Classification of Australian Waterbodies across a Wide Range of Optical Water Types
Abstract
1. Introduction
| Spectral Data | Classification | Dataset | Reference |
|---|---|---|---|
| SeaWiFS data | ED a Eigenvector | Northwest Atlantic | [42] |
| Normalized SeaWiFS data | ISODATA b | Global | [43] |
| In situ Rrs c | hierarchical | English Channel | [37] |
| In situ Rrs | FCM d | Global | [44] |
| In situ Rrs | FCM d | Chinese lakes | [45] |
| In situ Rrs | FCM d | Global lakes | [46] |
| In situ Rrs | thresholding | Yellow Sea | [47] |
| Normalized In situ Rrs | hierarchical | Eastern English Channel North Sea French Guiana | [33] |
| Normalized In situ Rrs | k-means | Global | [1] |
| Simulated rrs e | WFCM f | Estonia Finland | [48] |
- Develop a method to define distinct OWTs.
- Create a set of synthetic generalized inherent optical properties (GIOPs), based on the key features of each unique OWT.
- Present a case study as an example of a potential application of implementing the GIOPs water quality monitoring at a drainage basin scale.
2. Materials and Methods
2.1. In Situ Data
2.1.1. Datasets
2.1.2. Spectral Clustering
2.2. Satellite Data
- Create a mask of permanent waterbodies over the current (spatial) window by thresholding the WOfS dataset at the specified percentage limit (80%).
- Erode the water mask (by 2 pixels) and then re-expand it (by 2 pixels) in order to remove thin features connecting several main waterbodies: this allows for the selection of more than one sampling location where several waterbodies are connected by, e.g., thin river channels (would otherwise be counted as a single waterbody in the next step).
- Identify and count all the spatially distinct waterbodies in the current window. Discard any waterbody whose boundary extends beyond the edge of the window. The window size (1.0 × 1.0 degree) and overlap (0.7 degree) are selected such that these split waterbodies are ultimately processed (as a whole) in a different (overlapping) window, resulting in an unbiased selection of sampling location for these waterbodies. The window overlap is selected such that the largest waterbodies over the region of interest are properly captured.
- For each identified waterbody, erode the water mask (by 2 pixels) to remove the potential influence of nearby vegetation on the edges of the waterbody.
- Further erode the water mask (by 4 pixels) to ensure that the selected pixel is at the centre of a window of at least 9 × 9 pixels.
- For the remaining pixels, gradually erode the mask further until it cannot be eroded any more without removing all pixels. The resulting pixel(s) represent the most central location(s) for the considered waterbody. If more than one pixel remains, select the location closest to centre of gravity of the remaining pixels.
2.3. Data Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Acronym | Description |
|---|---|
| AOI | Area of Interest |
| ARD | Analysis ready data |
| DEA | Digital Earth Australia |
| ED | Euclidian Distance |
| EO | Earth Observation |
| FCM | Fuzzy-c Means |
| GIOP | Generalized Inherent Optical Properties |
| IOP | Inherent Optical Properties |
| IOSODATA | Iterative Self-Organizing Data Analysis Techniques |
| Kd | Vertical Attenuations |
| MDB | Murray-Darling Basin |
| NAP | Non-algal particulates |
| NCI | National Computing Infrastructure |
| NEC | North East Coastal |
| nSSM | normalized Spectral Similarity Metric |
| ODC | Open Data Cube |
| OWT | Optical Water Types |
| Rrs | Remote Sensing reflectance |
| rrs | Subsurface reflectance |
| SDG | Sustainable Development Goals |
| SDz | Secchi Depth |
| SEC | South East Coastal |
| SIOP | Specific Inherent Optical Properties |
| TOA | Top of Atmosphere |
| TSS | Total Suspended Solids |
| WFCM | Weighted Fuzzy-c Means |
| WOfS | Water Observations from Space |
| Parameter | Description | Unit |
|---|---|---|
| CCHL | Chlorophyll concentration (a proxy for phytoplankton) | μg L−1 |
| CDOM | Coloured dissolved organic matter | |
| CNAP | Non algal particulates concentration | mg L−1 |
| PHY | Phytoplankton | |
| a*PHY(440 nm) | Chlorophyll-a specific absorption at 440 nm | m2mg−1 |
| a*PHY(676 nm) | Chlorophyll-a specific absorption at 676 nm | m2mg−1 |
| γ aCDOM | Spectral slope constant of CDOM absorption coefficient | nm−1 |
| aCDOM(440 nm) | Absorption of CDOM at 440 nm | m−1 |
| a*NAP(440 nm) | Specific absorption of NAP at 440 nm | m2g−1 |
| γ aNAP | Spectral slope constant of NAP absorption coefficient | nm−1 |
| b*bNAP(555 nm) | Specific backscattering due to NAP at 555 nm | m2g−1 |
| γ bbNAP | Spectral slope constant of NAP backscattering coefficient | nm−1 |
| Basin | Area (km2) | Mean Rainfall (mm) | Mean Elevation (m) | Climate | Hydrogeology | Land Use |
|---|---|---|---|---|---|---|
| MDB | 1,061,000 | 458 | 260 | Range of climatic conditions: cool and humid eastern uplands; temperate southeast; subtropical northeast with monsoonal rain; hot, dry semi-arid; arid western plains. | Basinal aquifers in sedimentary deposits within the flatter landscapes; fractured rock aquifers and valley-fill alluvium in the highlands bordering the basin. | Dryland pasture, dryland and irrigated cropping, and urban land use. |
| NEC | 451,000 | 827 | 173 | Subtropical to tropical with hot, wet summers and cooler, dry winters. Monsoonal summer rainfall in the north, winter rainfall the south. | Topographically diverse terrain with high relief in coastal ranges and tablelands and coastal alluvial plains. Outcropping fractured basement rock, alluvial valley systems, and coastal sand deposits. | Native pasture, dryland and irrigated agriculture, and urban land use. |
| SEC | 129,500 | 995 | 323 | Warm temperate climate with moderate rainfall. | Outcropping fractured basement rock, alluvial valley, and coastal sand aquifers. | Nature conservation, dryland pasture, irrigated and dryland cropping, and urban land use |
References
- Spyrakos, E.; O’Donnell, R.; Hunter, P.D.; Miller, C.; Scott, M.; Simis, S.G.H.; Neil, C.; Barbosa, C.C.F.; Binding, C.E.; Bradt, S.; et al. Optical types of inland and coastal waters. Limnol. Oceanogr. 2018, 63, 846–870. [Google Scholar] [CrossRef]
- Tyler, A.N.; Hunter, P.D.; Spyrakos, E.; Groom, S.; Constantinescu, A.M.; Kitchen, J. Developments in earth observation for the assessment and monitoring of inland, transitional, coastal and shelf-sea waters. Sci. Total Environ. 2016, 572, 1307–1321. [Google Scholar] [CrossRef]
- Argent, R.M. Australia State of The Environment 2016: Inland Water, Independent Report to the Australian Government Minister for the Environment and Energy; Australian Government Department of the Environment and Energy: Canberra, Australia, 2017. [CrossRef]
- Dekker, A.G.; Hestir, E.L. Evaluating the Feasibility of Systematic Inland Water Quality Monitoring with Satellite Remote Sensing; CSIRO Water for a Healthy Country National Research Flagship: Canberra, Australia, 2012. [Google Scholar] [CrossRef]
- Malthus, T.J.; Hestir, E.L.; Dekker, A.G.; Brando, V.E. The case for a global inland water quality product. In Proceedings of the 2012 IEEE International Geoscience and Remote Sensing Symposium, Munich, Germany, 22–27 July 2012; pp. 5234–5237. [Google Scholar] [CrossRef]
- Matthews, M.W. A current review of empirical procedures of remote sensing in inland and near-coastal transitional waters. Int. J. Remote Sens. 2010, 32, 6855–6899. [Google Scholar] [CrossRef]
- Bonansea, M.; Rodriguez, M.C.; Pinotti, L.; Ferrero, S. Using multi-temporal Landsat imagery and linear mixed models for assessing water quality parameters in Rio Tercero reservoir (Aargentina). Remote Sens. Environ. 2015, 158, 28–41. [Google Scholar] [CrossRef]
- Torbick, N.; Hession, S.; Hagen, S.; Wiangwang, N.; Becker, B.; Qi, J.G. Mapping inland lake water quality across the lower peninsula of Michigan using Landsat TM imagery. Int. J. Remote Sens. 2013, 34, 7607–7624. [Google Scholar] [CrossRef]
- Brezonik, P.; Menken, K.D.; Bauer, M. Landsat-based remote sensing of lake water quality characteristics, including chlorophyll and colored dissolved organic matter (CDOM). Lake Reserv. Manag. 2005, 21, 373–382. [Google Scholar] [CrossRef]
- Dekker, A.G.; Vos, R.J.; Peters, S.W.M. Analytical algorithms for lake water TSM estimation for retrospective analyses of TM and SPOT sensor data. Int. J. Remote Sens. 2002, 23, 15–35. [Google Scholar] [CrossRef]
- Lymburner, L.; Botha, E.; Hestir, E.; Anstee, J.; Sagar, S.; Dekker, A.; Malthus, T. Landsat 8: Providing continuity and increased precision for measuring multi-decadal time series of total suspended matter. Remote Sens. Environ. 2016, 185, 108–118. [Google Scholar] [CrossRef]
- Hellweger, F.L.; Schlosser, P.; Lall, U.; Weissel, J.K. Use of satellite imagery for water quality studies in New York harbor. Estuar. Coast. Shelf Sci. 2004, 61, 437–448. [Google Scholar] [CrossRef]
- Hadjimitsis, D.G.; Hadjimitsis, M.G.; Clayton, C.; Clarke, B.A. Determination of turbidity in Kourris dam in Cyprus utilizing Landsat TM remotely sensed data. Water Resour. Manag. 2006, 20, 449–465. [Google Scholar] [CrossRef]
- Lehmann, M.K.; Nguyen, U.; Allan, M.; van der Woerd, H.J. Colour classification of 1486 lakes across a wide range of optical water types. Remote Sens. 2018, 10, 1273. [Google Scholar] [CrossRef]
- Van der Woerd, H.J.; Wernand, M.R. True colour classification of natural waters with medium-spectral resolution satellites: SeaWIFS, MODIS, MERIS and OLCI. Sensors 2015, 15, 25663–25680. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Lee, Z.; Shang, S.; Li, J.; Zhang, B.; Lin, G. Deriving inherent optical properties from classical water color measurements: Forel-Ule index and secchi disk depth. Opt. Express 2019, 27, 7642–7655. [Google Scholar] [CrossRef]
- Bugnot, A.B.; Lyons, M.B.; Scanes, P.; Clark, G.F.; Fyfe, S.K.; Lewis, A.; Johnston, E.L. A novel framework for the use of remote sensing for monitoring catchments at continental scales. J. Environ. Manag. 2018, 217, 939–950. [Google Scholar] [CrossRef]
- Heege, T.; Kiselev, V.; Wettle, M.; Hung, N.N. Operational multi-sensor monitoring of turbidity for the entire Mekong delta. Int. J. Remote Sens. 2014, 35, 2910–2926. [Google Scholar] [CrossRef]
- Olmanson, L.G.; Bauer, M.E.; Brezonik, P.L. A 20-year Landsat water clarity census of Minnesota’s 10,000 lakes. Remote Sens. Environ. 2008, 112, 4086–4097. [Google Scholar] [CrossRef]
- Lobo, F.L.; Costa, M.P.F.; Novo, E. Time-series analysis of Landsat-MSS/TM/OLI images over Amazonian waters impacted by gold mining activities. Remote Sens. Environ. 2015, 157, 170–184. [Google Scholar] [CrossRef]
- Bowling, L.; Baldwin, D.; Merrick, C.; Brayan, J.; Panther, J. Possible drivers of a chrysosporum ovalisporum bloom in the Murray River, Australia, in 2016. Mar. Freshw. Res. 2018, 69, 1649–1662. [Google Scholar] [CrossRef]
- Siegel, D.A.; Maritorena, S.; Nelson, N.B.; Behrenfeld, M.J.; McClain, C.R. Colored dissolved organic matter and its influence on the satellite-based characterization of the ocean biosphere. Geophys. Res. Lett. 2005, 32. [Google Scholar] [CrossRef]
- Dierssen, H.M.; Smith, R.C. Bio-optical properties and remote sensing ocean color algorithms for Antarctic peninsula waters. J. Geophys. Res. Ocean. 2000, 105, 26301–26312. [Google Scholar] [CrossRef]
- Otero, M.P.; Siegel, D.A. Spatial and temporal characteristics of sediment plumes and phytoplankton blooms in the Santa Barbara channel. Deep-Sea Res. Part II Top. Stud. Oceanogr. 2004, 51, 1129–1149. [Google Scholar] [CrossRef]
- Feng, H.; Campbell, J.W.; Dowell, M.D.; Moore, T.S. Modeling spectral reflectance of optically complex waters using bio-optical measurements from Tokyo bay. Remote Sens. Environ. 2005, 99, 232–243. [Google Scholar] [CrossRef]
- Santini, F.; Alberotanza, L.; Cavalli, R.M.; Pignatti, S. A two-step optimization procedure for assessing water constituent concentrations by hyperspectral remote sensing techniques: An application to the highly turbid Venice lagoon waters. Remote Sens. Environ. 2010, 114, 887–898. [Google Scholar] [CrossRef]
- Heege, T.F.J. Mapping of water constituents in Lake Constance using multispectral airborne scanner data and a physically based processing scheme. Can. J. Remote Sens. 2004, 30, 77–86. [Google Scholar] [CrossRef]
- Lee, Z.; Carder, K.L.; Arnone, R.A. Deriving inherent optical properties from water color: A multiband quasi-analytical algorithm for optically deep waters. Appl. Opt. 2002, 41, 5755–5772. [Google Scholar] [CrossRef]
- Brando, V.E.; Anstee, J.M.; Wettle, M.; Dekker, A.G.; Phinn, S.R.; Roelfsema, C. A physics based retrieval and quality assessment of bathymetry from suboptimal hyperspectral data. Remote Sens. Environ. 2009, 113, 755–770. [Google Scholar] [CrossRef]
- Brando, V.E.; Dekker, A.G.; Park, Y.J.; Schroeder, T. Adaptive semianalytical inversion of ocean color radiometry in optically complex waters. Appl. Opt. 2012, 51, 2808–2833. [Google Scholar] [CrossRef]
- Melin, F.; Vantrepotte, V.; Clerici, M.; D’Alimonte, D.; Zibordi, G.; Berthon, J.F.; Canuti, E. Multi-sensor satellite time series of optical properties and chlorophyll-a concentration in the Adriatic Sea. Prog. Oceanogr. 2011, 91, 229–244. [Google Scholar] [CrossRef]
- Shanmugam, P. A new bio-optical algorithm for the remote sensing of algal blooms in complex ocean waters. J. Geophys. Res. Ocean. 2011, 116, 12. [Google Scholar] [CrossRef]
- Vantrepotte, V.; Loisel, H.; Dessailly, D.; Meriaux, X. Optical classification of contrasted coastal waters. Remote Sens. Environ. 2012, 123, 306–323. [Google Scholar] [CrossRef]
- McKee, D.; Cunningham, A. Identification and characterisation of two optical water types in the Irish Sea from in situ inherent optical properties and seawater constituents. Estuar. Coast. Shelf Sci. 2006, 68, 305–316. [Google Scholar] [CrossRef]
- Spyrakos, E.; Vilas, L.G.; Palenzuela, J.M.T.; Barton, E.D. Remote sensing chlorophyll a of optically complex waters (Rias Baixas, NW Spain): Application of a regionally specific chlorophyll a algorithm for MERIS full resolution data during an upwelling cycle. Remote Sens. Environ. 2011, 115, 2471–2485. [Google Scholar] [CrossRef]
- Dzwonkowski, B.; Yan, X.H. Development and application of a neural network based ocean colour algorithm in coastal waters. Int. J. Remote Sens. 2005, 26, 1175–1200. [Google Scholar] [CrossRef]
- Lubac, B.; Loisel, H. Variability and classification of remote sensing reflectance spectra in the eastern English Channel and southern North Sea. Remote Sens. Environ. 2007, 110, 45–58. [Google Scholar] [CrossRef]
- IOCCG. Remote Sensing of Ocean Colour in Coastal, and Other Optically-Complex, Waters; IOCCG: Dartmouth, Canada, 2000. [Google Scholar] [CrossRef]
- Morel, A.; Prieur, L. Analysis of variations in ocean color. Limnol. Oceanogr. 1977, 22, 709–722. [Google Scholar] [CrossRef]
- Zibordi, G.; Holben, B.; Slutsker, I.; Giles, D.; D’Alimonte, D.; Melin, F.; Berthon, J.F.; Vandemark, D.; Feng, H.; Schuster, G.; et al. AERONET-OC: A network for the validation of ocean color primary products. J. Atmos. Ocean. Technol. 2009, 26, 1634–1651. [Google Scholar] [CrossRef]
- Zibordi, G.; Berthon, J.F.; Melin, F.; D’Alimonte, D. Cross-site consistent in situ measurements for satellite ocean color applications: The bioMap radiometric dataset. Remote Sens. Environ. 2011, 115, 2104–2115. [Google Scholar] [CrossRef]
- Traykovski, L.V.M.; Sosik, H.M. Feature-based classification of optical water types in the northwest Atlantic based on satellite ocean color data. J. Geophys. Res. Ocean. 2003, 108, 18. [Google Scholar] [CrossRef]
- Melin, F.; Vantrepotte, V. How optically diverse is the coastal ocean? Remote Sens. Environ. 2015, 160, 235–251. [Google Scholar] [CrossRef]
- Moore, T.S.; Dowell, M.D.; Bradt, S.; Verdu, A.R. An optical water type framework for selecting and blending retrievals from bio-optical algorithms in lakes and coastal waters. Remote Sens. Environ. 2014, 143, 97–111. [Google Scholar] [CrossRef]
- Shen, Q.; Li, J.S.; Zhang, F.F.; Sun, X.; Li, J.; Li, W.; Zhang, B. Classification of several optically complex waters in China using in situ remote sensing reflectance. Remote Sens. 2015, 7, 14731–14756. [Google Scholar] [CrossRef]
- Eleveld, M.A.; Ruescas, A.B.; Hommersom, A.; Moore, T.S.; Peters, S.W.M.; Brockmann, C. An optical classification tool for global lake waters. Remote Sens. 2017, 9, 24. [Google Scholar] [CrossRef]
- Ye, H.P.; Li, J.S.; Li, T.J.; Shen, Q.; Zhu, J.H.; Wang, X.Y.; Zhang, F.F.; Zhang, J.; Zhang, B. Spectral classification of the Yellow Sea and implications for coastal ocean color remote sensing. Remote Sens. 2016, 8, 23. [Google Scholar] [CrossRef]
- Reinart, A.; Herlevi, A.; Arst, H.; Sipelgas, L. Preliminary optical classification of lakes and coastal waters in Estonia and south Finland. J. Sea Res. 2003, 49, 357–366. [Google Scholar] [CrossRef]
- Blondeau-Patissier, D.; Brando, V.; Oubelkheir, K.; Dekker, A.; Clementson, L.; Daniel, P. Bio-optical variability of the absorption and scattering properties of the Queensland inshore and reef waters, Australia. J. Geophys. Res. Ocean. 2009, 114, 24. [Google Scholar] [CrossRef]
- Brando, V.E.; Blondeau-Patissier, D.; Dekker, A.G.; Daniel, P.J.; Wettle, M.; Oubelkheir, K.; Clementson, L. Bio-optical variability of Queensland coastal waters for parameterisation of coastal-reef algorithms. In Proceedings of the Ocean Optics XVIII, ONR-NASA, Montreal, QC, Canada, 9–13 October 2006. [Google Scholar] [CrossRef]
- Cherukuru, N.; Brando, V.; Schroeder, T.; Clementson, L.; Dekker, A. Influence of river discharge and ocean currents on coastal optical properties. Cont. Shelf Res. 2014, 84, 188–203. [Google Scholar] [CrossRef]
- Thompson, P.; Bonham, P.; Waite, A.; Clementson, L.; Cherukuru, N.; Hassler, C.; Doblin, M. Contrasting oceanographic conditions and phytoplankton communities on the east and west coasts of Australia. Deep-Sea Res. Part II Top. Stud. Oceanogr. 2011, 58, 645–663. [Google Scholar] [CrossRef]
- Hestir, E.L.; Brando, V.; Campbell, G.; Dekker, A.; Malthus, T. The relationship between dissolved organic matter absorption and dissolved organic carbon in reservoirs along a temperate to tropical gradient. Remote Sens. Environ. 2015, 156, 395–402. [Google Scholar] [CrossRef]
- Clementson, L.A.; Parslow, J.S.; Turnbull, A.R.; McKenzie, D.C.; Rathbone, C.E. Optical properties of waters in the Australasian sector of the Southern Ocean. J. Geophys. Res. 2001, 106, 31611–31625. [Google Scholar] [CrossRef]
- Brando, V.E.; Dekker, A.G. Satellite hyperspectral remote sensing for estimating estuarine and coastal water quality. IEEE Trans.Geosci. Remote Sens. 2003, 41, 1378–1387. [Google Scholar] [CrossRef]
- Botha, E.J.; Brando, V.E.; Dekker, A.G. Effects of per-pixel variability on uncertainties in bathymetric retrievals from high-resolution satellite images. Remote Sens. 2016, 8, 18. [Google Scholar] [CrossRef]
- Ruddick, K.G.; Voss, K.; Boss, E.; Castagna, A.; Frouin, R.; Gilerson, A.; Hieronymi, M.; Johnson, B.C.; Kuusk, J.; Lee, Z.; et al. A review of protocols for fiducial reference measurements of water-leaving radiance for validation of satellite remote-sensing data over water. Remote Sens. 2019, 11, 2198. [Google Scholar] [CrossRef]
- Torrecilla, E.; Stramski, D.; Reynolds, R.A.; Millan-Nunez, E.; Piera, J. Cluster analysis of hyperspectral optical data for discriminating phytoplankton pigment assemblages in the open ocean. Remote Sens. Environ. 2011, 115, 2578–2593. [Google Scholar] [CrossRef]
- Mobley, C.D. Hydrolight 3.0 Users’ Guide—Final Report—March 1995; SRI Project 5632, Contract N00014-94-C-0062; SRI International: Menlo Park, CA, USA, 1995. [Google Scholar]
- Mobley, C.D. Light and Water; Radiative Transfer in Natural Waters; Academic Press: London, UK, 1994. [Google Scholar]
- Jain, A.K.; Murty, M.N.; Flynn, P.J. Data clustering: A review. ACM Comput. Surv. 1999, 31, 264–323. [Google Scholar] [CrossRef]
- Ward, J.H. Hierarchical grouping to optimize an objective function. J. Am. Stat. Assoc. 1963, 58, 236–244. [Google Scholar] [CrossRef]
- Murtagh, F.; Contreras, P. Algorithms for hierarchical clustering: An overview, II. Wiley Interdiscip. Rev. Data Min. Knowl. Discov. 2017, 7, 16. [Google Scholar] [CrossRef]
- Benjamini, Y.; Braun, H.; John, W. Tukey’s contributions to multiple comparisons. Ann. Stat. 2002, 30, 1576–1594. [Google Scholar] [CrossRef]
- Wilson, C.O. Land use/land cover water quality nexus: Quantifying anthropogenic influences on surface water quality. Environ. Monit. Assess. 2015, 187. [Google Scholar] [CrossRef]
- Killough, B. Overview of the Open Data Cube Initiative. In 2018 IEEE International Geoscience and Remote Sensing Symposium; IEEE: New York, NY, USA, 2018; pp. 8629–8632. [Google Scholar] [CrossRef]
- Loveland, T.R.; Dwyer, J.L. Landsat: Building a strong future. Remote Sens. Environ. 2012, 122, 22–29. [Google Scholar] [CrossRef]
- Lewis, A.; Oliver, S.; Lymburner, L.; Evans, B.; Wyborn, L.; Mueller, N.; Raevski, G.; Hooke, J.; Woodcock, R.; Sixsmith, J.; et al. The Australian Geoscience Data Cube—Foundations and lessons learned. Remote Sens. Environ. 2017, 202, 276–292. [Google Scholar] [CrossRef]
- Li, F.Q.; Jupp, D.L.B.; Reddy, S.; Lymburner, L.; Mueller, N.; Tan, P.; Islam, A. An evaluation of the use of atmospheric and BRDF correction to standardize Landsat data. IEEE J. Sel. Toppics Appl. Earth Obs. Remote Sens. 2010, 3, 257–270. [Google Scholar] [CrossRef]
- Sixsmith, J.; Oliver, S.; Lymburner, L. IEEE A hybrid approach to automated Landsat pixel quality. In 2013 IEEE International Geoscience and Remote Sensing Symposium; IEEE: New York, NY, USA, 2013; pp. 4146–4149. [Google Scholar] [CrossRef]
- Irish, R.R.; Barker, J.L.; Goward, S.N.; Arvidson, T. Characterization of the Landsat-7 ETM+ automated cloud-cover assessment (ACCA) algorithm. Photogramm. Eng. Remote Sens. 2006, 72, 1179–1188. [Google Scholar] [CrossRef]
- Zhu, Z.; Woodcock, C.E. Object-based cloud and cloud shadow detection in Landsat imagery. Remote Sens. Environ. 2012, 118, 83–94. [Google Scholar] [CrossRef]
- Schroeder, T. Aquatic Atmospheric Correction—Aerosol Ancillary Data and Product Validation, Data Analysis Report, Prepared for Geoscience Australia; EP194931; CSIRO Oceans and Atmosphere: Canberra, Australia, 2019; p. 71. [Google Scholar]
- Mueller, N.; Lewis, A.; Roberts, D.; Ring, S.; Melrose, R.; Sixsmith, J.; Lymburner, L.; McIntyre, A.; Tan, P.; Curnow, S.; et al. Water observations from space: Mapping surface water from 25 years of Landsat imagery across Australia. Remote Sens. Environ. 2016, 174, 341–352. [Google Scholar] [CrossRef]
- Vanhellemont, Q.; Ruddick, K. Advantages of high quality SWIR bands for ocean colour processing: Examples from Landsat-8. Remote Sens. Environ. 2015, 161, 89–106. [Google Scholar] [CrossRef]
- Dyall, A.; Heap, A.D.; Tobin, G.; Bryce, S.; Ryan, D.A.; Galinec, V.; Creasey, J.; Gallagher, J.; Radke, L.; Smith, C.; et al. Australian Coastal Waterways Geomorphic Habitat Mapping (National Aggregated Product); Geoscience Australia: Canberra, Australia, 2005. Available online: http://metadata.imas.utas.edu.au/geonetwork/srv/en/metadata.show?uuid=9b403526-e386-47ef-8a78-be1ae179419d (accessed on 8 September 2019).
- Geoscience Australia. Geodata Topo 2.5 m 2003, 3rd ed.; Geoscience Australia: Canberra, Australia, 2003. Available online: http://pid.geoscience.gov.au/dataset/ga/60804 (accessed on 8 September 2019).
- Botha, E.J.; Brando, V.; Dekker, A.; Anstee, J.M.; Sagar, S. Increased spectral resolution enhances coral detection under varying water conditions. Remote Sens. Environ. 2013, 131, 247–261. [Google Scholar] [CrossRef]
- Babin, M.; Stramski, D.; Ferrari, G.M.; Claustre, H.; Bricaud, A.; Obolensky, G.; Hoepffner, N. Variations in the light absorption coefficients of phytoplankton, nonalgal particles, and dissolved organic matter in coastal waters around Europe. J. Geophys. Res. 2003, 108. [Google Scholar] [CrossRef]
- Slade, W.H.; Boss, E. Spectral attenuation and backscattering as indicators of average particle size. Appl. Opt. 2015, 54, 7264–7277. [Google Scholar] [CrossRef]
- Zanardi-Lamardo, E.; Moore, C.; Zika, R. Seasonal variation in molecular mass and optical properties of chromophoric dissolved organic material in coastal waters of southwest Florida. Mar. Chem. 2004, 89, 37–54. [Google Scholar] [CrossRef]
- Shi, K.; Li, Y.M.; Li, L.; Lu, H. Absorption characteristics of optically complex inland waters: Implications for water optical classification. J. Geophys. Res. Biogeosci. 2013, 118, 860–874. [Google Scholar] [CrossRef]
- Australian Bureau of Meteorology. Special Climate Statement 70—Drought Conditions in Australia and Impact on Water Resources in the Murray–Darling Basin; Australian Bureau of Meteorology: Melbourne, Australia, 2019. Available online: http://www.bom.gov.au/climate/current/statements/scs70.pdf (accessed on 12 September 2019).
- Australian Bureau of Meteorology. Australian Water Resources Assessment 2010; Bureau of Meteorology: Melbourne, Australia, 2011. Available online: http://www.bom.gov.au/water/awra/2010/documents/assessment-low.pdf on (accessed on 12 September 2019).
- Lewis, A.; Lymburner, L.; Purss, M.B.J.; Brooke, B.; Evans, B.; Ip, A.; Dekker, A.G.; Irons, J.R.; Minchin, S.; Mueller, N.; et al. Rapid, high-resolution detection of environmental change over continental scales from satellite data—The earth observation data cube. Int. J. Digit. Earth 2016, 9, 106–111. [Google Scholar] [CrossRef]
- Soomets, T.; Uudeberg, K.; Jakovels; Zagars, M.; Reinart, A.; Brauns, A.; Kutser, T. Comparison of lake optical water types derived from Sentinel-2 and Sentinel-3. Remote Sens. 2019, 11, 2883. [Google Scholar] [CrossRef]
- Schaeffer, B.; Greb, S.; Binding, C.; Urquhart, E.; Stumpf, R.; Tyler, A.; DiGiacomo, P.; Wang, M.; Odermatt, D.; Spyrakos, E.; et al. IOCCG Report: Earth Observations in Support of Global Water Quality Monitoring, 17th ed.; World Health Organization (WHO): Geneva, Switserland, 2018; p. 125. [Google Scholar] [CrossRef]
- Dekker, A.; Pinnel, N.; Gege, P.; Briottet, X.; Court, A.; Peters, S.; Turpie, K.; Sterckx, S.; Costa, M.; Giardino, C.; et al. Feasibility Study for an Aquatic Ecosystem Earth Observing System; EP183408; CSIRO for CEOS: Canberra, Australia, 2018. [Google Scholar]
- Shanmugam, S.; SrinivasaPerumal, P. Spectral matching approaches in hyperspectral image processing. Int. J. Remote Sens. 2014, 35, 8217–8251. [Google Scholar] [CrossRef]
- Botha, E.J.; Anstee, J.; Galvao Medeiros, T.A. DEA Final Report. Activity 2: Collation, Quality Assessment and Regionalisation of Ancillary Aquatic Data. Report to Geoscience Australia; EP19844; CSIRO: Canberra, Australia, 2019. [Google Scholar]
- Botha, E.J.; Anstee, J.M.; Lehmann, E. IWQual: Inland Water Quality, Aquatic Reflectance and bio-Optics Acquisition for Validation of AUSTRALIAN Inland Waters, Gap Analysis Report; EP197019; CSIRO Oceans and Atmosphere: Canberra, Australia, 2019; p. 57. [Google Scholar]




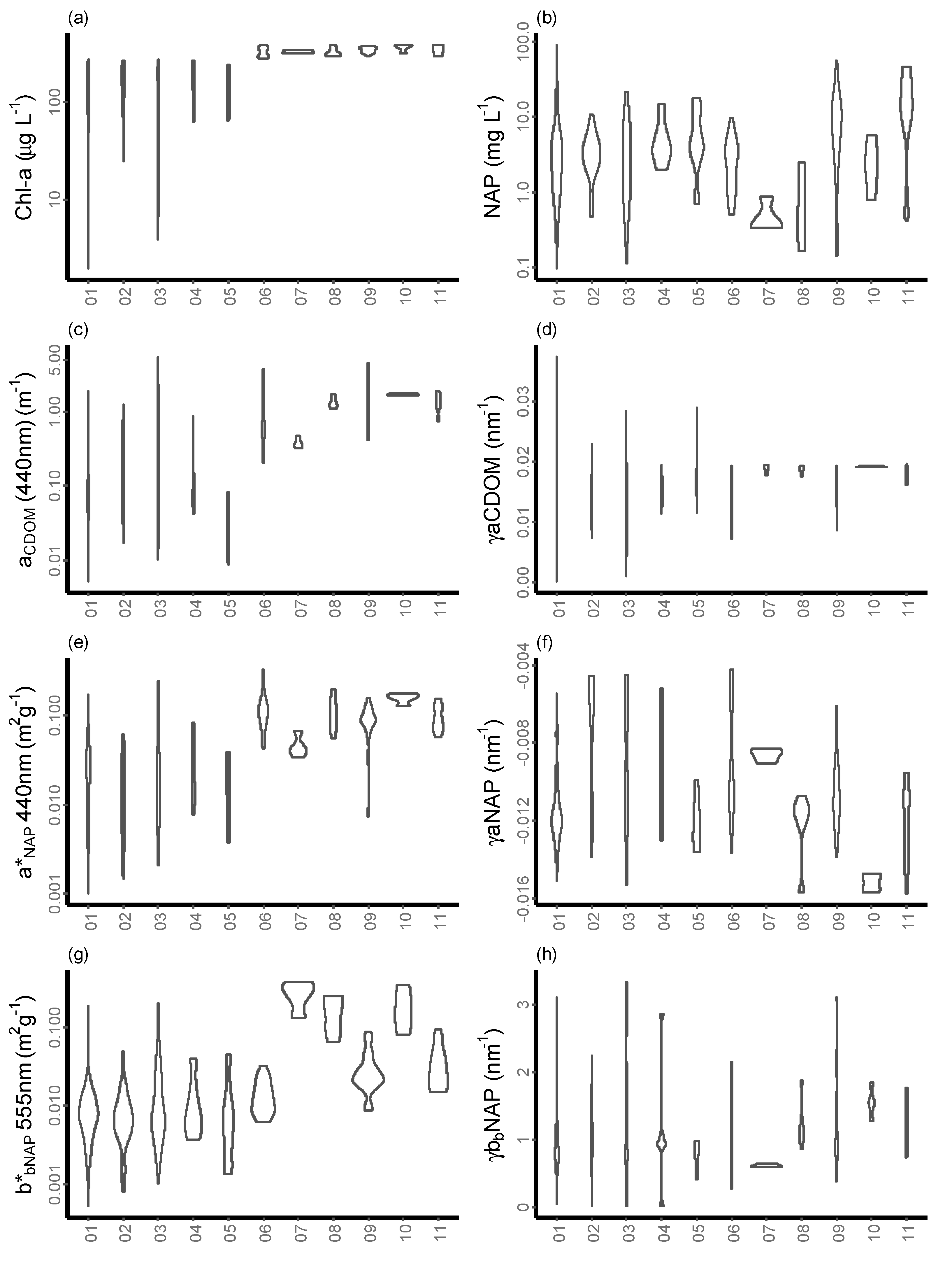
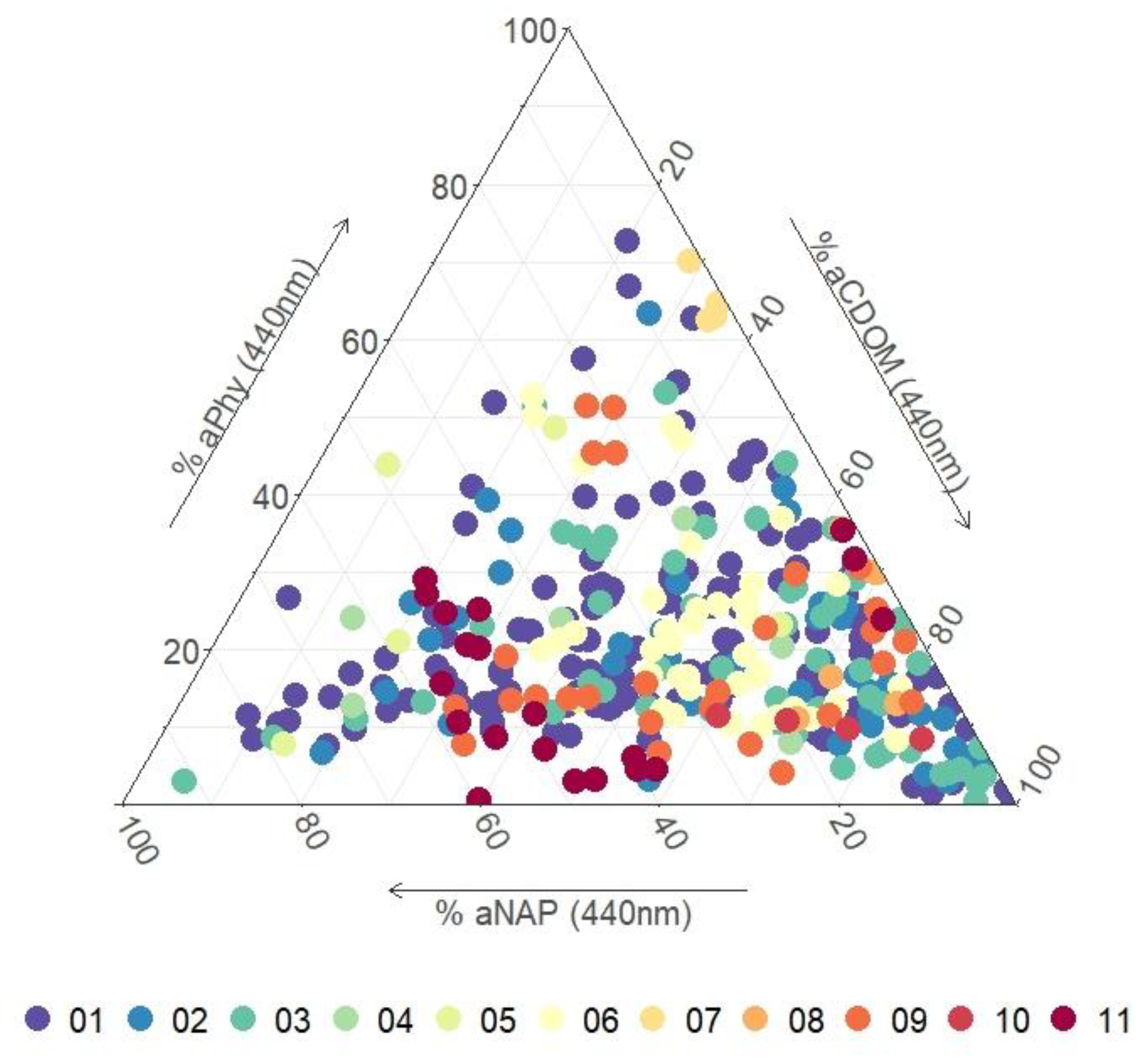
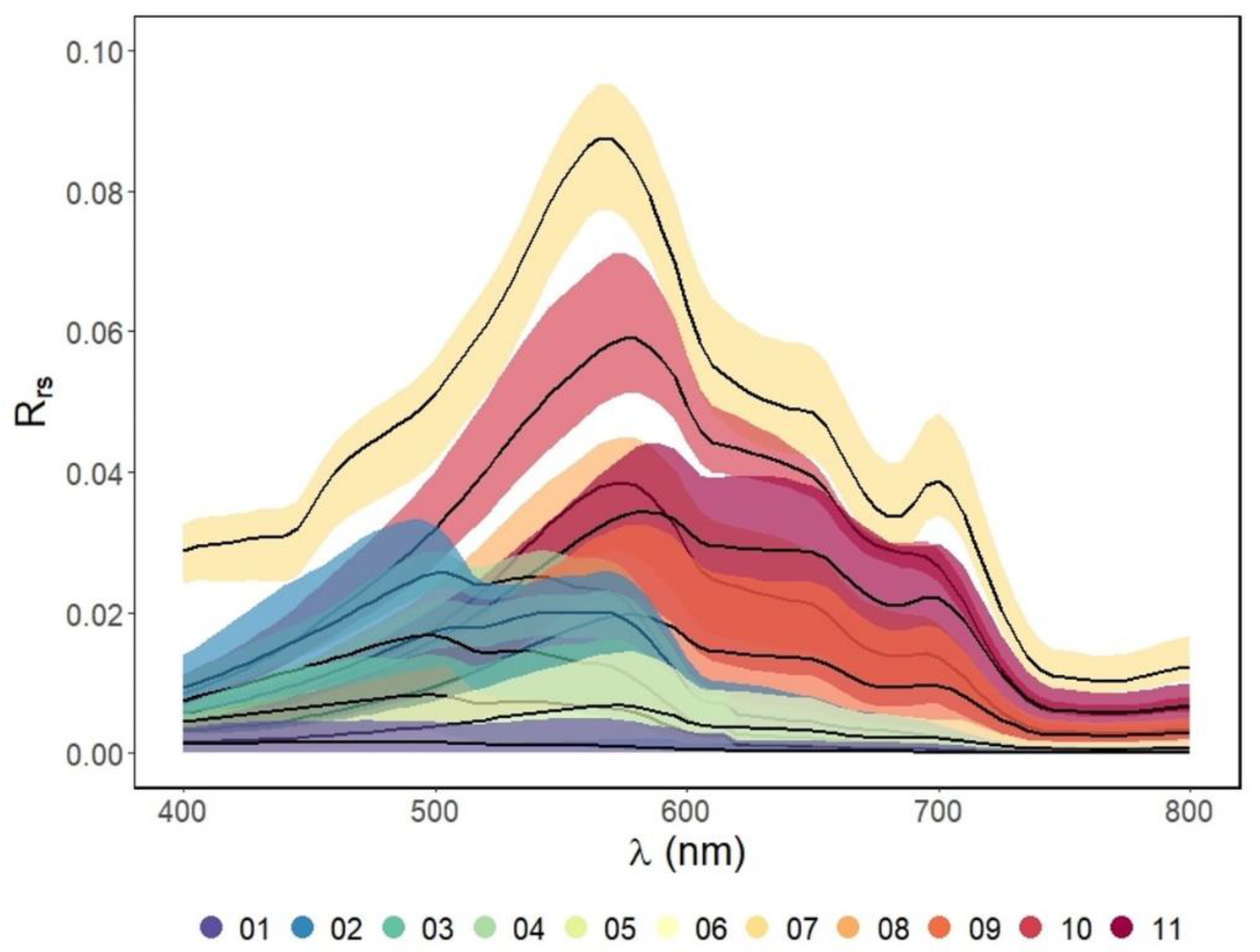

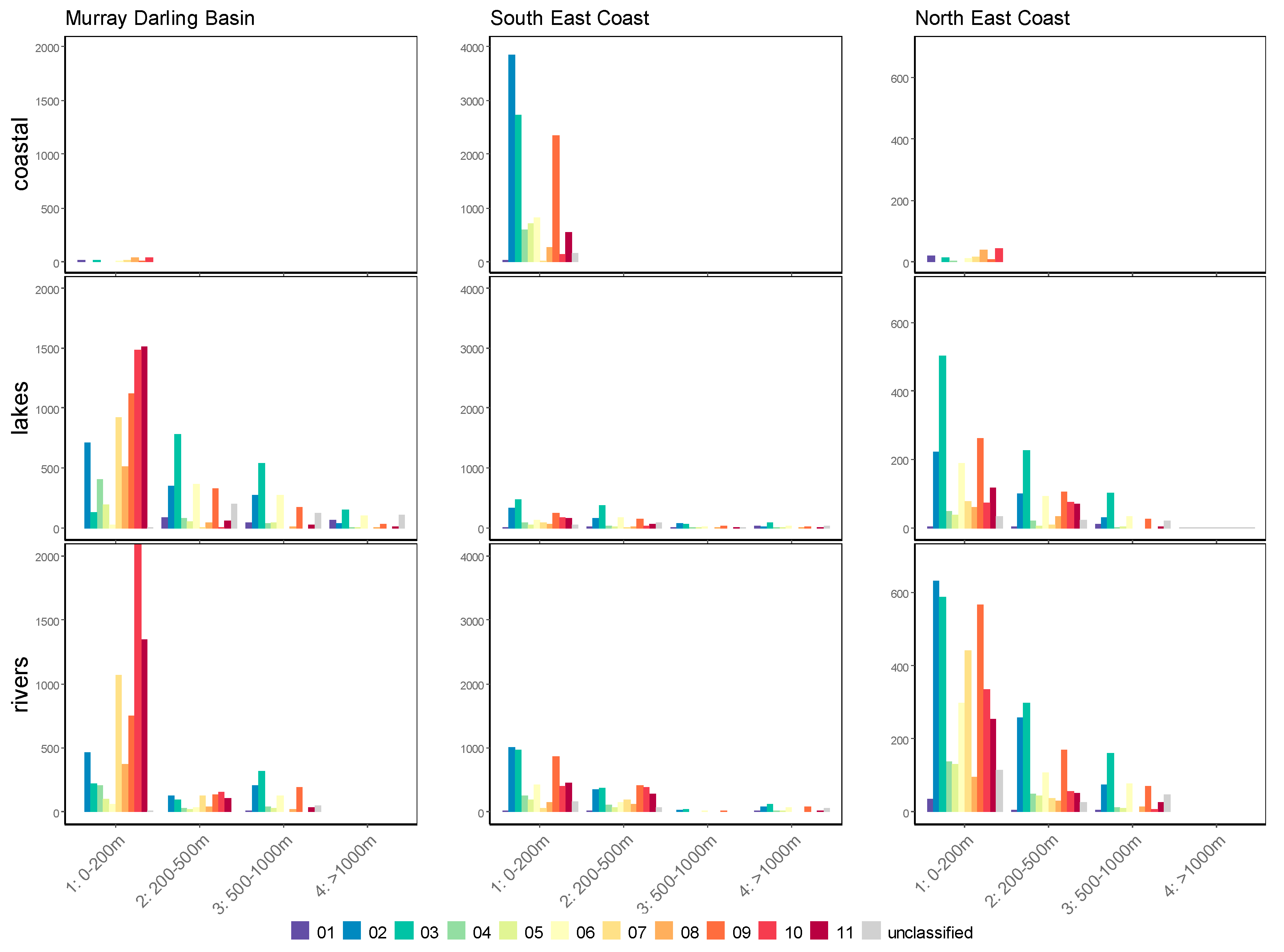
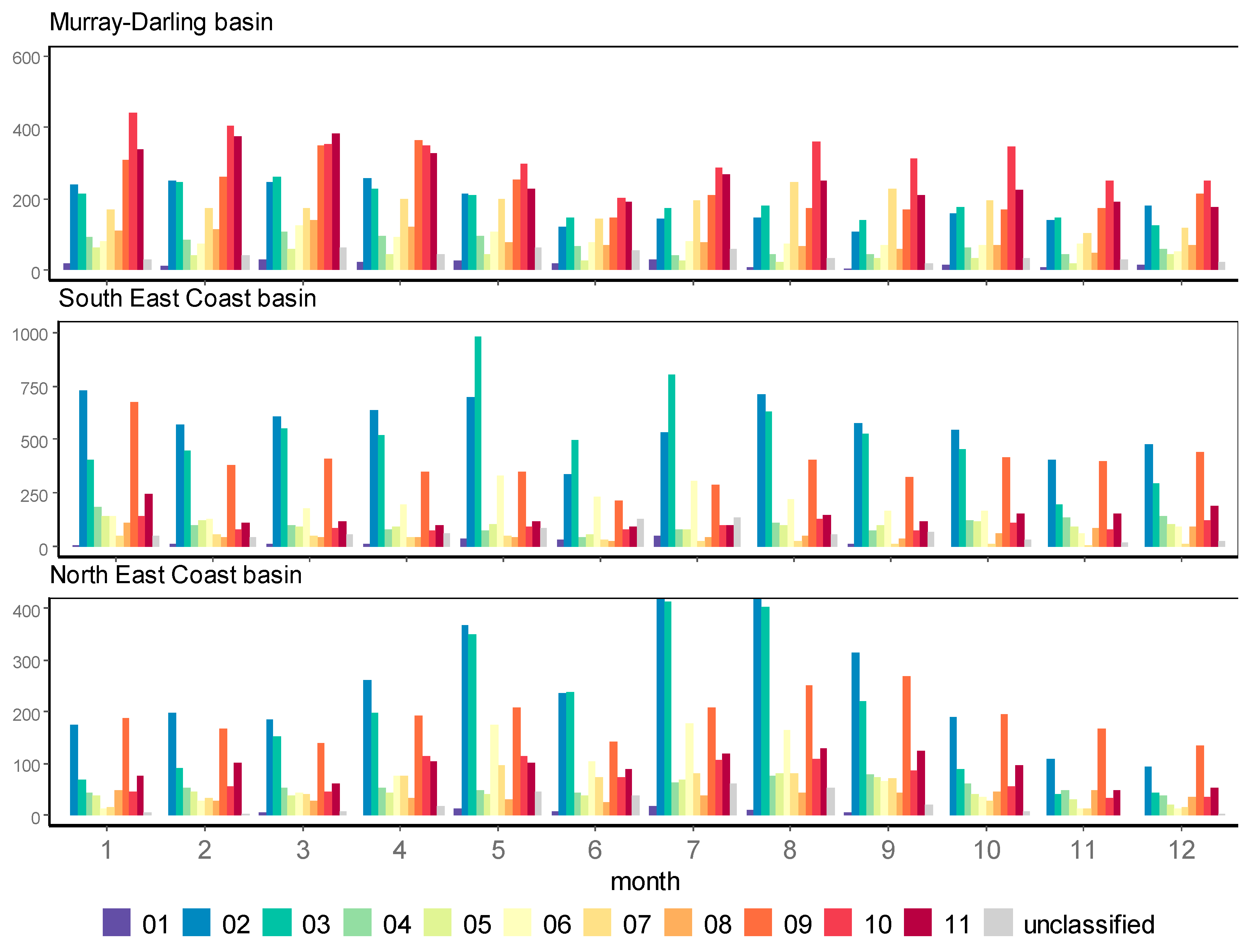
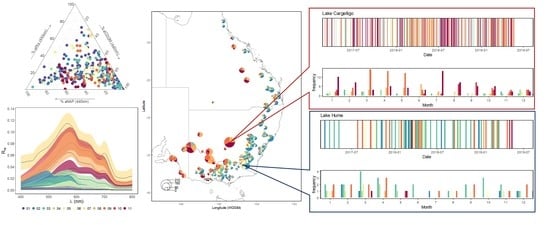
| Max | Min | Mean | Median | SD | |
|---|---|---|---|---|---|
| CCHL | 12.78 | 0.03 | 0.86 | 0.48 | 1.50 |
| 603.60 | 0.92 | 25.98 | 9.87 | 67.26 | |
| CNAP | 90.85 | 0.10 | 4.57 | 2.69 | 7.17 |
| 82.98 | 0.51 | 9.52 | 4.08 | 14.14 | |
| a*PHY(440 nm) | 0.4838 | 0.0192 | 0.0875 | 0.0728 | 0.0598 |
| 0.1953 | 0.0023 | 0.0365 | 0.0331 | 0.0249 | |
| a*PHY(676 nm) | 0.1907 | 0.0072 | 0.0293 | 0.0260 | 0.0148 |
| 0.1286 | 0.0013 | 0.0203 | 0.0190 | 0.0135 | |
| γ aCDOM | 0.0373 | 0.0002 | 0.0143 | 0.0147 | 0.0045 |
| 0.0211 | 0.0073 | 0.0170 | 0.0178 | 0.0026 | |
| aCDOM(440 nm) | 5.3877 | 0.0053 | 0.2452 | 0.0851 | 0.5299 |
| 4.4714 | 0.2023 | 1.0740 | 0.8383 | 0.7586 | |
| a*NAP(440 nm) | 0.2457 | 0.0010 | 0.0245 | 0.0170 | 0.0271 |
| 0.3342 | 0.0030 | 0.1009 | 0.0948 | 0.0480 | |
| γ aNAP | 0.0153 | 0.0044 | 0.0098 | 0.0111 | 0.0031 |
| 0.0158 | 0.0042 | 0.0103 | 0.0107 | 0.0027 | |
| b*bNAP(555 nm) | 0.1984 | 0.0005 | 0.0112 | 0.0075 | 0.0183 |
| 0.3767 | 0.0062 | 0.0564 | 0.0212 | 0.0821 | |
| γ bbNAP | −0.0220 | −3.3386 | −1.0197 | −0.9000 | 0.5804 |
| −0.2862 | −3.1108 | −1.1317 | −1.0313 | −0.4703 |
| N | Sum Sq | Mean Sq | F Value | Pr(>F) | |
|---|---|---|---|---|---|
| CCHL | 32 | 289.60 | 9.05 | 6.50 | <0.001 |
| 17 | 16796 | 988 | 0.19 | 1.000 | |
| CNAP | 32 | 1196 | 37.38 | 0.70 | 0.884 |
| 17 | 5159 | 303.45 | 3.42 | <0.001 | |
| a*PHY(440 nm) | 32 | 7.81E−02 | 2.44E−03 | 0.66 | 0.925 |
| 17 | 5.46E−03 | 3.21E−03 | 0.48 | 0.958 | |
| a*PHY(676 nm) | 32 | 2.82E−03 | 8.81E−05 | 0.37 | 0.999 |
| 17 | 5.64E−04 | 3.32E−05 | 0.157 | 1.000 | |
| γ aCDOM | 32 | 06.00E−04 | 1.87E−05 | 0.93 | 0.576 |
| 17 | 1.08E−04 | 6.36E−06 | 0.96 | 0.509 | |
| aCDOM(440 nm) | 32 | 19.62 | 0.6132 | 2.57 | <0.001 |
| 17 | 15.33 | 0.9016 | 1.75 | 0.047 | |
| a*NAP(440 nm) | 32 | 1.53E−02 | 4.79E−04 | 0.63 | 0.944 |
| 17 | 5.74E−02 | 3.38E−03 | 1.52 | 0.105 | |
| γ aNAP | 32 | 6.67E−4 | 2.09E−05 | 2.60 | <0.001 |
| 17 | 3.03E−4 | 1.78E−05 | 3.20 | <0.001 |
| Cluster# | N | Reflectivity | Rrs Characteristics 1 | SIOP Characteristics 2 | Dominant Absorber 3 | Description |
|---|---|---|---|---|---|---|
| 01 | 135 | low | broad plateau peaking between 400 nm and 500 nm | lowest Chl | CDOM | oligotrophic coastal waters with low amounts of suspended material |
| 02 | 39 | moderate | maximum peak between 480 nm and 500 nm smaller peak around 545 nm | highest aCDOM(440 nm) of coastal waters | CDOM | coastal waters with a strong estuarine influence |
| 03 | 55 | low | broad plateau peaking between 475 nm and 575 nm | lowest NAP of coastal waters | CDOM | open coastal waters with higher amounts of suspended organic material than c3 |
| 04 | 6 | moderate | broad plateau peaking between 500 nm and 600 nm | highest chl and a*PHY (440 nm) of coastal waters | PHY | eutrophic tropical coastal waters |
| 05 | 6 | moderate | steep increase from 350 nm to 560 nm followed by a sharp decrease to 600 nm | highest γ aNAP of coastal waters | NAP and CDOM | relatively turbid tropical coastal waters containing organic particulate material |
| 06 | 42 | low | broad plateau peaking between 500 nm and 600 nm | lowest b*bNAP (555 nm) of inland waters | CDOM | clear inland lake waters |
| 07 | 5 | high | steep increase from 350 nm to a peak at 570 nm, followed by a decrease to an absorption peak at 680 nm with a smaller peak around 700 nm | highest chl and very low NAP | PHY | eutrophic waters with high phytoplankton content |
| 08 | 6 | high | steep increase from 350 nm to a peak at 570 nm, followed by a gradual decrease to around 700 nm | low NAP and relatively high Chl | CDOM | CDOM rich waters |
| 09 | 27 | moderate | steep increase from 350 nm to a peak at 570 nm, followed by a gradual decrease to around 700 nm | relatively high NAP and low b*bNAP (555 nm) | CDOM and NAP | relatively clear inland waters with small suspended particles |
| 10 | 4 | high | steep increase from 350 nm to a peak at 580 nm, followed by a more gradual decrease to around 700 nm | high NAP, high aCDOM(440 nm) and largest γNAP | CDOM | sediment laden waters containing organic particulate material |
| 11 | 21 | moderate | steep increase from 350 nm to a peak at 590 nm, followed by a broad shoulder between 590–700 nm | Highest NAP, relatively low b*bNAP (555 nm) | NAP and CDOM | Sediment laden waters with small suspended particles |
| 01 | 02 | 03 | 04 | 05 | 06 | 07 | 08 | 09 | 10 | 11 | Accuracy (%) | Sensitivity | Precision (%) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 01 | 102 | 0 | 18 | 0 | 0 | 15 | 0 | 0 | 0 | 0 | 0 | 89 | 0.91 | 76 |
| 02 | 0 | 25 | 1 | 0 | 11 | 3 | 0 | 0 | 2 | 0 | 0 | 79 | 0.64 | 60 |
| 03 | 4 | 7 | 39 | 0 | 0 | 5 | 0 | 0 | 1 | 0 | 0 | 78 | 0.63 | 70 |
| 04 | 0 | 0 | 0 | 4 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 75 | 0.50 | 67 |
| 05 | 0 | 1 | 0 | 2 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 57 | 0.16 | 50 |
| 06 | 6 | 0 | 3 | 0 | 0 | 30 | 0 | 0 | 2 | 0 | 0 | 76 | 0.56 | 73 |
| 07 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 1 | 0 | 1 | 0 | 75 | 0.50 | 50 |
| 08 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 3 | 0 | 2 | 0 | 68 | 0.38 | 50 |
| 09 | 0 | 6 | 1 | 2 | 4 | 1 | 0 | 0 | 13 | 0 | 0 | 82 | 0.68 | 48 |
| 10 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 3 | 0 | 0 | 0 | 49 | 0.00 | 0 |
| 11 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 19 | 100 | 1.00 | 95 |
| Overall Accuracy: | 69% | |||||||||||||
| Kappa: | 62% | |||||||||||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Botha, E.J.; Anstee, J.M.; Sagar, S.; Lehmann, E.; Medeiros, T.A.G. Classification of Australian Waterbodies across a Wide Range of Optical Water Types. Remote Sens. 2020, 12, 3018. https://doi.org/10.3390/rs12183018
Botha EJ, Anstee JM, Sagar S, Lehmann E, Medeiros TAG. Classification of Australian Waterbodies across a Wide Range of Optical Water Types. Remote Sensing. 2020; 12(18):3018. https://doi.org/10.3390/rs12183018
Chicago/Turabian StyleBotha, Elizabeth J., Janet M. Anstee, Stephen Sagar, Eric Lehmann, and Thais A. G. Medeiros. 2020. "Classification of Australian Waterbodies across a Wide Range of Optical Water Types" Remote Sensing 12, no. 18: 3018. https://doi.org/10.3390/rs12183018
APA StyleBotha, E. J., Anstee, J. M., Sagar, S., Lehmann, E., & Medeiros, T. A. G. (2020). Classification of Australian Waterbodies across a Wide Range of Optical Water Types. Remote Sensing, 12(18), 3018. https://doi.org/10.3390/rs12183018