A MODIS Photochemical Reflectance Index (PRI) as an Estimator of Isoprene Emissions in a Temperate Deciduous Forest
Abstract
1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. Flux Data
2.3. Satellite Data
2.4. Statistical Analyses
3. Results
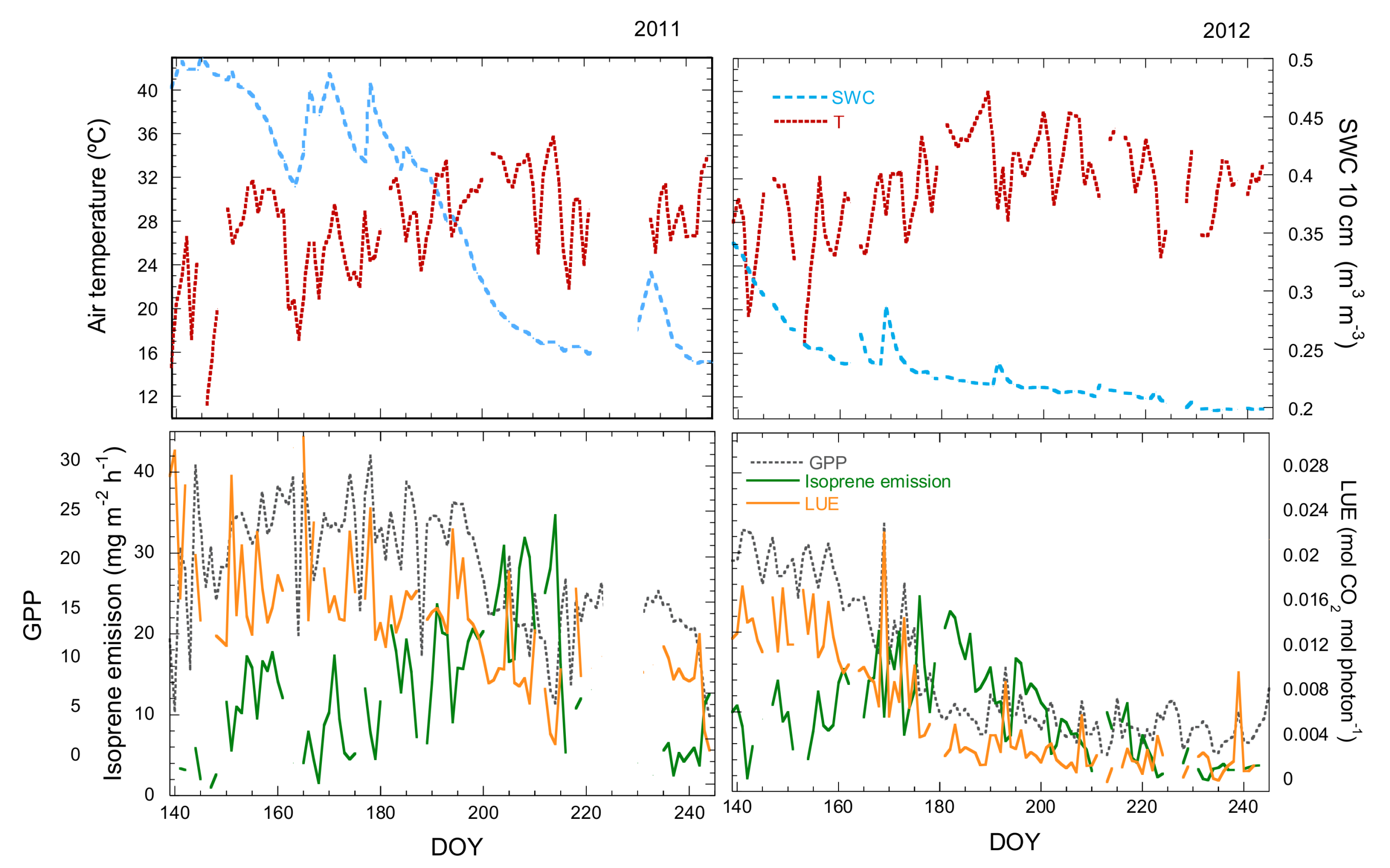
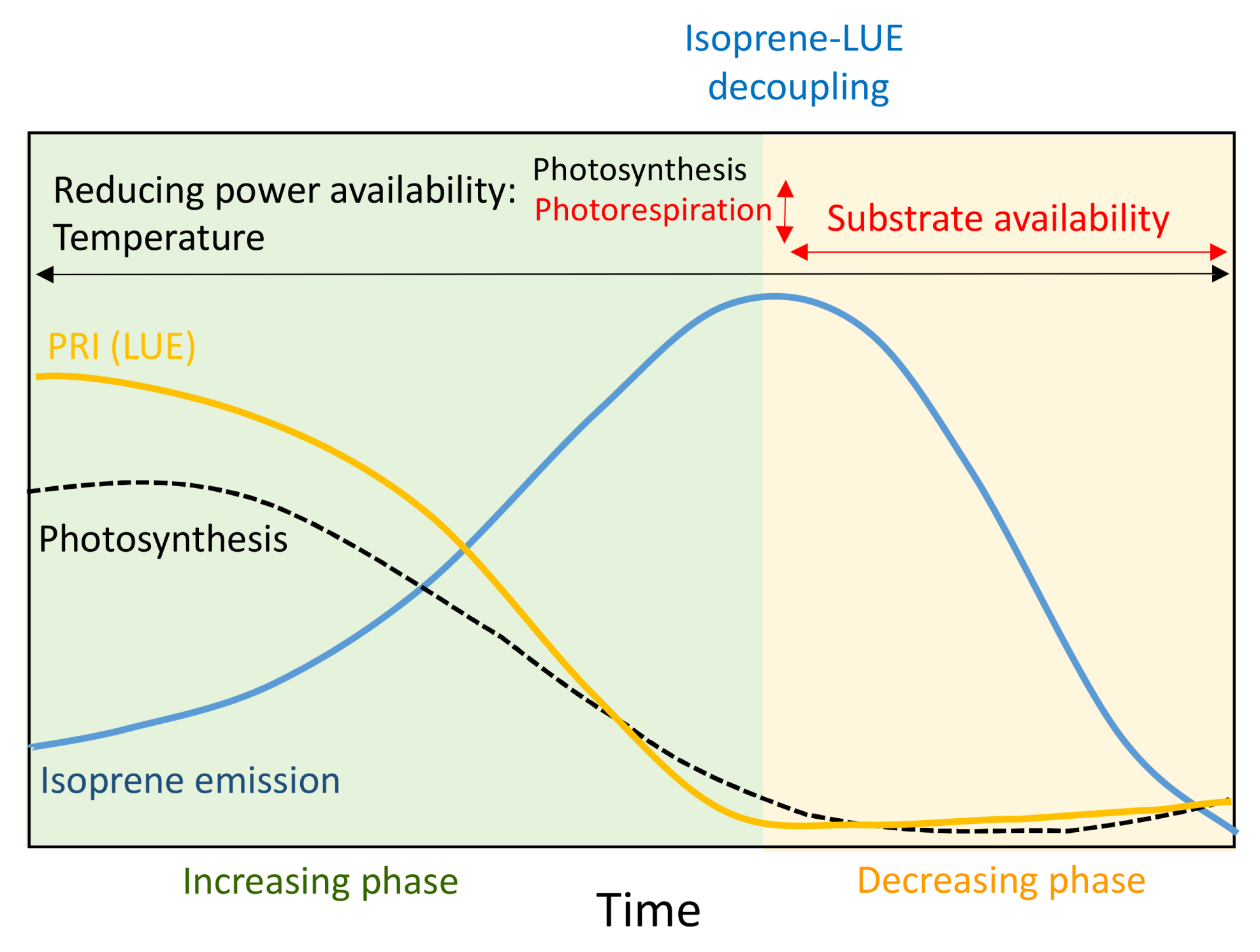
3.1. Seasonal Variation in Isoprene Emissions, Water Availability, Temperature and LUE
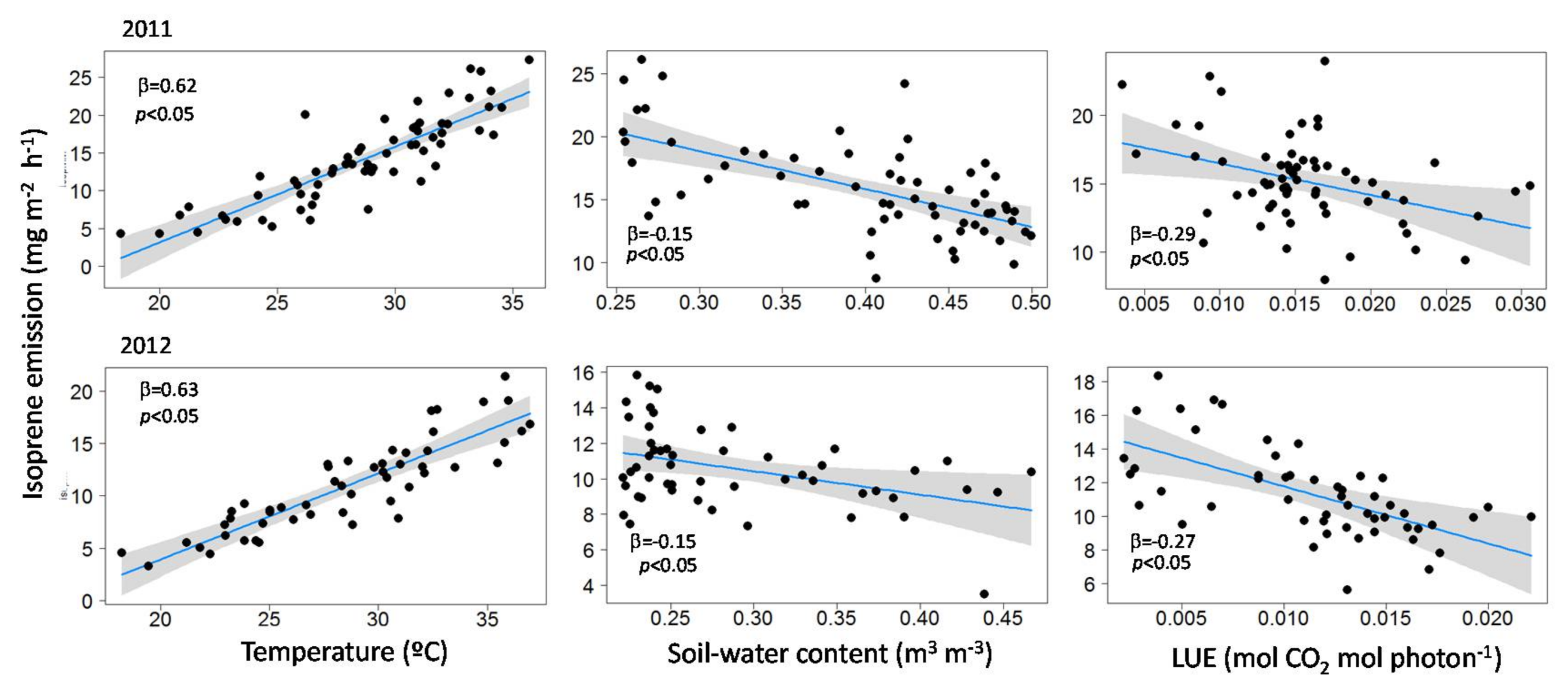
3.2. Influence of Temperature, LUE and SWC on Isoprene Emissions
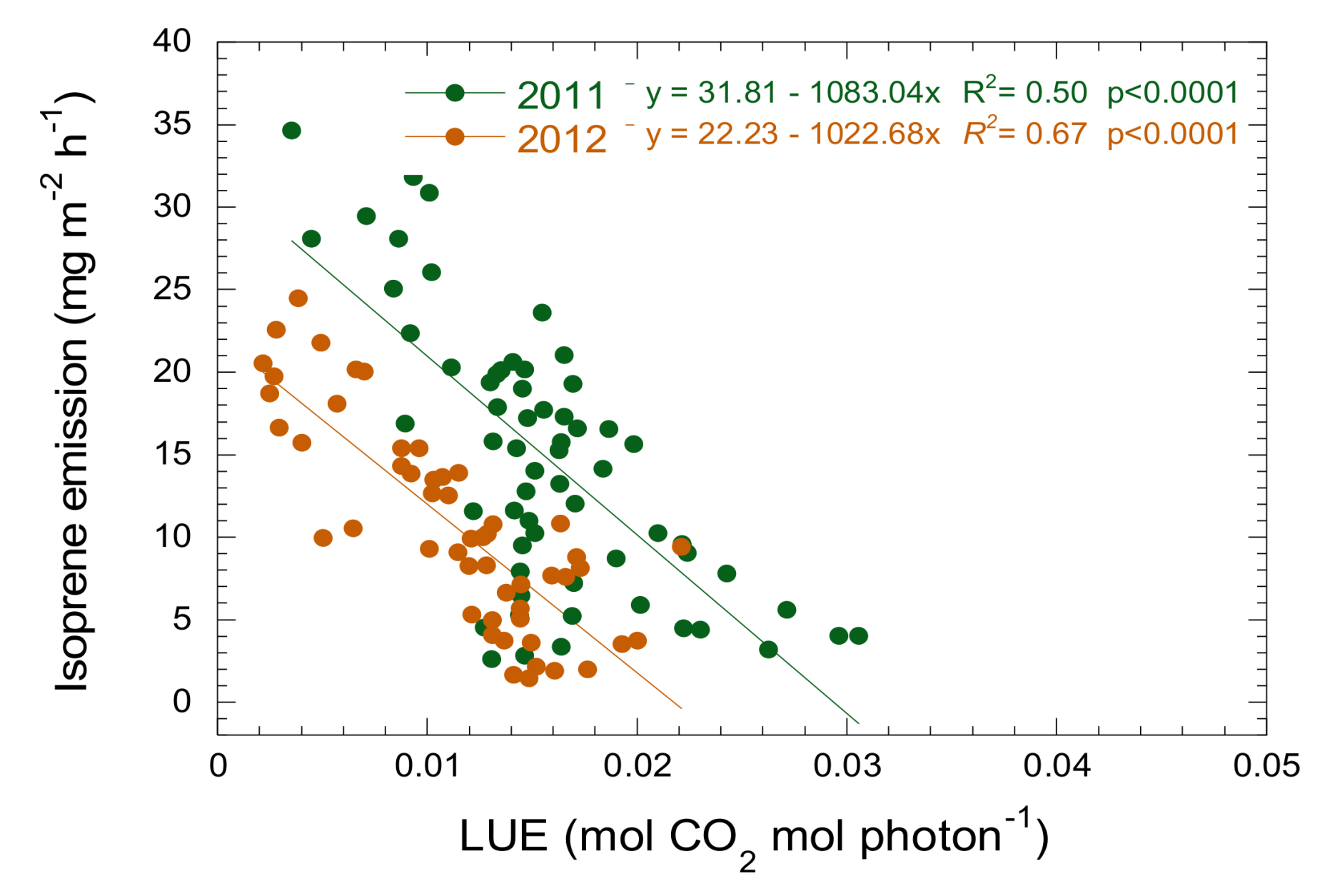
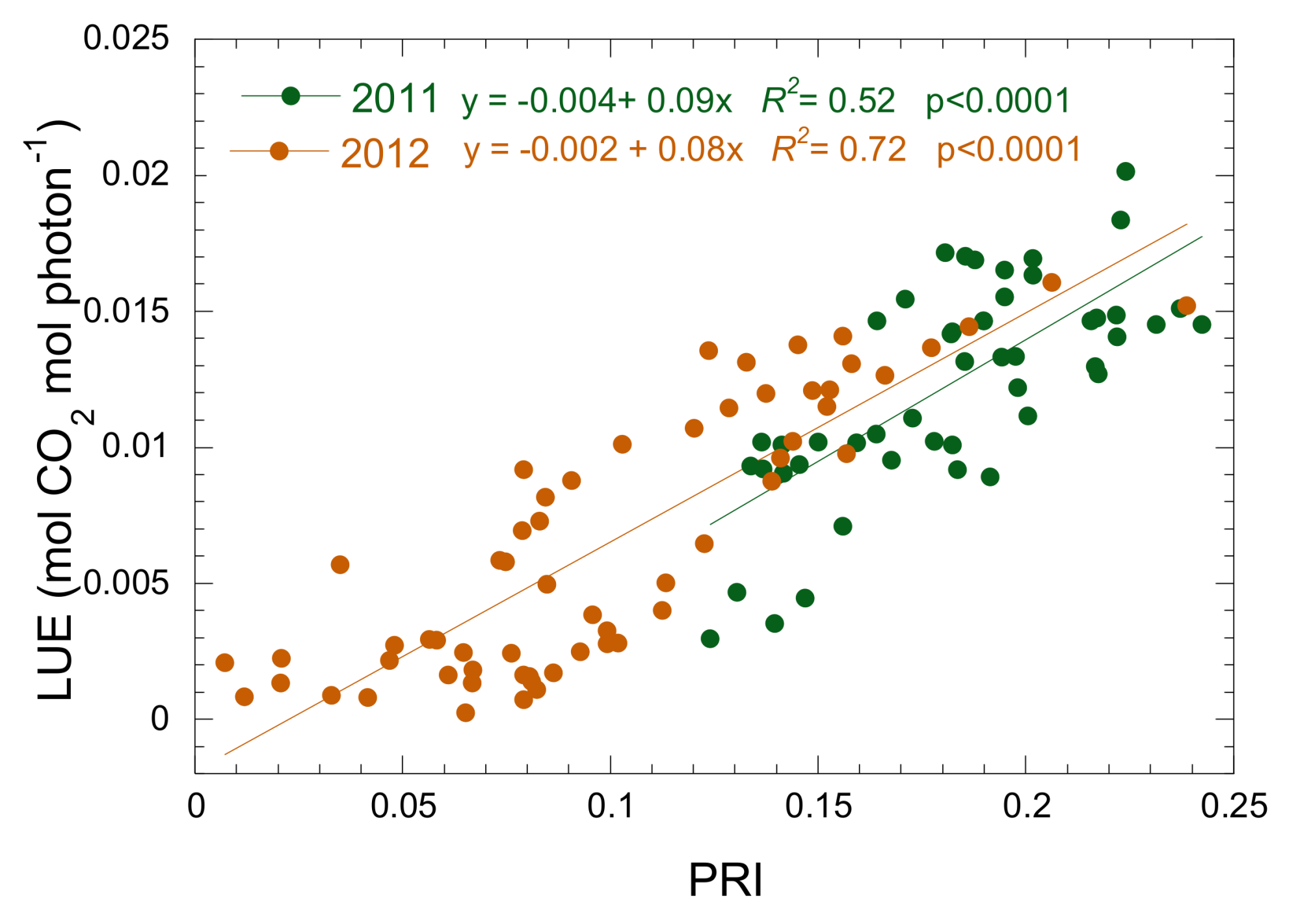
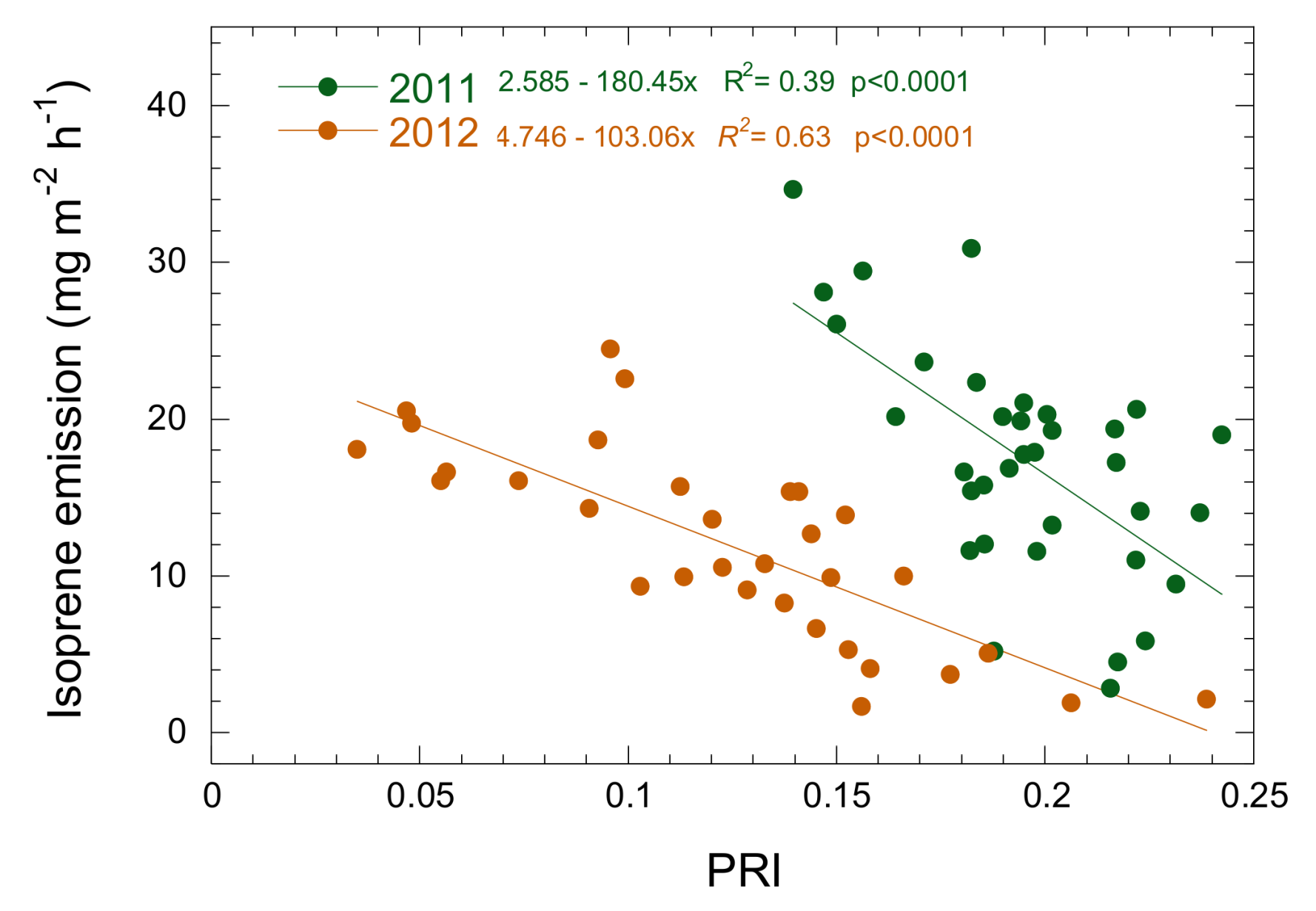
3.3. Relationship between Isoprene Emission, LUE and PRI
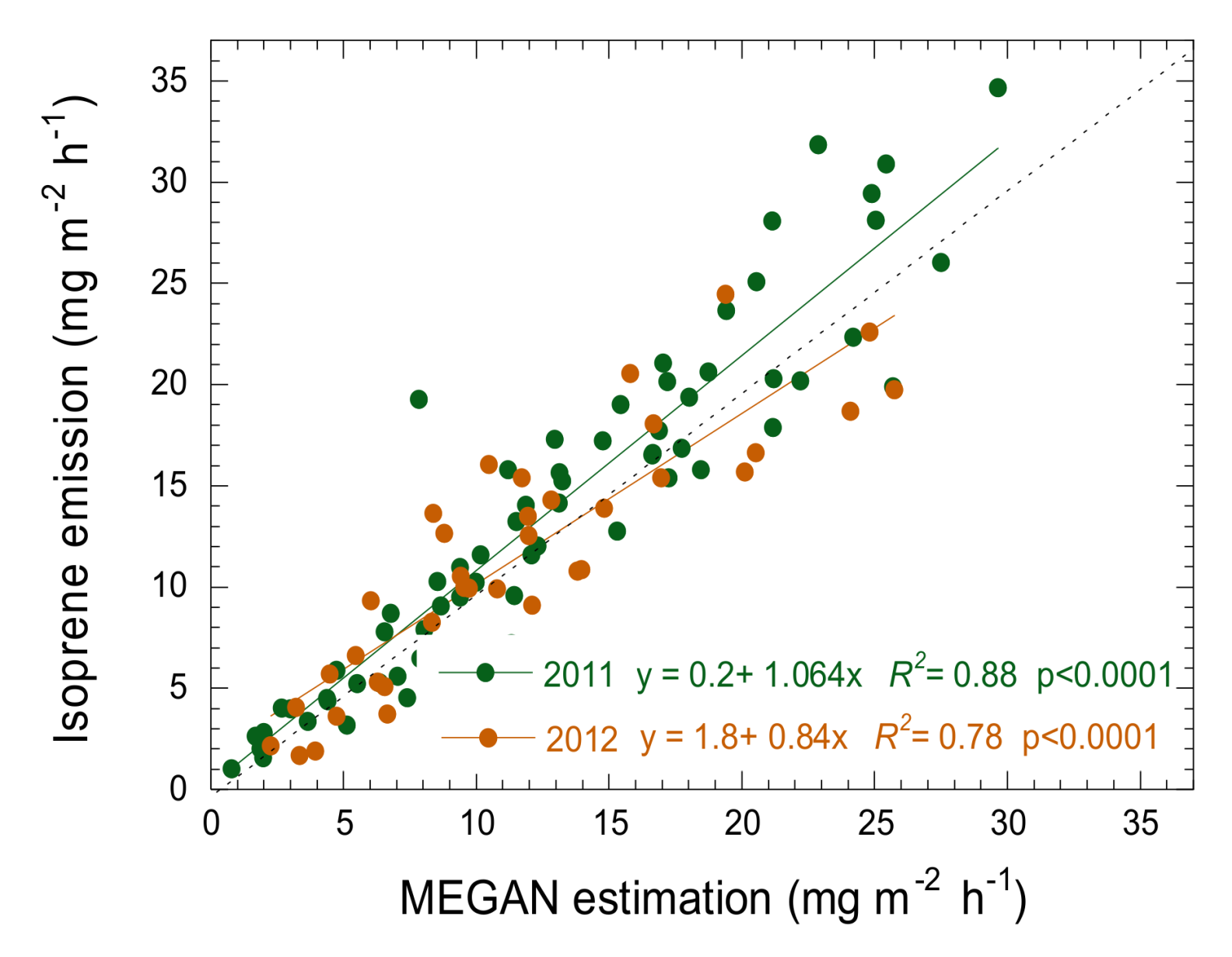
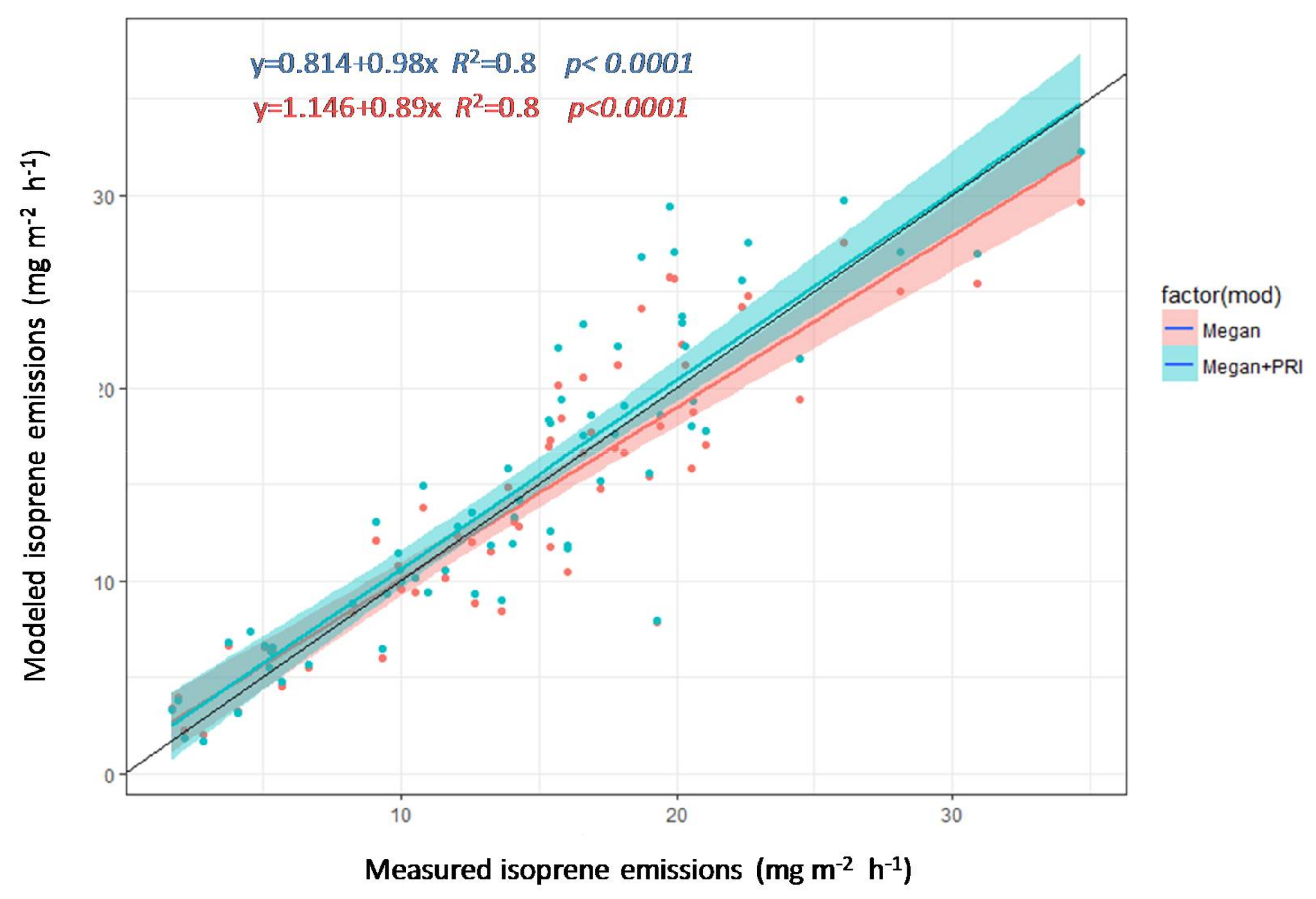
3.4. Estimation of Isoprene Emissions Using PRI and MEGAN
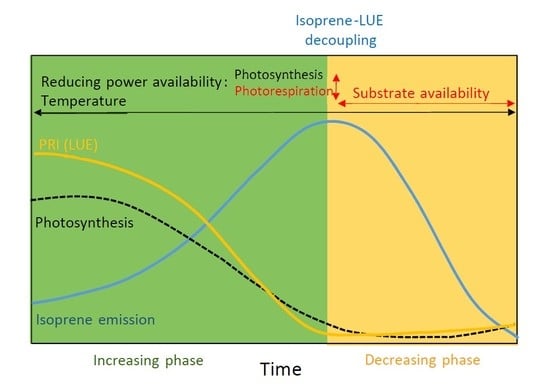
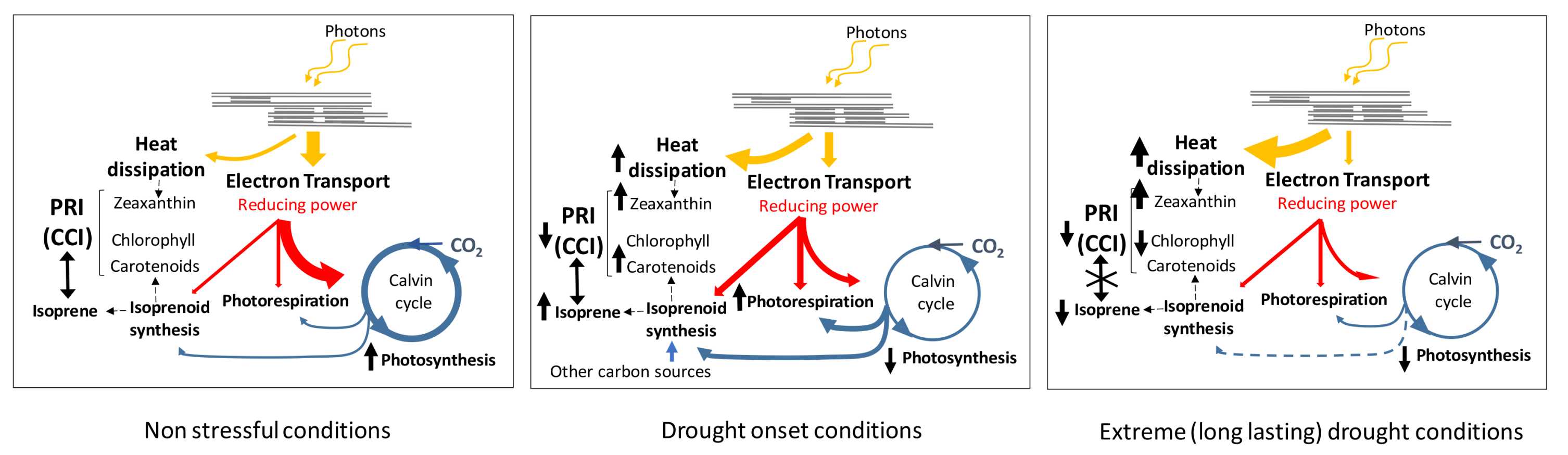
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Penuelas, J.; Llusia, J. BVOCs: Plant defense against climate warming? Trends Plant Sci. 2003, 8, 105–109. [Google Scholar] [CrossRef]
- Chameides, W.L.; Lindsay, R.W.; Richardson, J.; Kiang, C.S. The Role of Biogenic Hydrocarbons in Urban Photochemical Smog—Atlanta as a Case-Study. Science 1988, 241, 1473–1475. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, J.D.; Lerdau, M.; Atkinson, R.; Baldocchi, D.; Bottenheim, J.W.; Ciccioli, P.; Lamb, B.; Geron, C.; Gu, L.; Guenther, A.; et al. Biogenic Hydrocarbons in the Atmospheric Boundary Layer: A Review. Bull. Am. Meteorol. Soc. 2000, 81, 1537–1575. [Google Scholar] [CrossRef]
- Andreae, M.O. Atmospheric Aerosols: Biogeochemical Sources and Role in Atmospheric Chemistry. Science 1997, 276, 1052–1058. [Google Scholar] [CrossRef]
- Penuelas, J.; Staudt, M. BVOCs and global change. Trends Plant Sci. 2010, 15, 133–144. [Google Scholar] [CrossRef] [PubMed]
- Westberg, H.; Lamb, B.; Hafer, R.; Hills, A.; Shepson, P.; Vogel, C. Measurement of isoprene fluxes at the PROPHET site. J. Geophys. Res. 2001, 106, 24347–24358. [Google Scholar] [CrossRef]
- Spirig, C.; Neftel, A.; Ammann, C.; Dommen, J.; Grabmer, W.; Thielmann, A.; Schaub, A.; Beauchamp, J.; Wisthaler, A.; Hansel, A. Eddy covariance flux measurements of biogenic VOCs during ECHO 2003 using proton transfer reaction mass spectrometry. Atmos. Chem. Phys. 2005, 5, 465–481. [Google Scholar] [CrossRef]
- Gu, D.; Guenther, A.B.; Shilling, J.E.; Yu, H.; Huang, M.; Zhao, C.; Yang, Q.; Martin, S.T.; Artaxo, P.; Kim, S.; et al. Airborne observations reveal elevational gradient in tropical forest isoprene emissions. Nat. Commun. 2017, 8, 15541. [Google Scholar] [CrossRef] [PubMed]
- Guenther, A.B.; Jiang, X.; Heald, C.L.; Sakulyanontvittaya, T.; Duhl, T.; Emmons, L.K.; Wang, X. The Model of Emissions of Gases and Aerosols from Nature version 2.1 (MEGAN2.1): An extended and updated framework for modeling biogenic emissions. Geosci. Model Dev. 2012, 5, 1471–1492. [Google Scholar] [CrossRef]
- Guenther, A.B.; Zimmerman, P.R.; Harley, P.C.; Monson, R.K.; Fall, R. Isoprene and Monoterpene Emission Rate Variability—Model Evaluations and Sensitivity Analyses. J. Geophys. Res. 1993, 98, 12609–12617. [Google Scholar] [CrossRef]
- Niinemets, U.; Arneth, A.; Kuhn, U.; Monson, R.K.; Penuelas, J.; Staudt, M. The emission factor of volatile isoprenoids: Stress, acclimation, and developmental responses. Biogeosciences 2010, 7, 2203–2223. [Google Scholar] [CrossRef]
- Niinemets, U.; Monson, R.K.; Arneth, A.; Ciccioli, P.; Kesselmeier, J.; Kuhn, U.; Noe, S.M.; Penuelas, J.; Staudt, M. The leaf-level emission factor of volatile isoprenoids: Caveats, model algorithms, response shapes and scaling. Biogeosciences 2010, 7, 1809–1832. [Google Scholar] [CrossRef]
- Potosnak, M.J.; LeStourgeon, L.; Pallardy, S.G.; Hosman, K.P.; Gu, L.; Karl, T.; Geron, C.; Guenther, A.B. Observed and modeled ecosystem isoprene fluxes from an oak-dominated temperate forest and the influence of drought stress. Atmos. Environ. 2014, 84, 314–322. [Google Scholar] [CrossRef]
- Seco, R.; Karl, T.; Guenther, A.; Hosman, K.P.; Pallardy, S.G.; Gu, L.; Geron, C.; Harley, P.; Kim, S. Ecosystem-scale volatile organic compound fluxes during an extreme drought in a broadleaf temperate forest of the Missouri Ozarks (central USA). Glob. Chang. Biol. 2015, 21, 3657–3674. [Google Scholar] [CrossRef] [PubMed]
- IPCC. Climate Change 2014: Impacts, Adaptation, and Vulnerability. Part A: Global and Sectoral Aspects. Contribution of Working Group II to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change, Field, C.B., Barros, V.R., Dokken, D.J., Mach, K.J., Mastrandrea, M.D., Bilir, T.E., Chatterjee, M., Ebi, K.L., Estrada, Y.O., Genova, R.C., et al., Eds.; Cambridge University Press: Cambridge, UK, 2014.
- Monson, R.K.; Grote, R.; Niinemets, U.; Schnitzler, J.-P. Modeling the isoprene emission rate from leaves. New Phytol. 2012, 195, 541–559. [Google Scholar] [CrossRef] [PubMed]
- Morfopoulos, C.; Prentice, I.C.; Keenan, T.F.; Friedlingstein, P.; Medlyn, B.E.; Penuelas, J.; Possell, M. A unifying conceptual model for the environmental responses of isoprene emissions from plants. Ann. Bot. 2013, 112, 1223–1238. [Google Scholar] [CrossRef] [PubMed]
- Niinemets, U.; Tenhunen, J.D.; Harley, P.C.; Steinbrecher, R. A model of isoprene emission based on energetic requirements for isoprene synthesis and leaf photosynthetic properties for Liquidambar and Quercus. Plant Cell Environ. 1999, 22, 1319–1335. [Google Scholar] [CrossRef]
- Grote, R.; Morfopoulos, C.; Niinemets, U.; Sun, Z.; Keenan, T.F.; Pacifico, F.; Butler, T. A fully integrated isoprenoid emissions model coupling emissions to photosynthetic characteristics. Plant Cell Environ. 2014, 37, 1965–1980. [Google Scholar] [CrossRef] [PubMed]
- Palmer, P.I.; Jacob, D.J.; Fiore, A.M.; Martin, R.V.; Chance, K.; Kurosu, T.P. Mapping isoprene emissions over North America using formaldehyde column observations from space. J. Geophys. Res. 2003, 108. [Google Scholar] [CrossRef]
- Barkley, M.P.; Palmer, P.I.; Kuhn, U.; Kesselmeier, J.; Chance, K.; Kurosu, T.P.; Martin, R.V.; Helmig, D.; Guenther, A. Net ecosystem fluxes of isoprene over tropical South America inferred from Global Ozone Monitoring Experiment (GOME) observations of HCHO columns. J. Geophys. Res. 2008, 113. [Google Scholar] [CrossRef]
- Foster, P.N.; Prentice, I.C.; Morfopoulos, C.; Siddall, M.; van Weele, M. Isoprene emissions track the seasonal cycle of canopy temperature, not primary production: Evidence from remote sensing. Biogeosciences 2014, 11, 3437–3451. [Google Scholar] [CrossRef]
- Valin, L.C.; Fiore, A.M.; Chance, K.; Abad, G.G. The role of OH production in interpreting the variability of CH2O columns in the southeast U.S. J. Geophys. Res. Atmos. 2016, 478–493. [Google Scholar] [CrossRef]
- Penuelas, J.; Marino, G.; Llusia, J.; Morfopoulos, C.; Farre-Armengol, G.; Filella, I. Photochemical reflectance index as an indirect estimator of foliar isoprenoid emissions at the ecosystem level. Nat. Commun. 2013, 4. [Google Scholar] [CrossRef] [PubMed]
- Morfopoulos, C.; Sperlich, D.; Penuelas, J.; Filella, I.; Llusia, J.; Medlyn, B.E.; Niinemets, U.; Possell, M.; Sun, Z.; Prentice, I.C. A model of plant isoprene emission based on available reducing power captures responses to atmospheric CO2. New Phytol. 2014, 203, 125–139. [Google Scholar] [CrossRef] [PubMed]
- Penuelas, J.; Llusia, J. Plant VOC emissions: Making use of the unavoidable. Trends Ecol. Evol. 2004, 19, 402–404. [Google Scholar] [CrossRef] [PubMed]
- Owen, S.M.; Penuelas, J. Opportunistic emissions of volatile isoprenoids. Trends Plant Sci. 2005, 10, 420–426. [Google Scholar] [CrossRef] [PubMed]
- Penuelas, J.; Garbulsky, M.F.; Filella, I. Photochemical reflectance index (PRI) and remote sensing of plant CO2 uptake. New Phytol. 2011, 191, 596–599. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Filella, I.; Garbulsky, M.F.; Peñuelas, J. Affecting Factors and Recent Improvements of the Photochemical Reflectance Index (PRI) for Remotely Sensing Foliar, Canopy and Ecosystemic Radiation-Use Efficiencies. Remote Sens. 2016, 8, 677. [Google Scholar] [CrossRef]
- Penuelas, J.; Filella, I.; Gamon, J.A. Assessment of Photosynthetic Radiation-Use Efficiency with Spectral Reflectance. New Phytol. 1995, 131, 291–296. [Google Scholar] [CrossRef]
- Gamon, J.A.; Penuelas, J.; Field, C.B. A Narrow-Waveband Spectral Index That Tracks Diurnal Changes in Photosynthetic Efficiency. Remote Sens. Environ. 1992, 41, 35–44. [Google Scholar] [CrossRef]
- Sims, D.A.; Gamon, J.A. Relationships between leaf pigment content and spectral reflectance across a wide range of species, leaf structures and developmental stages. Remote Sens. Environ. 2002, 81, 337–354. [Google Scholar] [CrossRef]
- Stylinski, C.D.; Gamon, J.A.; Oechel, W.C. Seasonal patterns of reflectance indices, carotenoid pigments and photosynthesis of evergreen chaparral species. Oecologia 2002, 131, 366–374. [Google Scholar] [CrossRef] [PubMed]
- Filella, I.; Porcar-Castell, A.; Munne-Bosch, S.; Back, J.; Garbulsky, M.F.; Penuelas, J. PRI assessment of long-term changes in carotenoids/chlorophyll ratio and short-term changes in de-epoxidation state of the xanthophyll cycle. Int. J. Remote Sens. 2009, 30, 4443–4455. [Google Scholar] [CrossRef]
- Porcar-Castell, A.; Ignacio Garcia-Plazaola, J.; Nichol, C.J.; Kolari, P.; Olascoaga, B.; Kuusinen, N.; Fernandez-Marin, B.; Pulkkinen, M.; Juurola, E.; Nikinmaa, E. Physiology of the seasonal relationship between the photochemical reflectance index and photosynthetic light use efficiency. Oecologia 2012, 170, 313–323. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.Y.S.; Gamon, J.A. Three causes of variation in the photochemical reflectance index (PRI) in evergreen conifers. New Phytol. 2015, 206, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Gamon, J.A.; Huemmrich, K.F.; Wong, C.Y.S.; Ensminger, I.; Garrity, S.; Hollinger, D.Y.; Noormets, A.; Peñuelas, J. A remotely sensed pigment index reveals photosynthetic phenology in evergreen conifers. Proc. Natl. Acad. Sci. USA 2016, 113, 13087–13092. [Google Scholar] [CrossRef] [PubMed]
- Harris, A.; Owen, S.M.; Sleep, D.; Pereira, M.D.G.D.S. Constitutive changes in pigment concentrations: Implications for estimating isoprene emissions using the photochemical reflectance index. Physiol. Plant. 2016, 156, 190–200. [Google Scholar] [CrossRef] [PubMed]
- Owen, S.M.; Penuelas, J. Volatile isoprenoid emission potentials are correlated with essential isoprenoid concentrations in five plant species. Acta Physiol. Plant. 2013, 35, 3109–3125. [Google Scholar] [CrossRef]
- Harley, P.C.; Litvak, M.E.; Sharkey, T.D.; Monson, R.K. Isoprene Emission from Velvet Bean Leaves. Plant Physiol. 1994, 105, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Geron, C.; Guenther, A.; Sharkey, T.; Arnts, R.R. Temporal variability in basal isoprene emission factor. Tree Physiol. 2000, 20, 799–805. [Google Scholar] [CrossRef] [PubMed]
- Pressley, S.; Lamb, B.; Westberg, H.; Flaherty, J.; Chen, J.; Vogel, C. Long-term isoprene flux measurements above a northern hardwood forest. J. Geophys. Res. 2005, 110. [Google Scholar] [CrossRef]
- Gu, L.; Meyers, T.; Pallardy, S.G.; Hanson, P.J.; Yang, B.; Heuer, M.; Hosman, K.P.; Riggs, J.S.; Sluss, D.; Wullschleger, S.D. Direct and indirect effects of atmospheric conditions and soil moisture on surface energy partitioning revealed by a prolonged drought at a temperate forest site. J. Geophys. Res. 2006, 111. [Google Scholar] [CrossRef]
- Critchfield, H.J. General Climatology; Prentice-Hall: Englewood Cliffs, NJ, USA, 1966. [Google Scholar]
- Guenther, A.B.; Hills, A.J. Eddy covariance measurement of isoprene fluxes. J. Geophys. Res. 1998, 103, 13145–13152. [Google Scholar] [CrossRef]
- Karl, T.G.; Spirig, C.; Rinne, J.; Stroud, C.; Prevost, P.; Greenberg, J.; Fall, R.; Guenther, A. Virtual disjunct eddy covariance measurements of organic compound fluxes from a subalpine forest using proton transfer reaction mass spectrometry. Atmos. Chem. Phys. 2002, 2, 279–291. [Google Scholar] [CrossRef]
- Reichstein, M.; Falge, E.; Baldocchi, D.; Papale, D.; Aubinet, M.; Berbigier, P.; Bernhofer, C.; Buchmann, N.; Gilmanov, T.; Granier, A.; et al. On the separation of net ecosystem exchange into assimilation and ecosystem respiration: Review and improved algorithm. Glob. Chang. Biol. 2005, 11, 1424–1439. [Google Scholar] [CrossRef]
- Monteith, J.L. Using Tube Solarimeters to Measure Radiation Intercepted by Crop Canopies and to Analyse Stand Growth; Application Note: TSL-AN-4-1; Delta-T Devices: Cambridge, UK, 1993; pp. 1–11. [Google Scholar]
- Rahman, A.F.; Cordova, V.D.; Gamon, J.A.; Schmid, H.P.; Sims, D.A. Potential of MODIS ocean bands for estimating CO2 flux from terrestrial vegetation: A novel approach. Geophys. Res. Lett. 2004, 31. [Google Scholar] [CrossRef]
- Goerner, A.; Reichstein, M.; Rambal, S. Tracking seasonal drought effects on ecosystem light use efficiency with satellite-based PRI in a Mediterranean forest. Remote Sens. Environ. 2009, 113, 1101–1111. [Google Scholar] [CrossRef]
- Mac Nally, R.; Walsh, C.J. Hierarchical partitioning public-domain software. Biodivers. Conserv. 2004, 13, 659–660. [Google Scholar] [CrossRef]
- Breheny, P.; Burchett, W. Visualization of regression models using visreg. R Packag. 2013, 1–15. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing. R Found. Stat. Comput. 2015, 1, 1651. [Google Scholar]
- Garbulsky, M.F.; Penuelas, J.; Gamon, J.; Inoue, Y.; Filella, I. The photochemical reflectance index (PRI) and the remote sensing of leaf, canopy and ecosystem radiation use efficiencies: A review and meta-analysis. Remote Sens. Environ. 2011, 115, 281–297. [Google Scholar] [CrossRef]
- Niinemets, U. Mild versus severe stress and BVOCs: Thresholds, priming and consequences. Trends Plant Sci. 2010, 15, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Tani, A.; Tozaki, D.; Okumura, M.; Nozoe, S.; Hirano, T. Effect of drought stress on isoprene emission from two major Quercus species native to East Asia. Atmos. Environ. 2011, 45, 6261–6266. [Google Scholar] [CrossRef]
- Genard-Zielinski, A.-C.; Ormeno, E.; Boissard, C.; Fernandez, C. Isoprene Emissions from Downy Oak under Water Limitation during an Entire Growing Season: What Cost for Growth? PLoS ONE 2014, 9. [Google Scholar] [CrossRef] [PubMed]
- Dani, K.G.S.; Jamie, I.M.; Prentice, I.C.; Atwell, B.J. Increased Ratio of Electron Transport to Net Assimilation Rate Supports Elevated Isoprenoid Emission Rate in Eucalypts under Drought. Plant Physiol. 2014, 166, 1059–1072. [Google Scholar] [CrossRef] [PubMed]
- Dani, K.G.S.; Jamie, I.M.; Prentice, I.C.; Atwell, B.J. Species-specific photorespiratory rate, drought tolerance and isoprene emission rate in plants. Plant Signal. Behav. 2015, 10, e990830. [Google Scholar] [CrossRef] [PubMed]
- Fortunati, A.; Barta, C.; Brilli, F.; Centritto, M.; Zimmer, I.; Schnitzler, J.-P.; Loreto, F. Isoprene emission is not temperature-dependent during and after severe drought-stress: A physiological and biochemical analysis. Plant J. 2008, 55, 687–697. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Sharkey, T.D. Metabolic profiling of the methylerythritol phosphate pathway reveals the source of post-illumination isoprene burst from leaves. Plant Cell Environ. 2013, 36, 429–437. [Google Scholar] [CrossRef] [PubMed]
- Loreto, F.; Schnitzler, J.-P. Abiotic stresses and induced BVOCs. Trends Plant Sci. 2010, 15, 154–166. [Google Scholar] [CrossRef] [PubMed]
- Poorter, H.; Buehler, J.; van Dusschoten, D.; Climent, J.; Postma, J.A. Pot size matters: A meta-analysis of the effects of rooting volume on plant growth. Funct. Plant Biol. 2012, 39, 839–850. [Google Scholar] [CrossRef]
- Bertin, N.; Staudt, M. Effect of water stress on monoterpene emissions from young potted holm oak (Quercus ilex L.) trees. Oecologia 1996, 107, 456–462. [Google Scholar] [CrossRef] [PubMed]
- Brilli, F.; Barta, C.; Fortunati, A.; Lerdau, M.; Loreto, F.; Centritto, M. Response of isoprene emission and carbon metabolism to drought in white poplar (Populus alba) saplings. New Phytol. 2007, 175, 244–254. [Google Scholar] [CrossRef] [PubMed]
- Funk, J.L.; Jones, C.G.; Gray, D.W.; Throop, H.L.; Hyatt, L.A.; Lerdau, M.T. Variation in isoprene emission from Quercus rubra: Sources, causes, and consequences for estimating fluxes. J. Geophys. Res. 2005, 110. [Google Scholar] [CrossRef]
- Funk, J.L.; Mak, J.E.; Lerdau, M.T. Stress-induced changes in carbon sources for isoprene production in Populus deltoides. Plant Cell Environ. 2004, 27, 747–755. [Google Scholar] [CrossRef]
- Pegoraro, E.; Rey, A.; Greenberg, J.; Harley, P.; Grace, J.; Malhi, Y.; Guenther, A. Effect of drought on isoprene emission rates from leaves of Quercus virginiana Mill. Atmos. Environ. 2004, 38, 6149–6156. [Google Scholar] [CrossRef]
- Wu, C.; Pullinen, I.; Andres, S.; Carriero, G.; Fares, S.; Goldbach, H.; Hacker, L.; Kasal, T.; Kiendler-Scharr, A.; Kleist, E.; et al. Impacts of soil moisture on de novo monoterpene emissions from European beech, Holm oak, Scots pine, and Norway spruce. Biogeosciences 2015, 12, 177–191. [Google Scholar] [CrossRef]
- Sharkey, T.D.; Wiberley, A.E.; Donohue, A.R. Isoprene emission from plants: Why and how. Ann. Bot. 2008, 101, 5–18. [Google Scholar] [CrossRef] [PubMed]
- Velikova, V.; Sharkey, T.D.; Loreto, F. Stabilization of thylakoid membranes in isoprene-emitting plants reduces formation of reactive oxygen species. Plant Signal. Behav. 2012, 7, 139–141. [Google Scholar] [CrossRef] [PubMed]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Filella, I.; Zhang, C.; Seco, R.; Potosnak, M.; Guenther, A.; Karl, T.; Gamon, J.; Pallardy, S.; Gu, L.; Kim, S.; et al. A MODIS Photochemical Reflectance Index (PRI) as an Estimator of Isoprene Emissions in a Temperate Deciduous Forest. Remote Sens. 2018, 10, 557. https://doi.org/10.3390/rs10040557
Filella I, Zhang C, Seco R, Potosnak M, Guenther A, Karl T, Gamon J, Pallardy S, Gu L, Kim S, et al. A MODIS Photochemical Reflectance Index (PRI) as an Estimator of Isoprene Emissions in a Temperate Deciduous Forest. Remote Sensing. 2018; 10(4):557. https://doi.org/10.3390/rs10040557
Chicago/Turabian StyleFilella, Iolanda, Chao Zhang, Roger Seco, Mark Potosnak, Alex Guenther, Thomas Karl, John Gamon, Stephen Pallardy, Lianhong Gu, Saewung Kim, and et al. 2018. "A MODIS Photochemical Reflectance Index (PRI) as an Estimator of Isoprene Emissions in a Temperate Deciduous Forest" Remote Sensing 10, no. 4: 557. https://doi.org/10.3390/rs10040557
APA StyleFilella, I., Zhang, C., Seco, R., Potosnak, M., Guenther, A., Karl, T., Gamon, J., Pallardy, S., Gu, L., Kim, S., Balzarolo, M., Fernandez-Martinez, M., & Penuelas, J. (2018). A MODIS Photochemical Reflectance Index (PRI) as an Estimator of Isoprene Emissions in a Temperate Deciduous Forest. Remote Sensing, 10(4), 557. https://doi.org/10.3390/rs10040557