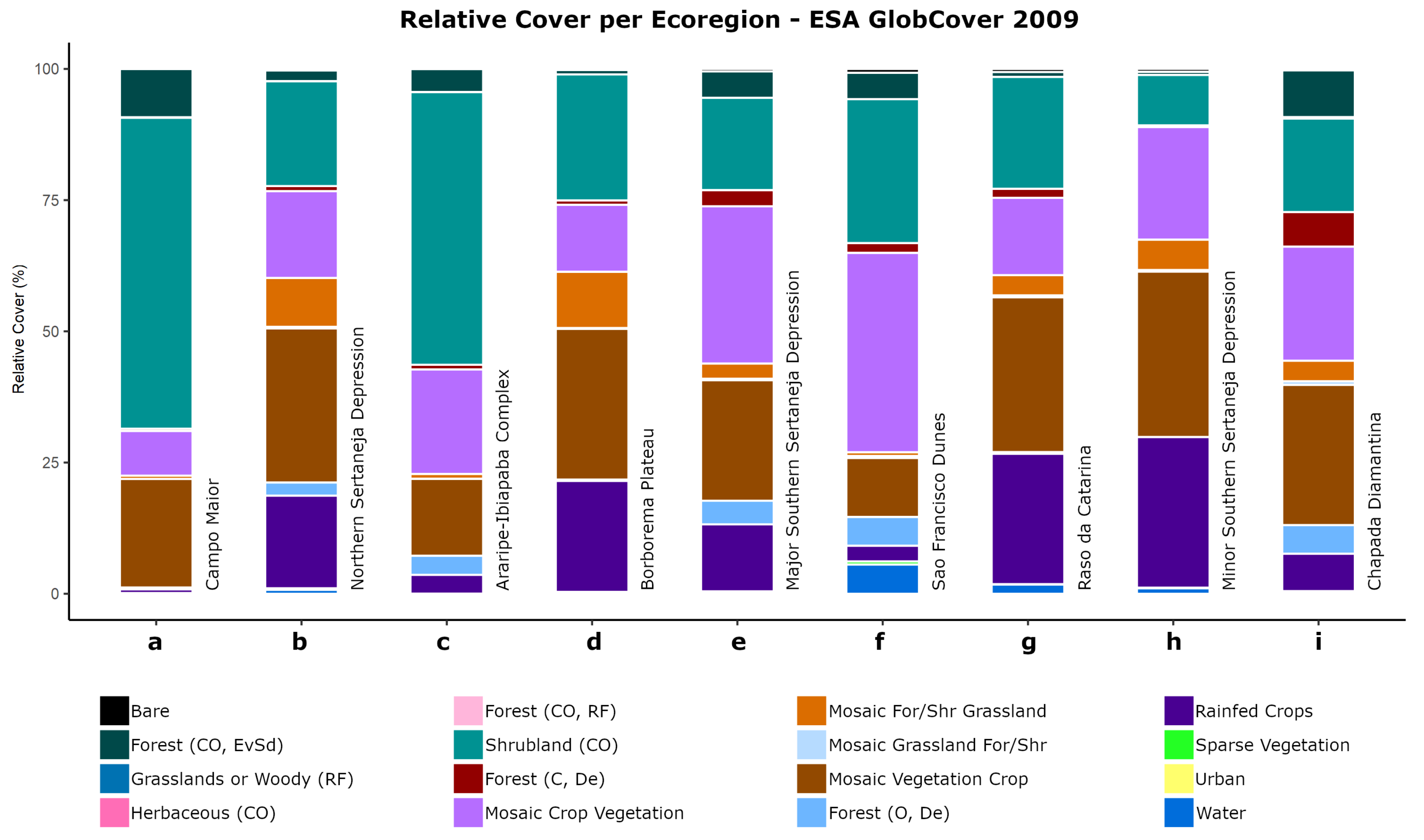
2.1. Study Area
2.2. Land Cover Classification Data
2.3. Environmental Indicators
2.4. MODIS-MAIAC Reflectance and Spectral Vegetation Indices
2.5. Sun-Induced Fluorescence
2.6. Statistics and Software
3. Results
3.1. Caatinga Ecoregion Land Cover Analysis
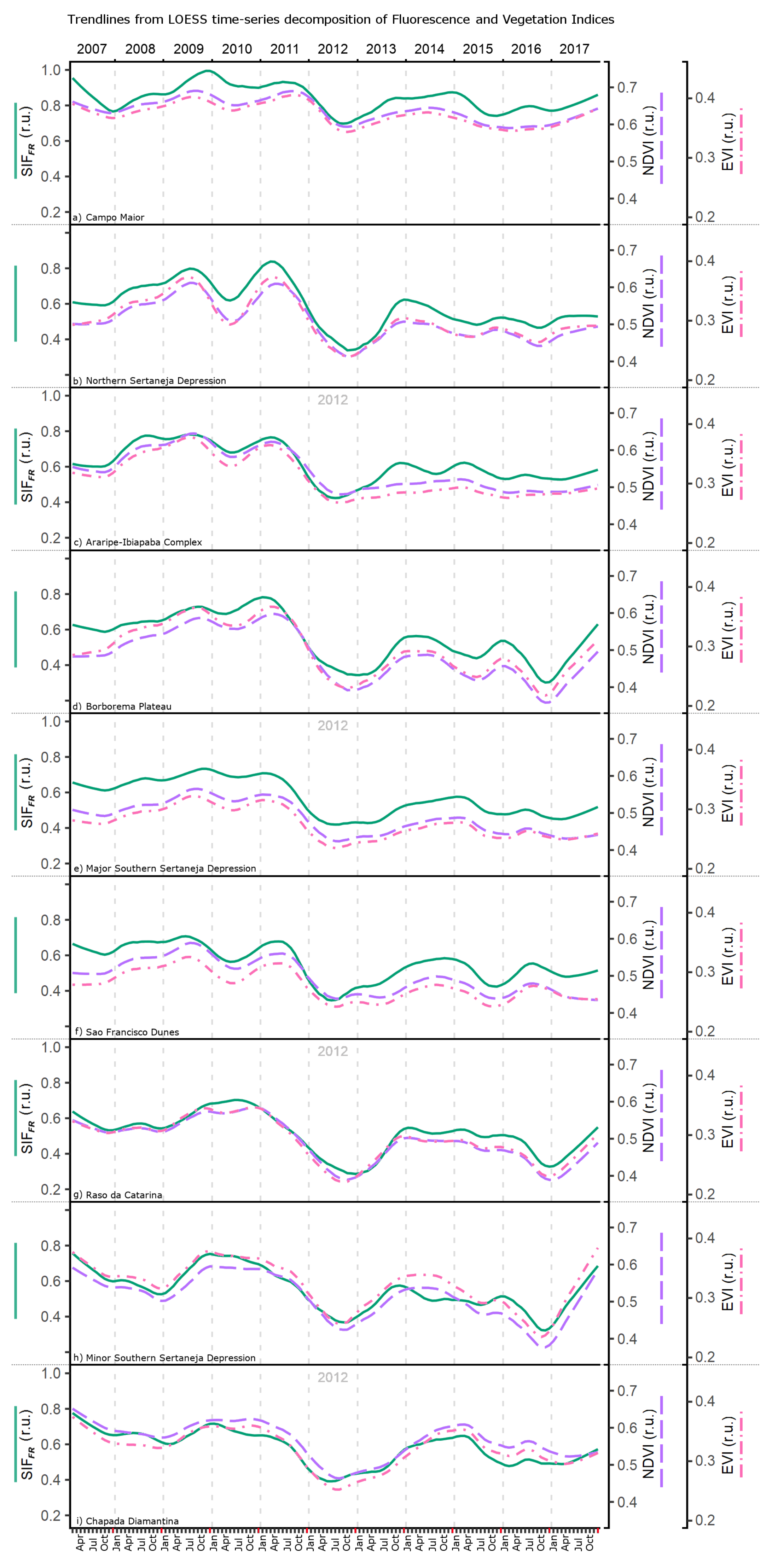
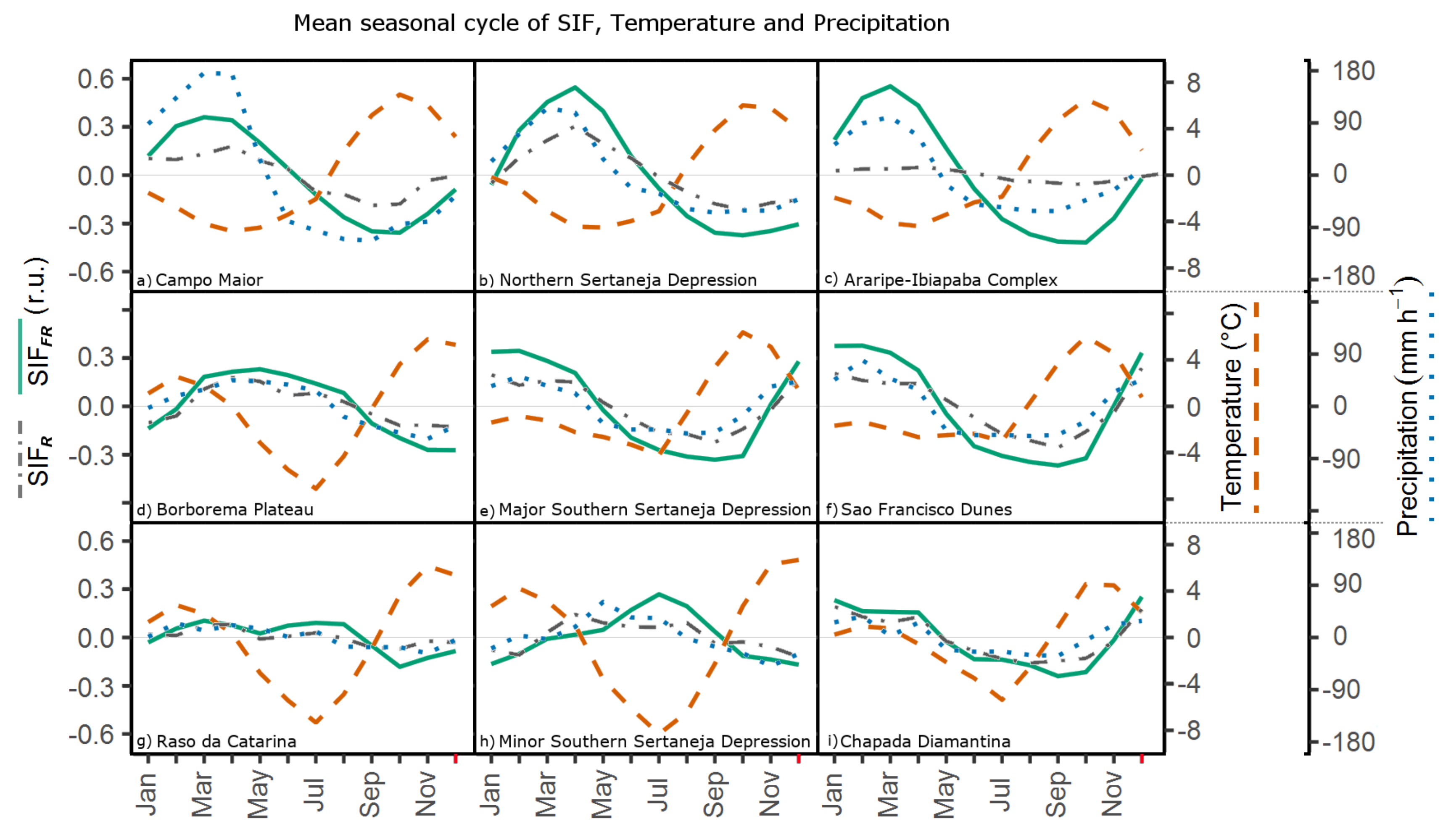
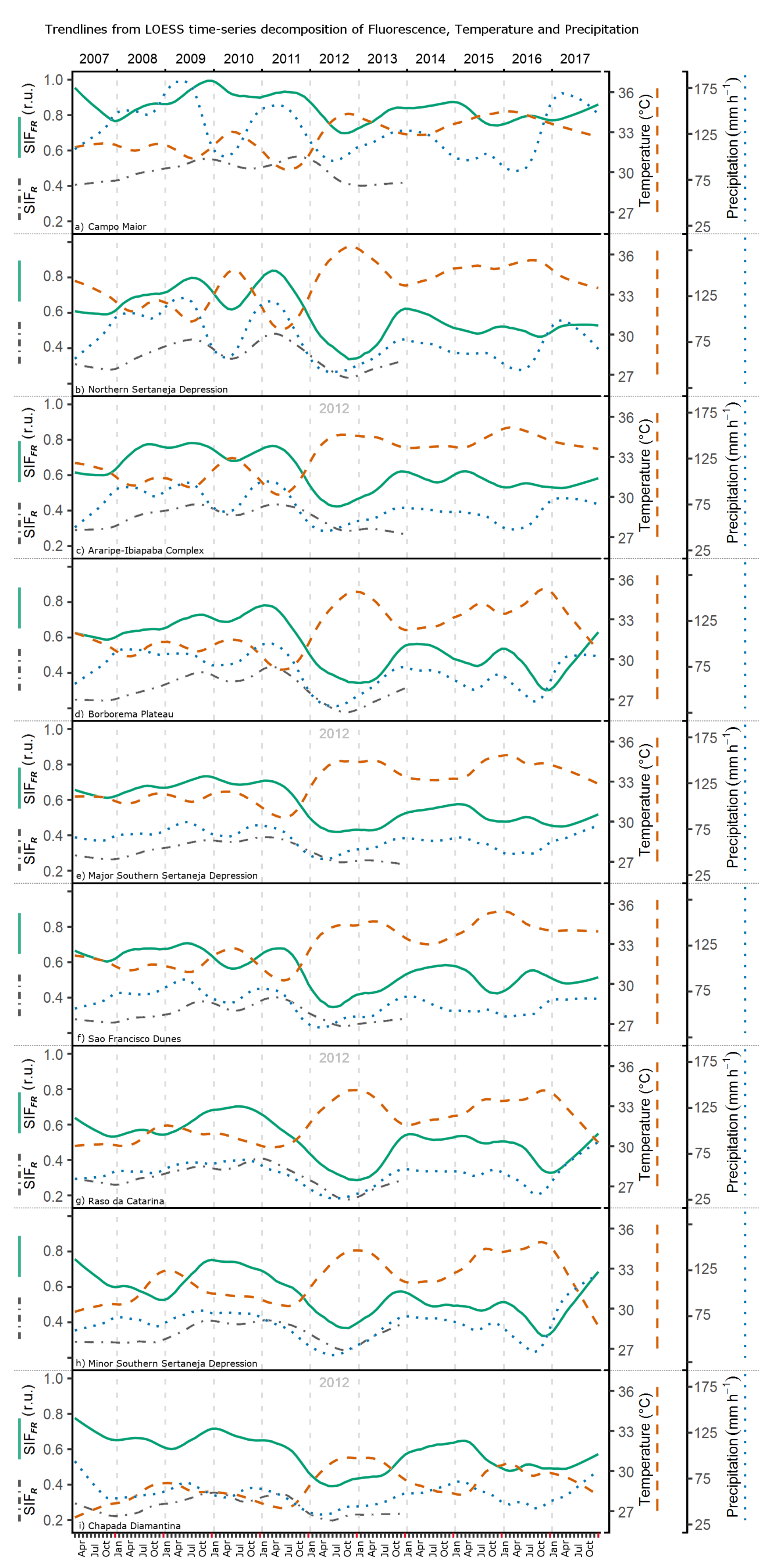
3.2. SIF and Climate: Seasonality and Trends
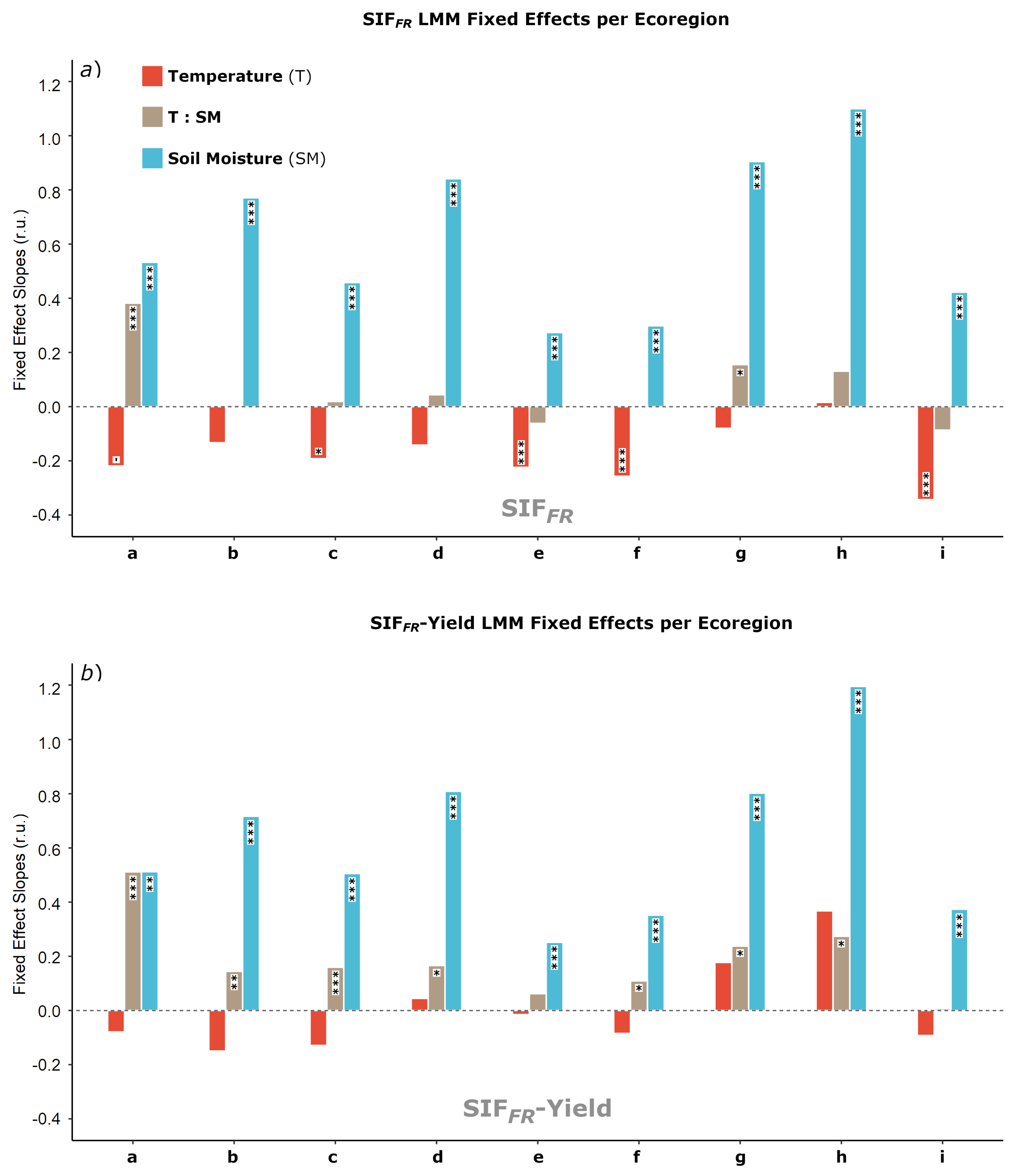
3.3. SIF and Climate: Linear Mixed Model Analysis
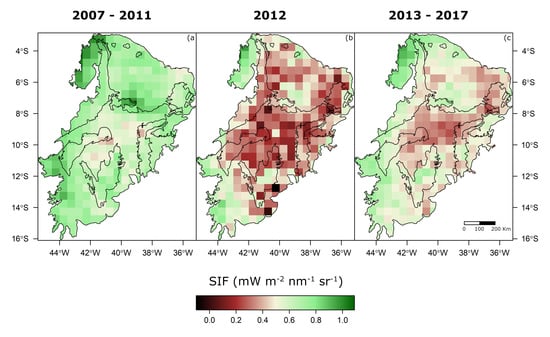
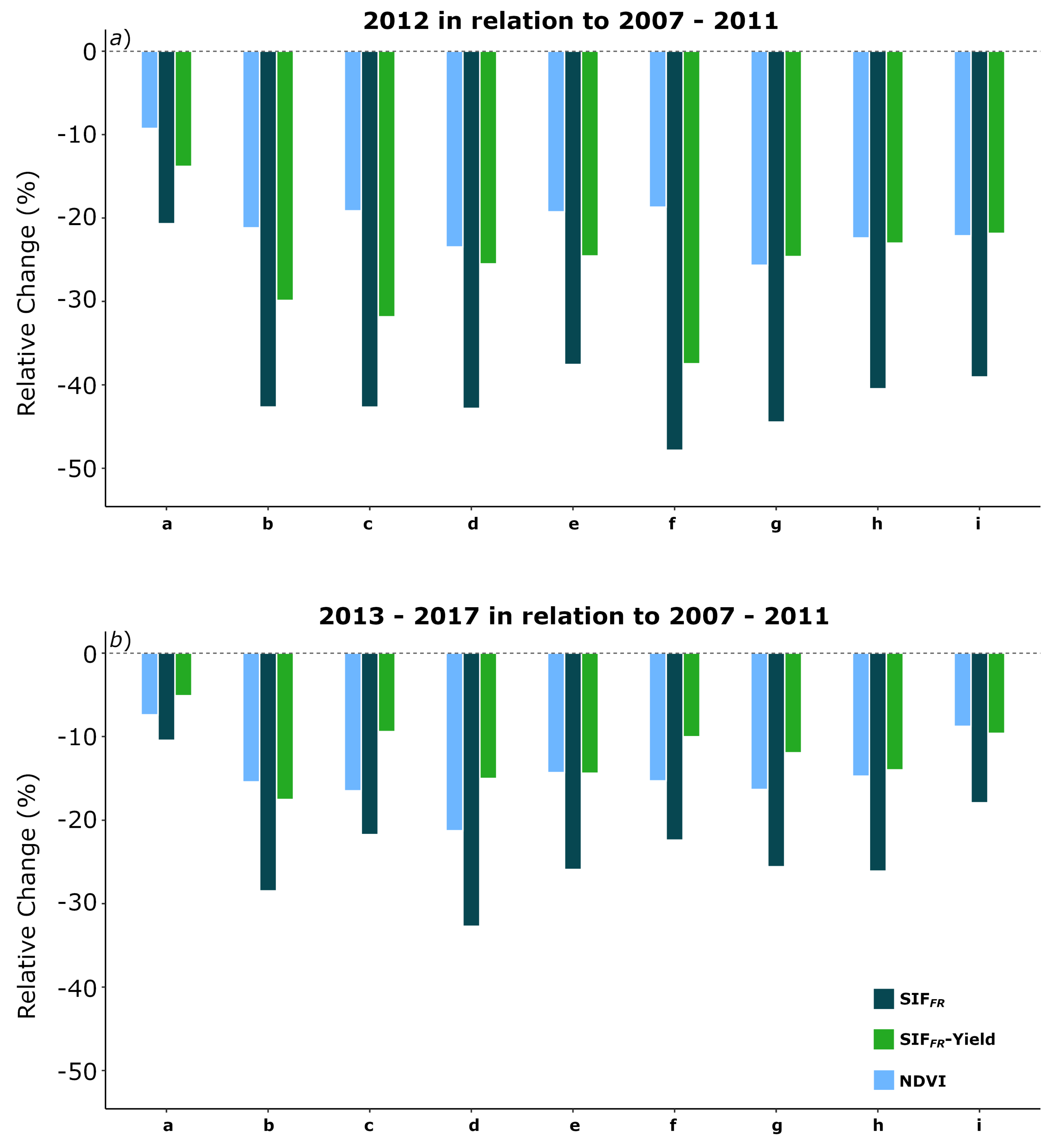
3.4. SIF and Climate: The 2012 Drought
4. Discussion
4.1. SIF Responses to Environmental Variation
4.2. SIF Seasonality and Phenological Processes
4.3. Apparent Influence of Vegetation Differences in SIF Responses
4.4. Linear Mixed Models and Our Adjustments to SIF
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Class Number | Class Name |
|---|---|
| 14 | Rainfed croplands |
| 20 | Mosaic cropland (50–70%)/vegetation (grassland/shrubland/forest) (20–50%) |
| 30 | Mosaic vegetation (grassland/shrubland/forest) (50–70%)/cropland (20–50%) |
| 40 | Closed to open (>15%) broadleaved evergreen or semi-deciduous forest (>5 m) |
| 50 | Closed (>40%) broadleaved deciduous forest (>5 m) |
| 60 | Open (15–40%) broadleaved deciduous forest/woodland (>5 m) |
| 110 | Mosaic forest or shrubland (50–70%)/grassland (20–50%) |
| 120 | Mosaic grassland (50–70%)/forest or shrubland (20–50%) |
| 130 | Closed to open (>15%) (broadleaved or needleleaved, evergreen or deciduous) shrubland (<5 m) |
| 140 | Closed to open (>15%) herbaceous vegetation (grassland, savannas or lichens/mosses) |
| 150 | Sparse (<15%) vegetation |
| 160 | Closed to open (>15%) broadleaved forest regularly flooded (semi-permanently or temporarily) |
| 170 | Closed (>40%) broadleaved forest or shrubland permanently flooded |
| 180 | Closed to open (>15%) grassland or woody vegetation on regularly flooded or waterlogged soil |
| 190 | Artificial surfaces and associated areas (Urban areas >50%) |
| 200 | Bare areas |
| 210 | Water bodies |
| Class Number | Ecoregion | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| a | b | c | d | e | f | g | h | i | |
| 14 | 0.70 | 17.65 | 3.58 | 21.15 | 12.76 | 2.99 | 24.84 | 28.70 | 7.13 |
| 20 | 8.52 | 16.54 | 19.92 | 12.76 | 29.96 | 37.99 | 14.73 | 21.45 | 21.74 |
| 30 | 20.67 | 29.36 | 14.66 | 28.73 | 22.97 | 11.24 | 29.49 | 31.51 | 26.72 |
| 40 | 9.19 | 2.03 | 4.35 | 0.83 | 5.02 | 5.02 | 0.92 | 0.60 | 8.91 |
| 50 | 0.43 | 0.96 | 0.88 | 0.83 | 3.08 | 1.86 | 1.73 | 0.32 | 6.58 |
| 60 | 0.35 | 2.51 | 3.64 | 0.26 | 4.50 | 5.48 | 0.32 | 0.02 | 5.45 |
| 110 | 0.62 | 9.33 | 0.90 | 10.72 | 2.90 | 0.72 | 3.85 | 5.79 | 3.91 |
| 120 | 0.01 | 0.28 | 0.02 | 0.15 | 0.27 | 0.37 | 0.37 | 0.30 | 0.69 |
| 130 | 59.25 | 19.99 | 51.96 | 24.01 | 17.58 | 27.43 | 21.32 | 9.61 | 17.80 |
| 140 | 0.01 | ||||||||
| 150 | 0.01 | 0.20 | 0.01 | 0.07 | 0.07 | 0.58 | 0.08 | 0.08 | 0.30 |
| 160 | 0.01 | 0.01 | |||||||
| 170 | 0.01 | ||||||||
| 180 | 0.10 | 0.01 | 0.01 | 0.01 | 0.30 | ||||
| 190 | 0.09 | 0.01 | 0.12 | 0.02 | 0.01 | 0.05 | |||
| 200 | 0.01 | 0.30 | 0.04 | 0.21 | 0.47 | 0.72 | 0.58 | 0.55 | 0.26 |
| 210 | 0.13 | 0.75 | 0.03 | 0.16 | 0.38 | 5.59 | 1.76 | 1.03 | 0.20 |
| SIF | dSIF | SIF-Prod | SIF-SZA | SIF-Yield | SIF | SIF | |
|---|---|---|---|---|---|---|---|
| Effect | Variance | Variance | Variance | Variance | Variance | Variance | Variance |
| Month:Year | 0.046 | 0.053 | 0.048 | 0.011 | 0.049 | 0.073 | 0.006 |
| Month | 0.161 | 0.192 | 0.129 | 0.021 | 0.218 | 0.172 | 0.017 |
| residual | 0.146 | 0.148 | 0.104 | 0.028 | 0.277 | 0.218 | 0.985 |
| Region | SIF and EVI | SIF and NDVI | SIF and EVI | SIF and NDVI |
|---|---|---|---|---|
| a | 0.42 *** | 0.47 *** | 0.25 *** | 0.26 *** |
| b | 0.72 *** | 0.73 *** | 0.65 *** | 0.68 *** |
| c | 0.54 *** | 0.57 *** | 0.50 *** | 0.59 *** |
| d | 0.70 *** | 0.71 *** | 0.54 *** | 0.54 *** |
| e | 0.65 *** | 0.65 *** | 0.54 *** | 0.56 *** |
| f | 0.58 *** | 0.59 *** | 0.37 *** | 0.42 *** |
| g | 0.64 *** | 0.64 *** | 0.28 *** | 0.30 *** |
| h | 0.46 *** | 0.47 *** | 0.31 *** | 0.34 *** |
| i | 0.42 *** | 0.44 *** | 0.25 *** | 0.25 *** |
| Caatinga | 0.57 *** | 0.58 *** | 0.41 *** | 0.43 *** |
References
- Sellers, P.J.; Schimel, D.S.; Moore, B.; Liu, J.; Eldering, A. Observing carbon cycle–climate feedbacks from space. Proc. Natl. Acad. Sci. USA 2018, 115, 7860–7868. [Google Scholar] [CrossRef] [PubMed]
- Porcar-Castell, A.; Tyystjärvi, E.; Atherton, J.; Van Der Tol, C.; Flexas, J.; Pfündel, E.E.; Moreno, J.; Frankenberg, C.; Berry, J.A. Linking chlorophyll a fluorescence to photosynthesis for remote sensing applications: Mechanisms and challenges. J. Exp. Bot. 2014, 65, 4065–4095. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, G.H.; Colombo, R.; Middleton, E.M.; Rascher, U.; van der Tol, C.; Nedbal, L.; Goulas, Y.; Pérez-Priego, O.; Damm, A.; Meroni, M.; et al. Remote sensing of solar-induced chlorophyll fluorescence (SIF) in vegetation: 50 years of progress. Remote Sens. Environ. 2019, 231, 111177. [Google Scholar] [CrossRef]
- Butler, W.L. Energy Distribution in the Photochemical Apparatus of Photosynthesis. Annu. Rev. Plant Physiol. 1978, 29, 345–378. [Google Scholar] [CrossRef]
- Demmig-Adams, B.; Adams, W.W. Photoprotection and Other Responses of Plants to High Light Stress. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1992, 43, 599–626. [Google Scholar] [CrossRef]
- Demmig-Adams, B.; Adams, W.W. Xanthophyll cycle and light stress in nature: Uniform response to excess direct sunlight among higher plant species. Planta 1996, 198, 460–470. [Google Scholar] [CrossRef]
- Maxwell, K.; Johnson, G.N. Chlorophyll fluorescence—A practical guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Baker, N.R. Chlorophyll Fluorescence: A Probe of Photosynthesis In Vivo. Annu. Rev. Plant Biol. 2008, 59, 89–113. [Google Scholar] [CrossRef]
- Govindje, E. Sixty-three years since Kautsky: Chlorophyll a Fluorescence. Aust. J. Plant Physiol. 1995, 22, 131–160. [Google Scholar] [CrossRef]
- Meroni, M.; Rossini, M.; Guanter, L.; Alonso, L.; Rascher, U.; Colombo, R.; Moreno, J. Remote sensing of solar-induced chlorophyll fluorescence: Review of methods and applications. Remote Sens. Environ. 2009, 113, 2037–2051. [Google Scholar] [CrossRef]
- Ryu, Y.; Berry, J.A.; Baldocchi, D.D. What is global photosynthesis? History, uncertainties and opportunities. Remote Sens. Environ. 2019, 223, 95–114. [Google Scholar] [CrossRef]
- Malenovský, Z.; Mishra, K.B.; Zemek, F.; Rascher, U.; Nedbal, L. Scientific and technical challenges in remote sensing of plant canopy reflectance and fluorescence. J. Exp. Bot. 2009, 60, 2987–3004. [Google Scholar] [CrossRef] [PubMed]
- Buschmann, C. Variability and application of the chlorophyll fluorescence emission ratio red/far-red of leaves. Photosynth. Res. 2007, 92, 261–271. [Google Scholar] [CrossRef] [PubMed]
- Rossini, M.; Nedbal, L.; Guanter, L.; Ac, A.; Alonso, L.; Burkart, A.; Cogliati, S.; Colombo, R.; Damm, A.; Drusch, M.; et al. Red and far red Sun-induced chlorophyll fluorescence as a measure of plant photosynthesis. Geophys. Res. Lett. 2015, 42, 1632–1639. [Google Scholar] [CrossRef]
- Van Wittenberghe, S.; Alonso, L.; Verrelst, J.; Moreno, J.; Samson, R. Bidirectional sun-induced chlorophyll fluorescence emission is influenced by leaf structure and light scattering properties—A bottom-up approach. Remote Sens. Environ. 2015, 158, 169–179. [Google Scholar] [CrossRef]
- Ač, A.; Malenovský, Z.; Olejníčková, J.; Gallé, A.; Rascher, U.; Mohammed, G. Meta-analysis assessing potential of steady-state chlorophyll fluorescence for remote sensing detection of plant water, temperature and nitrogen stress. Remote Sens. Environ. 2015, 168, 420–436. [Google Scholar] [CrossRef]
- Fournier, A.; Daumard, F.; Champagne, S.; Ounis, A.; Goulas, Y.; Moya, I. Effect of canopy structure on sun-induced chlorophyll fluorescence. ISPRS J. Photogramm. Remote Sens. 2012, 68, 112–120. [Google Scholar] [CrossRef]
- Verrelst, J.; Rivera, J.P.; van der Tol, C.; Magnani, F.; Mohammed, G.; Moreno, J. Global sensitivity analysis of the SCOPE model: What drives simulated canopy-leaving sun-induced fluorescence? Remote Sens. Environ. 2015, 166, 8–21. [Google Scholar] [CrossRef]
- Tucker, C.J. Red and Photographic Infrared Linear Combinations for Monitoring Vegetation. Remote Sens. Environ. 1979, 8, 127–150. [Google Scholar] [CrossRef]
- Huete, A.R.; Liu, H.; Batchily, K.; Van Leeuwen, W. A comparison of vegetation indices global set of TM images for EOS-MODIS. Remote Sens. Environ. 1997, 59, 440–451. [Google Scholar] [CrossRef]
- Huete, A.R.; Justice, C.; Leeuwen, W. MODIS Vegetation Index (MOD 13): Algorithm Theoretical Basis Document, version 3; Technical Report; NASA: Washington, DC, USA, 1999. [Google Scholar]
- Bannari, A.; Morin, D.; Bonn, F.; Huete, A.R. A review of vegetation indices. Remote Sens. Rev. 1995, 13, 95–120. [Google Scholar] [CrossRef]
- Entcheva-Campbell, P.; Middleton, E.; Corp, L.; Kim, M. Contribution of chlorophyll fluorescence to the apparent vegetation reflectance. Sci. Total. Environ. 2008, 404, 433–439. [Google Scholar] [CrossRef] [PubMed]
- Calderón, R.; Navas-Cortés, J.; Lucena, C.; Zarco-Tejada, P. High-resolution airborne hyperspectral and thermal imagery for early detection of Verticillium wilt of olive using fluorescence, temperature and narrow-band spectral indices. Remote Sens. Environ. 2013, 139, 231–245. [Google Scholar] [CrossRef]
- Yang, P.; van der Tol, C.; Verhoef, W.; Damm, A.; Schickling, A.; Kraska, T.; Muller, O.; Rascher, U. Using reflectance to explain vegetation biochemical and structural effects on sun-induced chlorophyll fluorescence. Remote Sens. Environ. 2018, 231, 110996. [Google Scholar] [CrossRef]
- Gitelson, A.; Buschmann, C.; Lichtenthaler, H.K. Leaf chlorophyll fluorescence corrected for re-absorption by means of absorption and reflectance measurements. J. Plant Physiol. 1998, 152, 283–296. [Google Scholar] [CrossRef]
- Joiner, J.; Guanter, L.; Lindstrot, R.; Voigt, M.; Vasilkov, A.P.; Middleton, E.M.; Huemmrich, K.F.; Yoshida, Y.; Frankenberg, C. Global monitoring of terrestrial chlorophyll fluorescence from moderate-spectral-resolution near-infrared satellite measurements: Methodology, simulations, and application to GOME-2. Atmos. Meas. Tech. 2013, 6, 2803–2823. [Google Scholar] [CrossRef]
- Joiner, J.; Yoshida, Y.; Guanter, L.; Middleton, E.M. New methods for retrieval of chlorophyll red fluorescence from hyper-spectral satellite instruments: Simulations and application to GOME-2 and SCIAMACHY. Atmos. Meas. Tech. Discuss. 2016, 9, 3939–3967. [Google Scholar] [CrossRef]
- Köhler, P.; Guanter, L.; Kobayashi, H.; Walther, S.; Yang, W. Assessing the potential of sun-induced fluorescence and the canopy scattering coefficient to track large-scale vegetation dynamics in Amazon forests. Remote Sens. Environ. 2018, 204, 769–785. [Google Scholar] [CrossRef]
- Sun, Y.; Fu, R.; Dickinson, R.; Joiner, J.; Frankenberg, C.; Gu, L.; Xia, Y.; Fernando, N. Drought onset mechanisms revealed by satellite solar-induced chlorophyll fluorescence: Insights from two contrasting extreme events. J. Geophys. Res. Biogeosci. 2015, 120, 2427–2440. [Google Scholar] [CrossRef]
- Yoshida, Y.; Joiner, J.; Tucker, C.; Berry, J.; Lee, J.E.; Walker, G.; Reichle, R.; Koster, R.; Lyapustin, A.; Wang, Y. The 2010 Russian drought impact on satellite measurements of solar-induced chlorophyll fluorescence: Insights from modeling and comparisons with parameters derived from satellite reflectances. Remote Sens. Environ. 2015, 166, 163–177. [Google Scholar] [CrossRef]
- Wang, S.; Huang, C.; Zhang, L.; Lin, Y.; Cen, Y.; Wu, T. Monitoring and assessing the 2012 drought in the great plains: Analyzing satellite-retrieved solar-induced chlorophyll fluorescence, drought indices, and gross primary production. Remote Sens. 2016, 8, 61. [Google Scholar] [CrossRef]
- Jiao, W.; Chang, Q.; Wang, L. The sensitivity of satellite solar-induced chlorophyll fluorescence (SIF) to meteorological drought. Earth Future 2019, 7, 558–573. [Google Scholar]
- Cunha, A.P.; Alvalá, R.C.; Nobre, C.A.; Carvalho, M.A. Monitoring vegetative drought dynamics in the Brazilian semiarid region. Agric. For. Meteorol. 2015, 214–215, 494–505. [Google Scholar] [CrossRef]
- Velloso, A.L.; Sampaio, E.V.S.B.; Pareyn, F.G.C. (Eds.) Ecoregiões Propostas Para o Bioma Caatinga (in Portuguese), 2nd ed.; Associação Plantas do Nordeste; Instituto de Conservação Ambiental—The Nature Conservancy do Brasil: Recife, Brazil, 2002; p. 75. [Google Scholar]
- Silva, J.M.C.; Leal, I.R.; Tabarelli, M. (Eds.) Caatinga—The Largest Tropical Dry Forest Region in South America; Springer International Publishing: Cham, Switzerland, 2017; p. 482. [Google Scholar]
- Andrade-Lima, D. The Caatingas Domain (original article in English). Revista Brasileira Botânica 1981, 4, 149–162. [Google Scholar]
- Breckle, S.W. Walter’s Vegetation of the Earth, 4th ed.; Springer: Berlin/Heidelberg, Germany, 2002; p. 525. [Google Scholar]
- Pennington, R.; Prado, D.E.; Pendry, C. Neotropical Seasonally Dry Forests and Quaternary Vegetation Changes. J. Biogeogr. 2000, 27, 261–273. [Google Scholar] [CrossRef]
- Pennington, R.; Lehmann, C.E.R.; Rowland, L.M. Tropical savannas and dry forests. Curr. Biol. 2018, 28, 542–545. [Google Scholar] [CrossRef]
- Santos, J.C.; Leal, I.R.; Almeida-Cortez, J.S.; Fernandes, G.W.; Tabarelli, M. Caatinga: The Scientific Negligence Experienced by a Dry Tropical Forest. Trop. Conserv. Sci. 2011, 4, 276–286. [Google Scholar] [CrossRef]
- Zscheischler, J.; Mahecha, M.D.; Harmeling, S.; Rammig, a.; Tomelleri, E.; Reichstein, M. Extreme events in gross primary production: A characterization across continents. Biogeosci. Discuss. 2014, 11, 1869–1907. [Google Scholar] [CrossRef]
- Zscheischler, J.; Mahecha, M.D.; Avitabile, V.; Calle, L.; Carvalhais, N.; Ciais, P.; Gans, F.; Gruber, N.; Hartmann, J.; Herold, M.; et al. Reviews and syntheses: An empirical spatiotemporal description of the global surface-atmosphere carbon fluxes: Opportunities and data limitations. Biogeosciences 2017, 14, 3685–3703. [Google Scholar] [CrossRef]
- Larcher, W. Physiological Plant Ecology, 4th ed.; Springer: Berlin/Heidelberg, 2003; p. 514. [Google Scholar]
- Arino, O.; Ramos Perez, J.J.; Kalogirou, V.; Bontemps, S.; Defourny, P.; Van Bogaert, E. Global Land Cover Map for 2009 (GlobCover 2009). European Space Agency (ESA) and Universite catholique de Louvain (UCL), PANGAEA. 2012. Available online: https://doi.org/doi:10.1002/9781118786352.wbieg0878 (accessed on 10 June 2019).
- Wan, Z.; Hook, S.; Hulley, G. MOD11C3 MODIS/Terra Land Surface Temperature/Emissivity Monthly L3 Global 0.05Deg CMG V006. 2015. Distributed by NASA EOSDIS Land Processes DAAC. Available online: https://doi.org/10.5067/MODIS/MOD11C3.006 (accessed on 5 August 2018).
- TRMM (TMPA/3B43) Rainfall Estimate L3 1 Month 0.25 Degree x 0.25 Degree V7. 2011. Available online: https://doi.org/10.5067/TRMM/TMPA/MONTH/7 (accessed on 6 August 2018).
- Parker, J.A.; Kenyon, R.V.; Troxel, D.E. Comparison of Interpolating Methods for Image Resampling. IEEE Trans. Med. Imaging 1983, 2, 31–39. [Google Scholar] [CrossRef]
- Zhu, A.X. Resampling, Raster. In International Encyclopedia of Geography: People, the Earth, Environment and Technology; Richardson, D., Castree, N., Goodchild, M.F., Kobayashi, A., Liu, W., Marston, R.A., Eds.; Wiley Online Library: Hoboken, NJ, USA, 2017. [Google Scholar] [CrossRef]
- Dorigo, W.; Wagner, W.; Albergel, C.; Albrecht, F.; Balsamo, G.; Brocca, L.; Chung, D.; Ertl, M.; Forkel, M.; Gruber, A.; et al. ESA CCI Soil Moisture for improved Earth system understanding: State-of-the art and future directions. Remote Sens. Environ. 2017, 203, 185–215. [Google Scholar] [CrossRef]
- Lyapustin, A.I.; Wang, Y. MCD19A1 MODIS/Terra+Aqua Land Surface BRF Daily L2G Global 500 m and 1 km SIN Grid V006. 2018, distributed by NASA EOSDIS Land Processes DAAC. Available online: https://doi.org/10.5067/MODIS/MCD19A1.006 (accessed on 2 June 2019).
- Lyapustin, A.I.; Wang, Y.; Laszlo, I.; Hilker, T.; Hall, F.G.; Sellers, P.J.; Tucker, C.J.; Korkin, S.V. Multi-angle implementation of atmospheric correction for MODIS (MAIAC): 3. Atmospheric correction. Remote Sens. Environ. 2012, 127, 385–393. [Google Scholar] [CrossRef]
- Dalagnol, R.; Wagner, F.H.; Galvão, L.S.; Nelson, B.W.; De Aragão, L.E.O.E. Life cycle of bamboo in the southwestern Amazon and its relation to fire events. Biogeosciences 2018, 15, 6087–6104. [Google Scholar] [CrossRef]
- Frankenberg, C.; O’Dell, C.; Berry, J.; Guanter, L.; Joiner, J.; Köhler, P.; Pollock, R.; Taylor, T.E. Prospects for chlorophyll fluorescence remote sensing from the Orbiting Carbon Observatory-2. Remote Sens. Environ. 2014, 147, 1–12. [Google Scholar] [CrossRef]
- Köehler, P.; Frankenberg, C.; Magney, T.S.; Guanter, L.; Joiner, J.; Landgraf, J. Global retrievals of solar induced chlorophyll fluorescence with TROPOMI: First results and inter-sensor comparison to OCO-2. Geophys. Res. Lett. 2018, 45, 10456–10463. [Google Scholar] [CrossRef]
- Du, S.; Liu, L.; Liu, X.; Zhang, X.; Zhang, X.; Bi, Y.; Zhang, L. Retrieval of global terrestrial solar-induced chlorophyll fluorescence from TanSat satellite. Sci. Bull. 2018, 63, 1502–1512. [Google Scholar] [CrossRef]
- Joiner, J.; Yoshida, Y.; Vasilkov, A.P.; Middleton, E.M.; Campbell, P.K.; Yoshida, Y.; Kuze, A.; Corp, L.A. Filling-in of near-infrared solar lines by terrestrial fluorescence and other geophysical effects: Simulations and space-based observations from SCIAMACHY and GOSAT. Atmos. Meas. Tech. 2012, 5, 809–829. [Google Scholar] [CrossRef]
- Tilstra, L.G.; Tuinder, O.N.E.; Stammes, P. A New Method for in-Flight Degradation Correction of GOME-2 Earth Reflectance Measurements, with Application to the Absorbing Aerosol Index; Technical Report; EUMETSAT: Darmstadt, Germany, 2012. [Google Scholar]
- Miao, G.; Guan, K.; Yang, X.; Bernacchi, C.J.; Berry, J.A.; DeLucia, E.H.; Wu, J.; Moore, C.E.; Meacham, K.; Cai, Y.; et al. Sun-Induced Chlorophyll Fluorescence, Photosynthesis, and Light Use Efficiency of a Soybean Field from Seasonally Continuous Measurements. J. Geophys. Res. Biogeosci. 2018, 123, 610–623. [Google Scholar] [CrossRef]
- Verma, M.; Schimel, D.; Evans, B.; Frankenberg, C.; Beringer, J.; Drewry, D.; Magney, T.; Marang, I.; Hutley, L.; Moore, C.; et al. Effect of environmental conditions on the relationship between solar-induced fluorescence and gross primary productivity at an OzFlux grassland site. J. Geophys. Res. Biogeosci. 2018, 122, 716–733. [Google Scholar] [CrossRef]
- Sellers, P.; Berry, J.; Collatz, G.; Field, C.; Hall, F. Canopy reflectance, photosynthesis, and transpiration. III. A reanalysis using improved leaf models and a new canopy integration scheme. Remote Sens. Environ. 2003, 42, 187–216. [Google Scholar] [CrossRef]
- Monteith, J.L. Solar Radiation and Productivity in Tropical Ecosystems. J. Appl. Ecol. 1972, 9, 747. [Google Scholar] [CrossRef]
- Monteith, J.L.; Moss, C.J. Climate and the Efficiency of Crop Production in Britain. Philos. Trans. R. Soc. B Biol. Sci. 1977, 281, 277–294. [Google Scholar]
- Gitelson, A.A.; Gamon, J.A. The need for a common basis for defining light-use efficiency: Implications for productivity estimation. Remote Sens. Environ. 2015, 156, 196–201. [Google Scholar] [CrossRef]
- Hilker, T.; Coops, N.C.; Wulder, M.A.; Black, T.A.; Guy, R.D. The use of remote sensing in light use efficiency based models of gross primary production: A review of current status and future requirements. Sci. Total. Environ. 2008, 404, 411–423. [Google Scholar] [CrossRef]
- Bontempo, E.; Valeriano, D. Sun-Induced Fluorescence’s Correlation to Carbon-Flux Increases When Raw Data is Adjusted to Account for Vegetation Biochemistry and Structure. Earth Space Sci. Open Arch. 2020. [Google Scholar] [CrossRef]
- Kendall, M.G.; Ord, K.J. The Advanced Theory of Statistics: Design and Analysis, and Time-Series; Hodder Arnold: London, UK, 1993; p. 296. [Google Scholar]
- Hyndman, R.; Athanasopoulos, G. Forecasting: Principles and Practice, 2nd ed.; OTexts: Melbourne, Australia, 2018; p. 380. [Google Scholar]
- Kendal, M.; Gibbons, J.D. Rank Correlation Methods, 5th ed.; Oxford University Press: Oxford, UK, 1990; p. 272. [Google Scholar]
- Schielzeth, H.; Forstmeier, W. Conclusions beyond support: Overconfident estimates in mixed models. Behav. Ecol. 2009, 20, 416–420. [Google Scholar] [CrossRef]
- Zuur, A.F.; Ieno, E.N. A protocol for conducting and presenting results of regression-type analyses. Methods Ecol. Evol. 2016, 7, 636–645. [Google Scholar] [CrossRef]
- Zuur, A.F.; Ieno, E.N.; Elphick, C.S. A protocol for data exploration to avoid common statistical problems. Methods Ecol. Evol. 2010, 1, 3–14. [Google Scholar] [CrossRef]
- Harrison, X.A.; Donaldson, L.; Correa-Cano, M.E.; Evans, J.; Fisher, D.N.; Goodwin, C.E.; Robinson, B.S.; Hodgson, D.J.; Inger, R. A brief introduction to mixed effects modelling and multi-model inference in ecology. PeerJ 2018, 6, e4794. [Google Scholar] [CrossRef]
- Nakagawa, S.; Schielzeth, H. A general and simple method for obtaining R2 from generalized linear mixed-effects models. Methods Ecol. Evol. 2013, 4, 133–142. [Google Scholar] [CrossRef]
- QGIS Development Team. QGIS Geographic Information System; Open Source Geospatial Foundation: Beaverton, OR, USA, 2019. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2013. [Google Scholar]
- Hafen, R.P. Local Regression Methods: Advancements, Aplications and New Methods. Ph.D. Thesis, University of Purdue, West Lafayette, IN, USA, 2010. [Google Scholar]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Fox, J.; Weisberg, S. An R Companion to Applied Regression, 3rd ed.; SAGE: Thousand Oaks, CA, USA, 2019. [Google Scholar]
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- Knowles, J.E.; Frederick, C. merTools: Tools for Analyzing Mixed Effect Regression Models. R Package Version 0.5.0. 2019. Available online: https://rdrr.io/cran/merTools/ (accessed on 28 August 2020).
- Hothorn, T.; Bretz, F.; Westfall, P. Simultaneous Inference in General Parametric Models. Biom. J. 2008, 50, 346–363. [Google Scholar] [CrossRef] [PubMed]
- Barton, K. MuMIn: Multi-Model Inference. R Package Version 1.43.6. 2019. Available online: https://rdrr.io/cran/MuMIn/ (accessed on 28 August 2020).
- Hijmans, R.J. Raster: Geographic Data Analysis and Modeling. R Package Version 2.9-23. 2019. Available online: https://rdrr.io/cran/raster/ (accessed on 28 August 2020).
- Perpiñán, O.; Hijmans, R. rasterVis: Visualization methods for the raster package. R Package Version 0.45. 2018. Available online: http://rasterVis.R-Forge.R-project.org/ (accessed on 28 August 2020).
- Bivand, R.; Keitt, T.; Rowlingson, B. Rgdal: Bindings for the ‘Geospatial’ Data Abstraction Library. R Package Version 1.4-4. 2019. Available online: http://rgdal.r-forge.r-project.org (accessed on 28 August 2020).
- Bontempo, E. Scripts used in article: Adjustments to SIF Aid the Interpretation of Drought Responses at the Caatinga of Northeast Brazil. Preprints 2020. [Google Scholar] [CrossRef]
- Lang, M.; Lichtenthaler, H.K.; Sowinska, M.; Heisel, F.; Miehé, J.A. Fluorescence imaging of water and temperature stress in plant leaves. J. Plant Physiol. 1996, 148, 613–621. [Google Scholar] [CrossRef]
- Prado, D. As caatingas da América do Sul. In Ecologia e Conservação das Caatingas; Leal, I., Tabarelli, M., Silva, J., Eds.; Universitaria UFPE: Recife, Brazil, 2003; p. 822. [Google Scholar]
- Bontempo e Silva, E.A.; Ono, K.; Sumida, A.; Uemura, S.; Hara, T. Contrasting traits, contrasting environments, and considerations on population dynamics under a changing climate: An ecophysiological field study of two co-dominant tree species. Plant Species Biol. 2014, 31, 38–49. [Google Scholar] [CrossRef]
- Santos, M.; Oliveira, M.; Figueiredo, K.; Falcao, H.; Arruda, E.; Almeida-Cortez, J.; Sampaio, E.; Ometto, J.; Menezes, R.; Oliveira, A.; et al. Caatinga, the Brazilian dry tropical forest: Can it tolerate climate changes? Theor. Exp. Plant Physiol. 2014, 26, 83–99. [Google Scholar] [CrossRef]
- Odum, E. Basic Ecology; CBS College Publishing: New York, NY, USA, 1983; p. 434. [Google Scholar]
- Gunderson, L.H. Ecological Resilience—In Theory and Application. Annu. Rev. Ecol. Syst. 2000, 31, 425–439. [Google Scholar] [CrossRef]

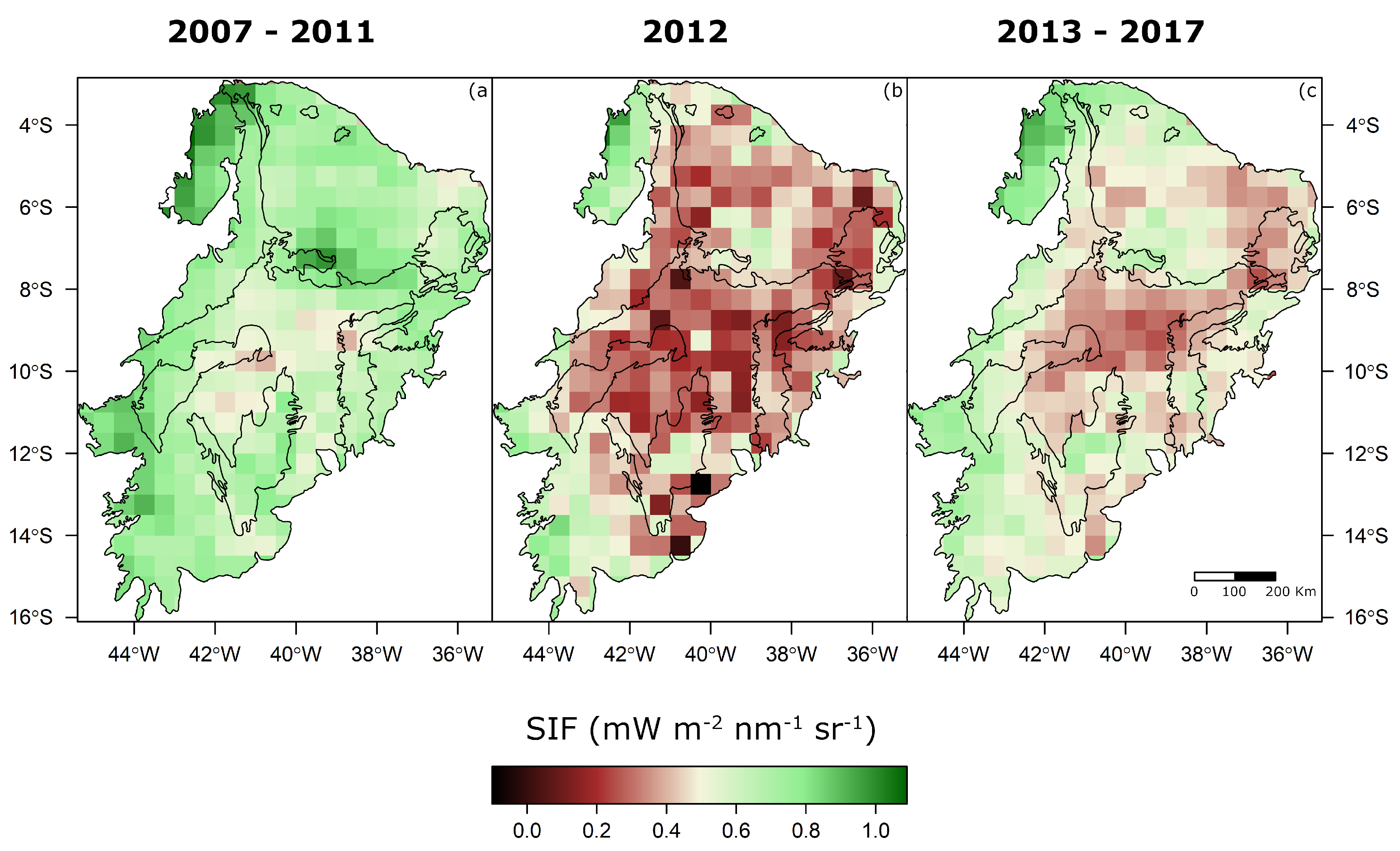
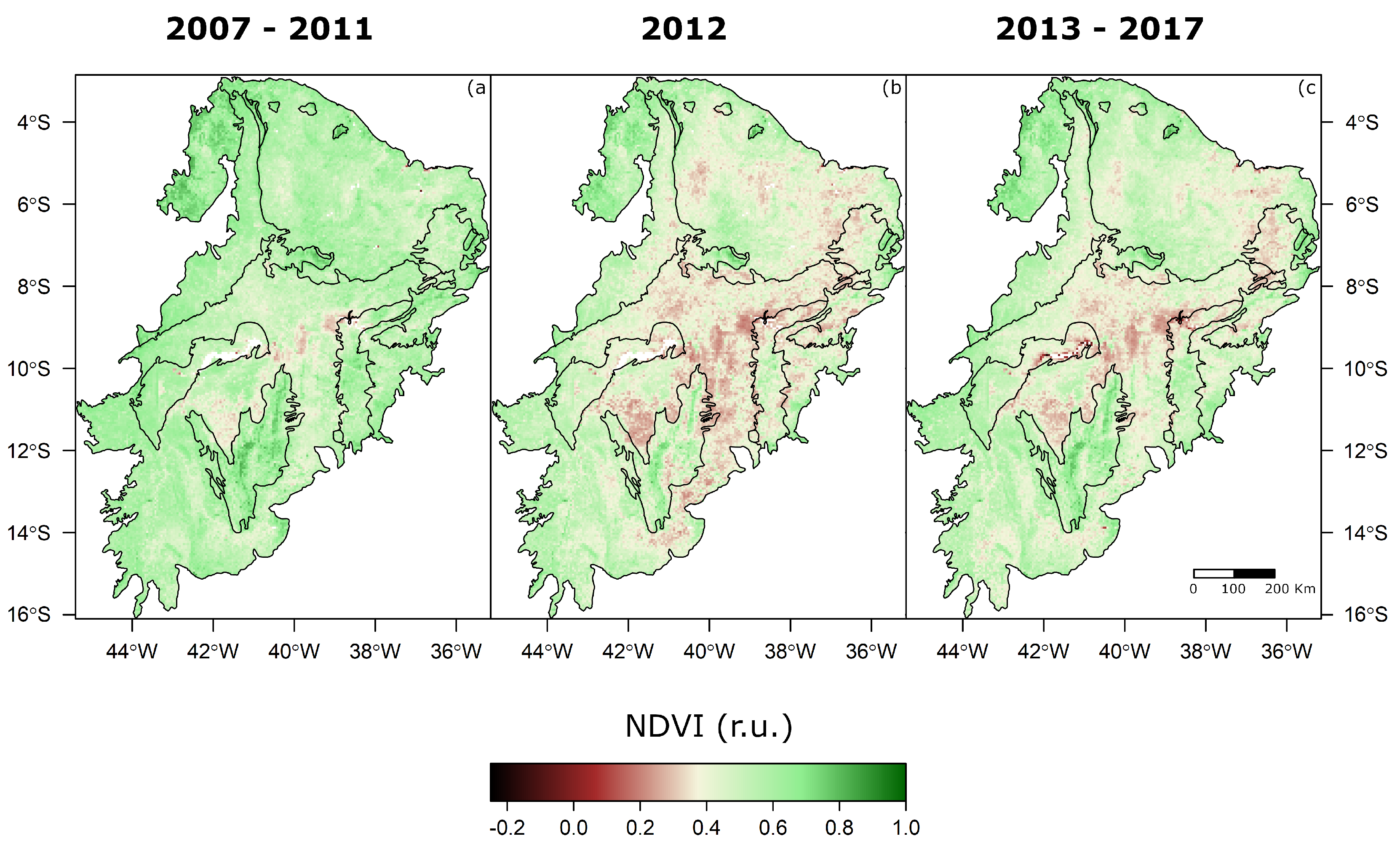
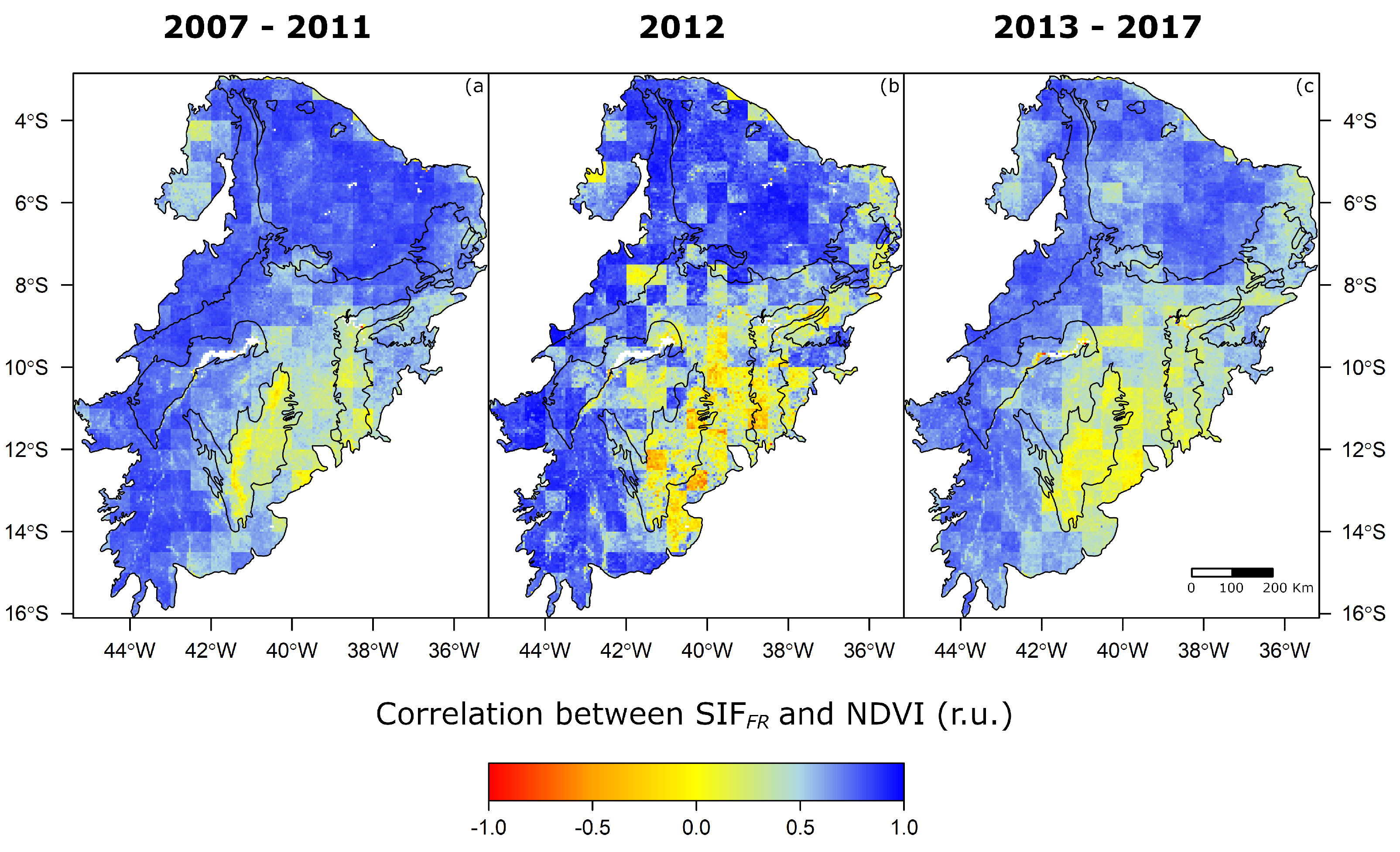
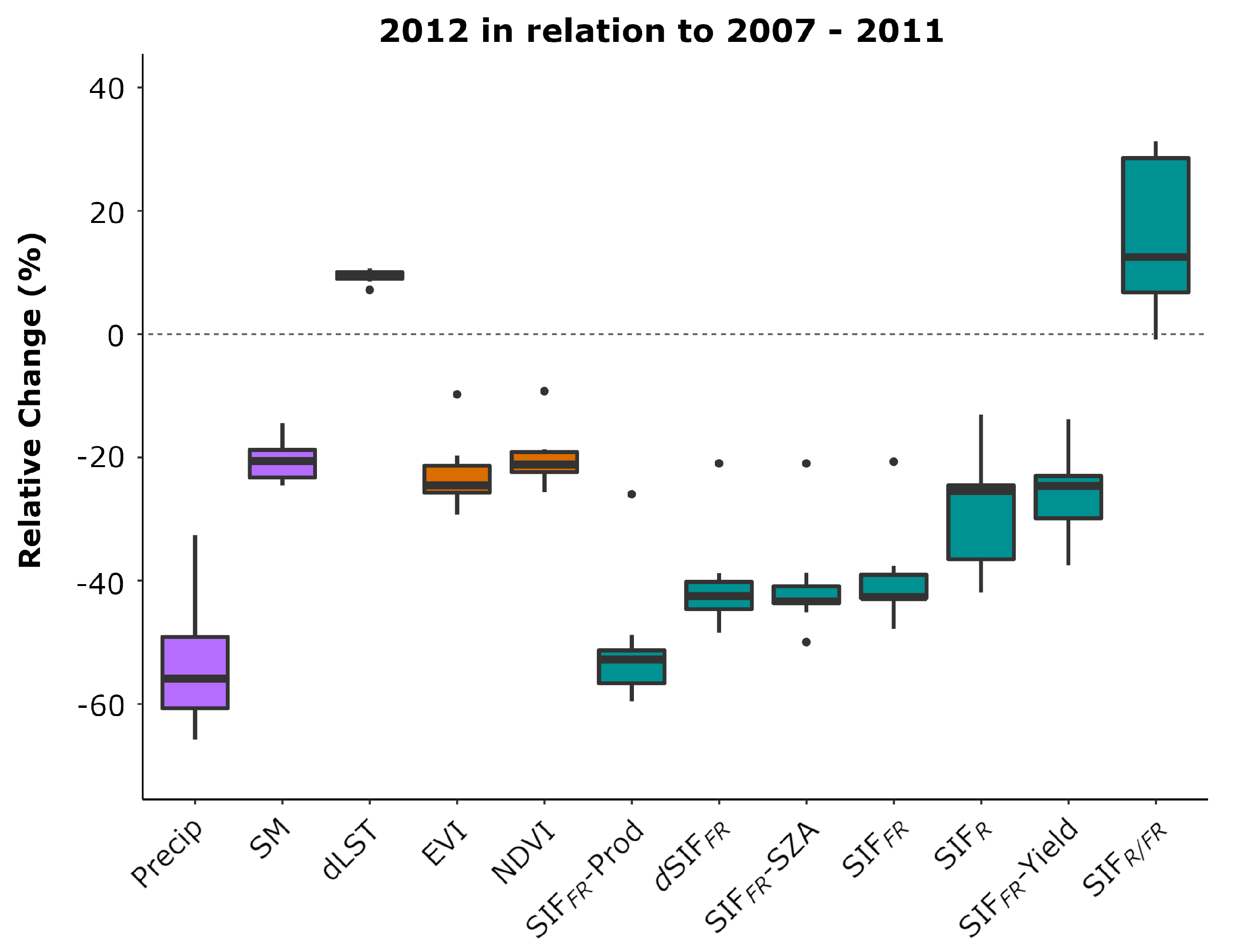
| Variable Label | Description | Data Source |
|---|---|---|
| SIF | Sun-induced chlorophyll fluorescence at the far-red wavelength peak. | GOME-2 MetOP-A + MetOP-B |
| SIF | Sun-induced chlorophyll fluorescence at the red wavelength peak. | GOME-2 MetOP-A |
| SIF | The ratio between SIF at both wavelength peaks. | GOME-2 MetOP-A |
| dSIF | Daily average of SIF based on a clear sky PAR proxy. | GOME-2 MetOP-A + MetOP-B |
| SIF-SZA | The quotient of SIF by the cosine of the Sun’s zenith angle (SZA). | GOME-2 MetOP-A + MetOP-B and MODIS |
| SIF-Yield | The quotient of SIF by NDVI—analog to “real fluorescence”. | GOME-2 MetOP-A + MetOP-B and MODIS |
| SIF-Prod | The product of SIF by NDVI—well correlated to GPP. | GOME-2 MetOP-A + MetOP-B and MODIS |
| Model | Fixed Effect | Estimate | SE | Chisq | df | p | |
|---|---|---|---|---|---|---|---|
| SIF | Temperature (T) | 0.039 | 76.887 | 1 | <0.001 | *** | |
| Soil Moisture (SM) | 0.614 | 0.032 | 395.087 | 1 | <0.001 | *** | |
| T: SM | 0.035 | 0.016 | 5.057 | 1 | 0.025 | * | |
| Ecoregion | 670.376 | 8 | <0.001 | *** | |||
| dSIF | Temperature (T) | 0.040 | 90.180 | 1 | <0.001 | *** | |
| Soil Moisture (SM) | 0.571 | 0.033 | 325.760 | 1 | <0.001 | *** | |
| T : SM | 0.015 | 5.070 | 1 | 0.0243 | * | ||
| Ecoregion | 645.610 | 8 | <0.001 | *** | |||
| SIF-Prod | Temperature (T) | 0.034 | 110.730 | 1 | <0.001 | *** | |
| Soil Moisture (SM) | 0.556 | 0.028 | 541.120 | 1 | <0.001 | *** | |
| T: SM | 0.013 | 16.290 | 1 | <0.001 | *** | ||
| Ecoregion | 1149.750 | 8 | <0.001 | *** | |||
| SIF-SZA | Temperature (T) | 0.017 | 101.921 | 1 | <0.001 | *** | |
| Soil Moisture (SM) | 0.239 | 0.014 | 325.115 | 1 | <0.001 | *** | |
| T: SM | 0.005 | 0.007 | 0.481 | 1 | 0.488 | ||
| Ecoregion | 599.259 | 8 | <0.001 | *** | |||
| SIF-Yield | Temperature (T) | 0.049 | 11.330 | 1 | 0.148 | ||
| Soil Moisture (SM) | 0.678 | 0.041 | 219.486 | 1 | <0.001 | *** | |
| T: SM | 0.150 | 0.021 | 51.682 | 1 | <0.001 | *** | |
| Ecoregion | 199.573 | 8 | <0.001 | *** | |||
| SIF | Temperature (T) | 0.057 | 21.645 | 1 | <0.001 | *** | |
| Soil Moisture (SM) | 0.542 | 0.048 | 126.721 | 1 | <0.001 | *** | |
| T: SM | 0.065 | 0.024 | 7.180 | 1 | 0.007 | * | |
| Ecoregion | 344.070 | 8 | <0.001 | *** | |||
| SIF | Temperature (T) | 0.003 | 0.068 | 0.000 | 1 | 0.985 | |
| Soil Moisture (SM) | 0.068 | 2.088 | 1 | 0.148 | |||
| T: SM | 0.004 | 0.045 | 0.009 | 1 | 0.925 | ||
| Ecoregion | 2.467 | 8 | 0.963 |
| Metric | SIF | dSIF | SIF-Prod | SIF-SZA | SIF-Yield | SIF | SIF |
|---|---|---|---|---|---|---|---|
| R (marginal) | 0.678 | 0.648 | 0.745 | 0.705 | 0.478 | 0.539 | 0.012 |
| R (conditional) | 0.867 | 0.867 | 0.905 | 0.862 | 0.735 | 0.783 | 0.035 |
| RMSE | 0.364 | 0.365 | 0.306 | 0.158 | 0.504 | 0.443 | 0.978 |
| REML | 1340 | 1366 | 982 | −588 | 2035 | 1165 | 2145 |
| BIC | 1446 | 1472 | 1088 | −482 | 2141 | 1265 | 2244 |
| Response Var. | Fixed Effect | p | ||
|---|---|---|---|---|
| SIF | Soil Moisture (SM) | 0.045 | * | |
| Temperature (T) | 0.025 | * | ||
| SM: T | 0.013 | * | ||
| SIF-Yield | Soil Moisture (SM) | 0.075 | ’ | |
| Temperature (T) | 0.119 | |||
| SM: T | 0.006 | ** |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bontempo, E.; Dalagnol, R.; Ponzoni, F.; Valeriano, D. Adjustments to SIF Aid the Interpretation of Drought Responses at the Caatinga of Northeast Brazil. Remote Sens. 2020, 12, 3264. https://doi.org/10.3390/rs12193264
Bontempo E, Dalagnol R, Ponzoni F, Valeriano D. Adjustments to SIF Aid the Interpretation of Drought Responses at the Caatinga of Northeast Brazil. Remote Sensing. 2020; 12(19):3264. https://doi.org/10.3390/rs12193264
Chicago/Turabian StyleBontempo, Edgard, Ricardo Dalagnol, Flavio Ponzoni, and Dalton Valeriano. 2020. "Adjustments to SIF Aid the Interpretation of Drought Responses at the Caatinga of Northeast Brazil" Remote Sensing 12, no. 19: 3264. https://doi.org/10.3390/rs12193264
APA StyleBontempo, E., Dalagnol, R., Ponzoni, F., & Valeriano, D. (2020). Adjustments to SIF Aid the Interpretation of Drought Responses at the Caatinga of Northeast Brazil. Remote Sensing, 12(19), 3264. https://doi.org/10.3390/rs12193264