Evaluation of Biomass Yield and Water Treatment in Two Aquaponic Systems Using the Dynamic Root Floating Technique (DRF)
Abstract
:1. Introduction
2. Experimental Section
2.1. Experimental System and Operation
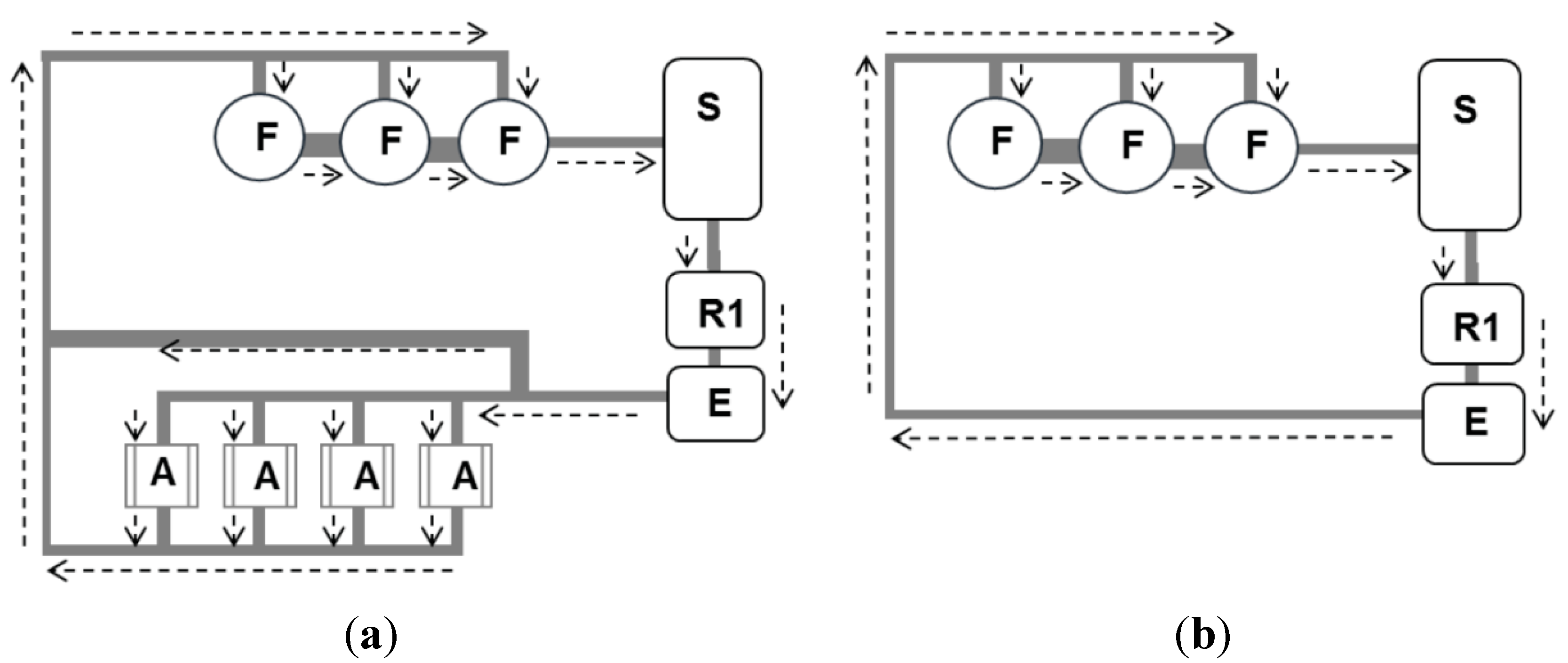
2.2. Experimental Conditions
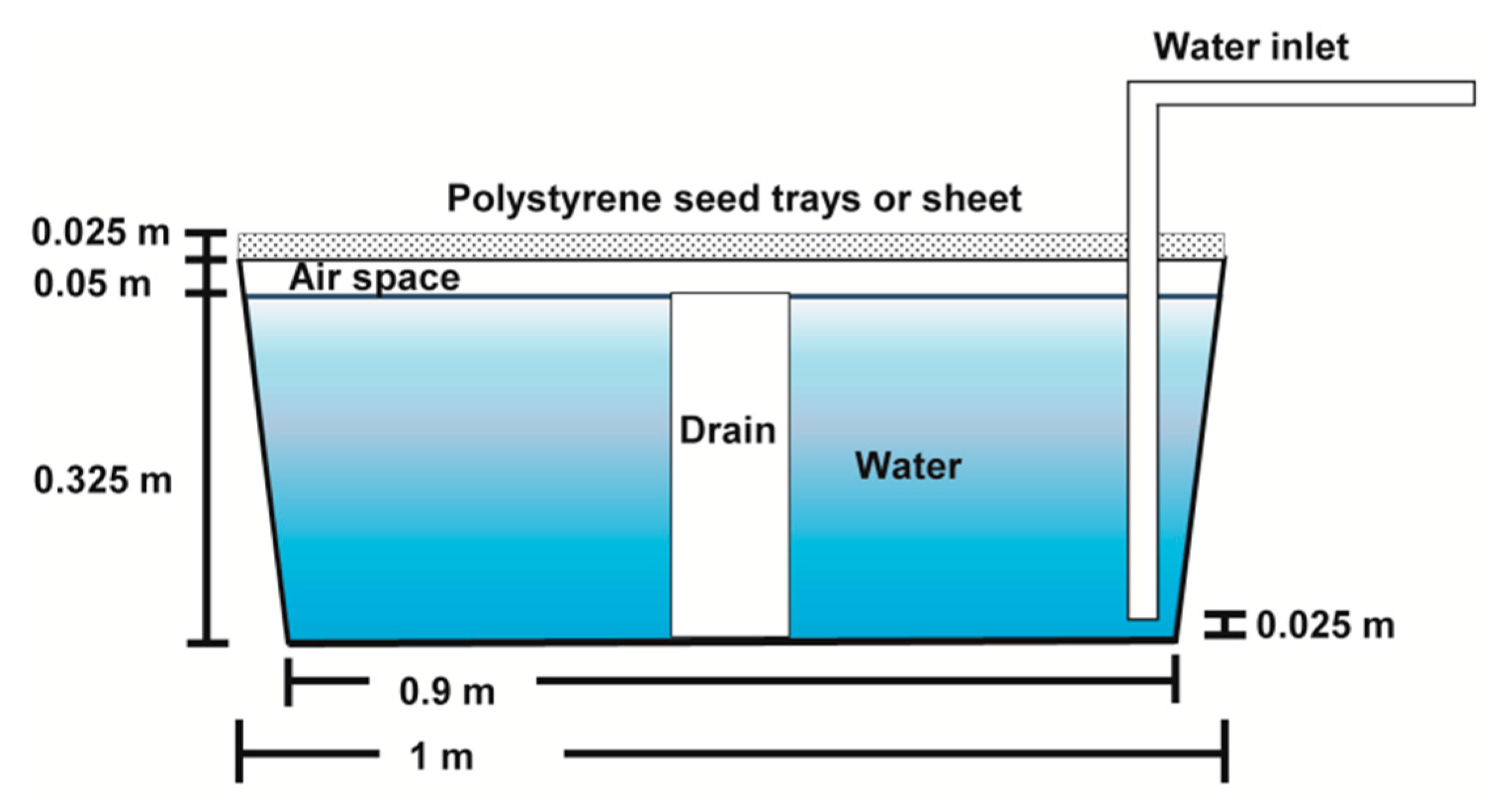
2.3. Water Quality and Nutrient Compounds
2.4. Statistical Analyses
3. Results
3.1. Food Production
| Variable | Unit | Treatment | ||
|---|---|---|---|---|
| COR | PAK | RAS | ||
| Initial weight | kg | 0.198 ± 1 × 10−4 | 0.198 ± 1 × 10−4 | 0.198 ± 1 × 10−4 |
| Final weight | kg | 0.311 ± 1× 10−2 | 0.301 ± 1.2 × 10−2 | 0.306 ± 1.1 × 10−2 |
| FCR | - | 1.8 ± 0.2 | 1.9 ± 0.1 | 1.9 ± 0.1 |
| Initial density | kg/m3 | 3.066 ± 0.002 | 3.065 ± 0.001 | 3.068 ± 0.005 |
| Final density | kg/m3 | 4.845 ± 0.357 | 4.816 ± 0.185 | 4.756 ± 0.092 |
| Fish biomass productivity | kg/m3 | 1.778 ± 0.355. | 1.751 ± 0.186 | 1.688 ± 0.096 |
| Average daily fish food provided | kg/tank | 0.0698 ± 6.6 × 10−3 | 0.0715 ± 2.3 × 10−3 | 0.0701 ± 2.6 × 10−3 |
| Ratio of fish food/growing plant area | kg/m2 | 0.0524 | 0.0537 | - |


| Parameter | Unit | Treatment | |
|---|---|---|---|
| PAK | COR | ||
| Edible fresh weight | kg/plant | 0.117 ± 0.061 | 2 × 10−4 ± 1.6 × 10−4 |
| Root fresh weight | kg/plant | 0.022 ± 0.011 | 2.3 × 10−4 ± 2.7 × 10−4 |
| Edible dry weight | kg/plant | 0.007 ± 0.003 | 3 × 10−5 ± 2 × 10−5 |
| Root dry weight | kg/plant | 0.002 ± 8 × 10−4 | 3 × 10−4 ± 5 × 10−4 |
| Leaf length | cm | 26.9 ± 4.3 | - |
3.2. Water Parameters
| Time | Treatment | Temperature (°C) | pH | D.O. (mg/L) | Conductivity (mS) |
|---|---|---|---|---|---|
| a.m. | COR | 29.20 ± 0.02 a,1 | 8.37 ± 0.03 a,1 | 6.16 ± 0.12 a,1 | 1.041 ± 4.4 × 10−4 ab,1 |
| PAK | 29.14 ± 0.07 a,1 | 8.47 ± 0.02 b,1 | 6.06 ± 0.20 a,1 | 1.036 ± 9.9 × 10−4 a,1 | |
| RAS | 29.14 ± 0.03 a,1 | 8.27 ± 0.02 c,1 | 6.03 ± 0.06 a,1 | 1.058 ± 1.2 × 10−4 b,1 | |
| p.m. | COR | 30.69 ± 0.09 ab,2 | 8.32 ± 0.04 a,2 | 5.61 ± 0.14 a,2 | 1.060 ± 4.6 × 10−4 a,1 |
| PAK | 30.75 ± 0.09 a,1 | 8.41 ± 0.03 b,2 | 5.43 ± 0.25 a,2 | 1.065 ± 3.2 × 10−3 ab,1 | |
| RAS | 30.28 ± 0.03 b,2 | 8.19 ± 0.02 c,2 | 5.38 ± 0.08 a,2 | 1.077 ± 1.2 × 10−3 b,1 |
| Time | Treatment | Temperature (°C) | pH | D.O. (mg/L) | Conductivity (mS) |
|---|---|---|---|---|---|
| a.m. | COR | 29.33 ± 0.10 a,1 | 8.32 ± 0.02 a,1 | 6.11 ± 0.11 a,1 | 1.042 ± 2.2 × 10−3 a,1 |
| PAK | 29.19 ± 0.10 a,1 | 8.40 ± 0.02 b,1 | 6.14 ± 0.13 a,1 | 1.033 ± 1.6 × 10−3 b,1 | |
| p.m. | COR | 31.04 ± 0.09 a,2 | 8.37 ± 0.02 a,2 | 6.03 ± 0.05 a,1 | 1.057 ± 5.7 × 10−4 a,2 |
| PAK | 31.06 ± 0.07 a,2 | 8.44 ± 0.01 b,2 | 5.91 ± 0.12 a,2 | 1.054 ± 5.4 × 10−4 b,2 |
3.3. Water Treatment
| Time | Treatment | TAN (mg/L) | NO2−–N (mg/L) | NO3−–N (mg/L) | PO43− (mg/L) |
|---|---|---|---|---|---|
| a.m. | COR | 0.104 ± 0.016 a,1 | 0.168 ± 0.006 b,1 | 17.810 ± 0.187 b,1 | 0.193 ± 0.005 b,1 |
| PAK | 0.117 ± 0.003 a,1 | 0.260 ± 0.005 c,1 | 15.906 ± 0.487 a,1 | 0.116 ± 0.006 a.1 | |
| RAS | 0.088 ± 0.015 a,1 | 0.153 ± 0.011 a,2 | 20.449 ± 0.252 b,1 | 0.864 ± 0.006 c,2 | |
| p.m. | COR | 0.125 ± 0.025 a,1 | 0.225 ± 0.031 a,1 | 17.155± 0.354 ab,2 | 0.214 ± 0.011 b,1 |
| PAK | 0.114± 0.010 a,1 | 0.266 ± 0.039 a,1 | 15.995 ± 0.128 a,1 | 0.118 ± 0.012 a,1 | |
| RAS | 0.126 ± 0.007 a,1 | 0.270 ± 0.009 a,1 | 18.667 ± 0.170 b,2 | 0.708 ± 0.030 c,1 |
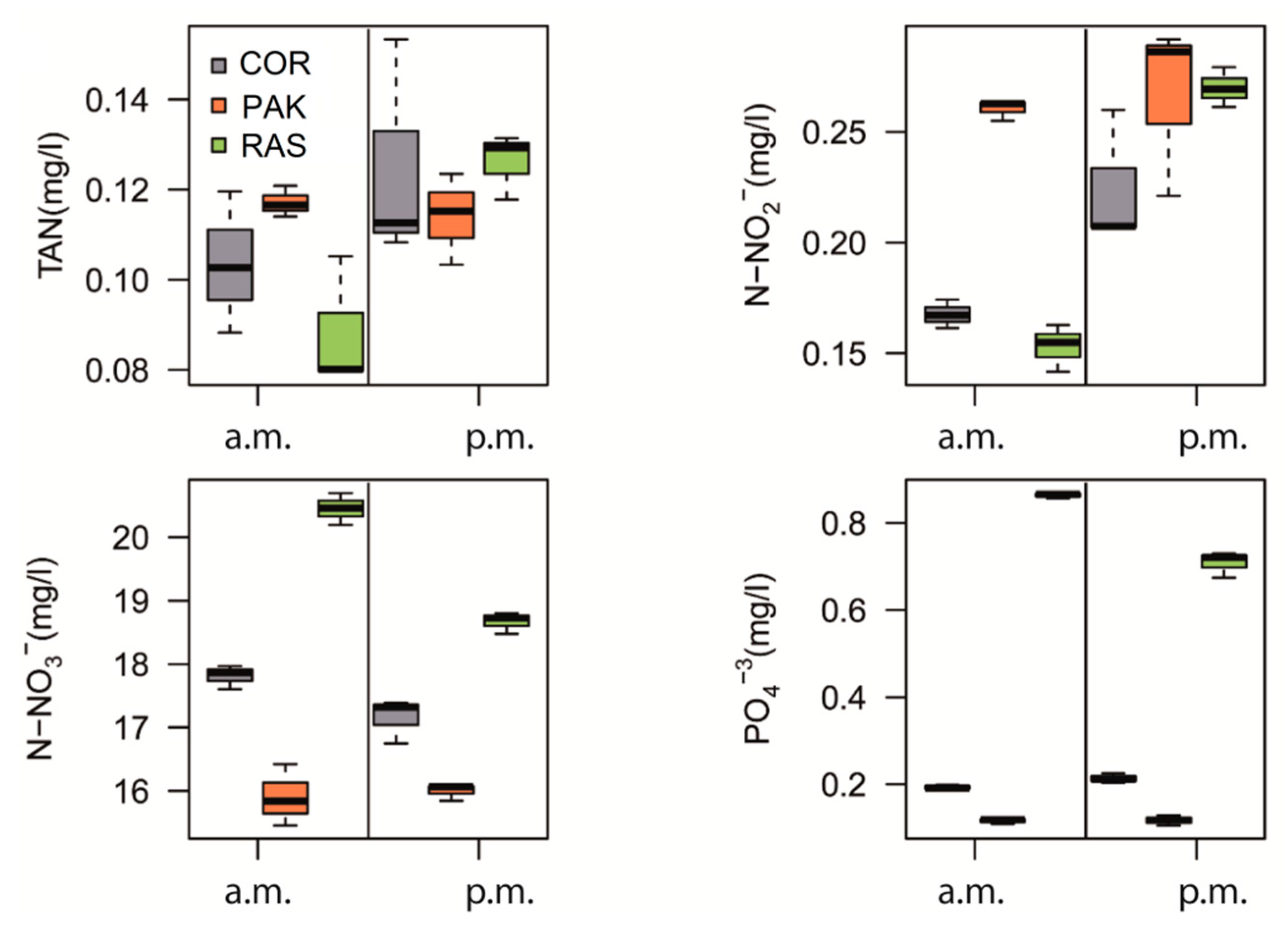
| Treatment | a.m. | p.m. |
|---|---|---|
| COR | 0.014 ± 0.001 ab,1 | 0.018 ± 0.004 a,1 |
| PAK | 0.018 ± 0.002 b,1 | 0.020 ± 0.003 a,1 |
| RAS | 0.009 ± 0.001 a,1 | 0.015 ± 0.001 a,2 |
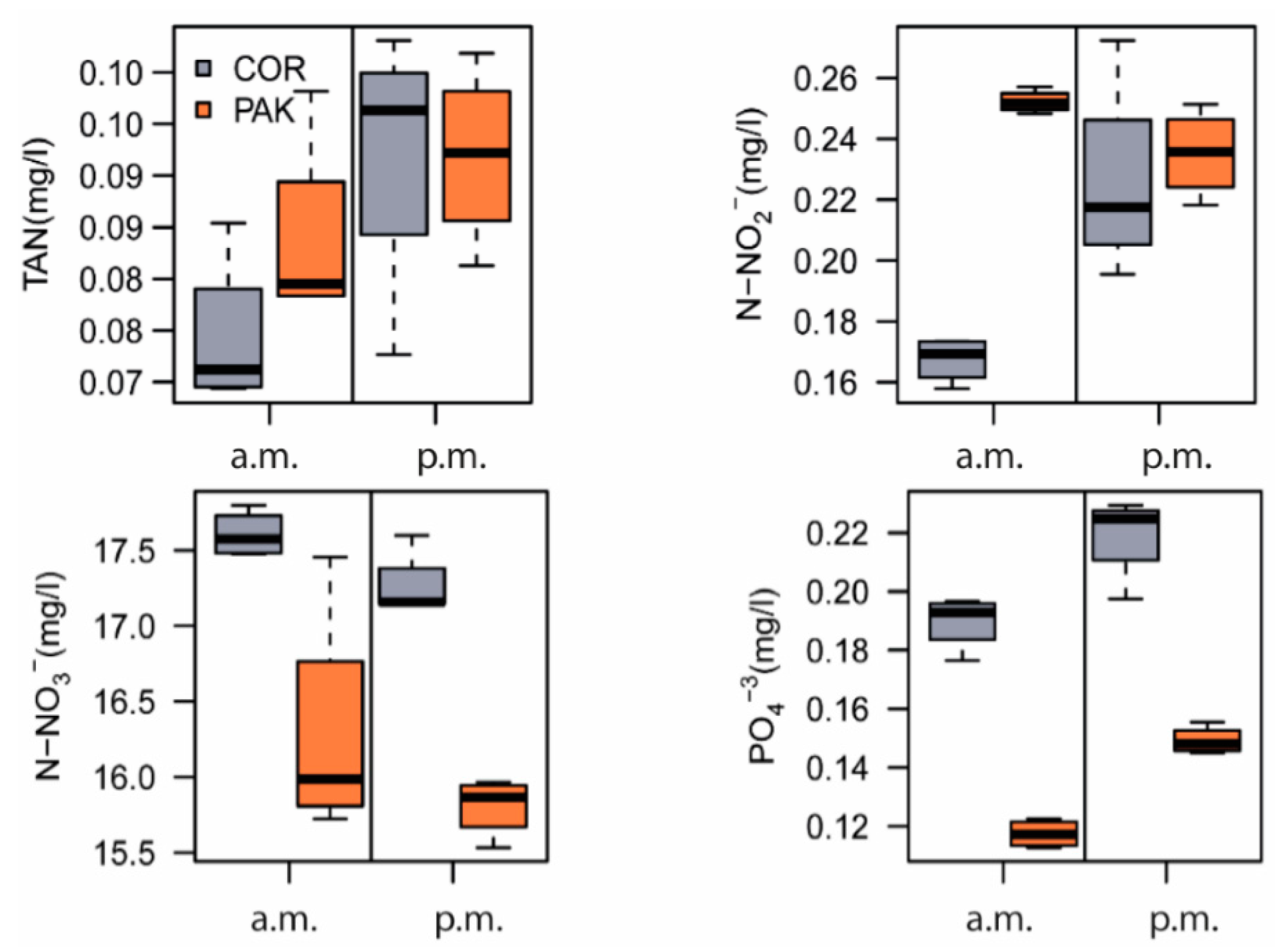
| Time | Treatment | TAN (mg/L) | NO2−–N (mg/L) | NO3−–N (mg/L) | PO43− (mg/L) |
|---|---|---|---|---|---|
| a.m. | COR | 0.079 ± 0.007 a,1 | 0.167 ± 0.008 a,1 | 17.606 ± 0.154 a,1 | 0.190 ± 0.009 b,2 |
| PAK | 0.089 ± 0.004 b,1 | 0.252 ± 0.010 b,1 | 16.287 ± 0.792 a,1 | 0.117 ± 0.005 a,2 | |
| p.m. | COR | 0.097 ± 0.033 a,1 | 0.226 ± 0.013 a,1 | 17.264 ± 0.224 b,1 | 0.219 ± 0.015 b,1 |
| PAK | 0.097 ± 0.014 a,1 | 0.235 ± 0.009 a,1 | 15.806 ± 0.195 a,1 | 0.149 ± 0.005 a,1 |
4. Discussion
4.1. Fish and Plant Production
4.2. Water Quality Parameters
4.3. Water Treatment and Nutrient Uptake
Fish Culture
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Turcios, A.E.; Papenbrock, J. Sustainable Treatment of Aquaculture Effluents—What Can We Learn from the Past for the Future? Sustainability 2014, 6, 836–856. [Google Scholar] [CrossRef]
- Frankic, A.; Hershner, C. Sustainable aquaculture: Developing the promise of aquaculture. Aquac. Int. 2003, 11, 517–530. [Google Scholar] [CrossRef]
- Poot-López, G.R.; Hernández, J.M.; Gasca-Leyva, E. Input management in integrated agriculture-aquaculture systems in Yucatan: Tree spinach leaves as a dietary supplement in Tilapia culture. Agric. Syst. 2010, 103, 98–104. [Google Scholar] [CrossRef]
- Altieri, M.A. Agroecology: Principles and Strategies for Designing Sustainable Farming Systems; Earthscan: London, UK, 2002; pp. 40–46. [Google Scholar]
- Rakocy, J.E.; Masser, M.P.; Losordo, T.M. Recirculating Aquaculture Tank Production Systems: Aquaponics-Integrating Fish and Plant Culture; SRAC Publication: Stoneville, MS, USA, 2006. [Google Scholar]
- Lennard, W.; Leonard, B. A Comparison of Three Different Hydroponic Sub-systems (gravel bed, floating and nutrient film technique) in an Aquaponic Test System. Aquac. Int. 2006, 14, 539–550. [Google Scholar] [CrossRef]
- Rakocy, J.E.; Bailey, D.S.; Shultz, R.C.; Thoman, E.S. Update on Tilapia and Vegetable Production in the UVI Aquaponic System. In Proceedings of the New Dimensions on Farmed Tilapia: Sixth International Symposium on Tilapia in Aquaculture, Manila, Philippines, 12–16 September 2004; pp. 12–16.
- Ghaly, A.E.; Kamal, M.; Mahmoud, N.S. Phytoremediation of aquaculture wastewater for water recycling and production of fish feed. Environ. Int. 2005, 31, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Al-Hafedh, Y.S.; Alam, A.; Beltagi, M.S. Food Production and Water Conservation in a Recirculating Aquaponic System in Saudi Arabia at Different Ratios of Fish Feed to Plants. J. World Aquac. Soc. 2008, 39, 510–520. [Google Scholar] [CrossRef]
- Resh, H.M. Cultivos Hidropónicos: Nuevas Técnicas de Producción; Mundi-Prensa: Madrid, Spain, 2001. [Google Scholar]
- Kratky, B. Three non-circulating hydroponic methods for growing lettuce. In Proceedings of the International Symposium on Soilless Culture and Hydroponics, Lima, Peru, 25–28 August 2008; Volume 843, pp. 65–72.
- Kao, T.-C.; Hsiang, T.; Changhua, R. The Dynamic Root Floating Hydroponic Technique: Year-Round Production of Vegetables in Roc on Taiwan; ASPAC Food & Fertilizer Technology Center: Taipei, Taiwan, 1991. [Google Scholar]
- Council of Agriculture, Executive Yuan, Republic of China. Dynamic Root Floating System for Leafy Vegetable Production. Available online: http://flora.coa.gov.tw/view_eng.php?id=244 (accessed on 15 July 2015).
- Ako, H. How to Build and Operate a Simple Small-to-Large Scale Aquaponics System; College of Tropical Agriculture and Human Resources (CTAHR) University of Hawaii: Honolulu, HI, USA, 2014. [Google Scholar]
- Remy, M.; Singh, B.K.; Taylor-Rieger, R. Evaluación de dos Técnicas Hidropónicas Adaptadas Para las Condiciones del Trópico Húmedo. Tierra Trop. 2005, 2, 29–35. [Google Scholar]
- Hazeri, N.; Valizadeh, J.; Shakeri, A.; Rajabpour, M. Evaluation of Essential Oil and Mineral Composition of Coriander (Coriandrum sativum L.) among Growth Conditions of Hydroponic, Field and Greenhouse. J. Essent. Oil Bear. Plants 2012, 15, 949–954. [Google Scholar] [CrossRef]
- Solano, K.D.G.; Mendoza, M.D.; Trejo-Téllez, L.I.; Cue, J.L.G.; Escudero, J.S. Efluente y té de vermicompost en la producción de hortalizas de hoja en sistema NFT. Interciencia 2013, 38, 863–869. [Google Scholar]
- Sánchez del Castillo, F.E.R.; Edgardo, R. Un Sistema de Producción de Plantas, Hidroponia: Principios y Métodos de Cultivo; Universidad Autónoma Chapingo: Chapingo, Mexico, 2006. [Google Scholar]
- USDA National Nutrient Database for Standard Reference, Release 27, Full Report (All Nutrients): 11116, Cabbage, Chinese (pak-choi), Raw. Available online: http://ndb.nal.usda.gov/ndb/foods/show/2931?fg=&man=&lfacet=&count=&max=35&sort=&qlookup=pak+choi&offset=&format=Full&new=&measureby= (accessed on 4 September 2015).
- Emerson, K.; Russo, R.C.; Lund, R.E.; Thurston, R.V. Aqueous Ammonia Equilibrium Calculations: Effect of pH and Temperature. J. Fish. Res. Board Can. 1975, 32, 2379–2383. [Google Scholar] [CrossRef]
- El-Shafai, S.A.; El-Gohary, F.A.; Nasr, F.A.; van der Steen, N.P.; Gijzen, H.J. Chronic ammonia toxicity to duckweed-fed tilapia (Oreochromis niloticus). Aquaculture 2004, 232, 117–127. [Google Scholar] [CrossRef]
- El-Sherif, M.; El-Feky, A.M. Effect of ammonia on Nile tilapia (O. niloticus) performance and some hematological and histological measures. In Proceedings of the 8th International Symposium on Tilapia in Aquaculture, Cairo, Egypt, 12–14 October 2008; pp. 513–530.
- Yildiz, H.Y.; Köksal, G.; Borazan, G.; Benli, Ç.K. Nitrite-induced methemoglobinemia in Nile tilapia, Oreochromis niloticus. J. Appl. Ichthyol. 2006, 22, 427–426. [Google Scholar] [CrossRef]
- Espinosa Moya, E.A.; Angel Sahagún, C.A.; Mendoza Carrillo, J.M.; Albertos Alpuche, P.J.; Álvarez-González, C.A.; Martínez-Yáñez, R. Herbaceous plants as part of biological filter for aquaponics system. Aquac. Res. 2014. [Google Scholar] [CrossRef]
- Hu, S.-Y. Food Plants of China; Chinese University Press: Hong Kong, China, 2005. [Google Scholar]
- Ronzio, R.A. The Encyclopedia of Nutrition and Good Health; Infobase Publishing: New York, NY, USA, 2003. [Google Scholar]
- FAO Ecocrop Database. Available online: http://ecocrop.fao.org/ecocrop/srv/en/cropView?id=547 (accessed on 17 July 2015).
- Kotzen, B.; Appelbaum, S. An Investigation of Aquaponics Using Brackish Water Resources in the Negev Desert. J. Appl. Aquac. 2010, 22, 297–320. [Google Scholar] [CrossRef]
- Garibaldi, A.; Gilardi, G.; Gullino, M.L. First Report of Collar and Root Rot Caused by Pythium ultimum on Coriander in Italy. Plant Dis. 2010, 94, 1167. [Google Scholar] [CrossRef]
- Madia, M.; Gaetan, S. Podredumbre basal y radicular del Coriandro causada por Rhizoctonia solani Khun. Bol. Sanid. Veg. Plagas (España) 1995, 21, 573–576. [Google Scholar]
- Endut, A.; Jusoh, A.; Ali, N.; Wan Nik, W.N.S.; Hassan, A. Effect of flow rate on water quality parameters and plant growth of water spinach (Ipomoea aquatica) in an aquaponic recirculating system. Desalination Water Treat. 2009, 5, 19–28. [Google Scholar] [CrossRef]
- Endut, A.; Jusoh, A.; Ali, N.; Wan Nik, W.B.; Hassan, A. A study on the optimal hydraulic loading rate and plant ratios in recirculation aquaponic system. Bioresour. Technol. 2010, 101, 1511–1517. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Lee, J.W.; Chandran, K.; Kim, S.; Brotto, A.C.; Khanal, S.K. Effect of plant species on nitrogen recovery in aquaponics. Bioresour. Technol. 2015, 188, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Hernández, M.; Gasca-Leyva, E.; Milstein, A. Polyculture of mixed-sex and male populations of Nile tilapia (Oreochromis niloticus) with the Mayan cichlid (Cichlasoma urophthalmus). Aquaculture 2014, 418–419, 26–31. [Google Scholar] [CrossRef]
- Roosta, H.R.; Mohsenian, Y. Effects of foliar spray of different Fe sources on pepper (Capsicum annum L.) plants in aquaponic system. Sci. Hortic. 2012, 146, 182–191. [Google Scholar] [CrossRef]
- Roosta, H.R.; Mohsenian, Y. Alleviation of Alkalinity-Induced Fe Deficiency in Eggplant (Solanum melongena L.) by Foliar Application of Different Fe Sources in Recirculating System. J. Plant Nutr. 2015, 38, 1768–1786. [Google Scholar] [CrossRef]
- Wanitprapha, K.; Huggins, C.A.; Nakamoto, S.T.; Won, B.; Pak, C. Economic Fact Sheet #18. Available online: http://www.ctahr.hawaii.edu/sustainag/extn_pub/veggie%20pubs/Oriental%20Vegetables/Won%20Bok%20and%20Pak%20Choi.pdf (accessed on 17 November 2015).
- Wong, J.W.C.; Li, G.X.; Wong, M.H. The growth of Brassica chinensis in heavy-metal-contaminated sewage sludge compost from Hong Kong. Bioresour. Technol. 1996, 58, 309–313. [Google Scholar] [CrossRef]
- Žutić, I.; Borošić, J.; Toth, N.; Novak, B.; Dobričević, N. Agronomic and Dietary Value of Pak Choy (Brassica rapa SSP. Chinensis) in Different Growing Periods. Acta Hortic. 2007, 729, 239–243. [Google Scholar] [CrossRef]
- El-Sayed, A.-F.M. Tilapia Culture; CABI: Wallingford, UK, 2006. [Google Scholar]
- Rakocy, J.E. Aquaponics: Vegetable hydroponics in recirculating systems. In Recirculating Aquaculture Systems, 2nd ed.; Timmons, M.B., Ebeling, J.M., Wheaton, F.W., Summerfelt, S.T., Vinci, B.J., Eds.; Northeast Regional Aquaculture Center: College Park, MD, USA, 2002; pp. 631–672. [Google Scholar]
- Antoniou, P.; Hamilton, J.; Koopman, B.; Jain, R.; Holloway, B.; Lyberatos, G.; Svoronos, S.A. Effect of temperature and ph on the effective maximum specific growth rate of nitrifying bacteria. Water Res. 1990, 24, 97–101. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, L.; Gasca-Leyva, E.; Escalante, E.; Fitzsimmons, K.M.; Lozano, D.V. Evaluation of Biomass Yield and Water Treatment in Two Aquaponic Systems Using the Dynamic Root Floating Technique (DRF). Sustainability 2015, 7, 15384-15399. https://doi.org/10.3390/su71115384
Silva L, Gasca-Leyva E, Escalante E, Fitzsimmons KM, Lozano DV. Evaluation of Biomass Yield and Water Treatment in Two Aquaponic Systems Using the Dynamic Root Floating Technique (DRF). Sustainability. 2015; 7(11):15384-15399. https://doi.org/10.3390/su71115384
Chicago/Turabian StyleSilva, Laura, Eucario Gasca-Leyva, Edgardo Escalante, Kevin M. Fitzsimmons, and David Valdés Lozano. 2015. "Evaluation of Biomass Yield and Water Treatment in Two Aquaponic Systems Using the Dynamic Root Floating Technique (DRF)" Sustainability 7, no. 11: 15384-15399. https://doi.org/10.3390/su71115384
APA StyleSilva, L., Gasca-Leyva, E., Escalante, E., Fitzsimmons, K. M., & Lozano, D. V. (2015). Evaluation of Biomass Yield and Water Treatment in Two Aquaponic Systems Using the Dynamic Root Floating Technique (DRF). Sustainability, 7(11), 15384-15399. https://doi.org/10.3390/su71115384






