Spectral Response Analysis: An Indirect and Non-Destructive Methodology for the Chlorophyll Quantification of Biocrusts
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Spectral Measurements and Biocrust Coverage Estimation
2.3. Chlorophyll a Extraction
2.4. Spectral Data Analysis
2.4.1. Data Transformation
2.4.2. Band Ratios and Standard Spectral Indexes
2.5. Statistical Analysis
3. Results
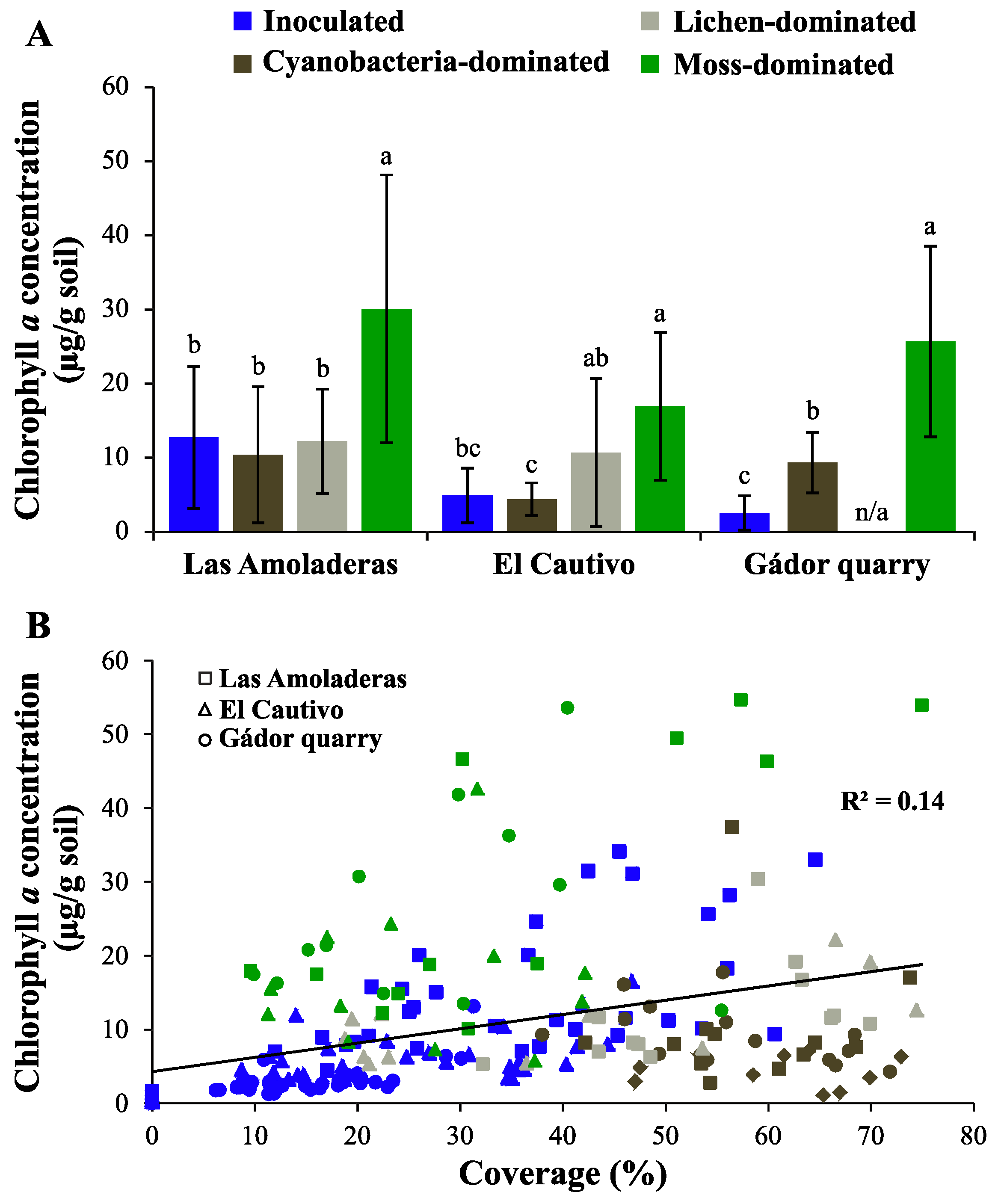
3.1. Chla of Different Biocrust Types
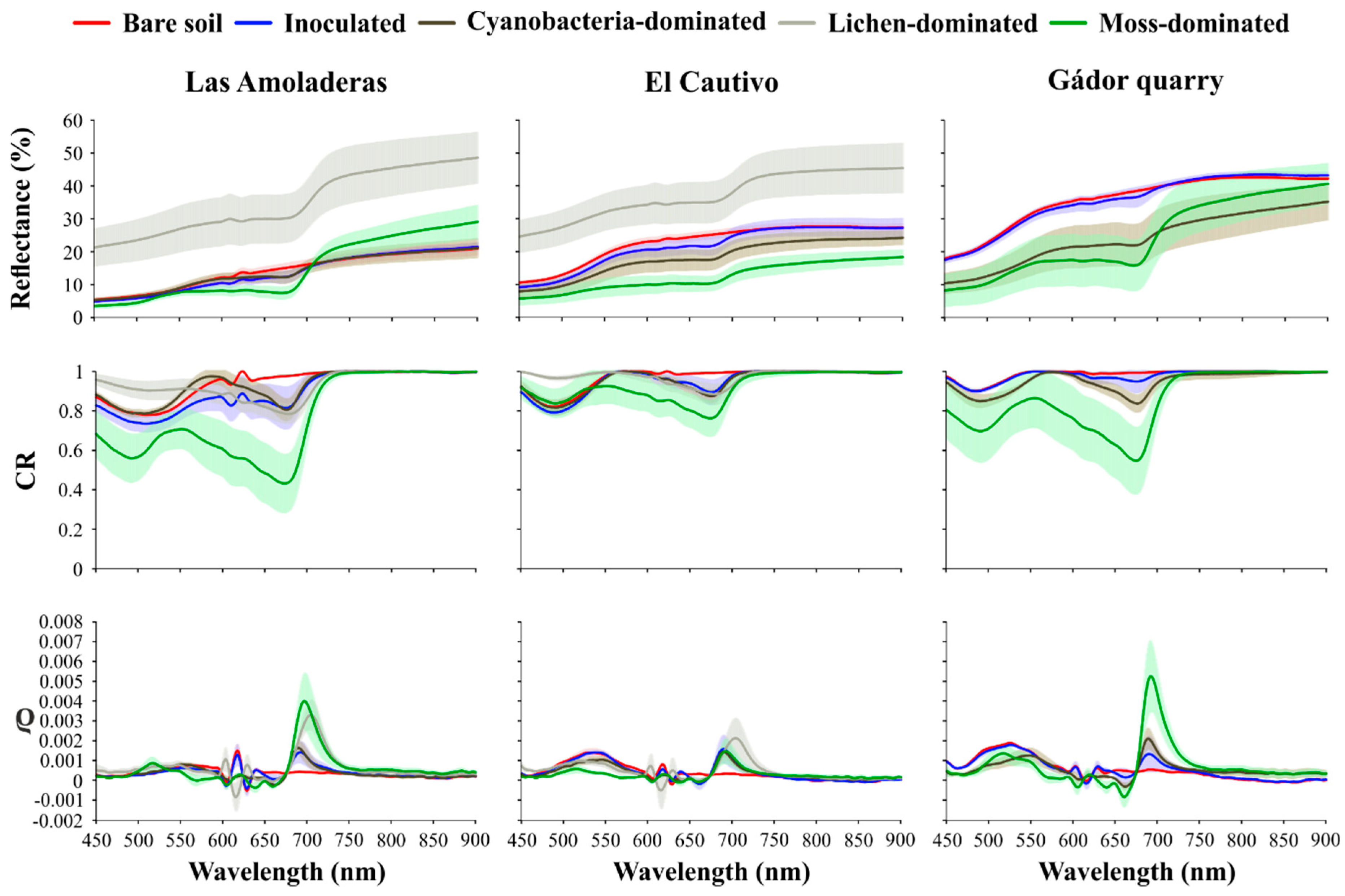
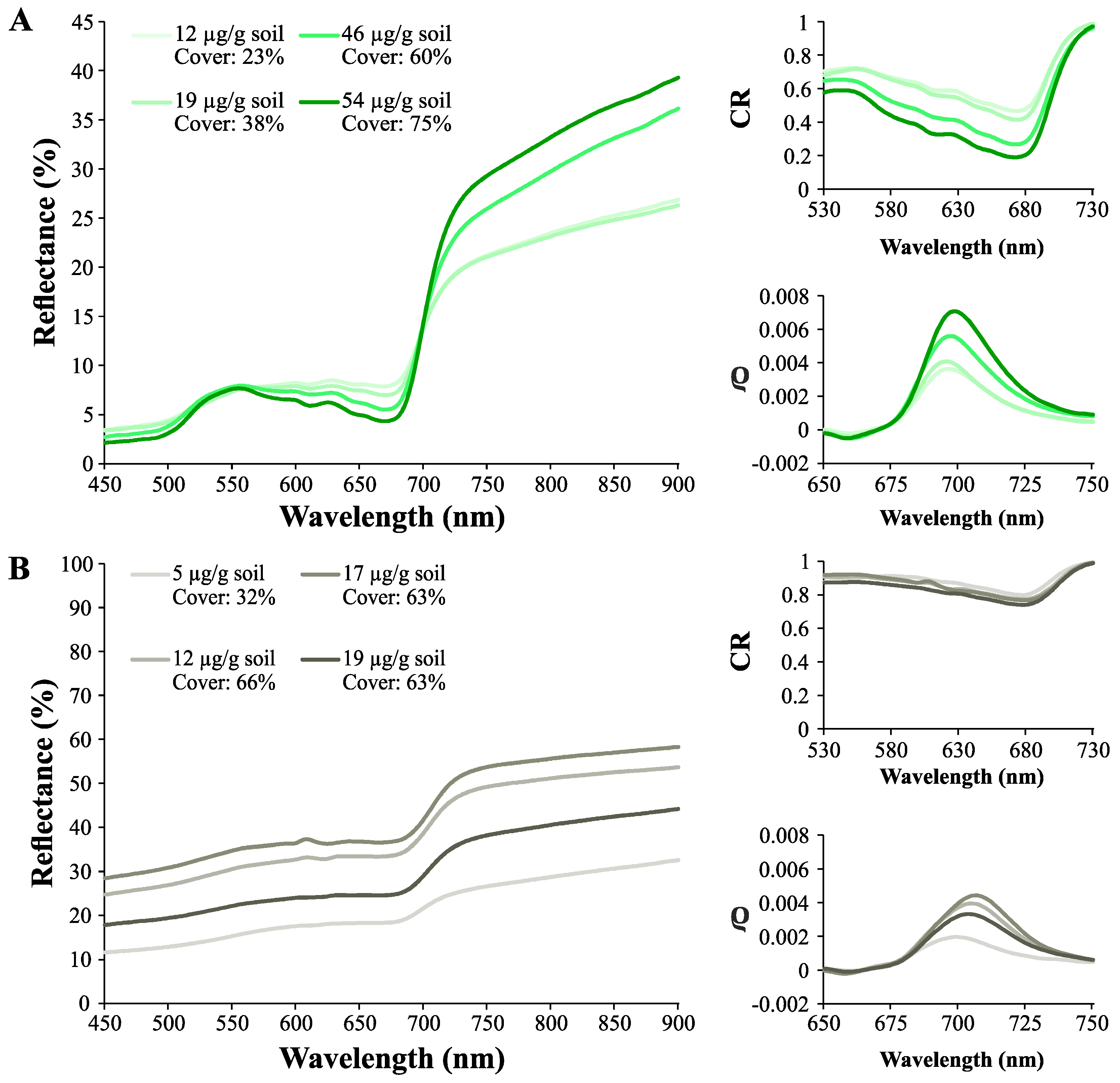
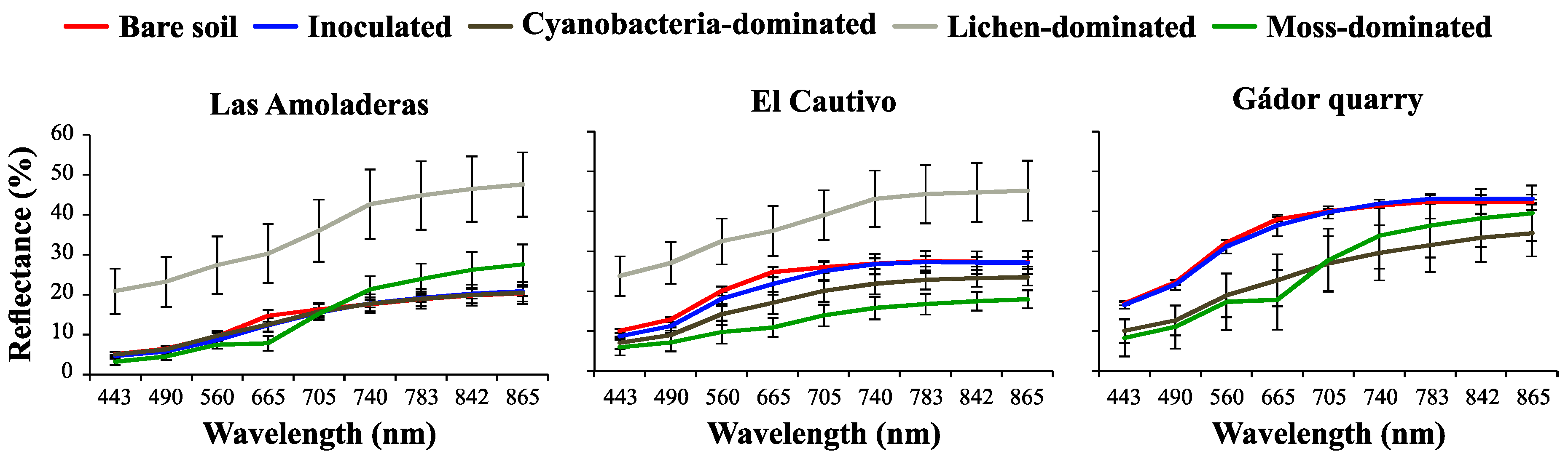
3.2. Effect of Biocrust and Chla on the Soil Surface Spectra
3.3. Chlorophyll a Predictions
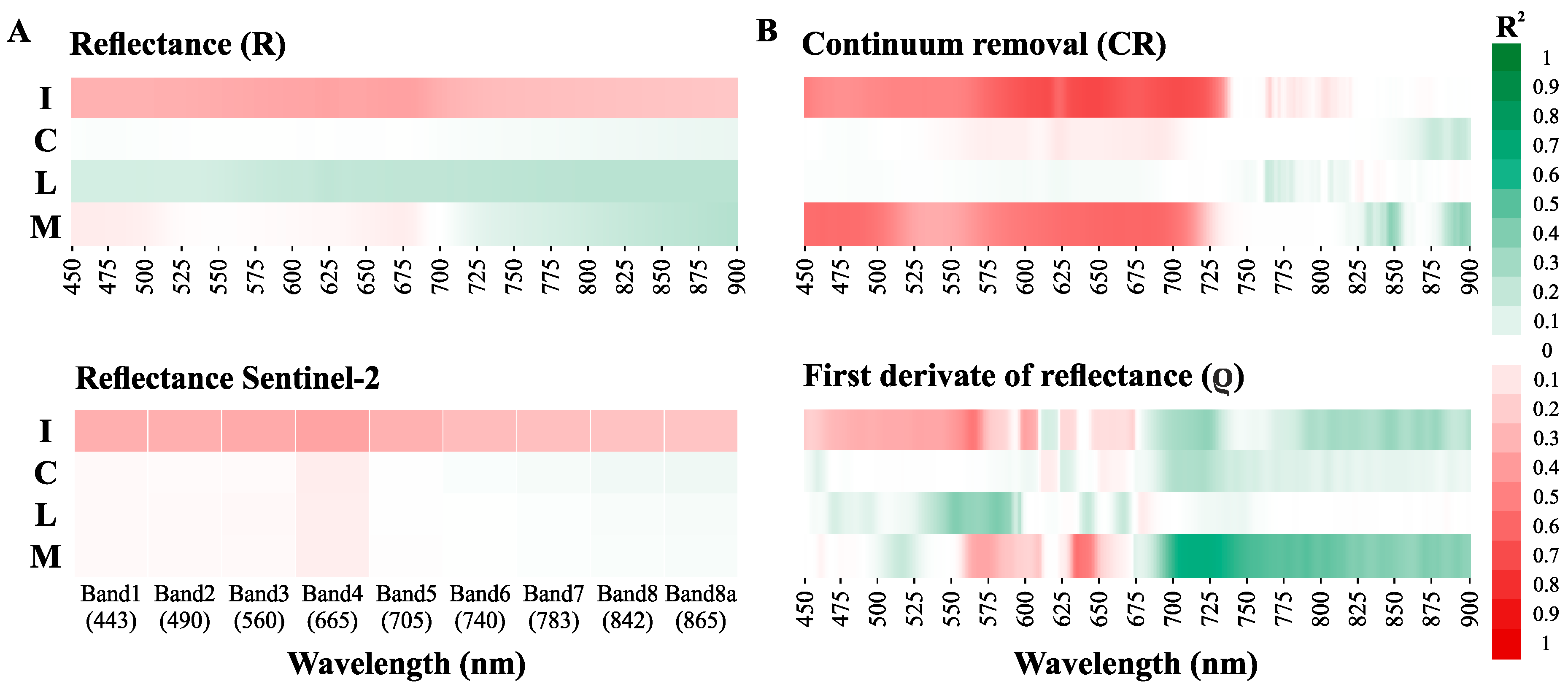
3.3.1. Direct Chla Predictions from the Surface Reflectance, CR, and ρ
3.3.2. Chlorophyll a Predictions from the Normalized Band Ratios
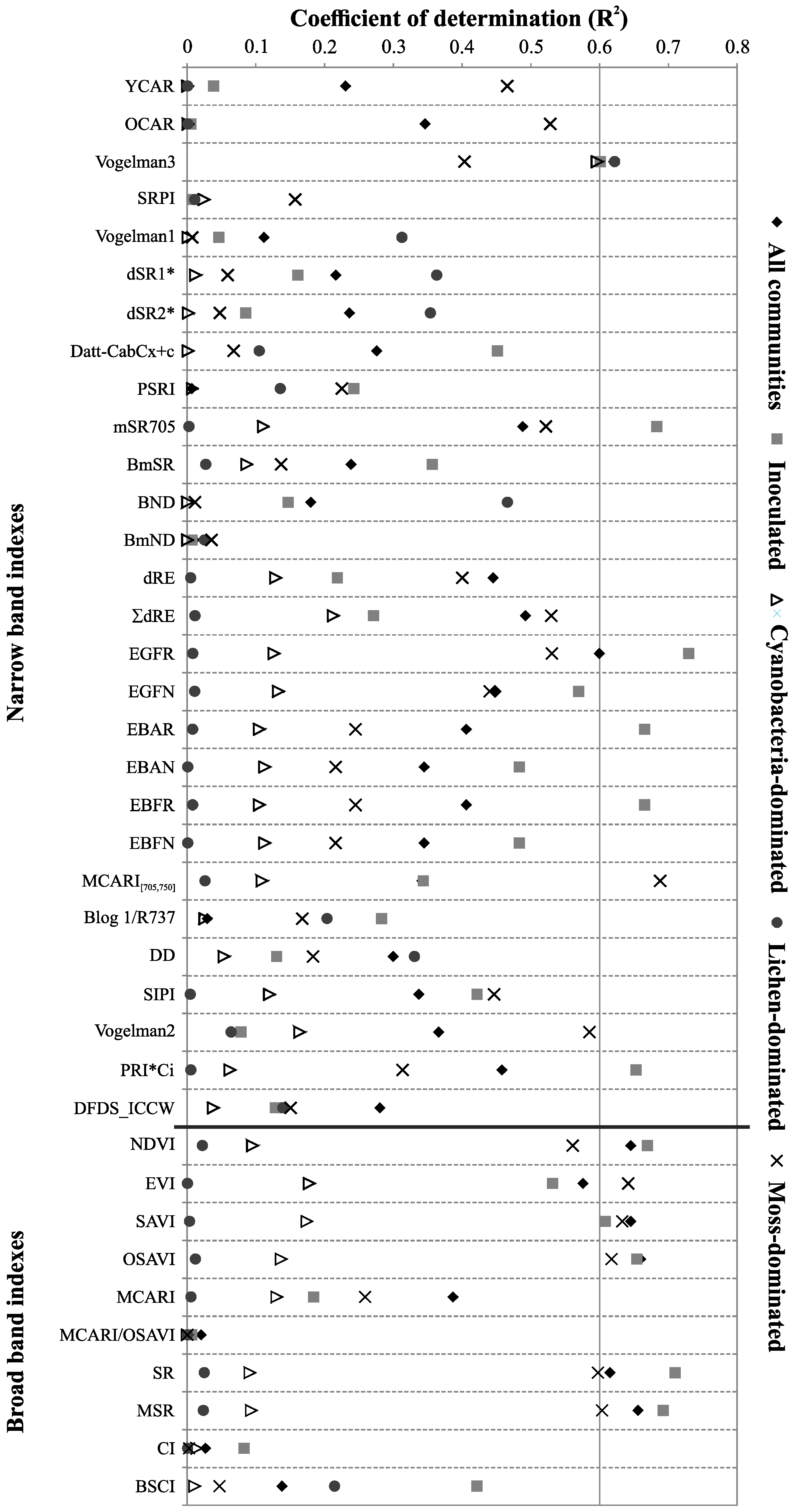
3.3.3. Sensitivity of the Standard Spectral Indexes to the Chlorophyll a Determination
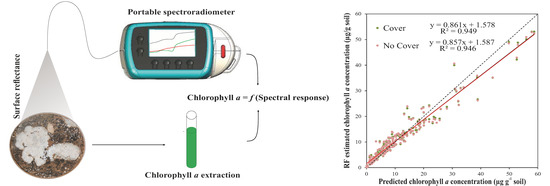
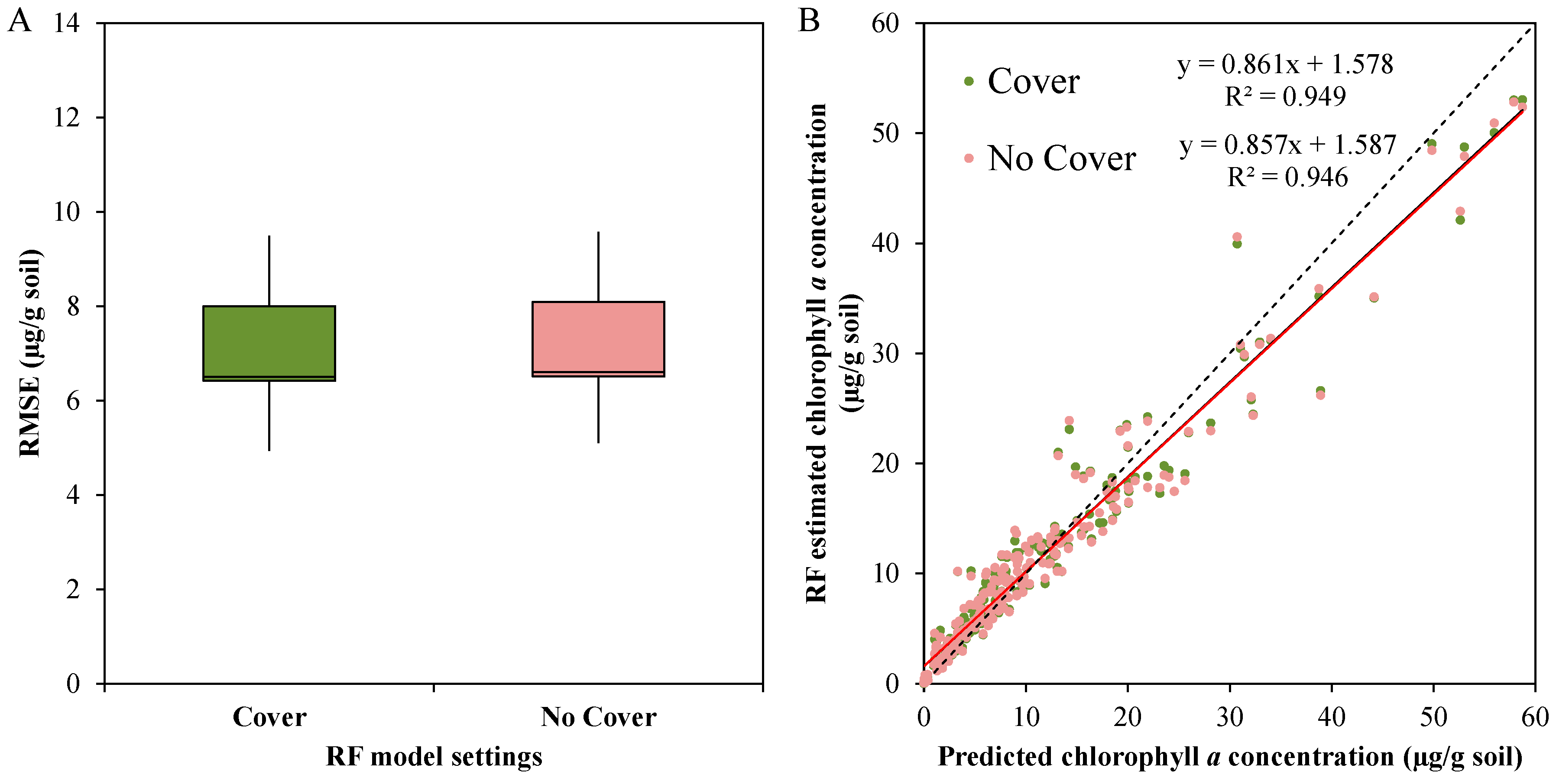
3.3.4. General Random Forest Model for Chla Prediction over a Wide Range of Biocrust Coverage and Composition Values
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pointing, S.B.; Belnap, J. Microbial colonization and controls in dryland systems. Nat. Rev. Microbiol. 2012, 10, 551–562. [Google Scholar] [CrossRef] [PubMed]
- Weber, B.; Hill, J. Remote Sensing of Biological Soil Crust at Different Scales. In Biological Soil Crust: An Organizing Principle in Drylands, 1st ed.; Weber, B., Büdel, B., Belnap, J., Eds.; Ecological Studies; Springer: Berlin, Germany, 2016; Volume 226, pp. 215–234. [Google Scholar]
- Büdel, B.; Williams, W.J.; Reichenberger, H. Annual net primary productivity of a cyanobacteria-dominated biological soil crust in the Gulf Savannah, Queensland, Australia. Biogeosciences 2018, 15, 491–505. [Google Scholar] [CrossRef] [Green Version]
- Williams, W.; Büdel, B.; Williams, S. Cyanobacterial species richness and Nostoc highly correlated to seasonal N enrichment in the northern Australian savannah. Biogeosci. Discuss. 2017, 1–24. [Google Scholar] [CrossRef]
- Maier, S.; Muggia, L.; Kuske, C.R.; Grube, M. Bacteria and Non-lichenized Fungi within Biological Soil Crusts. In Biological Soil Crust: An Organizing Principle in Drylands, 1st ed.; Weber, B., Büdel, B., Belnap, J., Eds.; Ecological Studies; Springer: Berlin, Germany, 2016; Volume 226, pp. 81–100. [Google Scholar]
- Bamforth, S.S. Protozoa of biological soil crusts of a cool desert in Utah. J. Arid Environ. 2008, 72, 722–729. [Google Scholar] [CrossRef]
- Darby, B.J.; Neher, D.A. Microfauna within Biological Soil Crusts. In Biological Soil Crust: An Organizing Principle in Drylands, 1st ed.; Weber, B., Büdel, B., Belnap, J., Eds.; Ecological Studies; Springer: Berlin, Germany, 2016; Volume 226, pp. 139–157. [Google Scholar]
- Chamizo, S.; Cantón, Y.; Miralles, I.; Domingo, F. Biological soil crust development affects physicochemical characteristics of soil surface in semiarid ecosystems. Soil Biol. Biochem. 2012, 49, 96–105. [Google Scholar] [CrossRef]
- Belnap, J.; Walker, B.J.; Munson, S.M.; Gill, R.A. Controls on sediment production in two U.S. deserts. Aeolian Res. 2014, 14, 15–24. [Google Scholar] [CrossRef]
- Rodríguez-Caballero, E.; Cantón, Y.; Jetten, V. Biological soil crust effects must be included to accurately model infiltration and erosion in drylands: An example from Tabernas Badlands. Geomorphology 2015, 241, 331–342. [Google Scholar] [CrossRef]
- Chamizo, S.; Rodríguez-Caballero, E.; Román, J.R.; Cantón, Y. Effects of biocrust on soil erosion and organic carbon losses under natural rainfall. Catena 2017, 148, 117–125. [Google Scholar] [CrossRef]
- Adessi, A.; Cruz de Carvalho, R.; De Philippis, R.; Branquinho, C.; Marques da Silva, J. Microbial extracellular polymeric substances improve water retention in dryland biological soil crusts. Soil Biol. Biochem. 2018, 116, 67–69. [Google Scholar] [CrossRef]
- Rodríguez-Caballero, E.; Castro, A.J.; Chamizo, S.; Quintas-Soriano, C.; Garcia-Llorente, M.; Cantón, Y.; Weber, B. Ecosystem services provided by biocrusts: From ecosystem functions to social values. J. Arid Environ. 2018, 159, 45–53. [Google Scholar] [CrossRef]
- Lange, O.L. Photosynthesis of Soil Crust Biota as Dependent on Environmental Factors. In Biological Soil Crust: Structure, Function and Management, 1st ed.; Belnap, J., Lange, O.L., Eds.; Ecological Studies; Springer: Berlin, Germany, 2001; Volume 150, pp. 217–240. [Google Scholar]
- Zaady, E.; Ben-David, E.A.; Sher, Y.; Tzirkin, R.; Nejidat, A. Inferring biological soil crust successional stage using combined PLFA, DGGE, physical and biophysiological analyses. Soil Biol. Biochem. 2010, 42, 842–849. [Google Scholar] [CrossRef]
- Lan, S.; Ouyang, H.; Wu, L.; Zhang, D.; Hu, C. Biological soil crust community types differ in photosynthetic pigment composition, fluorescence and carbon fixation in Shapotou region of China. Appl. Soil Ecol. 2017, 111, 9–16. [Google Scholar] [CrossRef]
- Bowker, M.A.; Reed, S.C.; Belnap, J.; Phillips, S.L. Temporal variation in community composition, pigmentation, and Fv/Fm of desert cyanobacterial soil crusts. Microb. Ecol. 2002, 43, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Belnap, J.; Phillips, S.L.; Smith, S.D. Dynamics of cover, UV-protective pigments, and quantum yield in biological soil crust communities of an undisturbed Mojave Desert shrubland. Flora Morphol. Distrib. Funct. Ecol. Plants 2007, 202, 674–686. [Google Scholar] [CrossRef]
- Kidron, G.J.; Vonshak, A.; Abeliovich, A. Recovery rates of microbiotic crusts within a dune ecosystem in the Negev Desert. Geomorphology 2008, 100, 444–452. [Google Scholar] [CrossRef]
- Dojani, S.; Budel, B.; Deutschewitz, K.; Weber, B. Rapid succession of Biological Soil Crusts after experimental disturbance in the Succulent Karoo, South Africa. Appl. Soil Ecol. 2011, 48, 263–269. [Google Scholar] [CrossRef]
- Ferrenberg, S.; Reed, S.C.; Belnap, J. Climate change and physical disturbance cause similar community shifts in biological soil crusts. Proc. Natl. Acad. Sci. USA 2015, 112, 12116–12121. [Google Scholar] [CrossRef] [Green Version]
- Rutherford, W.A.; Painter, T.H.; Ferrenberg, S.; Belnap, J.; Okin, G.S.; Flagg, C.; Reed, S.C. Albedo feedbacks to future climate via climate change impacts on dryland biocrusts. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef] [PubMed]
- Chamizo, S.; Mugnai, G.; Rossi, F.; Certini, G.; De Philippis, R. Cyanobacteria Inoculation Improves Soil Stability and Fertility on Different Textured Soils: Gaining Insights for Applicability in Soil Restoration. Front. Environ. Sci. 2018, 6. [Google Scholar] [CrossRef]
- Muñoz-Rojas, M.; Román, J.R.; Roncero-Ramos, B.; Erickson, T.E.; Merritt, D.J.; Aguila-Carricondo, P.; Cantón, Y. Cyanobacteria inoculation enhances carbon sequestration in soil substrates used in dryland restoration. Sci. Total Environ. 2018, 636, 1149–1154. [Google Scholar] [CrossRef] [PubMed]
- Castle, S.C.; Morrison, C.D.; Barger, N.N. Extraction of chlorophyll a from biological soil crusts: A comparison of solvents for spectrophotometric determination. Soil Biol. Biochem. 2011, 43, 853–856. [Google Scholar] [CrossRef]
- Lan, S.; Wu, L.; Zhang, D.; Hu, C.; Liu, Y. Ethanol outperforms multiple solvents in the extraction of chlorophyll-a from biological soil crusts. Soil Biol. Biochem. 2011, 43, 857–861. [Google Scholar] [CrossRef]
- Caesar, J.; Tamm, A.; Ruckteschler, N.; Lena Leifke, A.; Weber, B. Revisiting chlorophyll extraction methods in biological soil crusts - Methodology for determination of chlorophyll a and chlorophyll a Cb as compared to previous methods. Biogeosciences 2018, 15, 1415–1424. [Google Scholar] [CrossRef]
- Büdel, B.; Darienko, T.; Deutschewitz, K.; Dojani, S.; Friedl, T.; Mohr, K.I.; Salisch, M.; Reisser, W.; Weber, B. Southern african biological soil crusts are ubiquitous and highly diverse in drylands, being restricted by rainfall frequency. Microb. Ecol. 2009, 57, 229–247. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Caballero, E.; Knerr, T.; Weber, B. Importance of biocrusts in dryland monitoring using spectral indices. Remote Sens. Environ. 2015, 170, 32–39. [Google Scholar] [CrossRef]
- Rodriguez-Caballero, E.; Belnap, J.; Büdel, B.; Crutzen, P.J.; Andreae, M.O.; Pöschl, U.; Weber, B. Dryland photoautotrophic soil surface communities endangered by global change. Nat. Geosci. 2018, 11, 185–189. [Google Scholar] [CrossRef]
- Rodríguez-Caballero, E.; Paul, M.; Tamm, A.; Caesar, J.; Büdel, B.; Escribano, P.; Hill, J.; Weber, B. Biomass assessment of microbial surface communities by means of hyperspectral remote sensing data. Sci. Total Environ. 2017, 586, 1287–1297. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Pichel, F.; Belnap, J.; Neuer, S.; Schanz, F. Estimates of global cyanobacterial biomass and its distribution. Arch. Hydrobiol. Suppl. Algol. Stud. 2003, 109, 213–227. [Google Scholar] [CrossRef]
- Elbert, W.; Weber, B.; Burrows, S.; Steinkamp, J.; Büdel, B.; Andreae, M.O.; Pöschl, U. Contribution of cryptogamic covers to the global cycles of carbon and nitrogen. Nat. Geosci. 2012, 5, 459–462. [Google Scholar] [CrossRef]
- Porada, P.; Weber, B.; Elbert, W.; Pöschl, U.; Kleidon, A. Estimating impacts of lichens and bryophytes on global biogeochemical cycles. Glob. Biogeochem. Cycles 2014, 28, 71–85. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Gritz, Y.; Merzlyak, M.N. Relationships between leaf chlorophyll content and spectral reflectance and algorithms for non-destructive chlorophyll assessment in higher plant leaves. J. Plant Physiol. 2003, 160, 271–282. [Google Scholar] [CrossRef] [PubMed]
- Le Maire, G.; François, C.; Dufrêne, E. Towards universal broad leaf chlorophyll indices using PROSPECT simulated database and hyperspectral reflectance measurements. Remote Sens. Environ. 2004, 89, 1–28. [Google Scholar] [CrossRef]
- Zhao, D.; Reddy, K.R.; Kakani, V.G.; Read, J.J.; Koti, S. Selection of optimum reflectance ratios for estimating leaf nitrogen and chlorophyll concentrations of field-grown cotton. Agron. J. 2005, 97, 89–98. [Google Scholar] [CrossRef]
- Milton, E.J.; Schaepman, M.E.; Anderson, K.; Kneubühler, M.; Fox, N. Progress in field spectroscopy. Remote Sens. Environ. 2009, 113, 92–109. [Google Scholar] [CrossRef]
- Louchard, E.; Reid, R.; Stephens, C.; Davis, C.; Leathers, R.; Downes, T.; Maffione, R. Derivative analysis of absorption features in hyperspectral remote sensing data of carbonate sediments. Opt. Express 2002, 10, 1573–1584. [Google Scholar] [CrossRef]
- Stephens, F.C.; Louchard, E.M.; Reid, R.P.; Maffione, R.A. Effects of microalgal communities on reflectance spectra of carbonate sediments in subtidal optically shallow marine environments. Limnol. Oceanogr. 2003, 48, 535–546. [Google Scholar] [CrossRef]
- Tong, A.; He, Y. Estimating and mapping chlorophyll content for a heterogeneous grassland: Comparing prediction power of a suite of vegetation indices across scales between years. ISPRS J. Photogramm. Remote Sens. 2017, 126, 146–167. [Google Scholar] [CrossRef]
- Yoder, B.J.; Pettigrew-Crosby, R.E. Predicting nitrogen and chlorophyll content and concentrations from reflectance spectra (400–2500 nm) at leaf and canopy scales. Remote Sens. Environ. 1995, 53, 199–211. [Google Scholar] [CrossRef]
- Haboudane, D.; Miller, J.R.; Tremblay, N.; Zarco-Tejada, P.J.; Dextraze, L. Integrated narrow-band vegetation indices for prediction of crop chlorophyll content for application to precision agriculture. Remote Sens. Environ. 2002, 81, 416–426. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, J.M.; Miller, J.R.; Noland, T.L. Leaf chlorophyll content retrieval from airborne hyperspectral remote sensing imagery. Remote Sens. Environ. 2008, 112, 3234–3247. [Google Scholar] [CrossRef]
- Croft, H.; Chen, J.M.; Zhang, Y.; Simic, A. Modelling leaf chlorophyll content in broadleaf and needle leaf canopies from ground, CASI, Landsat TM 5 and MERIS reflectance data. Remote Sens. Environ. 2013, 133, 128–140. [Google Scholar] [CrossRef]
- Elarab, M.; Ticlavilca, A.M.; Torres-Rua, A.F.; Maslova, I.; McKee, M. Estimating chlorophyll with thermal and broadband multispectral high resolution imagery from an unmanned aerial system using relevance vector machines for precision agriculture. Int. J. Appl. Earth Obs. Geoinf. 2015, 43, 32–42. [Google Scholar] [CrossRef] [Green Version]
- Clevers, J.G.P.W.; Kooistra, L.; van den Brande, M.M.M. Using Sentinel-2 data for retrieving LAI and leaf and canopy chlorophyll content of a potato crop. Remote Sens. 2017, 9, 405. [Google Scholar] [CrossRef]
- Weber, B.; Olehowski, C.; Knerr, T.; Hill, J.; Deutschewitz, K.; Wessels, D.C.J.; Eitel, B.; Büdel, B. A new approach for mapping of Biological Soil Crusts in semidesert areas with hyperspectral imagery. Remote Sens. Environ. 2008, 112, 2187–2201. [Google Scholar] [CrossRef]
- Rodríguez-Caballero, E.; Escribano, P.; Olehowski, C.; Chamizo, S.; Hill, J.; Cantón, Y.; Weber, B. Transferability of multi- and hyperspectral optical biocrust indices. ISPRS J. Photogramm. Remote Sens. 2017, 126, 94–107. [Google Scholar] [CrossRef]
- Chamizo, S.; Stevens, A.; Cantón, Y.; Miralles, I.; Domingo, F.; Van Wesemael, B. Discriminating soil crust type, development stage and degree of disturbance in semiarid environments from their spectral characteristics. Eur. J. Soil Sci. 2012, 63, 42–53. [Google Scholar] [CrossRef]
- Alonso, M.; Rodríguez-Caballero, E.; Chamizo, S.; Escribano, P.; Cantón, Y. Evaluación de los diferentes índices para cartografiar biocostras a partir de información espectral. Rev. Teledetec. 2014, 79–98. [Google Scholar] [CrossRef]
- Rodríguez-Caballero, E.; Escribano, P.; Cantón, Y. Advanced image processing methods as a tool to map and quantify different types of biological soil crust. ISPRS J. Photogramm. Remote Sens. 2014, 90, 59–67. [Google Scholar] [CrossRef]
- Karnieli, A.; Kokaly, R.F.; West, N.E.; Clark, R.N. Remote Sensing of Biological Soil Crust. In Biological Soil Crust: Structure, Function and Management, 1st ed.; Belnap, J., Lange, O.L., Eds.; Ecological Studies; Springer: Berlin, Germany, 2001; Volume 150, pp. 431–455. [Google Scholar]
- Cantón, Y.; Solé-Benet, A.; Lázaro, R. Soil-geomorphology relations in gypsiferous materials of the tabernas desert (almería, se spain). Geoderma 2003, 115, 193–222. [Google Scholar] [CrossRef]
- Lázaro, R.; Cantón, Y.; Solé-Benet, A.; Bevan, J.; Alexander, R.; Sancho, L.G.; Puigdefábregas, J. The influence of competition between lichen colonization and erosion on the evolution of soil surfaces in the Tabernas badlands (SE Spain) and its landscape effects. Geomorphology 2008, 102, 252–266. [Google Scholar] [CrossRef]
- Luna, L.; Vignozzi, N.; Miralles, I.; Solé-Benet, A. Organic amendments and mulches modify soil porosity and infiltration in semiarid mine soils. Land Degrad. Dev. 2018, 29, 1019–1030. [Google Scholar] [CrossRef]
- Roncero-Ramos, B.; Muñoz-Martín, M.Á.; Chamizo, S.; Fernández-Valbuena, L.; Mendoza, D.; Perona, E.; Cantón, Y.; Mateo, P. Polyphasic evaluation of key cyanobacteria in biocrusts from the most arid region in Europe. PeerJ 2019, 7, e6169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Román, J.R.; Roncero-Ramos, B.; Chamizo, S.; Rodríguez-Caballero, E.; Cantón, Y. Restoring soil functions by means of cyanobacteria inoculation: Importance of soil conditions and species selection. Land Degrad. Dev. 2018, 29, 3184–3193. [Google Scholar] [CrossRef]
- Ritchie, R.J. Consistent sets of spectrophotometric chlorophyll equations for acetone, methanol and ethanol solvents. Photosynth. Res. 2006, 89, 27–41. [Google Scholar] [CrossRef] [PubMed]
- Savitzky, A.; Golay, M.J.E. Smoothing and Differentiation of Data by Simplified Least Squares Procedures. Anal. Chem. 1964, 36, 1627–1639. [Google Scholar] [CrossRef]
- Lehnert, L.W.; Meyer, H.; Obermeier, W.A.; Silva, B.; Regeling, B.; Thies, B.; Bendix, J. Hyperspectral Data Analysis in R: The hsdar-Package. arXiv 2018, arXiv:1805.05090. [Google Scholar] [CrossRef]
- Munden, R.; Curran, P.J.; Catt, J.A. The relationship between red edge and chlorophyll concentration in the broadbalk winter wheat experiment at rothamsted. Int. J. Remote Sens. 1994, 15, 705–709. [Google Scholar] [CrossRef]
- Clark, R.N.; Roush, T.L. Reflectance spectroscopy: Quantitative analysis techniques for remote sensing applications. J. Geophys. Res. Solid Earth 1984, 89, 6329–6340. [Google Scholar] [CrossRef]
- Mutanga, O.; Skidmore, A.K. Hyperspectral band depth analysis for a better estimation of grass biomass (Cenchrus ciliaris) measured under controlled laboratory conditions. Int. J. Appl. Earth Obs. Geoinf. 2004, 5, 87–96. [Google Scholar] [CrossRef]
- Filella, I.; Peñuelas, J. The red edge position and shape as indicators of plant chlorophyll content, biomass and hydric status. Int. J. Remote Sens. 1994, 15, 1459–1470. [Google Scholar] [CrossRef]
- Curran, P.J.; Dungan, J.L.; Peterson, D.L. Estimating the foliar biochemical concentration of leaves with reflectance spectrometry: Testing the Kokaly and Clark methodologies. Remote Sens. Environ. 2001, 76, 349–359. [Google Scholar] [CrossRef]
- Xue, L.; Yang, L. Deriving leaf chlorophyll content of green-leafy vegetables from hyperspectral reflectance. ISPRS J. Photogramm. Remote Sens. 2009, 64, 97–106. [Google Scholar] [CrossRef]
- Main, R.; Cho, M.A.; Mathieu, R.; O’Kennedy, M.M.; Ramoelo, A.; Koch, S. An investigation into robust spectral indices for leaf chlorophyll estimation. ISPRS J. Photogramm. Remote Sens. 2011, 66, 751–761. [Google Scholar] [CrossRef]
- Liaw, A.; Wiener, M. Classification and Regression by Random Forest. R News 2002, 2, 18–22. [Google Scholar]
- Wang, W.; Liu, Y.; Li, D.; Hu, C.; Rao, B. Feasibility of cyanobacterial inoculation for biological soil crusts formation in desert area. Soil Biol. Biochem. 2009, 41, 926–929. [Google Scholar] [CrossRef]
- Ayuso, S.V.; Silva, A.G.; Nelson, C.; Barger, N.N.; Garcia-Pichel, F. Microbial nursery production of high-quality biological soil crust biomass for restoration of degraded dryland soils. Appl. Environ. Microbiol. 2017. [Google Scholar] [CrossRef]
- Rock, B.N.; Hoshizaki, T.; Miller, J.R. Comparison of in situ and airborne spectral measurements of the blue shift associated with forest decline. Remote Sens. Environ. 1988, 24, 109–127. [Google Scholar] [CrossRef]
- Vogelmann, J.E.; Rock, B.N.; Moss, D.M. Red edge spectral measurements from sugar maple leaves. Int. J. Remote Sens. 1993, 14, 1563–1575. [Google Scholar] [CrossRef]
- Carter, G.A. Ratios of leaf reflectances in narrow wavebands as indicators of plant stress. Int. J. Remote Sens. 1994, 15, 517–520. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Merzlyak, M.N. Signature Analysis of Leaf Reflectance Spectra: Algorithm Development for Remote Sensing of Chlorophyll. J. Plant Physiol. 1996, 148, 494–500. [Google Scholar] [CrossRef]
- Darvishzadeh, R.; Skidmore, A.; Schlerf, M.; Atzberger, C.; Corsi, F.; Cho, M. LAI and chlorophyll estimation for a heterogeneous grassland using hyperspectral measurements. ISPRS J. Photogramm. Remote Sens. 2008, 63, 409–426. [Google Scholar] [CrossRef]
- Escribano, P.; Schmid, T.; Chabrillat, S. Optical Remote Sensing for Soil Mapping and Monitoring. In Mapping and Monitoring, Soil Mapping and Process Modeling for Sustainable Land Use Management, 1st ed.; Pereira, P., Brevik, E.C., Muñoz-Rojas, M., Miller, B.A., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; Chapter 4; pp. 87–125. [Google Scholar]
- Malenovský, Z.; Homolová, L.; Zurita-Milla, R.; Lukeš, P.; Kaplan, V.; Hanuš, J.; Gastellu-Etchegorry, J.P.; Schaepman, M.E. Retrieval of spruce leaf chlorophyll content from airborne image data using continuum removal and radiative transfer. Remote Sens. Environ. 2013, 131, 85–102. [Google Scholar] [CrossRef] [Green Version]
- Malenovský, Z.; Turnbull, J.D.; Lucieer, A.; Robinson, S.A. Antarctic moss stress assessment based on chlorophyll content and leaf density retrieved from imaging spectroscopy data. New Phytol. 2015, 208, 608–624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stefano, P.; Angelo, P.; Simone, P.; Filomena, R.; Federico, S.; Tiziana, S.; Umberto, A.; Vincenzo, C.; Acito, N.; Marco, D.; et al. The PRISMA Hyperspectral Mission: Science Activities and Opportunities for Agriculture and Land Monitoring. In Proceedings of the International Geoscience and Remote Sensing Symposium (IGARSS), Melbourne, VIC, Australia, 21–26 July 2013; pp. 4558–4561. [Google Scholar]
- Belnap, J.; Phillips, S.L.; Miller, M.E. Response of desert biological soil crusts to alterations in precipitation frequency. Oecologia 2004, 141, 306–316. [Google Scholar] [CrossRef] [PubMed]
- Wada, N.; Sakamoto, T.; Matsugo, S. Multiple roles of photosynthetic and sunscreen pigments in cyanobacteria focusing on the oxidative stress. Metabolites 2013, 3, 463–483. [Google Scholar] [CrossRef] [PubMed]
- Couradeau, E.; Karaoz, U.; Lim, H.C.; Nunes da Rocha, U.; Northen, T.; Brodie, E.; Garcia-Pichel, F. Bacteria increase arid-land soil surface temperature through the production of sunscreens. Nat. Commun. 2016, 7. [Google Scholar] [CrossRef] [PubMed]
- Pringault, O.; Garcia-Pichel, F. Hydrotaxis of Cyanobacteria in Desert Crusts. Microb. Ecol. 2004, 47, 366–373. [Google Scholar] [CrossRef] [PubMed]
- Rajeev, L.; Da Rocha, U.N.; Klitgord, N.; Luning, E.G.; Fortney, J.; Axen, S.D.; Shih, P.M.; Bouskill, N.J.; Bowen, B.P.; Kerfeld, C.A.; et al. Dynamic cyanobacterial response to hydration and dehydration in a desert biological soil crust. ISME J. 2013, 7, 2178–2191. [Google Scholar] [CrossRef] [Green Version]
- Raanan, H.; Felde, V.J.M.N.L.; Peth, S.; Drahorad, S.; Ionescu, D.; Eshkol, G.; Treves, H.; Felix-Henningsen, P.; Berkowicz, S.M.; Keren, N.; et al. Three-dimensional structure and cyanobacterial activity within a desert biological soil crust. Environ. Microbiol. 2016, 18, 372–383. [Google Scholar] [CrossRef]
- Breiman, L. Bagging Predictors. Mach. Learn. 1996, 24, 123–140. [Google Scholar] [CrossRef]


| R | CR | ρ | |||||
| Biocrust Type | Model | R2 | Model | R2 | Model | R2 | |
| (RMSE) | (RMSE) | (RMSE) | |||||
| Hyperspectral data | Inoculated | −43.91x + 17.25 | 0.37 | −86.71x + 85.51 | 0.71 | −32278x + 31.75 | 0.53 |
| (673) | (5.85) | (646) | (3.95) | (565) | (5.04) | ||
| Cyanobacteria | 21.89x + 2.29 | 0.07 | 1114x + 1101.50 | 0.2 | 20439x − 4.03 | 0.27 | |
| (900) | (6.03) | (894) | (5.59) | (720) | (5.33) | ||
| Lichen | 38.50x − 6.30 | 0.23 | 23062x + 23048 | 0.18 | 28969x − 0.13 | 0.41 | |
| (853) | (5.3) | (765) | (5.47) | (581) | (4.65) | ||
| Moss | 69.87x + 3.94 | 0.25 | −58.79x + 60.50 | 0.60 | 19148x − 3.22 | 0.65 | |
| (900) | (23.62) | (663) | (9.19) | (723) | (8.60) | ||
| All | −45.66x + 20.21 | 0.21 | −62.23x + 64.24 | 0.67 | 6084.40x + 0.49 | 0.51 | |
| (674) | (9.49) | (655) | (6.11) | (698) | (7.43) | ||
| Multispectral data | Inoculated | −43.98x + 17.28 | 0.36 | −221.66x + 224.83 | 0.67 | −17296x + 19.23 | 0.41 |
| (665) | (5.88) | (705) | (4.22) | (560) | (5.69) | ||
| Cyanobacteria | 9.30x + 6.67 | 0.01 | −36.45x + 40.61 | 0.08 | 20297x − 1.81 | 0.22 | |
| (665) | (6.23) | (665) | (5.99) | (740) | (5.52) | ||
| Lichen | 39.50x − 1.44 | 0.21 | −15.54x − 1.87 | 0.03 | 26617x − 2.24 | 0.26 | |
| (665) | (5.30) | (665) | (5.96) | (560) | (5.21) | ||
| Moss | −53.95x + 31.05 | 0.05 | −61.54x + 64.05 | 0.6 | 23194x + 2.47 | 0.64 | |
| (665) | (14.09) | (665) | (9.16) | (740) | (8.71) | ||
| All | −44.85x + 20.20 | 0.19 | −61.09x + 63.57 | 0.67 | 13415x − 1.40 | 0.47 | |
| (665) | (9.57) | (665) | (6.12) | (705) | (7.75) | ||
| Normalized Difference | Standard Vegetation Indexes | ||||||
| Biocrust Type | Model | R2 | Model | R2 | |||
| (RMSE) | (RMSE) | ||||||
| Hyperspectral data | Inoculated | 320.91x − 3.50 | 0.72 | 6.35x − 0.99 | 0.73 | ||
| (ND 730-704) | (3.90) | (EGFR) | (3.84) | ||||
| Cyanobacteria | 1182.20x − 9.36 | 0.26 | 154.82x − 2.68 | 0.21 | |||
| (ND 466-455) | (5.37) | (∑dRE) | (5.56) | ||||
| Lichen | −9702.90x + 19.02 | 0.19 | 74.14x + 32.80 | 0.46 | |||
| (ND 679-678) | (5.45) | (BND) | (4.42) | ||||
| Moss | 4757.60x − 0.95 | 0.65 | 1094.10x + 6.51 | 0.69 | |||
| (ND 519-518) | (8.57) | (MCARI[705,750]) | (8.09) | ||||
| All | 91.32x − 0.53 | 0.68 | 312.42x − 316.61 | 0.60 | |||
| (ND 734-687) | (6.02) | (Vogelman3) | (6.73) | ||||
| Multispectral data | Inoculated | 273.25x − 4.02 | 0.71 | 25.42x − 27.18 | 0.71 | ||
| (ND 740-705) | (3.96) | (SR) | (3.98) | ||||
| Cyanobacteria | 152.57x + 1.23 | 0.11 | 69.99x − 0.71 | 0.18 | |||
| (ND 740-705) | (6.10) | (EVI) | (5.68) | ||||
| Lichen | −55.45x + 16.45 | 0.05 | −4.79x + 24.52 | 0.21 | |||
| (ND 705-665) | (6.20) | (BSCI) | (5.36) | ||||
| Moss | 104.27x + 2.68 | 0.62 | 95.27x − 0.07 | 0.64 | |||
| (ND 705-665) | (9.57) | (EVI) | (8.67) | ||||
| All | 85.44x − 2.06 | 0.69 | 85.09x − 3.10 | 0.66 | |||
| (ND 740-665) | (6.28) | (OSAVI) | (6.21) | ||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Román, J.R.; Rodríguez-Caballero, E.; Rodríguez-Lozano, B.; Roncero-Ramos, B.; Chamizo, S.; Águila-Carricondo, P.; Cantón, Y. Spectral Response Analysis: An Indirect and Non-Destructive Methodology for the Chlorophyll Quantification of Biocrusts. Remote Sens. 2019, 11, 1350. https://doi.org/10.3390/rs11111350
Román JR, Rodríguez-Caballero E, Rodríguez-Lozano B, Roncero-Ramos B, Chamizo S, Águila-Carricondo P, Cantón Y. Spectral Response Analysis: An Indirect and Non-Destructive Methodology for the Chlorophyll Quantification of Biocrusts. Remote Sensing. 2019; 11(11):1350. https://doi.org/10.3390/rs11111350
Chicago/Turabian StyleRomán, José Raúl, Emilio Rodríguez-Caballero, Borja Rodríguez-Lozano, Beatriz Roncero-Ramos, Sonia Chamizo, Pilar Águila-Carricondo, and Yolanda Cantón. 2019. "Spectral Response Analysis: An Indirect and Non-Destructive Methodology for the Chlorophyll Quantification of Biocrusts" Remote Sensing 11, no. 11: 1350. https://doi.org/10.3390/rs11111350
APA StyleRomán, J. R., Rodríguez-Caballero, E., Rodríguez-Lozano, B., Roncero-Ramos, B., Chamizo, S., Águila-Carricondo, P., & Cantón, Y. (2019). Spectral Response Analysis: An Indirect and Non-Destructive Methodology for the Chlorophyll Quantification of Biocrusts. Remote Sensing, 11(11), 1350. https://doi.org/10.3390/rs11111350