Measuring Vegetation Phenology with Near-Surface Remote Sensing in a Temperate Deciduous Forest: Effects of Sensor Type and Deployment
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Field Measurements
2.2.1. Digital Camera Measurements
2.2.2. Spectroradiometer Measurements
2.2.3. Radiometer Measurements
2.3. Data Analysis
2.3.1. Vegetation Index from Digital Camera
2.3.2. Vegetation Index from Spectroradiometer
2.3.3. Vegetation Index from Radiometer
2.3.4. Phenophase Extraction
2.3.5. Statistical Analysis
3. Results and Discussion
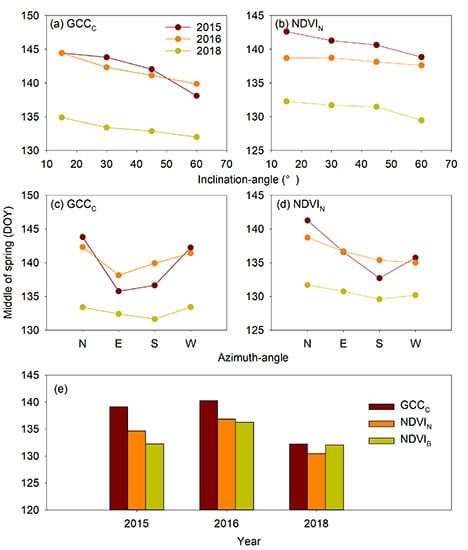
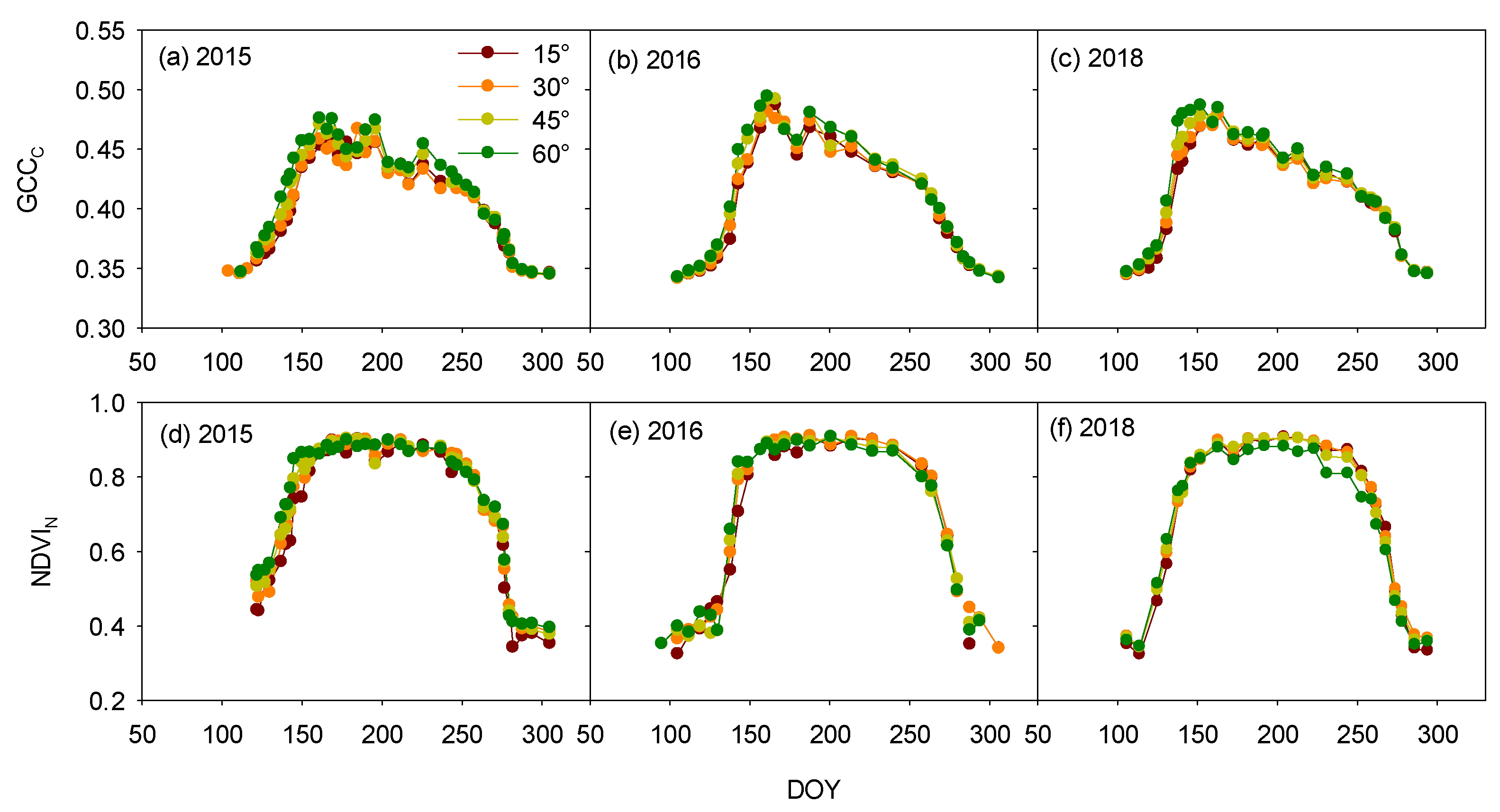
3.1. Inclination-Angle Effect on the Vegetation Indices and Phenophases
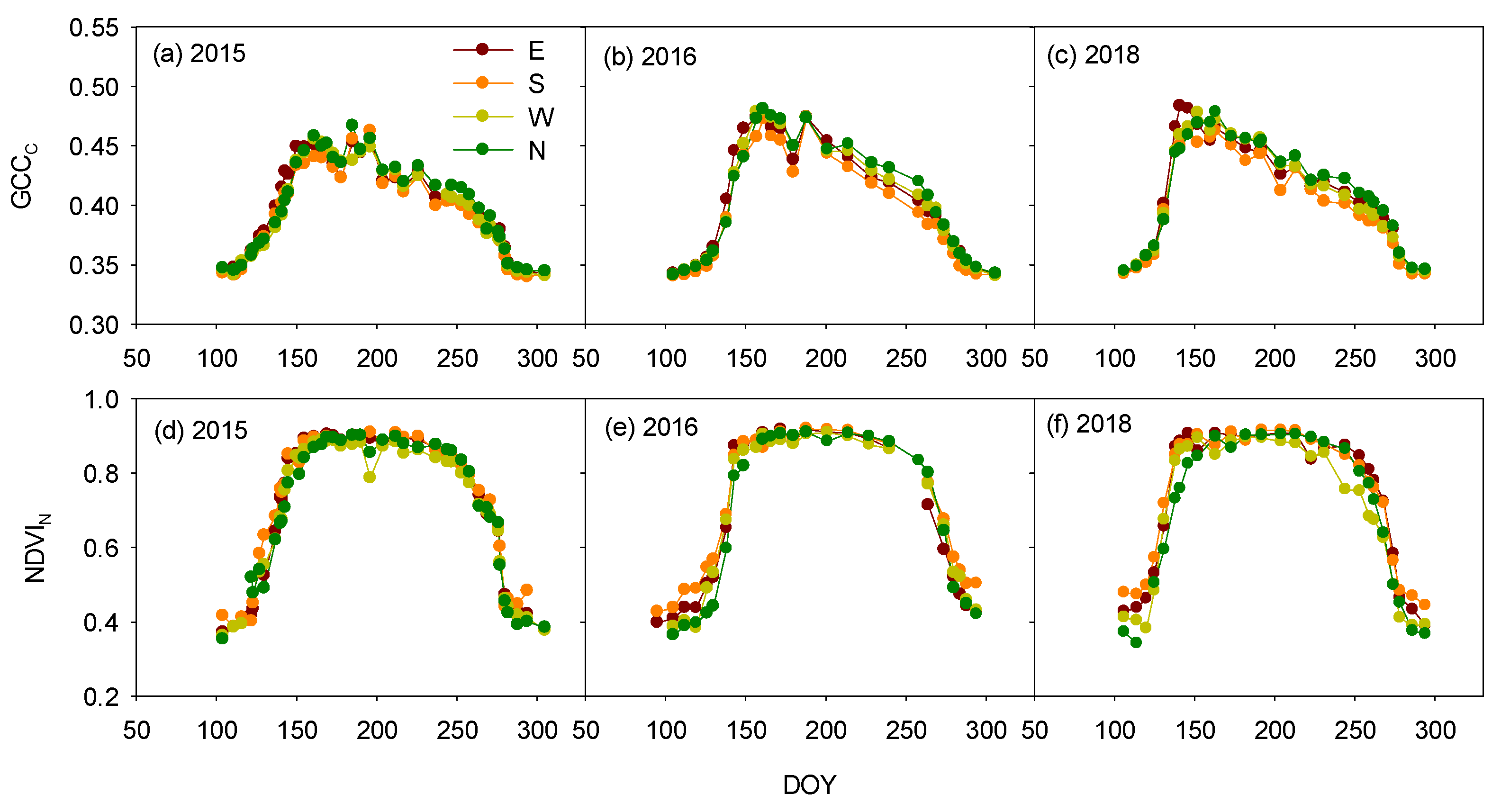
3.2. Azimuth-Angle Effect on the Vegetation Indices and Phenophases
3.3. Sensor-Type Effect on the Vegetation Indices and Phenophases
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Piao, S.L.; Tan, J.G.; Chen, A.P.; Fu, Y.H.; Ciais, P.; Liu, Q.; Janssens, I.A.; Vicca, S.; Zeng, Z.; Jeong, S.-J. Leaf onset in the northern hemisphere triggered by daytime temperature. Nat. Commun. 2015, 6, 6911. [Google Scholar] [CrossRef]
- Menzel, A.; Sparks, T.H.; Estrella, N.; Koch, E.; Aasa, A.; Ahas, R.; ALM-KÜBLER, K.; Bissolli, P.; Braslavská, O.G.; Briede, A. European phenological response to climate change matches the warming pattern. Glob. Chang. Biol. 2006, 12, 1969–1976. [Google Scholar] [CrossRef]
- Schwartz, M.D.; Ahas, R.; Aasa, A. Onset of spring starting earlier across the Northern Hemisphere. Glob. Chang. Biol. 2006, 12, 343–351. [Google Scholar] [CrossRef]
- Estiarte, M.; Peñuelas, J. Alteration of the phenology of leaf senescence and fall in winter deciduous species by climate change: Effects on nutrient proficiency. Glob. Chang. Biol. 2015, 21, 1005–1017. [Google Scholar] [CrossRef]
- Gill, A.L.; Gallinat, A.S.; Sanders-DeMott, R.; Rigden, A.J.; Gianotti, D.J.S.; Mantooth, J.A.; Templer, P.H. Changes in autumn senescence in northern hemisphere deciduous trees: A meta-analysis of autumn phenology studies. Ann. Bot. 2015, 116, 875–888. [Google Scholar] [CrossRef]
- Richardson, A.D.; Anderson, R.S.; Arain, M.A.; Barr, A.G.; Bohrer, G.; Chen, G.; Chen, J.M.; Ciais, P.; Davis, K.J.; Desai, A.R. Terrestrial biosphere models need better representation of vegetation phenology: Results from the North American Carbon Program Site Synthesis. Glob. Chang. Biol. 2012, 18, 566–584. [Google Scholar] [CrossRef]
- Barichivich, J.; Briffa, K.R.; Myneni, R.B.; Osborn, T.J.; Melvin, T.M.; Ciais, P.; Piao, S.; Tucker, C. Large-scale variations in the vegetation growing season and annual cycle of atmospheric CO2 at high northern latitudes from 1950 to 2011. Glob. Chang. Biol. 2013, 19, 3167–3183. [Google Scholar] [CrossRef]
- Piao, S.L.; Friedlingstein, P.; Ciais, P.; Viovy, N.; Demarty, J. Growing season extension and its impact on terrestrial carbon cycle in the Northern Hemisphere over the past 2 decades. Glob. Biogeochem. Cycle 2007, 21, 1148–1154. [Google Scholar] [CrossRef]
- Richardson, A.D.; Keenan, T.F.; Migliavacca, M.; Ryu, Y.; Sonnentag, O.; Toomey, M. Climate change, phenology, and phenological control of vegetation feedbacks to the climate system. Agric. For. Meteorol. 2013, 169, 156–173. [Google Scholar] [CrossRef]
- Wu, J.; Albert, L.; Lopes, A.; Restrepo-Coupe, N.; Hayek, M.; Wiedemann, K.; Guan, K.; Stark, S.; Christoffersen, B.; Prohaska, N. Leaf development and demography explain photosynthetic seasonality in Amazon evergreen forests. Science 2016, 351, 972–976. [Google Scholar] [CrossRef]
- Zhang, Y.L.; Song, C.H.; Band, L.E.; Sun, G.; Li, J.X. Reanalysis of global terrestrial vegetation trends from MODIS products: Browning or greening? Remote Sens. Environ. 2017, 191, 145–155. [Google Scholar] [CrossRef]
- Sparks, T.H.; Menzel, A. Observed changes in seasons: An overview. Int. J. Climatol. 2002, 22, 1715–1725. [Google Scholar] [CrossRef]
- Ahl, D.E.; Gower, S.T.; Burrows, S.N.; Shabanov, N.V.; Myneni, R.B.; Knyazikhin, Y. Monitoring spring canopy phenology of a deciduous broadleaf forest using MODIS. Remote Sens. Environ. 2006, 104, 88–95. [Google Scholar] [CrossRef]
- Balzarolo, M.; Vicca, S.; Nguy-Robertson, A.L.; Bonal, D.; Elbers, J.A.; Fu, Y.H.; Grünwald, T.; Horemans, J.A.; Papale, D.; Peñuelas, J. Matching the phenology of net ecosystem exchange and vegetation indices estimated with MODIS and FLUXNET in-situ observations. Remote Sens. Environ. 2016, 174, 290–300. [Google Scholar] [CrossRef]
- Richardson, A.D.; Klosterman, S.; Toomey, M. Near-surface sensor-derived phenology. In Phenology: An Integrative Environmental Science; Springer: Berlin/Heidelberg, Germany, 2013; pp. 413–430. [Google Scholar]
- Gamon, J.A.; Rahman, A.F.; Dungan, J.L.; Schildhauer, M.; Huemmrich, K.F. Spectral Network (SpecNet)-What is it and why do we need it? Remote Sens. Environ. 2006, 103, 227–235. [Google Scholar] [CrossRef]
- Brown, T.B.; Hultine, K.R.; Steltzer, H.; Denny, E.G.; Denslow, M.W.; Granados, J.; Henderson, S.; Moore, D.; Nagai, S.; Sanclements, M. Using phenocams to monitor our changing Earth: Toward a global phenocam network. Front. Ecol. Environ. 2016, 14, 84–93. [Google Scholar] [CrossRef]
- Richardson, A.D. Tracking seasonal rhythms of plants in diverse ecosystems with digital camera imagery. New Phytol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Sonnentag, O.; Hufkens, K.; Teshera-Sterne, C.; Young, A.M.; Friedl, M.; Braswell, B.H.; Milliman, T.; O’Keefe, J.; Richardson, A.D. Digital repeat photography for phenological research in forest ecosystems. Agric. For. Meteorol. 2012, 152, 159–177. [Google Scholar] [CrossRef]
- Liu, Y.; Hill, M.J.; Zhang, X.; Wang, Z.; Richardson, A.D.; Hufkens, K.; Filippa, G.; Baldocchi, D.D.; Ma, S.; Verfaillie, J. Using data from Landsat, MODIS, VIIRS and PhenoCams to monitor the phenology of California oak/grass savanna and open grassland across spatial scales. Agric. For. Meteorol. 2017, 237, 311–325. [Google Scholar] [CrossRef]
- Garrity, S.R.; Bohrer, G.; Maurer, K.D.; Mueller, K.L.; Vogel, C.S.; Curtis, P.S. A comparison of multiple phenology data sources for estimating seasonal transitions in deciduous forest carbon exchange. Agric. For. Meteorol. 2012, 151, 1741–1752. [Google Scholar] [CrossRef]
- Anderson, H.; Nilsen, L.; Tømmervik, H.; Karlsen, S.; Nagai, S.; Cooper, E. Using ordinary digital cameras in place of near-infrared sensors to derive vegetation indices for phenology studies of high Arctic vegetation. Remote Sens. 2016, 8, 847. [Google Scholar] [CrossRef]
- Sonnentag, O.; Detto, M.; Vargas, R.; Ryu, Y.; Runkle, B.; Kelly, M.; Baldocchi, D. Tracking the structural and functional development of a perennial pepperweed (Lepidium latifolium L.) infestation using a multi-year archive of webcam imagery and eddy covariance measurements. Agric. For. Meteorol. 2011, 151, 916–926. [Google Scholar] [CrossRef]
- Richardson, A.D.; Hufkens, K.; Milliman, T.; Aubrecht, D.M.; Chen, M.; Gray, J.M.; Johnston, M.R.; Keenan, T.F.; Klosterman, S.T.; Kosmala, M.; et al. Tracking vegetation phenology across diverse North American biomes using PhenoCam imagery. Sci. Data 2018, 5, 180028. [Google Scholar] [CrossRef]
- Gamon, J.A. Optical sampling of the flux tower footprint. Biogeosciences 2015, 12, 4509–4523. [Google Scholar] [CrossRef]
- Balzarolo, M.; Anderson, K.; Nichol, C.; Rossini, M.; Vescovo, L.; Arriga, N.; Wohlfahrt, G.; Calvet, J.-C.; Carrara, A.; Cerasoli, S. Ground-based optical measurements at European flux sites: A review of methods, instruments and current controversies. Sensors 2011, 11, 7954–7981. [Google Scholar] [CrossRef]
- Keenan, T.F.; Darby, B.; Felts, E.; Sonnentag, O.; Friedl, M.A.; Hufkens, K.; O’Keefe, J.; Klosterman, S.; Munger, J.W.; Toomey, M. Tracking forest phenology and seasonal physiology using digital repeat photography: A critical assessment. Ecol. Appl. 2014, 24, 1478–1489. [Google Scholar] [CrossRef]
- Moore, C.E.; Beringer, J.; Evans, B.; Hutley, L.B.; Tapper, N.J. Tree-grass phenology information improves light use efficiency modelling of gross primary productivity for an Australian tropical savanna. Biogeosciences 2017, 14, 1–38. [Google Scholar] [CrossRef]
- Vrieling, A.; Meroni, M.; Darvishzadeh, R.; Skidmore, A.K.; Wang, T.; Zurita-Milla, R.; Oosterbeek, K.; O’Connor, B.; Paganini, M. Vegetation phenology from Sentinel-2 and field cameras for a Dutch barrier island. Remote Sens. Environ. 2018, 215, 517–529. [Google Scholar] [CrossRef]
- Mizunuma, T.; Wilkinson, M.; Eaton, E.L.; Mencuccini, M.; Morison, J.I.L.; Grace, J. The relationship between carbon dioxide uptake and canopy colour from two camera systems in a deciduous forest in southern England. Funct. Ecol. 2013, 27, 196–207. [Google Scholar] [CrossRef]
- D’Odorico, P.; Gonsamo, A.; Gough, C.M.; Bohrer, G.; Morison, J.; Wilkinson, M.; Hanson, P.J.; Gianelle, D.; Fuentes, J.D.; Buchmann, N. The match and mismatch between photosynthesis and land surface phenology of deciduous forests. Agric. For. Meteorol. 2015, 214, 25–38. [Google Scholar] [CrossRef]
- Eklundh, L.; Jin, H.; Schubert, P.; Guzinski, R.; Heliasz, M. An optical sensor network for vegetation phenology monitoring and satellite data calibration. Sensors 2011, 11, 7678–7709. [Google Scholar] [CrossRef] [PubMed]
- Matzinger, N.; Andretta, M.; Gorsel, E.V.; Vogt, R.; Ohmura, A.; Rotach, M. Surface radiation budget in an Alpine valley. Q. J. R. Meteorol. Soc. 2003, 129, 877–895. [Google Scholar] [CrossRef]
- Tittebrand, A.; Spank, U.; Bernhofer, C. Comparison of satellite- and ground-based NDVI above different land-use types. Theor. Appl. Climatol. 2009, 98, 171–186. [Google Scholar] [CrossRef]
- Hilker, T.; Gitelson, A.; Coops, N.C.; Hall, F.G.; Black, T. Tracking plant physiological properties from multi-angular tower-based remote sensing. Oecologia 2011, 165, 865–876. [Google Scholar] [CrossRef] [PubMed]
- Moore, C.E.; Brown, T.; Keenan, T.F.; Duursma, R.A.; Dijk, A.I.J.M.V.; Beringer, J.; Culvenor, D.; Evans, B.; Huete, A.; Hutley, L.B. Australian vegetation phenology: New insights from satellite remote sensing and digital repeat photography. Biogeosciences 2016, 13, 5085–5102. [Google Scholar] [CrossRef]
- Ahrends, H.E.; Brügger, R.; Stöckli, R.; Schenk, J.; Michna, P.; Jeanneret, F.; Wanner, H.; Eugster, W. Quantitative phenological observations of a mixed beech forest in northern Switzerland with digital photography. J. Geophys. Res. Biogeosci. 2008, 113, G04004. [Google Scholar] [CrossRef]
- Richardson, A.D.; Jenkins, J.P.; Braswell, B.H.; Hollinger, D.Y.; Ollinger, S.V.; Smith, M.-L. Use of digital webcam images to track spring green-up in a deciduous broadleaf forest. Oecologia 2007, 152, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Saitoh, T.M.; Nagai, S.; Saigusa, N.; Kobayashi, H.; Suzuki, R.; Nasahara, K.N.; Muraoka, H. Assessing the use of camera-based indices for characterizing canopy phenology in relation to gross primary production in a deciduous broad-leaved and an evergreen coniferous forest in Japan. Ecol. Inform. 2012, 11, 45–54. [Google Scholar] [CrossRef]
- Wang, X.C.; Wang, C.K.; Li, Q.L. Wind regimes above and below a temperate deciduous forest canopy in complex terrain: Interactions between slope and valley winds. Atmosphere 2015, 6, 60–87. [Google Scholar] [CrossRef]
- Liu, F.; Wang, C.K.; Wang, X.C.; Zhang, J.S.; Zhang, Z.; Wang, J.J. Spatial patterns of biomass in the temperate broadleaved deciduous forest within the fetch of the Maoershan flux tower. Acta Ecol. Sin. 2016, 36, 6506–6519. [Google Scholar] [CrossRef]
- Richardson, A.D.; Braswell, B.H.; Hollinger, D.Y.; Jenkins, J.P.; Ollinger, S.V. Near-surface remote sensing of spatial and temporal variation in canopy phenology. Ecol. Appl. 2009, 19, 1417–1428. [Google Scholar] [CrossRef]
- Mizunuma, T.; Mencuccini, M.; Wingate, L.; Ogée, J.; Nichol, C.; Grace, J. Sensitivity of colour indices for discriminating leaf colours from digital photographs. Methods Ecol. Evol. 2014, 5, 1078–1085. [Google Scholar] [CrossRef]
- Filippa, G.; Cremonese, E.; Migliavacca, M.; Galvagno, M.; Forkel, M.; Wingate, L.; Tomelleri, E.; Cella, U.M.D.; Richardson, A.D. Phenopix: A R package for image-based vegetation phenology. Agric. For. Meteorol. 2016, 220, 141–150. [Google Scholar] [CrossRef]
- Ross, J.; Sulev, M. Sources of errors in measurements of PAR. Agric. For. Meteorol. 2000, 100, 103–125. [Google Scholar] [CrossRef]
- Huemmrich, K.F.; Black, T.A.; Jarvis, P.G.; McCaughey, J.; Hall, F.G. High temporal resolution NDVI phenology from micrometeorological radiation sensors. J. Geophys. Res. Atmos. 1999, 104, 27935–27944. [Google Scholar] [CrossRef]
- Hufkens, K.; Friedl, M.; Sonnentag, O.; Braswell, B.H.; Milliman, T.; Richardson, A.D. Linking near-surface and satellite remote sensing measurements of deciduous broadleaf forest phenology. Remote Sens. Environ. 2012, 117, 307–321. [Google Scholar] [CrossRef]
- Liu, F.; Wang, C.K.; Wang, X.C. Application of near-surface remote sensing in monitoring dynamics of forest canopy phenology. Chin. J. Appl. Ecol. 2018, 29, 1768–1778. [Google Scholar]
- Zhang, X.Y.; Friedl, M.A.; Schaaf, C.B.; Strahler, A.H.; Hodges, J.C.F.; Gao, F.; Reed, B.C.; Huete, A. Monitoring vegetation phenology using MODIS. Remote Sens. Environ. 2003, 84, 471–475. [Google Scholar] [CrossRef]
- Elmore, A.J.; Guinn, S.M.; Minsley, B.J.; Richardson, A.D. Landscape controls on the timing of spring, autumn, and growing season length in mid-Atlantic forests. Glob. Chang. Biol. 2012, 18, 656–674. [Google Scholar] [CrossRef]
- Klosterman, S.; Hufkens, K.; Gray, J.; Melaas, E.; Sonnentag, O.; Lavine, I.; Mitchell, L.; Norman, R.; Friedl, M.; Richardson, A. Evaluating remote sensing of deciduous forest phenology at multiple spatial scales using PhenoCam imagery. Biogeosciences 2014, 11, 4305–4320. [Google Scholar] [CrossRef]
- Delpierre, N.; Guillemot, J.; Dufrêne, E.; Cecchini, S.; Nicolas, M. Tree phenological ranks repeat from year to year and correlate with growth in temperate deciduous forests. Agric. For. Meteorol. 2017, 234–235, 1–10. [Google Scholar] [CrossRef]
- Augspurger, C.K.; Salk, C.F. Constraints of cold and shade on the phenology of spring ephemeral herb species. J. Ecol. 2016, 105, 246–254. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, C.; Chen, J.M.; Wang, X.; Jin, G. Empirical models for tracing seasonal changes in leaf area index in deciduous broadleaf forests by digital hemispherical photography. For. Ecol. Manag. 2015, 351, 67–77. [Google Scholar] [CrossRef]
- Yang, X.; Tang, J.W.; Mustard, J.F. Beyond leaf color: Comparing camera-based phenological metrics with leaf biochemical, biophysical, and spectral properties throughout the growing season of a temperate deciduous forest. J. Geophys. Res. Biogeosci. 2014, 119, 181–191. [Google Scholar] [CrossRef]
- Wingate, L.; Ogée, J.; Cremonese, E.; Filippa, G.; Mizunuma, T.; Migliavacca, M.; Moisy, C.; Wilkinson, M.; Moureaux, C.; Wohlfahrt, G. Interpreting canopy development and physiology using a European phenology camera network at flux sites. Biogeosciences 2015, 12, 5995–6015. [Google Scholar] [CrossRef]
- Brown, L.A.; Dasha, J.; Ogutua, B.O.; Richardson, A.D. On the relationship between continuous measures of canopy greenness derived using near-surface remote sensing and satellite-derived vegetation products. Agric. For. Meteorol. 2017, 247, 280–292. [Google Scholar] [CrossRef]
- Peltoniemi, M.; Aurela, M.; Böttcher, K.; Kolari, P.; Loehr, J.; Hokkanen, T.; Karhu, J.; Linkosalmi, M.; Tanis, C.M.; Metsämäki, S. Networked web-cameras monitor congruent seasonal development of birches with phenological field observations. Agric. For. Meteorol. 2018, 249, 335–347. [Google Scholar] [CrossRef]
- Nasahara, K.N.; Nagai, S. Review: Development of an in situ observation network for terrestrial ecological remote sensing: The Phenological Eyes Network (PEN). Ecol. Res. 2015, 30, 211–223. [Google Scholar] [CrossRef]
- Ryu, Y.; Lee, G.; Jeon, S.; Song, Y.; Kimm, H. Monitoring multi-layer canopy spring phenology of temperate deciduous and evergreen forests using low-cost spectral sensors. Remote Sens. Environ. 2014, 149, 227–238. [Google Scholar] [CrossRef]
- Ge, Q.; Wang, H.; Dai, J. Phenological response to climate change in China: A meta-analysis. Glob. Chang. Biol. 2015, 21, 265–274. [Google Scholar] [CrossRef]
- Fisher, J.I.; Mustard, J.F.; Vadeboncoeur, M.A. Green leaf phenology at Landsat resolution: Scaling from the field to the satellite. Remote Sens. Environ. 2006, 100, 265–279. [Google Scholar] [CrossRef]
- Scheifinger, H.; Menzel, A.; Koch, E.; Peter, C.; Ahas, R. Atmospheric mechanisms governing the spatial and temporal variability of phenological phases in central Europe. Int. J. Climatol. 2002, 22, 1739–1755. [Google Scholar] [CrossRef]
- Fisher, J.I.; Mustard, J.F. Cross-scalar satellite phenology from ground, Landsat, and MODIS data. Remote Sens. Environ. 2007, 109, 261–273. [Google Scholar] [CrossRef]
- Inoue, T.; Nagai, S.; Saitoh, T.M.; Muraoka, H.; Nasahara, K.N.; Koizumi, H. Detection of the different characteristics of year-to-year variation in foliage phenology among deciduous broad-leaved tree species by using daily continuous canopy surface images. Ecol. Inform. 2014, 22, 58–68. [Google Scholar] [CrossRef]
- Toomey, M.; Friedl, M.A.; Frolking, S.; Hufkens, K.; Klosterman, S.; Sonnentag, O.; Baldocchi, D.D.; Bernacchi, C.J.; Biraud, S.C.; Bohrer, G. Greenness indices from digital cameras predict the timing and seasonal dynamics of canopy-scale photosynthesis. Ecol. Appl. 2015, 25, 99–115. [Google Scholar] [CrossRef] [PubMed]
- Hufkens, K.; Filippa, G.; Cremonese, E.; Migliavacca, M.; D’Odorico, P.; Peichl, M.; Gielen, B.; Hörtnagl, L.; Soudani, K.; Papale, D.; et al. Assimilating phenology datasets automatically across ICOS ecosystem stations. Int. Agrophys. 2018, 32, 677–687. [Google Scholar] [CrossRef]
- Petach, A.R.; Toomey, M.; Aubrecht, D.M.; Richardson, A.D. Monitoring vegetation phenology using an infrared-enabled security camera. Agric. For. Meteorol. 2014, 195, 143–151. [Google Scholar] [CrossRef]
- Filippa, G.; Cremonese, E.; Galvagno, M.; Migliavacca, M.; Sonnentag, O.; Hufkens, K.; Ryu, Y.; Humphreys, E.; Umberto, M.D.C.; Richardson, A.D. NDVI derived from IR-enabled digital cameras: Applicability across different plant functional types. Agric. For. Meteorol. 2018, 249, 275–285. [Google Scholar] [CrossRef]
- Toda, M.; Richardson, A.D. Estimation of plant area index and phenological transition dates from digital repeat photography and radiometric approaches in a hardwood forest in the Northeastern United States. Agric. For. Meteorol. 2018, 249, 457–466. [Google Scholar] [CrossRef]


| VI | Inclination Angle | 2015 | 2016 | 2018 | |||
|---|---|---|---|---|---|---|---|
| Spring | Autumn | Spring | Autumn | Spring | Autumn | ||
| GCCC | 30° | 0.987 | 0.993 | 0.991 | 1.000 | 1.000 | 1.000 |
| 45° | 0.982 | 0.984 | 1.000 | 1.000 | 0.991 | 1.000 | |
| 60° | 0.982 | 0.989 | 1.000 | 1.000 | 0.891 | 1.000 | |
| NDVIN | 30° | 0.984 | 0.951 | 1.000 | 1.000 | 1.000 | 1.000 |
| 45° | 0.982 | 0.945 | 0.817 | 1.000 | 1.000 | 1.000 | |
| 60° | 0.973 | 0.951 | 0.830 | 0.964 | 1.000 | 0.983 | |
| NDVIB | 81° | 0.980 | 0.992 | ||||
| VI | Year | Inclination Angle | SOS | MOS | POS | SOF | MOF | EOF | LOS1 | LOS2 |
|---|---|---|---|---|---|---|---|---|---|---|
| GCCC | 2015 | 30° | 0 ± 5 | −1 ± 2 | −1 ± 5 | 4 ± 7 | 2 ± 3 | 1 ± 7 | 3 ± 3 | 1 ± 9 |
| 45° | −5 ± 7 | −2 ± 2 | 0 ± 7 | 0 ± 8 | 1 ± 3 | 2 ± 8 | 3 ± 4 | 7 ± 10 | ||
| 60° | −12 ± 8 * | −6 ± 4 * | −1 ± 8 | −11 ± 12 | −2 ± 5 | 6 ± 12 | 4 ± 6 | 18 ± 15 | ||
| 2016 | 30° | −2 ± 8 | −2 ± 3 | −3 ± 8 | −1 ± 4 | 1 ± 2 | 3 ± 4 | 3 ± 4 | 5 ± 9 | |
| 45° | −3 ± 8 | −3 ± 3 | −4 ± 8 | 0 ± 4 | 1 ± 2 | 1 ± 4 | 4 ± 4 | 4 ± 9 | ||
| 60° | −3 ± 8 | −5 ± 3 | −6 ± 8 | −3 ± 5 | 1 ± 2 | 5 ± 5 | 6 ± 4 | 8 ± 10 | ||
| 2018 | 30° | 0 ± 5 | −1 ± 2 | −3 ± 5 | 0 ± 8 | 0 ± 3 | 0 ± 8 | 2 ± 4 | 1 ± 10 | |
| 45° | 0 ± 4 | −2 ± 2 | −4 ± 4 | 0 ± 8 | 0 ± 3 | 0 ± 8 | 2 ± 4 | 0 ± 9 | ||
| 60° | 1 ± 4 | −3 ± 2 * | −7 ± 4 * | −2 ± 9 | 0 ± 4 | 2 ± 9 | 3 ± 4 | 1 ± 10 | ||
| NDVIN | 2015 | 30° | 3 ± 12 | −1 ± 4 | −6 ± 12 | −8 ± 9 | −1 ± 4 | 7 ± 9 | 0 ± 6 | 3 ± 15 |
| 45° | 4 ± 11 | −2 ± 4 | −8 ± 11 | −8 ± 10 | −1 ± 4 | 7 ± 10 | 1 ± 6 | 3 ± 15 | ||
| 60° | 6 ± 10 | −4 ± 4 | −14 ± 10 * | 2 ± 7 | 1 ± 3 | 0 ± 7 | 5 ± 5 | −6 ± 12 | ||
| 2016 | 30° | 7 ± 12 | 0 ± 5 | −7 ± 12 | −4 ± 11 | −1 ± 5 | 3 ± 11 | −1 ± 7 | −5 ± 16 | |
| 45° | 10 ± 12 | −1 ± 5 | −12 ± 12 | −6 ± 11 | −1 ± 5 | 4 ± 11 | −1 ± 7 | −6 ± 16 | ||
| 60° | 12 ± 12 | −1 ± 5 | −14 ± 12 | −3 ± 11 | −2 ± 5 | 0 ± 11 | −1 ± 7 | −12 ± 16 | ||
| 2018 | 30° | −3 ± 7 | −1 ± 3 | 2 ± 7 | −2 ± 6 | −1 ± 3 | −1 ± 6 | −1 ± 4 | 2 ± 10 | |
| 45° | −2 ± 6 | −1 ± 3 | 1 ± 6 | −4 ± 8 | −2 ± 3 | −1 ± 8 | −1 ± 5 | 2 ± 10 | ||
| 60° | −3 ± 6 | −3 ± 3 | −3 ± 6 | 0 ± 8 | −1 ± 3 | −2 ± 8 | 1 ± 4 | 1 ± 10 | ||
| NDVIB | 2016 | 81° | 0 ± 4 | 0 ± 2 | 1 ± 4 | 1 ± 11 | −2 ± 5 | −5 ± 11 | −2 ± 6 | −5 ± 12 |
| Year | Inclination Angle | SOS | MOS | POS | SOF | MOF | EOF | LOS1 | LOS2 |
|---|---|---|---|---|---|---|---|---|---|
| 2015 | 30° | −1 ± 7 | 2 ± 2 | 2 ± 7 | 0 ± 11 | 0 ± 5 | 0 ± 11 | −2 ± 5 | −1 ± 13 |
| 45° | −3 ± 11 | 3 ± 3 | 8 ± 11 | −3 ± 12 | −1 ± 4 | 1 ± 12 | −4 ± 6 | 3 ± 16 | |
| 60° | −10 ± 10 | −1 ± 3 | 8 ± 10 | −2 ± 17 | −1 ± 6 | 1 ± 17 | 0 ± 7 | 10 ± 19 | |
| 2016 | 30° | 2 ± 13 | −1 ± 6 | −4 ± 13 | 0 ± 8 | 0 ± 3 | 0 ± 8 | 1 ± 7 | −1 ± 15 |
| 45° | −1 ± 11 | −2 ± 5 | −4 ± 11 | 0 ± 8 | 1 ± 3 | 2 ± 8 | 3 ± 6 | 2 ± 13 | |
| 60° | −1 ± 11 | −3 ± 5 | −4 ± 11 | 0 ± 9 | 1 ± 4 | 3 ± 9 | 4 ± 6 | 4 ± 14 | |
| 2018 | 30° | 0 ± 8 | 0 ± 3 | 0 ± 8 | −1 ± 9 | 0 ± 3 | 0 ± 9 | 0 ± 5 | 1 ± 12 |
| 45° | 1 ± 7 | −1 ± 3 | −3 ± 7 | 0 ± 8 | 0 ± 3 | 1 ± 8 | 2 ± 4 | 0 ± 11 | |
| 60° | 0 ± 7 | −2 ± 3 | −5 ± 7 | 0 ± 11 | 1 ± 4 | 2 ± 11 | 3 ± 5 | 2 ± 13 |
| VI | Azimuth Angle | 2015 | 2016 | 2018 | |||
|---|---|---|---|---|---|---|---|
| Spring | Autumn | Spring | Autumn | Spring | Autumn | ||
| GCCC | E | 0.970 | 0.993 | 0.991 | 1.000 | 0.745 | 1.000 |
| S | 0.994 | 0.974 | 1.000 | 0.986 | 0.945 | 0.967 | |
| W | 0.997 | 1.000 | 0.991 | 1.000 | 0.955 | 0.983 | |
| NDVIN | E | 0.951 | 0.991 | 0.976 | 1.000 | 0.855 | 1.000 |
| S | 0.956 | 0.965 | 0.988 | 0.976 | 0.855 | 1.000 | |
| W | 0.979 | 0.996 | 0.952 | 1.000 | 0.900 | 0.983 | |
| VI | Year | Azimuth Angle | SOS | MOS | POS | SOF | MOF | EOF | LOS1 | LOS2 |
|---|---|---|---|---|---|---|---|---|---|---|
| GCCC | 2015 | E | −12 ± 6 * | −8 ± 3 * | −4 ± 6 | 1 ± 12 | 2 ± 5 | 4 ± 12 | 11 ± 5 * | 16 ± 13 |
| S | −17 ± 7 * | −7 ± 3 * | 3 ± 7 | 2 ± 10 | 1 ± 4 | 1 ± 10 | 9 ± 5 | 18 ± 13 | ||
| W | −6 ± 7 | −2 ± 2 | 3 ± 7 | −7 ± 13 | −1 ± 5 | 5 ± 13 | 1 ± 5 | 11 ± 14 | ||
| 2016 | E | 0 ± 6 | −4 ± 3 * | −9 ± 6 | −4 ± 9 | 2 ± 3 | 8 ± 9 | 6 ± 4 | 7 ± 11 | |
| S | 0 ± 7 | −2 ± 3 | −5 ± 7 | −7 ± 13 | −2 ± 5 | 2 ± 13 | 0 ± 6 | 2 ± 15 | ||
| W | 2 ± 6 | −1 ± 2 | −4 ± 6 | −1 ± 8 | 1 ± 3 | 3 ± 8 | 2 ± 4 | 1 ± 10 | ||
| 2018 | E | 2 ± 5 | −1 ± 2 | −4 ± 5 | 1 ± 10 | 1 ± 4 | 1 ± 10 | 2 ± 4 | −1 ± 11 | |
| S | 1 ± 5 | −2 ± 2 | −4 ± 5 | 2 ± 8 | 0 ± 3 | −2 ± 8 | 1 ± 4 | −3 ± 9 | ||
| W | 1 ± 5 | 0 ± 2 | −1 ± 5 | −3 ± 10 | −1 ± 4 | 2 ± 10 | −1 ± 4 | 1 ± 11 | ||
| NDVIN | 2015 | E | −3 ± 8 | −5 ± 3 * | −7 ± 8 | −3 ± 11 | 0 ± 5 | 2 ± 11 | 4 ± 6 | 5 ± 14 |
| S | −10 ± 10 | −9 ± 4 * | −7 ± 10 | 1 ± 13 | 0 ± 6 | −2 ± 13 | 8 ± 7 | 8 ± 16 | ||
| W | −10 ± 9 | −6 ± 3 * | −1 ± 9 | 0 ± 10 | 1 ± 4 | 1 ± 10 | 6 ± 5 | 11 ± 14 | ||
| 2016 | E | −2 ± 7 | −2 ± 3 | −2 ± 7 | −11 ± 10 | −4 ± 4 | 2 ± 10 | −2 ± 5 | 5 ± 12 | |
| S | −6 ± 7 | −3 ± 3 | 0 ± 7 | −4 ± 12 | −2 ± 5 | −1 ± 12 | 1 ± 6 | 6 ± 14 | ||
| W | −6 ± 6 | −4 ± 3 | −1 ± 6 | −1 ± 12 | 0 ± 5 | 2 ± 12 | 4 ± 6 | 8 ± 13 | ||
| 2018 | E | 8 ± 6 | −1 ± 3 | −10 ± 6 | 5 ± 7 | 2 ± 3 | 0 ± 7 | 3 ± 4 | −8 ± 9 | |
| S | 6 ± 6 | −2 ± 3 | −10 ± 6 | 7 ± 6 | 2 ± 3 | −3 ± 6 | 4 ± 4 | −9 ± 8 | ||
| W | 7 ± 6 | −2 ± 3 | −10 ± 6 | 4 ± 9 | 1 ± 4 | −2 ± 9 | 2 ± 5 | −9 ± 11 |
| VI | Year | SOS | MOS | POS | SOF | MOF | EOF | LOS1 | LOS2 |
|---|---|---|---|---|---|---|---|---|---|
| GCCC vs. NDVIN | 2015 | 1 ± 7 | 4 ± 3 * | 8 ± 7 | −4 ± 14 | −1 ± 6 | 3 ± 14 | −5 ± 7 | 1 ± 16 |
| 2016 | 2 ± 6 | 3 ± 3 * | 5 ± 6 | −5 ± 9 | 0 ± 4 | 4 ± 9 | −4 ± 5 | 2 ± 11 | |
| 2018 | 4 ± 3 * | 2 ± 1 | −1 ± 3 | 4 ± 8 | 3 ± 3 | 3 ± 8 | 2 ± 3 | −2 ± 8 | |
| GCCC vs. NDVIB | 2015 | 14 ± 9 * | 7 ± 3 * | −1 ± 9 | 7 ± 11 | 3 ± 5 | 0 ± 11 | −4 ± 6 | −15 ± 15 |
| 2016 | 4 ± 5 | 4 ± 1 * | 4 ± 5 | −9 ± 11 | 0 ± 5 | 9 ± 11 | −4 ± 6 | 5 ± 12 | |
| 2018 | 4 ± 3 | 0 ± 1 | −4 ± 3 | 18 ± 8 * | 8 ± 4 * | −3 ± 8 | 7 ± 4 * | −7 ± 9 | |
| NDVIN vs. NDVIB | 2015 | 13 ± 10 | 2 ± 4 | −8 ± 10 | 10 ± 11 | 4 ± 5 | −3 ± 11 | 1 ± 6 | −16 ± 14 |
| 2016 | 2 ± 6 | 1 ± 3 | −1 ± 6 | −4 ± 11 | 0 ± 6 | 5 ± 11 | 0 ± 6 | 3 ± 13 | |
| 2018 | 0 ± 2 | −2 ± 1 * | −3± 2 | 14 ± 8 * | 4 ± 3 | −6 ± 8 | 6 ± 4 | −5 ± 8 |
| VI | Year | SOS | MOS | POS | SOF | MOF | EOF | LOS1 | LOS2 |
|---|---|---|---|---|---|---|---|---|---|
| GCCN vs. GCCC | 2015 | 1 ± 7 | −2 ± 3 | −5 ± 7 | 5 ± 15 | 2 ± 6 | −1 ± 15 | 4 ± 6 | −2 ± 16 |
| 2016 | 1 ± 5 | −3 ± 2 | −6 ± 5 | −3 ± 11 | −1 ± 4 | 2 ± 11 | 2 ± 5 | 1 ± 12 | |
| 2018 | −2 ± 3 | 0 ± 2 | 1 ± 3 | 1 ± 8 | 0 ± 3 | −1 ± 8 | 0 ± 4 | 1 ± 9 | |
| NDVIBS vs. NDVIB | 2015 | 23 ± 11 * | 13 ± 4 * | 3 ± 11 | 1 ± 14 | −1 ± 6 | 4 ± 14 | −15 ± 7 * | −27 ± 18 |
| 2016 | 12 ± 5 * | 5 ± 2 * | −2 ± 5 | −15 ± 17 | −5 ± 7 | −4 ± 17 | −10 ± 7 * | −8 ± 18 | |
| 2018 | 5 ± 5 | 1 ± 2 | −3 ± 5 | 8 ± 10 | 2 ± 5 | 5 ± 10 | 1 ± 5 | −10 ± 11 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, F.; Wang, X.; Wang, C. Measuring Vegetation Phenology with Near-Surface Remote Sensing in a Temperate Deciduous Forest: Effects of Sensor Type and Deployment. Remote Sens. 2019, 11, 1063. https://doi.org/10.3390/rs11091063
Liu F, Wang X, Wang C. Measuring Vegetation Phenology with Near-Surface Remote Sensing in a Temperate Deciduous Forest: Effects of Sensor Type and Deployment. Remote Sensing. 2019; 11(9):1063. https://doi.org/10.3390/rs11091063
Chicago/Turabian StyleLiu, Fan, Xingchang Wang, and Chuankuan Wang. 2019. "Measuring Vegetation Phenology with Near-Surface Remote Sensing in a Temperate Deciduous Forest: Effects of Sensor Type and Deployment" Remote Sensing 11, no. 9: 1063. https://doi.org/10.3390/rs11091063
APA StyleLiu, F., Wang, X., & Wang, C. (2019). Measuring Vegetation Phenology with Near-Surface Remote Sensing in a Temperate Deciduous Forest: Effects of Sensor Type and Deployment. Remote Sensing, 11(9), 1063. https://doi.org/10.3390/rs11091063