Abstract
Sulfated polysaccharides from marine macroalgae have been shown to possess a variety of biological activities against fungi, bacteria, viruses, and protozoa. In this study, the in vitro activity of algal polysaccharides against Leishmania infantum (Kinetoplastida: Trypanosomatidae) was investigated. The polysaccharides were extracted from different macroalgae of the Mediterranean Sea: Chaetomorpha linum, Agardhiella subulata, Gracilaria viridis, Gracilaria bursa-pastoris, Hypnea cornuta, Sargassum muticum, and Undaria pinnatifida. Preliminary results showed a good anti-leishmanial activity of the investigated species, encouraging the focus on their use as natural resources in order to match integrated management strategies for the employment of local macroalgae.
1. Introduction
Marine algae are great sources of natural products that play an invaluable role in the drug discovery process. Many reports have been published about isolated compounds from algae with biological activity, demonstrating their ability to produce metabolites different from those found in terrestrial plants, with high complexity and unlimited diversity of pharmacological and/or biological properties [1]. Among these, sulphated polysaccharides are widely used in the food and cosmetic industries, but also acknowledged as endowed with a rather low toxicity and numerous biological activities, including antiviral, anticoagulant, anti-tumoral, antimetastatic, and anti-inflammatory effects, worthwhile for clinical uses [2,3,4]. Therefore, applications of algal products are increasingly frequent both in human and veterinary medicine.
Among the different diseases of interest for scientific research, leishmaniasis represents an important problem for public health and, at present, few works have demonstrated the anti-leishmanial activity of seaweed extracts [5,6,7,8,9]. Leishmaniasis is a disease with a worldwide distribution affecting both humans and animals. There is increasing awareness that drug treatment can be complicated by variation in the sensitivity of Leishmania species to drugs, variation in pharmacokinetics, and variation in drug-host immune response interaction [10]. Leishmaniasis has several diverse clinical manifestations: ulcerative skin lesions, destructive mucosal inflammation, and disseminated visceral infection (Kala Azar). Epidemiology, immunopathology, and outcome are similarly diverse, since infection occurs in multiple endemic regions, in both children and adults, and is caused by nearly two dozen distinct Leishmania species [11]. Approximately 0.2–0.4 million visceral leishmaniasis cases and 0.7–1.2 million cutaneous leishmaniasis cases occur each year. Cutaneous leishmaniasis is more widely distributed, with about one-third of cases occurring in each of three regions, the Americas, the Mediterranean basin, and Western Asia from the Middle East to Central Asia [12].
Leishmaniasis is associated with about 2–4 million disability-adjusted life years and around 70,000 deaths per year. Ninety percent of cutaneous leishmaniasis (CL) infections develop in Afghanistan, Pakistan, Syria, Saudi Arabia, Algeria, Iran, Brazil, and Peru; 90% of visceral leishmaniasis (VL) occurs in India, Bangladesh, Nepal, Sudan, and Brazil [11]. In view of this geography, leishmaniasis remains embedded in poverty as a neglected disease [13]. Except in Southern Europe, barebones national health services in endemic regions block access to ready diagnosis, affordable treatments, and effective disease control. With little prospect for financial return, antileishmanial drug development remains stalled [14].
In this study, sulphated polysaccharides were extracted from different species of Mediterranean macroalgae and were tested against the protozoan L. infantum (MHOM/IT/80/IPT1), the agent of leishmaniasis in the Mediterranean basin. The experiment was carried out at the National Reference Centre for Leishmaniasis of Palermo (Italy) according to national protocols.
2. Materials and Methods
2.1. Algal Materials and Phycocolloid Extractions
Samples of red, green, and brown macroalgae were collected in Venice Lagoon and Cape Peloro Lagoon in Sicily (Italy) during the spring and summer of 2013: the green alga Chaetomorpha linum (O.F. Müller) Kützing, the red algae Gracilaria bursa-pastoris (S.G. Gmelin) P.C. Silva, Gracilaria viridis Sfriso, Wolf, Sciuto, M. Morabito, Andreoli & Moro, Agardhiella subulata (C. Agardh) Kraft & M.J. Wynne, Hypnea cornuta (Kützing) J. Agardh, and the brown algae Sargassum muticum (Yendo) Fensholt and Undaria pinnatifida (Harvey) Suringar.
According to literature data [15,16,17], phycocolloid extraction protocol was performed in order to find an easy and affordable method. Algae were washed with clean water, sun dried, pulverized and macerated in absolute ethanol and successively in acetone, in order to eliminate pigments and lipids. Subsequently, they were incubated in distilled water at 70 °C for 24 h. The residue was removed via centrifugation, and the supernatant was added with one volume of 96% ethanol to obtain the crude extract through precipitation. Details for the extraction of phycocolloid types from different macroalgae are reported in Figure 1.
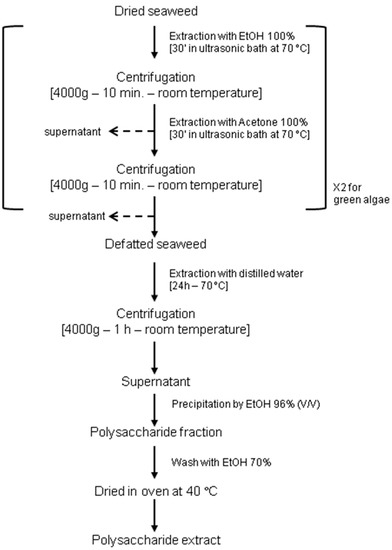
Figure 1.
Phycocolloid extraction protocol performed in this study.
2.2. Cytotoxic Essay
Before anti-leishmanial assays, potential cytotoxic action of the crude extracts was checked by the MTT viability assay on DH82, VERO, L929, MDCK, and U937 cell lines [18].
The VERO cell line (CCL-81) was obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA). The cell line was cultured in Eagle’s minimum essential medium (MEM, Gibco, Grand Island, NY, USA), supplemented with 10% fetal bovine serum (FBS, Gibco), penicillin (100 IU/mL), and streptomycin (100 mg/mL). DH82 cells (ATCC CRL-10389) were propagated in minimum essential medium (MEM) with non-essential aminoacids, 2 mM L-glutamine, and 10% FBS (MEM growth media). Cells were grown at 37 °C in 5% CO2 and passaged semi-weekly. Mouse fibroblast cells of the permanent cell line L929 (ATCC CCL 1) were routinely cultivated in MEM containing 10% fetal bovine serum, penicillin (100 U/mL), and streptomycin (100 μg/mL), at 37 °C in an air atmosphere containing 5% CO2.
Canine kidney epithelial MDCK cells were purchased from the American Type Tissue Collection (ATCC) and cultured at 37 °C in a humidified incubator with 5% CO2, and DMEM media were supplemented with 10% fetal bovine serum, 2 mM glutamine, 100 units/mL penicillin, and 100 mg/mL streptomycin (Life Technologies Inc., Carlsbad, CA, USA).
2.3. Activity of Phycocolloids in L. infantum Promastigote Cultures
In each experiment, exponentially growing cells were plated in 100 μL aliquots of growth medium into 96-well plates at 105 cells per well, respectively, and incubated for 24 h. For loading of crude extracts, the cells (in 96-well plates) were incubated with the crude extracts (at concentration of 20, 40, 80, and 160 μg/mL). After 48 h incubation, the MTT solution (5 mg/mL) was added to each well, and the formazan precipitate was dissolved in 100 μL dimethyl sulfoxide after 4 h incubation. The absorbance was measured in an ELISA reader (Thermo Molecular Devices Co., Union City, CA, USA) at 570 nm. The cell viability ratio was calculated as a percentage in reference to the control using Equation (1):
where AS is the absorbance sample, AB is the absorbance blank, and AC is the absorbance control; blank is MTT plus acidic isopropanol (0.1 N HCl in absolute isopropanol).
Cell viability ratio = [(AS − AB)/(AC − AB)] × 100,
L. infantum promastigotes (IPT1/MON1, Higher Institute of Health—Rome, Italy) were treated in phosphate buffered saline (PBS) and cultured at 25 °C and pH 7.18 in RPMI-PY medium [19], which consisted of RPMI 1640 (Sigma R0883) supplemented with equal volume of Pepton-yeast medium [20], 10% FBS, 1% glutamine, 250 μg/mL gentamicin, and 500 μg/mL of 5-fluorocytosine. Temperature, differentiation time, and acidification of the medium were used as variables for the preconditioning of the promastigote cultures of L. infatum. The influence of temperature was evaluated by incubating the promastigotes from 25 °C at 37 °C.
Flasks containing 5 mL of culture medium RPMI-PY were inoculated with 4 × 106 promastigotes/mL and treated with serial concentrations of the polysaccharide extract (20, 40, 80, and 160 µg/mL). After 48 h of treatment at 24 °C, we proceeded with the evaluation of the percentage of Leishmania viability via cell counting and a comparison with the control culture (100% viability).
To evaluate the leishmanicidal activity of phycocolloid compounds, the percentage of live and dead parasites was determined morphologically after labeling with acridine orange (100 μg/mL) and ethidium bromide (100 μg/mL). After 48 h of exposure to each compound, parasites (1 × 106) were centrifuged and the pellet was resuspended in 25 μL of the dye mixture. Subsequently, 10 μL of the mixture was examined in oil immersion with a 100× objective using a fluorescence microscope Nikon Eclipse E200. Live parasites were determined by the uptake of acridine orange (green fluorescence) and exclusion of ethidium bromide (red fluorescence). Dead parasites were determined by the uptake of ethidium bromide (red fluorescence).
The leishmanicidal effect of polysaccharide extract was further verified evaluating the morphological characteristics of L. infantum via staining via May–Grünwald–Giemsa staining, after 48 h of treatment.
2.4. Activity of Phycocolloids in Trypanosoma cruzi Cultures
Trypomastigotes of the Y strain of Trypanosoma cruzi was also used in the experiments to evaluate the toxic action of the compounds on another class of parasites belonging to the same family of Leishmania (Trypanosomatidae). They were treated in phosphate buffered saline (PBS) and cultured at 25 °C and pH 7.18 in RPMI-PY medium. Then, 4 × 106 promastigotes/mL were treated with the same serial concentrations of the polysaccharide extract (20, 40, 80, and 160 µg/mL). After 48 h of treatment at 24 °C, we proceeded with the evaluation of the percentage of viability via cell counting and a comparison with the control culture (100% viability).
2.5. Statistical Analysis
The experiment was performed by 2 observers in 3 replicates and repeated with 3 new batches of parasites. Obtained data were compared by statistical analysis through a t-test (p-value < 0.05).
3. Results
Seven species of macroalgae were tested to evaluate the anti-leishmanial activity of polysaccharide extract: the green alga Chaetomorpha linum, the red algae Gracilaria bursa-pastoris, Gracilaria viridis, Agardhiella subulata, Hypnea cornuta, and the brown algae Sargassum muticum and Undaria pinnatifida. According to literature data, phycocolloids found in marine algae include fucoidan and laminarans from brown algae, carrageenan from red algae, and ulvan from green algae.
The polysaccharide extract were inoculated into DH82, VERO, L929, MDCK, and U937 cell lines cultures; the MTT viability assay showed that tested concentrations of algal extracts did not have a cytotoxic effect.
The antileishmanial activity of phycocolloids is reported in Table 1 and shown as dose-response curves in Figure 2.

Table 1.
Viability of Leishmania infantum (%) cultured with tested phycocolloids.
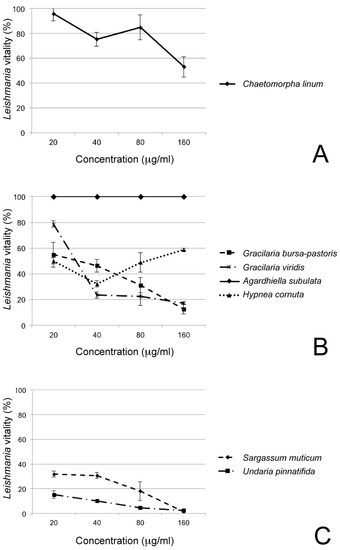
Figure 2.
Dose-response curve of polysaccharide extracts from (a) the green alga Chaetomorpha linum; (b) the red algae Gracilaria bursa-pastoris, G. viridis, Agardhiella subulata, and Hypnea cornuta; (c) the brown algae Sargassum muticum and Undaria pinnatifida.
All samples of macroalgae, except Agardhiella subulata and Hypnea cornuta, showed a positive effect against L. infantum because increasing the polysaccharide extract concentration the protozoan viability decreased proportionally. The Agardhiella subulata polysaccharides extract did not show any anti-leishmanial effect: indeed, the viability of the parasites remains always at 100%. Instead, the polysaccharides of Hypnea cornuta seemed to show a temporary effect against L. infantum: at 40 µg/mL polysaccharide concentration, the vitality of Leishmania decreased and resumed at 80–160 µg/mL concentration. Currently there are not many studies on phycocolloids from Hypnea cornuta, nor even of Hypnea phycocolloids against Leishmania species. It can be assumed that there is a compound that, at higher concentrations, interferes with the antiprotozoan activity of the phycocolloids. Finally, the remaining species were particularly active against the protozoan, and the brown seaweeds Undaria pinnatifida and Sargassum muticum showed a great anti-leishmanial activity: at the maximum concentration of inoculated polysaccharides, the mortality of the parasite was 100%. In these last samples, the morphological observations of L. infantum by May–Grünwald–Giemsa staining clearly showed a decrease in the number of promastigotes, compared with the control, at 20 µg/mL polysaccharide concentration, and they were characterized by an inhibition of growth and by the presence of abnormal and roundish form of promastigotes, at 40 µg/mL concentration. In the cultures with 80 µg/mL of polysaccharide extract, L. infantum cells were aggregates, rounds, and without flagella. Finally, in L. infantum cultures with 160 µg/mL polysaccharide concentration, it was not possible to find whole forms of protozoa but apoptotic bodies (Figure 3).
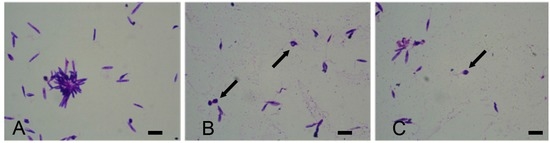
Figure 3.
May–Grünwald–Giemsa stained micrographs of Leishmania infantum: (A) Control; (B) Promastigotes exposed to 160 µg/mL of Undaria pinnatifida polysaccharide extract; (C) Promastigotes exposed to 160 µg/mL of Sargassum muticum polysaccharide extract. Note in (B,C) the presence of suffering cells and apoptotic bodies (arrows) in cultures incubated with derivatives of seaweed extracts. Scale bars: 10 µm.
Parasites exposed for 48 h to phycocolloids and stained with the acridine orange and ethidium bromide mixture (Figure 4) showed changes in morphology (such as loss of cell volume and nuclear condensation), sharing many characteristics with metazoans apoptotic death.
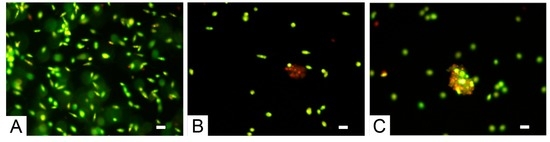
Figure 4.
Morphologic changes observed in Leishmania infantum stained with the acridine orange and ethidium bromide: (A) Control; (B) Promastigotes exposed to 160 µg/mL of Undaria pinnatifida polysaccharide extract; (C) Promastigotes exposed to 160 µg/mL of Sargassum muticum polysaccharide extract. Scale bars: 10 µm.
Instead, the analysis of the same concentrations of Undaria pinnatifida and Sargassum muticum did not show any interesting activity against Trypanosoma cruzi cultures, suggesting their specific toxic action to the genus Leishmania.
4. Discussion
A total of 98 countries and 3 territories on 5 continents reported endemic leishmaniasis transmission. In terms of global disease load, the leishmaniasis is the third most important vector-borne disease, after malaria and lymphatic filariasis [21].
The primary reservoir hosts of Leishmania are sylvatic mammals, such as forest rodents and wild canids. With the increasing process of domiciliation of the zoonotic cycle of transmission of leishmaniases, synanthropic and domestic animals have assumed an important role as reservoirs of infection. Control of leishmaniasis transmission is thus focused on vector control and, in some areas, culling of infected dogs; there is currently no vaccine, and treatment of infected dogs is not usually curative [22,23].
Present therapeutic regimes for leishmanial diseases rely on pentavalent antimonials, such as Pentamidine and Amphotericine B, which show high toxicity at the effective therapeutic doses [24]. Therefore, the research of new antileishmanial drugs from natural resources is urgent [9].
Natural polysaccharides play a relevant role in biomedical and pharmaceutical applications, particularly in the field of drug delivery, for their intrinsic biocompatibility and potential low cost [25]. However, seaweeds have not been receiving appropriate attention in the past and the availability of algal pharmaceutical data is still scarce compared to that of terrestrial plants [26]. Previous investigation in order to measure the antileishmanial potential from marine algae is extremely limited, being restricted to some species with the test organisms Leishmania donovani [7,27], L. maxicana [6], L. major [9], and L. amazonensis [8], while studies on the Mediterranean species L. infantum are still missing.
Preliminary results obtained in this work showed that the phycocolloids, extracts from different species of macroalgae collected in the Mediterranean Sea, had remarkable activity against L. infantum, revealing the investigated species as a great source of natural antiprotozoal products and contributing to give a new impetus to deepen their use in medicinal therapy. Although the literature has not yet clarified the relation between polysaccharide structures and biological activities, further studies will be required to evaluate phycocolloids, whether they can be used as anti-leishmanial compounds or as fortifying agents with existing synthetic compounds for the development of anti-leishmanial agents. Biological activities of phycocolloids depend on chemical structure, molecular weight, and chain conformations [28]. Given the positive results obtained from some algal extracts, according to the National Reference Centre for Leishmaniasis, the next research step involves selecting the phycocolloid activities and to investigate their action on L. infantum infected macrophages. Furthermore, it would be very interesting to isolate or synthesize structurally related compounds in order to establish structure–activity relationships, considering that the determination of the mechanism of action of the natural compounds against Leishmania is currently still unknown.
Acknowledgments
This paper was supported by the Italian Flagship project RITMARE, supported by the Ministry of Education, University and Research (MIUR). The authors would particularly like to thank Fabio Trincardi (current director of the Institute of Marine Sciences of the CNR) for his support and encouragement. We thank the associate editor and two anonymous referees for their fruitful comments, which helped us to improve the manuscript.
Author Contributions
Simona Armeli Minicante, Fabrizio Vitale and Giuseppa Genovese conceived and designed the experiments; Simona Armeli Minicante, Germano Castelli, Federica Bruno and Silvia Michelet performed the experiments; Germano Castelli, Federica Bruno, Silvia Michelet and Marina Morabito analyzed the data; Adriano Sfriso and Fabrizio Vitale contributed reagents/materials/analysis tools; Simona Armeli Minicante wrote the first draft of the manuscript, and Simona Armeli Minicante, Marina Morabito, Giuseppa Genovese, Fabrizio Vitale, Germano Castelli and Federica Bruno contributed to the editing of the manuscript. All authors have read and approved the final manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Chanda, S.; Dave, R.; Kaneria, M.; Nagani, K. Seaweeds: A novel, untapped source of drugs from sea to combat Infectious diseases, in Current Research, Technology and Education Topics. In Applied Microbiology and Microbial Biotechnology; Méndez-Vilas, A., Ed.; Formatex: Badajoz, Spain, 2010; pp. 473–480. [Google Scholar]
- Genovese, G.; Leitner, S.; Armeli Minicante, S.; Lass-Flörl, C. The Mediterranean red alga Asparagopsis taxiformis has antifungal activity against Aspergillus species. Mycoses 2013, 56, 516–519. [Google Scholar] [CrossRef] [PubMed]
- Jiao, G.; Yu, G.; Zhang, J.; Ewart, H.S. Chemical Structures and Bioactivities of Sulfated Polysaccharides from Marine Algae. Mar. Drugs 2011, 9, 196–223. [Google Scholar] [CrossRef] [PubMed]
- Parthiban, C.; Parameswari, K.; Saranya, C.; Hemalatha, A.; Anantharaman, P. Production of Sodium Alginate from Selected Seaweeds and Their Physiochemical and Biochemical Properties. Asian Pac. J. Trop. Biomed. 2012, 2012, 1–4. [Google Scholar]
- Fouladvand, M.; Barazesh, A.; Farokhzad, F.; Malekizadeh, H.; Sartavi, K. Evaluation of in vitro anti-Leishmanial activity of some brown, green and red algae from the Persian Gulf. Eur. Rev. Med. Pharmacol. Sci. 2011, 15, 597–600. [Google Scholar] [PubMed]
- Freile-Pelegrin, Y.; Robledo, D.; Chan-Bacab, M.J.; Ortega-Morales, B.O. Antileishmanial properties of tropical marine algae extracts. Fitoterapia 2008, 79, 374–377. [Google Scholar] [CrossRef] [PubMed]
- Genovese, G.; Tedone, L.; Hamann, M.T.; Morabito, M. The Mediterranean Red Alga Asparagopsis: A Source of Compounds against Leishmania. Mar. Drugs 2009, 7, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Lehnhardt Pires, C.; Rodrigues, S.; Bristot, D.; Gaeta, H.; de Oliveira Toyama, D.; Lobo Farias, W.; Toyama, M. Evaluation of Macroalgae Sulfated Polysaccharides on the Leishmania (L.) amazonensis Promastigote. Mar. Drugs 2013, 11, 934–943. [Google Scholar] [CrossRef] [PubMed]
- Sabina, H.; Aliya, R. Bioactive assessment of selected marine red algae against Leishmania major and chemical constituents of Osmundea pinnatifida. Pak. J. Bot. 2011, 43, 3053–3056. [Google Scholar]
- Manna, L.; Reale, S.; Vitale, F.; Gravino, A.E. Evidence for a relationship between Leishmania load and clinical manifestations. Res. Vet. Sci. 2009, 87, 76–78. [Google Scholar] [CrossRef] [PubMed]
- Desjeux, P. Leishmaniasis: Current situation and new perspectives. Comp. Immunol. Microbiol. Infect. Dis. 2004, 27, 305–318. [Google Scholar] [CrossRef] [PubMed]
- Alvar, J.; Vélez, I.D.; Bern, C.; Herrero, M.; Desjeux, P.; Cano, J.; Jannin, J.; den Boer, M. Leishmaniasis Worldwide and Global Estimates of Its Incidence. PLoS ONE 2012, 7, e35671. [Google Scholar] [CrossRef] [PubMed]
- Yamey, G. The world’s most neglected diseases. BMJ 2002, 325, 176–177. [Google Scholar] [CrossRef] [PubMed]
- Guerin, P.J.; Olliaro, P.; Sundar, S.; Boelaert, M.; Croft, S.L.; Desjeux, P.; Wasunna, M.K.; Bryceson, A.D.M. Visceral leishmaniasis: Current status of control, diagnosis, and treatment, and a proposed research and development agenda. Lancet Infect. Dis. 2002, 2, 494–501. [Google Scholar] [CrossRef]
- Athukorala, Y.; Lee, K.-W.; Kim, S.-K.; Jeon, Y.-J. Anticoagulant activity of marine green and brown algae collected from Jeju Island in Korea. Bioresour. Thecnol. 2007, 98, 1711–1716. [Google Scholar] [CrossRef] [PubMed]
- Matsubara, K.; Matsuura, Y.; Bacic, A.; Liao, M.; Hori, K.; Miyazawa, K. Anticoagulant properties of a sulfated galactan preparation from a marine green alga Codium cylindricum. Int. J. Biol. Macromol. 2001, 28, 395–399. [Google Scholar] [CrossRef]
- Minghou, J. Processing and Extraction of Phycocolloids. FAO Corporate Document Repository. Available online: http://www.fao.org/docrep/field/003/ab728e/ab728e08.htm (accessed on 23 October 2016).
- Carmichael, J.; Degraff, W.G.; Gazdar, A.F.; Minna, J.D.; Mitchell, J.B. Evaluation of a tetrazolium-based semiautomated colorimetric assay: Assessment of chemosensitivity testing. Cancer Res. 1987, 47, 936–942. [Google Scholar] [PubMed]
- Castelli, G.; Galante, A.; Verde, V.L.; Migliazzo, A.; Reale, S.; Lupo, T.; Piazza, M.; Vitale, F.; Bruno, F. Evaluation of Two Modified Culture Media for Leishmania infantum Cultivation Versus Different Culture Media. J. Parasitol. 2014, 100, 228–230. [Google Scholar] [CrossRef] [PubMed]
- Limoncu, M.E.; Balcioglu, I.; Yereli, K.; Ozbel, Y.; Ozbilgin, A. A new experimental in vitro culture medium for cultivation of Leishmania species. J. Clin. Microbiol. 1997, 35, 2430–2431. [Google Scholar] [PubMed]
- Solano-Gallego, L.; Rodríguez-Cortés, A.; Iniesta, L.; Quintana, J.; Pastor, J.; Espada, Y.; Portús, M.; Alberola, J. Cross-sectional serosurvey of feline leishmaniasis in ecoregions around the northwestern Mediterranean. Am. J. Trop. Med. Hyg. 2007, 76, 676–680. [Google Scholar] [PubMed]
- Baneth, G.; Shaw, S.E. Chemotherapy of canine leishmaniosis. Vet. Parasitol. 2002, 106, 315–324. [Google Scholar] [CrossRef]
- Quinnell, R.J.; Courtenay, O. Transmission, reservoir hosts and control of zoonotic visceral leishmaniasis. Parasitology 2009, 136, 1915–1934. [Google Scholar] [CrossRef] [PubMed]
- Deniz, T.; Kaiser, M.; Brun, R.; Yardely, V.; Schmidt, T.J.; Tosun, F.; Ruedi, P. Antitrypanosomal and antileishmanial activities of flavonoids and their analogues: In vitro, in vivo, structure-activity relationship and quantitative structure—Activity relationship studies. Antimicrob. Agents Chemother. 2006, 50, 1352–1364. [Google Scholar]
- Laurienzo, P. Marine Polysaccharides in Pharmaceutical Applications: An Overview. Mar. Drugs 2010, 8, 2435–2465. [Google Scholar] [CrossRef] [PubMed]
- Mayer, A.M.S.; Rodríguez, A.D.; Berlinck, R.G.S.; Fusetani, N. Marine pharmacology in 2007–8: Marine compounds with antibacterial, anticoagulant, antifungal, anti-inflammatory, antimalarial, antiprotozoal, antituberculosis, and antiviral activities; affecting the immune and nervous system, and other miscellaneous mechanisms of action. Comp. Biochem. Physiol. C 2011, 153, 191–222. [Google Scholar]
- Orhan, I.; Sener, B.; Atici, T.; Brun, R.; Perozzo, R.; Tasdemir, D. Turkish freshwater and marine macrophyte extracts show In vitro antiprotozoal activity and inhibit FabI, a key enzyme of Plasmodium falciparum fatty acid biosynthesis. Phytomedicine 2006, 13, 388–393. [Google Scholar] [CrossRef] [PubMed]
- Ye, H.; Wang, K.; Zhou, C.; Liu, J.; Zeng, X. Purification, antitumor and antioxidant activities in vitro of polysaccharides from the brown seaweed Sargassum pallidum. Food Chem. 2008, 111, 428–432. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).