UAV and Ground Image-Based Phenotyping: A Proof of Concept with Durum Wheat
Abstract
1. Introduction
2. Materials and Methods
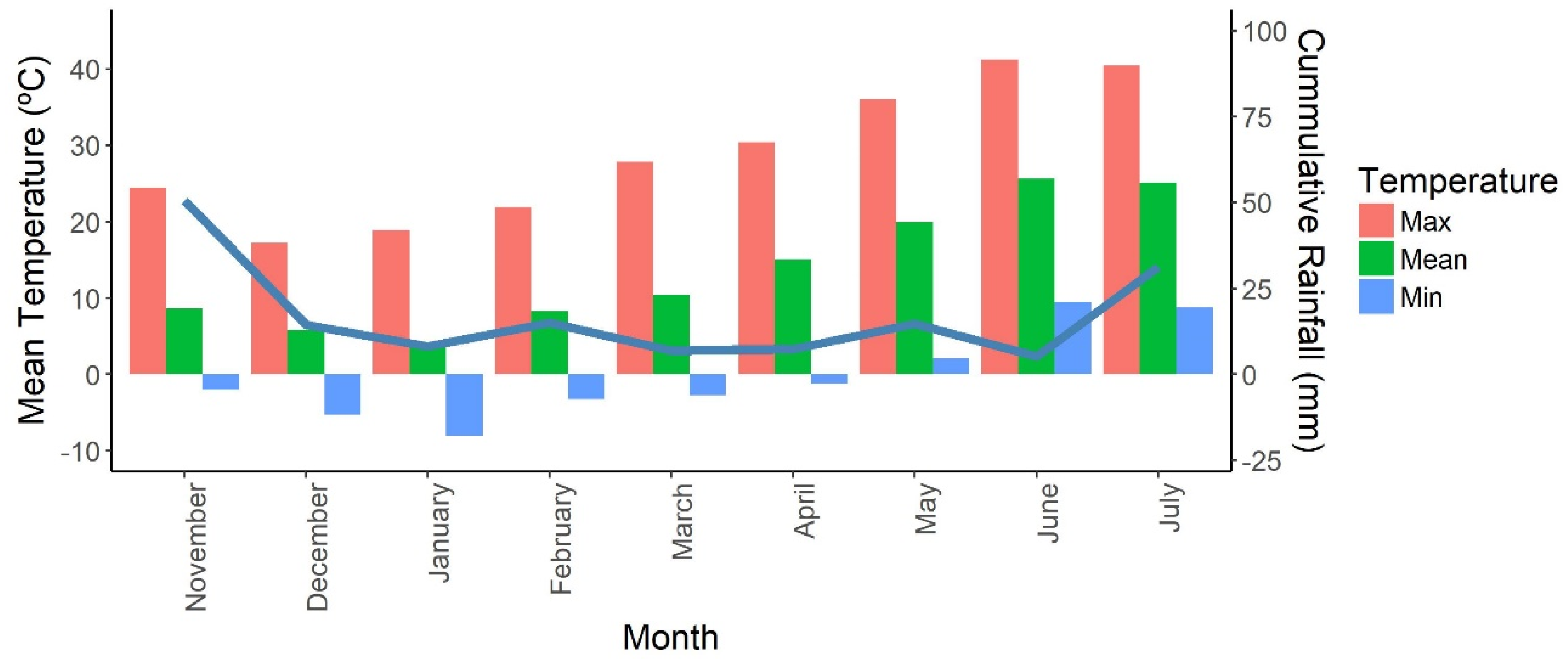
2.1. Plant Material, Site Description and Growing Conditions
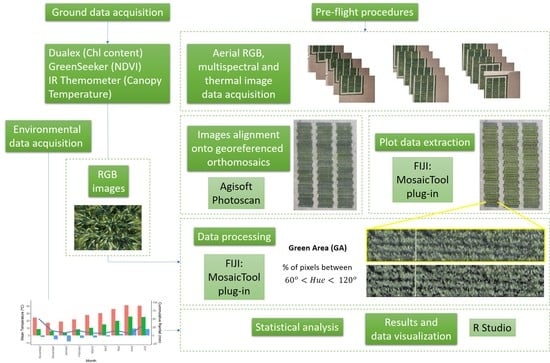
2.2. Aerial Platform Description and Orthomosaic Reconstruction Procedure
2.3. RGB Vegetation Indixes
2.4. Multispectral Vegetation Indexes
2.5. Canopy Temperature
2.6. Leaf Pigment Assessment
2.7. Statistical Analysis
3. Results
3.1. Effects of the Growing Conditions on Yield
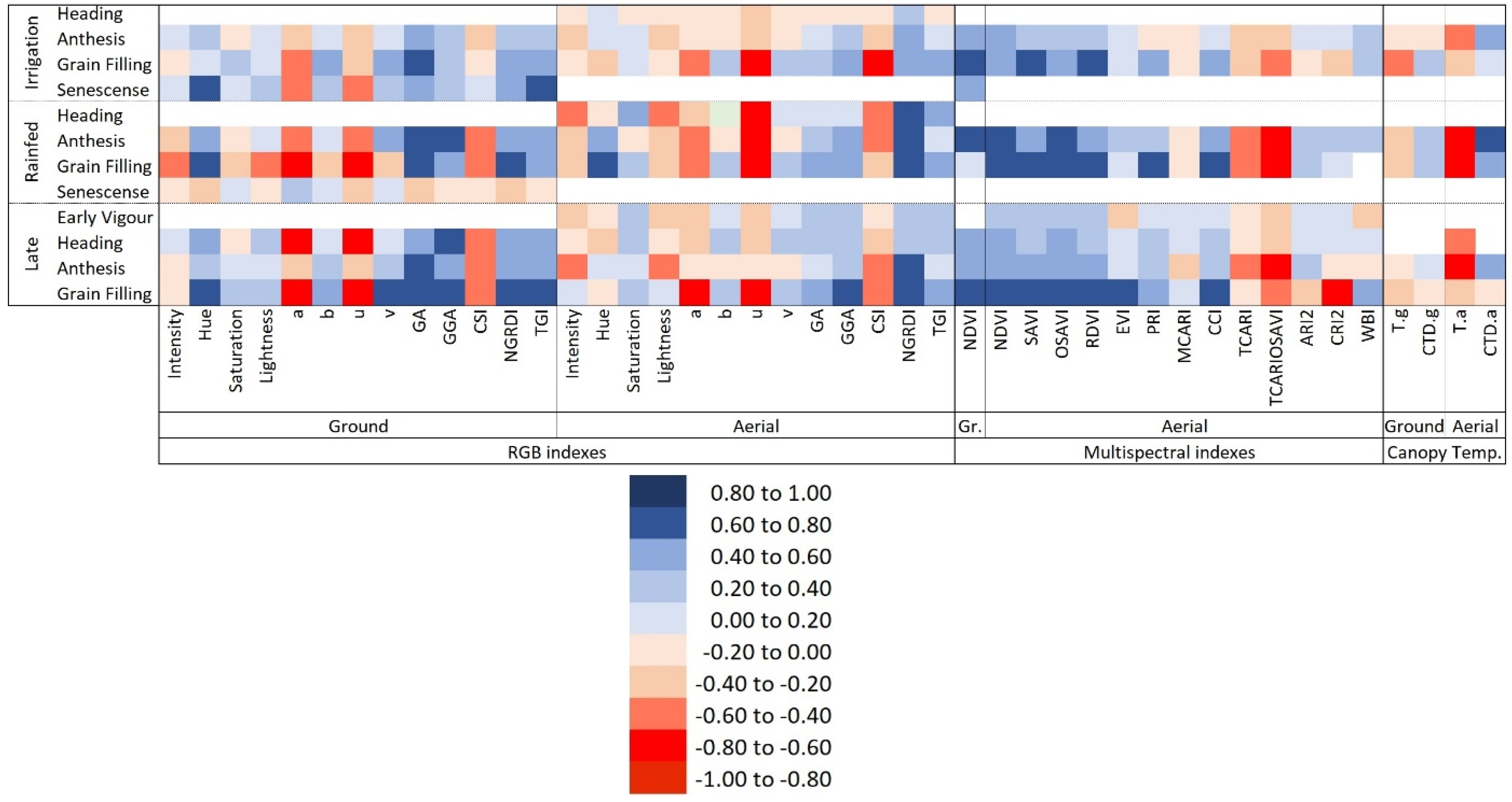
3.2. Phenotypic Variability of the Vegetation Indexes, Canopy Temperature, and Pigment Measurements Assessing GY Differences
3.3. Evaluation of GY and Remote Sensing Traits Heritability
3.4. GY Predictive Models
4. Discussion
4.1. Implications of Growing Conditions on Final GY
4.2. Ability of the Remote Sensing Measurements to Assess Genotypic Differences in Yield under Different Growing Conditions
4.3. Comparative Performance of Ground Versus Aerially Assessed Indexes
4.4. Repeatability and Applicability of Remote Sensing Measurements for Assessing GY
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| MAPAMA | Ministerio de Agricultura y Pesca Alimentación y Medio Ambiente |
| UAV | Unmanned Aerial Vehicle |
| RGB | Red-Green-Blue |
| NDVI | Normalized Difference Vegetation Index |
| GA | Green Area |
| CCI | Chlorophyll Content Index |
| TGI | Triangular Greenness Index |
| GY | Grain Yield |
| HTPP | High-Throughput Plant Phenotyping |
| MET | Multi-Environment Trials |
| INIA | Instituto Nacional de Investigación y Tecnología Agraria y Alimentaria |
| SIAR | Servicio de Informacion Agroclimática para el Regadio |
| NBI | Nitrogen Balance Index |
| CT | Canopy Temperature |
| CTD | Canopy Temperature Depression |
| ANOVA | Analysis of Variance |
| H2 | Heritability |
| rg | Genetic Correlations |
| σ2g | Genotype Variance |
| σ2g | Genotype Variance |
| σ2e | Error variance |
| n | Number of Replicates |
References
- Diffenbaugh, N.S.; Giorgi, F. Climate change hotspots in the CMIP5 global climate model ensemble. Clim. Chang. 2012, 114, 813–822. [Google Scholar] [CrossRef]
- Asseng, S.; Ewert, F.; Martre, P.; Rötter, R.P.; Lobell, D.B.; Cammarano, D.; Kimball, B.A.; Ottman, M.J.; Wall, G.W.; White, J.W.; et al. Rising temperatures reduce global wheat production. Nat. Clim. Chang. 2015, 5, 143–147. [Google Scholar] [CrossRef]
- Vicente-Serrano, S.M.; Lopez-Moreno, J.I.; Beguería, S.; Lorenzo-Lacruz, J.; Sanchez-Lorenzo, A.; García-Ruiz, J.M.; Azorin-Molina, C.; Morán-Tejeda, E.; Revuelto, J.; Trigo, R.; et al. Evidence of increasing drought severity caused by temperature rise in southern Europe. Environ. Res. Lett. 2014, 9, 1–9. [Google Scholar] [CrossRef]
- Royo, C.; Soriano, J.M.; Alvaro, F. Wheat: A Crop in the Bottom of the Mediterranean Diet Pyramid. In Mediterranean Identities—Environment, Society, Culture; IntechOpen: London, UK, 2017. [Google Scholar] [CrossRef]
- Reynolds, M.; Langridge, P. Physiological breeding. Curr. Opin. Plant Biol. 2016, 31, 162–171. [Google Scholar] [CrossRef] [PubMed]
- Quintero, A.; Molero, G.; Reynolds, M.P.; Calderini, D.F. Trade-o ff between grain weight and grain number in wheat depends on GxE interaction: A case study of an elite CIMMYT panel (CIMCOG). Eur. J. Agron. 2018, 92, 17–29. [Google Scholar] [CrossRef]
- Araus, J.L.; Cairns, J.E. Field high-throughput phenotyping: The new crop breeding frontier. Trends Plant Sci. 2014, 19, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Cobb, J.N.; DeClerck, G.; Greenberg, A.; Clark, R.; McCouch, S. Next-generation phenotyping: Requirements and strategies for enhancing our understanding of genotype-phenotype relationships and its relevance to crop improvement. Theor. Appl. Genet. 2013, 126, 867–887. [Google Scholar] [CrossRef]
- Araus, L.; Kefauver, S.C. Breeding to adapt agriculture to climate change: Affordable phenotyping solutions. Curr. Opin. Plant. Biol. 2018, 45, 237–247. [Google Scholar] [CrossRef] [PubMed]
- Atzberger, C. Advances in Remote Sensing of Agriculture: Context Description, Existing Operational Monitoring Systems and Major Information Needs. Remote Sens. 2013, 5, 949–981. [Google Scholar] [CrossRef]
- Araus, J.L.; Kefauver, S.C.; Zaman-allah, M.; Olsen, M.S.; Cairns, J.E. Translating High-Throughput Phenotyping into Genetic Gain. Trends Plant Sci. 2018, 23, 451–466. [Google Scholar] [CrossRef]
- Deery, D.M.; Rebetzke, G.J.; Jimenez-Berni, J.A.; James, R.A.; Condon, A.G.; Bovill, W.D.; Hutchinson, P.; Scarrow, J.; Davy, R.; Furbank, R.T. Methodology for High-Throughput Field Phenotyping of Canopy Temperature Using Airborne Thermography. Front. Plant Sci. 2016, 7, 1808. [Google Scholar] [CrossRef]
- Yang, G.; Liu, J.; Zhao, C.; Li, Z.; Huang, Y. Unmanned Aerial Vehicle Remote Sensing for Field-Based Crop Phenotyping: Current Status and Perspectives. Front. Plant Sci. 2017, 8, 1111. [Google Scholar] [CrossRef]
- Berni, J.A.J.; Member, S.; Zarco-tejada, P.J.; Suárez, L.; Fereres, E. Thermal and Narrowband Multispectral Remote Sensing for Vegetation Monitoring From an Unmanned Aerial Vehicle. IEEE Trans. Geosci. Remote Sens. 2009, 47, 1–17. [Google Scholar] [CrossRef]
- Yousfi, S.; Kellas, N.; Saidi, L.; Benlakehal, Z.; Chaou, L.; Siad, D.; Herda, F.; Karrou, M.; Vergara, O.; Gracia, A.; et al. Comparative performance of remote sensing methods in assessing wheat performance under Mediterranean conditions. Agric. Water Manag. 2016, 164, 137–147. [Google Scholar] [CrossRef]
- Vergara-Díaz, O.; Zaman-allah, M.A.; Masuka, B.; Hornero, A.; Zarco-Tejada, P.; Prasanna, B.M.; Cairns, J.E.; Araus, J.L. A Novel Remote Sensing Approach for Prediction of Maize Yield Under Different Conditions of Nitrogen Fertilization. Front. Plant Sci. 2016, 7, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Vergara-Díaz, O.; Kefauver, S.C.; Elazab, A.; Nieto-Taladriz, M.T.; Araus, J.L. Grain yield losses in yellow-rusted durum wheat estimated using digital and conventional parameters under field conditions. Crop J. 2015, 3, 200–210. [Google Scholar] [CrossRef]
- Fernandez-Gallego, J.A.; Kefauver, S.C.; Gutiérrez, N.A.; Nieto-Taladriz, M.T.; Araus, J.L. Wheat ear counting in-field conditions: High throughput and low-cost approach using RGB images. Plant Methods 2018, 14, 22. [Google Scholar] [CrossRef] [PubMed]
- Condorelli, G.E.; Maccaferri, M.; Newcomb, M.; Andrade-Sanchez, P.; White, J.W.; French, A.N.; Sciara, G.; Ward, R.; Tuberosa, R. Comparative Aerial and Ground Based High Throughput Phenotyping for the Genetic Dissection of NDVI as a Proxy for Drought Adaptive Traits in Durum Wheat. Front. Plant Sci. 2018, 9, 1–17. [Google Scholar] [CrossRef]
- Tucker, C.J. Red and photographic infrared linear combinations for monitoring vegetation. Remote Sens. Environ. 1979, 8, 127–150. [Google Scholar] [CrossRef]
- Peñuelas, J.; Filella, I.; Biel, C.; Serrano, L.; Savé, R. The reflectance at the 950–970 nm region as an indicator of plant water status. Int. J. Remote Sens. 1993, 14, 1887–1905. [Google Scholar] [CrossRef]
- Daughtry, C.; Walthall, C.L.; Kim, M.S.; Brown de Colstoun, E.; McMurtrey, J.E. Estimating Corn Leaf Chlorophyll Concentration from Leaf and Canopy Reflectance. Remote Sens. Environ. 2000, 74, 229–239. [Google Scholar] [CrossRef]
- Jackson, R.D.; Reginato, R.J.; Idso, S.B. Wheat canopy temperature: A practical tool for evaluating water requirements. Water Resour. Res. 1988, 13, 651–656. [Google Scholar] [CrossRef]
- Moran, M.S.; Clarke, T.R.; Inoue, Y.; Vidal, A. Estimating crop water deficit using the relation between surface-air temperature and spectral vegetation index. Remote Sens. Environ. 1994, 49, 246–263. [Google Scholar] [CrossRef]
- Casadesús, J.; Villegas, D. Conventional Digital Cameras as a Tool for Assessing LAI and Biomass for Cereal Breeding. New Technol. 2013, 56, 1–27. [Google Scholar]
- Gracia-Romero, A.; Kefauver, S.C.; Vergara-Díaz, O.; Zaman-Allah, M.A.; Prasanna, B.M.; Cairns, J.E.; Araus, J.L. Comparative Performance of Ground vs. Aerially Assessed RGB and Multispectral Indices for Early-Growth Evaluation of Maize Performance under Phosphorus Fertilization. Front. Plant Sci. 2017, 8, 2004. [Google Scholar] [CrossRef]
- Zadoks, J.C.; Chang, T.T.; Konzak, C.F. A decimal code for the growth stages of cereals. Weed Res. 1974, 14, 415–421. [Google Scholar] [CrossRef]
- Bendig, J.; Bolten, A.; Bennertz, S.; Broscheit, J.; Eichfuss, S.; Bareth, G. Estimating biomass of barley using crop surface models (CSMs) derived from UAV-based RGB imaging. Remote Sens. 2014, 6, 10395–10412. [Google Scholar] [CrossRef]
- Casadesus, J.; Kaya, Y.; Bort, J.; Nachit, M.M.; Araus, J.L.; Amor, S.; Ferrazzano, G.; Maalouf, F. Using vegetation indices derived from conventional digital cameras as selection criteria for wheat breeding in water-limited environments. Ann. Appl. Biol. 2007, 150, 227–236. [Google Scholar] [CrossRef]
- Zaman-Allah, M.; Vergara, O.; Araus, J.L.; Tarekegne, A.; Magorokosho, C.; Zarco-Tejada, P.J.; Hornero, A.; Albà, A.H.; Das, B.; Craufurd, P.; et al. Unmanned aerial platform-based multi-spectral imaging for field phenotyping of maize. Plant Methods 2015, 11, 35. [Google Scholar] [CrossRef] [PubMed]
- Pointer, M.R. A comparison of the CIE 1976 colour spaces. Color Res. Appl. 1981, 6, 108–118. [Google Scholar] [CrossRef]
- Hunt, R.; Cavigelli, M.; Daughtry, C.; McMurtrey, J.E.; Walthall, C.L. Evaluation of Digital Photography from Model Aircraft for Remote Sensing of Crop Biomass and Nitrogen Status. Precis. Agric. 2005, 6, 359–378. [Google Scholar] [CrossRef]
- Hunt, E.R.; Doraiswamy, P.C.; Mcmurtrey, J.E.; Daughtry, C.S.T.; Perry, E.M.; Akhmedov, B. A visible band index for remote sensing leaf chlorophyll content at the canopy scale. Int. J. Appl. Earth Obser. Geoinf. 2013, 21, 103–112. [Google Scholar] [CrossRef]
- Rouse, J.W.; Hass, R.H.; Schell, J.A.; Deering, D.W. Monitoring vegetation systems in the great plains with ERTS. In Proceedings of the Third Earth Resources Technology Satellite (ERTS) Symposium, Washington, DC, USA, 10–14 December 1973; Volume 1, pp. 309–317. [Google Scholar]
- Huete, A. A soil-adjusted vegetation index (SAVI). Remote Sens. Environ. 1988, 25, 295–309. [Google Scholar] [CrossRef]
- Rondeaux, G.; Steven, M.; Baret, F. Optimization of soil-adjusted vegetation indices. Remote Sens. Environ. 1996, 55, 95–107. [Google Scholar] [CrossRef]
- Roujean, J.-L.; Breon, F.-M. Estimating PAR absorbed by vegetation from bidirectional reflectance measurements. Remote Sens. Environ. 1995, 51, 375–384. [Google Scholar] [CrossRef]
- Huete, A.; Didan, K.; Miura, T.; Rodriguez, E.P.; Gao, X.; Ferreira, L.G. Overview of the radiometric and biophysical performance of the MODIS vegetation indices. Remote Sens. Environ. 2002, 83, 195–213. [Google Scholar] [CrossRef]
- Haboudane, D.; Miller, J.R.; Tremblay, N.; Zarco-Tejada, P.J.; Dextraze, L. Integrated narrow-band vegetation indices for prediction of crop chlorophyll content for application to precision agriculture. Remote Sens. Environ. 2002, 81, 416–426. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Merzlyak, M.N.; Chivkunova, O.B. Optical Properties and Nondestructive Estimation of Anthocyanin Content in Plant Leaves. Photochem. Photobiol. 2001, 74, 38–45. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Zur, Y.; Chivkunova, O.B.; Merzlyak, M.N. Assessing carotenoid content in plant leaves with reflectance spectroscopy. Photochem. Photobiol. 2002, 75, 272–281. [Google Scholar] [CrossRef]
- Gamon, J.A.; Peñuelas, J.; Field, C.B. A narrow-waveband spectral index that tracks diurnal changes in photosynthetic efficiency. Remote Sens. Environ. 1992, 41, 35–44. [Google Scholar] [CrossRef]
- Gamon, J.A.; Huemmrich, K.F.; Wong, C.Y.S.; Ensminger, I.; Garrity, S.; Hollinger, D.Y.; Noormets, A.; Peñuelas, J. A remotely sensed pigment index reveals photosynthetic phenology in evergreen conifers. Proc. Natl. Acad. Sci. USA 2016, 113, 13087–13092. [Google Scholar] [CrossRef]
- Costa, J.M.; Grant, O.M.; Chaves, M.M. Thermography to explore plant-environment interactions. J. Exp. Bot. 2013, 64, 3937–3949. [Google Scholar] [CrossRef]
- Kefauver, S.C.; Vicente, R.; Vergara-Díaz, O.; Fernandez-Gallego, J.A.; Kerfal, S.; Lopez, A.; Melichar, J.P.E.; Serret Molins, M.D.; Araus, J.L. Comparative UAV and Field Phenotyping to Assess Yield and Nitrogen Use Efficiency in Hybrid and Conventional Barley. Front. Plant Sci. 2017, 8, 1–15. [Google Scholar] [CrossRef]
- Cerovic, Z.G.; Masdoumier, G.; Ghozlen, N.B.; Latouche, G. A new optical leaf-clip meter for simultaneous non-destructive assessment of leaf chlorophyll and epidermal flavonoids. Physiol. Plant. 2012, 146, 251–260. [Google Scholar] [CrossRef]
- Cerovic, Z.G.; Ghozlen, N.B.; Milhade, C.; Obert, M.; Debuisson, S.; Le Moigne, M. Nondestructive Diagnostic Test for Nitrogen Nutrition of Grapevine (Vitis vinifera L.) Based on Dualex Leaf-Clip Measurements in the Field. J. Agric. Food Chem. 2015, 63, 3669–3680. [Google Scholar] [CrossRef]
- RStudio Team. RStudio: Integrated Development for R; RStudio, Inc.: Boston, MA, USA; Available online: http://www.rstudio.com/ (accessed on 12 September 2018).
- R Core Team R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2017.
- Alvarado, G.; López, M.; Vargas, M.; Pacheco, Á.; Rodríguez, F.; Burgueño, J.; Crossa, J. META-R (Multi Environment Trail Analysis with R for Windows) Version 6.01. 2017. Available online: https://data.cimmyt.org/dataset.xhtml?persistentId=hdl:11529/10201/ (accessed on 16 December 2018).
- Prasad, P.V.V.; Staggenborg, S. Impacts of drought and/or heat stress on physiological, developmental, growth, and yield processes of crop plants. In Response of Crops to Limited Water: Understanding and Modeling Water Stress Effects on Plant Growth Processes Response of Crops; American Society of Agronomy: Madison, WI, USA, 2008; pp. 301–355. [Google Scholar]
- Farooq, M.; Bramley, H.; Palta, J.A.; Siddique, K.H.M. Heat Stress in Wheat during Reproductive and Grain-Filling Phases. Crit. Rev. Plant Sci. 2011, 30, 491–507. [Google Scholar] [CrossRef]
- Pinto, R.S.; Molero, G.; Reynolds, M.P. Identification of heat tolerant wheat lines showing genetic variation in leaf respiration and other physiological traits. Euphytica 2017, 213, 1–15. [Google Scholar] [CrossRef]
- Rashid, M.A.; Andersen, M.N.; Wollenweber, B.; Kørup, K.; Zhang, X.; Olesen, J.E. Impact of heat-wave at high and low VPD on photosynthetic components of wheat and their recovery. Environ. Exp. Bot. 2018, 147, 138–146. [Google Scholar] [CrossRef]
- Long, S.P.; Ort, D.R. More than taking the heat: Crops and global change. Curr. Opin. Plant Biol. 2010, 13, 241–248. [Google Scholar] [CrossRef]
- Torriani, D.S.; Calanca, P.; Schmid, S.; Beniston, M.; Fuhrer, J. Potential effects of changes in mean climate and climate variability on the yield of winter and spring crops in Switzerland. Clim. Chang. 2007, 34, 59–69. [Google Scholar] [CrossRef]
- Ghahramani, A.; Kokic, P.N.; Moore, A.D.; Zheng, B.; Chapman, S.C.; Howden, M.S.; Crimp, S.J. The Value of Adapting to Climate Change in Australian Wheat Farm Systems: Farm to Cross-Regional Scale. Agric. Ecosyst. Environ. 2015, 211, 112–125. [Google Scholar]
- Nouri, M.; Homaee, M.; Bannayan, M.; Hoogenboom, G. Towards shifting planting date as an adaptation practice for rainfed wheat response to climate change. Agric. Water Manag. 2017, 186, 108–119. [Google Scholar] [CrossRef]
- Weiss, A.; Hays, C.J.; Won, J. Assessing winter wheat responses to climate change scenarios: A simultation study in the U.S. Great Plains. 2003, 58, 119–147. [Google Scholar]
- Altenbach, S.B.; DuPont, F.M.; Kothari, K.M.; Chan, R.; Johnson, E.L.; Lieu, D. Temperature, water and fertilizer influence the timing of key events during grain development in a US spring wheat. J. Cereal Sci. 2003, 37, 9–20. [Google Scholar] [CrossRef]
- Fernandez-gallego, J.A.; Kefauver, S.C.; Vatter, T. Low-cost assessment of grain yield in durum wheat using RGB images. Eur. J. Agron. 2019, 105, 146–156. [Google Scholar] [CrossRef]
- Snape, J.W.; Butterworth, K.; Whitechurch, E.; Worland, A.J. Waiting for fine times: Genetics of flowering time in wheat. Euphytica 2001, 119, 185–190. [Google Scholar] [CrossRef]
- Asana, R.D.; Mani, V.S. Studies in Physiological Analysis of Yield. II. Further Observations on Varietal Differences in Photosynthesis in the Leaf, Stem and Ear of Wheat. Physiol. Plant. 1955, 8, 8–19. [Google Scholar] [CrossRef]
- Ugarte, C.; Calderini, D.F.; Slafer, G.A. Grain weight and grain number responsiveness to pre-anthesis temperature in wheat, barley and triticale. Field Crops Res. 2007, 100, 240–248. [Google Scholar] [CrossRef]
- González, F.G.; Slafer, G.A.; Miralles, D.J. Grain and floret number in response to photoperiod during stem elongation in fully and slightly vernalized wheats. Field Crops Res. 2003, 81, 17–27. [Google Scholar] [CrossRef]
- Lukina, E.V.; Stone, M.L.; Raun, W.R. Estimating vegetation coverage in wheat using digital images. J. Plant Nut. 1999, 22, 341–350. [Google Scholar] [CrossRef]
- Cabrera-Bosquet, L.; Molero, G.; Stellacci, A.M.; Bort, J.; Nogues, S.; Araus, J.L. NDVI as a potential tool for predicting biomass, plant nitrogen content and growth in wheat genotypes subjected to different water and nitrogen conditions. Cereal Res. Commun. 2011, 39, 147–159. [Google Scholar] [CrossRef]
- Duan, T.; Chapman, S.C.; Guo, Y.; Zheng, B. Field Crops Research Dynamic monitoring of NDVI in wheat agronomy and breeding trials using an unmanned aerial vehicle. Field Crops Res. 2017, 210, 71–80. [Google Scholar] [CrossRef]
- Gregersen, P.L.; Culetic, A.; Boschian, L.; Krupinska, K. Plant senescence and crop productivity. Plant Mol. Biol. 2013, 82, 603–622. [Google Scholar] [CrossRef] [PubMed]
- Thomas, H.; Ougham, H. The stay-green trait. J. Exp. Bot. 2014, 65, 3889–3900. [Google Scholar] [CrossRef]
- Christopher, J.; Veyradier, M.; Borrell, A.; Harvey, G.; Fletcher, S.; Chenu, K. Phenotyping novel stay-green traits to capture genetic variation in senescence dynamics. Funct. Plant Biol. 2014, 41, 1035–1048. [Google Scholar] [CrossRef]
- Christopher, J.T.; Christopher, M.J.; Borrell, A.K.; Fletcher, S.; Chenu, K. Stay-green traits to improve wheat adaptation in well- watered and water-limited environments. J. Exp. Bot. 2016, 67, 5159–5172. [Google Scholar] [CrossRef]
- Spano, G.; Di Fonzo, N.; Perrotta, C.; Platani, C.; Ronga, G.; Lawlor, D.W.; Napier, J.A. Physiological characterization of stay green mutants in durum wheat. J. Exp. Bot. 2003, 54, 1415–1420. [Google Scholar] [CrossRef] [PubMed]
- Crain, J.; Reynolds, M.; Poland, J. Utilizing High-Throughput Phenotypic Data for Improved Phenotypic Selection of Stress-Adaptive Traits in Wheat. Crop Sci. 2017, 659, 648–659. [Google Scholar] [CrossRef]
- Lopes, M.S.; Reynolds, M.P. Stay-green In Posidonia in oceanica spring wheat cadmium can be induces determined changes by in spectral DNA reflectance methylation measurements and chromatin (normalized patterning difference vegetation index) independently from phenology. J. Exp. Bot. 2012, 63, 3789–3798. [Google Scholar] [CrossRef] [PubMed]
- Kichey, T.; Hirel, B.; Heumez, E. In winter wheat (Triticum aestivum L.), post-anthesis nitrogen uptake and remobilisation to the grain correlates with agronomic traits and nitrogen physiological markers. Field Crop Res. 2007, 102, 22–32. [Google Scholar] [CrossRef]
- Derkx, A.P.; Orford, S.; Griffiths, S.; Foulkes, M.J.; Hawkesford, M.J. Identification of Differentially Senescing Mutants of Wheat and Impacts on Yield, Biomass and Nitrogen. J. Integr. Plant Biol. 2012, 54, 555–566. [Google Scholar] [CrossRef]
- Buchaillot, M.L.; Gracia-Romero, A.; Vergara-Diaz, O.; Zaman-Allah, M.A.; Tarekegne, A.; Cairns, J.E.; Prasanna, B.M.; Araus, J.L.; Kefauver, S.C. Evaluating Maize Genotype Performance under Low Nitrogen Conditions Using RGB UAV Phenotyping Techniques. Sensor 2019, 19, 1815. [Google Scholar] [CrossRef] [PubMed]
- Monostori, I.; Árendás, T.; Hoffman, B.; Galiba, G.; Gierczik, K.; Szira, F.; Vágújfalvi, A. Relationship between SPAD value and grain yield can be affected by cultivar, environment and soil nitrogen content in wheat. Euphytica 2016, 211, 103–112. [Google Scholar] [CrossRef]
- Gamon, J.A.; Serrano, L.; Surfus, J.S. The photochemical reflectance index: An optical indicator of photosynthetic radiation use efficiency across species, functional types, and nutrient levels. Oecologia 1997, 112, 492. [Google Scholar] [CrossRef] [PubMed]
- Peñuelas, J.; Garbulsky, M.F.; Filella, I. Photochemical reflectance index (PRI) and remote sensing of plant CO2 uptake. New Phytol. 2011, 191, 596–599. [Google Scholar] [CrossRef] [PubMed]
- Magney, T.S.; Vierling, L.A.; Eitel, J.U.H.; Huggins, D.R.; Garrity, S.R. Response of high frequency Photochemical Reflectance Index (PRI) measurements to environmental conditions in wheat. Remote Sens. Environ. 2016, 173, 84–97. [Google Scholar] [CrossRef]
- Loss, S.P.; Siddique, K.H.M. Morphological and Physiological Traits Associated with Wheat Yield Increases in Mediterranean Environments; Sparks, D.L., Ed.; Academic Press: Boston, MA, USA, 1994; Volume 52, pp. 229–276. [Google Scholar]
- Villegas, D.; Aparicio, N.; Royo, C. Assessment of durum wheat yield and carbon isotope discrimination by reflectance indices WI and PRI. In Cereal Science and Technology for Feeding Ten Billion People: Genomics Era and beyond; Options Méditerranéennes: Série A. Séminaires Méditerranéens: Zaragoza, Spain, 2008; Volume 81, pp. 403–405. [Google Scholar]
- Araus, J.L.; Bort, J.; Steduto, P.; Villegas, D.; Royo, C. Breeding cereals for Mediterranean conditions: Ecophysiological clues for biotechnology application. Ann. Appl. Biol. 2003, 142, 129–141. [Google Scholar] [CrossRef]
- Fischer, R.A.; Rees, D.; Sayre, K.D.; Lu, Z.-M.; Condon, A.G.; Saavedra, A.L. Wheat Yield Progress Associated with Higher Stomatal Conductance and Photosynthetic Rate, and Cooler Canopies. Crop Sci. 1998, 38, 1467–1475. [Google Scholar] [CrossRef]
- Berger, B.; Parent, B.; Tester, M. High-throughput shoot imaging to study drought responses. J. Exp. Bot. 2010, 61, 3519–3528. [Google Scholar] [CrossRef]
- Aparicio, N.; Villegas, D.; Casadesus, J.; Araus, J.L.; Royo, C. Spectral vegetation indices as nondestructive tools for determining durum wheat yield. Agron. J. 2000, 92, 83–91. [Google Scholar] [CrossRef]
- Crain, J.; Mondal, S.; Rutkoski, J.; Singh, R.P.; Poland, J. Combining High-Throughput Phenotyping and Genomic Information to Increase Prediction and Selection Accuracy in Wheat Breeding. Plant Genome 2018, 11, 1–14. [Google Scholar] [CrossRef]
- Petersen, L.K. Real-Time Prediction of Crop Yields From MODIS Relative Vegetation Health: A Continent-Wide Analysis of Africa. Remote Sens. 2018, 10, 1726. [Google Scholar] [CrossRef]
- Torres-Sánchez, J.; Peña, J.M.; Castro, A.I. Multi-temporal mapping of the vegetation fraction in early-season wheat fields using images from UAV. Comput. Electron. Agric. 2014, 103, 104–113. [Google Scholar] [CrossRef]
- Stöcker, C.; Bennett, R.; Nex, F.; Gerke, M.; Zevenbergen, J. Review of the current state of UAV regulations. Remote Sens. 2017, 9, 459. [Google Scholar] [CrossRef]
- Gago, J.; Douthe, C.; Coopman, R.E.; Gallego, P.P.; Ribas-Carbo, M.; Flexas, J.; Escalona, J.; Medrano, H. UAVs challenge to assess water stress for sustainable agriculture. Agric. Water Manag. 2015, 153, 9–19. [Google Scholar] [CrossRef]
- Sudhakar, P.; Latha, P.; Reddy, P.V. Phenotyping Crop Plants for Physiological and Biochemical Traits; Academic Press: Boston, MA, USA, 2016. [Google Scholar]
- Piepho, H.P.; Möhring, J. Computing heritability and selection response from unbalanced plant breeding trials. Genetics 2007, 177, 1881–1888. [Google Scholar] [CrossRef]



| Sampling | Date | DAS | GDD | Zadocks Scale | Phen. Stage | |
|---|---|---|---|---|---|---|
| Supplementary Irrigation | 1st | 26/04/2017 | 125 | 2224.05 | 55–59 | Heading |
| 2nd | 04/05/2017 | 133 | 2399.68 | 61 | Anthesis | |
| 3rd | 18/05/2017 | 147 | 2767.24 | 75 | Milk Grain Filling | |
| 4th | 06/06/2017 | 166 | 3377.17 | 87 | Senescence | |
| Rainfed | 1st | 26/04/2017 | 125 | 2224.05 | 55–57 | Heading |
| 2nd | 04/05/2017 | 133 | 2399.68 | 61–65 | Anthesis | |
| 3rd | 18/05/2017 | 147 | 2767.24 | 77–79 | Late Grain Filling | |
| 4th | 06/06/2017 | 166 | 3377.17 | 90–99 | Senescence | |
| Late-Planting | 1st | 26/04/2017 | 56 | 1270.57 | 30–32 | Stem Elongation |
| 2nd | 04/05/2017 | 64 | 1446.21 | 45–47 | Booting | |
| 3rd | 18/05/2017 | 78 | 1813.76 | 58–59 | Heading | |
| 4th | 06/06/2017 | 97 | 2423.69 | 75–79 | Milk Grain Filling |
| Date of Sampling | RGB | Multispectral | Thermal |
|---|---|---|---|
| 26/04/2017 | 133 | 24 * | 543 * |
| 04/05/2017 | 184 | 61 | 605 |
| 18/05/2017 | 182 | 71 | 804 |
| 06/06/2017 | 97 * | 36 * | 585 * |
| Measure | Sensor/Camera and Approximated Cost | Image | Major Specifications |
|---|---|---|---|
| RGB indexes | Sony ILCE-QX1 <500 € |  | 20.1 Megapixel. Sensor size: 23.20 × 15.40 mm. Focal length: 35 mm. Trigged and exposure time. programed in automatic mode. |
| Panasonic Lumix GX7 <500 € |  | 16 Megapixels. Sensor size: 17.3 × 13.0 mm. Focal length: 35 mm. Trigged and exposure time programed in automatic mode. | |
| Multispect. indexes | Tetracam micro-MCA12 <25,000 € |  | Incident Light Sensor (ILS). 15.6 Megapixels. Sensor size: 6.66 × 5.32 mm. Wavelengh range: 450 to 950 nm. |
| Trimble GreenSeeker Handheld Crop Sensor <500 € |  | Wavelength range: 670 and 840 nm. Field of view: 25 cm (1 m from the canopy). | |
| Canopy temperature | Raytek PhotoTempTM MXSTM TD infrared thermometer <300 € |  | Temperature range: −30 to 900 °C. Wavelength range: 8 to 14 µm. |
| FLIR Tau2 640 thermal imaging camera < 8000 € |  | With a VOx uncooled microbolometer equipped with a TeAx Thermal Capture 2.0. Temperature range: −55 to 95 °C. Wavelength range: 7.5 to 13.5 µm. | |
| Pigment content | Dualex Force-A <4000 € |  | Measured area: 5 mm in diameter Sample thickness: 1 mm maximum Light sources: 5 LED; 1 UV-A, 1 red and 2 near NIR (near-infrared) |
| Target Group | Index | Formula | Type; Bands | Ref |
|---|---|---|---|---|
| Vegetation cover | Green Area (GA) | RGB; HIS color model | [29] | |
| Greener Area (GGA) | RGB; HIS color model | [29] | ||
| Greenness | Crop Senescence Index (CSI) | RGB; HIS color model | [30] | |
| a*; b* | RGB; CIElab color model | [29] | ||
| u*; v* | RGB; CIEluv color model | [29] | ||
| Normalized Green-Red Difference Index (NGRDI) | RGB; Red and Green bands | [33] | ||
| Triangular Greenness Index (TGI) | RGB; Red, Green and Blue bands | [33] | ||
| Normalized Difference Vegetation Index (NDVI) | Multispectral; Red, NIR | [34] | ||
| Soil Adjusted Vegetation Index (SAVI) | Intermediate vegetation, L = 0.5 | Multispectral; Red, NIR | [35] | |
| Optimized soil-adjusted vegetation index (OSAVI) | Multispectral; Red, NIR | [36] | ||
| Renormalized Difference Vegetation Index (RDVI) | Multispectral; Red, NIR | [37] | ||
| Enhanced Vegetation Index (EVI) | Multispectral; Blue, Red, NIR | [38] | ||
| Leaf Pigments | Modified Chlorophyll Absorption Ratio Index (MCARI) | Multispectral; Green, Red, NIR | [22] | |
| Transformed Chlorophyll Absorption Index (TCARI) | Multispectral; Green, Red, NIR | [39] | ||
| TCARI/OSAVI ratio | Multispectral; Green, Red, NIR | [39] | ||
| Anthocyanin Reflectance Index 2 (ARI2) | Multispectral; Blue, Red, NIR | [40] | ||
| Carotenoid Reflectance Index 2 (CRI2) | Multispectral; Blue, Red | [41] | ||
| Photosynthetic Activity | Photochemical Reflectance Index (PRI)* | Multispectral; Green | [42] | |
| Chlorophyll Carotenoid Index (CCI) | Multispectral; Green, NIR | [43] | ||
| Water content | Water Band Index (WBI) | Multispectral; NIR | [21] |
| Supplementary Irrigation | Rainfed | Late-Planting | |||
|---|---|---|---|---|---|
| Olivadur Burgos Sculpur Euroduro Iberus Claudio Vitron Athoris Kiko Nick Regallo Dorondon Pedroso Amilcar Avispa Saragolla Gallareta Mexa Sole D. Ricardo Simeto D. Norman Arcobaleno Core Mean ANOVA | 6.03 ± 0.18 a 5.67 ± 0.23 ab 5.34 ± 0.03 abc 5.31 ± 0.12 abc 5.21 ± 0.06 abc 5.19 ± 0.07 abc 5.14 ± 0.12 abc 5.08 ± 0.22 abc 5.07 ± 0.19 abc 5.02 ± 0.14 abc 4.96 ± 0.16 abcd 4.92 ± 0.22 abcd 4.80 ± 0.11 abcd 4.76 ± 0.16 abcd 4.74 ± 0.17 abcd 4.71 ± 0.25 abcd 4.59 ± 0.13 abcd 4.55 ± 0.17 abcd 4.48 ± 0.06 bcd 4.11 ± 0.14 cd 4.10 ± 0.09 cd 4.05 ± 0.11 cd 3.46 ± 0.04 d 4.84 ± 0.04 0.003 ** | Olivadur Athoris Claudio Kiko Nick Avispa Burgos Amilcar Dorondon Sculpur Regallo Vitron Iberus D. Ricardo Euroduro D. Norman Simeto Gallareta Mexa Pedroso Arcobaleno Saragolla Solea Core Mean ANOVA | 3.58 ± 0.13 a 3.28 ± 0.08 ab 3.22 ± 0.10 ab 3.14 ± 0.14 ab 3.08 ± 0.17 ab 3.06 ± 0.11 ab 3.06 ± 0.06 ab 3.04 ± 0.07 ab 2.88 ± 0.05 abc 2.83 ± 0.15 abc 2.81 ± 0.08 abc 2.73 ± 0.15 abcd 2.72 ± 0.14 abcd 2.65 ± 0.10 abcd 2.63 ± 0.12 abcd 2.59 ± 0.20 abcd 2.57 ± 0.06 abcd 2.52 ± 0.12 abcd 2.50 ± 0.11 abcd 2.34 ± 0.15 bcd 2.27 ± 0.14 bcd 1.82 ± 0.06 cd 1.61 ± 0.08 d 2.74 ± 0.04 0.002 ** | Euroduro Burgos Claudio Olivadur Sculpur Iberus Athoris Solea D. Norman Regallo Vitron Saragolla D. Ricardo Dorondon Kiko Nick Gallareta Avispa Amilcar Mexa Arcobaleno Pedroso Simeto Core Mean ANOVA | 5.06 ± 0.07 a 4.87 ± 0.12 ab 4.62 ± 0.08 abc 4.44 ± 0.09 abcd 4.31 ± 0.05 abcd 4.21 ± 0.13 bcde 4.19 ± 0.04 bcde 4.01 ± 0.12 cdef 3.98 ± 0.03 cdef 3.89 ± 0.11 cdefg 3.78 ± 0.02 cdefg 3.74 ± 0.17 defgh 3.69 ± 0.11 defghi 3.50 ± 0.10 efghi 3.45 ± 0.10 efghi 3.43 ± 0.11 efghi 3.24 ± 0.05 fghi 3.18 ± 0.06 ghi 3.08 ± 0.13 hi 3.08 ± 0.05 hi 3.06 ± 0.07 hi 2.95 ± 0.08 i 3.05 ± 0.18 hi 3.78 ± 0.04 0.000 *** |
| Trial | Phenological Stage | Equation | R2 | RSE | p-Value | PS% |
|---|---|---|---|---|---|---|
| Supplementary Irrigation | Anthesis | GY = 64.84 NGRDI + 1.68 | 0.254 | 0.652 | 0.000 | 60 |
| Grain Filling | GY = 8.69 GA − 2.85 | 0.468 | 0.551 | 0.000 | 60 | |
| GY = 0.00096 TGI − 2.00 | 0.270 | 0.645 | 0.000 | 40 | ||
| GY = 12.19 SAVI − 1.83 | 0.423 | 0.573 | 0.000 | 80 | ||
| GY = 26.29 PRI − 0.32 | 0.287 | 0.637 | 0.000 | 60 | ||
| Senescence | GY = 0.07 Hue + 1.98 | 0.361 | 0.603 | 0.000 | 60 | |
| GY = −0.14 a* + 2.43 | 0.201 | 0.675 | 0.000 | 60 | ||
| Combination | GY = 33.64 NGRDI.A + 11.72 PRI.GF + 0.04 Hue.LGF − 0.92 | 0.421 | 0.583 | 0.000 | 80 | |
| Rainfed | Heading | GY = 0.0009 TGI + 2.00 | 0.270 | 0.645 | 0.000 | 80 |
| GY = −0.466 u* − 0.63 | 0.371 | 0.478 | 0.000 | 40 | ||
| GY = 63.80 NGRDI + 0.29 | 0.468 | 0.440 | 0.000 | 60 | ||
| Anthesis | GY = −0.08 u* + 1.83 | 0.340 | 0.490 | 0.000 | 60 | |
| GY = 4.06 GA − 0.28 | 0.442 | 0.450 | 0.000 | 60 | ||
| GY = 42.97 NGRDI + 1.59 | 0.453 | 0.446 | 0.000 | 60 | ||
| GY = 7.95 NDVI − 3.56 | 0.440 | 0.451 | 0.000 | 60 | ||
| GY = −0.36 CT + 11.71 | 0.581 | 0.390 | 0.000 | 40 | ||
| Grain Filling | GY = −0.10 a* + 2.40 | 0.413 | 0.462 | 0.000 | 60 | |
| GY = 2.22 GA + 2.02 | 0.413 | 0.462 | 0.000 | 60 | ||
| GY = 7.79 NGRDI + 3.12 | 0.386 | 0.472 | 0.000 | 60 | ||
| GY = 5.18 NDVI + 0.07 | 0.489 | 0.431 | 0.000 | 60 | ||
| GY = 28.12 PRI − 1.67 | 0.438 | 0.452 | 0.000 | 60 | ||
| GY = 8.88 CCI + 1.76 | 0.433 | 0.454 | 0.000 | 60 | ||
| GY = −2.94 TCARIO/SAVI + 4.45 | 0.488 | 0.431 | 0.000 | 80 | ||
| GY = 22.09 NGRDI.A − 0.28 CT.GF − 0.57 NDVI.GF + 9.45 | 0.632 | 0.371 | 0.000 | 60 | ||
| Combination | ||||||
| Late-Planting | Heading | GY = −0.11 u* + 1.88 | 0.366 | 0.555 | 0.000 | 60 |
| GY = 5.90 GGA − 0.63 | 0.376 | 0.551 | 0.000 | 60 | ||
| GY = 9.19 NGRDI + 1.95 | 0.349 | 0.563 | 0.000 | 60 | ||
| Anthesis | GY = 7.16 GA − 2.38 | 0.434 | 0.524 | 0.000 | 60 | |
| GY = 64.79 NGRDI + 0.43 | 0.398 | 0.541 | 0.000 | 80 | ||
| GY = 11.52 SAVI − 2.27 | 0.406 | 0.537 | 0.000 | 80 | ||
| GY = −0.43 CT + 15.46 | 0.414 | 0.533 | 0.000 | 60 | ||
| Grain Filling | GY = −0.15 a* + 2.46 | 0.588 | 0.447 | 0.000 | 80 | |
| GY = 2.82 GA + 2.16 | 0.559 | 0.463 | 0.000 | 80 | ||
| GY = 0.0009 TGI + 1.80 | 0.533 | 0.476 | 0.000 | 80 | ||
| GY = 9.29 SAVI − 0.19 | 0.563 | 0.461 | 0.000 | 80 | ||
| GY = 13.89 CCI + 0.56 | 0.568 | 0.458 | 0.000 | 100 | ||
| GY = −1.08 CRI2 + 6.45 | 0.488 | 0.499 | 0.000 | 80 | ||
| Combination | GY = 1.13 GGA.H + 4.03 SAVI.A + 10.05 CCI.GF − 1.51 | 0.625 | 0.433 | 0.000 | 80 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gracia-Romero, A.; Kefauver, S.C.; Fernandez-Gallego, J.A.; Vergara-Díaz, O.; Nieto-Taladriz, M.T.; Araus, J.L. UAV and Ground Image-Based Phenotyping: A Proof of Concept with Durum Wheat. Remote Sens. 2019, 11, 1244. https://doi.org/10.3390/rs11101244
Gracia-Romero A, Kefauver SC, Fernandez-Gallego JA, Vergara-Díaz O, Nieto-Taladriz MT, Araus JL. UAV and Ground Image-Based Phenotyping: A Proof of Concept with Durum Wheat. Remote Sensing. 2019; 11(10):1244. https://doi.org/10.3390/rs11101244
Chicago/Turabian StyleGracia-Romero, Adrian, Shawn C. Kefauver, Jose A. Fernandez-Gallego, Omar Vergara-Díaz, María Teresa Nieto-Taladriz, and José L. Araus. 2019. "UAV and Ground Image-Based Phenotyping: A Proof of Concept with Durum Wheat" Remote Sensing 11, no. 10: 1244. https://doi.org/10.3390/rs11101244
APA StyleGracia-Romero, A., Kefauver, S. C., Fernandez-Gallego, J. A., Vergara-Díaz, O., Nieto-Taladriz, M. T., & Araus, J. L. (2019). UAV and Ground Image-Based Phenotyping: A Proof of Concept with Durum Wheat. Remote Sensing, 11(10), 1244. https://doi.org/10.3390/rs11101244