1. Introduction
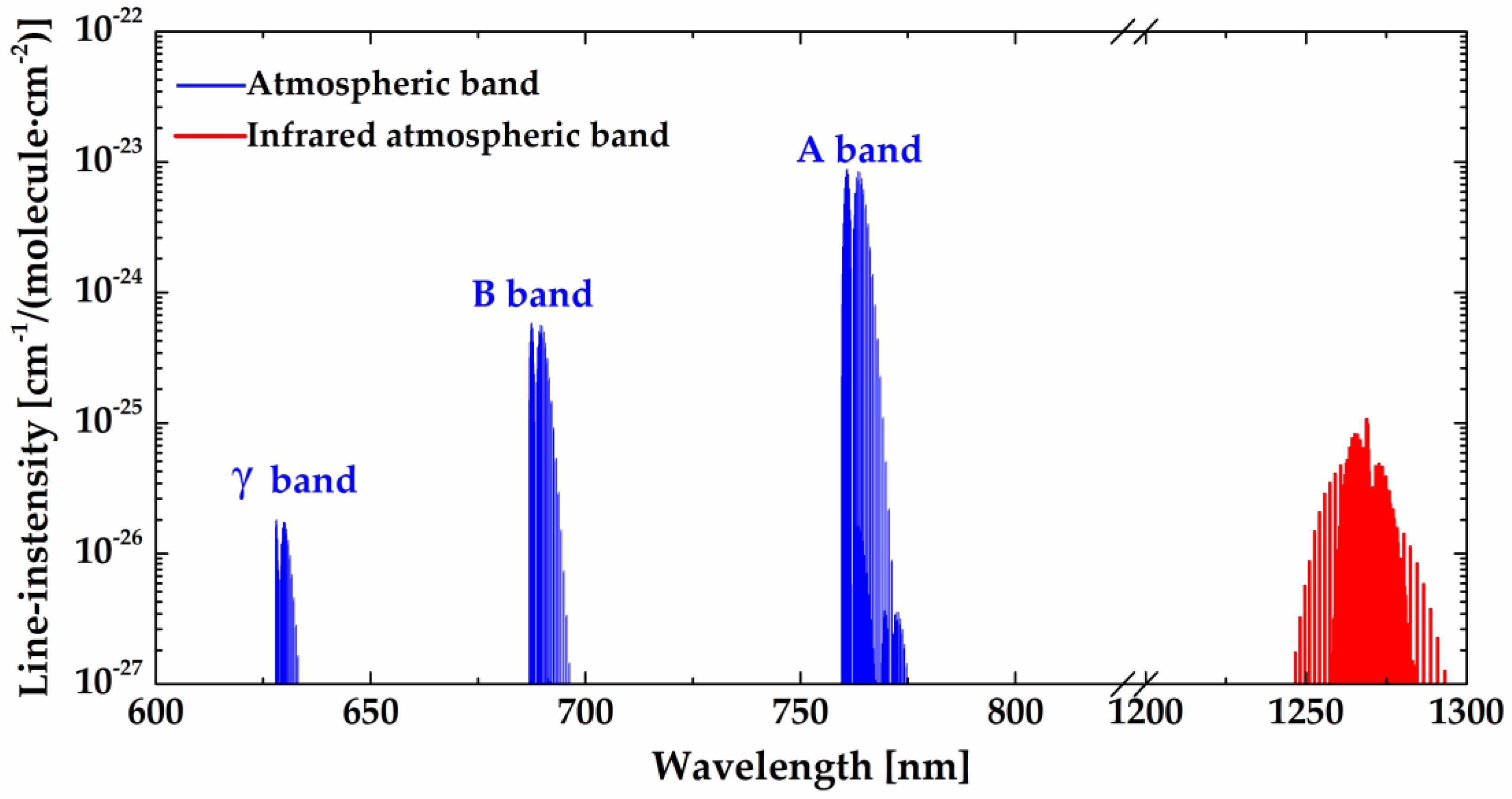
Measurements of radiation of electronically excited molecular oxygen in the atmospheric band O
2 (
) at 762 nm and in the infrared atmospheric band O
2 (
) at 1.27 µm provide fundamental probes into energy transfer, compositional structures, and thermodynamic and dynamic features of the upper stratosphere, mesosphere, and lower thermosphere. Compared with the atmospheric band, the infrared atmospheric band has a greater advantage in bright signal (the strongest radiative signal in the visible and near-infrared region of the spectrum) and extended altitude (from 20 to 120 km). Therefore, the O
2(a
1Δ
g) airglow is selected as the target source by many space-based instruments aboard rockets and satellites. Mesosphere-Thermosphere Emissions for Ozone Remote Sensing (METEORS) [
1], Optical Spectrograph and Infrared Imager System (OSIRIS) [
2], and Sounding of the Atmosphere using Broadband Emission Radiometry (SABER) [
3] prove that the O
3 altitude distribution can be extracted by incorporating O
2(a
1Δ
g) dayglow observations. The remarkable success of Spectroscopy for the Investigation of the Characteristics of the Atmosphere of Mars (SPICAM) instrument onboard the Mars Express orbiter has stimulated interest in taking measurements of O
2(a
1Δ
g) dayglow emission and their comparison with photochemical models to yield the Martian water cycle [
4]. The Mesospheric Imaging Michelson Interferometer (MIMI) supported by Canada’s StaSci program and the Waves Michelson Interferometer (WAMI) funded by NASA’s MIDEX program use a Michelson interferometer to observe the strong and weak groups of emission lines in the O
2 infrared atmospheric band to achieve simultaneous measurements of the Earth temperature and wind [
5]. More recently, the the Near-space Wind and Temperature Sensing Interferometer (NWTSI) instrument, which closely follows the WAMI and MIMI concepts, was designed ingeniously to improve the accuracies of wind and temperature measurements in the near space [
6]. Last year, Wu et al. reported the application of O
2(a
1Δ
g) dayglow for wind observation with a Doppler Asymmetric Spatial Heterodyne (DASH) type instrument from a limb-viewing satellite by measuring Doppler shifts of the emission line O
19P
18, which were detectable due to their weak self-absorption, bright radiation intensity, and large spectral separation range [
7].
High-resolution spectra of the reflected and scattered sunlight near the O
2 infrared atmospheric band also play a key role in atmospheric remote sensing, especially for the determination of CO
2 mixing ratio columns from space. Several infrared CO
2 absorption bands (e.g., 15, 4.3, 2.7, 2.0, and 1.6 µm) can be used for CO
2 mixing ratio retrievals by the use of atmospheric pressure and temperature, which can be retrieved from the O
2 absorption bands (e.g., 0.76 or 1.27 µm) [
8]. The O
2 absorption band around 1.27 µm is much weaker than the 0.76 µm band, so the radiative transfer modeling will be more accurate. In addition, the 1.27 µm band is much closer in wavelength to the CO
2 absorption bands (1.6 and 2.0 µm), which reduces uncertainties caused by spectral variations in the atmospheric path. Sun et al. evaluated the use of O
2(a
1∆
g) band in spaceborne remote sensing of greenhouse gas with modern spaceborne spectrometers [
9]. Dobler et al. discussed the feasibility of determining surface pressure by measuring atmospheric O
2 near the 1.27 µm band for determination of CO
2 mixing ratio columns with a laser absorption spectrometer [
10].
Depending on the atmospheric region, the O
2(a
1∆
g) band can be observed both in emission and absorption, with the latter dominating below about 30 km. The absorption and emission spectral features also interact with each other in atmospheric remote sensing. Sharp et al. reported the impact of ambient O
2(a
1Δ
g) on O
2 column remote sensing with satellite-based laser using absorption lines in the 1.27 µm band [
11]. Bertaux et al. evaluated the contribution of O
2(a
1Δ
g) airglow to the retrieval accuracy of CO
2 mixing ratio columns in the MicroCarb optical concept [
12]. Wu et al. analyzed the spectral interference of scattered sunlight on the O
2(a
1Δ
g) dayglow observations and evaluated wind precision with and without the effect of spectral interference [
7].
In this paper, we report the radiative transfer characteristics of the O
2 infrared atmospheric band in limb-viewing geometry.
Section 2 describes the photochemical reaction mechanism of the O
2 infrared atmospheric band, the O
2(a
1Δ
g, v’ = 0) emission rates, and the O
2(a
1Δ
g, v’ = 0, J) rotational distribution.
Section 3 presents the results of the limb spectral radiance and the line shape simulations of the O
2(a
1Δ
g) emission spectrum and multiple scattering spectrum as well as the total spectral radiance. The effects of solar zenith angle (SZA), surface albedo, and aerosol loading on the line shapes are also discussed in this Section. A concluding summary in
Section 4 completes this paper.
3. Limb Spectral Radiance
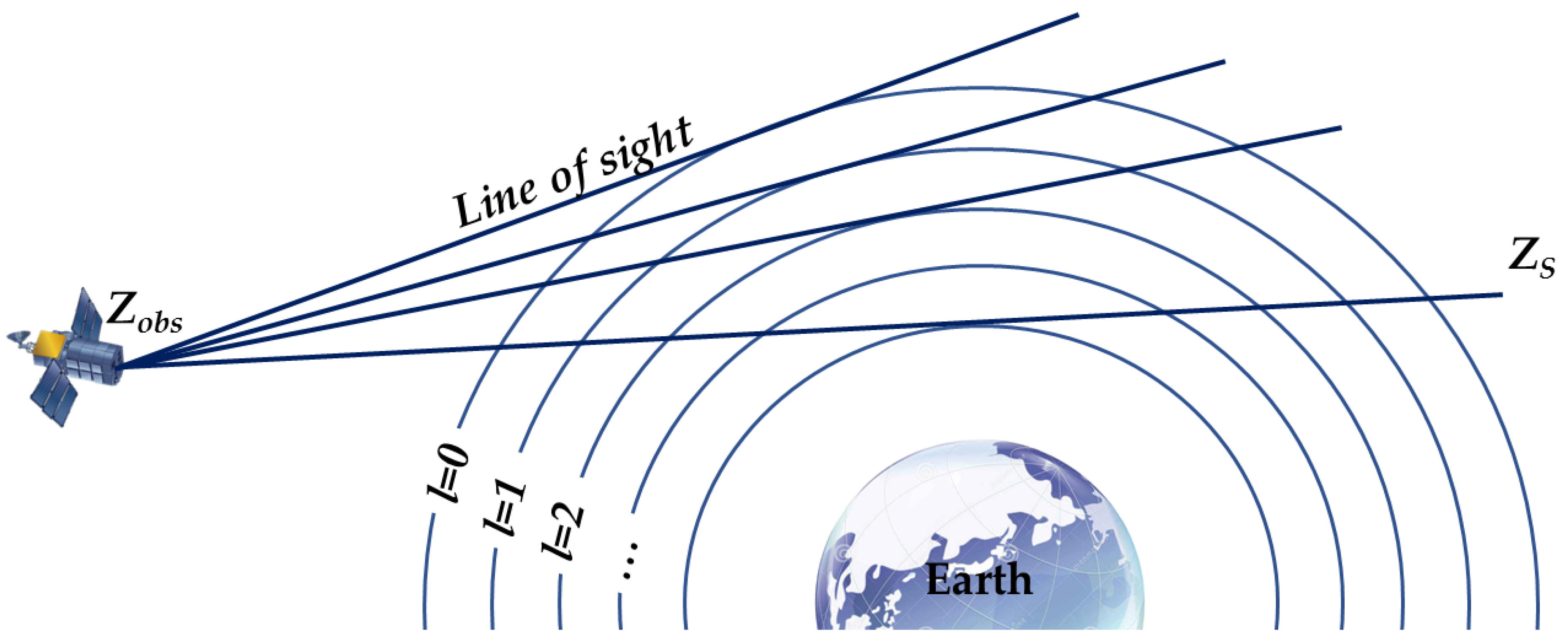
Figure 7 illustrates the construction of the path model in limb-viewing geometry. For the O
2 infrared atmospheric band, a strong self-absorption appears because of the relatively large line strength of ground state. Therefore, the limb spectral radiance is not simply a straightforward Abel-type integration of the VER profile along the path between the emitter and the observer. The brightness is modified by the attenuation along the line-of-sight path. In addition, limb brightness measurements unavoidably include Rayleigh scattering and Mie scattering, which will be subject to the extinction process in the O
2 infrared atmospheric band spectral region. For accurate calculation of the spectral brightness of the O
2 infrared atmospheric band, the absorption for both emission lines and the scattered sunlight continuum background must be taken into consideration, especially at low tangent heights.
3.1. O2(a1Δg, v’ = 0) Emission Spectrum
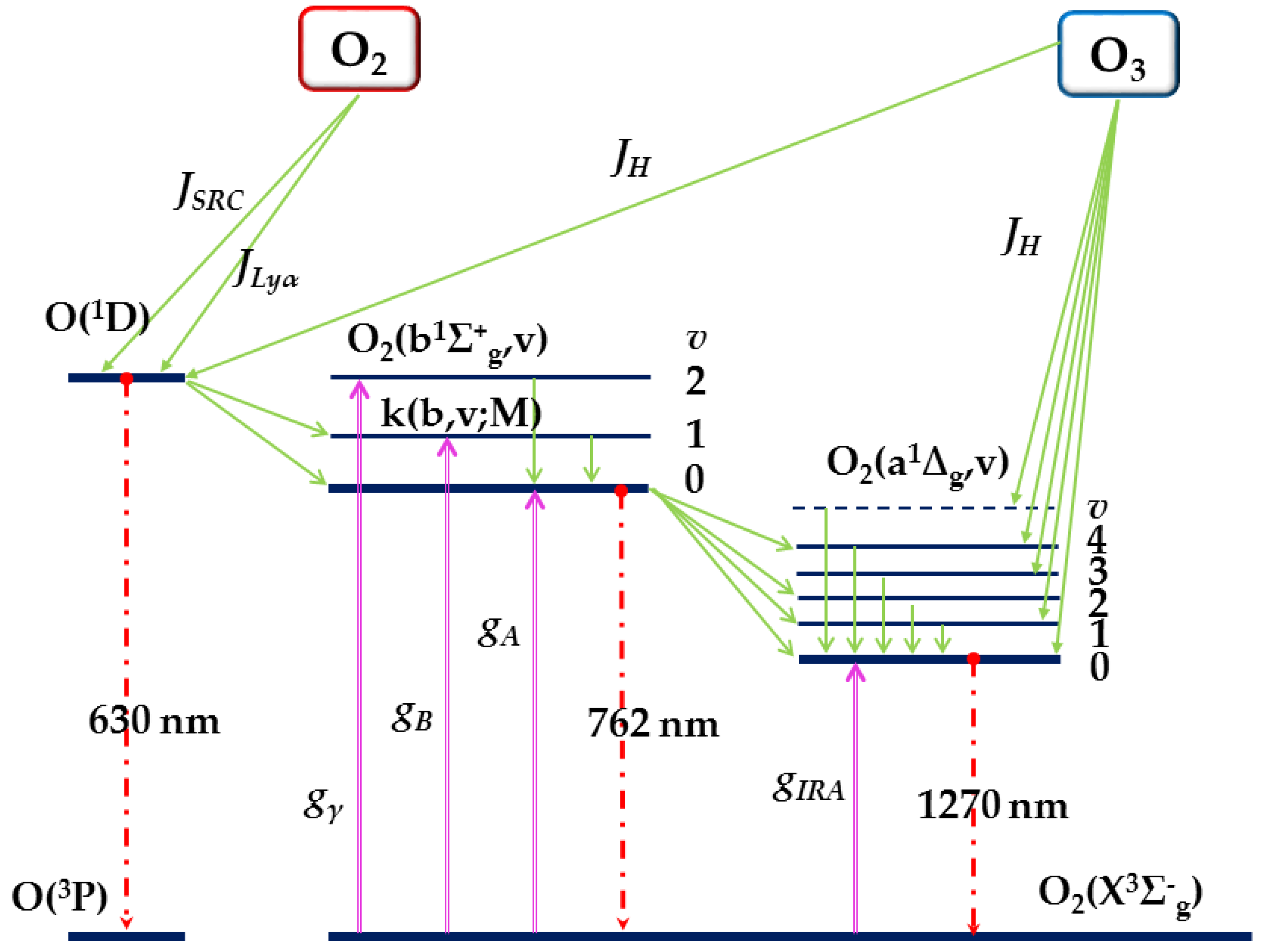
As described above, O
2(a
1Δ
g,
v’ = 0) distribution is in collisional and chemical balance. At lower altitude, the quenching-related decay of O
2(a
1Δ
g,
v’ = 0) is significant because the probability of quenching determines the lifetime of O
2(a
1Δ
g,
v’ = 0), while at higher altitude, the local thermodynamic equilibrium (LTE) is broken because of the presence of constant sources of excited molecular oxygen due to O
2 and O
3 photodissociation. O
2(a
1Δ
g) is formed mainly by photolysis of O
3 in the Hartley band below about 100 km and by energy transfer from O(
1D) above that altitude [
20,
22]. The collisional quenching of O
2(b
1Σ
g) with N
2 and O
2 also contributes to the production of O
2(a
1Δ
g), especially at 65–85 km. Thus, the distribution of O
2(a
1Δ
g,
v’ = 0) is not strongly connected to the kinetic temperature and cannot be given by the Boltzmann distribution. This behavior is referred to as nonlocal thermal equilibrium (non-LTE).
In the non-LTE case, the source function of the
transition is given by [
23]
where
is the value of the Planck function at wavenumber
and temperature
TK,
is the vibrational energy of the
state,
is the vibrational temperature and is expressed as
Here, and refer to the statistical weight of the and the states. It is important to note that the vibrational temperature, which, being substituted in Boltsmann distribution, gives the vibrational level population. Correspondingly, at the LTE conditions, the vibrational temperature becomes equal to a kinetic one.
When the self-absorption process is considered, the limb spectral brightness of any given rotational line in the O
2 infrared atmospheric band can be carried out numerically by [
23]
where
z is a certain position along the line of sight between the observation point
and the point
at the furthest extent of the limb;
and
are the upper and lower boundaries of layer
l, respectively;
α(
ν) is the molecular absorption coefficient;
n(
z) is the number density of O
2 molecules in the ground state;
is the layer absorber amount of layer
l; and
is the atmospheric transmission caused by self-absorption effect between
z and
zobs, which can be given by
It is obvious that the forward model for the spectral brightness has a strong dependence on the atmospheric temperature, which is concretely embodied in the self-absorption cross section, the line strength, and the Doppler width. In addition, the spectral brightness has a linear dependence on the VER profile.
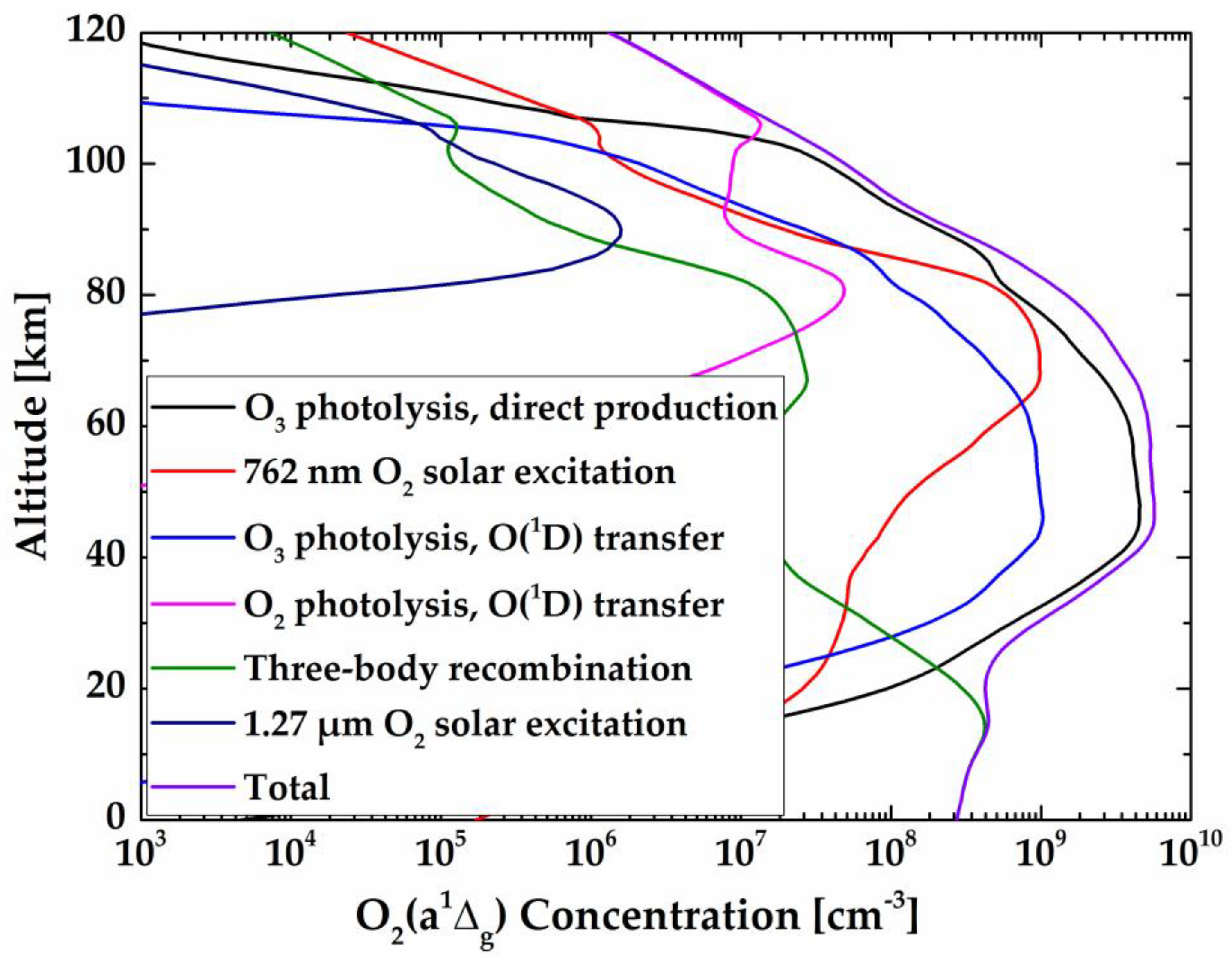
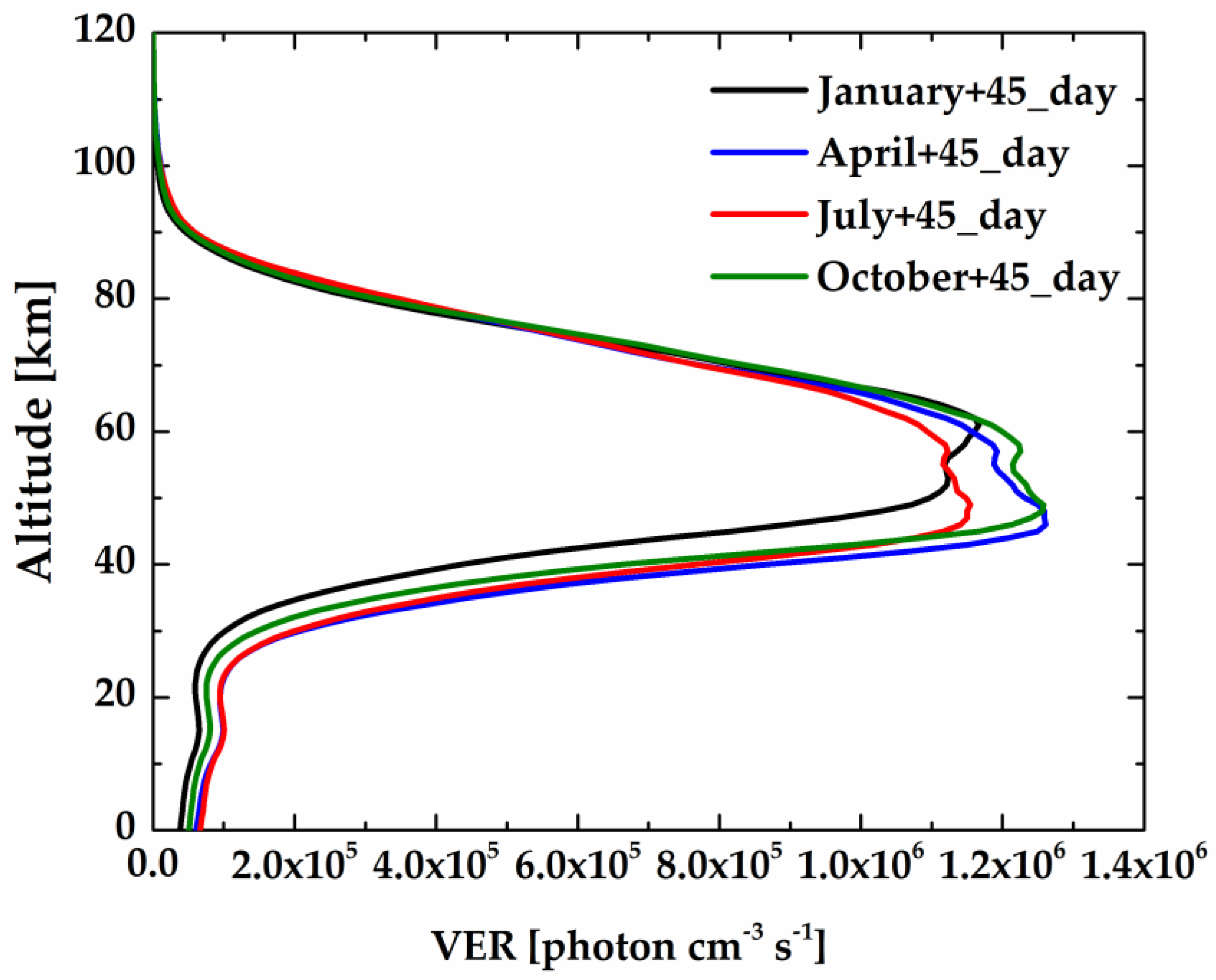
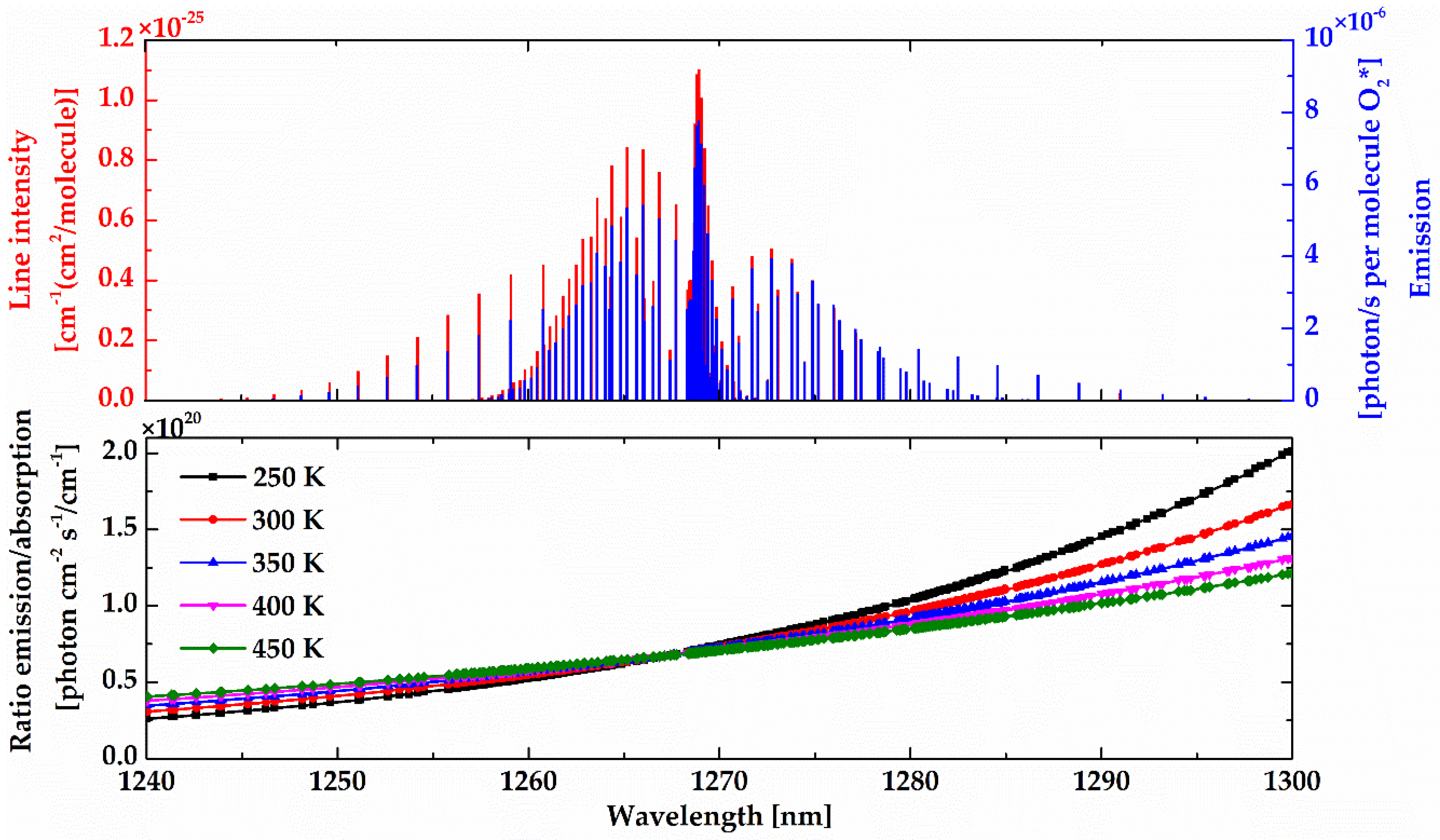
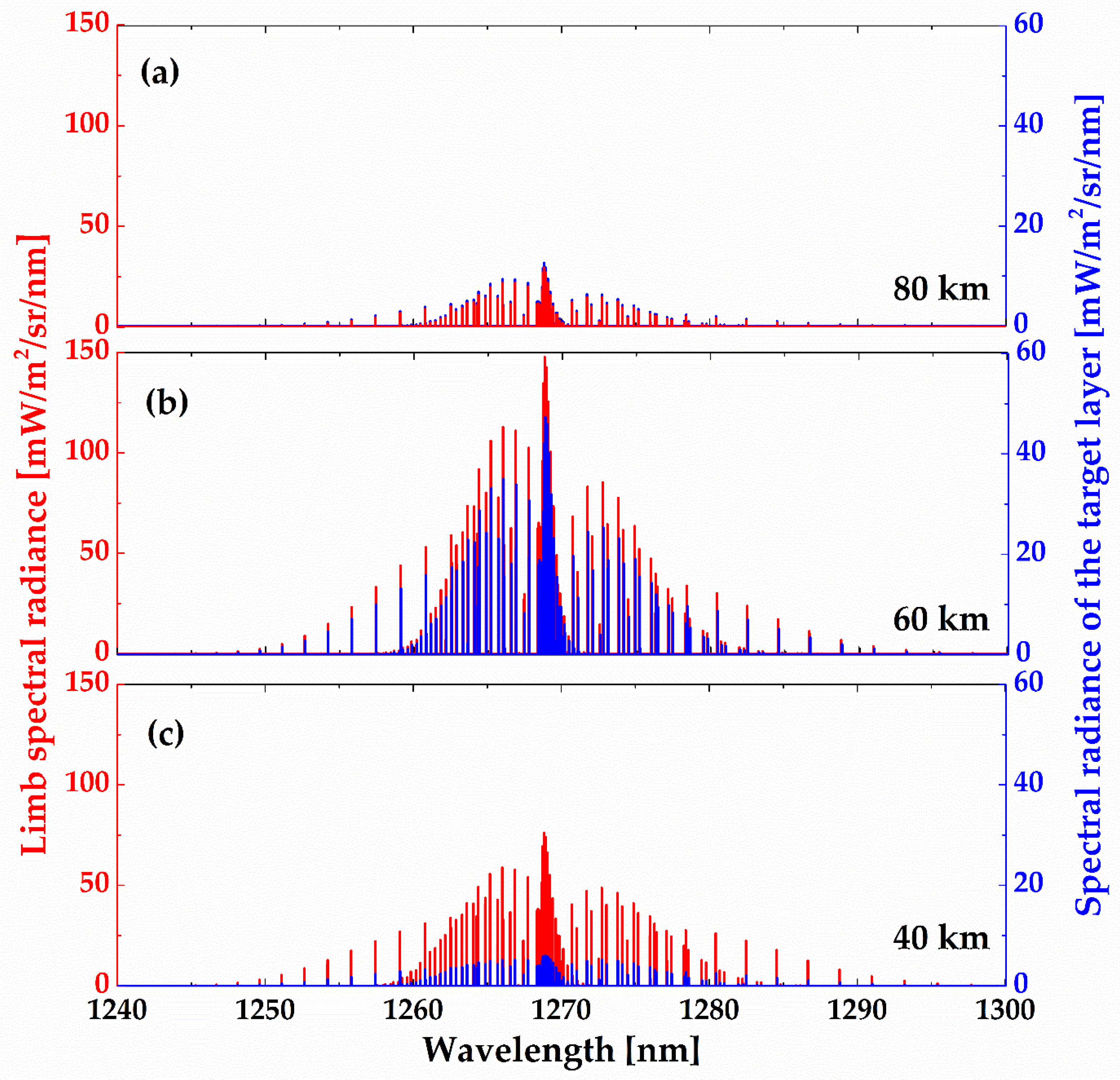
Figure 8 shows the calculated limb spectral brightness and the spectral radiance of the target layer at different tangent heights using the non-LTE model. As illustrated, the spectral radiance of the target layer comes closer to the limb spectral brightness at relatively high tangent heights (up to ~60 km), while at low altitude, the target layer radiance is relatively small compared with the limb brightness. Two factors explain this phenomenon: (1) O
2 number density leads to a strong self-absorption at low altitude and (2) the lower the altitude, the smaller the O
2(a
1Δ
g) VER.
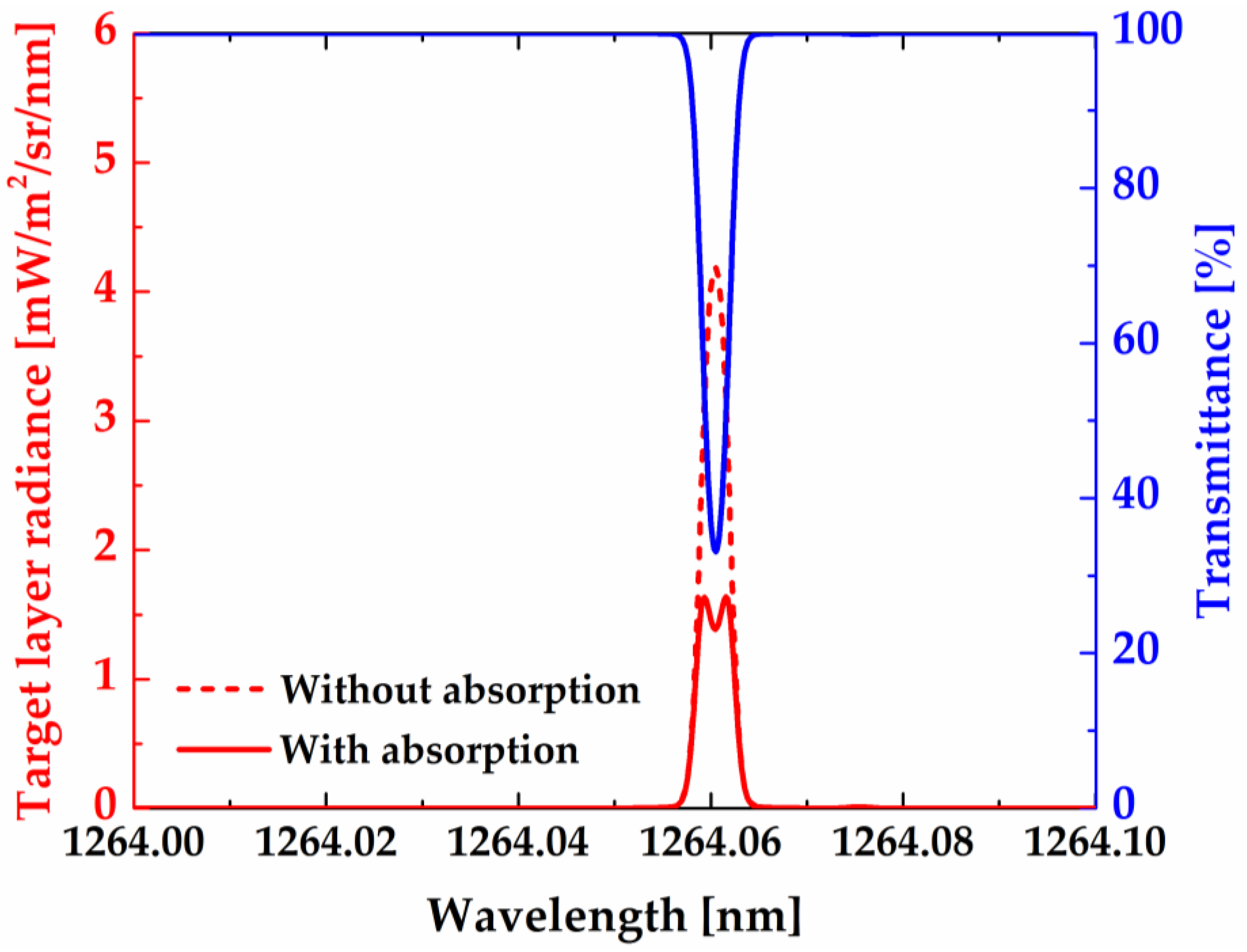
Figure 9 shows the spectral brightness and the atmospheric transmittance of a relatively strong line (R
9Q
10 at 1264.06 nm) of O
2 infrared atmospheric band at the tangent height of 50 km. For comparison, the spectral line brightness neglecting self-absorption is also displayed in the same figure. As illustrated, the self-absorption of the R
9Q
10 line is significant even at 50 km tangent height. As a type of Doppler line shape, the value of the absorption cross section is large at the line center and decreases toward the two wings, so the process of self-absorption leads to a spectral line broadening for the emission line. Due to this broadening effect, the WAMI, MIMI, and NWTSI instruments cannot provide meaningful Doppler temperature, and the rotational temperatures need to be measured by observing relative radiation intensity of three rotational lines.
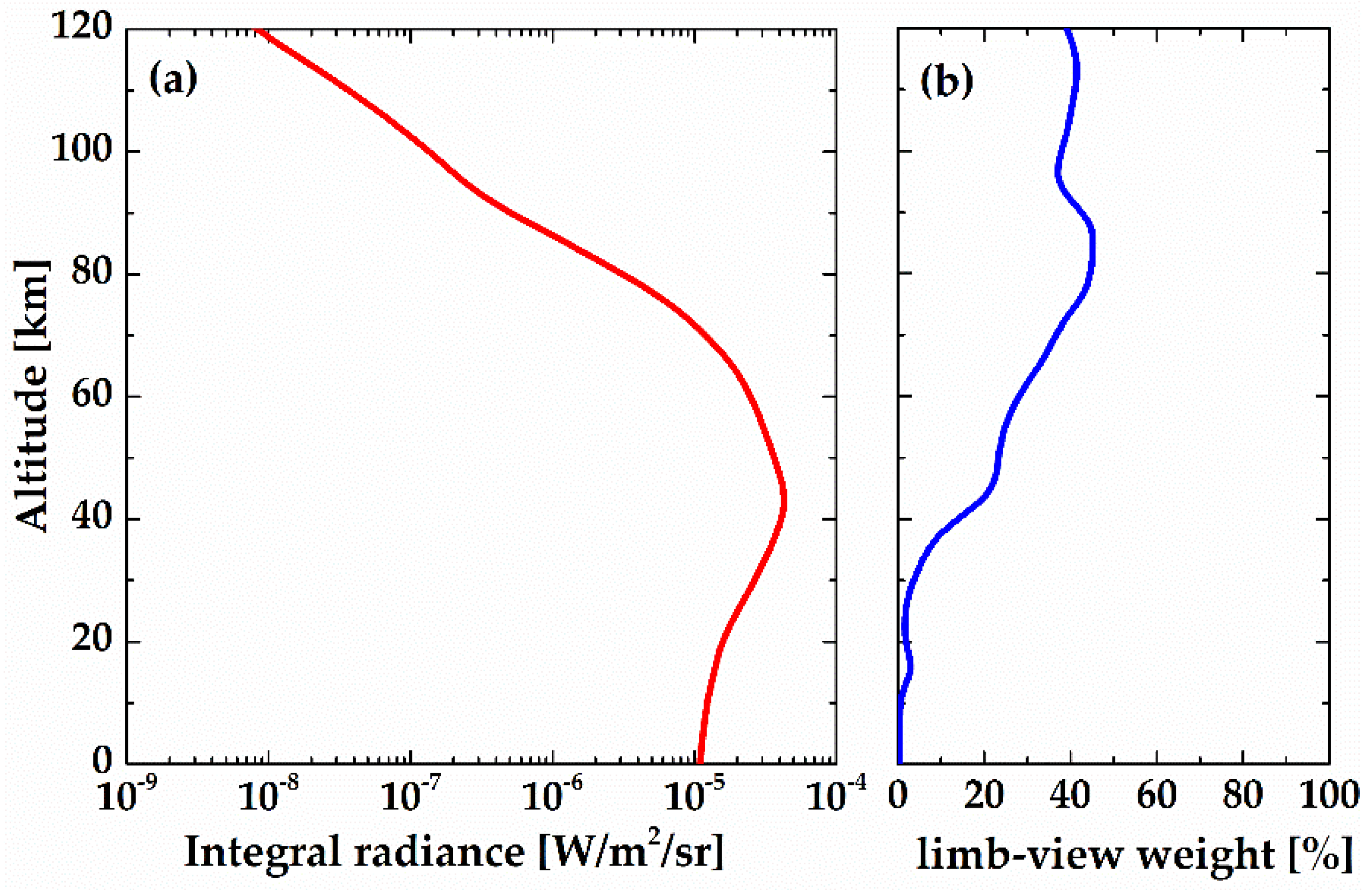
The integral radiance and limb-view weight work together to affect the retrieval accuracy in satellite remote sensing applications. The radiance values and weight profile of the R
9Q
10 line varying with the tangent altitude are shown in
Figure 10. At lower heights, the contribution of the target layer is very small because of the small VER and strong self-absorption. As the tangent height increases, the limb-view weight also increases, which leads to an increase in measurement precision. However, at higher altitudes, the integral radiance goes down exponentially with height, which is certain to cause decrease in the signal-to-noise ratio. Therefore, if the R
9Q
10 line is used as the target source, the atmospheric information of the tangent point can be obtained from 20 to 90 km, where both the integral radiance and the weight value are large enough.
3.2. Scattering Absorption Spectrum
The brightness spectrum of the O
2 infrared atmospheric band scattered by the Earth’s atmosphere is strongly marked by rotational lines of molecular oxygen. Considering source terms reflected by clouds and the Earth’s surface, and scattered by molecules and particles, the limb brightness spectrum with absorption characteristics can be expressed as [
24]
where
Here,
is the Rayleigh or Mie scattering rate per unit volume,
is the total Rayleigh or Mie scattering cross section,
is the attenuated solar flux,
is the top-of-atmosphere solar spectral irradiance,
is the SZA, and
is the volume scattering rate at the scattering altitude due to ground reflected light. For our study, we adopted the expression of
provided by Hays, et al. [
25].
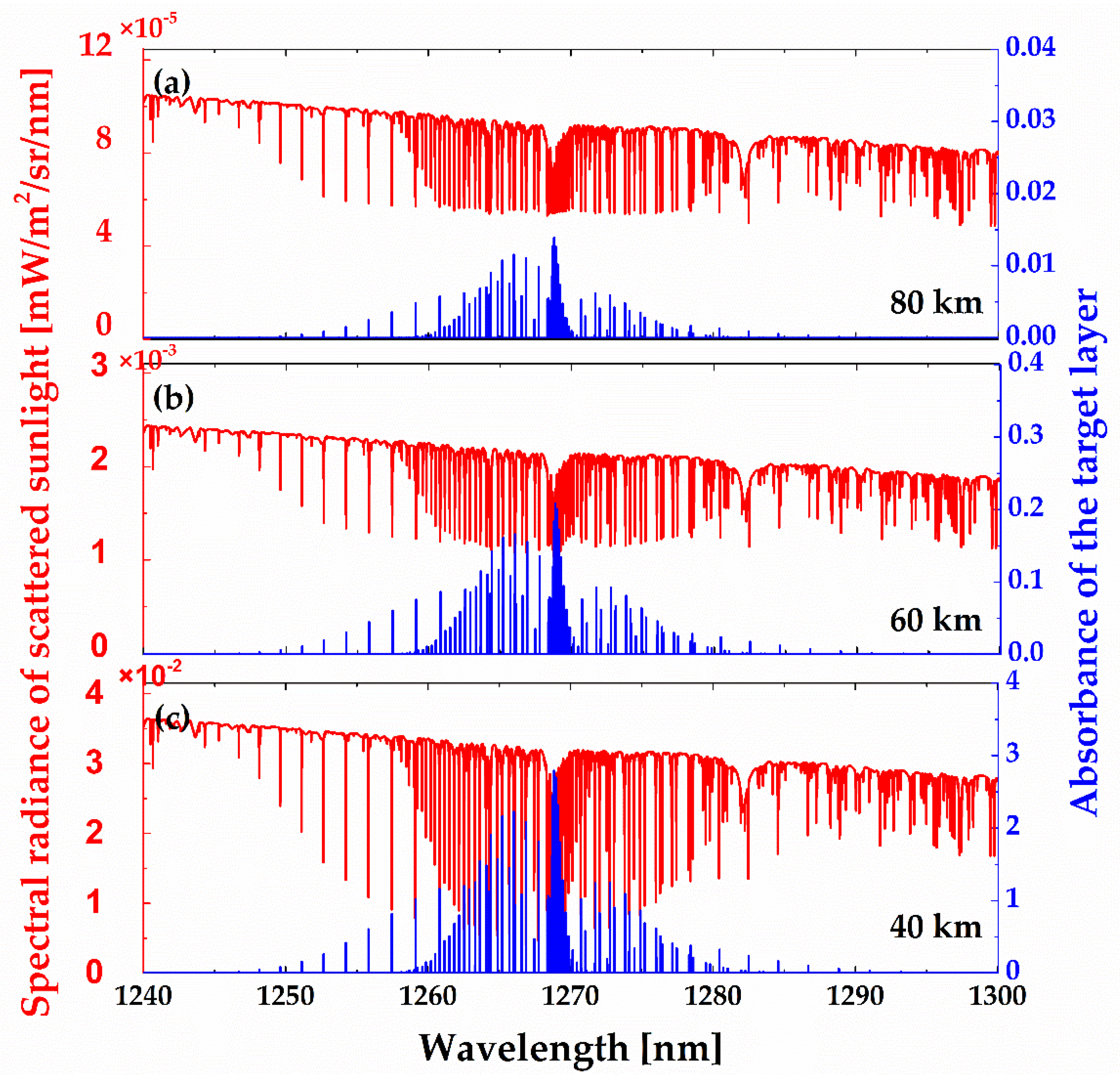
Figure 11 shows the limb spectral radiance of the scattered sunlight at 1.27 μm and the absorbance of the target layer at tangent heights of 40, 60, and 80 km. The spherical adding and doubling methods developed by Abreu, et al. were adopted in this work [
26]. As illustrated, the scattered sunlight radiance as well as the absorbance of the target layer goes down exponentially with tangent height. The O
2 absorption spectrum is quite similar to limb emission spectrum in shape (shown in
Figure 8), while emission lines are much narrower than absorption lines because pressure broadening can be ignored at high altitude. They also have different distributions in spectrum because the emission rate and the absorption cross section are influenced differently by atmospheric temperature due to their different populations of rotational levels (as noted before).
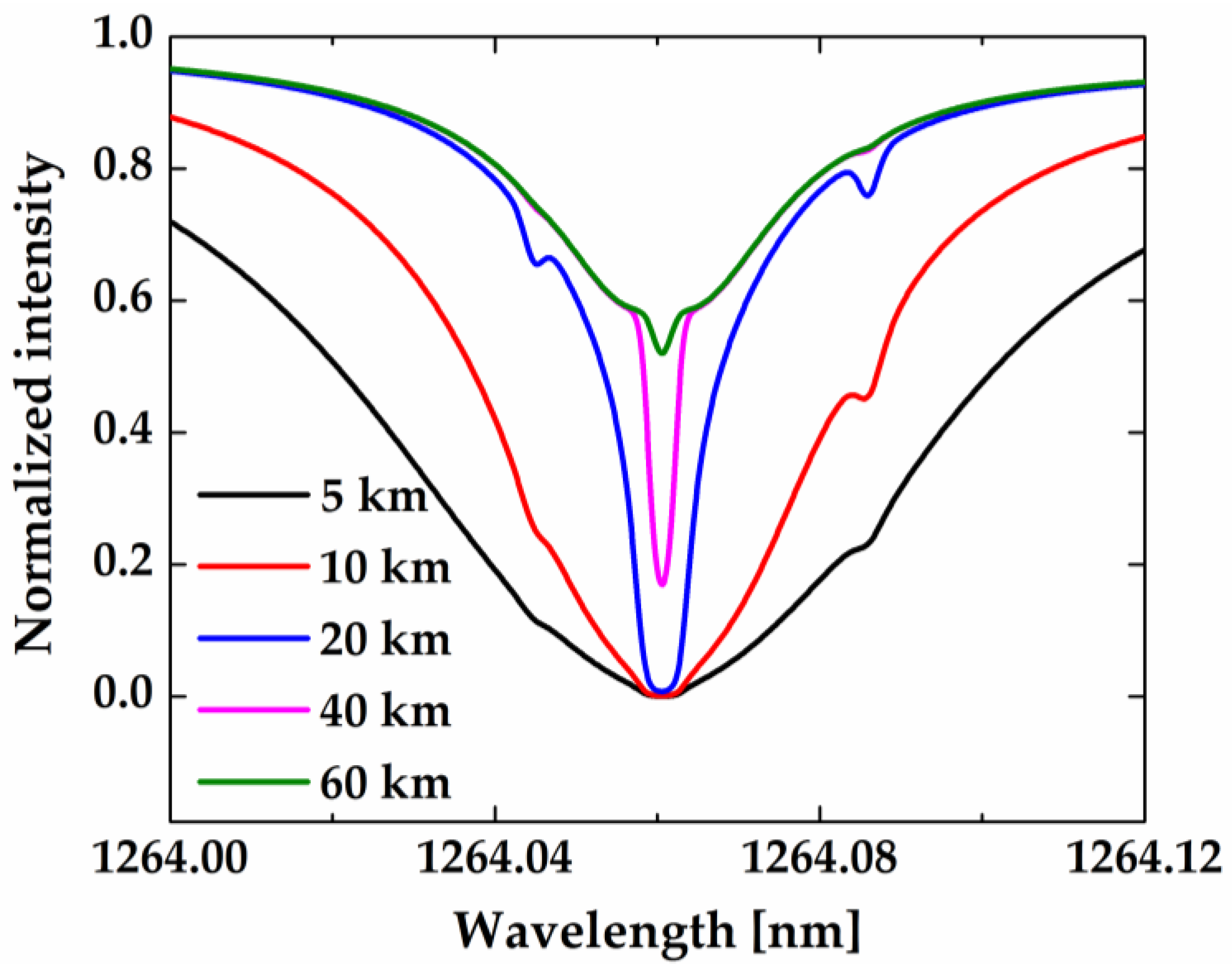
In order to better follow the spectral shape development as a function of tangent height, a close-up of one of these lines (red in
Figure 11) is shown in
Figure 12 after normalization to the maximum intensity (the asymptotic value). As can be seen, the absorption lines are very broad at tangent heights of 5 and 10 km because of the atmospheric scattering from low altitudes, where collision broadening dominates. At tangent heights higher than 20 km, the whole line shape becomes a superposition of two components: (1) a narrow one, which is sensitive to the atmospheric condition at the tangent point, and (2) a much broader component, which is contributed by the reflection and scattering taking place near the ground and the lower atmosphere.
3.3. Total Spectral Radiance
The spectral brightness that would be observed either in absorption or in emission can be calculated using the above-described airglow emission or scattering absorption radiative transfer models, respectively. The scattering absorption spectrum
is obtained separately using the line-by-line multiple scattering radiative transfer model. The emission brightness
is calculated under the assumption that the attenuation along the line-of-sight path is caused by single scattering and self-absorption. Total spectral radiance
in both emission and absorption can be obtained by combining these two components together simply because the scattering and emission processes are independent of each other in both physical and mathematical models [
27]:
The surface characteristics play a key role in determining the line shape of the total spectral brightness for limb observation.
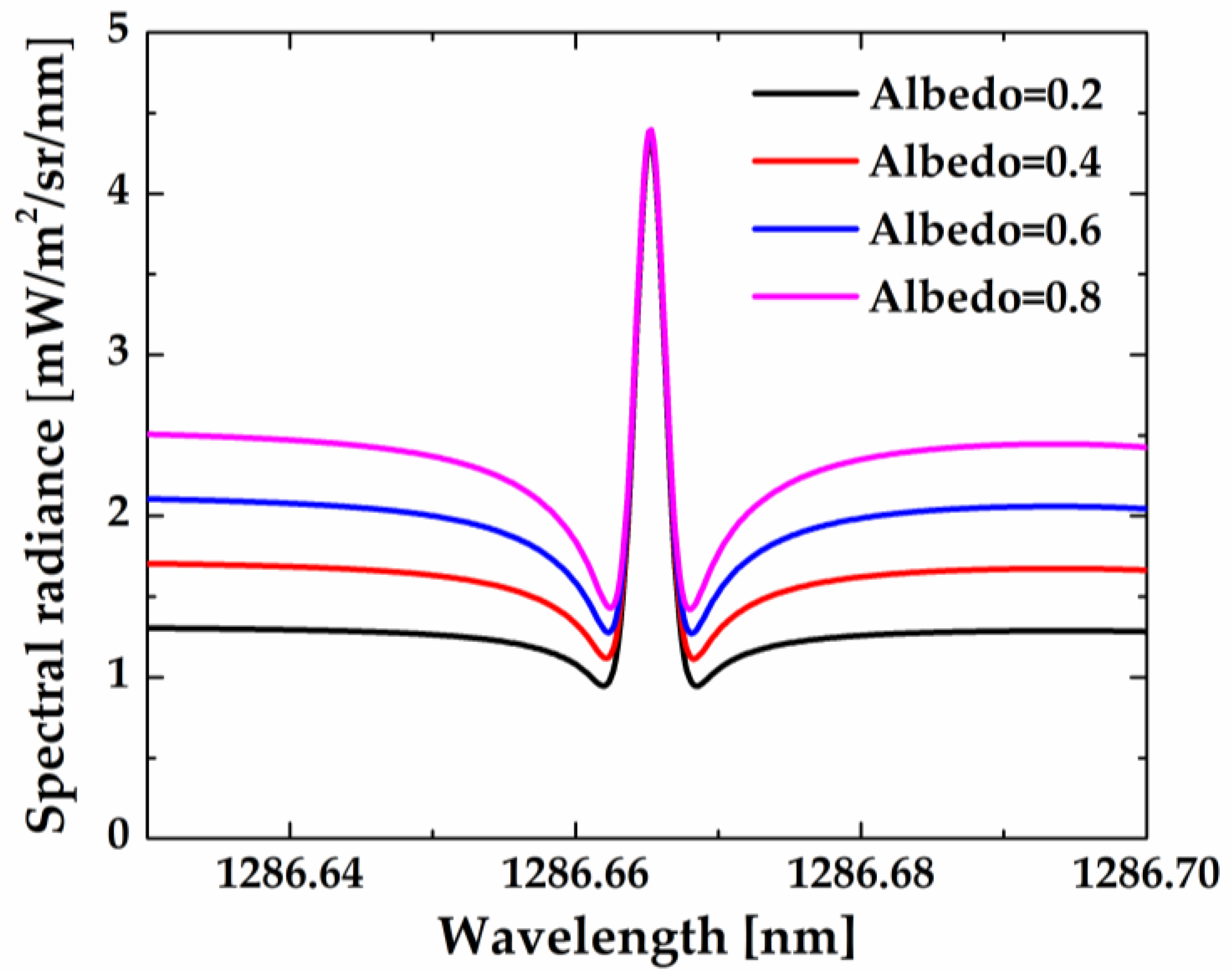
Figure 13 shows the asymptotic value and line center intensity as a function of albedos at the tangent height of 15 km. The asymptotic value increases with increasing albedo, while the line center intensity remains the same.
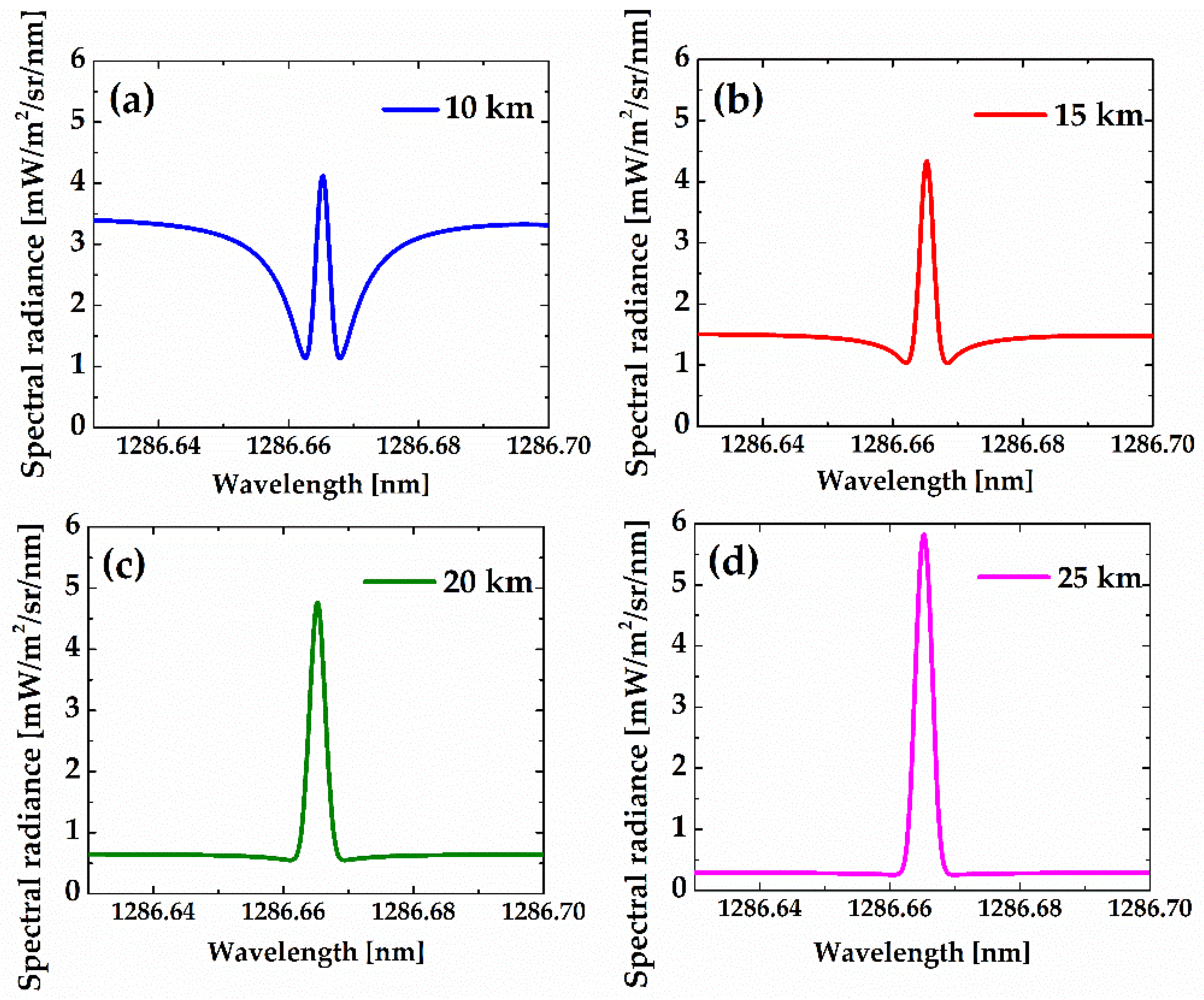
Figure 14 shows the total spectral brightness of the R
9Q
10 line for albedos equal to 0.2 at different tangent heights. As illustrated, the total spectral brightness can be observed both in absorption and emission, with the latter dominating above 20 km. The O
2 infrared atmospheric band line is very broad at low altitudes but observable in narrow emission at higher altitude because thermal motion rather than collision dominates the broadening process. Note that the wings of the line vary significantly with tangent height, both in width and magnitude. The line center, however, develops a peak in the absorption valley. At higher tangent heights (>20 km), the asymptotic value goes down exponentially, but the general shape of the lines remains unchanged.
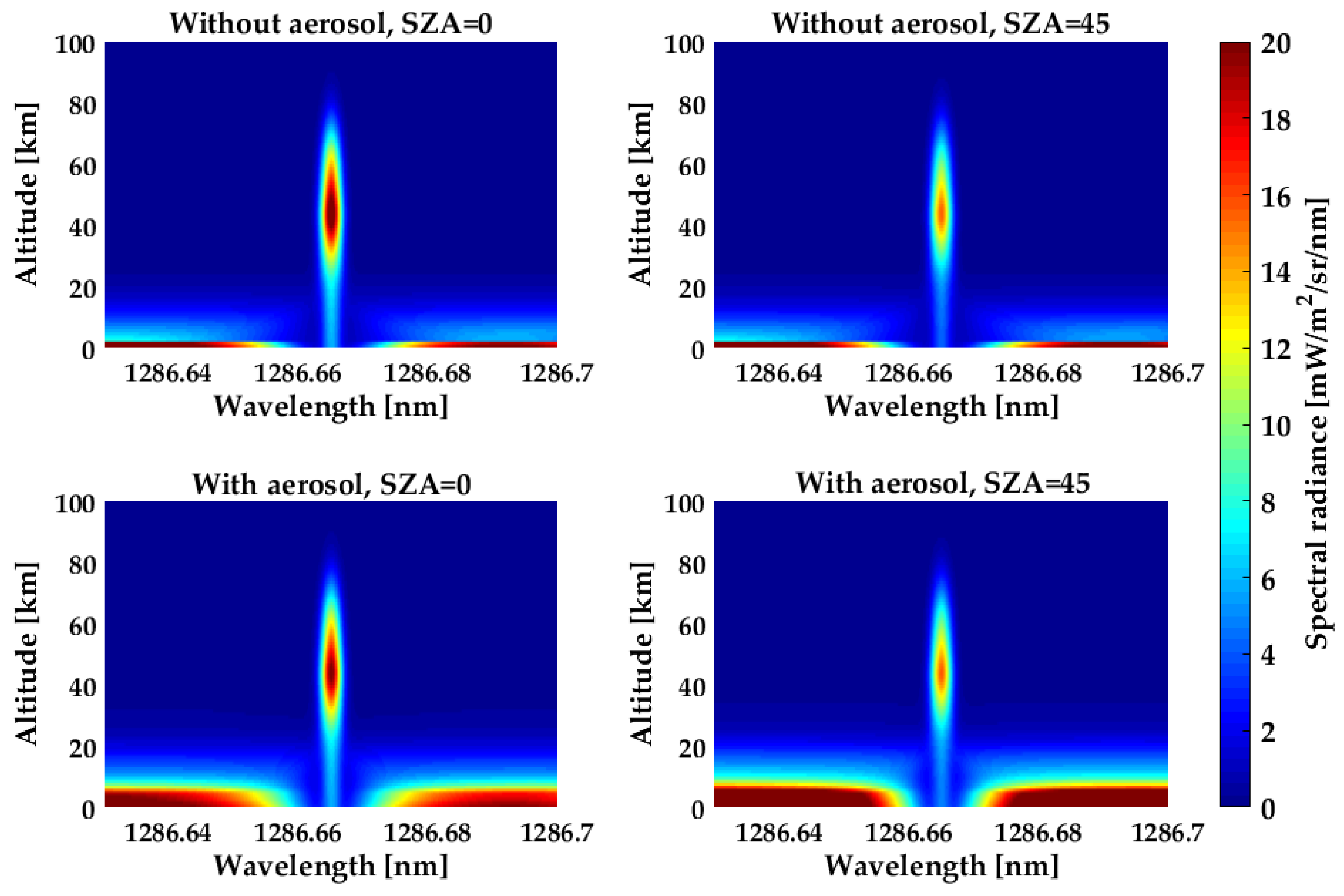
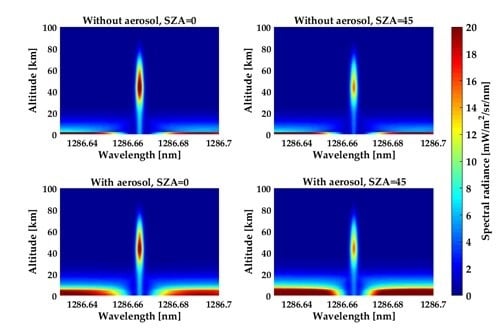
The SZA and atmospheric aerosol have significant influence on both the asymptotic value and line center intensity.
Figure 15 shows the variations in total spectral brightness with the SZA and atmospheric aerosol at the tangent heights from 0 to 100 km. Wavelength is the abscissa, tangent height is the ordinate, and the scale of the spectral brightness is made by the pseudo color intensity in each graph. Several features are apparent in this figure. The asymptotic intensity increases by approximately two times when the SZA grows from zero to 45°. The impact of this effect can be explained by Mie and Rayleigh scattering phase functions: scattering angle becomes smaller as the SZA increases, and the decreasing scattering angle leads to an increase in scattered signal. The variation in the line center is not as large as it is in the asymptote. This behavior is due to the fact that the intensity in the line center depends not only on the amount of scattered light but also on absorption and emission. The variation in the SZA changes the path length of the solar radiation passing through, which causes a change in the solar energy being deposited at different heights. As the column densities through the O
2 and O
3 increase, the optical depth increases, while the photolysis rate decreases. At tangent heights above 80 km, the SZA has a smaller effect on the O
2(a
1Δ
g) concentration because of the gradual disappearance of O
3. The production of O(
1D) becomes the dominant source of O
2(a
1Δ
g), and this production steadily decreases with the SZA. The atmospheric aerosol is important in determining the asymptotic intensity. Similar to SZA, the center intensity is not a strong function of the atmospheric aerosol. The center intensity variations with Mie scattering are mostly due to changes in extinction coefficient caused by the existence of aerosol. Changes in the intensity of the absorption feature at low tangent height lines of sight are masked by the dominant emission feature of the mesosphere.
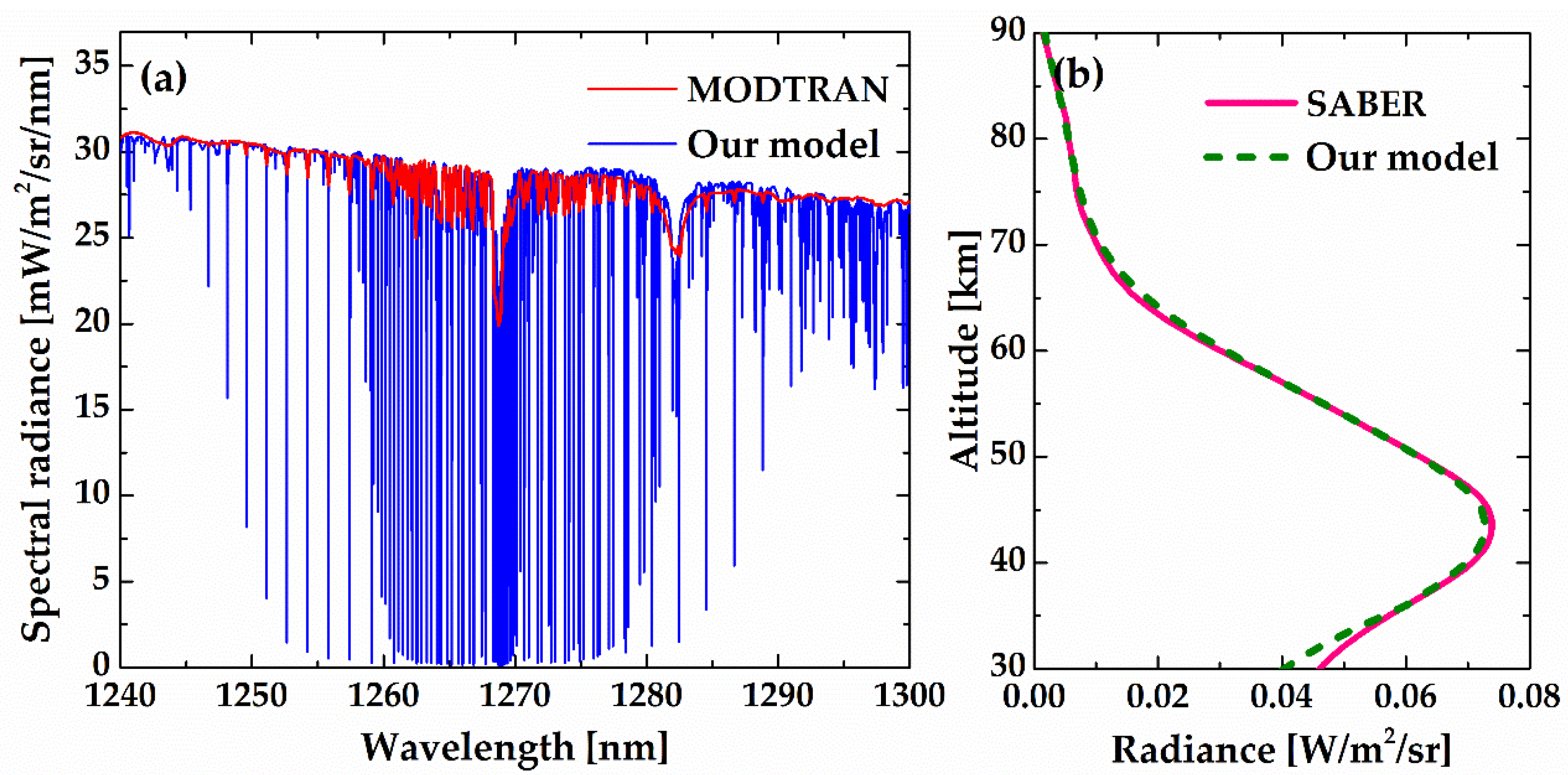
In order to validate the emission–absorption transfer algorithm, we compared our model results with those obtained from MODTRAN4 [
28] and the TIMED–SABER measurements [
29,
30] for the same latitude (48.9°N), date (10 July) and SZA (35.5°).
Figure 16a shows the comparison between our model and the MODTRAN4 result at tangent height of 20 km with a surface albedo of 0.2 and an aerosol model of stratospheric background, which validates the multiple scattering algorithm. Due to the resolution limitation of MODTRAN4, the MODTRAN result was calculated at 1 cm
−1. The spectral resolution can reach 0.001 cm
−1 with our model, in which the multiple scattering algorithm is achieved by means of a line-by-line approach.
Figure 16b shows the O
2(a
1Δ
g) band brightness calculated by our model and that measured by the TIMED–SABER. The agreement between the observed band brightness and model prediction is remarkable, which suggests that the emission algorithm, including the non-LTE source function and self-absorption calculation, is very sound.
4. Conclusions
We have presented simulations of the radiative transfer characteristics of the O2 infrared atmospheric band in limb-viewing geometry. Both the non-LTE effect and multiple scattering were taken into account for developing the radiative transfer model. This model incorporates the most recent cross sections, spectroscopic parameters, rate constants, and solar fluxes. The limb spectral brightness observed in both absorption and emission was first calculated separately. The scattering absorption spectrum was carried out using the line-by-line multiple scattering radiative transfer model. The emission brightness was calculated based on the non-LTE theory, assuming that the attenuation along the line-of-sight path is caused by single scattering and self-absorption. Total spectral radiance was then obtained by combining these two components together. For the absorption spectrum simulation, the spherical adding and doubling methods were used. In the calculation of emission brightness, the source function under condition of non-LTE was used, which differed greatly from the Planck’s law. The emission rates for calculation of source function were carried out based on six major O2(a1Δg) production mechanisms, and the vibrational temperature was obtained to describe the excitation degree of the excited vibrational state relative to the ground state. The variations in the limb spectral brightness with surface albedo, atmospheric aerosol, and SZA were simulated, and their basic characteristics were discussed. The surface characteristics, SZA, and atmospheric aerosol play key roles in determining the asymptotic value of the line shape, while the line center intensity is just sensitive to changes in atmospheric aerosol and SZA. It is expected that a better understanding of the radiative transfer characteristics of the O2 infrared atmospheric band will certainly improve the accuracy of near-infrared spaceborne instruments, such as WAMI, MIMI, and NWTSI, for which such new knowledge of non-LTE and multiple scattering could be used for an improved retrieval of atmospheric temperature and wind field.