Genetically-Improved Tilapia Strains in Africa: Potential Benefits and Negative Impacts
Abstract
:1. The Importance of Aquaculture to Africa’s Development
2. Tilapia Characteristics and Production
3. Social Benefits of Tilapia
4. Distribution of Tilapias
5. Background to Genetic Improvement of Tilapias
6. The Genetic Improvement of Farmed Tilapia (GIFT) Project
7. The GIFT/GenoMar Supreme (GST) Strain
8. Dissemination of GIFT/GST Strains in Africa
Sharing the benefits arising from the utilization of genetic resources in a fair and equitable way, including by appropriate access to the genetic resources and by appropriate transfer of relevant technologies, taking into account all rights over those resources and to technologies, and by appropriate funding, thereby contributing to the conservation of biological diversity and the sustainable use of its components.[43]
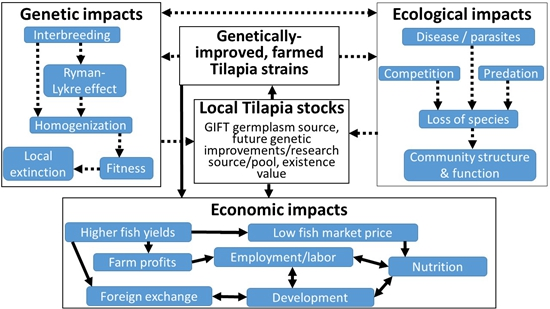
9. Potential Ecological Impacts of Tilapia Introductions
10. Potential Genetic Impacts of Tilapia Genetic Improvement on Native Populations
11. Potential Economic Benefits of the GIFT Strain in Africa; Case Study of Ghana
11.1. Model
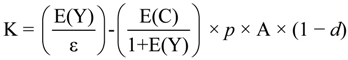
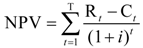
11.2. Data and Key Assumptions
11.3. Analysis
| Variable | Minimum | Most likely | Maximum |
|---|---|---|---|
| Recurrent costs ($) | 30,000 | 60,000 | 90,000 |
| Increase in production costs (%) | 10 | 20 | 30 |
| 2012 Nile tilapia aquaculture production (metric tons) | 26,200 | 28,200 | 30,200 |
| Peak adoption rate (%) | 60 | 70 | 80 |
| Yield change (%) | 24 | 41.4 | 61 |
11.4. Results
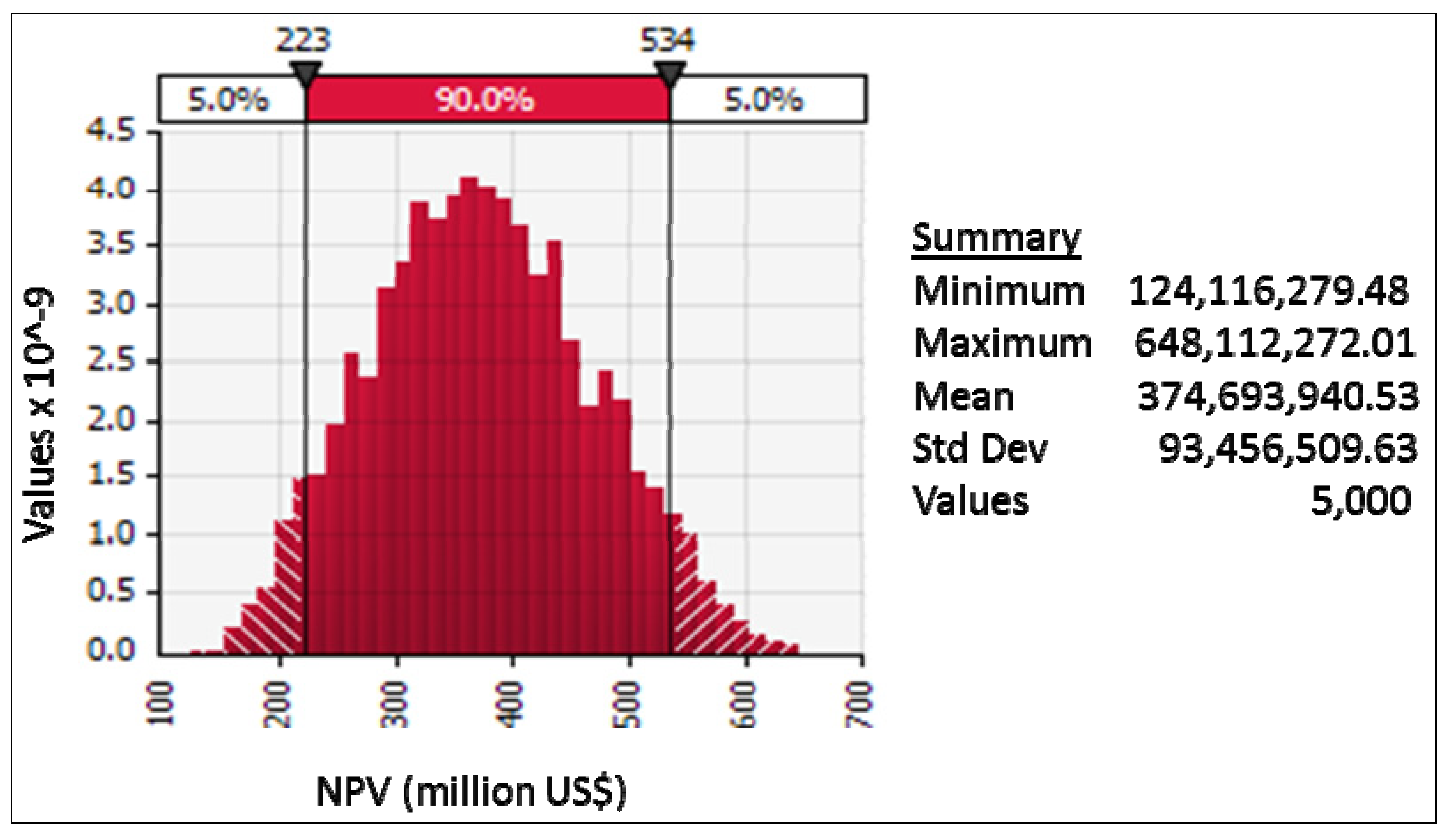
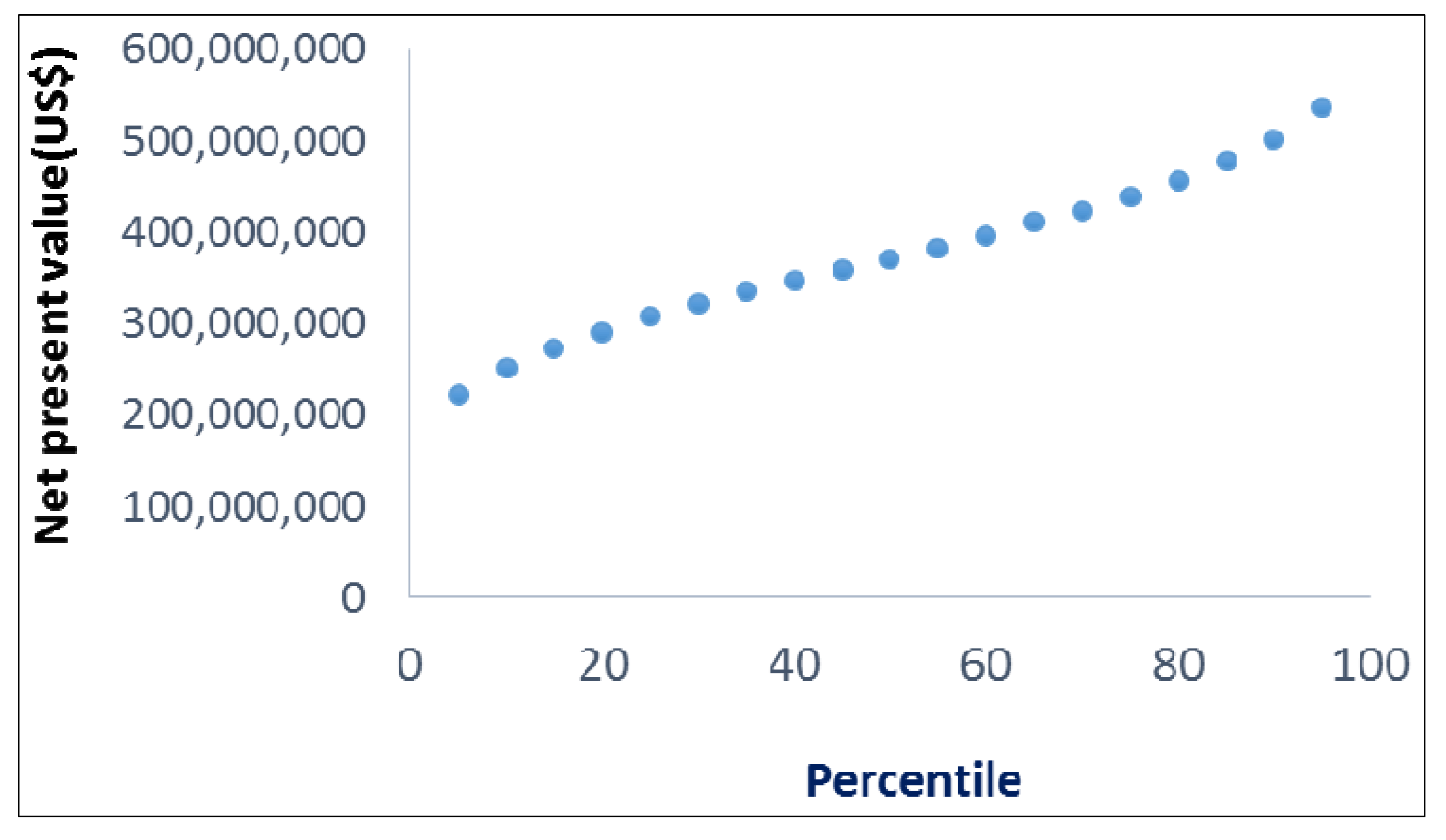
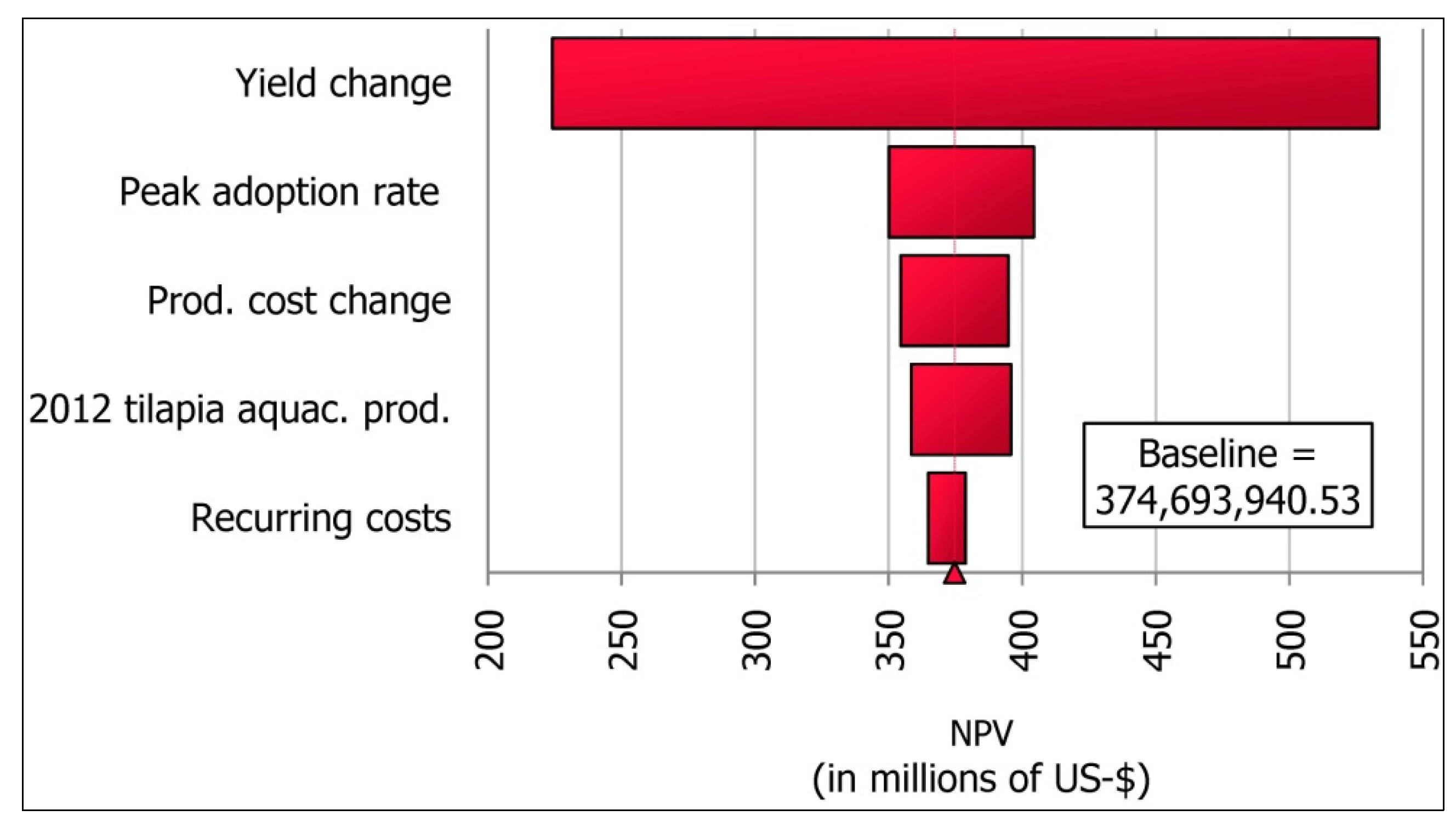
11.5. Discussion
12. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References and Notes
- Subasinghe, R.; Soto, D.; Jia, J. Global aquaculture and its role in sustainable development. Rev. Aquac. 2009, 1, 2–9. [Google Scholar] [CrossRef]
- Béné, C.; Heck, S. Fish and food security in Africa. NAGA WorldFish Center Q. 2005, 28, 8–13. [Google Scholar]
- Brummett, R.E.; Angoni, D.E.; Pouomogne, V. On-farm and on-station comparison of wild and domesticated Cameroonian populations of Oreochromis niloticus. Aquaculture 2004, 242, 157–164. [Google Scholar] [CrossRef]
- Lind, C.E.; Brummett, R.E.; Ponzoni, R.W. Exploitation and conservation of fish genetic resources in Africa: Issues and priorities for aquaculture development and research. Rev. Aquac. 2012, 4, 125–141. [Google Scholar] [CrossRef]
- Ponzoni, R.W.; Nguyen, N.H.; Khaw, H.L.; Hamzah, A.; Bakar, K.R.A.; Yee, H.Y. Genetic improvement of Nile tilapia (Oreochromis niloticus) with special reference to the work conducted by the Worldfish Center with the GIFT strain. Rev. Aquac. 2011, 3, 27–41. [Google Scholar] [CrossRef]
- Greer, D.S.; Harvey, B.J. Blue Genes: Sharing and Conserving the World’s Aquatic Biodiversity; Earthscan: London, UK, 2004. [Google Scholar]
- Teichert-Coddington, D.; Popma, T.; Lovshin, L. Attributes of tropical pond-cultured fish. In Dynamics of Pond Aquaculture; Egna, H.S., Boyd, C.E., Eds.; CRC Press: Boca Raton, FL, USA, 1997; pp. 183–198. [Google Scholar]
- El-Sayed, A.F.M. Tilapia Culture; CABI: Cambridge, MA, USA, 2006. [Google Scholar]
- Fitzsimmons, K. Potential to increase global tilapia production. In Global Outlook for Aquaculture Leadership; GOAL Conference: Kuala Lumpur, Malaysia, 2010. [Google Scholar]
- Food and Agriculture Organization. The State of World Fisheries and Aquaculture; Food and Agriculture Organization (United Nations): Rome, Italy, 2010; p. 218. [Google Scholar]
- Shelton, W.L.; Popma, T.J. Biology. In Tilapia: Biology, Culture, and Nutrition; Lim, C.E., Webster, C.D., Eds.; Haworth Press: Binghamton, NY, USA, 2006; pp. 1–49. [Google Scholar]
- Food and Agriculture Organization. State of World Fisheries and Aquaculture; Food and Agriculture Organisation: Rome, Italy, 2012; p. 209. [Google Scholar]
- Fitzsimmons, K. Prospect and potential for global production. In Tilapia: Biology, Culture, and Nutrition; Lim, C., Webster, C., Eds.; Harworth Press: Binghamton, NY, USA, 2006; pp. 51–72. [Google Scholar]
- Pullin, R. Tilapia: Everyman’s fish. Biologist 1985, 32, 84–88. [Google Scholar]
- Gjoen, H. GIFT Program Continues: Distribution of Fast-Growing Tilapia to Expand. Available online: http://pdf.gaalliance.org/pdf/GAA-Gj%F8en-Dec01.pdf (accessed on 30 May 2014).
- McAndrew, B.J. Evolution, phylogenetic relationships and biogeography. In Tilapias: Biology and Exploitation; Beveridge, M.C.M., McAndrew, B.J., Eds.; Kluwer Academic: Dordrecht, The Netherlands, 2000; pp. 1–32. [Google Scholar]
- Pillay, T.V.R.; Kutty, M. Aquaculture: Principles and Practices; Blackwell Publishing: Oxford, UK, 2005. [Google Scholar]
- Canonico, G.C.; Arthington, A.; McCrary, J.K.; Thieme, M.L. The effects of introduced tilapias on native biodiversity. Aquat. Conserv.: Mar. Freshw. Ecosyst. 2005, 15, 463–483. [Google Scholar] [CrossRef]
- Fish Base Tilapia spp. Available online: http://www.fishbase.org/Nomenclature/ScientificNameSearchList.php? (accessed on 14 December 2013).
- Courtenay, W. Tilapias as non-indigenous species in the Americas: Environmental, regulatory and legal issues. Tilapia Aquac. Am. 1997, 1, 18–33. [Google Scholar]
- Pullin, R.S.V.; Palomares, M.; Casal, C.; Dey, M.; Pauly, D. Environmental impacts of tilapia. In Fourth International Symposium on Tilapia in Aquaculture; Fitzsimmons, K., Ed.; Northeast Regional Aquacultural Engineering Services: Ithaca, NY, USA, 1997; pp. 554–570. [Google Scholar]
- De Silva, S.S.; Subasinghe, R.P.; Bartley, D.M.; Lowther, A. Tilapias as Alien Aquatics in Asia and the Pacific: A Review; Food and Agriculture Organization: Rome, Italy, 2004; Volume 453. [Google Scholar]
- Ponzoni, R.W.; Nguyen, N.H.; Khaw, H.L. Investment appraisal of genetic improvement programs in Nile tilapia (Oreochromis niloticus). Aquaculture 2007, 269, 187–199. [Google Scholar] [CrossRef]
- Lutz, C.G. Recent directions in genetics. In Tilapia: Biology, Culture, and Nutrition; Lim, C.E., Webster, C.D., Eds.; Haworth Press: Binghamton, NY, USA, 2006; pp. 139–180. [Google Scholar]
- Poompuang, S.; Hallerman, E.M. Toward detection of quantitative trait loci and marker-assisted selection in fish. Rev. Fish. Sci. 1997, 5, 253–277. [Google Scholar] [CrossRef]
- Attipoe, F.Y.K.; Agyakwah, S.K. Status of Catfish Farming and Research in Ghana. In Proceedings of the a Workshop on the Development of a Genetic Improvement Program for African Catfish Clarias gariepinus, Accra, Ghana, 5–9 November 2007; Ponzoni, R.W., Nguyen, N.H., Eds.; p. 23.
- Attipoe, F.Y.; Blay Jnr, J.; Agyakwah, S.; Ponzoni, R.W.; Khaw, H.L.; Abban, E.K. Genetic parameters and response to selection in the development of Akosombo strain of the Nile tilapia (Oreochromis niloticus) in the Volta Basin, Ghana. In Proceedings of the International Symposium on Tilapia in Aquaculture, Jerusalem, Palestine, 6–10 October 2013.
- Ofori, J.; Abban, E.; Karikari, A.; Brummett, R. Production parameters and economics of small-scale tilapia cage aquaculture in the Volta Lake, ghana. J. Appl. Aquac. 2010, 22, 337–351. [Google Scholar] [CrossRef]
- Bentsen, H.B.; Gjerde, B.; Nguyen, N.H.; Rye, M.; Ponzoni, R.W.; Palada de Vera, M.S.; Bolivar, H.L.; Velasco, R.R.; Danting, J.C.; Dionisio, E.E. Genetic improvement of farmed tilapias: Genetic parameters for body weight at harvest in Nile tilapia (Oreochromis niloticus) during five generations of testing in multiple environments. Aquaculture 2012, 338, 56–65. [Google Scholar]
- Dey, M.M.; Gupta, M.V. Socioeconomics of disseminating genetically improved Nile tilapia in Asia: An introduction. Aquac. Econ. Manag. 2000, 4, 5–11. [Google Scholar] [CrossRef]
- Eknath, A.; Acosta, B. Genetic Improvement of Farmed Tilapias (GIFT) Project: Final Report, March 1988 to December 1997; World Fish Center: Penang, Malaysia, 1998. [Google Scholar]
- Asian Development Bank. An Impact Evaluation of the Development of Genetically Improved Farmed Tilapia and Their Dissemination in Selected Countries; Asian Development Bank: Manila, Phillippines, 2005. [Google Scholar]
- Asian Development Bank. Overview of Freshwater Aquaculture of Tilapia in the Philippines; Asian Development Bank: Manila, Phillippines, 2004. [Google Scholar]
- Eknath, A.E.; Tayamen, M.M.; Palada-de Vera, M.S.; Danting, J.C.; Reyes, R.A.; Dionisio, E.E.; Capili, J.B.; Bolivar, H.L.; Abella, T.A.; Circa, A.V. Genetic improvement of farmed tilapias: The growth performance of eight strains of Oreochromis niloticus tested in different farm environments. Aquaculture 1993, 111, 171–188. [Google Scholar] [CrossRef]
- Gupta, M.V.; Acosta, B.O.; Eknath, A.E.; Dey, M.M. Breakthrough in genetic improvement of tropical finfish through partnership between ICLARM, ASI and developing country NARS. Available online: http://www.fao.org/docs/eims/upload/206603/3_8_cases.PDF (accessed on 30 May 2014).
- Acosta, B.O.; Gupta, M.V. The genetic improvement of farmed tilapias project: Impact and lessons learned. In Success Stories in Asian Aquaculture; De Silva, S.S., Davy, F.B., Eds.; Springer: London, UK, 2010; pp. 149–171. [Google Scholar]
- Dey, M.M. The impact of genetically improved farmed Nile tilapia in Asia. Aquac. Econ. Manag. 2000, 4, 107–124. [Google Scholar] [CrossRef]
- Gupta, M.; Acosta, B. From drawing board to dining table: The success story of the GIFT project. NAGA WorldFish Center Q. 2004, 27, 4–14. [Google Scholar]
- Dey, M.M.; Eknath, A.E. Current Trends in the Asian Tilapia Industry and the Significance of Genetically Improved Tilapia Breeds; INFOFISH: Kuala Lumpur, Malaysia, 1997. [Google Scholar]
- Gupta, M.V.; Bartley, D.M.; Acosta, B.O. Use of Genetically Improved and Alien Species for Aquaculture and Conservation of Aquatic Biodiversity in Africa; The WorldFish Center: Penang, Malaysia, 2004; Volume 68. [Google Scholar]
- Gupta, M.V. Genetic enhancement and conservation of aquatic biodiversity in Africa. NAGA WorldFish Center Q. 2002, 25, 48–53. [Google Scholar]
- WorldFish Center. Policy on the transfer of genetically improved farm tilapia (GIFT) from Asia to Africa by the Worldfish Center. In The WorldFish Center Working Papers; WorldFish Center: Penang, Malaysia, 2007. [Google Scholar]
- Convention on Biological Diversity. The Nagoya Protocol on Access and Benefit-Sharing. Available online: http://www.cbd.int/abs/ (accessed on 21 March 2014).
- Ansah, Y.B.; Frimpong, E.A.; Amisah, S. Biological assessment of aquaculture effects on effluent-receiving streams in Ghana using structural and functional composition of fish and macroinvertebrate assemblages. Environ. Manag. 2012, 50, 166–180. [Google Scholar] [CrossRef]
- Volpe, J.P.; Taylor, E.B.; Rimmer, D.W.; Glickman, B.W. Evidence of natural reproduction of aquaculture-escaped Atlantic salmon in a coastal British Columbia river. Conserv. Biol. 2000, 14, 899–903. [Google Scholar] [CrossRef]
- Naylor, R.L.; Williams, S.L.; Strong, D.R. Aquaculture—A gateway for exotic species. Science 2001, 294, 1655–1656. [Google Scholar] [CrossRef]
- Breder, C.M.; Rosen, D.E. Modes of Reproduction in Fishes; The American Museum of Natural History: New York, NY, USA, 1966. [Google Scholar]
- Balon, E.K. Reproductive guilds of fishes: A proposal and definition. J. Fish. Res. Board Can. 1975, 32, 821–864. [Google Scholar] [CrossRef]
- Arthington, A.; Blüdhorn, D.; Kennard, M. Food resource partitioning by the introduced cichlid, Oreochromis mossambicus, and two native fishes in a subtropical Australian impoundment. In Proceedings of the Third Asian Fisheries Forum, Singapore, 26–30 October 1994; pp. 425–428.
- Crutchfield, J.U., Jr. Establishment and expansion of redbelly tilapia and blue tilapia in a power plant cooling reservoir. Am. Fish. Soc. Symp. 1995, 15, 452–461. [Google Scholar]
- McCrary, J.; van den Berghe, E.; McKaye, K.; Lopez Perez, L. Tilapia cultivation: A threat to native fish species in Nicaragua. Encuentro 2001, 58, 9–19. [Google Scholar]
- Starling, F.; Lazzaro, X.; Cavalcanti, C.; Moreira, R. Contribution of omnivorous tilapia to eutrophication of a shallow tropical reservoir: Evidence from a fish kill. Freshw. Biol. 2002, 47, 2443–2452. [Google Scholar] [CrossRef]
- Moreau, J. A review of introductions of tilapia in open waters of Africa, their influence on ecology and fisheries. In Proceedings of the International Symposium on Tilapia in Aquaculture; Fishelson, L., Yaron, Z., Eds.; Tel Aviv University Press: Nazareth, Israel, 1983; pp. 77–85. [Google Scholar]
- Vareschi, E.; Jacobs, J. The ecology of Lake Nakuru. Oecologia 1985, 65, 412–424. [Google Scholar] [CrossRef]
- Noga, E.J. Fish Disease: Diagnosis and Treatment, 2nd ed.; John Wiley & Sons: Ames, IA, USA, 2010; p. 519. [Google Scholar]
- Zambrano, L.; Martínez-Meyer, E.; Menezes, N.; Peterson, A.T. Invasive potential of common carp (Cyprinus carpio) and Nile tilapia (oreochromis niloticus) in American freshwater systems. Can. J. Fish. Aquat. Sci. 2006, 63, 1903–1910. [Google Scholar] [CrossRef]
- OIE Aquatic animal health. Available online: http://www.oie.int/en/international-standard-setting/aquatic-code/access-online/ (accessed on 20 March 2014).
- Carvalho, G.R.; Hauser, L. Genetic impacts of fish introductions: A perspective on African lakes. In The Impact of Species Changes in African Lakes; Chapman and Hall: London, UK, 1995; pp. 457–493. [Google Scholar]
- Ryman, N.; Laikre, L. Effects of supportive breeding on the genetically effective population size. Conserv. Biol. 1991, 5, 325–329. [Google Scholar] [CrossRef]
- Hallerman, E.M. Population Genetics: Principles and Applications for Fisheries Scientists; Hallerman, E.M., Ed.; American Fisheries Society: Bethesda, MD, USA, 2003; Hallerman, E.M., Ed.; pp. 239–259. [Google Scholar]
- Ferguson, A.; Fleming, I.A.; Hindar, K.; Skaala, O.; McGinnity, P.; Cross, T.; Prodohl, P. The Atlantic Salmon: Genetic Conservation and Management. Blackwell Publishing: Oxford, UK, 2007; Verspoor, E., Stradmeyer, L., Nielsen, J.L., Eds.; pp. 357–398. [Google Scholar]
- McGinnity, P.; Prodöhl, P.; Ferguson, A.; Hynes, R.; ó Maoiléidigh, N.; Baker, N.; Cotter, D.; O’Hea, B.; Cooke, D.; Rogan, G. Fitness reduction and potential extinction of wild populations of Atlantic salmon, Salmo salar, as a result of interactions with escaped farm salmon. Proc. R. Soc. Lond. Ser. B: Biol. Sci. 2003, 270, 2443–2450. [Google Scholar] [CrossRef]
- Araki, H.; Cooper, B.; Blouin, M.S. Genetic effects of captive breeding cause a rapid, cumulative fitness decline in the wild. Science 2007, 318, 100–103. [Google Scholar] [CrossRef]
- Araki, H.; Cooper, B.; Blouin, M.S. Carry-over effect of captive breeding reduces reproductive fitness of wild-born descendants in the wild. Biol. Lett. 2009, 5, 621–624. [Google Scholar] [CrossRef]
- Hindar, K.; Ryman, N.; Utter, F. Genetic effects of cultured fish on natural fish populations. Can. J. Fish. Aquat. Sci. 1991, 48, 945–957. [Google Scholar] [CrossRef]
- Tufto, J. Effects of releasing maladapted individuals: A demographic-evolutionary model. Am. Nat. 2001, 158, 331–340. [Google Scholar] [CrossRef]
- Tufto, J. Gene flow from domesticated species to wild relatives: Migration load in a model of multivariate selection. Evolution 2010, 64, 180–192. [Google Scholar] [CrossRef]
- Garant, D.; Forde, S.E.; Hendry, A.P. The multifarious effects of dispersal and gene flow on contemporary adaptation. Funct. Ecol. 2007, 21, 434–443. [Google Scholar]
- Storfer, A. Gene flow and endangered species translocation. Biol. Conserv. 1999, 87, 173–180. [Google Scholar] [CrossRef]
- Tallmon, D.A.; Luikart, G.; Waples, R.S. The alluring simplicity and complex reality of genetic rescue. Trends Ecol. Evol. 2004, 19, 489–495. [Google Scholar] [CrossRef]
- Hallerman, E. Application of risk analysis to genetic issues in aquaculture. In Understanding and Applying Risk Analysis in Aquaculture; FAO Fisheries and Aquaculture Technical Paper; Bondad-Reantaso, M.G., Arthur, J.R., Subasinghe, R.P., Eds.; Food and Agriculture Organization: Rome, Italy, 2008; Volume 519, pp. 47–66. [Google Scholar]
- Seward, N.W.; VerCauteren, K.C.; Witmer, G.W.; Engeman, R.M. Feral swine impacts on agriculture and the environment. Sheep Goat Res. J. 2004, 19, 34–40. [Google Scholar]
- McLeod, R.; Norris, A. Counting the Cost: Impact of Invasive Animals in Australia, 2004; Cooperative Research Centre for Pest Animal Control Canberra: Canberra, Australia, 2004. [Google Scholar]
- Muir, W.; Howard, R. Fitness components and ecological risk of transgenic release: A model using Japanese medaka (Oryzias latipes). Am. Nat. 2001, 158, 1–16. [Google Scholar]
- Pimm, S.L. The complexity and stability of ecosystems. Nature 1984, 307, 321–326. [Google Scholar] [CrossRef]
- Moyle, P.B.; Light, T. Biological invasions of fresh water: Empirical rules and assembly theory. Biol. Conserv. 1996, 78, 149–161. [Google Scholar] [CrossRef]
- Brummett, R.E.; Ponzoni, R.W. Concepts, alternatives, and environmental considerations in the development and use of improved strains of tilapia in African aquaculture. Rev. Fish. Sci. 2009, 17, 70–77. [Google Scholar] [CrossRef]
- Hallerman, E.; Hilsdorf, A.W.S. Conservation genetics of tilapias: Seeking to define appropriate units for management. Isr. J. Aquac.—Bamidgeh 2014, in press. [Google Scholar]
- Trewavas, E. Tilapiine Fishes of the Genera Sarotherodon, Oreochromis and Danakilia; British Museum (Natural History): London, UK, 1983. [Google Scholar]
- Philippart, J.-C.; Ruwet, J.-C. Ecology and distribution of tilapias. Biol. Cult. Tilapias 1982, 7, 15–60. [Google Scholar]
- Bezault, E.; Balaresque, P.; Toguyeni, A.; Fermon, Y.; Araki, H.; Baroiller, J.-F.; Rognon, X. Spatial and temporal variation in population genetic structure of wild Nile tilapia (Oreochromis niloticus) across Africa. BMC Genet. 2011, 12. [Google Scholar] [CrossRef] [Green Version]
- Nyingi, D.; de Vos, L.; Aman, R.; Agnèse, J.-F. Genetic characterization of an unknown and endangered native population of the Nile tilapia (Oreochromis niloticus (Linnaeus, 1758) (Cichlidae; Teleostei) in the Loboi Swamp (Kenya). Aquaculture 2009, 297, 57–63. [Google Scholar] [CrossRef]
- Navarro-Martín, L.; Vinas, J.; Ribas, L.; Diaz, N.; Gutierrez, A.; di Croce, L.; Piferrer, F. DNA methylation of the gonadal aromatase (cyp19a) promoter is involved in temperature-dependent sex ratio shifts in the European sea bass. PLoS Genet. 2011, 7, e1002447. [Google Scholar] [CrossRef] [Green Version]
- Hassanien, H.A.; Gilbey, J. Genetic diversity and differentiation of Nile tilapia (Oreochromis niloticus) revealed by DNA microsatellites. Aquac. Res. 2005, 36, 1450–1457. [Google Scholar] [CrossRef]
- Eknath, A.E.; Hulata, G. Use and exchange of genetic resources of Nile tilapia (Oreochromis niloticus). Rev. Aquac. 2009, 1, 197–213. [Google Scholar] [CrossRef]
- Convention on Biological Diversity—The Cartegena Protocol on Biosafety. Available online: http://bch.cbd.int/protocol/default.shtml (accessed on 20 March 2014).
- Kapuscinski, A.R.; Hayes, K.R.; Li, S.; Dana, G. Environmental Risk Assessment of Genetically Modified Organisms Volume 3: Methodologies for Transgenic Fish; CABI: Cambridge, MA, USA, 2007. [Google Scholar]
- Masters, A.; Coulibaly, B.; Sanogo, D.; Sidibé, M.; Williams, A. The Economic Impact of Agricultural Research: A Practical Guide; Department of Agricultural Economics, Purdue University: West Lafayette, IN, USA, 1996. [Google Scholar]
- Alston, J.M.; Norton, G.W.; Pardey, P.G. International Service for National Agricultural Research. In Science under Scarcity: Principles and Practice for Agricultural Research Evaluation and Priority Setting; Cornell University Press: Ithaca, NY, USA, 1998. [Google Scholar]
- Alpuerto, V.L.E.B.; Norton, G.W.; Alwang, J.; Ismail, A.M. Economic impact analysis of marker-assisted breeding for tolerance to salinity and phosphorous deficiency in rice. Appl. Econ. Perspect. Policy 2009, 31, 779–792. [Google Scholar]
- Rudi, N.; Norton, G.W.; Alwang, J.; Asumugha, G. Economic impact analysis of marker-assisted breeding for resistance to pests and post-harvest deterioration in cassava. Afr. J. Agric. Resour. Econ. 2010, 4, 110–122. [Google Scholar]
- Food and Agriculture Organization (FAO). FAOSTAT; FAO: Rome, Italy, 2012. [Google Scholar]
- Food and Agriculture Organization (FAO). Global Aquaculture Production 2013; FAO: Rome, Italy, 2013. [Google Scholar]
- Engle, C.R.; Neira, I. Tilapia Farm Business Management and Economics; Aquaculture CRSP, Oregon State University: Corvallis, OR, USA, 2005. [Google Scholar]
- Asian Development Bank. Special Evaluation Study on Small-Scale Freshwater Rural Aquaculture Development for Poverty Reduction; Asian Development Bank: Manila, Phillippines, 2004; p. 67. [Google Scholar]
- Padi, J.; ARDEC, Water Research Institute, Akosombo, Ghana. Personal communication, 2012.
- Palisade Corporation. @Risk, Edition 6 ed; Palisade Corporation: Ithaca, NY, USA, 2013. [Google Scholar]
- World Bank Data by Country; Ghana. The World Bank Group, 2013. Available online: http://data.worldbank.org/country/ghana (accessed on 6 July 2013).
- Overseas Development Institute. Ghana’s Sustained Agricultural Growth: Putting Underused Resources to Work; ODI Publications: London, UK, 2011; p. 27. [Google Scholar]
- Ghana Fisheries Commission. Ghana National Aquaculture Development Plan; Ghana Ministry of Food and Agriculture: Accra, Ghana, 2012; p. 85. [Google Scholar]
- Frimpong, E.A.; Anane-Taabeah, G. Social and Economic Performance of Tilapia Farming in Ghana; Junning, C., Quagrainie, K.K., Hishamunda, N., Eds.; FAO: Rome, Italy, 2014; in press. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Ansah, Y.B.; Frimpong, E.A.; Hallerman, E.M. Genetically-Improved Tilapia Strains in Africa: Potential Benefits and Negative Impacts. Sustainability 2014, 6, 3697-3721. https://doi.org/10.3390/su6063697
Ansah YB, Frimpong EA, Hallerman EM. Genetically-Improved Tilapia Strains in Africa: Potential Benefits and Negative Impacts. Sustainability. 2014; 6(6):3697-3721. https://doi.org/10.3390/su6063697
Chicago/Turabian StyleAnsah, Yaw B., Emmanuel A. Frimpong, and Eric M. Hallerman. 2014. "Genetically-Improved Tilapia Strains in Africa: Potential Benefits and Negative Impacts" Sustainability 6, no. 6: 3697-3721. https://doi.org/10.3390/su6063697
APA StyleAnsah, Y. B., Frimpong, E. A., & Hallerman, E. M. (2014). Genetically-Improved Tilapia Strains in Africa: Potential Benefits and Negative Impacts. Sustainability, 6(6), 3697-3721. https://doi.org/10.3390/su6063697