Ethanol Production from Enzymatically Treated Dried Food Waste Using Enzymes Produced On-Site
Abstract
:1. Introduction
2. Results
2.1. Evaluation of the Production of Thermophilic Enzymes Using HFW as Raw Material
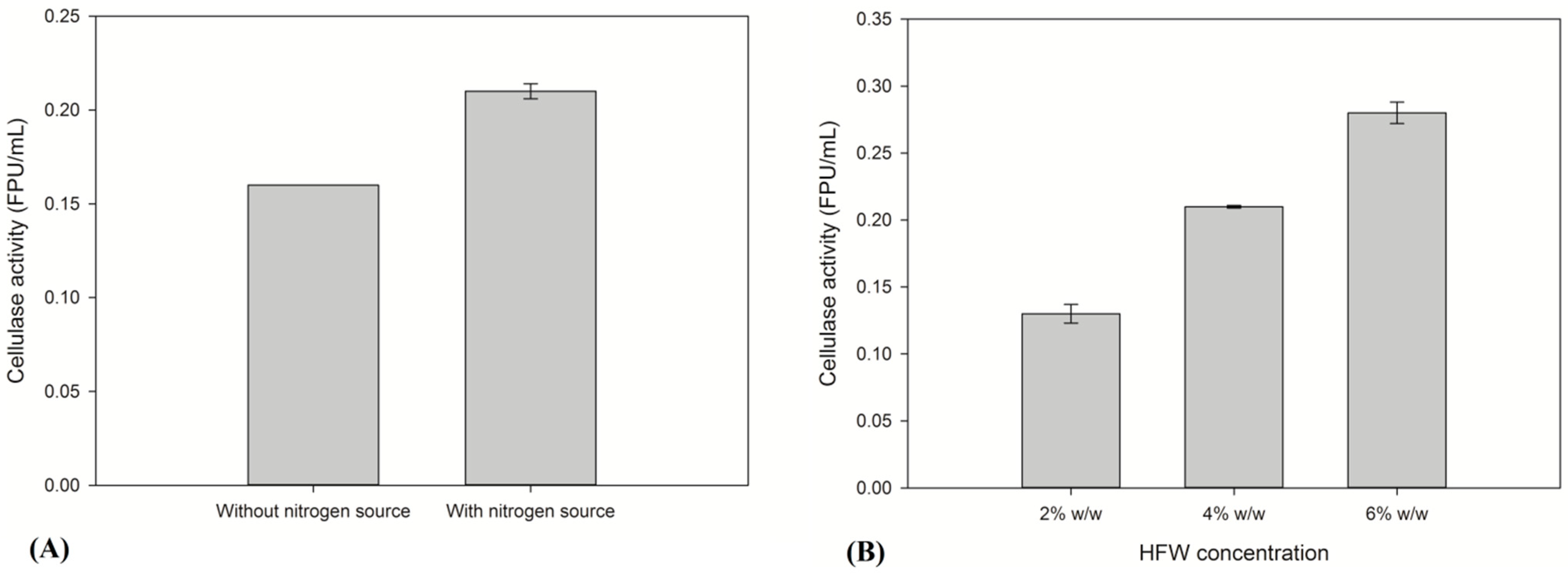
2.2. Saccharification of HFW and Subsequent Ethanol Production
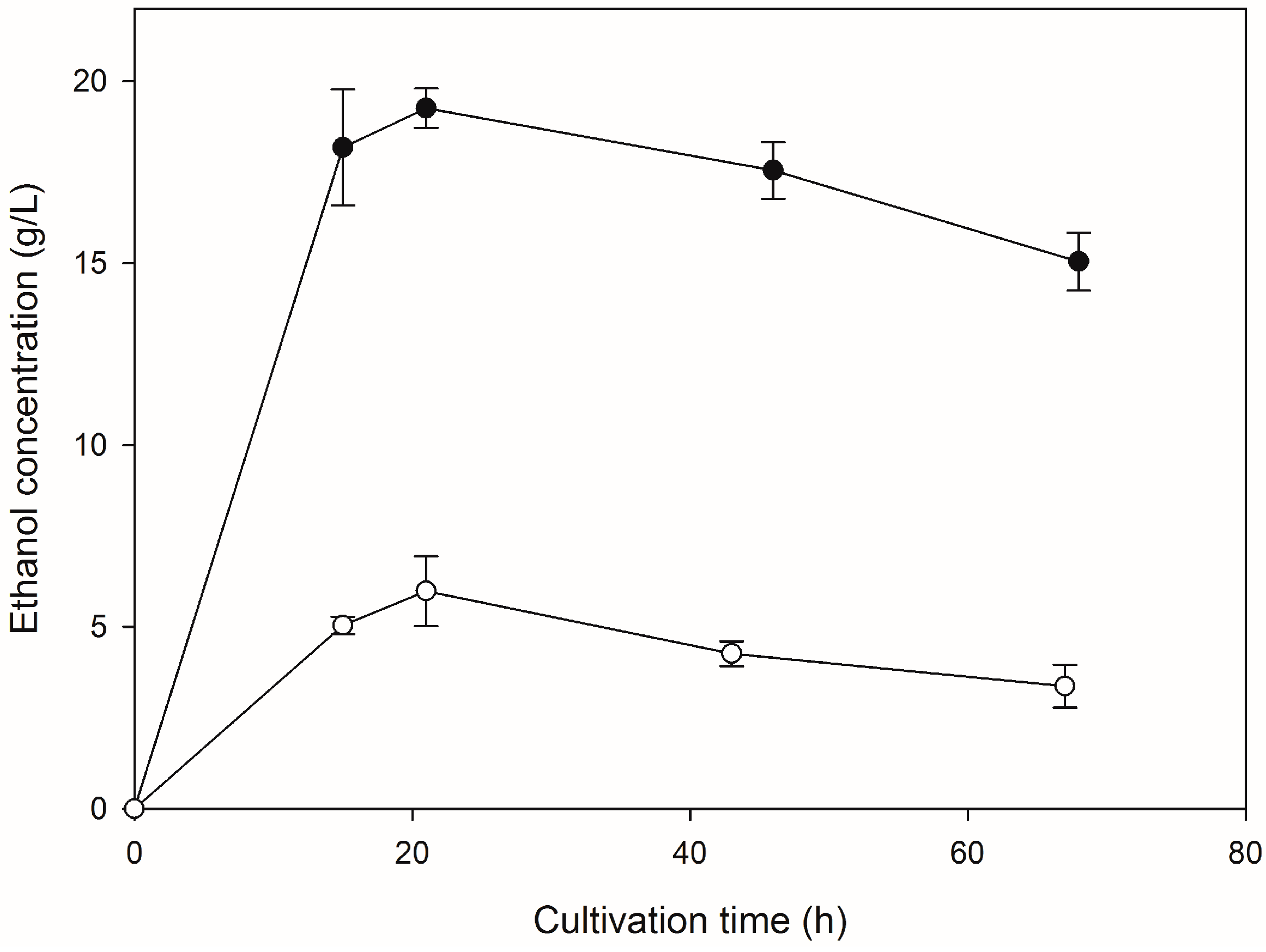
3. Discussion
| Microorganism | Raw Material | Enzyme Activity (FPU/mL) | Enzyme Yield (FPU/g) | Reference |
|---|---|---|---|---|
| Trichoderma asperellum | Wheat bran | n.a. | 2.2 | [41] |
| Neurospora sitophila | Steam exploded wheat straw | n.a. | 6.4 | [42] |
| Trichoderma reesei | Alkali treated sugarcane bagasse | 0.09 | n.a. | [43] |
| Trichoderma reesei | Avicel | 0.16 | n.a. | [43] |
| Fomitopsis sp. | Wheat bran | n.a. | 6.8 | [44] |
| Bacillus subtilis | Banana wastes | n.a. | 2.8 | [27] |
| Trichoderma viride | Banana peel | n.a. | 5.6 | [28] |
| M. thermophila | Household food wastes | 0.28 | 4.7 | Present work |
4. Materials and Methods
4.1. Raw Materials and Microorganisms
4.2. Cultivation of Myceliophthora Thermophila
4.3. Enzyme Production and Concentration
4.4. Saccharification and Ethanol Fermentation of HFW
4.5. Analytical Methods
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Shen, F.; Saddler, J.N.; Liu, R.; Lin, L.; Deng, S.; Zhang, Y.; Yang, G.; Xiao, H.; Li, Y. Evaluation of steam pretreatment on sweet sorghum bagasse for enzymatic hydrolysis and bioethanol production. Carbohyd. Polym. 2011, 86, 1542–1548. [Google Scholar] [CrossRef]
- Kachrimanidou, V.; Kopsahelis, N.; Chatzifragkou, A.; Papanikolaou, S.; Yanniotis, S.; Kookos, I.; Koutinas, A.A. Utilization of by-products from sunflower-based biodiesel production processes for production of fermentation feedstocks. Waste Biomass Valor. 2013, 4, 529–537. [Google Scholar] [CrossRef]
- Matsakas, L.; Rova, U.; Christakopoulos, P. Evaluation of dried sweet sorghum stalks as raw material for methane production. Biomed. Res. Int. 2014. [Google Scholar] [CrossRef] [Green Version]
- Matsakas, L.; Christakopoulos, P. Optimization of ethanol production from high dry matter liquefied dry sweet sorghum stalks. Biomass Bioenerg. 2013, 51, 91–98. [Google Scholar] [CrossRef]
- Wang, M.; Wang, J.; Tan, J.X.; Sun, J.F.; Mou, J.L. Optimization of ethanol fermentation from sweet sorghum juice using response surface methodology. Energy Source 2011, 33, 1139–1146. [Google Scholar] [CrossRef]
- Matsakas, L.; Christakopoulos, P. Fermentation of liquefacted hydrothermally pretreated sweet sorghum bagasse to ethanol at high-solids content. Bioresour. Technol. 2013, 127, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Pimentel, D.; Marklein, A.; Toth, M.A.; Karpoff, M.N.; Paul, G.S.; McCormack, R.; Kyriazis, J.; Krueger, T. Food versus biofuels: Environmental and economic costs. Hum. Ecol. 2009, 37, 1–12. [Google Scholar] [CrossRef]
- European Communities: EC preparatory study on food waste in the EU27. Available online: http://ec.europa.eu/environment/eussd/pdf/bio_foodwaste_report.pdf (accessed on 16 January 2015).
- Melikoglu, M.; Lin, C.S.K.; Webb, C. Analysing global food waste problem: pinpointing the facts and estimating the energy content. Cent. Eur. J. Eng. 2013, 3, 157–164. [Google Scholar] [CrossRef]
- Moon, H.C.; Song, I.S.; Kim, J.C.; Shirai, Y.; Lee, D.H.; Kim, J.K.; Chung, S.O.; Kim, D.H.; Oh, K.K.; Cho, Y.S. Enzymatic hydrolysis of food waste and ethanol fermentation. Int. J. Energy Res. 2009, 33, 164–172. [Google Scholar] [CrossRef]
- Lin, C.S.K.; Pfaltzgraff, L.A.; Herrero-Davila, L.; Mubofu, E.B.; Abderrahim, S.; Clark, J.H.; Koutinas, A.A.; Kopsahelis, N.; Stamatelatou, K.; Dickson, F.; et al. Food waste as a valuable resource for the production of chemicals, materials and fuels. Current situation and global perspective. Energy Environ. Sci. 2013, 6, 426–464. [Google Scholar] [CrossRef]
- Adhikari, B.K.; Barrington, S.; Martinez, J. Predicted growth of world urban food waste and methane production. Waste Manag. Res. 2006, 24, 421–433. [Google Scholar] [CrossRef] [PubMed]
- Kiran, E.U.; Trzcinski, A.P.; Ng, W.J.; Liu, Y. Enzyme Production from Food Wastes Using a Biorefinery Concept. Waste Biomass. Valor. 2014, 5, 903–917. [Google Scholar] [CrossRef]
- Yan, S.; Li, J.; Chen, X.; Wu, J.; Wang, P.; Ye, J.; Yao, J. Enzymatical hydrolysis of food waste and ethanol production from the hydrolysate. Renew. Energy 2011, 36, 1259–1265. [Google Scholar] [CrossRef]
- Uncu, O.N.; Cekmecelioglu, D. Cost-effective approach to ethanol production and optimization by response surface methodology. Waste Manag. 2011, 31, 636–643. [Google Scholar] [CrossRef] [PubMed]
- Luque, R.; Clark, J. Valorisation of food residues: Waste to wealth using green chemical technologies. Sustain. Chem. Process. 2013. [Google Scholar] [CrossRef]
- Arancon, R.A.D.; Lin, C.S.K.; Chan, K.M.; Kwan, T.H.; Luque, R. Advances on waste valorization: New horizons for a more sustainable society. Energy Sci. Eng. 2013, 1, 53–71. [Google Scholar] [CrossRef]
- Zhang, R.; El-Mashad, H.; Hartman, K.; Wang, F.; Liu, G.; Choate, C.; Gamble, P. Characterization of food waste as feedstock for anaerobic digestion. Bioresour. Technol. 2007, 98, 929–935. [Google Scholar] [CrossRef] [PubMed]
- Banks, C.J.; Chesshire, M.; Heaven, S.; Arnold, R. Anaerobic digestion of source-segregated domestic food waste: Performance assessment by mass and energy balance. Bioresour. Technol. 2011, 102, 612–620. [Google Scholar] [CrossRef] [PubMed]
- Forster-Carneiro, T.; Pérez, M.; Romero, L.I. Influence of total solid and inoculum contents on performance of anaerobic reactors trating food waste. Bioresour. Technol. 2008, 99, 6994–7002. [Google Scholar] [CrossRef] [PubMed]
- Matsakas, L.; Kekos, D.; Loizidou, M.; Christakopoulos, P. Utilization of household food waste for the production of ethanol at high dry material content. Biotechnol. Biofuels 2014. [Google Scholar] [CrossRef]
- Cekmecelioglu, D.; Uncu, O.N. Kinetic modeling of enzymatic hydrolysis of pretreated kitchen wastes for enhancing bioethanol production. Waste Manag. 2013, 33, 735–739. [Google Scholar] [CrossRef] [PubMed]
- Shukla, J.; Kar, R. ; Potato peel as a solid state substrate for thermostable alpha amylase production by thermophilic Bacillus isolates. World J. Microbiol. Biotechnol. 2006, 22, 417–422. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, X.; Wang, X.; Ma, H. Glucoamylase production from food waste by Aspergillus. niger under submerged fermentation. Process. Biochem. 2008, 43, 280–286. [Google Scholar] [CrossRef]
- Jellouli, K.; Bayoudh, A.; Manni, L.; Agrebi, R.; Nasri, M. Purification, biochemical and molecular characterization of a metalloprotease from Pseudomonas aeruginosa MN7 grown on shrimp wastes. Appl. Microbiol. Biotechnol. 2008, 79, 989–999. [Google Scholar] [CrossRef] [PubMed]
- Afify, M.M.; Abd El-Ghany, T.M.; Alawlaqi, M.M. Microbial utilization of potato wastes for protease production and their using as biofertilizer. Aust. J. Basic Appl. Sci. 2011, 5, 308–315. [Google Scholar]
- Chundakkadu, K. Production of bacterial cellulases by solid state bioprocessing of banana wastes. Bioresour. Technol. 1999, 69, 231–239. [Google Scholar] [CrossRef]
- Sun, H.Y.; Li, J.; Zhao, P.; Peng, M. Banana peel: A novel substrate for cellulase production under solid-state fermentation. Afr. J. Biotechnol. 2011, 10, 17887–17890. [Google Scholar] [CrossRef]
- Dominguez, A.; Deive, F.J.; Sanroman, M.A.; Longo, M.A. Biodegradation and utilization of waste cooking oil by Yarrowia lipolytica CECT 1240. Eur. J. Lipid Sci. Technol. 2010, 112, 1200–1208. [Google Scholar] [CrossRef]
- Papanikolaou, S.; Dimou, A.; Fakas, S.; Diamantopoulou, P.; Philippoussis, A.; Galiotou-Panayotou, M.; Aggelis, G. Biotechnological conversion of waste cooking olive oil into lipid rich biomass using Aspergillus and Penicillium strains. J. Appl. Microbiol. 2011, 110, 1138–1150. [Google Scholar] [CrossRef] [PubMed]
- Garzon, C.G.; Hours, R.A. Citrus waste: an alternative substrate for pectinase production in solid-state culture. Bioresource. Technol. 1992, 39, 93–95. [Google Scholar] [CrossRef]
- Martínez Sabajanes, M.; Yáñez, R.; Alonso, J.L.; Parajó, J.C. Pectic oligosaccharides production from orange peel waste by enzymatic hydrolysis. Int. J. Food Sci. Technol. 2012, 47, 747–754. [Google Scholar] [CrossRef]
- Matsakas, L.; Sterioti, A.A.; Rova, U.; Christakopoulos, P. Use of dried sorghum for the efficient production of lipids by the yeast Lipomyces starkeyi CBS 1807. Ind. Crop. Prod. 2014, 62, 367–372. [Google Scholar] [CrossRef]
- Topakas, E.; Katapodis, P.; Kekos, D.; Macris, B.J.; Christakopoulos, P. Production and partial characterization of xylanase by Sporotrichum thermophile under solid-state fermentation. World J. Microb. Biot. 2003, 19, 195–198. [Google Scholar] [CrossRef]
- Viikari, L.; Alapuranen, M.; Puranen, T.; Vehmaanperä, J.; Siika-Aho, M. Thermostable enzymes in lignocellulose hydrolysis. Adv. Biochem. Eng. Biotechnol. 2007, 108, 121–145. [Google Scholar] [PubMed]
- Kallioinen, A.; Puranen, T.; Siika-aho, M. Mixtures of thermostable enzymes show high performance in biomass saccharification. Appl. Biochem. Biotechnol. 2014, 173, 1038–1056. [Google Scholar] [CrossRef] [PubMed]
- Bhat, K.M.; Maheshwari, R. Sporotrichum thermophile growth, cellulose degradation, and cellulase activity. Appl. Environ. Microbiol. 1987, 53, 2175–2182. [Google Scholar] [PubMed]
- Maheshwari, R.; Bharadwaj, G.; Bhat, M.K. Thermophilic Fungi: Their Physiology and Enzymes. Microbiol. Mol. Biol. Rev. 2000, 64, 461–488. [Google Scholar] [CrossRef] [PubMed]
- Dimarogona, M.; Topakas, E.; Olsson, L.; Christakopoulos, P. Lignin boosts the cellulase performance of a GH-61 enzyme from Sporotrichum thermophile. Bioresour. Technol. 2012, 110, 480–487. [Google Scholar] [CrossRef] [PubMed]
- Katapodis, P.; Christakopoulou, V.; Christakopoulos, P. Optimization of xylanase production by Sporotrichum thermophile using corn cobs and response surface methodology. Eng. Life Sci. 2006, 6, 410–415. [Google Scholar] [CrossRef]
- Raghuwanshi, S.; Deswal, D.; Karp, M.; Kuhad, R.C. Bioprocessing of enhanced cellulase production from a mutant of Trichoderma asperellum RCK2011 and its application in hydrolysis of cellulose. Fuel 2014, 124, 183–189. [Google Scholar] [CrossRef]
- Li, Y.; Peng, X.; Chen, H. Comparative characterization of proteins secreted by Neurospora sitophila in solid-state and submerged fermentation. J. Biosci. Bioeng. 2013, 116, 493–498. [Google Scholar] [CrossRef] [PubMed]
- Aiello, C.; Ferrer, A.; Ledesma, A. Effect of alkaline treatments at various temperatures on cellulose and biomass production using submerged sugarcane bagasse fermentation with Trichoderma reesei QM 9414. Bioresour. Technol. 1996, 57, 13–18. [Google Scholar] [CrossRef]
- Deswal, D.; Khasa, Y.P.; Kuhad, R.C. Optimization of cellulose production by a brown rot fungus Fomitopsis. sp. RCK2010 under solid state fermentation. Bioresour. Technol. 2011, 102, 6065–6072. [Google Scholar] [CrossRef] [PubMed]
- Szijártó, N.; Horan, E.; Zhang, J.; Puranen, T.; Siika-aho, M.; Viikari, L. Thermostable endoglucanases in the liquefaction of hydrothermally pretreated wheat straw. Biotechnol. Biofuels 2011. [Google Scholar] [CrossRef]
- Jensen, J.W.; Felby, C.; Jørgensen, H.; Rønsch, G.Ø.; Nøholm, N.D. Enzymatic processing of municipal solid waste. Waste Manag. 2010, 30, 2497–2503. [Google Scholar] [CrossRef] [PubMed]
- Sarris, D.; Matsakas, L.; Aggelis, G.; Koutinas, A.A.; Papanikolaou, S. Aerated vs non-aerated conversions of molasses and olive mill wastewaters blends into bioethanol by Saccharomyces cerevisiae under non-aseptic conditions. Ind. Crop. Prod. 2014, 56, 83–93. [Google Scholar] [CrossRef]
- Piškur, J.; Rozpędowska, E.; Polakova, S.; Merico, A.; Compagno, C. How did Saccharomyces evolve to become a good brewer? Trends Genet. 2006, 22, 183–186. [Google Scholar] [CrossRef] [PubMed]
- Ratledge, C. Yeast physiology—A micro-synopsis. Bioprocess. Biosyst. Eng. 1991, 6, 195–203. [Google Scholar] [CrossRef]
- Soni, S.K.; Batra, N.; Bansal, N.; Soni, R. Bioconversion of sugarcane bagasse into second generation bioethanol after enzymatic hydrolysis with in-house produced cellulases from Aspergillus sp. S4B2F. Bioresources 2010, 5, 741–758. [Google Scholar]
- Sukumaran, R.K.; Singhania, R.R.; Mathew, G.M.; Pandey, A. Cellulase production using biomass feed stock and its application in lignocellulose saccharification for bio-ethanol production. Renew. Energy 2009, 34, 421–424. [Google Scholar] [CrossRef]
- Walker, K.; Vadlani, P.; Madl, R.; Ugorowski, P.; Hohn, K.L. Ethanol fermentation from food processing waste. Envviron. Prog. Sustain. Energy 2012, 32, 1280–1283. [Google Scholar] [CrossRef]
- Klein-Marcuschamer, D.; Oleskowicz-Popiel, P.; Simmons, B.A.; Blanch, H.W. The challenge of enzyme cost in the production of lignocellulosic biofuels. Biotechnol. Bioeng. 2012, 109, 1083–1087. [Google Scholar] [CrossRef] [PubMed]
- LIFE 08/ENV/GR/000566. Available online: http://www.uest.gr/drywaste/site/index.htm (accessed on 15 January 2015).
- Ghose, T.K. Measurement of cellulase activities. Pure Appl. Chem. 1987, 59, 257–268. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matsakas, L.; Christakopoulos, P. Ethanol Production from Enzymatically Treated Dried Food Waste Using Enzymes Produced On-Site. Sustainability 2015, 7, 1446-1458. https://doi.org/10.3390/su7021446
Matsakas L, Christakopoulos P. Ethanol Production from Enzymatically Treated Dried Food Waste Using Enzymes Produced On-Site. Sustainability. 2015; 7(2):1446-1458. https://doi.org/10.3390/su7021446
Chicago/Turabian StyleMatsakas, Leonidas, and Paul Christakopoulos. 2015. "Ethanol Production from Enzymatically Treated Dried Food Waste Using Enzymes Produced On-Site" Sustainability 7, no. 2: 1446-1458. https://doi.org/10.3390/su7021446
APA StyleMatsakas, L., & Christakopoulos, P. (2015). Ethanol Production from Enzymatically Treated Dried Food Waste Using Enzymes Produced On-Site. Sustainability, 7(2), 1446-1458. https://doi.org/10.3390/su7021446







