Invasive Eichhornia crassipes Affects the Capacity of Submerged Macrophytes to Utilize Nutrients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Species and Sampling
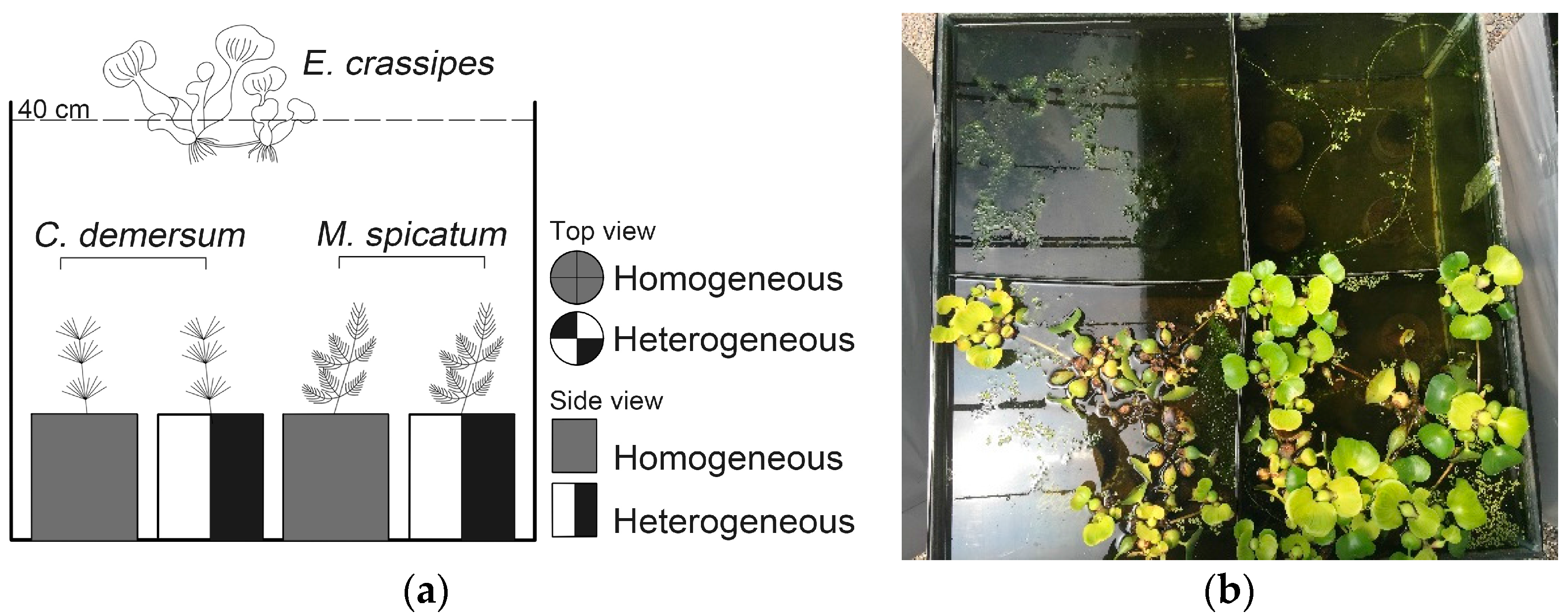
2.2. Experimental Design
2.3. Measurements and Data Analysis
3. Results
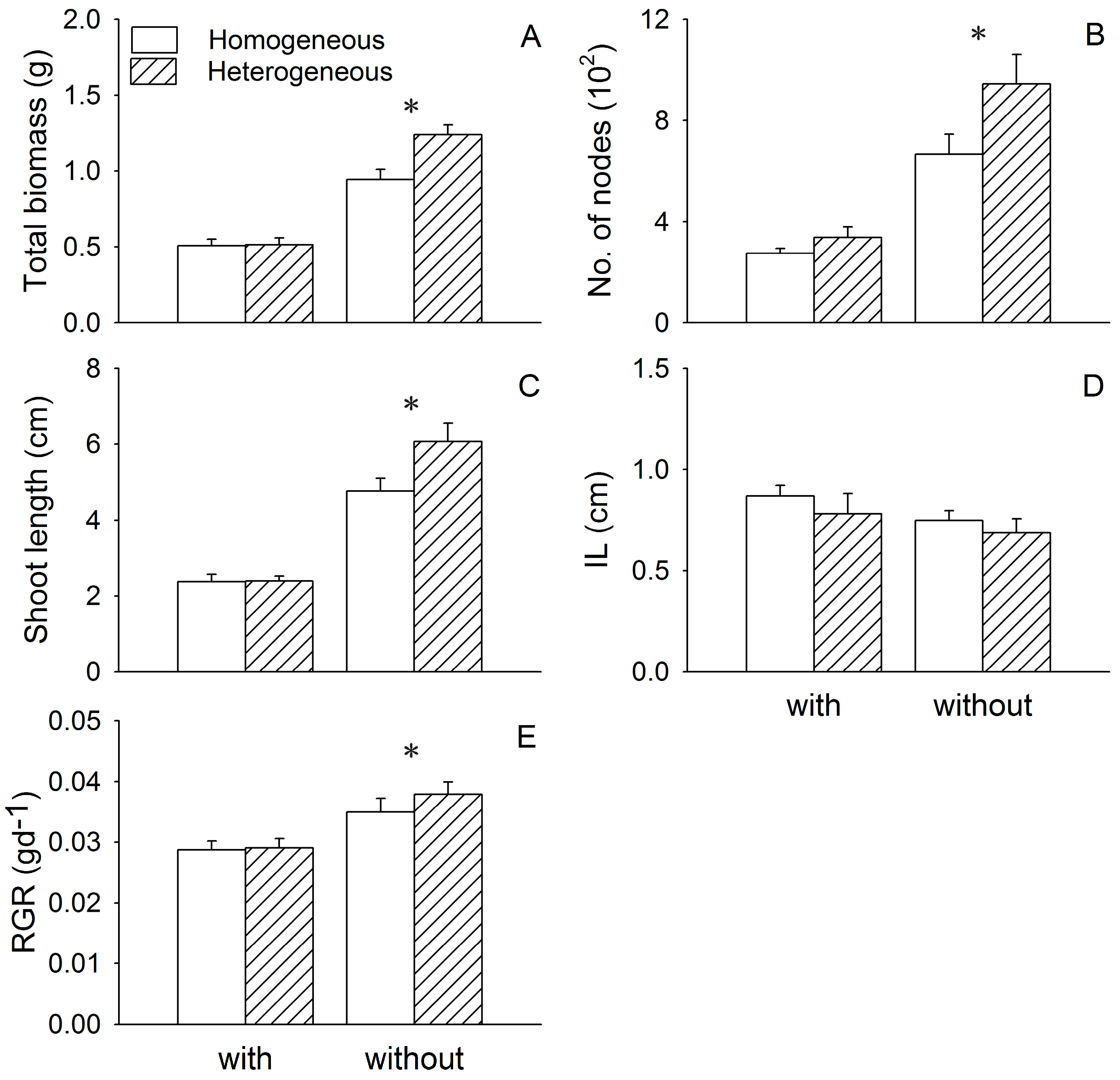
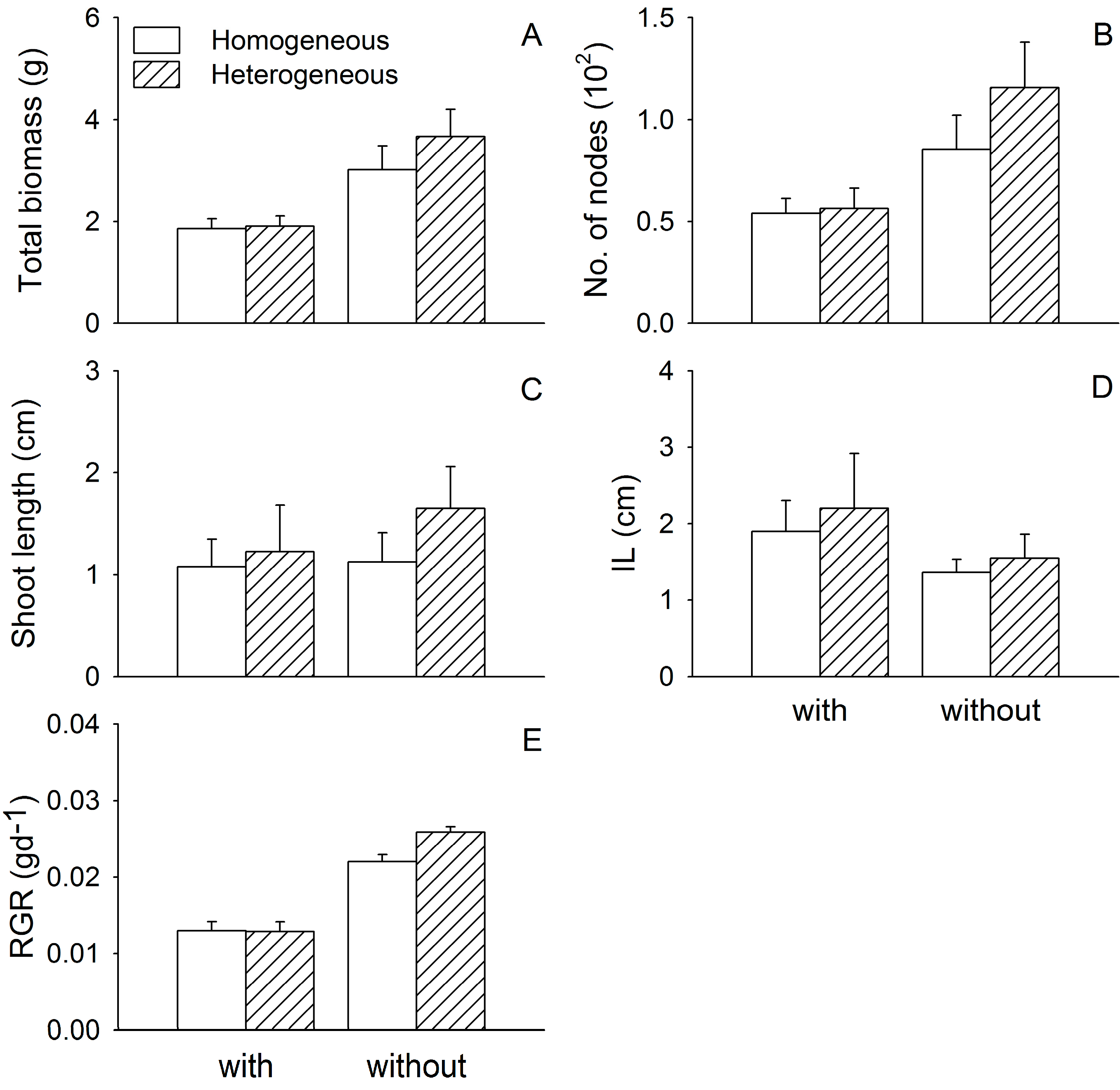
3.1. Growth of Two Submerged Species
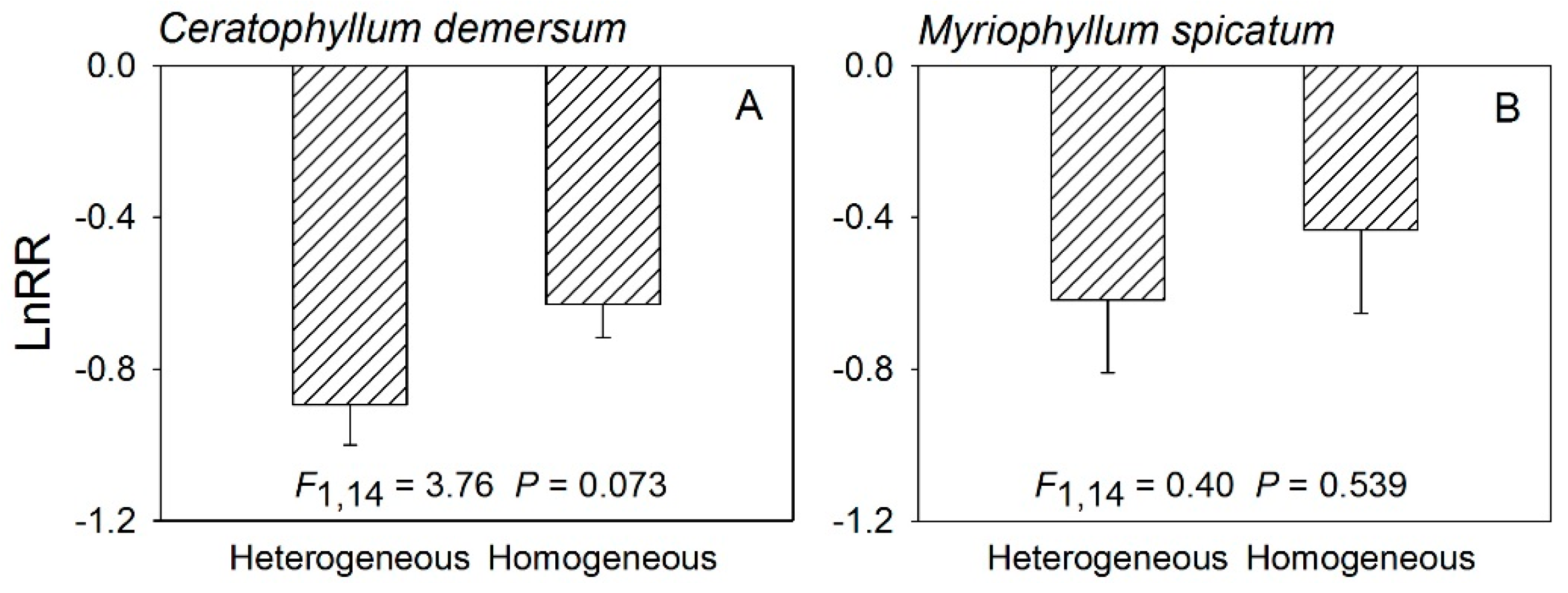
3.2. Results of LnRR
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Villamagna, A.M.; Murphy, B.R. Ecological and socio-economic impacts of invasive water hyacinth (Eichhornia crassipes): A review. Freshw. Boil. 2010, 55, 282–298. [Google Scholar] [CrossRef]
- Schultz, R.; Dibble, E. Effects of invasive macrophytes on freshwater fish and macroinvertebrate communities: The role of invasive plant traits. Hydrobiologia 2012, 684, 1–14. [Google Scholar] [CrossRef]
- Hamelin, C.; Gagnon, D.; Truax, B. Exotic Invasive Shrub Glossy Buckthorn Reduces Restoration Potential for Native Forest Herbs. Sustainability 2017, 9, 249. [Google Scholar] [CrossRef]
- Rodríguez, M.; Brisson, J.; Rueda, G.; Rodríguez, M.S. Water quality improvement of a reservoir invaded by an exotic macrophyte. Invasions Plant Sci. Mana 2012, 5, 290–299. [Google Scholar] [CrossRef]
- Ceschin, S.; Bella, V.D.; Piccari, F.; Abati, S. Colonization dynamics of the alien macrophyte Lemna minuta Kunth: A case study from a semi-natural pond in Appia Antica Regional Park (Rome, Italy). Fund. Appl. Limnol. 2016, 188, 93–101. [Google Scholar] [CrossRef]
- Adams, C.S.; Boar, R.R.; Hubble, D.S.; Gikungu, M.; Harper, D.M.; Hickley, P.; Tarras-Wahlberg, N. The dynamics and ecology of exotic tropical species in floating plant mats: Lake Naivasha, Kenya. Hydrobiologia 2002, 488, 115–122. [Google Scholar] [CrossRef]
- Shanab, S.M.M.; Shalaby, E.A.; Lightfoot, D.A.; Ei-Shemy, H.A. Allelopathic effects of water hyacinth [Eichhornia crassipes]. PLoS ONE 2010, 5, e13200. [Google Scholar] [CrossRef] [PubMed]
- O’Farrell, I.; de Tezanos Pinto, P.; Rodriguez, P.L.; Chaparro, G.; Pizarro, H.N. Experimental evidence of the dynamic effect of free-floating plants on phytoplankton ecology. Freshw. Biol. 2009, 54, 363–375. [Google Scholar] [CrossRef]
- Li, H.L.; Xu, Y.S.; Wang, Y.Y.; Yu, N.Q.; Zhang, M.X.; Lei, G.C.; Yu, F.H. Does clonal fragmentation of the floating plant Eichhornia crassipes affect the growth of submerged macrophyte communities? Folia Geobot. 2015, 50, 283–291. [Google Scholar] [CrossRef]
- De Tezanos Pinto, P.; O’Farrell, I. Regime shifts between free-floating plants and phytoplankton: A review. Hydrobiologia 2014, 740, 13–24. [Google Scholar] [CrossRef]
- Ceschin, S.; Abati, S.; Leacche, I.; Iamonico, D.; Iberite, M.; Zuccarello, V. Does the alien Lemna minuta show an invasive behavior outside its original range? Evidence of antagonism with the native L. minor in central Italy. Int. Rev. Hydrobiol. 2016, 101, 173–181. [Google Scholar] [CrossRef]
- Carr, G.M. Macrophyte growth and sediment phosphorus and nitrogen in a Canadian prairie river. Freshw. Biol. 1998, 39, 525–536. [Google Scholar] [CrossRef]
- Lu, J.; Wang, Z.; Xing, W.; Liu, G.H. Effects of substrate and shading on the growth of two submerged macrophytes. Hydrobiologia 2013, 700, 157–167. [Google Scholar] [CrossRef]
- Wang, S.; Jin, X.; Jiao, L.; Wu, F.C. Response in root morphology and nutrient contents of Myriophyllum spicatum to sediment type. Ecol. Eng. 2009, 35, 1264–1270. [Google Scholar] [CrossRef]
- Liu, L.; Bu, X.Q.; Wan, J.Y.; Dong, B.C.; Luo, F.L.; Li, H.L.; Yu, F.H. Impacts of sediment type on the performance and composition of submerged macrophyte communities. Aquat. Ecol. 2017, 51, 167–176. [Google Scholar] [CrossRef]
- Jarvis, J.C.; Moore, K.A. Effects of seed source, sediment type, and burial depth on mixed-annual and perennial Zostera marina L. seed germination and seedling establishment. Estuar. Coast. 2015, 38, 964–978. [Google Scholar] [CrossRef]
- Ramsey, M.H.; Argyraki, A. Estimation of measurement uncertainty from field sampling: Implications for the classification of contaminated land. Sci. Total Environ. 1997, 198, 243–257. [Google Scholar] [CrossRef]
- Roiloa, S.R.; Sánchez-Rodríguez, P.; Retuerto, R. Heterogeneous distribution of soil nutrients increase intra-specific competition in the clonal plant Glechoma hederacea. Plant Ecol. 2014, 215, 863–873. [Google Scholar] [CrossRef]
- De Kroon, H.; Visser, E.J.W.; Huber, H.; Mommer, L.; Hutchings, M.J. A modular concept of plant foraging behaviour: The interplay between local responses and systemic control. Plant Cell Environ. 2009, 32, 704–712. [Google Scholar] [CrossRef] [PubMed]
- García-Palacios, P.; Maestre, F.T.; Gallardo, A. Soil nutrient heterogeneity modulates ecosystem responses to changes in the identity and richness of plant functional groups. J. Ecol. 2011, 99, 551–562. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Dong, B.C.; Alpert, P.; Li, H.L.; Zhang, M.X.; Lei, G.C.; Yu, F.H. Effects of soil nutrient heterogeneity on intraspecific competition in the invasive, clonal plant Alternanthera philoxeroides. Ann. Bot. 2012, 109, 813–818. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.J.; Shi, X.P.; Meng, X.F.; Wu, X.J.; Luo, F.L.; Yu, F.H. Effects of spatial patch arrangement and scale of covarying resources on growth and intraspecific competition of a clonal plant. Front. Plant Sci. 2016, 7. [Google Scholar] [CrossRef] [PubMed]
- Day, K.J.; John, E.A.; Hutchings, M.J. The effects of spatially heterogeneous nutrient supply on yield, intensity of competition and root placement patterns in Briza media and Festuca ovina. Funct. Ecol. 2003, 17, 454–463. [Google Scholar] [CrossRef]
- Center, T.D.; Dray, F.A.; Jubinsky, G.P.; Grodowitz, M.J. Biological control of water hyacinth under conditions of maintenance management: Can herbicides and insects be integrated? Environ. Manag. 1999, 23, 241–256. [Google Scholar] [CrossRef]
- Malik, A. Environmental challenge vis a vis opportunity: The case of water hyacinth. Environ. Int. 2007, 33, 122–138. [Google Scholar] [CrossRef] [PubMed]
- Cronk, J.K.; Fennessy, M.S. Wetland Plants: Biology and Ecology; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Dibble, K.L.; Pooler, P.S.; Meyerson, L.A. Impacts of plant invasions can be reversed through restoration: A regional meta-analysis of faunal communities. Biol. Invasions 2013, 15, 1725–1737. [Google Scholar] [CrossRef]
- Zhou, J.; Li, H.L.; Alpert, P.; Zhang, M.X.; Yu, F.H. Fragmentation of the invasive, clonal plant Alternanthera philoxeroides decreases its growth but not its competitive effect. Flora 2017, 228, 17–23. [Google Scholar] [CrossRef]
- Cahill, J.F. Fertilization effects on interactions between above- and belowground competition in an old field. Ecology 1999, 80, 466–480. [Google Scholar] [CrossRef]
- Hussner, A.; Meyer, C.; Busch, J. The influence of water level and nutrient availability on growth and root system development of Myriophyllum aquaticum. Weed Res. 2009, 49, 73–80. [Google Scholar] [CrossRef]
- Zhang, Q.; Dong, B.C.; Li, H.L.; Liu, R.H.; Luo, F.L.; Zhang, M.X.; Lei, G.C.; Yu, F.H. Does light heterogeneity affect structure and biomass of submerged macrophyte communities? Bot. Stud. 2012, 53, 377–385. [Google Scholar]
- De Tezanos Pinto, P.; Allende, L.; O’farrell, I. Influence of free-floating plants on the structure of a natural phytoplankton assemblage: An experimental approach. J. Plankton Res. 2007, 29, 47–56. [Google Scholar] [CrossRef]
- Xie, D.; Yu, D.; You, W.H.; Wang, L.G. Morphological and physiological responses to sediment nutrients in the submerged macrophyte Myriophyllum spicatum. Wetlands 2013, 33, 1095–1102. [Google Scholar] [CrossRef]
- You, W.; Yu, D.; Liu, C.; Xie, D.; Xiong, W. Clonal integration facilitates invasiveness of the alien aquatic plant Myriophyllum aquaticum L. under heterogeneous water availability. Hydrobiologia 2013, 718, 27–39. [Google Scholar] [CrossRef]
- Wijesinghe, D.K.; Hutchings, M.J. The effects of environmental heterogeneity on the performance of Glechoma hederacea: The interactions between patch contrast and patch scale. J. Ecol. 1999, 87, 860–872. [Google Scholar] [CrossRef]
- Hutchings, M.J.; John, E.A. The effects of environmental heterogeneity on root growth and root/shoot partitioning. Ann. Bot. 2004, 94, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Dong, B.C.; Wang, J.Z.; Liu, R.H.; Zhang, M.X.; Luo, F.L.; Yu, F.H. Soil heterogeneity affects ramet placement of Hydrocotyle vulgaris. J. Plant Ecol. 2015, 8, 91–100. [Google Scholar] [CrossRef]
- Scheffer, M.; Szabó, S.; Gragnani, A.; van Nes, E.H.; Rinaldi, S.; Kautsky, N.; Norberg, J.; Roijackers, R.M.M.; Franken, R.J.M. Floating plant dominance as a stable state. Proc. Natl. Acad. Sci. USA 2003, 100, 4040–4045. [Google Scholar] [CrossRef] [PubMed]
- Fetene, M. Intra-and inter-specific competition between seedlings of Acacia etbaica and a perennial grass (Hyparrenia hirta). J. Arid Environ. 2003, 55, 441–451. [Google Scholar] [CrossRef]
- Yuan, Y.; Wang, K.; Li, D.; Pan, Y.; Lv, Y.; Zhao, M.; Gao, J. Interspecific interactions between Phragmites australis and Spartina alterniflora along a tidal gradient in the Dongtan Wetland, Eastern China. PLoS ONE 2013, 8, e53843. [Google Scholar] [CrossRef] [PubMed]
- Li, H.L.; Wang, Y.Y.; An, S.Q.; Zhi, Y.B.; Lei, G.C.; Zhang, M.X. Sediment type affects competition between a native and an exotic species in coastal China. Sci. Rep. 2014, 4, 6748. [Google Scholar] [CrossRef] [PubMed]
- Van Gerven, L.P.A.; de Klein, J.J.M.; Gerla, D.J.; Kooi, B.W.; Kuiper, J.J.; Mooij, W.M. Competition for light and nutrients in layered communities of aquatic plants. Am. Nat. 2015, 186, 72–83. [Google Scholar] [CrossRef] [PubMed]
- Forchhammer, N.C. Production potential of aquatic plants in systems mixing floating and submerged macrophytes. Freshw. Biol. 1999, 41, 183–191. [Google Scholar] [CrossRef]
- Netten, J.J.C.; Arts, G.H.P.; Gylstra, R.; van Nes, E.H.; Scheffer, M.; Roijacker, R.M.M. Effect of temperature and nutrients on the competition between free-floating Salvinia natans and submerged Elodea nuttallii in mesocosms. Fund. Appl. Limnol. 2010, 177, 125–132. [Google Scholar] [CrossRef]
| Trait | Eichhornia crassipes (E) | Heterogeneity (H) | I × H | |||
|---|---|---|---|---|---|---|
| F1,28 | P | F1,28 | P | F1,28 | P | |
| Ceratophyllum demersum | ||||||
| Total biomass | 108.61 | <0.001 | 7.34 | 0.011 | 6.63 | 0.016 |
| No. of nodes 1 | 68.73 | <0.001 | 5.03 | 0.033 | 0.56 | 0.460 |
| Shoot length 1 | 132.23 | <0.001 | 3.36 | 0.078 | 2.27 | 0.143 |
| IL | 2.37 | 0.135 | 1.14 | 0.295 | 0.03 | 0.865 |
| RGR | 113.05 | <0.001 | 3.29 | 0.081 | 3.74 | 0.063 |
| Myriophyllum spicatum | ||||||
| Total biomass 1 | 7.89 | 0.009 | 2.31 | 0.140 | 0.01 | 0.941 |
| No. of nodes 1 | 13.65 | 0.001 | 0.13 | 0.718 | 0.10 | 0.757 |
| Shoot length | 0.41 | 0.528 | 0.85 | 0.364 | 0.27 | 0.609 |
| IL | 1.77 | 0.194 | 0.30 | 0.588 | 0.02 | 0.900 |
| RGR | 16.41 | <0.001 | 0.78 | 0.386 | 0.50 | 0.484 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, J.; Pan, X.; Xu, H.; Wang, Q.; Cui, L. Invasive Eichhornia crassipes Affects the Capacity of Submerged Macrophytes to Utilize Nutrients. Sustainability 2017, 9, 565. https://doi.org/10.3390/su9040565
Zhou J, Pan X, Xu H, Wang Q, Cui L. Invasive Eichhornia crassipes Affects the Capacity of Submerged Macrophytes to Utilize Nutrients. Sustainability. 2017; 9(4):565. https://doi.org/10.3390/su9040565
Chicago/Turabian StyleZhou, Jian, Xu Pan, Haiting Xu, Qi Wang, and Lijuan Cui. 2017. "Invasive Eichhornia crassipes Affects the Capacity of Submerged Macrophytes to Utilize Nutrients" Sustainability 9, no. 4: 565. https://doi.org/10.3390/su9040565
APA StyleZhou, J., Pan, X., Xu, H., Wang, Q., & Cui, L. (2017). Invasive Eichhornia crassipes Affects the Capacity of Submerged Macrophytes to Utilize Nutrients. Sustainability, 9(4), 565. https://doi.org/10.3390/su9040565





